A Novel Wearable Device for Continuous Blood Pressure Monitoring Utilizing Strain Gauge Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subject Recruitment
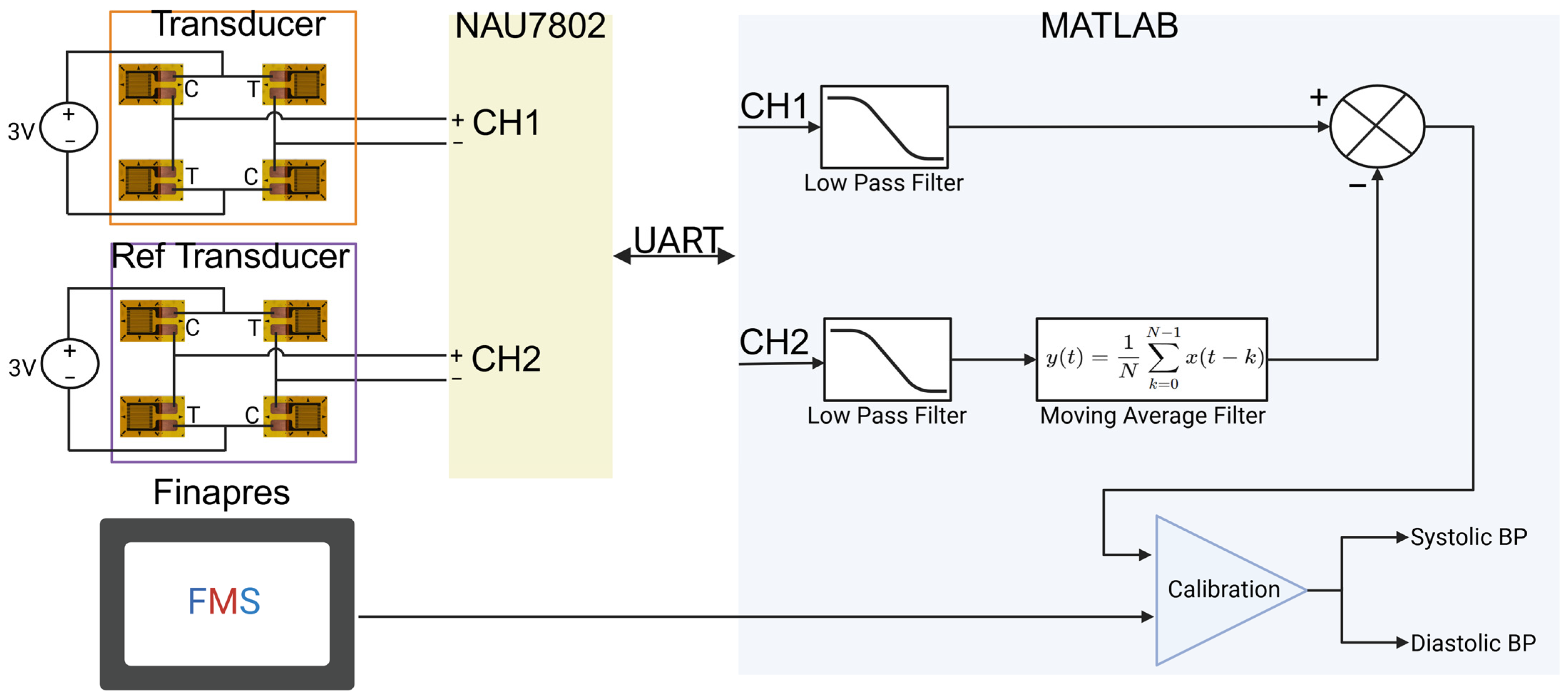
2.2. Design and Fabrication
2.3. System Calibration
2.4. Transducer Calibration and Characterization
2.5. Physics
2.6. Data Collection
2.6.1. Pre-Testing
2.6.2. Data Collection/Static Testing
2.6.3. Data Collection/Dynamic Testing
2.7. Data Processing
2.7.1. System Characterization
2.7.2. Dynamic Testing
3. Results
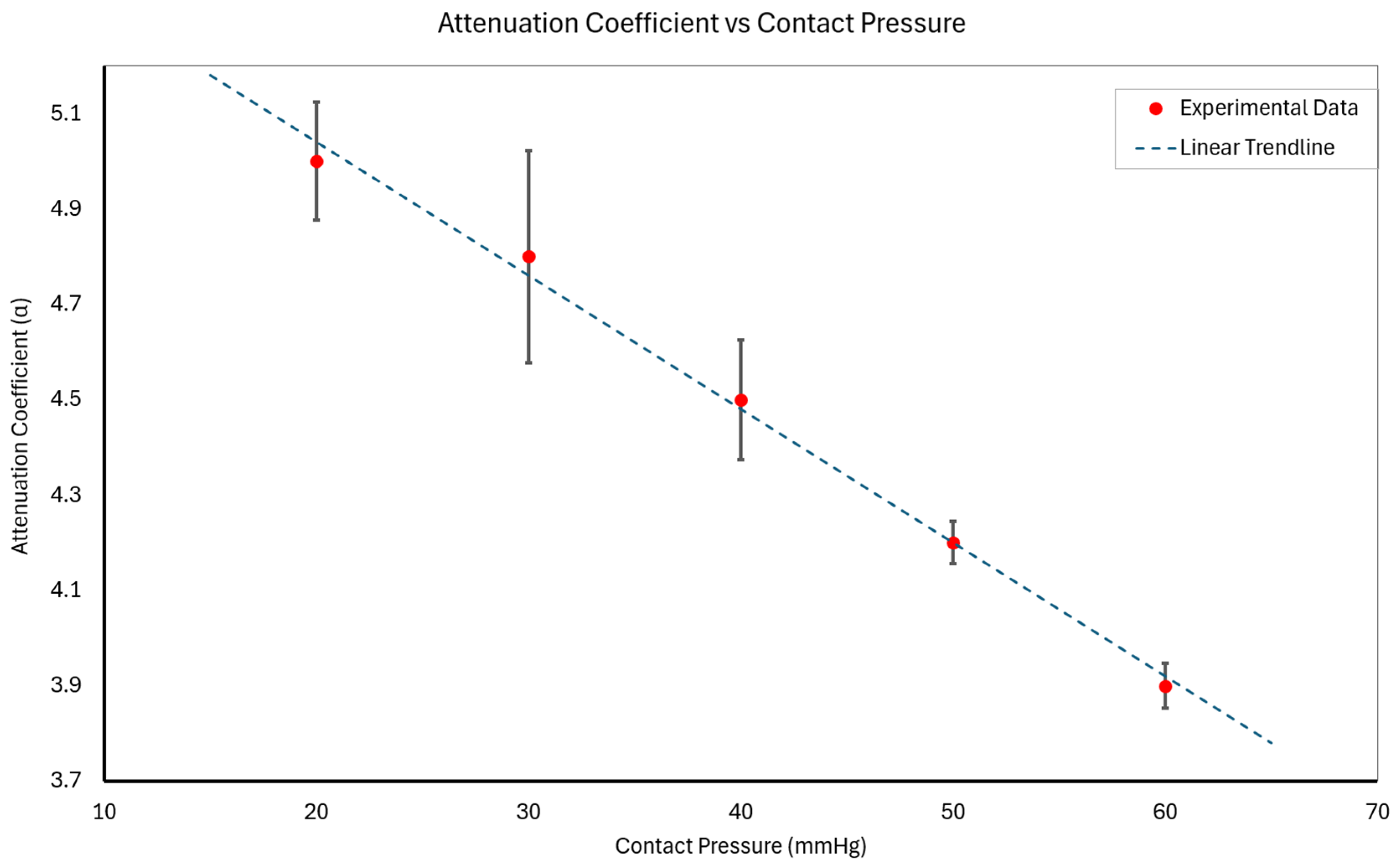
3.1. Static Contact Pressure Analysis and Attenuation Assessment
- At 20 mmHg: α20 = 5.0 ± 0.1;
- At 30 mmHg: α30 = 4.8 ± 0.2;
- At 40 mmHg: α40 = 4.5 ± 0.1;
- At 50 mmHg: α50 = 4.2 ± 0.1;
- At 60 mmHg: α60 = 3.9 ± 0.1;
- where X and Y represent the mean and standard deviation of α across subjects, respectively.
3.2. Model-Based Analysis
3.3. Assessment of Device Performance Under Dynamic Physiological Conditions
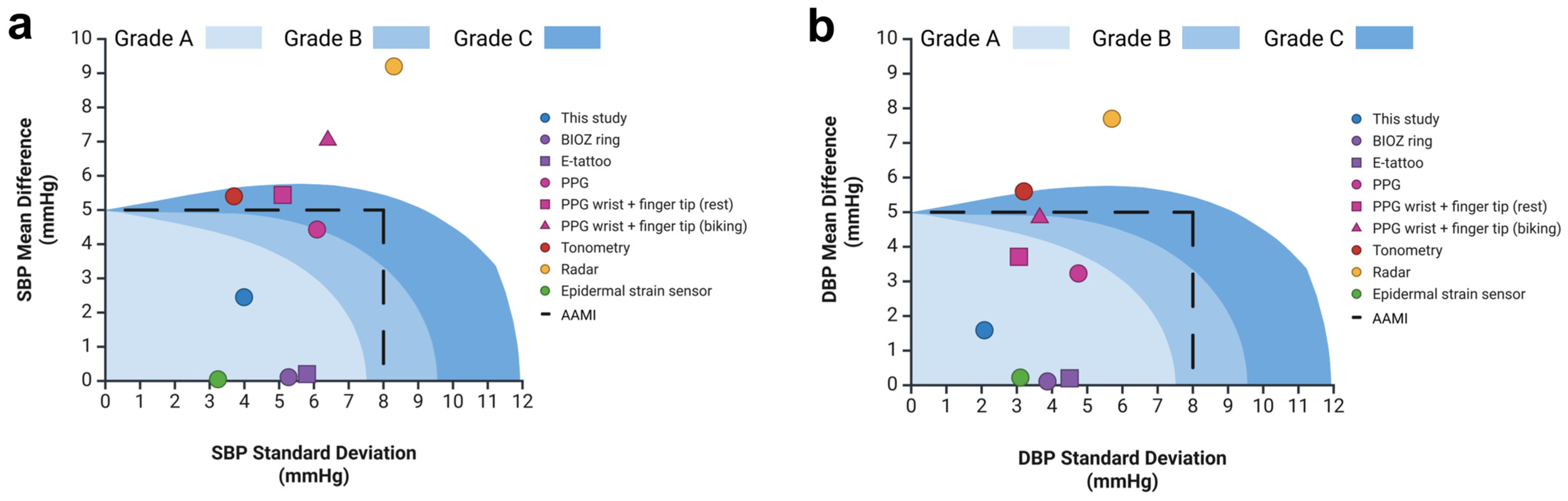
3.4. Comparison of Device Accuracy with Reference Standard
4. Discussion
4.1. Comparison to Prior Work and State-of-the-Art Devices
4.2. Limitations
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Analog-to-Digital Converter |
| AC | Alternating Current |
| AAMI | Association for the Advancement of Medical Instrumentation |
| BMI | Body Mass Index |
| BP | Blood Pressure |
| BHS | British Hypertension Society |
| BIOZ | Bioimpedance |
| CVD | Cardiovascular Disease |
| DBP | Diastolic Blood Pressure |
| DC | Direct Current |
| FMS | Finapres Medical System |
| I2C | Inter-Integrated Circuit |
| IRB | Institutional Review Board |
| LoA | Limits of Agreement |
| MAE | Mean Absolute Error |
| MmHg | Millimeters of Mercury |
| NOVA | Noninvasive Vascular Analyzer |
| PPG | Photoplethysmography |
| PTT | Pulse Transit Time |
| Pc | Contact Pressure |
| SBP | Systolic Blood Pressure |
| UART | Universal Asynchronous Receiver/Transmitter |
| Z | Mechanical Impedance |
References
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J. The heart of the world. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Tackling, G.; Borhade, M.B. Hypertensive heart disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Powell-Wiley, T.M.; Baumer, Y.; Baah, F.O.; Baez, A.S.; Farmer, N.; Mahlobo, C.T.; Pita, M.A.; Potharaju, K.A.; Tamura, K.; Wallen, G.R. Social determinants of cardiovascular disease. Circ. Res. 2022, 130, 782–799. [Google Scholar] [CrossRef] [PubMed]
- Charchar, F.J.; Prestes, P.R.; Mills, C.; Ching, S.M.; Neupane, D.; Marques, F.Z.; Sharman, J.E.; Vogt, L.; Burrell, L.M.; Korostovtseva, L. Lifestyle management of hypertension: International Society of Hypertension position paper endorsed by the World Hypertension League and European Society of Hypertension. J. Hypertens. 2024, 42, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Ogedegbe, G.; Pickering, T. Principles and techniques of blood pressure measurement. Cardiol. Clin. 2010, 28, 571–586. [Google Scholar] [CrossRef]
- Nitzan, M.; Slotki, I.; Shavit, L. More accurate systolic blood pressure measurement is required for improved hypertension management: A perspective. Med. Devices Evid. Res. 2017, 10, 157–163. [Google Scholar] [CrossRef]
- Mirdamadi, A.; Etebari, M. Comparison of manual versus automated blood pressure measurement in intensive care unit, coronary care unit, and emergency room. ARYA Atheroscler. 2017, 13, 29. [Google Scholar]
- Khan Mamun, M.M.R.; Sherif, A. Advancement in the cuffless and noninvasive measurement of blood pressure: A review of the literature and open challenges. Bioengineering 2022, 10, 27. [Google Scholar] [CrossRef]
- Bilo, G.; Sala, O.; Perego, C.; Faini, A.; Gao, L.; Głuszewska, A.; Ochoa, J.E.; Pellegrini, D.; Lonati, L.M.; Parati, G. Impact of cuff positioning on blood pressure measurement accuracy: May a specially designed cuff make a difference? Hypertens. Res. 2017, 40, 573–580. [Google Scholar] [CrossRef]
- Mahe, G.; Comets, E.; Nouni, A.; Paillard, F.; Dourmap, C.; Le Faucheur, A.; Jaquinandi, V. A minimal resting time of 25 min is needed before measuring stabilized blood pressure in subjects addressed for vascular investigations. Sci. Rep. 2017, 7, 12893. [Google Scholar] [CrossRef]
- Zheng, D.; Giovannini, R.; Murray, A. Effect of respiration, talking and small body movements on blood pressure measurement. J. Hum. Hypertens. 2012, 26, 458–462. [Google Scholar] [CrossRef]
- Pioli, M.R.; Ritter, A.M.; de Faria, A.P.; Modolo, R. White coat syndrome and its variations: Differences and clinical impact. Integr. Blood Press. Control 2018, 11, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Kuruvilla, S.; Ogedegbe, G.; Gerin, W.; Schwartz, J.E.; Pickering, T.G. What is the optimal interval between successive home blood pressure readings using an automated oscillometric device? J. Hypertens. 2009, 27, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Barbosa, K.; Ho, V.; Griggs, D.; Ghirmai, T.; Krishnan, S.K.; Hsiai, T.K.; Chiao, J.-C.; Cao, H. Cuff-less and continuous blood pressure monitoring: A methodological review. Technologies 2017, 5, 21. [Google Scholar] [CrossRef]
- Konstantinidis, D.; Iliakis, P.; Tatakis, F.; Thomopoulos, K.; Dimitriadis, K.; Tousoulis, D.; Tsioufis, K. Wearable blood pressure measurement devices and new approaches in hypertension management: The digital era. J. Hum. Hypertens. 2022, 36, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Charlton, P.H.; Allen, J.; Bailón, R.; Baker, S.; Behar, J.A.; Chen, F.; Clifford, G.D.; Clifton, D.A.; Davies, H.J.; Ding, C. The 2023 wearable photoplethysmography roadmap. Physiol. Meas. 2023, 44, 111001. [Google Scholar] [CrossRef]
- Elgendi, M.; Fletcher, R.; Liang, Y.; Howard, N.; Lovell, N.H.; Abbott, D.; Lim, K.; Ward, R. The use of photoplethysmography for assessing hypertension. npj Digit. Med. 2019, 2, 60. [Google Scholar] [CrossRef]
- Schutte, A.E.; Kollias, A.; Stergiou, G.S. Blood pressure and its variability: Classic and novel measurement techniques. Nat. Rev. Cardiol. 2022, 19, 643–654. [Google Scholar] [CrossRef]
- Scardulla, F.; Cosoli, G.; Spinsante, S.; Poli, A.; Iadarola, G.; Pernice, R.; Busacca, A.; Pasta, S.; Scalise, L.; D’Acquisto, L. Photoplethysmograhic sensors, potential and limitations: Is it time for regulation? A comprehensive review. Measurement 2023, 218, 113150. [Google Scholar] [CrossRef]
- Fine, J.; Branan, K.L.; Rodriguez, A.J.; Boonya-Ananta, T.; Ajmal; Ramella-Roman, J.C.; McShane, M.J.; Cote, G.L. Sources of inaccuracy in photoplethysmography for continuous cardiovascular monitoring. Biosensors 2021, 11, 126. [Google Scholar] [CrossRef]
- Rodriguez, A.J.; Boonya-Ananta, M.T.; Gonzalez, M.; Le, V.N.D.; Fine, J.; Palacios, C.; McShane, M.J.; Coté, G.L.; Ramella-Roman, J.C. Skin optical properties in the obese and their relation to body mass index: A review. J. Biomed. Opt. 2022, 27, 030902. [Google Scholar] [CrossRef]
- Moraes, J.L.; Rocha, M.X.; Vasconcelos, G.G.; Vasconcelos Filho, J.E.; De Albuquerque, V.H.C.; Alexandria, A.R. Advances in photopletysmography signal analysis for biomedical applications. Sensors 2018, 18, 1894. [Google Scholar] [CrossRef]
- Finnegan, E.; Davidson, S.; Harford, M.; Watkinson, P.; Tarassenko, L.; Villarroel, M. Features from the photoplethysmogram and the electrocardiogram for estimating changes in blood pressure. Sci. Rep. 2023, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- El-Hajj, C.; Kyriacou, P.A. A review of machine learning techniques in photoplethysmography for the non-invasive cuff-less measurement of blood pressure. Biomed. Signal Process. Control 2020, 58, 101870. [Google Scholar] [CrossRef]
- Ibrahim, B.; Jafari, R. Cuffless blood pressure monitoring from an array of wrist bio-impedance sensors using subject-specific regression models: Proof of concept. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Vysotskaya, N.; Will, C.; Servadei, L.; Maul, N.; Mandl, C.; Nau, M.; Harnisch, J.; Maier, A. Continuous non-invasive blood pressure measurement using 60 GHz-radar—A feasibility study. Sensors 2023, 23, 4111. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Sun, G.; Hoshiga, M.; Kotani, K.; Asao, T. Noncontact Monitoring of Relative Changes in Blood Pressure Using Microwave Radar Sensors. J. Biomed. Sci. Eng. 2022, 15, 51–65. [Google Scholar] [CrossRef]
- Sharma, P.; Imtiaz, S.A.; Rodriguez-Villegas, E. Acoustic sensing as a novel wearable approach for cardiac monitoring at the wrist. Sci. Rep. 2019, 9, 20079. [Google Scholar] [CrossRef]
- Sel, K.; Osman, D.; Huerta, N.; Edgar, A.; Pettigrew, R.I.; Jafari, R. Continuous cuffless blood pressure monitoring with a wearable ring bioimpedance device. npj Digit. Med. 2023, 6, 59. [Google Scholar] [CrossRef]
- Naranjo-Hernández, D.; Reina-Tosina, J.; Min, M. Fundamentals, recent advances, and future challenges in bioimpedance devices for healthcare applications. J. Sens. 2019, 2019, 9210258. [Google Scholar] [CrossRef]
- Goyal, K.; Borkholder, D.A.; Day, S.W. Dependence of skin-electrode contact impedance on material and skin hydration. Sensors 2022, 22, 8510. [Google Scholar] [CrossRef]
- Ibrahim, B.; McMurray, J.; Jafari, R. A wrist-worn strap with an array of electrodes for robust physiological sensing. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA; 2018; pp. 4313–4317. [Google Scholar]
- Alvarez, J.T.; Gerez, L.F.; Araromi, O.A.; Hunter, J.G.; Choe, D.K.; Payne, C.J.; Wood, R.J.; Walsh, C.J. Towards soft wearable strain sensors for muscle activity monitoring. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, G.; Zhu, K.; Liu, S.; Guo, W.; Jiang, Z.; Li, Z. Materials, devices, and systems of on-skin electrodes for electrophysiological monitoring and human–machine interfaces. Adv. Sci. 2021, 8, 2001938. [Google Scholar] [CrossRef] [PubMed]
- Viana, G.; Costa, M.; Banea, M.; Da Silva, L. A review on the temperature and moisture degradation of adhesive joints. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2017, 231, 488–501. [Google Scholar] [CrossRef]
- Lai, H.; Liu, Y.; Cheng, Y.; Shi, L.; Wang, R.; Sun, J. Temperature-Triggered Adhesive Bioelectric Electrodes with Long-Term Dynamic Stability and Reusability. Adv. Sci. 2023, 10, 2300793. [Google Scholar] [CrossRef]
- Kummerow, C.D.; Poczatek, J.C.; Almond, S.; Berg, W.; Jarrett, O.; Jones, A.; Kantner, M.; Kuo, C.-P. Hyperspectral microwave sensors—Advantages and limitations. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2021, 15, 764–775. [Google Scholar] [CrossRef]
- Alahnomi, R.A.; Zakaria, Z.; Yussof, Z.M.; Althuwayb, A.A.; Alhegazi, A.; Alsariera, H.; Rahman, N.A. Review of recent microwave planar resonator-based sensors: Techniques of complex permittivity extraction, applications, open challenges and future research directions. Sensors 2021, 21, 2267. [Google Scholar] [CrossRef]
- Islam, S.M.M. Radar-based remote physiological sensing: Progress, challenges, and opportunities. Front. Physiol. 2022, 13, 955208. [Google Scholar] [CrossRef]
- Mehta, S.; Kwatra, N.; Jain, M.; McDuff, D. Examining the challenges of blood pressure estimation via photoplethysmogram. Sci. Rep. 2024, 14, 18318. [Google Scholar] [CrossRef]
- Xu, J.; Harpe, P.; Van Hoof, C. An energy-efficient and reconfigurable sensor IC for bio-impedance spectroscopy and ECG recording. IEEE J. Emerg. Sel. Top. Circuits Syst. 2018, 8, 616–626. [Google Scholar] [CrossRef]
- Kong, F.; Zou, Y.; Li, Z.; Deng, Y. Advances in portable and wearable acoustic sensing devices for human health monitoring. Sensors 2024, 24, 5354. [Google Scholar] [CrossRef]
- Brown, C.V.; Martin, M.J.; Shoemaker, W.C.; Wo, C.C.; Chan, L.; Azarow, K.; Demetriades, D. The effect of obesity on bioimpedance cardiac index. Am. J. Surg. 2005, 189, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, H.; Zhou, J.; Huang, X.; Xu, L.; Jia, S.; Gao, Z.; Yao, K.; Li, D.; Zhang, B. Thin, soft, wearable system for continuous wireless monitoring of artery blood pressure. Nat. Commun. 2023, 14, 5009. [Google Scholar] [CrossRef] [PubMed]
- Van Helleputte, N.; Konijnenburg, M.; Pettine, J.; Jee, D.-W.; Kim, H.; Morgado, A.; Van Wegberg, R.; Torfs, T.; Mohan, R.; Breeschoten, A. A 345 µW multi-sensor biomedical SoC with bio-impedance, 3-channel ECG, motion artifact reduction, and integrated DSP. IEEE J. Solid-State Circuits 2014, 50, 230–244. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef]
- Wang, G.; Atef, M.; Lian, Y. Towards a continuous non-invasive cuffless blood pressure monitoring system using PPG: Systems and circuits review. IEEE Circuits Syst. Mag. 2018, 18, 6–26. [Google Scholar] [CrossRef]
- Kireev, D.; Sel, K.; Ibrahim, B.; Kumar, N.; Akbari, A.; Jafari, R.; Akinwande, D. Continuous cuffless monitoring of arterial blood pressure via graphene bioimpedance tattoos. Nat. Nanotechnol. 2022, 17, 864–870. [Google Scholar] [CrossRef]
- Mercier, L.; Langø, T.; Lindseth, F.; Collins, D.L. A review of calibration techniques for freehand 3-D ultrasound systems. Ultrasound Med. Biol. 2005, 31, 449–471. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Chen, C.-H.; Sue, C.-Y.; Lu, W.-H.; Chiou, Y.-H. Estimation of blood pressure in the radial artery using strain-based pulse wave and photoplethysmography sensors. Micromachines 2018, 9, 556. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Ma, W.; Qiu, L.; Xia, K.; Zhang, Y.; Lu, H.; Zhu, M.; Liang, X.; Wu, X.-E. Monitoring blood pressure and cardiac function without positioning via a deep learning–assisted strain sensor array. Sci. Adv. 2023, 9, eadh0615. [Google Scholar] [CrossRef]
- Hu, J.-R.; Martin, G.; Iyengar, S.; Kovell, L.C.; Plante, T.B.; van Helmond, N.; Dart, R.A.; Brady, T.M.; Turkson-Ocran, R.-A.N.; Juraschek, S.P. Validating cuffless continuous blood pressure monitoring devices. Cardiovasc. Digit. Health J. 2023, 4, 9–20. [Google Scholar] [CrossRef]
- de Sousa, N.M.; Magosso, R.F.; Dipp, T.; Plentz, R.D.; Marson, R.A.; Montagnolli, A.N.; Martins, R.A.; Perez, S.E.; Baldissera, V. Continuous blood pressure response at different intensities in leg press exercise. Eur. J. Prev. Cardiol. 2014, 21, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, S.; Zhu, R. A new method of continuous blood pressure monitoring using multichannel sensing signals on the wrist. Microsyst. Nanoeng. 2023, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Scardulla, F.; D’Acquisto, L.; Colombarini, R.; Hu, S.; Pasta, S.; Bellavia, D. A study on the effect of contact pressure during physical activity on photoplethysmographic heart rate measurements. Sensors 2020, 20, 5052. [Google Scholar] [CrossRef]
- Imholz, B.P.; Montfrans, G.A.V.; Settels, J.J.; Hoeven, G.M.V.D.; Karemaker, J.M.; Wieling, W. Continuous non-invasive blood pressure monitoring: Reliability of Finapres device during the Valsalva manoeuvre. Cardiovasc. Res. 1988, 22, 390–397. [Google Scholar] [CrossRef]
- Silke, B.; McAuley, D. Accuracy and precision of blood pressure determination with the Finapres: An overview using re-sampling statistics. J. Hum. Hypertens. 1998, 12, 403–409. [Google Scholar] [CrossRef]
- Ismail, S.N.A.; Nayan, N.A.; Jaafar, R.; May, Z. Recent advances in non-invasive blood pressure monitoring and prediction using a machine learning approach. Sensors 2022, 22, 6195. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Cho, S.-Y.; Correia, R.; Morgan, S.P. Non-invasive cuff-less blood pressure estimation using a hybrid deep learning model. Opt. Quantum Electron. 2021, 53, 1–20. [Google Scholar] [CrossRef]
- Kemmotsu, O.; Ueda, M.; Otsuka, H.; Yamamura, T.; Okamura, A.; Ishikawa, T.; Winter, D.C.; Eckerle, J.S. Blood Pressure Measurement by Arterial Tonometry in Controlled Hypotension. Anesthesia Analg. 1991, 73, 54–58. [Google Scholar] [CrossRef]
- Hines, E.A.; Brown, G.E. The cold pressor test for measuring the reactibility of the blood pressure: Data concerning 571 normal and hypertensive subjects. Am. Hear. J. 1936, 11, 1–9. [Google Scholar] [CrossRef]
- Hanson, P.; Nagle, F. Isometric Exercise: Cardiovascular Responses in Normal and Cardiac Populations. Cardiol. Clin. 1987, 5, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.S.; Lind, A.R. Aging, isometric strength and endurance, and cardiovascular responses to static effort. J. Appl. Physiol. 1975, 38, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.E.; Shaver, J.A.; Leon, D.F.; Thompson, M.E.; Reddy, P.S.; Leonard, J.J. Autonomic Mechanisms in Hemodynamic Responses to Isometric Exercise. J. Clin. Investig. 1974, 54, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.S.; Burse, R.L.; Lind, A.R. Comparison of physiological responses of women and men to isometric exercise. J. Appl. Physiol. 1975, 38, 863–868. [Google Scholar] [CrossRef]
- Parati, G.; Casadei, R.; Groppelli, A.; Di Rienzo, M.; Mancia, G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 1989, 13, 647–655. [Google Scholar] [CrossRef]
- Mukkamala, R.; Hahn, J.-O.; Inan, O.T.; Mestha, L.K.; Kim, C.-S.; Toreyin, H.; Kyal, S. Toward Ubiquitous Blood Pressure Monitoring via Pulse Transit Time: Theory and Practice. IEEE Trans. Biomed. Eng. 2015, 62, 1879–1901. [Google Scholar] [CrossRef]






| Feature | Strain Gauge-Based Device | PPG-Based Devices | BioZ-Based Devices | Acoustic Devices |
|---|---|---|---|---|
| Accuracy and Sensitivity | High | Low [40] | Moderate [41] | Low [42] |
| Impact of Skin Tone | None | Yes [20] | None * | None * |
| Influence of BMI | Moderate | High [20] | Moderate [43] | Moderate [44] |
| Motion Artifact Sensitivity | Moderate | High [40] | Moderate [45] | High [42] |
| Suitability for Continuous Monitoring | High | Moderate [20] | Moderate [29] | Moderate [46] |
| Need for Recalibration | No | Yes [47] | Yes [48] | Yes [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McMurray, J.P.; DeVries, A.; Frazee, K.; Sizemore, B.; Branan, K.L.; Jennings, R.; Coté, G.L. A Novel Wearable Device for Continuous Blood Pressure Monitoring Utilizing Strain Gauge Technology. Biosensors 2025, 15, 413. https://doi.org/10.3390/bios15070413
McMurray JP, DeVries A, Frazee K, Sizemore B, Branan KL, Jennings R, Coté GL. A Novel Wearable Device for Continuous Blood Pressure Monitoring Utilizing Strain Gauge Technology. Biosensors. 2025; 15(7):413. https://doi.org/10.3390/bios15070413
Chicago/Turabian StyleMcMurray, Justin P., Aubrey DeVries, Kendall Frazee, Bailey Sizemore, Kimberly L. Branan, Richard Jennings, and Gerard L. Coté. 2025. "A Novel Wearable Device for Continuous Blood Pressure Monitoring Utilizing Strain Gauge Technology" Biosensors 15, no. 7: 413. https://doi.org/10.3390/bios15070413
APA StyleMcMurray, J. P., DeVries, A., Frazee, K., Sizemore, B., Branan, K. L., Jennings, R., & Coté, G. L. (2025). A Novel Wearable Device for Continuous Blood Pressure Monitoring Utilizing Strain Gauge Technology. Biosensors, 15(7), 413. https://doi.org/10.3390/bios15070413






