Recent Advances in Flexible Sensors for Neural Interfaces: Multimodal Sensing, Signal Integration, and Closed-Loop Feedback
Abstract
1. Introduction
2. Recent Advances in Materials and Structural Designs for Flexible Sensors in Neural Interfaces
2.1. Flexible Functional Materials
2.1.1. Carbon-Based Materials
2.1.2. Metal-Based Materials
2.1.3. Polymer Composite Materials
| Materials | Mechanical Properties | Electrochemical Properties | Lifespan | Advantage | Cite | |||
|---|---|---|---|---|---|---|---|---|
| Material Resilience | Target Object | Sensitivity | Detection Limit (LOD) | Impedance/Resistance | ||||
| GFMEs(rGO) | / | DA | 1.54 nA/μM | / | / | / | Anti-pollution | [21] |
| CFMEs | / | DA | 0.41 nA/μM | / | / | / | Can be surface treated | [22] |
| AuNR@ZIF-8 | / | DA | / | 0.03 μM | / | / | High selectivity | [23] |
| pBDD/AuNPs | / | DA | 1.5 μA·μM−1·cm−2 | 68 nM | / | 6 months | Anti-pollution | [37] |
| rGO/EGaIn/AuNPs | 30% | DA | / | 0.105 μM | / | / | Stretch impedance stability | [27] |
| PEDOT:BF4/EGaIn | ~600% | / | / | / | 3 kΩ (1 kHz) | 4 weeks | Electrochemically stabilized liquid metal | [28] |
| EGaIn | 480% | / | / | / | 3.54 mΩ/square | 7000 strain cycles | Temperature and pressure resistance | [38] |
| GaIn/Pt | 100% | / | / | / | 250 ± 40 kΩ | 2000 strain cycles | Printable | [39] |
| PPY/PEDOT:PSS/GCE | / | ST | 7.4248 μA/μM cm−2 | 45 pM | Rct = 5.21 Ω | 100 CV cycles | High sensitivity and high selection | [33] |
| PEGDA/MXene | / | DA | / | 2.55 μM | / | 40 days | Breathable and translucent | [34] |
2.2. Structural Design of Flexible Neuroelectrodes
2.2.1. Thin Film Electrodes
2.2.2. Miniaturized Probes

3. Neurotransmitter Sensing Techniques
3.1. Electrochemical Detection
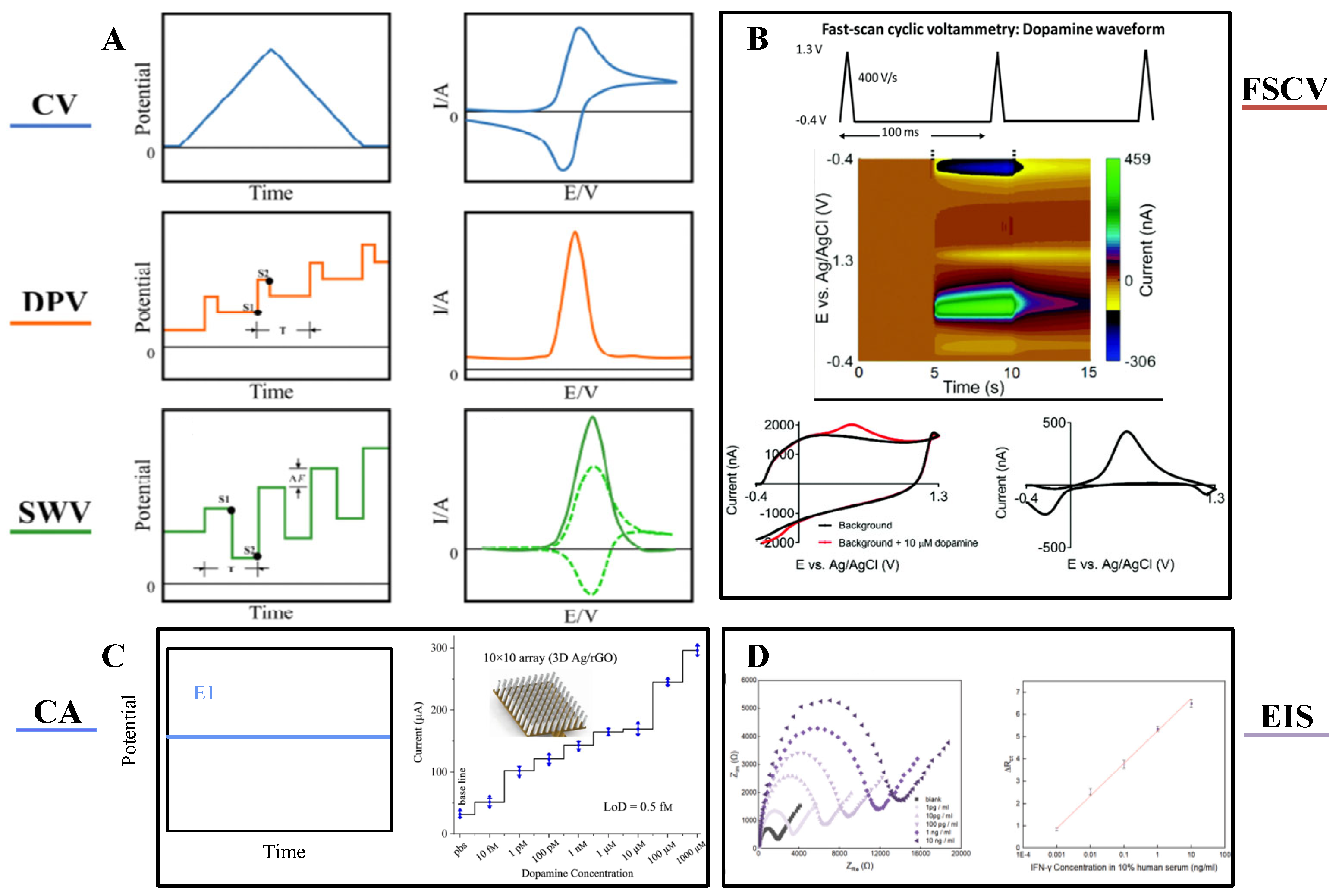
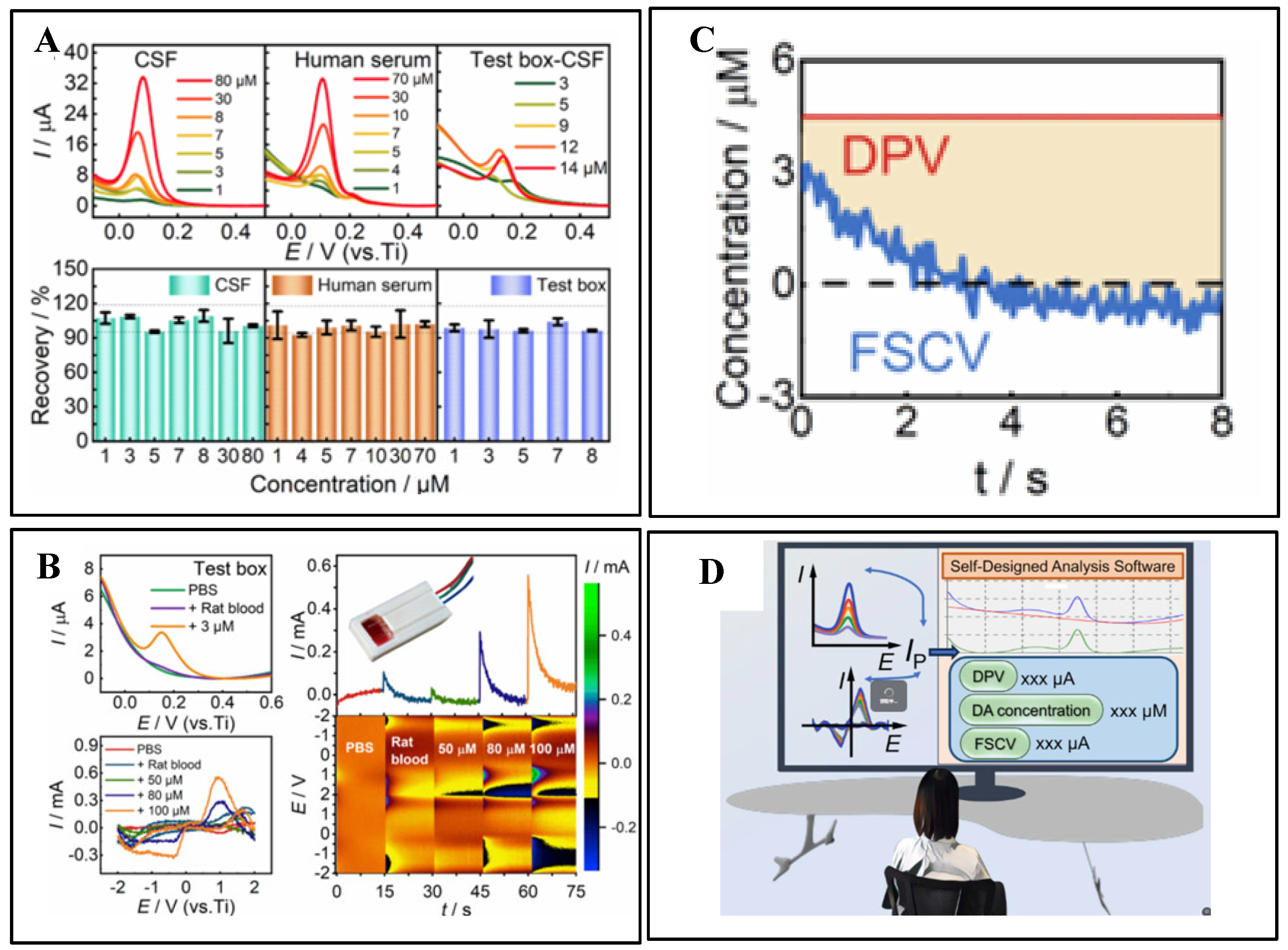
| Technology | Electrode Type | Materials | Target Object | Sensitivity | Detection Limit (LOD) | Linear Range | Selectivity | Temporal Resolution | Stability | Robustness | BIOCOMPATIBILITY | Cite |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPV | Flexible | PEDOT–titania–poly(dimethylsiloxane) | EP | 100 nM ± 5 | 20–1000 μM | The EP and DA peaks are placed together | Minute-level | 50 consecutive scans, the percentage decrease in current is less than 5% | [65] | |||
| Rigid | Hybrid Multi-walled Carbon Nanotubes-Supernano Diamond | DA | 36 ± 2% μA/μM/cm2 | 9.5 ± 1.2% nM. | 33 nM to 1 μM | AA, DA, and 5-HT can be distinguished | Minute-level | 5-h electrochemical cycle | Suitable for acute chemical sensing | [66] | ||
| SWV | Flexible | PEDOT/CNT | DA | <0.1 µM | 0.5–10 µM | Prevents DOPAC, AA, and negatively charged interfering molecules from approaching the electrode surface and generating SWV currents | Second-level | The electrode was placed in the mouse brain and repeated continuously for 90 min | In vivo validation | [67] | ||
| Rigid | Nanoporous diamond/gold particles | DA | 0.28 μA/μM | 68 nM | 3–100 μM | Using Nafion membrane to interfere with AA, L-DOPA, DOPAC, and UA | Second-level | >6 months (room temperature) still retains 95.3% of the average SWV response current | High (20 individual tests (approx. 4% current fluctuation (except for the highest) | Excellent, good stain resistance | [37] | |
| FSCV | Flexible | Metal-complexed polyimide | DA | 0.1 nA/fM | 5.6 nM | 10 nM to 1 μM | High | Millisecond-level | In vivo validation | [59] | ||
| Rigid | CNS–Ta | DA | 0.002 nA/µM µm2 | 8 nM | 100 nM–100 μM | Dopamine, uric acid, and ascorbic acid at different potentials | Millisecond-level | 10 days | Long-term continuous application of the potential waveform RSD is 3.7% ± 0.8% | [68] | ||
| CA | Flexible | rGO/PEDOT:PSS-modified polyimide | DA | 15 pA/μM | 192 ± 29 nM | 1–96 μM | With Nafion membranes, it is possible to resist AA and UA interference, but it is not possible to completely distinguish between NAs | Minute-level | >6 weeks (in vivo) | High (48 weeks electrophysiology) | Excellent (low inflammatory response) | [52] |
| Rigid | La/MWCNT | 5-HT | 13 nM | 0.04 µM–0.89 mM | No multiple compounds were found to be likely to interfere | Minute-level | 15 days | 10 consecutive CV measurements were performed with an RSD < 4.2% | [69] | |||
| EIS | Flexible | Au microgap electrodes are made up of aptamers/MXene | IFN-γ | 0.26 pg/mL | 1 pg/mL to 10 ng/mL | High selectivity for each target protein, | Minute-level | 4 days | Well | [61] | ||
| Rigid | Gold electrode/self-assembled monolayer | IgG | 0.5 μg/L | 5–400 μg/L | High | Minute-level | [70] |
3.2. Spectroscopic Detection
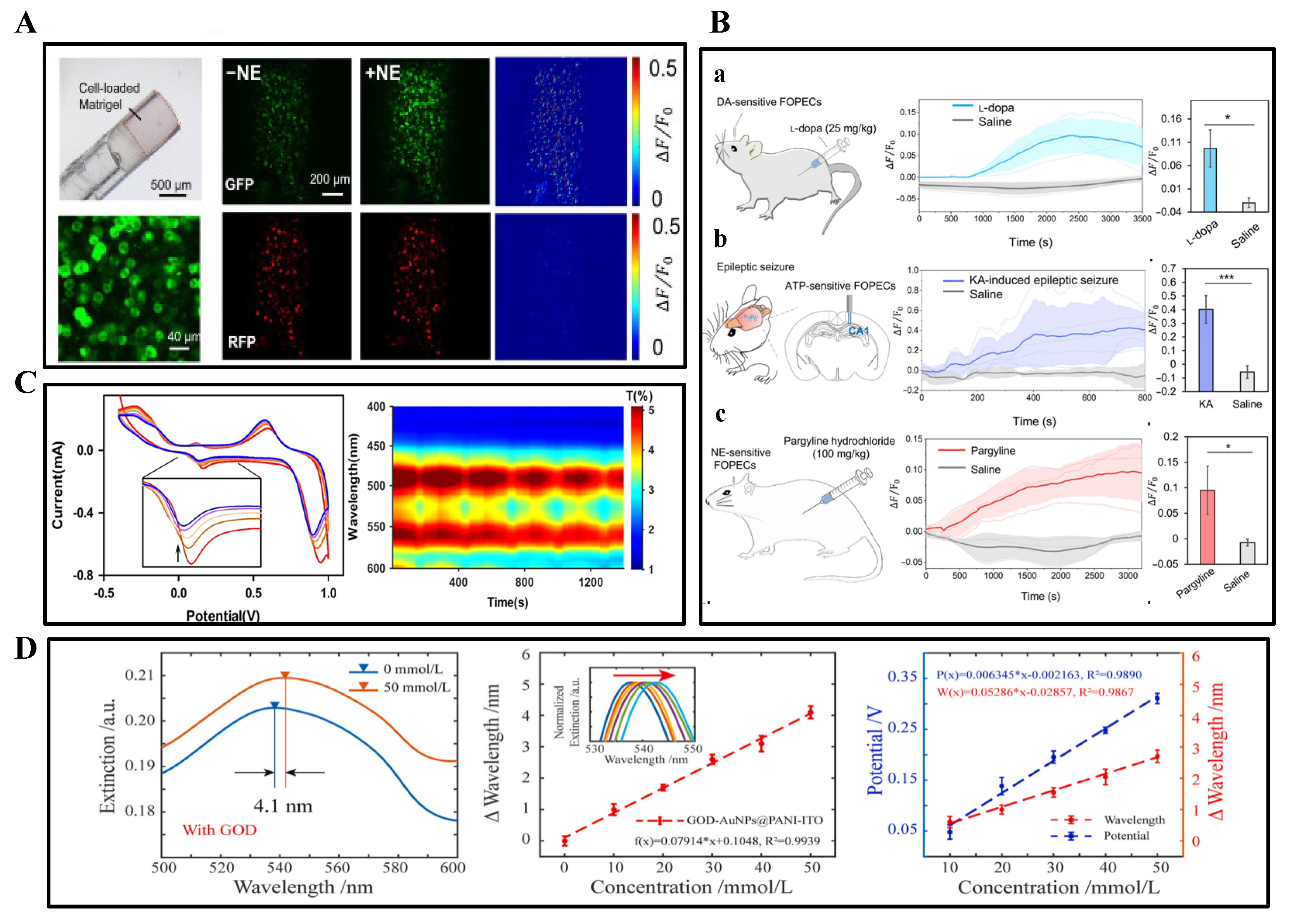
3.3. Sampling Techniques

4. Integration of Multimodal Sensing Systems for Neural Interfaces
4.1. Electrophysiological-Chemical Signal Co-Recording
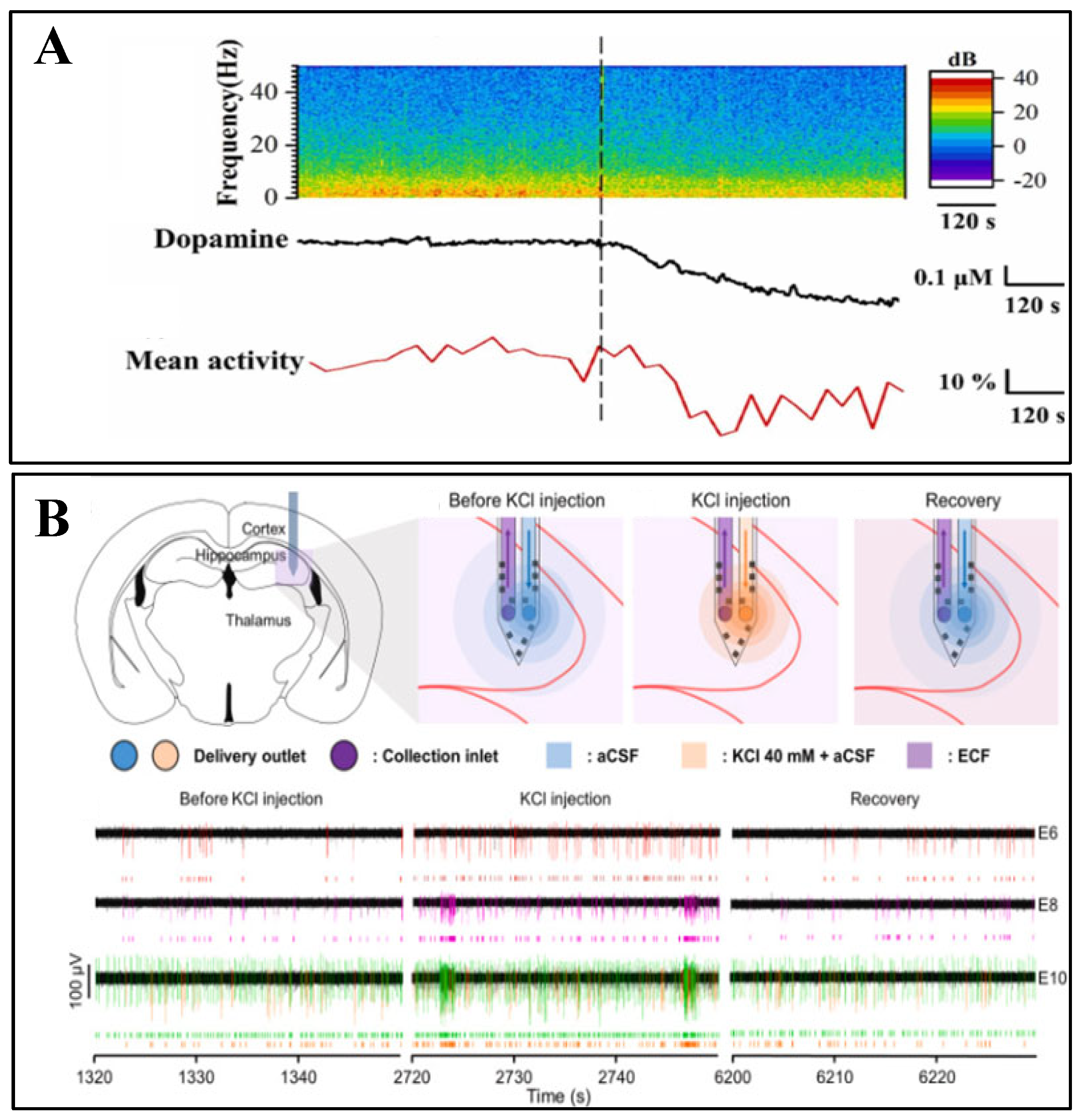
4.2. Optical-Electrochemical Co-Monitoring

4.3. Auxiliary Role of Pressure and Electrophysiological Sensors
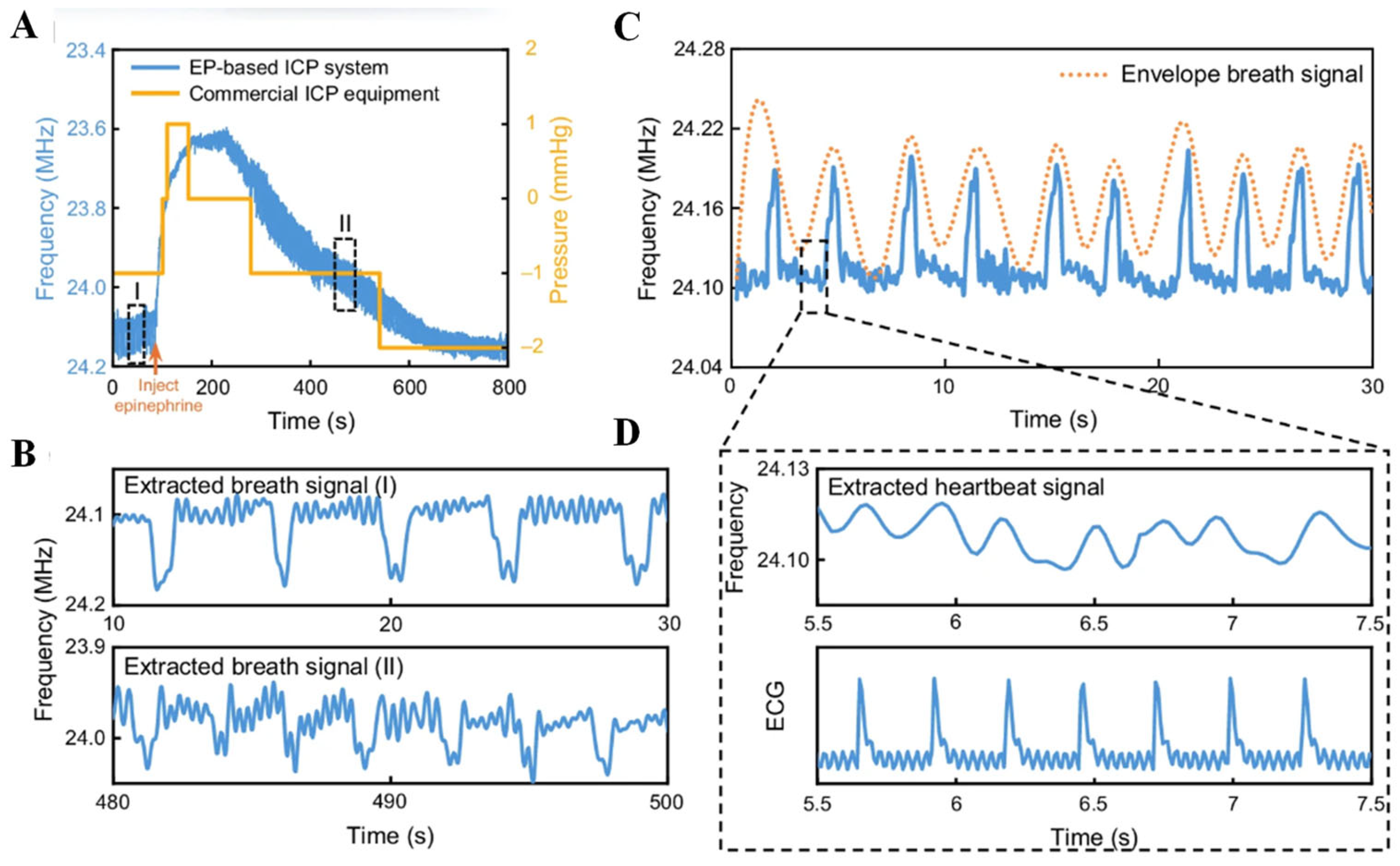
5. Closed-Loop Neuromodulation System
5.1. System Circuitry
5.2. Closed-Loop Diagnosis and Treatment
5.2.1. Closed-Loop Regulation of Drug Release via Electrochemical Sensing
5.2.2. Closed-Loop Regulation via Electrophysiological Detection and Stimulation

5.2.3. Algorithm-Driven Regulation of Mechanical Signal Sensing
5.3. Machine Learning and Signal Optimization
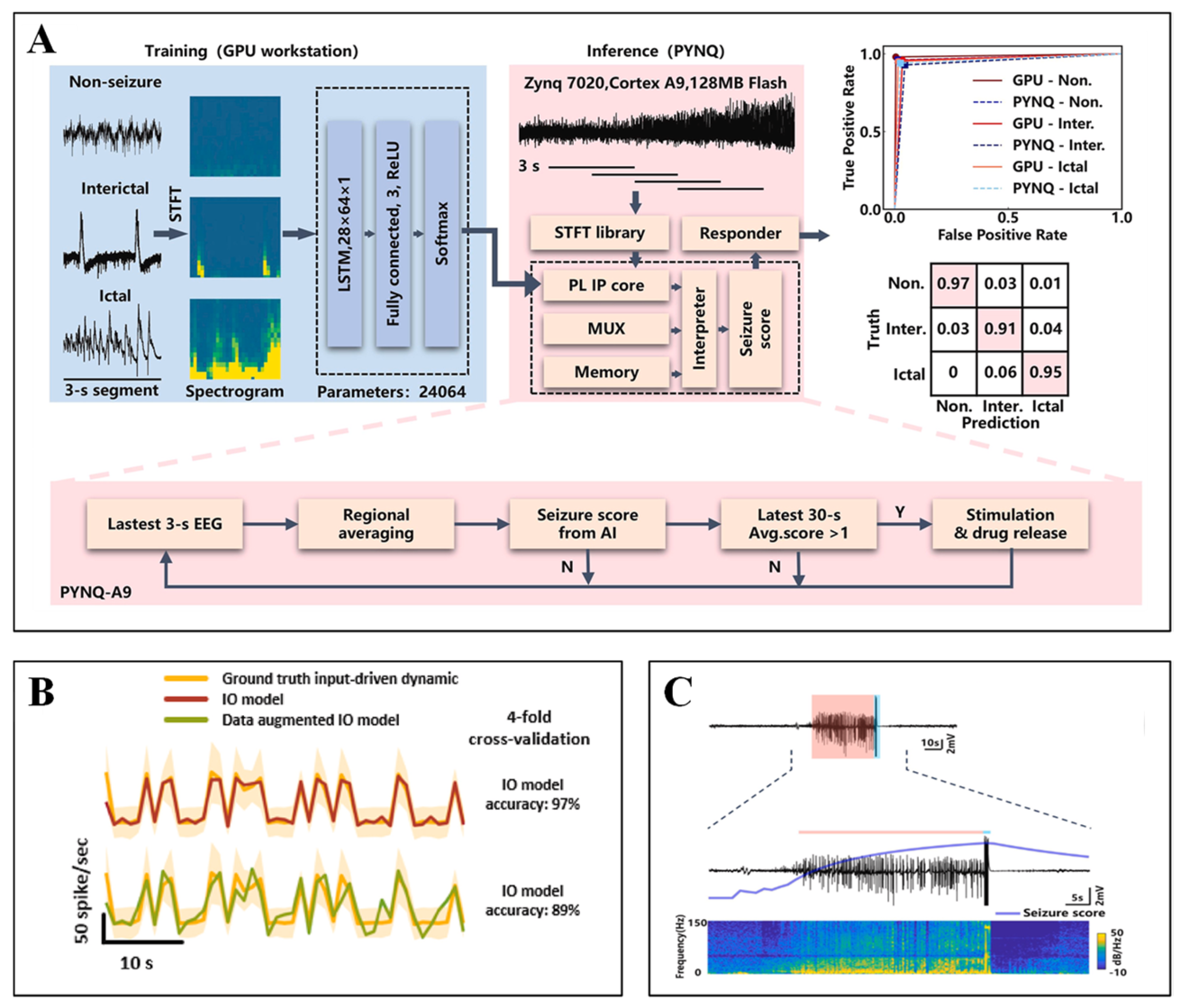
6. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Gong, Y.; Jiang, Z.; Stevens, T.; Li, W. Flexible high-density microelectrode arrays for closed-loop brain–machine interfaces: A review. Front. Neurosci. 2024, 18, 1348434. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lu, Y.; Wang, L. Neurotransmitter Sensing in Diagnosis and Research of Neurological Diseases. Chin. J. Anal. Chem. 2019, 47, 1651–1663. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, Y.; Yu, P.; Mao, L. Brain Electrochemistry. J. Electrochem. 2022, 28, 2108551. [Google Scholar] [CrossRef]
- Zhao, C.; Park, J.; Root, S.E.; Bao, Z. Skin-inspired soft bioelectronic materials, devices and systems. Nat. Rev. Bioeng. 2024, 2, 671–690. [Google Scholar] [CrossRef]
- Rousche, P.J.; Pellinen, D.S.; Pivin, D.P.; Williams, J.C.; Vetter, R.J.; Kipke, D.R. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans. Biomed. Eng. 2001, 48, 361–371. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Liu, H.; Wang, K.; Moses, K.; Feng, Z.; Li, P.; Huang, W. Flexible Electrodes for Brain–Computer Interface System. Adv. Mater. 2023, 35, 2211012. [Google Scholar] [CrossRef]
- Song, X.; Gu, Y.; Wang, S.; Wang, J.; Yu, L. Nanowire-Based Flexible Sensors for Wearable Electronics, Brain–Computer Interfaces, and Artificial Skins. Electron 2025, 3, e77. [Google Scholar] [CrossRef]
- Chai, C.; Yang, X.; Zheng, Y.; Heyat, M.B.B.; Li, Y.; Yang, D.; Chen, Y.; Sawan, M. Multimodal fusion of magnetoencephalography and photoacoustic imaging based on optical pump: Trends for wearable and noninvasive Brain–Computer interface. Biosens. Bioelectron. 2025, 278, 117321. [Google Scholar] [CrossRef]
- Sha, B.; Du, Z. Neural repair and regeneration interfaces: A comprehensive review. Biomed. Mater. 2024, 19, 022002. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Wu, H.; Beier, K.T.; Sawan, M. Challenges and future trends in wearable closed-loop neuromodulation to efficiently treat methamphetamine addiction. Front. Psychiatry 2023, 14, 1085036. [Google Scholar] [CrossRef]
- Al-Mufti, F.; Kim, M.; Dodson, V.; Sursal, T.; Bowers, C.; Cole, C.; Scurlock, C.; Becker, C.; Gandhi, C.; Mayer, S.A. Machine Learning and Artificial Intelligence in Neurocritical Care: A Specialty-Wide Disruptive Transformation or a Strategy for Success. Curr. Neurol. Neurosci. 2019, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, B.; Zhao, W.; Sheng, D.; Yin, L.; Sheng, X.; Yao, D. Multimodal Technologies for Closed-Loop Neural Modulation and Sensing. Adv. Healthc. Mater. 2024, 13, 2303289. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.A.R.; Goshi, N.; Seker, E. Multifunctional Neural Interfaces for Closed-Loop Control of Neural Activity. Adv. Funct. Mater. 2018, 28, 1703523. [Google Scholar] [CrossRef]
- Delong, L.M.; Witt, C.E.; Pennell, M.; Ross, A.E. A microfluidic chip for sustained oxygen gradient formation in the intestine ex vivo. Lab A Chip 2024, 24, 1918–1929. [Google Scholar] [CrossRef]
- Zaaimi, B.; Turnbull, M.; Hazra, A.; Wang, Y.; Gandara, C.; McLeod, F.; McDermott, E.E.; Escobedo-Cousin, E.; Idil, A.S.; Bailey, R.G.; et al. Closed-loop optogenetic control of the dynamics of neural activity in non-human primates. Nat. Biomed. Eng. 2023, 7, 559–575. [Google Scholar] [CrossRef]
- Gebodh, N.; Miskovic, V.; Laszlo, S.; Datta, A.; Bikson, M. A Scalable Framework for Closed-Loop Neuromodulation with Deep Learning. bioRxiv 2023, 2021–2023. [Google Scholar] [CrossRef]
- Oehrn, C.R.; Cernera, S.; Hammer, L.H.; Shcherbakova, M.; Yao, J.; Hahn, A.; Wang, S.; Ostrem, J.L.; Little, S.; Starr, P.A. Chronic adaptive deep brain stimulation versus conventional stimulation in Parkinson’s disease: A blinded randomized feasibility trial. Nat. Med. 2024, 30, 3345–3356. [Google Scholar] [CrossRef]
- Qi, X.; Du, Z.J.; Zhu, L.; Liu, X.; Xu, H.; Zhou, Z.; Zhong, C.; Li, S.; Wang, L.; Zhang, Z. The Glutamatergic Postrhinal Cortex–Ventrolateral Orbitofrontal Cortex Pathway Regulates Spatial Memory Retrieval. Neurosci. Bull. 2019, 35, 447–460. [Google Scholar] [CrossRef]
- Alba, N.; Du, Z.; Catt, K.; Kozai, T.; Cui, X. In Vivo Electrochemical Analysis of a PEDOT/MWCNT Neural Electrode Coating. Biosensors 2015, 5, 618–646. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Gu, M.; Yang, Z.; Zhan, L.; Du, Z. Sensing and Stimulation Applications of Carbon Nanomaterials in Implantable Brain-Computer Interface. Int. J. Mol. Sci. 2023, 24, 5182. [Google Scholar] [CrossRef]
- Li, Y.; Jarosova, R.; Weese-Myers, M.E.; Ross, A.E. Graphene-Fiber Microelectrodes for Ultrasensitive Neurochemical Detection. Anal. Chem. 2022, 94, 4803–4812. [Google Scholar] [CrossRef] [PubMed]
- Hanser, S.M.; Shao, Z.; Zhao, H.; Venton, B.J. Electrochemical treatment in KOH improves carbon nanomaterial performance to multiple neurochemicals. Analyst 2024, 149, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Niu, G.; Gao, F.; Lu, K.; Sun, Z.; Li, H.; Stenzel, M.; Liu, C.; Jiang, Y. Gold Nanorods (AuNRs) and Zeolitic Imidazolate Framework-8 (ZIF-8) Core–Shell Nanostructure-Based Electrochemical Sensor for Detecting Neurotransmitters. ACS Omega 2021, 6, 33149–33158. [Google Scholar] [CrossRef] [PubMed]
- Foremny, K.; Nagels, S.; Kreienmeyer, M.; Doll, T.; Deferme, W. Biocompatibility Testing of Liquid Metal as an Interconnection Material for Flexible Implant Technology. Nanomaterials 2021, 11, 3251. [Google Scholar] [CrossRef]
- Zhao, Z.; Soni, S.; Lee, T.; Nijhuis, C.A.; Xiang, D. Smart Eutectic Gallium–Indium: From Properties to Applications. Adv. Mater. 2023, 35, 2203391. [Google Scholar] [CrossRef]
- Li, J.; Mo, D.; Hu, J.; Wang, S.; Gong, J.; Huang, Y.; Li, Z.; Yuan, Z.; Xu, M. PEDOT:PSS-based bioelectronics for brain monitoring and modulation. Microsyst. Nanoeng. 2025, 11, 87. [Google Scholar] [CrossRef]
- Lee, D.; Park, S.; Seo, J.; Lee, W.; Kim, M.; Kim, J. Functionalized EGaIn Electrodes with Tunable Reduced-Graphene-Oxide Assembled EGaIn Core–Shell Particles for Soft and Deformable Electrochemical Biosensors. Adv. Funct. Mater. 2024, 34, 2311696. [Google Scholar] [CrossRef]
- Lim, T.; Kim, M.; Akbarian, A.; Kim, J.; Tresco, P.A.; Zhang, H. Conductive Polymer Enabled Biostable Liquid Metal Electrodes for Bioelectronic Applications. Adv. Healthc. Mater. 2022, 11, 2102382. [Google Scholar] [CrossRef]
- Du, Z.J.; Luo, X.; Weaver, C.; Cui, X.T. Poly (3, 4-ethylenedioxythiophene)-ionic liquid coating improves neural recording and stimulation functionality of MEAs. J. Mater. Chem. C 2015, 3, 6515–6524. [Google Scholar] [CrossRef]
- Du, Z.J.; Kolarcik, C.L.; Kozai, T.D.Y.; Luebben, S.D.; Sapp, S.A.; Zheng, X.S.; Nabity, J.A.; Cui, X.T. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017, 53, 46–58. [Google Scholar] [CrossRef]
- Golabchi, A.; Wu, B.; Du, Z.; Cui, X. Long-Term Neural Recording Performance of PEDOT/CNT/Dexamethasone-Coated Electrode Array Implanted in Visual Cortex of Rats. Adv. Nanobiomed Res. 2025, 5, 2400114. [Google Scholar] [CrossRef]
- Wu, B.; Castagnola, E.; McClung, C.A.; Cui, X.T. PEDOT/CNT Flexible MEAs Reveal New Insights into the Clock Gene’s Role in Dopamine Dynamics. Adv. Sci. 2024, 11, 2308212. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Alagumalai, K.; Kim, S.; Almutairi, T.M. Hybrid polypyrrole/poly (3,4-ethylenedioxythiophene) polystyrene sulfonate flexible electrode for monoamine transmitter serotonin in human fluids. Chem. Eng. J. 2025, 503, 158341. [Google Scholar] [CrossRef]
- Mun, T.J.; Yang, E.; Moon, J.; Kim, S.; Park, S.G.; Kim, M.; Choi, N.; Lee, Y.; Kim, S.J.; Seong, H. Silane-Functionalized MXene-PEGDA Hydrogel for Enhanced Electrochemical Sensing of Neurotransmitters and Antioxidants. ACS Appl. Polym. Mater. 2024, 6, 9533–9544. [Google Scholar] [CrossRef]
- Taylor, I.M.; Du, Z.; Bigelow, E.T.; Eles, J.R.; Horner, A.R.; Catt, K.A.; Weber, S.G.; Jamieson, B.G.; Cui, X.T. Aptamer-functionalized neural recording electrodes for the direct measurement of cocaine in vivo. J. Mater. Chem. B 2017, 5, 2445–2458. [Google Scholar] [CrossRef]
- Zeng, Z.; Fang, X.; Miao, W.; Liu, Y.; Maiyalagan, T.; Mao, S. Electrochemically Sensing of Trichloroacetic Acid with Iron (II) Phthalocyanine and Zn-Based Metal Organic Framework Nanocomposites. ACS Sens. 2019, 4, 1934–1941. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Jiao, Z.; Zhu, R.; Ma, L.; Zhou, K.; Yu, Z.; Wei, Q. Engineering a Au-NPs/Nafion modified nanoporous diamond sensing interface for reliable voltammetric quantification of dopamine in human serum. Chem. Eng. J. 2022, 446, 136927. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Zhu, B.; Chen, Q.; Zhang, Y.; Xu, Z.; Sun, D.; Lin, L.; Wu, D. Self-Patterning of Highly Stretchable and Electrically Conductive Liquid Metal Conductors by Direct-Write Super-Hydrophilic Laser-Induced Graphene and Electroless Copper Plating. ACS Appl. Mater. Inter. 2023, 15, 4713–4723. [Google Scholar] [CrossRef]
- Dong, R.; Wang, L.; Hang, C.; Chen, Z.; Liu, X.; Zhong, L.; Qi, J.; Huang, Y.; Liu, S.; Wang, L.; et al. Printed Stretchable Liquid Metal Electrode Arrays for In Vivo Neural Recording. Small 2021, 17, 2006612. [Google Scholar] [CrossRef]
- Du, Z.; Lu, Y.; Wei, P.; Deng, C.; Li, X. Progress in Devices and Materials for Implantable Multielectrode Arrays. Acta Phys. Chim. Sin. 2020, 36, 2007004. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, J.; Li, B.; Cao, Z.; Dong, S. Multimodal integrated flexible neural probe for in situ monitoring of EEG and lactic acid. RSC Adv. 2024, 14, 35520–35528. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Lu, W.; Zhang, Y.; Liu, Y.; Kim, J.U.; Shen, H.; Wu, Y.; Luan, H.; Kilner, K.; Lee, S.P.; et al. A wireless and battery-less implant for multimodal closed-loop neuromodulation in small animals. Nat. Biomed. Eng. 2023, 7, 1252–1269. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Ling, W.; Chen, Y.; Li, C.; Huang, X. Construction of a Flexible Optogenetic Device for Multisite and Multiregional Optical Stimulation Through Flexible µ-LED Displays on the Cerebral Cortex. Small 2023, 19, 2302241. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, M.; Vázquez-Guardado, A.; Wegener, A.J.; Grajales-Reyes, J.G.; Deng, Y.; Wang, T.; Avila, R.; Moreno, J.A.; Minkowicz, S.; et al. Wireless multilateral devices for optogenetic studies of individual and social behaviors. Nat. Neurosci. 2021, 24, 1035–1045. [Google Scholar] [CrossRef]
- Ling, W.; Yu, J.; Ma, N.; Li, Y.; Wu, Z.; Liang, R.; Hao, Y.; Pan, H.; Liu, W.; Fu, B.; et al. Flexible Electronics and Materials for Synchronized Stimulation and Monitoring in Multi-Encephalic Regions. Adv. Funct. Mater. 2020, 30, 2002644. [Google Scholar] [CrossRef]
- Yang, S.; Yang, S.; Li, P.; Gou, S.; Cheng, Y.; Jia, Q.; Du, Z. Advanced neuroprosthetic electrode design optimized by electromagnetic finite element simulation: Innovations and applications. Front. Bioeng. Biotech. 2024, 12, 1476447. [Google Scholar] [CrossRef]
- Zhao, C.; Cheung, K.M.; Huang, I.; Yang, H.; Nakatsuka, N.; Liu, W.; Cao, Y.; Man, T.; Weiss, P.S.; Monbouquette, H.G.; et al. Implantable aptamer–field-effect transistor neuroprobes for In Vivo neurotransmitter monitoring. Sci. Adv. 2021, 7, eabj7422. [Google Scholar] [CrossRef]
- Hemed, N.M.; Hwang, F.; Zhao, E.T.; Ding, J.B.; Melosh, N.A. Multiplexed neurochemical sensing with sub-nM sensitivity across 2.25 mm2 area. Biosens. Bioelectron. 2024, 261, 116474. [Google Scholar] [CrossRef]
- Masvidal-Codina, E.; Illa, X.; Dasilva, M.; Calia, A.B.; Dragojević, T.; Vidal-Rosas, E.E.; Prats-Alfonso, E.; Martínez-Aguilar, J.; De la Cruz, J.M.; Garcia-Cortadella, R.; et al. High-resolution mapping of infraslow cortical brain activity enabled by graphene microtransistors. Nat. Mater. 2019, 18, 280–288. [Google Scholar] [CrossRef]
- Lucarini, I.; Maita, F.; Conte, G.; Saracino, E.; Formaggio, F.; Palmieri, E.; Fabbri, R.; Konstantoulaki, A.; Lazzarini, C.; Caprini, M.; et al. Silicon Nanowire Mats Enable Advanced Bioelectrical Recordings in Primary DRG Cell Cultures. Adv. Healthc. Mater. 2025, 121, 2500379. [Google Scholar] [CrossRef]
- Gou, S.; Li, P.; Yang, S.; Bi, G.; Du, Z. High-Performance MXene/PEDOT-PSS Microscale Fiber Electrodes for Neural Recording and Stimulation. Adv. Funct. Mater. 2025, 35, 2424236. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Yang, H.; Jiang, W.; Jiang, J.; Zou, D.; Zhu, Z.; Tao, C.; Ni, S.; Zhou, Z.; et al. Ultraflexible Neural Electrodes Enabled Synchronized Long-Term Dopamine Detection and Wideband Chronic Recording Deep in Brain. ACS Nano 2024, 18, 34272–34287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, W.; Li, H.; Xuan, X.; Li, C.; Li, M. Flexible Au–Pt–vertical graphene neural microelectrode for the rapid detection of steady-state and transient dopamine in rats. Biosens. Bioelectron. 2025, 278, 117282. [Google Scholar] [CrossRef]
- Marken, F.; Neudeck, A.; Bond, A.M. Cyclic Voltammetry. In Electroanalytical Methods: Guide to Experiments and Applications; Scholz, F., Bond, A.M., Compton, R.G., Fiedler, D.A., Inzelt, G., Kahlert, H., Komorsky-Lovrić, Š., Lohse, H., Lovrić, M., Marken, F., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 57–106. [Google Scholar]
- Mirceski, V.; Skrzypek, S.; Stojanov, L. Square-wave voltammetry. Chemtexts 2018, 4, 17. [Google Scholar] [CrossRef]
- Alyamni, N.; Abot, J.L.; Zestos, A.G. Perspective—Advances in Voltammetric Methods for the Measurement of Biomolecules. ECS Sens. Plus 2024, 3, 27001. [Google Scholar] [CrossRef]
- Venton, B.J.; Cao, Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 2020, 145, 1158–1168. [Google Scholar] [CrossRef]
- Shin, M.; Venton, B.J. Fast-Scan Cyclic Voltammetry (FSCV) Reveals Behaviorally Evoked Dopamine Release by Sugar Feeding in the Adult Drosophila Mushroom Body. Angew. Chem. Int. Ed. 2022, 61, e202207399. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Yuan, L.; Zhang, B.; Bishop, E.S.; Wang, K.; Tang, J.; Zheng, Y.; Xu, W.; Niu, S.; et al. A tissue-like neurotransmitter sensor for the brain and gut. Nature 2022, 606, 94–101. [Google Scholar] [CrossRef]
- Ali, M.A.; Hu, C.; Yuan, B.; Jahan, S.; Saleh, M.S.; Guo, Z.; Gellman, A.J.; Panat, R. Breaking the barrier to biomolecule limit-of-detection via 3D printed multi-length-scale graphene-coated electrodes. Nat. Commun. 2021, 12, 7077. [Google Scholar] [CrossRef]
- Noh, S.; Lee, H.; Kim, J.; Jang, H.; An, J.; Park, C.; Lee, M.; Lee, T. Rapid electrochemical dual-target biosensor composed of an Aptamer/MXene hybrid on Au microgap electrodes for cytokines detection. Biosens. Bioelectron. 2022, 207, 114159. [Google Scholar] [CrossRef]
- El Ichi, S.; Zebda, A.; Laaroussi, A.; Reverdy-Bruas, N.; Chaussy, D.; Naceur Belgacem, M.; Cinquin, P.; Martin, D.K. Chitosan improves stability of carbon nanotube biocathodes for glucose biofuel cells. Chem. Commun. 2014, 50, 14535–14538. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tseng, H.; Chen, B.; Lo, Y.; Shao, H.; Wu, Y.; Li, S.; Chang, C.; Liu, T.; Hsieh, F.; et al. Proof of Concept for Sustainable Manufacturing of Neural Electrode Array for In Vivo Recording. Biosensors 2023, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, R.; Li, X.; Dong, N.; Zhu, B.; Wang, J.; Lin, X.; Su, B. COF-Coated Microelectrode for Space-Confined Electrochemical Sensing of Dopamine in Parkinson’s Disease Model Mouse Brain. J. Am. Chem. Soc. 2023, 145, 23727–23738. [Google Scholar] [CrossRef] [PubMed]
- Aashish, A.; Sadanandhan, N.K.; Ganesan, K.P.; Saraswathy Hareesh, U.N.; Muthusamy, S.; Devaki, S.J. Flexible Electrochemical Transducer Platform for Neurotransmitters. ACS Omega 2018, 3, 3489–3500. [Google Scholar] [CrossRef]
- Taylor, I.M.; Patel, N.A.; Freedman, N.C.; Castagnola, E.; Cui, X.T. Direct In Vivo Electrochemical Detection of Resting Dopamine Using Poly (3,4-ethylenedioxythiophene)/Carbon Nanotube Functionalized Microelectrodes. Anal. Chem. 2019, 91, 12917–12927. [Google Scholar] [CrossRef]
- Tan, C.; Dutta, G.; Yin, H.; Siddiqui, S.; Arumugam, P.U. Detection of neurochemicals with enhanced sensitivity and selectivity via hybrid multiwall carbon nanotube-ultrananocrystalline diamond microelectrodes. Sens. Actuators B Chem. 2018, 258, 193–203. [Google Scholar] [CrossRef]
- Zestos, A.G.; Yang, C.; Jacobs, C.B.; Hensley, D.; Venton, B.J. Carbon nanospikes grown on metal wires as microelectrode sensors for dopamine. Analyst 2015, 140, 7283–7292. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, R.; Chai, Y.; Zhang, Y.; Chen, S. A simple strategy based on lanthanum–multiwalled carbon nanotube nanocomposites for simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Sens. Actuators B Chem. 2012, 166–167, 601–607. [Google Scholar] [CrossRef]
- Holzer, B.; Manoli, K.; Ditaranto, N.; Macchia, E.; Tiwari, A.; Di Franco, C.; Scamarcio, G.; Palazzo, G.; Torsi, L. Characterization of Covalently Bound Anti-Human Immunoglobulins on Self-Assembled Monolayer Modified Gold Electrodes. Adv. Biosyst. 2017, 1, 1700055. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, N.; Yetisen, A.K. Brain neurochemical monitoring. Biosens. Bioelectron. 2021, 189, 113351. [Google Scholar] [CrossRef]
- Zhou, B.; Fan, K.; Guo, J.; Feng, J.; Yang, C.; Li, Y.; Shi, S.; Kong, L. Plug-and-play fiber-optic sensors based on engineered cells for neurochemical monitoring at high specificity in freely moving animals. Sci. Adv. 2023, 9, eadg218. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, H.; Zhao, X.; Wu, J.; Muhammad, F.; Lin, S.; He, J.; Zhou, L.; Zhang, C.; Deng, Y.; et al. Surface-Enhanced Raman Scattering Active Gold Nanoparticles with Enzyme-Mimicking Activities for Measuring Glucose and Lactate in Living Tissues. ACS Nano 2017, 11, 5558–5566. [Google Scholar] [CrossRef] [PubMed]
- Pisano, F.; Masmudi-Martín, M.; Andriani, M.S.; Cid, E.; Kazemzadeh, M.; Pisanello, M.; Balena, A.; Collard, L.; Parras, T.J.; Bianco, M.; et al. Vibrational fiber photometry: Label-free and reporter-free minimally invasive Raman spectroscopy deep in the mouse brain. Nat. Methods 2025, 22, 371–379. [Google Scholar] [CrossRef]
- Paria, D.; Convertino, A.; Mussi, V.; Maiolo, L.; Barman, I. Silver-Coated Disordered Silicon Nanowires Provide Highly Sensitive Label-Free Glycated Albumin Detection through Molecular Trapping and Plasmonic Hotspot Formation. Adv. Healthc. Mater. 2021, 10, 2001110. [Google Scholar] [CrossRef]
- Chang, H.; Hur, W.; Kang, H.; Jun, B. In vivo surface-enhanced Raman scattering techniques: Nanoprobes, instrumentation, and applications. Light Sci. Appl. 2025, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, F.; Li, Y.; Shan, J.; Lu, Y.; Liu, Q. Bio-electron transfer modulated localized surface plasmon resonance biosensing with charge density monitoring. Biosens. Bioelectron. 2022, 201, 113956. [Google Scholar] [CrossRef]
- Li, N.; Lu, Y.; Li, S.; Zhang, Q.; Wu, J.; Jiang, J.; Liu, G.L.; Liu, Q. Monitoring the electrochemical responses of neurotransmitters through localized surface plasmon resonance using nanohole array. Biosens. Bioelectron. 2017, 93, 241–249. [Google Scholar] [CrossRef]
- Soltani, S. Microdialysis sampling in the brain: Analytical approaches and challenges. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Zetterström, T.; Ungerstedt, U. Effects of apomorphine on the in vivo release of dopamine and its metabolites, studied by brain dialysis. Eur. J. Pharmacol. 1984, 97, 29–36. [Google Scholar] [CrossRef]
- Kennedy, R.T. Emerging trends in in vivo neurochemical monitoring by microdialysis. Curr. Opin. Chem. Biol. 2013, 17, 860–867. [Google Scholar] [CrossRef]
- Robbins, E.M.; Jaquins-Gerstl, A.S.; Okonkwo, D.O.; Boutelle, M.G.; Michael, A.C. Dexamethasone-Enhanced Continuous Online Microdialysis for Neuromonitoring of O2 after Brain Injury. ACS Chem. Neurosci. 2023, 14, 2476–2486. [Google Scholar] [CrossRef]
- Petit-Pierre, G.; Colin, P.; Laurer, E.; Déglon, J.; Bertsch, A.; Thomas, A.; Schneider, B.L.; Renaud, P. In Vivo neurochemical measurements in cerebral tissues using a droplet-based monitoring system. Nat. Commun. 2017, 8, 1239. [Google Scholar] [CrossRef] [PubMed]
- Samper, I.C.; Gowers, S.A.N.; Rogers, M.L.; Murray, D.R.K.; Jewell, S.L.; Pahl, C.; Strong, A.J.; Boutelle, M.G. 3D printed microfluidic device for online detection of neurochemical changes with high temporal resolution in human brain microdialysate. Lab. A Chip 2019, 19, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Chae, U.; Shin, H.; Choi, N.; Ji, M.; Park, H.; Lee, S.H.; Woo, J.; Cho, Y.; Kim, K.; Yang, S.; et al. Bimodal neural probe for highly co-localized chemical and electrical monitoring of neural activities in vivo. Biosens. Bioelectron. 2021, 191, 113473. [Google Scholar] [CrossRef]
- Fan, P.; Wang, Y.; Dai, Y.; Jing, L.; Liang, W.; Lu, B.; Yang, G.; Song, Y.; Wu, Y.; Cai, X. Flexible microelectrode array probe for simultaneous detection of neural discharge and dopamine in striatum of mice aversion system. Sens. Actuators B Chem. 2023, 390, 133990. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, D.; Li, M.; Xuan, X.; Li, H. Brain–Computer Interface and Electrochemical Sensor Based on Boron–Nitrogen Co-Doped Graphene–Diamond Microelectrode for EEG and Dopamine Detection. ACS Sens. 2025, 10, 868–880. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Xia, J.; Zhao, W.; Dong, S.; Ye, Z.; Pan, G.; Luo, J.; Zhang, S. Multimodal Electrocorticogram Active Electrode Array Based on Zinc Oxide-Thin Film Transistors. Adv. Sci. 2023, 10, 2204467. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Cai, X.; Xie, Y.; Wang, T.; Cheng, D.; Li, L.; Li, R.; Deng, Y.; Ding, H.; et al. A wireless, implantable optoelectrochemical probe for optogenetic stimulation and dopamine detection. Microsyst. Nanoeng. 2020, 6, 64. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Xia, X.; Zhang, K.; Wu, J.; Liu, Y.; Zhang, C.; Cai, X.; Lu, J.; Xu, L.; et al. An ultrasensitive multimodal intracranial pressure biotelemetric system enabled by exceptional point and iontronics. Nat. Commun. 2024, 15, 9557. [Google Scholar] [CrossRef]
- Du, Z.J.; Bi, G.Q.; Cui, X.T. Electrically Controlled Neurochemical Release from Dual-Layer Conducting Polymer Films for Precise Modulation of Neural Network Activity in Rat Barrel Cortex. Adv. Funct. Mater. 2018, 28, 1703988. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.S. Closed-loop neural interfaces for pain: Where do we stand? Cell Rep. Med. 2024, 5, 101662. [Google Scholar] [CrossRef]
- Nong, H.; Jin, M.; Pan, C.; Zhou, H.; Zhang, C.; Pan, X.; Chen, Y.; Wei, X.; Lu, Y.; Zhao, K.; et al. Intelligent Sensing Technologies Based on Flexible Wearable Sensors: A Review. IEEE Sens. J. 2024, 24, 22197–22217. [Google Scholar] [CrossRef]
- Costa, J.C.; Spina, F.; Lugoda, P.; Garcia-Garcia, L.; Roggen, D.; Munzenrieder, N. Flexible Sensors—From Materials to Applications. Technologies 2019, 7, 35. [Google Scholar] [CrossRef]
- Luo, Y.; Abidian, M.R.; Ahn, J.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.; Berggren, M.; Berkey, C.A.; Bettinger, C.J.; et al. Technology Roadmap for Flexible Sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An Overview of the Development of Flexible Sensors. Adv. Mater. 2017, 29, 1700375. [Google Scholar] [CrossRef]
- Liu, S.; Guo, W.; Chen, H.; Yin, Z.; Tang, X.; Sun, Q. Recent Progress on Flexible Self-Powered Tactile Sensing Platforms for Health Monitoring and Robotics. Small 2024, 20, 2405520. [Google Scholar] [CrossRef]
- Wu, G.; Li, X.; Bao, R.; Pan, C. Innovations in Tactile Sensing: Microstructural Designs for Superior Flexible Sensor Performance. Adv. Funct. Mater. 2024, 34, 2405722. [Google Scholar] [CrossRef]
- Tang, J.; Gou, K.; Wang, C.; Wei, M.; Tan, Q.; Weng, G. Self-Powered and 3D Printable Soft Sensor for Human Health Monitoring, Object Recognition, and Contactless Hand Gesture Recognition. Adv. Funct. Mater. 2024, 34, 2411172. [Google Scholar] [CrossRef]
- Roberts, J.G.; Sombers, L.A. Fast-Scan Cyclic Voltammetry: Chemical Sensing in the Brain and Beyond. Anal. Chem. 2018, 90, 490–504. [Google Scholar] [CrossRef]
- Ruckodanov, D.A.; Maidment, N.T.; Monbouquette, H.G. Electrochemical Sensing of Dopamine with an Implantable Microelectrode Array Microprobe Including an On-Probe Iridium Oxide Reference Electrode. ACS Chem. Neurosci. 2025, 16, 642–648. [Google Scholar] [CrossRef]
- Siwakoti, U.; Jones, S.A.; Kumbhare, D.; Cui, X.T.; Castagnola, E. Recent Progress in Flexible Microelectrode Arrays for Combined Electrophysiological and Electrochemical Sensing. Biosensors 2025, 15, 100. [Google Scholar] [CrossRef]
- Kook, G.; Lee, S.W.; Lee, H.C.; Cho, I.; Lee, H.J. Neural Probes for Chronic Applications. Micromachines 2016, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Xu, Z.; Mo, F.; Wang, Y.; Duan, Y.; Liu, Y.; Jing, L.; Shan, J.; Jia, Q.; Wang, M.; et al. Long-term stability strategies of deep brain flexible neural interface. NPJ Flex. Electron. 2025, 9, 40. [Google Scholar] [CrossRef]
- Gupta, B.; Perillo, M.L.; Siegenthaler, J.R.; Christensen, I.E.; Welch, M.P.; Rechenberg, R.; Banna, G.M.H.U.; Galstyan, D.; Becker, M.F.; Li, W.; et al. In Vitro Biofouling Performance of Boron-Doped Diamond Microelectrodes for Serotonin Detection Using Fast-Scan Cyclic Voltammetry. Biosensors 2023, 13, 576. [Google Scholar] [CrossRef]
- Qi, Y.; Kang, S.; Fang, H.; Guest, E. Advanced materials for implantable neuroelectronics. MRS Bull. 2023, 48, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, R.; Zhang, H.; Cao, P.; Liu, Z.; Li, Y. Research Progress on the Flexibility of an Implantable Neural Microelectrode. Micromachines 2022, 13, 386. [Google Scholar] [CrossRef]
- Shi, D.; Narayanan, S.; Woeppel, K.; Cui, X.T. Improving the Biocompatibility and Functionality of Neural Interface Devices with Silica Nanoparticles. Acc. Chem. Res. 2024, 57, 1684–1695. [Google Scholar] [CrossRef]
- Vomero, M.; Ciarpella, F.; Zucchini, E.; Kirsch, M.; Fadiga, L.; Stieglitz, T.; Asplund, M. On the longevity of flexible neural interfaces: Establishing biostability of polyimide-based intracortical implants. Biomaterials 2022, 281, 121372. [Google Scholar] [CrossRef]
- Capuani, S.; Malgir, G.; Chua, C.Y.X.; Grattoni, A. Advanced strategies to thwart foreign body response to implantable devices. Bioeng. Transl. Med. 2022, 7, e10300. [Google Scholar] [CrossRef]
- Gori, M.; Vadalà, G.; Giannitelli, S.M.; Denaro, V.; Di Pino, G. Biomedical and Tissue Engineering Strategies to Control Foreign Body Reaction to Invasive Neural Electrodes. Front. Bioeng. Biotechnol. 2021, 9, 659033. [Google Scholar] [CrossRef]
- Merolla, A.; Michetti, C.; Moschetta, M.; Vacca, F.; Ciano, L.; Emionite, L.; Astigiano, S.; Romei, A.; Horenkamp, S.; Berglund, K.; et al. A pH-sensitive closed-loop nanomachine to control hyperexcitability at the single neuron level. Nat. Commun. 2024, 15, 5609. [Google Scholar] [CrossRef]
- Krishnan, A.; Forssell, M.; Du, Z.; Cui, X.T.; Fedder, G.K.; Kelly, S.K. Residual voltage as an ad-hoc indicator of electrode damage in biphasic electrical stimulation. J. Neural Eng. 2021, 18, 0460c1. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tian, Y.; Jiang, L.; Jin, S.; Ye, Y.; Lu, Y.; Su, H.; Yang, Y.; Wei, X.; Zhou, Z.; et al. A bimodal closed-loop neuromodulation implant integrated with ultraflexible probes to treat epilepsy. Biosens. Bioelectron. 2025, 271, 117071. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chen, H.; Chen, X.; Lin, Z.; Yang, Q.; Tie, C.; Wang, H.; Niu, L.; Guo, Y.; Zheng, H. Noninvasive closed-loop acoustic brain-computer interface for seizure control. Theranostics 2024, 14, 5965–5981. [Google Scholar] [CrossRef] [PubMed]
- Topalovic, U.; Barclay, S.; Ling, C.; Alzuhair, A.; Yu, W.; Hokhikyan, V.; Chandrakumar, H.; Rozgic, D.; Jiang, W.; Basir-Kazeruni, S.; et al. A wearable platform for closed-loop stimulation and recording of single-neuron and local field potential activity in freely moving humans. Nat. Neurosci. 2023, 26, 517–527. [Google Scholar] [CrossRef]
- Johnson, G.W.; Doss, D.J.; Makhoul, G.S.; Cai, L.Y.; Bibro, C.E.; Paulo, D.L.; Reddy, S.B.; Naftel, R.P.; Haas, K.F.; Wallace, M.T.; et al. Brain-State Modeling Using Electroencephalography: Application to Adaptive Closed-Loop Neuromodulation for Epilepsy; Cold Spring Harbor Laboratory: Cold Spring, NY, USA, 2024. [Google Scholar]
- Lee, S.; Park, S.; Park, J.; Lee, J.Y. Implantable polypyrrole bioelectrodes inducing anti-inflammatory macrophage polarization for long-term In Vivo signal recording. Acta Biomater. 2023, 168, 458–469. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, M.; Pan, S.J.; Loy, L.Y.; Guo, W.; Zhang, Z.; Chin, P.L.; Guan, C.; King, N.K.K.; Ang, B.T. Artificial neural network based intracranial pressure mean forecast algorithm for medical decision support. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 7111–7114. [Google Scholar]
- Motamedi-Khozani, R.; Abbasi-Moayed, S.; Hormozi-Nezhad, M.R. Single-Component Double-Emissive Ratiometric Probe: Toward Machine Learning Driven Detection and Discrimination of Neurological Biomarkers. Anal. Chem. 2025, 97, 8248–8257. [Google Scholar] [CrossRef]
- Wang, J.; Huang, D.; Zheng, D.; Shen, F.; Zhang, Y. Selectively Quantify Toxic Pollutants in Water by Machine Learning Empowered Electrochemical Biosensors. Env. Sci. Technol. 2025, 59, 616–627. [Google Scholar] [CrossRef]
- Mun, J.; Lee, J.; Park, S. Real-time closed-loop brainstem stimulation modality for enhancing temporal blood pressure reduction. Brain Stimul. Basic. Transl. Clin. Res. Neuromodulation 2024, 17, 826–835. [Google Scholar] [CrossRef]
- Gou, S.; Yang, S.; Cheng, Y.; Yang, S.; Liu, H.; Li, P.; Du, Z. Applications of 2D Nanomaterials in Neural Interface. Int. J. Mol. Sci. 2024, 25, 8615. [Google Scholar] [CrossRef]
- Musk, E. An Integrated Brain-Machine Interface Platform with Thousands of Channels. J. Med. Internet Res. 2019, 21, e16194. [Google Scholar] [CrossRef]
- Oxley, T.J.; Yoo, P.E.; Rind, G.S.; Ronayne, S.M.; Lee, C.M.S.; Bird, C.; Hampshire, V.; Sharma, R.P.; Morokoff, A.; Williams, D.L.; et al. Motor neuroprosthesis implanted with neurointerventional surgery improves capacity for activities of daily living tasks in severe paralysis: First in-human experience. Br. Med. J. Publ. Group 2021, 13, 102–108. [Google Scholar] [CrossRef]
- Hochberg, L.R.; Bacher, D.; Jarosiewicz, B.; Masse, N.Y.; Simeral, J.D.; Vogel, J.; Haddadin, S.; Liu, J.; Cash, S.S.; van der Smagt, P.; et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 2012, 485, 372–375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Qiao, X.; Ma, J.; Yang, Z.; Luo, X.; Du, Z. Recent Advances in Flexible Sensors for Neural Interfaces: Multimodal Sensing, Signal Integration, and Closed-Loop Feedback. Biosensors 2025, 15, 424. https://doi.org/10.3390/bios15070424
Yang S, Qiao X, Ma J, Yang Z, Luo X, Du Z. Recent Advances in Flexible Sensors for Neural Interfaces: Multimodal Sensing, Signal Integration, and Closed-Loop Feedback. Biosensors. 2025; 15(7):424. https://doi.org/10.3390/bios15070424
Chicago/Turabian StyleYang, Siyi, Xiujuan Qiao, Junlong Ma, Zhihao Yang, Xiliang Luo, and Zhanhong Du. 2025. "Recent Advances in Flexible Sensors for Neural Interfaces: Multimodal Sensing, Signal Integration, and Closed-Loop Feedback" Biosensors 15, no. 7: 424. https://doi.org/10.3390/bios15070424
APA StyleYang, S., Qiao, X., Ma, J., Yang, Z., Luo, X., & Du, Z. (2025). Recent Advances in Flexible Sensors for Neural Interfaces: Multimodal Sensing, Signal Integration, and Closed-Loop Feedback. Biosensors, 15(7), 424. https://doi.org/10.3390/bios15070424






