Mechanically Tunable Composite Hydrogel for Multi-Gesture Motion Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Components
2.2. Fabrication of Devices Utilizing PVA-ATMP Hydrogel
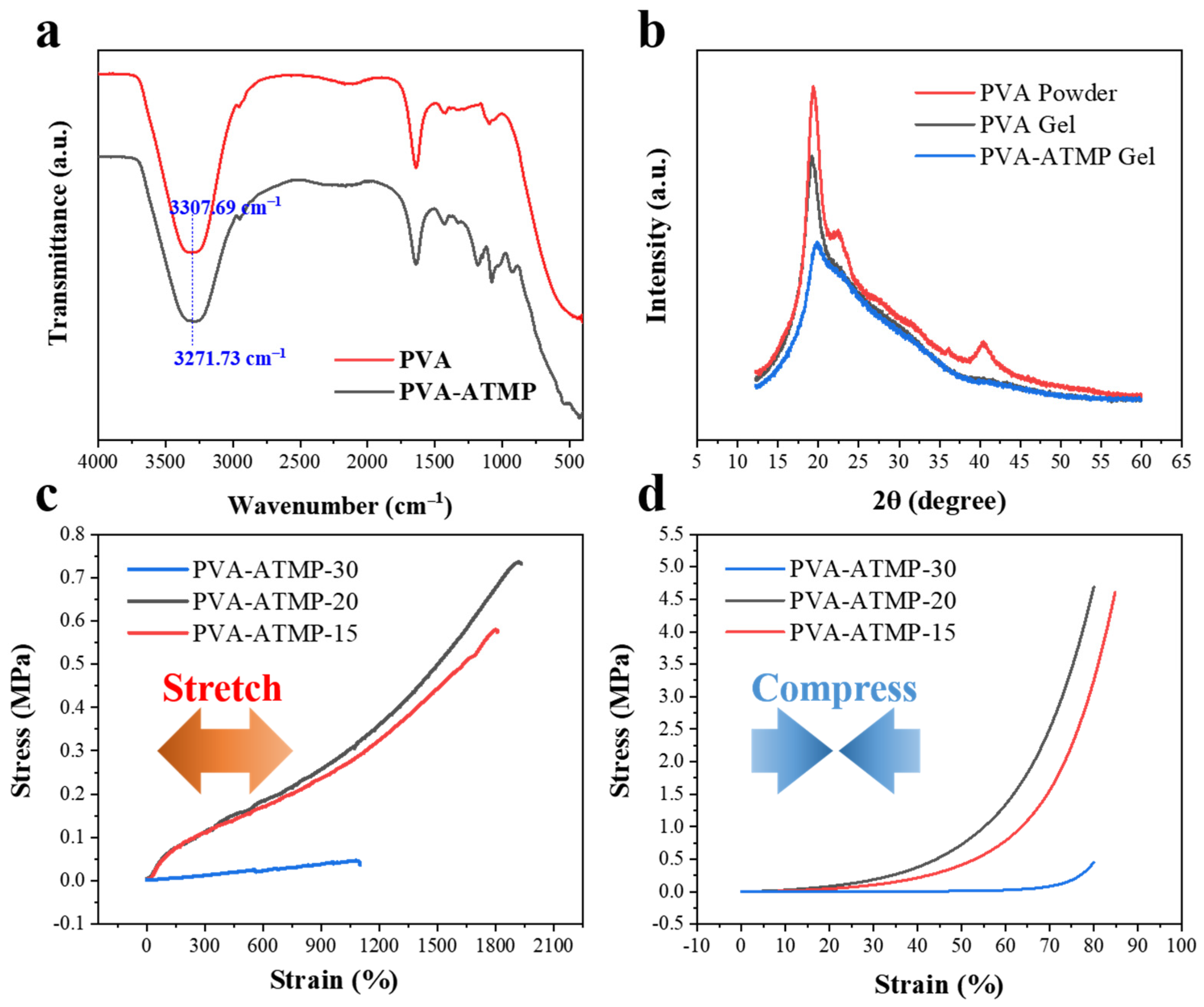
2.3. Analysis of PVA-ATMP Hydrogel
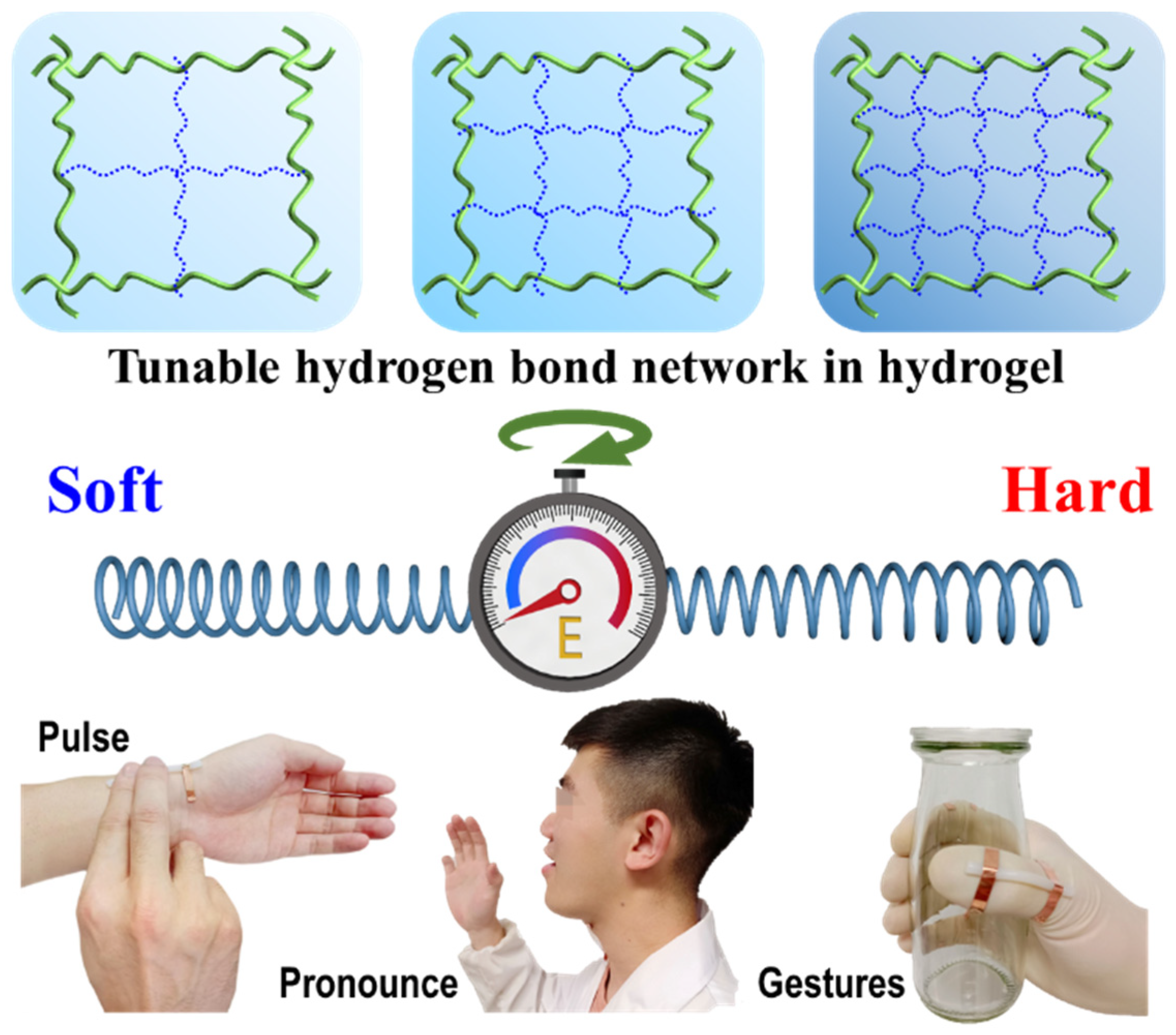
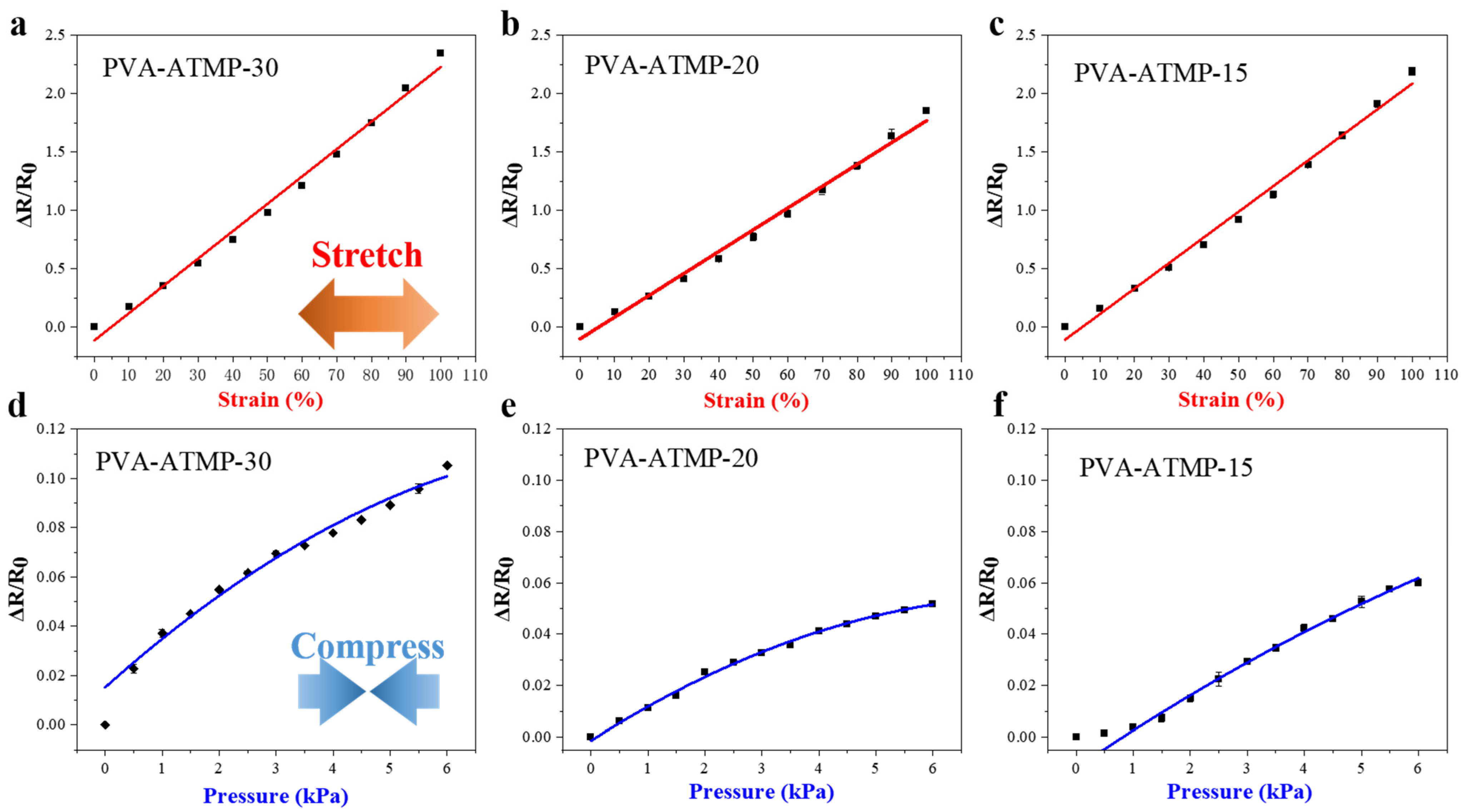
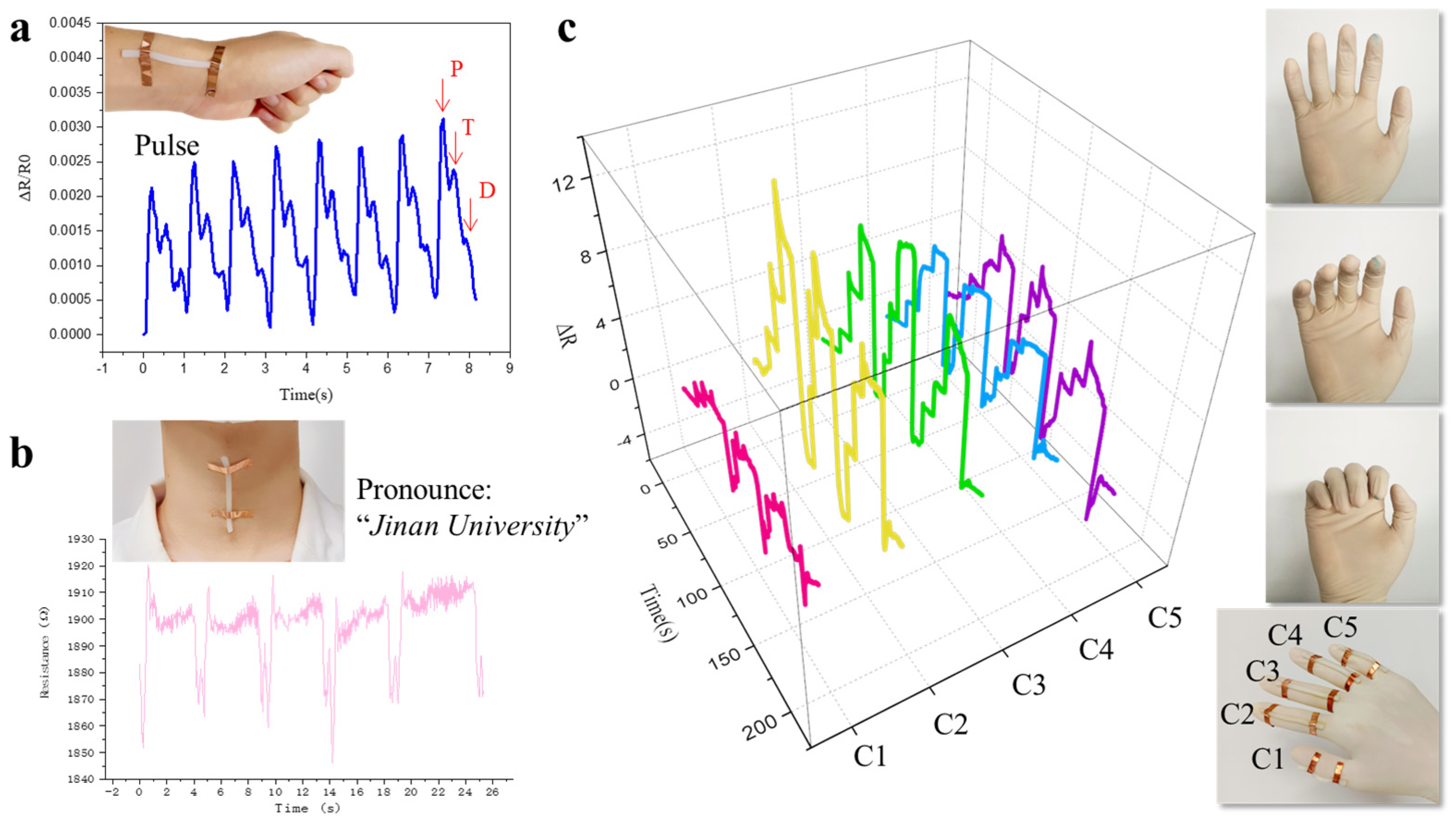
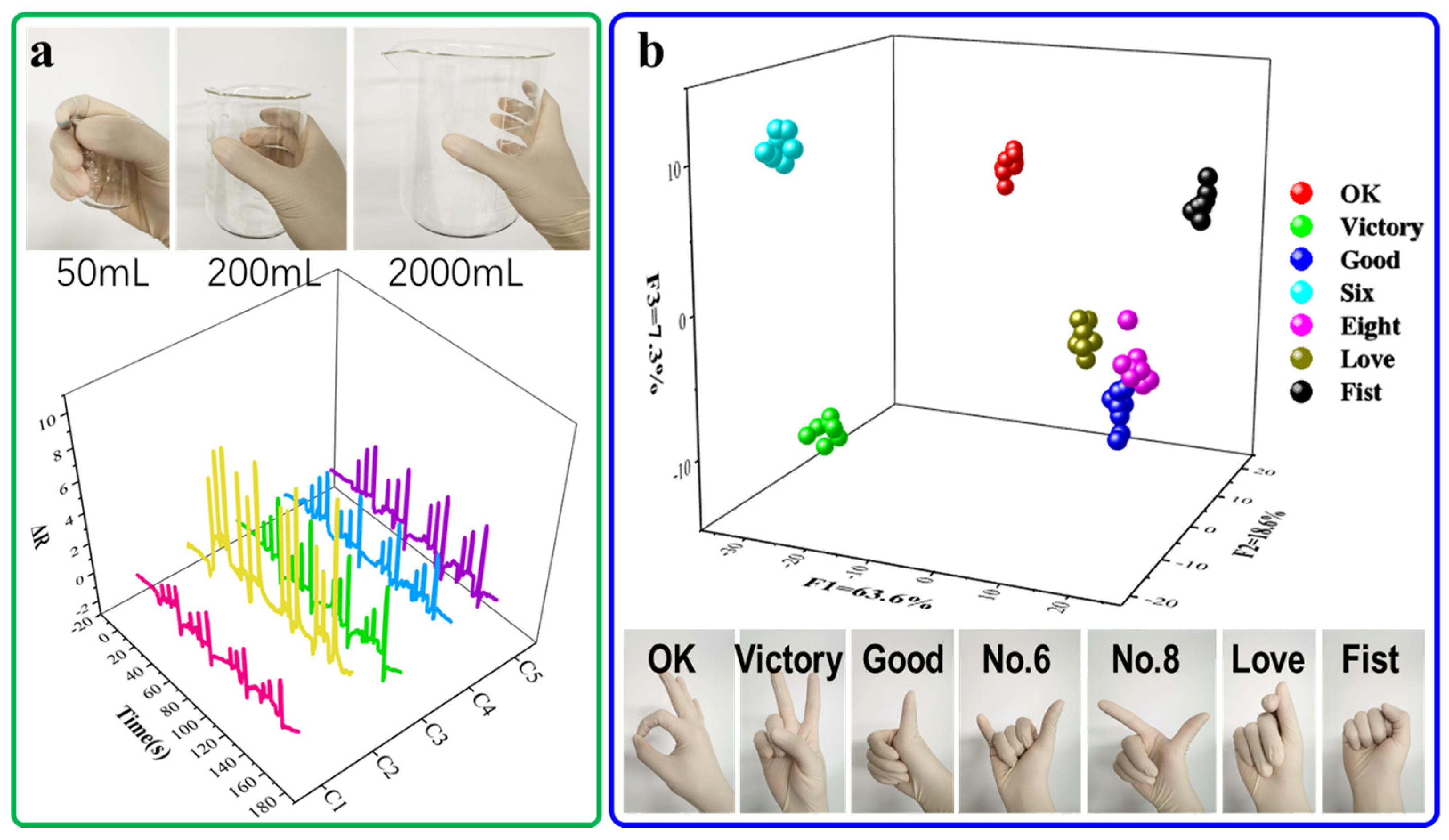
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATMP | Amino trimethylene phosphonic acid |
| PVA | Poly(vinyl alcohol) |
| GF | Gauge factor |
| LDA | Linear discriminant analysis |
References
- Sui, X.; Mu, Q.; Li, J.; Zhao, B.; Gu, H.; Yu, H.; Du, J.; Ren, L.; Hu, D. High-Performance Flexible PLA/BTO-Based Pressure Sensor for Motion Monitoring and Human–Computer Interaction. Biosensors 2024, 14, 508. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Afshari, R.; Jain, S.; Zheng, Y.; Lin, M.; Zenkar, S.; Yin, J.; Chen, J.; Peppas, N.; Annabi, N. Advances in conducting nanocomposite hydrogels for wearable biomonitoring. Chem. Soc. Rev. 2025, 54, 2595. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Zhao, C.; Li, W.; Dong, E.; Xu, M.; Huang, H.; Yang, Y.; Li, L.; Zheng, L.; et al. Breaking the Saturation of Sensitivity for Ultrawide Range Flexible Pressure Sensors by Soft-Strain Effect. Adv. Mater. 2024, 36, 2405405. [Google Scholar] [CrossRef]
- Song, J.; Chen, S.; Sun, L.; Guo, Y.; Zhang, L.; Wang, S.; Xuan, H.; Guan, Q.; You, Z. Mechanically and Electronically Robust Transparent Organohydrogel Fibers. Adv. Mater. 2020, 32, 1906994. [Google Scholar] [CrossRef]
- Chen, G.; Wang, G.; Tan, X.; Hou, K.; Meng, Q.; Zhao, P.; Wang, S.; Zhang, J.; Zhou, Z.; Chen, T.; et al. Integrated dynamic wet spinning of core-sheath hydrogel fibers for optical-to-brain/tissue communications. Natl. Sci. Rev. 2021, 8, nwaa209. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.; Li, D.; Yuan, J.; Huang, D.; Zhang, C.; Song, W.; Wang, C.; Wang, J.; Liu, L.; et al. Fiber-Shaped, Stretchable Strain Sensors with High Linearity by One-Step Injection Molding for Structural Health Monitoring. Adv. Funct. Mater. 2025, 2500701, publisher preview. [Google Scholar] [CrossRef]
- Lee, D.H.; Miyashita, T.; Xuan, Y.; Takei, K. Ultrasensitive and Stretchable Strain SensorsBased on Laser-Induced Graphene With ZnO Nanoparticles. ACS Nano 2024, 18, 32255–32265. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, D.; Yan, F. Wearable Resistive-Type Stretchable Strain Sensors: Materials and Applications. Adv. Mater. 2025, 37, 2413929. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Z.; Ji, X.; Xu, X.; Chen, F.; Pan, X.; Fu, Z.; Chen, Y.; Zhang, Z.; Liu, H.; et al. Ag–thiolate interactions to enable an ultrasensitive and stretchable MXene strain sensor with high temporospatial resolution. Nat. Commun. 2024, 15, 5354. [Google Scholar] [CrossRef]
- Yoon, K.; Lee, S.; Kwon, C.; Won, C.; Cho, S.; Lee, S.; Lee, M.; Lee, J.; Lee, H.; Jang, K.I.; et al. Highly Stretchable Thermoelectric Fiber with Embedded Copper(I) Iodide Nanoparticles for a Multimodal Temperature, Strain, and Pressure Sensor in Wearable Electronics. Adv. Funct. Mater. 2024, 35, 2407759. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, T.; Song, N.; Dai, J.; Qian, G.; Ding, P. Oriented BN/BNNT heterostructure constructed by interface engineering strategy for polyamide-imide composite film with advanced flexibility and thermally conductive properties. Chem. Eng. J. 2024, 481, 148653. [Google Scholar] [CrossRef]
- Li, M.; Wu, H.; Gao, K.; Wang, Y.; Hu, J.; Guo, Z.; Hu, R.; Zhang, M.; Pang, X.; Guo, M.; et al. Smart Implantable Hydrogel for Large Segmental Bone Regeneration. Adv. Healthc. Mater. 2024, 13, 2402916. [Google Scholar] [CrossRef]
- Hua, J.; Su, M.; Sun, X.; Li, J.; Sun, Y.; Qiu, H.; Shi, Y.; Pan, L. Hydrogel-Based Bioelectronics and Their Applications in Health Monitoring. Biosensors 2023, 13, 696. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, M.; Wan, P. MXene hydrogel for wearable electronics. Matter 2021, 4, 2582–2685. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, X.; Zheng, M.; Yue, O.; Huang, M.; Zou, X.; Cui, B.; Xie, L.; Dong, S.; Shang, J.; et al. Mechanically Robust and Transparent Organohydrogel-Based E-Skin Nanoengineered from Natural Skin. Adv. Funct. Mater. 2023, 33, 2212856. [Google Scholar] [CrossRef]
- Sun, Z.; Dong, C.; Chen, B.; Li, W.; Hu, H.; Zhou, J.; Li, C.; Huang, Z. Strong, Tough, and Anti-Swelling Supramolecular Conductive Hydrogels for Amphibious Motion Sensors. Small 2023, 19, 2303612. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, J.; Ji, T.; Xue, Y.; Zhao, L.; Zhao, K.; Jia, B.; Wang, B.; Wang, J.; Zhang, S.; et al. Self-Healing Hydrogel Bioelectronics. Adv. Mater. 2024, 36, 2306350. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.C.; Nie, Y.; Li, H.N.; Li, W.L.; Lin, W.T.; Xue, Y.R.; Li, K.; Fang, Y.; Liang, H.Q.; Yang, H.C.; et al. Supergravity-Steered Generic Manufacturing of Nanosheets-Embedded Nanocomposite Hydrogel with Highly Oriented, Heterogeneous Architecture. Adv. Mater. 2024, 36, 2400075. [Google Scholar] [CrossRef]
- Yuan, T.; Li, C.; Kolinski, J.M.; Amstad, E. Electrostatically Reinforced Double Network Granular Hydrogels. Adv. Sci. 2025, 12, 2412566. [Google Scholar] [CrossRef]
- Ai, Z.; Li, L.; Huang, M.; Su, X.; Gao, Y.; Wu, J. An Ultrafast, High-Loading, and Durable Poly(p-aminoazobenzene)/Reduced Graphene Oxide Composite Electrode for Supercapacitors. Adv. Funct. Mater. 2023, 33, 2211057. [Google Scholar] [CrossRef]
- Lopez-Larrea, N.; Wustoni, S.; Peñas, M.I.; Uribe, J.; Dominguez-Alfaro, A.; Gallastegui, A.; Inal, S.; Mecerreyes, D. PNIPAM/PEDOT:PSS Hydrogels for Multifunctional Organic Electrochemical Transistors. Adv. Funct. Mater. 2024, 34, 2403708. [Google Scholar] [CrossRef]
- Li, J.; Du, C.; Yang, X.; Yao, Y.; Qin, D.; Meng, F.; Yang, S.; Tan, Y.; Chen, X.; Jiang, W.; et al. Instantaneous Self-Healing Chitosan Hydrogels with Enhanced Drug Leakage Resistance for Infected Stretchable Wounds Healing. Small 2025, 21, 2409641. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, Y.; Yang, R.; Fang, W.; Zhang, K.; Liu, M.; Wang, Y.; Yang, M.; Fu, Q. Multilayered hydrogel scaffold construct with native tissue matched elastic modulus: A regenerative microenvironment for urethral scar-free healing. Biomaterials 2025, 312, 122711. [Google Scholar] [CrossRef]
- Pu, L.; Yuan, Z.; Cai, Y.; Li, X.; Xue, Z.; Niu, Y.; Li, D.; Ma, S.; Xu, W. Multiperformance PAM/PVA/CaCO3 Hydrogel for Flexible Sensing and Information Encryption. ACS Appl. Mater. Interfaces 2024, 16, 32762–32772. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, L.; Guo, Y.; Li, M.; Hu, L.; Wu, H.; Cui, W.; Ran, R. Extreme Toughening of Conductive Hydrogels Through Synergistic Effects of Mineralization, Salting-Out, and Ion Coordination Induced by Multivalent Anions. Small 2025, 21, 2409565. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Ji, T.; Bai, R.; Zhu, H. Highly Ionic Conductive, Stretchable, and Tough Ionogel for Flexible Solid-State Supercapacitor. Small 2024, 20, 2307019. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Griffin, A.; Qian, J.; Qiang, Z.; Sun, B.; Ye, C.; Zhu, M. Mechanically Strong and Tough Organohydrogels for Wide Temperature Tolerant, Flexible Solid-State Supercapacitors. Adv. Funct. Mater. 2024, 34, 2405962. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, M.; Huang, C.; Li, Z.; Yuan, Y.; Hu, S.; Zhang, L.; Wan, P. Flexible antibacterial degradable bioelastomer nanocompositesfor ultrasensitive human–machine interaction sensing enabled bymachine learning. Aggregate 2024, 5, e522. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, X.; Illeperuma, W.; Chaudhuri, O.; Oh, K.; Mooney, D.; Vlassak, J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Rumon, M.M.H. Synthesis of PVA-Based Hydrogels for Biomedical Applications: Recent Trends and Advances. Gels 2025, 11, 88. [Google Scholar] [CrossRef]
- Ren, T.; Gan, J.; Zhou, L.; Chen, H. Physically Crosslinked Hydrogels Based on Poly (Vinyl Alcohol) and Fish Gelatin for Wound Dressing Application: Fabrication and Characterization. Polymers 2020, 12, 1729. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yin, J.; Liu, S.; Xue, B.; Shokoohi, C.; Ge, G.; Hu, M.; Li, T.; Tao, X.; Rao, Z.; et al. Learning Hand Kinematics for Parkinson’s Disease Assessment Using a Multimodal Sensor Glove. Adv. Sci. 2023, 10, 2206982. [Google Scholar] [CrossRef]
- Li, T.; Wang, Q.; Cao, Z.; Zhu, J.; Wang, N.; Li, R.; Meng, W.; Liu, Q.; Yu, S.; Liao, X.; et al. Nerve-Inspired Optical Waveguide Stretchable Sensor Fusing Wireless Transmission and AI Enabling Smart Tele-Healthcare. Adv. Sci. 2025, 12, 2410395. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kantarcigl, C.; Kim, B.; Baruah, R.; Maity, S.; Park, Y.; Kim, K.; Lee, S.; Malandraki, J.; Avlani, S.; et al. Flexible submental sensor patch with remote monitoring controls for management of oropharyngeal swallowing disorders. Sci. Adv. 2019, 5, eaay3210. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, X.; Yuan, L.; Ming, H.; Li, Z.; Li, J.; Luo, F.; Tan, H. Bioinspired Injectable Polyurethane Underwater Adhesive with Fast Bonding and Hemostatic Properties. Adv. Sci. 2024, 11, 2308538. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, B.; Yang, J.; Zhou, J.; Xu, Y. Linear discriminant analysis. Nat. Rev. Methods Primers 2024, 4, 70. [Google Scholar] [CrossRef]
- Huang, Y.; Li, F.Y.; Qin, M.; Jiang, L.; Song, Y.L. A Multi-stopband Photonic-Crystal Microchip for High-Performance Metal-Ion Recognition Based on Fluorescent Detection. Angew. Chem. Int. Ed. 2013, 52, 7296–7299. [Google Scholar] [CrossRef]
- Fu, Q.; Tang, J.; Wang, W.; Wang, R. Biocomposite Polyvinyl Alcohol/Ferritin Hydrogels with Enhanced Stretchability and Conductivity for Flexible Strain Sensors. Gels 2025, 11, 59. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Z.; Li, K.; Ma, C.; Zhou, W.; Lin, T.; Xu, J.; Liu, X. Self-Healable PEDOT:PSS-PVA Nanocomposite Hydrogel Strain Sensor for Human Motion Monitoring. Nanomaterials 2023, 13, 2465. [Google Scholar] [CrossRef]
- Hu, J.; Wu, Y.; Yang, Q.; Zhou, Q.; Hui, L.; Liu, Z.; Xu, F.; Ding, D. One-pot Freezing-thawing Preparation of Cellulose Nanofibrils Reinforced Polyvinyl Alcohol Based Ionic Hydrogel Strain Sensor for Human Motion Monitoring. Carbohydr. Polym. 2021, 275, 118697. [Google Scholar] [CrossRef]
- Di, X.; Ma, Q.; Xu, Y.; Yang, M.; Wu, G.; Sun, P. High-performance ionic conductive poly(vinyl alcohol) hydrogels for flexible strain sensors based on a universal soaking strategy. Mater. Chem. Front. 2021, 5, 315–323. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2018, 29, 1806220. [Google Scholar] [CrossRef]
- Li, Y.; Ren, P.; Sun, Z.; Xue, R.; Ding, D.; Tian, W.; Ren, F.; Jin, Y.; Chen, Z.; Zhu, G. High-strength, anti-fatigue, cellulose nanofiber reinforced polyvinyl alcohol based ionic conductive hydrogels for flexible strain/pressure sensors and triboelectric nanogenerators. J. Colloid Interface Sci. 2024, 669, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, R.; Liu, H.; Li, D.; Fu, S.; Jin, K.; Cheng, Y.; Fu, Z.; Xing, F.; Tian, Y. Tough, high conductivity pectin polysaccharide-based hydrogel for strain sensing and real-time information transmission. Int. J. Biol. Macromol. 2024, 257, 128575. [Google Scholar] [CrossRef]
- Wei, J.; Wang, R.; Pan, F.; Fu, Z. Polyvinyl Alcohol/Graphene Oxide Conductive Hydrogels via the Synergy of Freezing and Salting Out for Strain Sensors. Sensors 2022, 22, 3015. [Google Scholar] [CrossRef]
- Filipecka-Szymczyk, K.; Makowska-Janusik, M.; Marczak, W. Molecular Dynamics Simulations of HEMA-Based Hydrogels for Ophthalmological Applications. Molecules 2024, 29, 5784. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, D.; Zheng, Z.; Wang, X. Tough double network hydrogels with rapid self-reinforcement and low hysteresis based on highly entangled networks. Nat. Commun. 2024, 15, 1344. [Google Scholar] [CrossRef]
- Zhu, L.; Qiu, J.; Sakai, E. A high modulus hydrogel obtained from hydrogen bond reconstruction and its application in vibration damper. RSC Adv. 2017, 7, 43755–43763. [Google Scholar] [CrossRef]
- She, W.; Shen, C.; Xue, Z.; Zhang, B.; Zhang, G.; Meng, Q. Hydrogel Strain Sensors for Integrating Into Dynamic Organ-on-a-Chip. Small 2025, 21, e2407704. [Google Scholar] [CrossRef]
- Lv, J.; Kong, C.; Yang, C.; Yin, L.; Jeerapan, I.; Pu, F.; Zhang, X.; Yang, S.; Yang, Z. Wearable, stable, highly sensitive hydrogel–graphene strain sensors. Beilstein J. Nanotechnol. 2019, 10, 475–480. [Google Scholar] [CrossRef]
- Zhou, Y.; Lian, H.; Li, Z.; Yin, L.; Ji, Q.; Li, K.; Qi, F.; Huang, Y. Crack engineering boosts the performance of flexible sensors. VIEW 2022, 3, 20220025. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Fei, X.; Tian, J.; Xu, L.Q.; Li, Y. A ionic liquid enhanced conductive hydrogel for strain sensing applications. J. Colloid Interface Sci. 2022, 606, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Xu, W.; Wang, Y.; Xiong, W.; Xiong, C.; You, L.; Wang, S. High-conductivity and long-term stability strain sensor based on silk fibroin and polyvinyl alcohol hydrogels. Mater. Today Commun. 2024, 38, 108465. [Google Scholar] [CrossRef]
- Wang, H.; Lin, H.; Hu, X.; Zhou, Z.; Chen, Q.; Hong, M.; Fu, H. Highly Flexible, Freezing-Resistant, Anisotropically Conductive Sandwich-Shaped Composite Hydrogels for Strain Sensors. Ind. Eng. Chem. Res. 2023, 62, 5563–5573. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, Y.; Xu, J.; Qian, R.; Yu, Z.; Ye, C. Stretchable, adhesive and self-healing conductive hydrogels based on PEDOT:PSS-stabilized liquid metals for human motion detection. Chem. Eng. J. 2024, 494, 152971. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Z.; Ruan, H.; Li, Y. High conductivity, low-hysteresis, flexible PVA hydrogel multi-functional sensors: Wireless wearable sensor for health monitoring. Chem. Eng. J. 2025, 505, 158877. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Shi, X.Y.; Qiao, F.H.; Sun, J.Z.; Hu, Q.L. Multistimuli-Responsive PNIPAM-Based Double Cross-Linked Conductive Hydrogel with Self-Recovery Ability for Ionic Skin and Smart Sensor. Biomacromolecules 2022, 23, 5239–5252. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Liao, W.; Zhang, D.; Dai, Y.; Wu, C.; Wen, J.; Zeng, W. Stretchable conductive hydrogels integrated with microelectronic devices for strain sensing. J. Mater. Chem. C 2023, 11, 15873. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Yue, C.; Song, Y.; Manzo, M.; Huang, Z.; Cai, L. Stretchable, sensitive, and environment-tolerant ionic conductive organohydrogel reinforced with cellulose nanofibers for human motion monitoring. Cellulose 2022, 29, 1897–1909. [Google Scholar] [CrossRef]
- Tian, X.; Niu, B.; Hua, T.; Yang, M.Y.; Yang, Y.Y.; Dong, S.S. Continuous Fabrication of a Highly Integrated, User-Friendly, and Low-Cost Triboelectric Yarn/Fabric for Diverse Sensing Applications. ACS Sustain. Chem. Eng. 2023, 11, 16087–16097. [Google Scholar] [CrossRef]
- Qin, Z.H.; Liu, S.D.; Bai, J.H.; Yin, J.J.; Li, N.; Jiao, T.F. Ionic conductive hydroxypropyl methyl cellulose reinforced hydrogels with extreme stretchability, self-adhesion and anti-freezing ability for highly sensitive skin-like sensors. Int. J. Biol. Macromol. 2022, 220, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Jiang, H.; Li, G.; Fu, B.; Bao, X.; Wang, Z.; Hu, Q. Stretchable, conductive PAni-PAAm-GOCS hydrogels with excellent mechanical strength, strain sensitivity and skin affinity. Chem. Eng. J. 2020, 394, 124901. [Google Scholar] [CrossRef]
- Cao, Q.; Shu, Z.; Zhang, T.; Ji, W.; Chen, J.; Wei, Y. Magnetic nanocomposite hydrogel with ultra-stretchable as strain sensors for monitoring human motion and the change of magnetic field. J. Appl. Polym. Sci. 2024, 141, e54934. [Google Scholar] [CrossRef]


| Hydrogel | PVA (g) | ATMP (mL) | DI Water (mL) |
|---|---|---|---|
| PVA-ATMP-15 | 3 | 10 | 15 |
| PVA-ATMP-20 | 4 | 10 | 20 |
| PVA-ATMP-30 | 4 | 10 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; He, Z.; Shen, B.; Li, J.; Tang, Y.; Pang, S.; Tian, X.; Wang, S.; Li, F. Mechanically Tunable Composite Hydrogel for Multi-Gesture Motion Monitoring. Biosensors 2025, 15, 412. https://doi.org/10.3390/bios15070412
Zhang J, He Z, Shen B, Li J, Tang Y, Pang S, Tian X, Wang S, Li F. Mechanically Tunable Composite Hydrogel for Multi-Gesture Motion Monitoring. Biosensors. 2025; 15(7):412. https://doi.org/10.3390/bios15070412
Chicago/Turabian StyleZhang, Jiabing, Zilong He, Bin Shen, Jiang Li, Yongtao Tang, Shuhuai Pang, Xiaolin Tian, Shuang Wang, and Fengyu Li. 2025. "Mechanically Tunable Composite Hydrogel for Multi-Gesture Motion Monitoring" Biosensors 15, no. 7: 412. https://doi.org/10.3390/bios15070412
APA StyleZhang, J., He, Z., Shen, B., Li, J., Tang, Y., Pang, S., Tian, X., Wang, S., & Li, F. (2025). Mechanically Tunable Composite Hydrogel for Multi-Gesture Motion Monitoring. Biosensors, 15(7), 412. https://doi.org/10.3390/bios15070412





