Peroxidase-Mimicking Nanozymes of Nitrogen Heteroatom-Containing Graphene Oxide for Biomedical Applications
Abstract
1. Introduction
2. Overview of the Graphene Oxide, Its Derivatives, and Peroxidase-like Activity
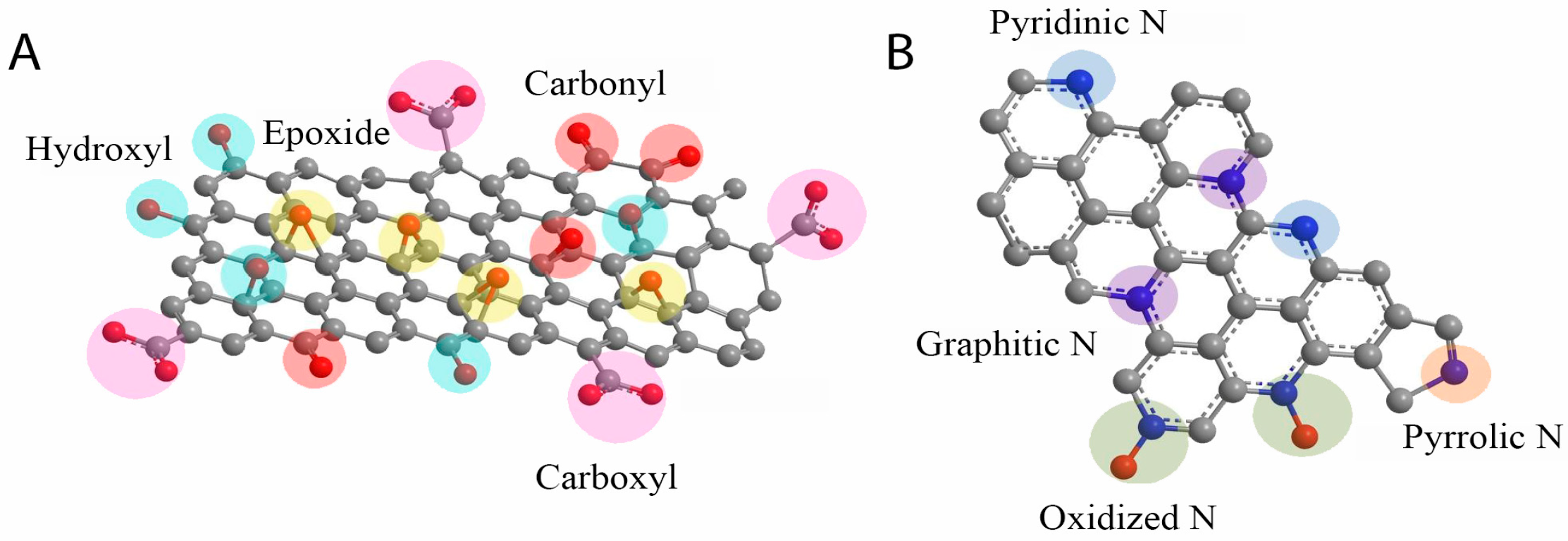
2.1. Overview of Graphene Oxide
2.2. Overview of Peroxidase-like Activity
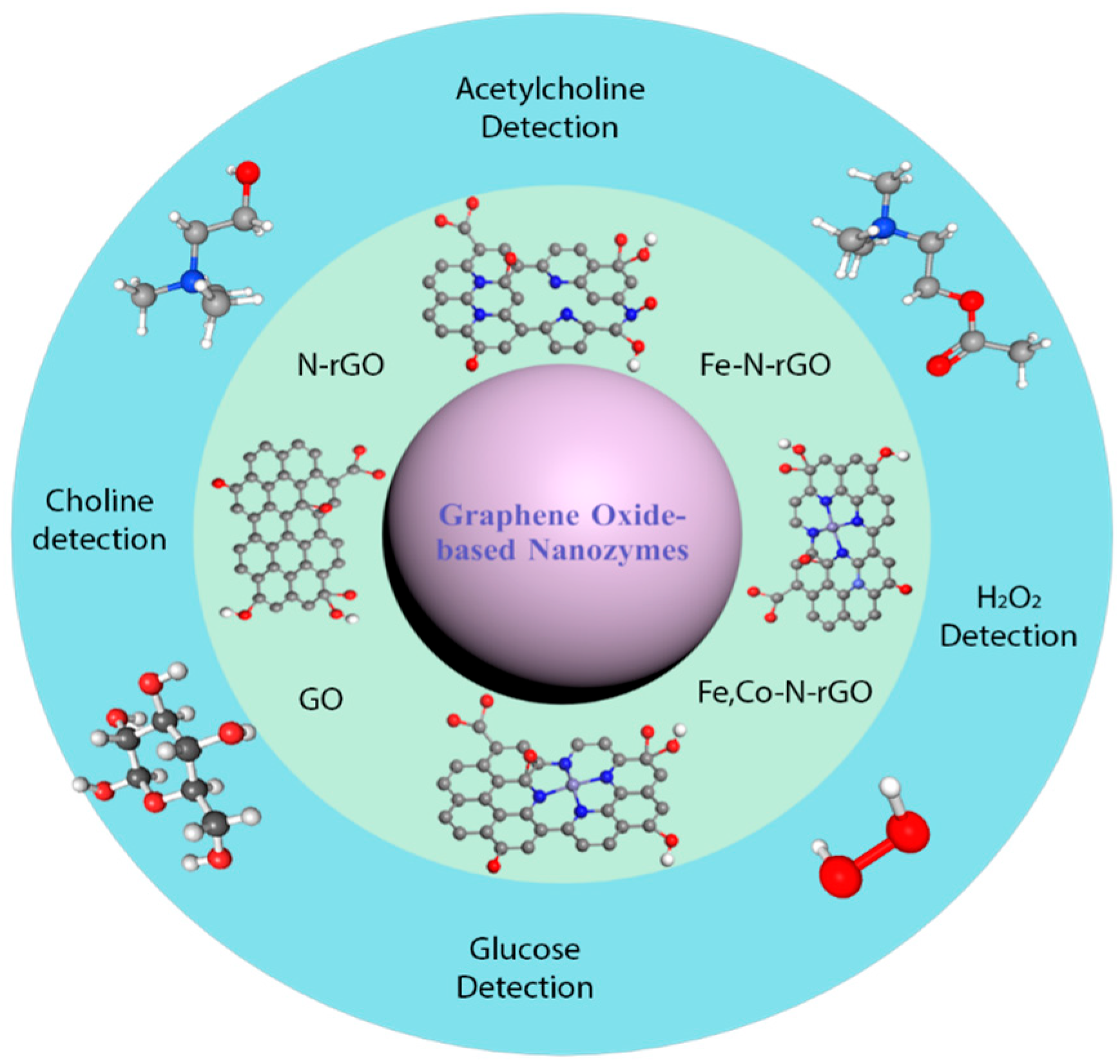
3. Recently Advanced Development and Progression of the N Heteroatom-Containing GO-Based Nanozymes
3.1. Graphene Oxide Nanozymes and Their Biomedical Application
3.2. Nitrogen-Doped Graphene Oxide Nanozymes and Their Biomedical Application
3.2.1. Synthesis of N-Doped Graphene Oxide
3.2.2. Peroxidase-like Activity of N-Doped Graphene Oxide and Their Biomedical Application
3.2.3. N, B-Co-Doped Graphene Oxide Nanozymes and Their Biomedical Applications
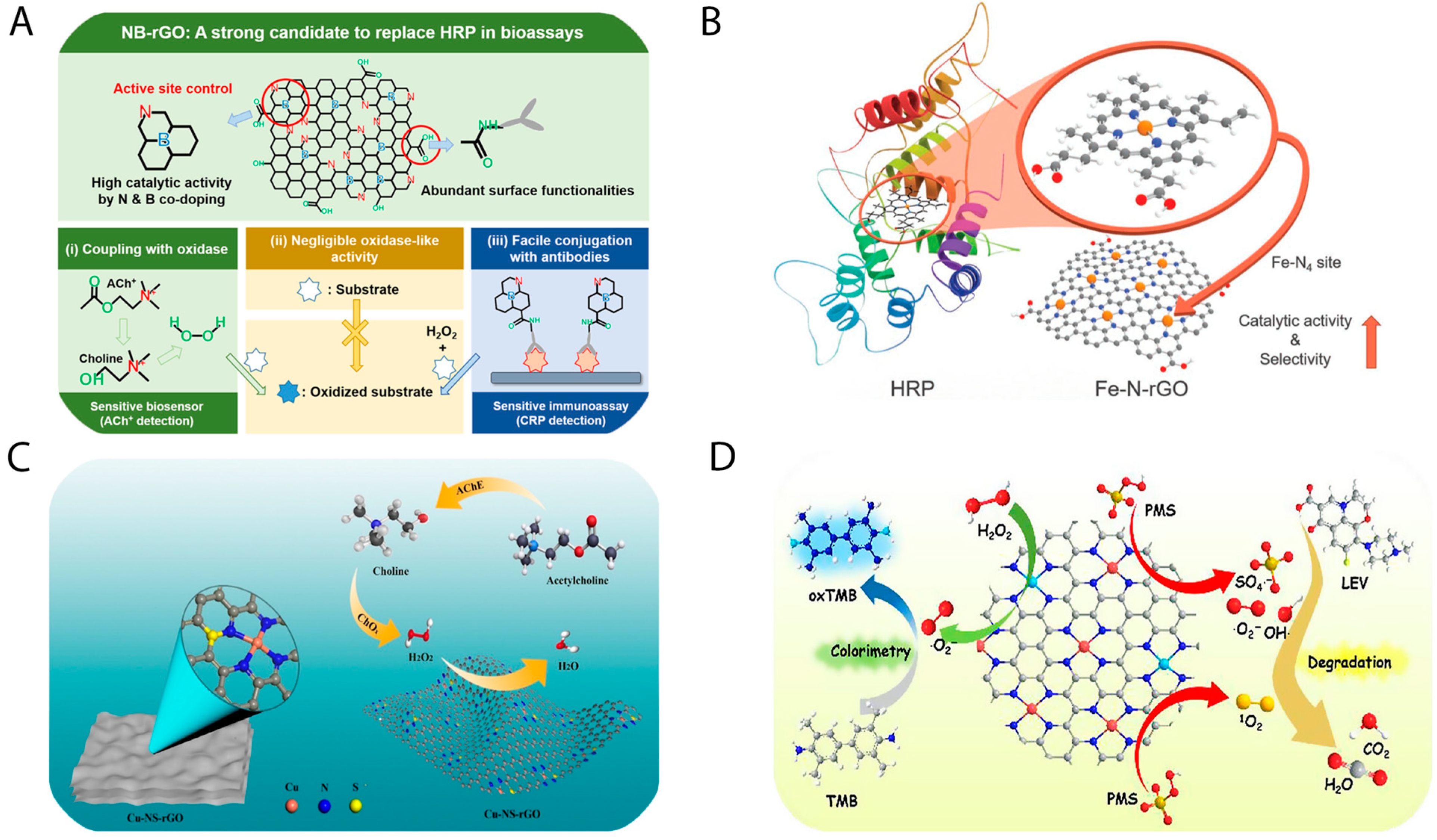
3.2.4. Peroxidase-Mimicking Activity of Metal (M, M’) and N Co-Doped GO Nanozymes and Their Biomedical Applications
4. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-Nanoparticle-Based Transphosphorylation Catalysts. Angew. Chem. Int. Ed. 2004, 43, 6165–6169. [Google Scholar] [CrossRef]
- Lévy, R. Peptide-Capped Gold Nanoparticles: Towards Artificial Proteins. ChemBioChem 2006, 7, 1141–1145. [Google Scholar] [CrossRef]
- Pengo, P.; Baltzer, L.; Pasquato, L.; Scrimin, P. Substrate Modulation of the Activity of an Artificial Nanoesterase Made of Peptide-Functionalized Gold Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 400–404. [Google Scholar] [CrossRef]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. Acid Catalysis in Basic Solution: A Supramolecular Host Promotes Orthoformate Hydrolysis. Science 2007, 316, 85–88. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Li, S.; Reynolds, A.D.; Mosley, R.L.; Bronich, T.K.; Kabanov, A.V.; Gendelman, H.E. A Macrophage–Nanozyme Delivery System for Parkinson’s Disease. Bioconjugate Chem. 2007, 18, 1498–1506. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Le, P.G.; Kim, M.I. Research Progress and Prospects of Nanozyme-Based Glucose Biofuel Cells. Nanomaterials 2021, 11, 2116. [Google Scholar] [CrossRef]
- Prakobkij, A.; Saenmuangchin, R.; Chunta, S.; Amatatongchai, M.; Citterio, D.; Jarujamrus, P. Peroxidase-like Activity of Aptamer-Gold Nanoparticles for Selective and Sensitive Fluorescence Detection of Low-Density Lipoproteins. ACS Appl. Nano Mater. 2024, 7, 12356–12365. [Google Scholar] [CrossRef]
- Singh, A.K.; Bijalwan, K.; Kaushal, N.; Kumari, A.; Saha, A.; Indra, A. Oxidase-like Nanozyme Activity of Manganese Metal–Organic Framework Nanosheets for Colorimetric and Fluorescence Sensing of l-Cysteine. ACS Appl. Nano Mater. 2023, 6, 8036–8045. [Google Scholar] [CrossRef]
- Fang, G.; Kang, R.; Chong, Y.; Wang, L.; Wu, C.; Ge, C. MOF-based DNA hydrolases optimized by atom engineering for the removal of antibiotic-resistant genes from aquatic environment. Appl. Catal. B Environ. 2023, 320, 121931. [Google Scholar] [CrossRef]
- Lucecki, C.A.; Durigon, D.C.; Terenzi, H.; Bortoluzzi, A.J.; Neves, A.; Peralta, R.A. Improving the hydrolase-like activity of a lanthanum (III) complex through second coordination sphere. Inorganica Chim. Acta 2025, 579, 122588. [Google Scholar] [CrossRef]
- Wang, M.; Qi, W. 16—Assembled peptides for biomimetic catalysis. In Artificial Protein and Peptide Nanofibers; Wei, G., Kumbar, S.G., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 383–413. [Google Scholar] [CrossRef]
- Zhang, N.; Meng, X.-G.; Wu, Y.-Y.; Song, H.-J.; Huang, H.; Wang, F.; Lv, J. Highly Selective Isomerization of Glucose into Fructose Catalyzed by a Mimic Glucose Isomerase. ChemCatChem 2019, 11, 2355–2361. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, Y.; Zhang, Y.; Zhang, A.; Jia, M.; Yin, J.; Shen, G. Nanozymes: A bibliometrics review. J. Nanobiotechnology 2024, 22, 704. [Google Scholar] [CrossRef]
- Ornelas-González, A.; Rito-Palomares, M.; González-González, M. TMB vs ABTS: Comparison of multi-enzyme-based approaches for the colorimetric quantification of salivary glucose. J. Chem. Technol. Biotechnol. 2022, 97, 2720–2727. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Lee, J.; Cho, A.; Kim, M.S.; Choi, D.; Han, J.W.; Kim, M.I.; Lee, J. Rational Development of Co-Doped Mesoporous Ceria with High Peroxidase-Mimicking Activity at Neutral pH for Paper-Based Colorimetric Detection of Multiple Biomarkers. Adv. Funct. Mater. 2022, 32, 2112428. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Lee, D.H.; Nguyen, P.T.; Le, P.G.; Kim, M.I. Foldable paper microfluidic device based on single iron site-containing hydrogel nanozyme for efficient glucose biosensing. Chem. Eng. J. 2023, 454, 140541. [Google Scholar] [CrossRef]
- Le, P.G.; Jung, S.-C.; Han, J.-H.; Cho, S. Nanozyme-integrated paper chip based on high peroxidase-like activity of Zn-doped reduced graphene oxide for glucose sensing. Microchem. J. 2025, 213, 113895. [Google Scholar] [CrossRef]
- Das, B.; Lou-Franco, J.; Gilbride, B.; Ellis, M.G.; Stewart, L.D.; Grant, I.R.; Balasubramanian, P.; Cao, C. Peroxidase-Mimicking Activity of Biogenic Gold Nanoparticles Produced from Prunus nepalensis Fruit Extract: Characterizations and Application for the Detection of Mycobacterium bovis. ACS Appl. Biomater. 2022, 5, 2712–2725. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Q.; Wang, F.; Xiao, Z.; He, L.; He, D.; Deng, L. Gold–Platinum Nanodots with High-Peroxidase-like Activity and Photothermal Conversion Efficiency for Antibacterial Therapy. ACS Appl. Mater. Interfaces 2021, 13, 37535–37544. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Chen, W.; Sun, S.; Tang, H.; Li, Y. Perovskite mesoporous LaFeO3 with peroxidase-like activity for colorimetric detection of gallic acid. Sens. Actuators B Chem. 2020, 321, 128642. [Google Scholar] [CrossRef]
- Dong, H.; Du, W.; Dong, J.; Che, R.; Kong, F.; Cheng, W.; Ma, M.; Gu, N.; Zhang, Y. Depletable peroxidase-like activity of Fe3O4 nanozymes accompanied with separate migration of electrons and iron ions. Nat. Commun. 2022, 13, 5365. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Q.; Liu, M.; Xue, L.; Wang, G.; Zhang, S.; Hu, W. N, P, S Codoped Carbon Nanozymes with Enhanced Peroxidase-like Activity and Binding Affinity for Total Antioxidant Capacity Assay. ACS Appl. Nano Mater. 2023, 6, 23303–23312. [Google Scholar] [CrossRef]
- Le, P.G.; Le, X.A.; Duong, H.S.; Jung, S.H.; Kim, T.; Kim, M.I. Ultrahigh peroxidase-like catalytic performance of Cu–N4 and Cu–N4S active sites-containing reduced graphene oxide for sensitive electrochemical biosensing. Biosens. Bioelectron. 2024, 255, 116259. [Google Scholar] [CrossRef]
- Yi, Y.; Zhou, X.; Liao, D.; Hou, J.; Liu, H.; Zhu, G. High Peroxidase-Mimicking Metal–Organic Frameworks Decorated with Platinum Nanozymes for the Colorimetric Detection of Acetylcholine Chloride and Organophosphorus Pesticides via Enzyme Cascade Reaction. Inorg. Chem. 2023, 62, 13929–13936. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Wan, C.-Q.; Wang, Y.-X.; Shen, X.-F.; Pang, Y.-H. Bismuth-based metal-organic framework peroxidase-mimic nanozyme: Preparation and mechanism for colorimetric-converted ultra-trace electrochemical sensing of chromium ion. J. Hazard. Mater. 2023, 451, 131148. [Google Scholar] [CrossRef]
- Chen, Y.; Rong, C.; Gao, W.; Luo, S.; Guo, Y.; Gu, Y.; Yang, G.; Xu, W.; Zhu, C.; Qu, L.-L. Ag-MXene as peroxidase-mimicking nanozyme for enhanced bacteriocide and cholesterol sensing. J. Colloid Interface Sci. 2024, 653, 540–550. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. MXene-Based Composites as Nanozymes in Biomedicine: A Perspective. Nano-Micro Lett. 2022, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Zhang, N.; Yan, W.; Guo, Y.; Tan, H.; Yang, C.; Wang, F.; Yao, H. Bimetallic Cu@Co-MOFs Mimic Peroxidase for Colorimetric Detection of Glutathione. ACS Appl. Nano Mater. 2024, 7, 24683–24696. [Google Scholar] [CrossRef]
- Sruthi, V.P.; Senthilkumar, S. Prudently designed Se@fMWCNT as a peroxidase mimicking nanozyme for distinctive electrochemical detection of H2O2 and glutathione. J. Mater. Chem. C 2024, 12, 8924–8934. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Zhu, R.; Wang, B.; Yang, J.; Xu, F.; Ramaswamy, S.; Zhang, X. Fe3+-Doped Aminated Lignin as Peroxidase-Mimicking Nanozymes for Rapid and Durable Colorimetric Detection of H2O2. ACS Sustain. Chem. Eng. 2021, 9, 12833–12843. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, J.; Kim, H.S.; Cho, A.; Shim, K.H.; Le, T.N.; An, S.S.A.; Han, J.W.; Kim, M.I.; Lee, J. Heme Cofactor-Resembling Fe–N Single Site Embedded Graphene as Nanozymes to Selectively Detect H2O2 with High Sensitivity. Adv. Funct. Mater. 2020, 30, 1905410. [Google Scholar] [CrossRef]
- Kim, M.S.; Cho, S.; Joo, S.H.; Lee, J.; Kwak, S.K.; Kim, M.I.; Lee, J. N- and B-Codoped Graphene: A Strong Candidate to Replace Natural Peroxidase in Sensitive and Selective Bioassays. ACS Nano 2019, 13, 4312–4321. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.S.; Ahmad, M.A.; Nawaz, M.H.; Hayat, A.; Nasir, M. Nitrogen-doped graphene oxide as a catalyst for the oxidation of Rhodamine B by hydrogen peroxide: Application to a sensitive fluorometric assay for hydrogen peroxide. Microchim. Acta 2019, 187, 47. [Google Scholar] [CrossRef]
- Varodi, C.; Pogăcean, F.; Coros, M.; Magerusan, L.; Stefan-van Staden, R.-I.; Pruneanu, S. Hydrothermal Synthesis of Nitrogen, Boron Co-Doped Graphene with Enhanced Electro-Catalytic Activity for Cymoxanil Detection. Sensors 2021, 21, 6630. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.K.; Knaus, T.; Garcia, A.; Yan, N.; Mutti, F.G. Bacterial Peroxidase on Electrochemically Reduced Graphene Oxide for Highly Sensitive H2O2 Detection. ChemBioChem 2022, 23, e202200346. [Google Scholar] [CrossRef]
- Xie, J.; Cao, H.; Jiang, H.; Chen, Y.; Shi, W.; Zheng, H.; Huang, Y. Co3O4-reduced graphene oxide nanocomposite as an effective peroxidase mimetic and its application in visual biosensing of glucose. Anal. Chim. Acta 2013, 796, 92–100. [Google Scholar] [CrossRef]
- Zhuang, J.; Midgley, A.C.; Wei, Y.; Liu, Q.; Kong, D.; Huang, X. Machine-Learning-Assisted Nanozyme Design: Lessons from Materials and Engineered Enzymes. Adv. Mater. 2024, 36, 2210848. [Google Scholar] [CrossRef]
- Xuan, W.; Li, X.; Gao, H.; Zhang, L.; Hu, J.; Sun, L.; Kan, H. Artificial intelligence driven platform for rapid catalytic performance assessment of nanozymes. Sci. Rep. 2025, 15, 13305. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Z.; Gao, X.J.; Gao, X. Reaction Mechanisms and Kinetics of Nanozymes: Insights from Theory and Computation. Adv. Mater. 2024, 36, 2211151. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Li, J.; Zhao, L.; Tuersuntuoheti, T.; Mehmood, A.; Zhou, N.; Hao, S.; Wang, C.; Guo, Y.; Lin, W. A molecular docking and molecular dynamics simulation study on the interaction between cyanidin-3-O-glucoside and major proteins in cow’s milk. J. Food Biochem. 2021, 45, e13570. [Google Scholar] [CrossRef]
- Wang, P.; Linares-Pastén, J.A.; Zhang, B. Synthesis, Molecular Docking Simulation, and Enzymatic Degradation of AB-Type Indole-Based Polyesters with Improved Thermal Properties. Biomacromolecules 2020, 21, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Poh, H.L.; Šaněk, F.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Pumera, M. Graphenes prepared by Staudenmaier, Hofmann and Hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 2012, 4, 3515–3522. [Google Scholar] [CrossRef]
- Moo, J.G.S.; Khezri, B.; Webster, R.D.; Pumera, M. Graphene Oxides Prepared by Hummers’, Hofmann’s, and Staudenmaier’s Methods: Dramatic Influences on Heavy-Metal-Ion Adsorption. ChemPhysChem 2014, 15, 2922–2929. [Google Scholar] [CrossRef] [PubMed]
- Anegbe, B.; Ifijen, I.H.; Maliki, M.; Uwidia, I.E.; Aigbodion, A.I. Graphene oxide synthesis and applications in emerging contaminant removal: A comprehensive review. Environ. Sci. Eur. 2024, 36, 15. [Google Scholar] [CrossRef]
- Feicht, P.; Biskupek, J.; Gorelik, T.E.; Renner, J.; Halbig, C.E.; Maranska, M.; Puchtler, F.; Kaiser, U.; Eigler, S. Brodie’s or Hummers’ Method: Oxidation Conditions Determine the Structure of Graphene Oxide. Chem.—A Eur. J. 2019, 25, 8955–8959. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Qadoos, A.; Rashid, M.; Naeem, M.N.; Jiang, Z.; Moin, M.; Babar, M. Bandgap engineering in graphene oxide (GO) via integrating DFT calculations with atmospheric pressure microplasma (AMP) treatment for optoelectronic applications. Hybrid Adv. 2025, 8, 100353. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Viswanath, P.; Rao, V.K.; Suzuki, S.; Yoshimura, M. New Insight into the Characterization of Graphene Oxide and Reduced Graphene Oxide Monolayer Flakes on Si-Based Substrates by Optical Microscopy and Raman Spectroscopy. J. Phys. Chem. C 2021, 125, 7791–7798. [Google Scholar] [CrossRef]
- Lee, A.Y.; Yang, K.; Anh, N.D.; Park, C.; Lee, S.M.; Lee, T.G.; Jeong, M.S. Raman study of D* band in graphene oxide and its correlation with reduction. Appl. Surf. Sci. 2021, 536, 147990. [Google Scholar] [CrossRef]
- Sharma, M.; Rani, S.; Pathak, D.K.; Bhatia, R.; Kumar, R.; Sameera, I. Temperature dependent Raman modes of reduced graphene oxide: Effect of anharmonicity, crystallite size and defects. Carbon 2021, 184, 437–444. [Google Scholar] [CrossRef]
- Parpal, M.; El Sachat, A.; Sotomayor Torres, C.M.; Gómez-Romero, P.; Rueda-García, D.; Chavez-Angel, E. In situ Raman analysis of reduced-graphene oxide-based electroactive nanofluids. Diam. Relat. Mater. 2024, 141, 110541. [Google Scholar] [CrossRef]
- Wu, W.; Ranasinghe, J.C.; Chatterjee, A.; Huang, S. Recent advances on Raman spectroscopy of graphene: Towards biosensing applications. Mater. Chem. Phys. 2024, 318, 129281. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibers. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Wu, J.; Lin, H.; Moss, D.J.; Loh, K.P.; Jia, B. Graphene oxide for photonics, electronics and optoelectronics. Nat. Rev. Chem. 2023, 7, 162–183. [Google Scholar] [CrossRef]
- Ferrari, I.; Motta, A.; Zanoni, R.; Scaramuzzo, F.A.; Amato, F.; Dalchiele, E.A.; Marrani, A.G. Understanding the nature of graphene oxide functional groups by modulation of the electrochemical reduction: A combined experimental and theoretical approach. Carbon 2023, 203, 29–38. [Google Scholar] [CrossRef]
- Khine, Y.Y.; Wen, X.; Jin, X.; Foller, T.; Joshi, R. Functional groups in graphene oxide. Phys. Chem. Chem. Phys. 2022, 24, 26337–26355. [Google Scholar] [CrossRef]
- Hartmann, S.J.; Iurchenkova, A.A.; Kallio, T.; Fedorovskaya, E.O. Electrochemical Properties of Nitrogen and Oxygen Doped Reduced Graphene Oxide. Energies 2020, 13, 312. [Google Scholar] [CrossRef]
- Ruiz-Marizcal, J.M.; Paez-Ornelas, J.I.; Fernández-Escamilla, H.N.; Murillo-Bracamontes, E.A.; Alonso-Núñez, G.; Perez-Tijerina, E.G.; Takeuchi, N.; Romo-Herrera, J.M. From Graphene Oxide to N-Doped Graphene: Understanding the Doping Process. Adv. Energy Sustain. Res. 2025, 6, 2400310. [Google Scholar] [CrossRef]
- Prakash, D.; Manivannan, S. N, B co-doped and Crumpled Graphene Oxide Pseudocapacitive Electrode for High Energy Supercapacitor. Surf. Interfaces 2021, 23, 101025. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Wang, D.; Song, X.; Li, P.; Gao, X.J.; Gao, X. Origins of the peroxidase mimicking activities of graphene oxide from first principles. J. Mater. Chem. B 2020, 8, 9028–9034. [Google Scholar] [CrossRef]
- Yokwana, K.; Ntsendwana, B.; Nxumalo, E.N.; Mhlanga, S.D. Recent advances in nitrogen-doped graphene oxide nanomaterials: Synthesis and applications in energy storage, sensor electrochemical applications and water treatment. J. Mater. Res. 2023, 38, 3239–3263. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, X.J.; Zhu, Y.; Muhammad, F.; Tan, S.; Cao, W.; Lin, S.; Jin, Z.; Gao, X.; Wei, H. Nitrogen-Doped Carbon Nanomaterials as Highly Active and Specific Peroxidase Mimics. Chem. Mater. 2018, 30, 6431–6439. [Google Scholar] [CrossRef]
- Liang, D.; Yang, Y.; Li, G.; Wang, Q.; Chen, H.; Deng, X. Endogenous H2O2-Sensitive and Weak Acidic pH-Triggered Nitrogen-Doped Graphene Nanoparticles (N-GNMs) in the Tumor Microenvironment Serve as Peroxidase-Mimicking Nanozymes for Tumor-Specific Treatment. Materials 2021, 14, 1933. [Google Scholar] [CrossRef]
- Luo, S.; Sha, M.; Tian, F.; Li, X.; Fu, L.; Gu, Y.; Qu, L.-L.; Yang, G.-H.; Zhu, C. Nitrogen and boron co-doped graphene nanoribbons as peroxidase-mimicking nanozymes for enhanced biosensing. Chin. Chem. Lett. 2022, 33, 344–348. [Google Scholar] [CrossRef]
- Li, D.; Fu, J.; Guo, S.; Cao, J.; Liu, Z.; Zhao, R. Theoretical insights into the peroxidase-like activity of N-doped, B-doped and B/N-Codoped graphene. Chem. Phys. Lett. 2025, 876, 142193. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, Y.; Fan, Y.; Jiang, L.; Liu, W.; Yang, X. Multifunctional Fe/Cu Dual-Single Atom Nanozymes with Enhanced Peroxidase Activity for Isoniazid Detection and Levofloxacin Degradation. Langmuir 2024, 40, 12671–12680. [Google Scholar] [CrossRef]
- Jiao, L.; Wu, J.; Zhong, H.; Zhang, Y.; Xu, W.; Wu, Y.; Chen, Y.; Yan, H.; Zhang, Q.; Gu, W.; et al. Densely Isolated FeN4 Sites for Peroxidase Mimicking. ACS Catal. 2020, 10, 6422–6429. [Google Scholar] [CrossRef]
| Nanozymes | Sensor Types | Application | Targets | Environments | Linear Ranges (M) | Detection Limits (M) | Ref. |
|---|---|---|---|---|---|---|---|
| GO-COOH | Colorimetric sensor | Diabetic mellitus | H2O2 | Phosphate buffer | 5 × 10−8–10−6 | 5 × 10−8 | [64] |
| Glucose | 10−6–2 × 10−5 | 10−6 | |||||
| N-rGO | Colorimetric sensor | Tumor treatment | H2O2 | KH2PO4/Na2HPO4 | 10−4–10−3 | NA ** | [68] |
| N-rGO | Colorimetric sensor | Decomposition of Rhodamine B | H2O2 | PBS * | 10−9–10−6 | 94 × 10−12 | [36] |
| N, B-rGO | Fluorescent sensor | Neurotransmitter | H2O2 | Tris-acetate buffer | 5 × 10−7–3 × 10−5 | 10−7 | [35] |
| Choline | 5 × 10−8–5 × 10−6 | 10−8 | |||||
| Acetylcholine | 5 × 10−8–5 × 10−6 | 3 × 10−8 | |||||
| Inflammatory marker | C-reactive protein | Bovine serum albumin | 1–5000 ng.ml−1 | 5 ng.ml−1 | |||
| N,B-rGO | Colorimetric sensor | Cytokine detection | Interleukin-6 | PBS | 0.001–1000 ng.ml−1 | 0.3 pgmL−1 | [69] |
| Fe-N4-C | Colorimetric sensor | Neurotransmitter | Choline | Tris-acetate buffer | 5 × 10−8–10−6 | 10−8 | [34] |
| Choline | Tris-acetate buffer | 5 × 10−8–10−6 | 2 × 10−8 | ||||
| Acetyl Choline | Tris-acetate buffer | 5 × 10−9–10−7 | 2 × 10−8 | ||||
| Cu-N4 and Cu-N4-S | Electrochemical sensor | Neurotransmitter | H2O2 | PBS | 10−14–10−12 | 5.0 × 10−14 | [26] |
| Choline | Human serum | 10−9–10−7 | 2.5 × 10−9 | ||||
| Acetyl Choline | Human serum | 2 × 10−8–10−7 | 5.0 × 10−9 | ||||
| Zn-N4-C | Colorimetric sensor | Diabetic Mellitus | H2O2 | PBS | 10−7–10−6 | 1.47 × 10−9 | [20] |
| Colorimetric sensor | Glucose | Human serum | 2.5 × 10−4–1.5 × 10−3 | 0.12 × 10−3 | |||
| Glucose paper chip | Glucose | Human serum | 10−3–3 × 10−2 | 0.78 × 10−3 | |||
| FeCu-NC | Colorimetric sensor | Antibiotic detection | Isoniazid | Sodium Acetate buffer | 9 × 10−7–10−5 | 3 × 10−7 | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, P.G.; Kim, D.; Chung, J.-P.; Cho, S. Peroxidase-Mimicking Nanozymes of Nitrogen Heteroatom-Containing Graphene Oxide for Biomedical Applications. Biosensors 2025, 15, 435. https://doi.org/10.3390/bios15070435
Le PG, Kim D, Chung J-P, Cho S. Peroxidase-Mimicking Nanozymes of Nitrogen Heteroatom-Containing Graphene Oxide for Biomedical Applications. Biosensors. 2025; 15(7):435. https://doi.org/10.3390/bios15070435
Chicago/Turabian StyleLe, Phan Gia, Daesoo Kim, Jae-Pil Chung, and Sungbo Cho. 2025. "Peroxidase-Mimicking Nanozymes of Nitrogen Heteroatom-Containing Graphene Oxide for Biomedical Applications" Biosensors 15, no. 7: 435. https://doi.org/10.3390/bios15070435
APA StyleLe, P. G., Kim, D., Chung, J.-P., & Cho, S. (2025). Peroxidase-Mimicking Nanozymes of Nitrogen Heteroatom-Containing Graphene Oxide for Biomedical Applications. Biosensors, 15(7), 435. https://doi.org/10.3390/bios15070435






