Recent Advances in Antibody Discovery Using Ultrahigh-Throughput Droplet Microfluidics: Challenges and Future Perspectives
Abstract
1. Introduction
2. Fundamentals of Droplet Microfluidics
2.1. Droplet Generation Chips and Parallelization
2.2. Intermediate On-Chip Microfluidic Operations to Achieve On-Chip Workflow Functionality
2.2.1. Pico-Injection and Pico-Washing
2.2.2. On-Chip Incubation
2.2.3. Deterministic Lateral Displacement (DLD)
2.2.4. Droplet Merging: Passive and Active (Electrocoalescence)
2.2.5. Droplet Splitting
2.2.6. Sample Enrichment by Ion Enrichment
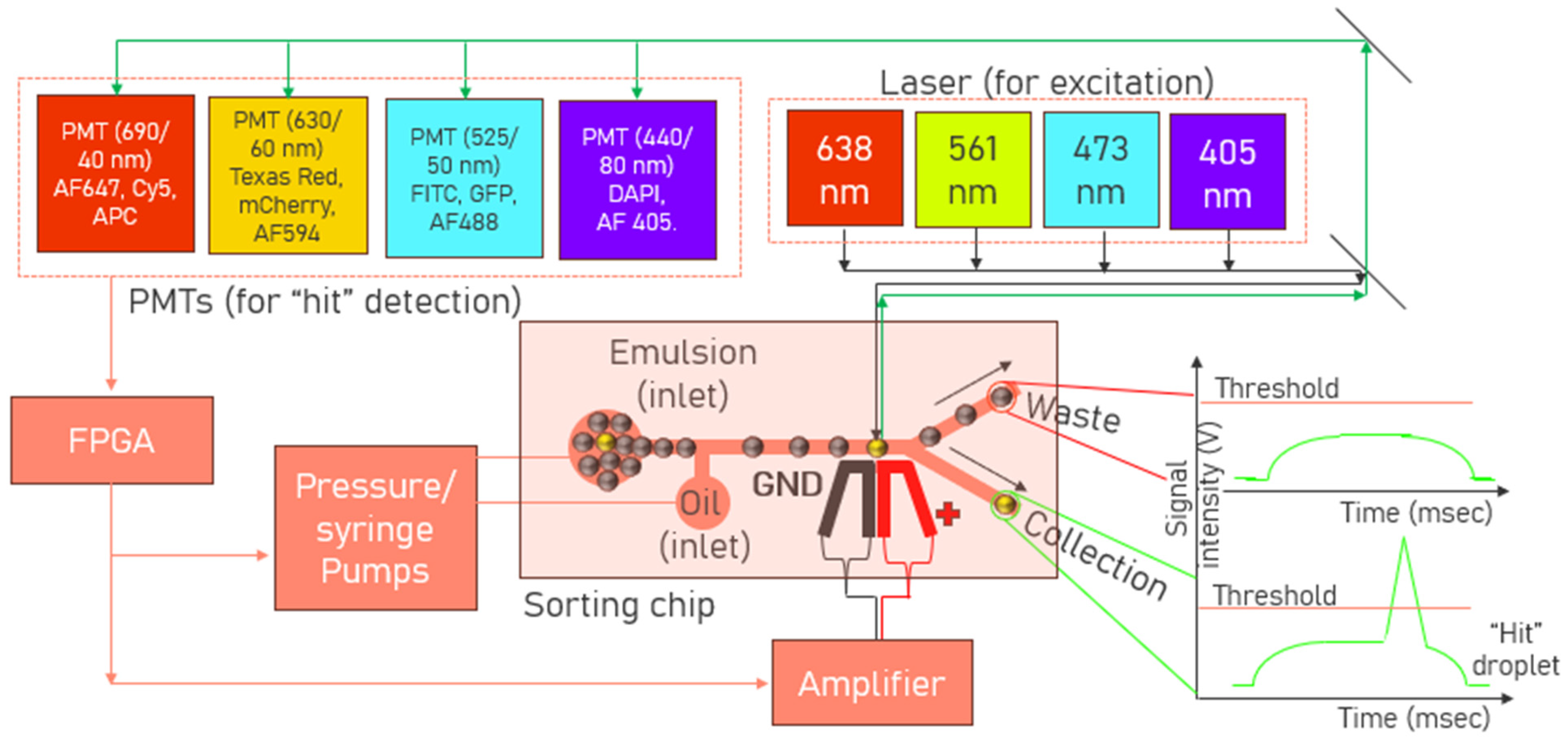
2.3. Sorting Chip
3. Types of Assays
3.1. Binding Assays
3.2. Functional Assays
3.3. Förster Resonance Energy Transfer (FRET) Assays
3.4. Internalization Assays
3.5. Neutralization and Infection Assays
4. New Developments for Improved High Throughput Screening
4.1. High Cell and Bead Encapsulation in Droplets
4.2. Microfluidics Workstation Design Considerations for High-Throughput Droplet Sorting
4.3. Scope of Automation
5. Commercial Landscape
6. Current Challenges
6.1. Translation from Benchtop to Droplet Format
6.2. Defining and Identifying “Hit” Droplets
6.3. Addressing Inter-Droplet Diffusion Reduction
7. Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Antibody Therapeutics Approved or in Regulatory Review in the EU or US. The Antibody Society. Available online: https://www.antibodysociety.org/resources/approved-antibodies/ (accessed on 12 January 2025).
- Crescioli, S.; Kaplon, H.; Wang, L.; Visweswaraiah, J.; Kapoor, V.; Reichert, J.M. Antibodies to watch in 2025. mAbs 2024, 17, 2443538. [Google Scholar] [CrossRef] [PubMed]
- Coulson, A.; Levy, A.; Gossell-Williams, M. Monoclonal Antibodies in Cancer Therapy: Mechanisms, Successes and Limitations. West. Indian. Med. J. 2014, 63, 650–654. [Google Scholar] [CrossRef]
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018, 18, 46–61. [Google Scholar] [CrossRef]
- Paul, S.; Konig, M.F.; Pardoll, D.M.; Bettegowda, C.; Papadopoulos, N.; Wright, K.M.; Gabelli, S.B.; Ho, M.; van Elsas, A.; Zhou, S. Cancer therapy with antibodies. Nat. Rev. Cancer 2024, 24, 399–426. [Google Scholar] [CrossRef]
- Jin, S.; Sun, Y.; Liang, X.; Gu, X.; Ning, J.; Xu, Y.; Chen, S.; Pan, L. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 39. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Q.; Huang, L. B cell depletion therapies in autoimmune diseases: Monoclonal antibodies or chimeric antigen receptor-based therapy? Front. Immunol. 2023, 14, 1126421. [Google Scholar] [CrossRef]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to combat viral infections: Development strategies and progress. Nat. Rev. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Bradbury, A.R.M.; Sidhu, S.; Dübel, S.; McCafferty, J. Beyond natural antibodies: The power of in vitro display technologies. Nat. Biotechnol. 2011, 29, 245–254. [Google Scholar] [CrossRef]
- Sormanni, P.; Aprile, F.A.; Vendruscolo, M. Third generation antibody discovery methods: In silico rational design. Chem. Soc. Rev. 2018, 47, 9137–9157. [Google Scholar] [CrossRef]
- Kellermann, S.-A.; Green, L.L. Antibody discovery: The use of transgenic mice to generate human monoclonal antibodies for therapeutics. Curr. Opin. Biotechnol. 2002, 13, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandley, P.; Rohatgi, S. Recent Advances in the Development of Monoclonal Antibodies and Next-Generation Antibodies. ImmunoHorizons 2023, 7, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; de Villavicencio Diaz, T.N.; Lange, V.; Wu, L.; Le Bihan, T.; Ma, B. Exploring the sheep (Ovis aries) immunoglobulin repertoire by next generation sequencing. Mol. Immunol. 2023, 156, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ismanto, H.S.; Zhou, H.; Saputri, D.S.; Sugihara, F.; Standley, D.M. Advances in antibody discovery from human BCR repertoires. Front. Bioinform. 2022, 2, 1044975. [Google Scholar] [CrossRef]
- Adler, A.S.; Bedinger, D.; Adams, M.S.; Asensio, M.A.; Edgar, R.C.; Leong, R.; Leong, J.; Mizrahi, R.A.; Spindler, M.J.; Bandi, S.R.; et al. A natively paired antibody library yields drug leads with higher sensitivity and specificity than a randomly paired antibody library. MAbs 2018, 10, 431–443. [Google Scholar] [CrossRef]
- Grzeschik, J.; Yanakieva, D.; Roth, L.; Krah, S.; Hinz, S.C.; Elter, A.; Zollmann, T.; Schwall, G.; Zielonka, S.; Kolmar, H. Yeast Surface Display in Combination with Fluorescence-activated Cell Sorting Enables the Rapid Isolation of Antibody Fragments Derived from Immunized Chickens. Biotechnol. J. 2019, 14, 1800466. [Google Scholar] [CrossRef]
- Gangwal, A.; Lavecchia, A. Unleashing the power of generative AI in drug discovery. Drug Discov. Today 2024, 29, 103992. [Google Scholar] [CrossRef]
- DePalma, A. Antibody Discovery Taps AI for Multidimensional Optimization. Available online: https://www.genengnews.com/topics/artificial-intelligence/antibody-discovery-taps-ai-for-multidimensional-optimization/ (accessed on 3 May 2025).
- Hoogenboom, H.R. Selecting and screening recombinant antibody libraries. Nat. Biotechnol. 2005, 23, 1105–1116. [Google Scholar] [CrossRef]
- Slavny, P.; Hegde, M.; Doerner, A.; Parthiban, K.; McCafferty, J.; Zielonka, S.; Hoet, R. Advancements in mammalian display technology for therapeutic antibody development and beyond: Current landscape, challenges, and future prospects. Front. Immunol. 2024, 15, 1469329. [Google Scholar] [CrossRef]
- Boder, E.T.; Wittrup, K.D. Yeast surface display for screening combinatorial polypeptide libraries. Nat. Biotechnol. 1997, 15, 553–557. [Google Scholar] [CrossRef]
- Van Lent, J.; Breukers, J.; Ven, K.; Ampofo, L.; Horta, S.; Pollet, F.; Imbrechts, M.; Geukens, N.; Vanhoorelbeke, K.; Declerck, P.; et al. Miniaturized single-cell technologies for monoclonal antibody discovery. Lab Chip 2021, 21, 3627–3654. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Packard, T.A.; O’Neill, S.K.; Hinman, R.M.; Rihanek, M.; Gottlieb, P.A.; Cambier, J.C. Detection and Enrichment of Rare Antigen-specific B Cells for Analysis of Phenotype and Function. J. Vis. Exp. 2017, 120, 55382. [Google Scholar] [CrossRef]
- ⅄PEXTM—Human mAb Discovery and Production Technology Suite. Available online: https://www.aridispharma.com/%E2%85%84pex/ (accessed on 1 March 2024).
- Rettig, J.R.; Folch, A. Large-Scale Single-Cell Trapping And Imaging Using Microwell Arrays. Anal. Chem. 2005, 77, 5628–5634. [Google Scholar] [CrossRef]
- Shembekar, N.; Chaipan, C.; Utharala, R.; Merten, C.A. Droplet-based microfluidics in drug discovery, transcriptomics and high-throughput molecular genetics. Lab Chip 2016, 16, 1314–1331. [Google Scholar] [CrossRef]
- Shembekar, N.; Hu, H.; Eustace, D.; Merten, C.A. Single-cell droplet microfluidic screening for antibodies specifically binding to target cells. Cell Rep. 2018, 22, 2206–2215. [Google Scholar] [CrossRef]
- Fischer, K.; Lulla, A.; So, T.Y.; Pereyra-Gerber, P.; Raybould, M.I.J.; Kohler, T.N.; Yam-Puc, J.C.; Kaminski, T.S.; Hughes, R.; Pyeatt, G.L.; et al. Rapid discovery of monoclonal antibodies by microfluidics-enabled FACS of single pathogen-specific antibody-secreting cells. Nat. Biotechnol. 2024, 43, 960–970. [Google Scholar] [CrossRef]
- Matuła, K.; Rivello, F.; Huck, W.T.S. Single-Cell Analysis Using Droplet Microfluidics. Adv. Biosyst. 2020, 4, 1900188. [Google Scholar] [CrossRef]
- Autour, A.; Merten, C.A. Fluorescence-activated droplet sequencing (FAD-seq) directly provides sequences of screening hits in antibody discovery. Proc. Natl. Acad. Sci. USA 2024, 121, e2405342121. [Google Scholar] [CrossRef]
- Sun, H.; Hu, N.; Wang, J. Application of microfluidic technology in antibody screening. Biotechnol. J. 2022, 17, 2100623. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in microfluidic materials, functions, integration, and applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.R.; Poitzsch, A.; Hwang, Y.; Lee, J.; Czerwinska, J.; Weitz, D.A. Impact of inlet channel geometry on microfluidic drop formation. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2009, 80, 26310. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Neild, A.; deMello, A.; Liu, A.-Q.; Ai, Y. The Poisson distribution and beyond: Methods for microfluidic droplet production and single cell encapsulation. Lab Chip 2015, 15, 3439–3459. [Google Scholar] [CrossRef]
- Harrington, J.; Esteban, L.B.; Butement, J.; Vallejo, A.F.; Lane, S.I.R.; Sheth, B.; Jongen, M.S.A.; Parker, R.; Stumpf, P.S.; Smith, R.C.G.; et al. Dual dean entrainment with volume ratio modulation for efficient droplet co-encapsulation: Extreme single-cell indexing. Lab Chip 2021, 21, 3378–3386. [Google Scholar] [CrossRef]
- Hashimoto, M.; Shevkoplyas, S.S.; Zasońska, B.; Szymborski, T.; Garstecki, P.; Whitesides, G.M. Formation of Bubbles and Droplets in Parallel, Coupled Flow-Focusing Geometries. Small 2008, 4, 1795–1805. [Google Scholar] [CrossRef]
- Nisisako, T.; Torii, T. Microfluidic large-scale integration on a chip for mass production of monodisperse droplets and particles. Lab Chip 2008, 8, 287–293. [Google Scholar] [CrossRef]
- Ofner, A.; Moore, D.G.; Rühs, P.A.; Schwendimann, P.; Eggersdorfer, M.; Amstad, E.; Weitz, D.A.; Studart, A.R. High-Throughput Step Emulsification for the Production of Functional Materials Using a Glass Microfluidic Device. Macromol. Chem. Phys. 2017, 218, 1600472. [Google Scholar] [CrossRef]
- Abate, A.R.; Hung, T.; Mary, P.; Agresti, J.J.; Weitz, D.A. High-throughput injection with microfluidics using picoinjectors. Proc. Natl. Acad. Sci. USA 2010, 107, 19163–19166. [Google Scholar] [CrossRef]
- Eastburn, D.J.; Sciambi, A.; Abate, A.R. Picoinjection Enables Digital Detection of RNA with Droplet RT-PCR. PLoS ONE 2013, 8, e62961. [Google Scholar] [CrossRef]
- Lin, W.N.; Tay, M.Z.; Wong, J.X.E.; Lee, C.Y.; Fong, S.-W.; Wang, C.-I.; Ng, L.F.P.; Renia, L.; Chen, C.-H.; Cheow, L.F. Rapid microfluidic platform for screening and enrichment of cells secreting virus neutralizing antibodies. Lab Chip 2022, 22, 2578–2589. [Google Scholar] [CrossRef]
- Mutafopulos, K.; Lu, P.J.; Garry, R.; Spink, P.; Weitz, D.A. Selective cell encapsulation, lysis, pico-injection and size-controlled droplet generation using traveling surface acoustic waves in a microfluidic device. Lab Chip 2020, 20, 3914–3921. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Weitz, D. Manipulating the duration of picoinjection controls the injected volume of individual droplets. Biomicrofluidics 2024, 18, 044102. [Google Scholar] [CrossRef] [PubMed]
- Siedlik, M.J.; Issadore, D. Pico-washing: Simultaneous liquid addition and removal for continuous-flow washing of microdroplets. Microsyst. Nanoeng. 2022, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Frenz, L.; Blank, K.; Brouzes, E.; Griffiths, A.D. Reliable microfluidic on-chip incubation of droplets in delay-lines. Lab Chip 2009, 9, 1344–1348. [Google Scholar] [CrossRef]
- Mirkale, K.; Chatterjee, D. The effect of microfluidic chip geometry on droplet clustering in a high throughput droplet incubation platform for single-cell analysis. Phys. Fluids 2024, 36, 011914. [Google Scholar] [CrossRef]
- Joensson, H.N.; Uhlén, M.; Svahn, H.A. Droplet size based separation by deterministic lateral displacement—Separating droplets by cell-induced shrinking. Lab Chip 2011, 11, 1305–1310. [Google Scholar] [CrossRef]
- Marquet, C. Shear Stress in Microfluidic Devices. Available online: https://blog.darwin-microfluidics.com/shear-stress-in-microfluidic-devices/ (accessed on 26 April 2025).
- Huang, L.R.; Cox, E.C.; Austin, R.H.; Sturm, J.C. Continuous particle separation through deterministic lateral displacement. Science 2004, 304, 987–990. [Google Scholar] [CrossRef]
- Tottori, N.; Hatsuzawa, T.; Nisisako, T. Separation of main and satellite droplets in a deterministic lateral displacement microfluidic device. RSC Adv. 2017, 7, 35516–35524. [Google Scholar] [CrossRef]
- Fallah-Araghi, A.; Baret, J.-C.; Ryckelynck, M.; Griffiths, A.D. A completely in vitro ultrahigh-throughput droplet-based microfluidic screening system for protein engineering and directed evolution. Lab Chip 2012, 12, 882–891. [Google Scholar] [CrossRef]
- Maenaka, H.; Yamada, M.; Yasuda, M.; Seki, M. Continuous and Size-Dependent Sorting of Emulsion Droplets Using Hydrodynamics in Pinched Microchannels. Langmuir 2008, 24, 4405–4410. [Google Scholar] [CrossRef]
- Fidalgo, L.M.; Whyte, G.; Bratton, D.; Kaminski, C.F.; Abell, C.; Huck, W.T.S. From Microdroplets to Microfluidics: Selective Emulsion Separation in Microfluidic Devices. Angew. Chem. Int. Ed. 2008, 47, 2042–2045. [Google Scholar] [CrossRef] [PubMed]
- ESI-Mine: A Label-Free Platform for High-Throughput Miniaturized Electrospray Ionization Mass Spectrometry (ESI-MS). Available online: https://www.spectraresearch.com/wp-content/uploads/2020/12/Sphere-Fluidics-ESI-Mine-Brochure-2020-1.pdf (accessed on 1 April 2025).
- Li, X.; Brown, M.J.; Smith, C.A.; Cooper, G.; Liu, X.; Dossang, A.; Pawate, V.; Bridges, A.; Holmes, D.; Leavens, B. Picodroplet Mass Spectrometry for Miniaturized High Throughput Analysis of Synthetic Biology Microbial Clones. Available online: https://www.spherefluidics.com/wp-content/uploads/2017/10/2016-12-Picodroplet-mass-spectrometry-for-miniaturized-high-throughput-analysis-of-synthetic-biology-microbial-clones.pdf (accessed on 1 April 2025).
- Christopher, G.F.; Bergstein, J.; End, N.B.; Poon, M.; Nguyen, C.; Anna, S.L. Coalescence and splitting of confined droplets at microfluidic junctions. Lab Chip 2009, 9, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, L.; Liao, M.; He, L.; Wu, P. Multiple splitting of droplets using multi-furcating microfluidic channels. Biomicrofluidics 2019, 13, 024112. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, H.; Han, S.-I.; Han, A. Cell Washing and Solution Exchange in Droplet Microfluidic Systems. Anal. Chem. 2021, 93, 8622–8630. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.-y.; Hu, S.-l.; Lu, J.-q.; Liu, C.; Liu, J.-s. Electrokinetic concentrating with a nanofluidic device for magnetic beads-based antigen–antibody immunoassay. Microsyst. Technol. 2016, 22, 283–286. [Google Scholar] [CrossRef]
- Lu, B.; Chen, J.W.; Maharbiz, M.M. Ion concentration polarization (ICP) of proteins at silicon micropillar nanogaps. PLoS ONE 2020, 15, e0229405. [Google Scholar] [CrossRef]
- Park, S.; Yossifon, G. Combining dielectrophoresis and concentration polarization-based preconcentration to enhance bead-based immunoassay sensitivity. Nanoscale 2019, 11, 9436–9443. [Google Scholar] [CrossRef]
- Xi, H.-D.; Zheng, H.; Guo, W.; Gañán-Calvo, A.M.; Ai, Y.; Tsao, C.-W.; Zhou, J.; Li, W.; Huang, Y.; Nguyen, N.-T.; et al. Active droplet sorting in microfluidics: A review. Lab Chip 2017, 17, 751–771. [Google Scholar] [CrossRef]
- Schütz, S.S.; Beneyton, T.; Baret, J.-C.; Schneider, T.M. Rational design of a high-throughput droplet sorter. Lab Chip 2019, 19, 2220–2232. [Google Scholar] [CrossRef]
- Richter, E.S.; Link, A.; McGrath, J.S.; Sparrow, R.W.; Gantz, M.; Medcalf, E.J.; Hollfelder, F.; Franke, T. Acoustic sorting of microfluidic droplets at kHz rates using optical absorbance. Lab Chip 2023, 23, 195–202. [Google Scholar] [CrossRef]
- Abate, A.R.; Agresti, J.J.; Weitz, D.A. Microfluidic sorting with high-speed single-layer membrane valves. Appl. Phys. Lett. 2010, 96, 203509. [Google Scholar] [CrossRef]
- Sciambi, A.; Abate, A.R. Accurate microfluidic sorting of droplets at 30 kHz. Lab Chip 2015, 15, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, A.; Nakagawa, Y.; Loo, M.H.; Shibata, Y.; Tanaka, N.; Setyaningrum, D.L.; Park, J.W.; Shirasaki, Y.; Mikami, H.; Huang, D.; et al. Sequentially addressable dielectrophoretic array for high-throughput sorting of large-volume biological compartments. Sci. Adv. 2020, 6, eaba6712. [Google Scholar] [CrossRef] [PubMed]
- Vyawahare, S.; Brundage, M.; Kijac, A.; Gutierrez, M.; de Geus, M.; Sinha, S.; Homyk, A. Sorting droplets into many outlets. Lab Chip 2021, 21, 4262–4273. [Google Scholar] [CrossRef]
- Caen, O.; Schütz, S.; Jammalamadaka, M.S.S.; Vrignon, J.; Nizard, P.; Schneider, T.M.; Baret, J.-C.; Taly, V. High-throughput multiplexed fluorescence-activated droplet sorting. Microsyst. Nanoeng. 2018, 4, 33. [Google Scholar] [CrossRef]
- Isozaki, A.; Huang, D.; Nakagawa, Y.; Goda, K. Dual sequentially addressable dielectrophoretic array for high-throughput, scalable, multiplexed droplet sorting. Microfluid. Nanofluidics 2021, 25, 32. [Google Scholar] [CrossRef]
- Zhang, H.; Gupte, R.; Li, Y.; Huang, C.; Guzman, A.R.; Han, J.J.; Jung, H.; Sabnis, R.; de Figueiredo, P.; Han, A. NOVAsort for error-free droplet microfluidics. Nat. Commun. 2024, 15, 9444. [Google Scholar] [CrossRef]
- Mazutis, L.; Gilbert, J.; Ung, W.L.; Weitz, D.A.; Griffiths, A.D.; Heyman, J.A. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. [Google Scholar] [CrossRef]
- Chokkalingam, V.; Tel, J.; Wimmers, F.; Liu, X.; Semenov, S.; Thiele, J.; Figdor, C.G.; Huck, W.T.S. Probing cellular heterogeneity in cytokine-secreting immune cells using droplet-based microfluidics. Lab Chip 2013, 13, 4740–4744. [Google Scholar] [CrossRef]
- El Debs, B.; Utharala, R.; Balyasnikova, I.V.; Griffiths, A.D.; Merten, C.A. Functional single-cell hybridoma screening using droplet-based microfluidics. Proc. Natl. Acad. Sci. USA 2012, 109, 11570–11575. [Google Scholar] [CrossRef]
- Yuan, Y.; Brouchon, J.; Calvo-Calle, J.M.; Xia, J.; Sun, L.; Zhang, X.; Clayton, K.L.; Ye, F.; Weitz, D.A.; Heyman, J.A. Droplet encapsulation improves accuracy of immune cell cytokine capture assays. Lab Chip 2020, 20, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, R.; Shen, B.; Li, N.; Zhou, H.; Wang, W.; Zhao, Y.; Huang, M.; Fang, P.; Wang, S.; et al. High-throughput functional screening for next-generation cancer immunotherapy using droplet-based microfluidics. Sci. Adv. 2021, 7, eabe3839. [Google Scholar] [CrossRef]
- Day, R.N.; Periasamy, A.; Schaufele, F. Fluorescence resonance energy transfer microscopy of localized protein interactions in the living cell nucleus. Methods 2001, 25, 4–18. [Google Scholar] [CrossRef]
- Josephides, D.; Davoli, S.; Whitley, W.; Ruis, R.; Salter, R.; Gokkaya, S.; Vallet, M.; Matthews, D.; Benazzi, G.; Shvets, E.; et al. Cyto-Mine: An Integrated, Picodroplet System for High-Throughput Single-Cell Analysis, Sorting, Dispensing, and Monoclonality Assurance. SLAS Technol. 2020, 25, 177–189. [Google Scholar] [CrossRef]
- Rutkauskaite, J.; Berger, S.; Stavrakis, S.; Dressler, O.; Heyman, J.; Casadevall I Solvas, X.; deMello, A.; Mazutis, L. High-throughput single-cell antibody secretion quantification and enrichment using droplet microfluidics-based FRET assay. iScience 2022, 25, 104515. [Google Scholar] [CrossRef]
- Gaa, R.; Menang-Ndi, E.; Pratapa, S.; Nguyen, C.; Kumar, S.; Doerner, A. Versatile and rapid microfluidics-assisted antibody discovery. mAbs 2021, 13, 1978130. [Google Scholar] [CrossRef]
- Li, Y.; Liu, P.C.; Shen, Y.; Snavely, M.D.; Hiraga, K. A Cell-Based Internalization and Degradation Assay with an Activatable Fluorescence-Quencher Probe as a Tool for Functional Antibody Screening. J. Biomol. Screen. 2015, 20, 869–875. [Google Scholar] [CrossRef]
- Riedl, T.; van Boxtel, E.; Bosch, M.; Parren, P.W.H.I.; Gerritsen, A.F. High-Throughput Screening for Internalizing Antibodies by Homogeneous Fluorescence Imaging of a pH-Activated Probe. J. Biomol. Screen. 2015, 21, 12–23. [Google Scholar] [CrossRef]
- von Bredow, B.; Arias, J.F.; Heyer, L.N.; Gardner, M.R.; Farzan, M.; Rakasz, E.G.; Evans, D.T. Envelope Glycoprotein Internalization Protects Human and Simian Immunodeficiency Virus-Infected Cells from Antibody-Dependent Cell-Mediated Cytotoxicity. J. Virol. 2015, 89, 10648–10655. [Google Scholar] [CrossRef]
- Safa, N.; Vaithiyanathan, M.; Sombolestani, S.; Charles, S.; Melvin, A.T. Population-based analysis of cell-penetrating peptide uptake using a microfluidic droplet trapping array. Anal. Bioanal. Chem. 2019, 411, 2729–2741. [Google Scholar] [CrossRef]
- Gérard, A.; Woolfe, A.; Mottet, G.; Reichen, M.; Castrillon, C.; Menrath, V.; Ellouze, S.; Poitou, A.; Doineau, R.; Briseno-Roa, L.; et al. High-throughput single-cell activity-based screening and sequencing of antibodies using droplet microfluidics. Nat. Biotechnol. 2020, 38, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Miksa, M.; Komura, H.; Wu, R.; Shah, K.G.; Wang, P. A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. J. Immunol. Methods 2009, 342, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, B.; He, M.; Li, X.; Chen, P.; Hu, B. Study on uptake of gold nanoparticles by single cells using droplet microfluidic chip-inductively coupled plasma mass spectrometry. Talanta 2019, 200, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Chaipan, C.; Pryszlak, A.; Dean, H.; Poignard, P.; Benes, V.; Griffiths, A.D.; Merten, C.A. Single-Virus Droplet Microfluidics for High-Throughput Screening of Neutralizing Epitopes on HIV Particles. Cell Chem. Biol. 2017, 24, 751–757.e753. [Google Scholar] [CrossRef]
- Wippold, J.A.; Wang, H.; Tingling, J.; Leibowitz, J.L.; de Figueiredo, P.; Han, A. PRESCIENT: Platform for the rapid evaluation of antibody success using integrated microfluidics enabled technology. Lab Chip 2020, 20, 1628–1638. [Google Scholar] [CrossRef]
- Konry, T.; Dominguez-Villar, M.; Baecher-Allan, C.; Hafler, D.A.; Yarmush, M.L. Droplet-based microfluidic platforms for single T cell secretion analysis of IL-10 cytokine. Biosens. Bioelectron. 2010, 26, 2707–2710. [Google Scholar] [CrossRef]
- Khajvand, T.; Huang, P.; Li, L.; Zhang, M.; Zhu, F.; Xu, X.; Huang, M.; Yang, C.; Lu, Y.; Zhu, Z. Interfacing droplet microfluidics with antibody barcodes for multiplexed single-cell protein secretion profiling. Lab Chip 2021, 21, 4823–4830. [Google Scholar] [CrossRef]
- Singhal, A.; Haynes, C.A.; Hansen, C.L. Microfluidic Measurement of Antibody−Antigen Binding Kinetics from Low-Abundance Samples and Single Cells. Anal. Chem. 2010, 82, 8671–8679. [Google Scholar] [CrossRef]
- Dimatteo, R.; Di Carlo, D. IL-2 secretion-based sorting of single T cells using high-throughput microfluidic on-cell cytokine capture. Lab Chip 2022, 22, 1576–1583. [Google Scholar] [CrossRef]
- Mazutis, L.; Vasiliauskas, R.; Weitz, D.A. Microfluidic Production of Alginate Hydrogel Particles for Antibody Encapsulation and Release. Macromol. Biosci. 2015, 15, 1641–1646. [Google Scholar] [CrossRef]
- Kemna, E.W.M.; Schoeman, R.M.; Wolbers, F.; Vermes, I.; Weitz, D.A.; van den Berg, A. High-yield cell ordering and deterministic cell-in-droplet encapsulation using Dean flow in a curved microchannel. Lab Chip 2012, 12, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, P.; Luo, Z.; Wang, L.; Ding, W.; Wu, T.; Chen, J.; He, J.; He, Y.; Wang, H.; et al. Dean Flow Assisted Single Cell and Bead Encapsulation for High Performance Single Cell Expression Profiling. ACS Sens. 2019, 4, 1299–1305. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Hyun, K.A.; Jung, H.-I. Microfluidic recapitulation of circulating tumor cell–neutrophil clusters via double spiral channel-induced deterministic encapsulation. Lab Chip 2021, 21, 3483–3497. [Google Scholar] [CrossRef]
- Link, A.; McGrath, J.S.; Zaimagaoglu, M.; Franke, T. Active single cell encapsulation using SAW overcoming the limitations of Poisson distribution. Lab Chip 2022, 22, 193–200. [Google Scholar] [CrossRef]
- Panwar, J.; Autour, A.; Merten, C.A. Design and construction of a microfluidics workstation for high-throughput multi-wavelength fluorescence and transmittance activated droplet analysis and sorting. Nat. Protoc. 2023, 18, 1090–1136. [Google Scholar] [CrossRef]
- Panwar, J.; Utharala, R.; Fennelly, L.; Frenzel, D.; Merten, C.A. iSort enables automated complex microfluidic droplet sorting in an effort to democratize technology. Cell Rep. Methods 2023, 3, 100478. [Google Scholar] [CrossRef]
- Cole, R.H.; Tang, S.-Y.; Siltanen, C.A.; Shahi, P.; Zhang, J.Q.; Poust, S.; Gartner, Z.J.; Abate, A.R. Printed droplet microfluidics for on demand dispensing of picoliter droplets and cells. Proc. Natl. Acad. Sci. USA 2017, 114, 8728–8733. [Google Scholar] [CrossRef]
- Kintses, B.; Hein, C.; Mohamed, M.F.; Fischlechner, M.; Courtois, F.; Lainé, C.; Hollfelder, F. Picoliter Cell Lysate Assays in Microfluidic Droplet Compartments for Directed Enzyme Evolution. Chem. Biol. 2012, 19, 1001–1009. [Google Scholar] [CrossRef]
- Baret, J.-C.; Miller, O.J.; Taly, V.; Ryckelynck, M.; El-Harrak, A.; Frenz, L.; Rick, C.; Samuels, M.L.; Hutchison, J.B.; Agresti, J.J.; et al. Fluorescence-activated droplet sorting (FADS): Efficient microfluidic cell sorting based on enzymatic activity. Lab Chip 2009, 9, 1850–1858. [Google Scholar] [CrossRef]
- Obexer, R.; Pott, M.; Zeymer, C.; Griffiths, A.D.; Hilvert, D. Efficient laboratory evolution of computationally designed enzymes with low starting activities using fluorescence-activated droplet sorting. Protein Eng. Des. Sel. 2016, 29, 355–366. [Google Scholar] [CrossRef]
- Lombardo, J.A.; Aliaghaei, M.; Nguyen, Q.H.; Kessenbrock, K.; Haun, J.B. Microfluidic platform accelerates tissue processing into single cells for molecular analysis and primary culture models. Nat. Commun. 2021, 12, 2858. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Lombardo, J.A.; Westerhof, T.M.; Pennell, M.; Ng, A.; Alshetaiwi, H.; Luna, B.M.; Nelson, E.L.; Kessenbrock, K.; Hui, E.E.; et al. Microfluidic filter device with nylon mesh membranes efficiently dissociates cell aggregates and digested tissue into single cells. Lab Chip 2018, 18, 2776–2786. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Kim, S.C.; Modavi, C.; Abate, A.R. Robotic automation of droplet microfluidics. Biomicrofluidics 2022, 16, 014102. [Google Scholar] [CrossRef]
- Wohnhaas, C.T.; Leparc, G.G.; Fernandez-Albert, F.; Kind, D.; Gantner, F.; Viollet, C.; Hildebrandt, T.; Baum, P. DMSO cryopreservation is the method of choice to preserve cells for droplet-based single-cell RNA sequencing. Sci. Rep. 2019, 9, 10699. [Google Scholar] [CrossRef]
- Schneider, G. Automating drug discovery. Nat. Rev. Drug Discov. 2018, 17, 97–113. [Google Scholar] [CrossRef]
- McGrath, J.; Jimenez, M.; Bridle, H. Deterministic lateral displacement for particle separation: A review. Lab Chip 2014, 14, 4139–4158. [Google Scholar] [CrossRef]
- Müller, T.; Ruggeri, F.S.; Kulik, A.J.; Shimanovich, U.; Mason, T.O.; Knowles, T.P.J.; Dietler, G. Nanoscale spatially resolved infrared spectra from single microdroplets. Lab Chip 2014, 14, 1315–1319. [Google Scholar] [CrossRef]
- Zhu, Y.; Fang, Q. Integrated Droplet Analysis System with Electrospray Ionization-Mass Spectrometry Using a Hydrophilic Tongue-Based Droplet Extraction Interface. Anal. Chem. 2010, 82, 8361–8366. [Google Scholar] [CrossRef]
- Anagnostidis, V.; Sherlock, B.; Metz, J.; Mair, P.; Hollfelder, F.; Gielen, F. Deep learning guided image-based droplet sorting for on-demand selection and analysis of single cells and 3D cell cultures. Lab Chip 2020, 20, 889–900. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Huang, C.-H.; Pai, P.-C.; Seo, J.; Lei, K.F. A Review on Microfluidics-Based Impedance Biosensors. Biosensors 2023, 13, 83. [Google Scholar] [CrossRef]
- Tong, Z.; Shen, C.; Li, Q.; Yin, H.; Mao, H. Combining sensors and actuators with electrowetting-on-dielectric (EWOD): Advanced digital microfluidic systems for biomedical applications. Analyst 2023, 148, 1399–1421. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, L. Drug Intelligence Science (DIS®): Pioneering a high-resolution translational platform to enhance the probability of success for drug discovery and development. Drug Discov. Today 2023, 28, 103795. [Google Scholar] [CrossRef] [PubMed]
- Koehler, C.; Sauter, P.F.; Klasen, B.; Waldmann, C.; Pektor, S.; Bausbacher, N.; Lemke, E.A.; Miederer, M. Genetic Code Expansion for Site-Specific Labeling of Antibodies with Radioisotopes. ACS Chem. Biol. 2023, 18, 443–448. [Google Scholar] [CrossRef]
- Arlene, W.; Angus, L. The Top 10 Antibody-Drug Conjugate Contenders in 2021. Fierce Pharma. Available online: https://www.fiercepharma.com/special-report/top-10-antibody-drug-conjugate-contenders-2021 (accessed on 12 January 2024).
- Theocharopoulos, C.; Lialios, P.P.; Samarkos, M.; Gogas, H.; Ziogas, D.C. Antibody-Drug Conjugates: Functional Principles and Applications in Oncology and Beyond. Vaccines 2021, 9, 1111. [Google Scholar] [CrossRef]
- Courtois, F.; Olguin, L.F.; Whyte, G.; Theberge, A.B.; Huck, W.T.S.; Hollfelder, F.; Abell, C. Controlling the Retention of Small Molecules in Emulsion Microdroplets for Use in Cell-Based Assays. Anal. Chem. 2009, 81, 3008–3016. [Google Scholar] [CrossRef]
- Skhiri, Y.; Gruner, P.; Semin, B.; Brosseau, Q.; Pekin, D.; Mazutis, L.; Goust, V.; Kleinschmidt, F.; El Harrak, A.; Hutchison, J.B.; et al. Dynamics of molecular transport by surfactants in emulsions. Soft Matter 2012, 8, 10618–10627. [Google Scholar] [CrossRef]
- Zinchenko, A.; Devenish, S.R.A.; Hollfelder, F. Rapid quantitative assessment of small molecule leakage from microdroplets by flow cytometry and improvement of fluorophore retention in biochemical assays. bioRxiv 2023. [Google Scholar] [CrossRef]
- Omidfar, K.; Kashanian, S. A mini review on recent progress of microfluidic systems for antibody development. J. Diabetes Metab. Disord. 2024, 23, 323–331. [Google Scholar] [CrossRef]
- Kim, J.; McFee, M.; Fang, Q.; Abdin, O.; Kim, P.M. Computational and artificial intelligence-based methods for antibody development. Trends Pharmacol. Sci. 2023, 44, 175–189. [Google Scholar] [CrossRef]
- Bai, G.; Sun, C.; Guo, Z.; Wang, Y.; Zeng, X.; Su, Y.; Zhao, Q.; Ma, B. Accelerating antibody discovery and design with artificial intelligence: Recent advances and prospects. Semin. Cancer Biol. 2023, 95, 13–24. [Google Scholar] [CrossRef]
- Környei, Z.; Beke, S.; Mihálffy, T.; Jelitai, M.; Kovács, K.J.; Szabó, Z.; Szabó, B. Cell sorting in a Petri dish controlled by computer vision. Sci. Rep. 2013, 3, 1088. [Google Scholar] [CrossRef] [PubMed]
- Brower, K.K.; Khariton, M.; Suzuki, P.H.; Still, C.I.I.; Kim, G.; Calhoun, S.G.K.; Qi, L.S.; Wang, B.; Fordyce, P.M. Double Emulsion Picoreactors for High-Throughput Single-Cell Encapsulation and Phenotyping via FACS. Anal. Chem. 2020, 92, 13262–13270. [Google Scholar] [CrossRef] [PubMed]
- Brower, K.K.; Carswell-Crumpton, C.; Klemm, S.; Cruz, B.; Kim, G.; Calhoun, S.G.K.; Nichols, L.; Fordyce, P.M. Double emulsion flow cytometry with high-throughput single droplet isolation and nucleic acid recovery. Lab Chip 2020, 20, 2062–2074. [Google Scholar] [CrossRef] [PubMed]


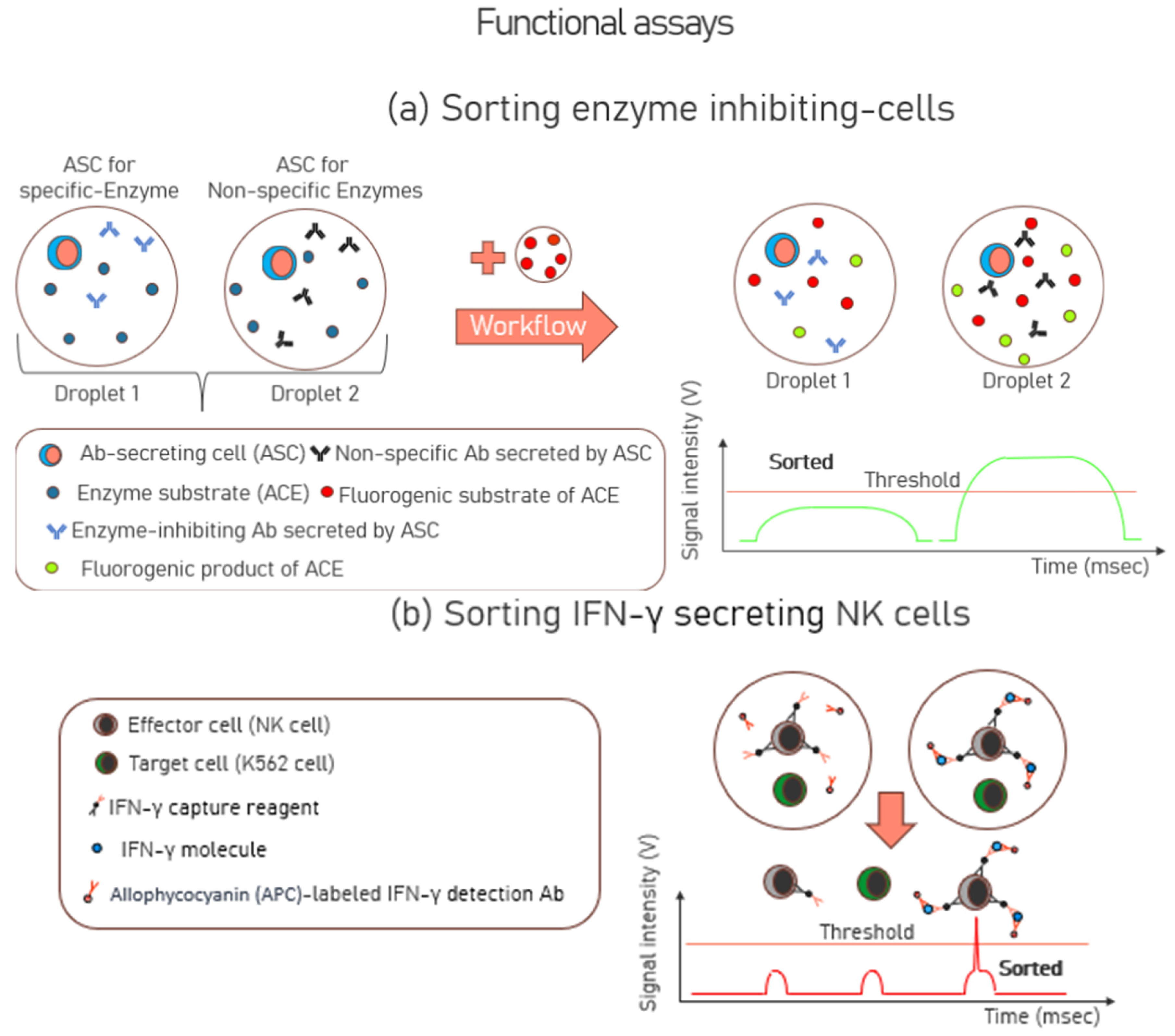
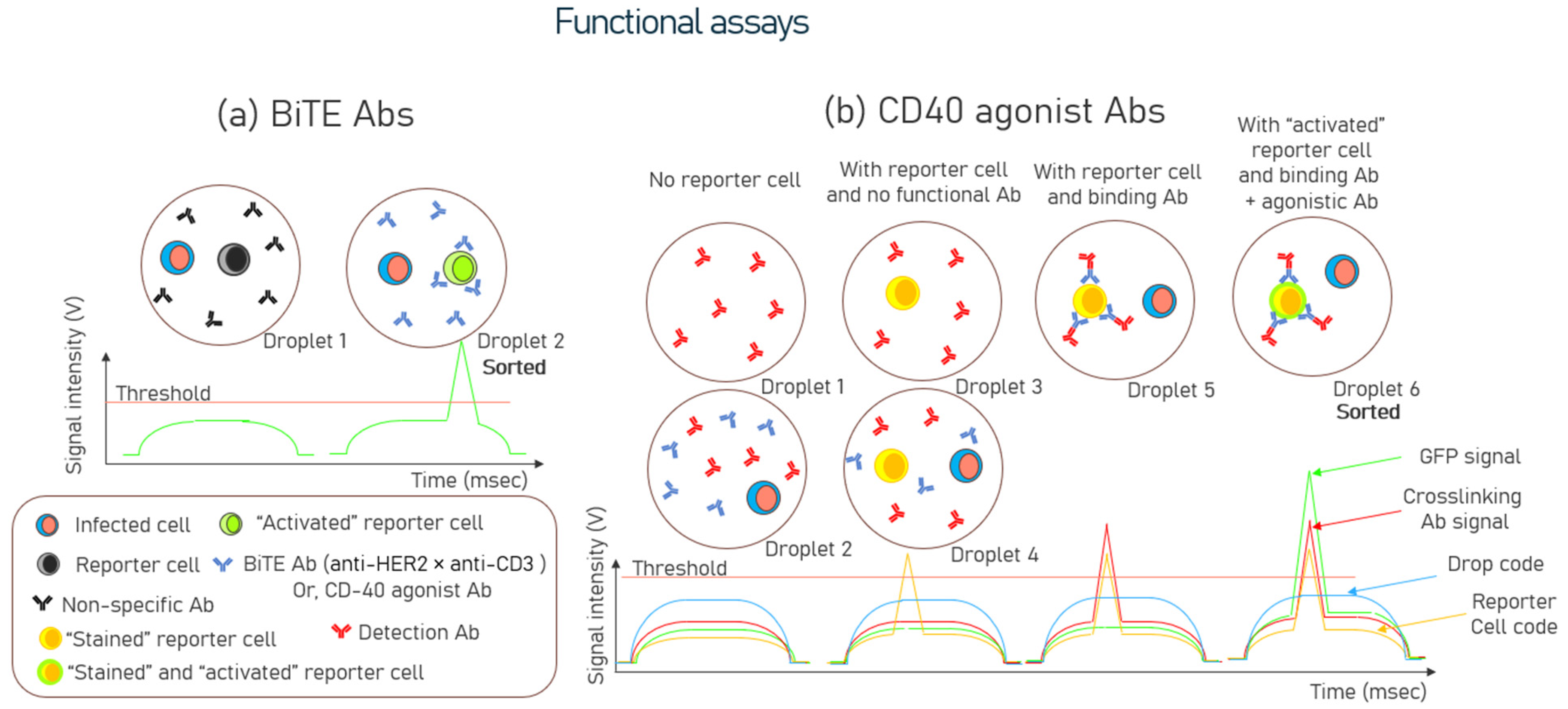
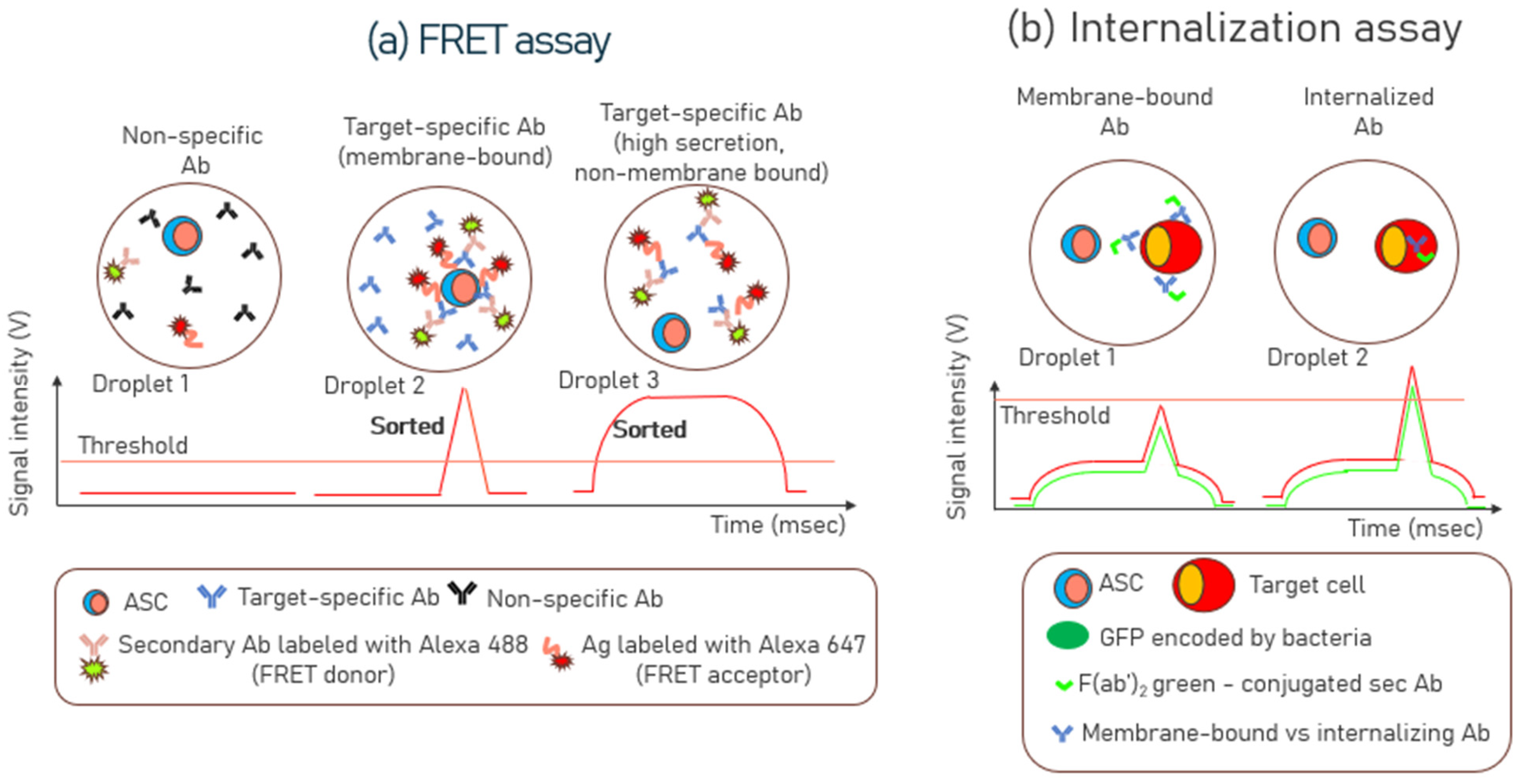

| Immunoassay Strategy [Bead (Material)—Diameter (μm)/Coating] | Cell Types/Populations | Primary Ab/Cytokine | Ag/Capture Ab—Reagent | Detection Ab | Microfluidic Platform | References |
|---|---|---|---|---|---|---|
| Streptavidin-coated polystyrene (PS) beads—diameter 6 μm | 9E10 cells secreting IgG antibodies against human c-MYC protein | IgG | Biotinylated Goat anti-mouse IgG | DyLight 488 Affini- Goat anti-Mouse IgG, F(ab’)2 Fragment Specific | Droplet Generation + FADS | Singleplexing [74] |
| Avidin Coated Particles, diameter 0.9 μm | CD4 + CD25 + regulatory T cells | human IL-10 | Biotinylated IL-10 mAb (Invitrogen AHC7109) | rat anti-human IL-10 FITC conjugated antibodies | Droplet Generation + FACS | Singleplexing [92] |
| Barcode array on glass substrate (width—10 µm, pitch—25 µm) | U937-derived macrophage cells, human tumor cell lines (U87 and SCC6 cells) | (i) TNF-alpha, (ii) MIP-1b, (iii) MCP-1, (iv) IL-8, (v) IL-10 | (i) Mouse IgG1, κ/Mab11, (ii) Mouse IgG1 kappa/A174E18A7, (iii) Mouse IgG1, κ/5D3-F7, (iv) Mouse IgG1, κ/H8A5, (v) Rat IgG2a, κ/JES3-12G8 | (i) Mouse IgG1, κ/MAb1, (ii) Mouse IgG2B/24006, (iii) Armenian Hamster IgG/2H5 (iv) Mouse IgG1, κ/E8N1, (v) Rat IgG1, κ/JES3-9D7 | Droplet-based barcoded microarrays | Multiplexing [93] |
| PS/biotinylated mAbs specific for IL-2, TNF-a, or IFN-gamma | T cells (4 M/mL) stimulated by phorbol 12-myristate 13-acetate (PMA, 1 µg/mL) and ionomycin (0.2 µg/mL) | IL-2, IFN-gamma, TNF-alpha | anti-CD3, anti-CD69, anti-IL-2, anti-TNF-a and anti-IFN-g- | anti-CD3-PE/FITC, anti-CD69-PerCP®(both BD Pharmingen), anti-IL-2-Alexa Fluor® 488, anti-TNF-a-PE and anti-IFN-g-Alexa®Fluor 647® | Droplet Generation + FACS | Multiplexing [75] |
| Protein A-coated PS, capture Ab, diameter 5.5 μm | D1.3 Hybridoma cells | D1.3, HyHEL-5, and LGB-1 | Rabbit or goat anti-mouse pAb | HEL-Dylight488, HEL-Dylight633 for D1.3; HEL-Dylight488 for HyHEL-5, EGFP for LGB-1 | Microfluidic flow channels for trapping beads | Multiplexing [94] |
| NA | T cells stimulated by phorbol 12-myristate 13-acetate (PMA) and ionomycin | human IL-10 | Miltenyi IL-2 capture reagent | Miltenyi phycoerythrin—conjugated anti IL-2 Ab | Parallelized Droplet Generation + FACS | On-cell membrane [95] |
| Target cells: K562 cells | OKT9 hybridoma cells | IgG | NA | goat anti-mouse IgG Alexa 488 antibody | Droplet Generation + FADS | Cellular Binding (Cell surface) [28] |
| Target cells: EGFR-positive A431 cells | mAb108 hybridoma cells | EFGR-specific IgG | NA | Alexa 488 AffiniPure Fab goat anti-mouse IgG (H + L) | Cytomine | Cellular Binding (Cell surface) [82] |
| Animal Model | Immunogen | Cell Types/Populations | Primary Ab | Donor | Acceptor | Microfluidic Platform | References |
|---|---|---|---|---|---|---|---|
| Mouse | human TNF-alpha | DG44 CHO cell line | human IgG4 | Green fluorophore-conjugated human TNF-alpha | Red fluorophore-conjugated anti-mouse IgG-Fc Ab | Cytomine | [80] |
| NA | c-myc peptide | 9E10 cells secret IgG antibodies against human c-MYC protein | IgG | Alexa Fluor 488-conjugated anti-mouse F(ab’)2-specific pAb | Alexa Fluor 647-conjugated c-myc peptide | Droplet Generation + FADS | [81] |
| Human, mouse | Tetanus Toxoid | Plasma cells (bone marrow, lymph nodes, splenocytes) | IgG | Goat Anti-Human IgG Fc-DyLight® 488 | Goat F(ab’)2 Anti-Human IgG—(Fab’)2 (DyLight® 594), pre-adsorbed | Cytomine | [82] |
| Target Cells | Cell Types/Populations | Reporter Cells | Primary Ab | Detection Criteria | Microfluidic Platform | References |
|---|---|---|---|---|---|---|
| K562 cells | NK-92 MI cells (coated with IFN-γ capture reagent) | NA | Anti IFN-γ Ab | APC signal from IFN-γ detection Ab | Droplet Generation + FACS | [77] |
| NA | OKT3 hybridoma cells | Jurkat-GFP cells | anti-CD3 Ab | GFP signal from reporter cells | Cytomine | [82] |
| NA | Hybridoma cells expressing Ab, 4E3 which inhibits ACE-1 | NA | Antibodies that target and inhibit ACE-1 | Droplets with low fluorescence intensity were sorted which indicated the presence of ACE-1 inhibitory Ab | Integrated chip (Generator + fusion + on-chip incubation + FADS) | [76] |
| NA | K562-Her2 cells (positive control), HEK293FT cells infected with lentivirus | Jurkat/NF-κB-GFP, Jurkat/pIL2-eGFP | Anti-Her2 × anti-CD3 bispecific Ab | Reporter cells’s Cell Trace Yellow signal and K562-Her2 cells’s Cell Trace Violet signal | Droplet Generation + FADS | [78] |
| Manufacturer | Wavelengths (nm) | Power (mW) | Beam Diameter (mm) | Directly Passes Through OL (Y/N) | OL’s Magnification | Droplet Volume (pL) | References |
|---|---|---|---|---|---|---|---|
| Changchun Dragon Lasers | 405 | 50 | 1.2 | Y | 10×, 20× | 140 | [101] |
| 473 | 100 | 2 | Y | 10×, 20× | |||
| 561 | 50 | NA | Y | 10×, 20× | |||
| Melles-Griot | 488 | 50 | 0.7 | CL -> OL | 20× | 50 | [74] |
| Omicron (PhoxX+ 488-100) | 488 | 100 | 0.7–1 | CL -> OL | 20× | 40 | [81] |
| Changchun New Industries (CNI) | 473 | NA | <1.2 | NA | NA | 8 | [68] |
| Changchun New Industries (CNI) | 473, 532, 640 (aligned) | 100 | <1.2 | NA | NA | 270 | [103] |
| Picarro Cyan | 488 | 20 | NA | Y | 40× | ~24 | [104] |
| Newport-Spectraphysics | 488 | 20 | 1.3 ± 0.3 | CL -> PCL -> OL | 40× | 12 | [105] |
| Omicron (combiner) Laserage GmbH | 365 + 488 + 561 | NA | 0.7 | OL | NA | ~17 | [106] |
| PMT—Model, Company | Sorting Speed (Hz) | Frequency BW (kHz) | Gain | Wavelength Detected (min, max) | Wavelength Detected (peak) | References |
|---|---|---|---|---|---|---|
| H10722-20, Hamamatsu | 100–200 | 20 | 2 × 106 | 230, 920 | 630 | [101] |
| 500 | [81] | |||||
| 1000 | [106] | |||||
| H5784-20, Hamamatsu | 200 | 5 × 105 | 230, 920 | 630 | [74] | |
| 300 | [105] | |||||
| PMM02, Thorlabs | 30,000 | 5.1 × 105 | 300, 800 | 420 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, D.; McGrath, J.S.; Moore, J.H.; Gardner, J.; Blom, D. Recent Advances in Antibody Discovery Using Ultrahigh-Throughput Droplet Microfluidics: Challenges and Future Perspectives. Biosensors 2025, 15, 409. https://doi.org/10.3390/bios15070409
Das D, McGrath JS, Moore JH, Gardner J, Blom D. Recent Advances in Antibody Discovery Using Ultrahigh-Throughput Droplet Microfluidics: Challenges and Future Perspectives. Biosensors. 2025; 15(7):409. https://doi.org/10.3390/bios15070409
Chicago/Turabian StyleDas, Dhiman, John Scott McGrath, John Hudson Moore, Jason Gardner, and Daniël Blom. 2025. "Recent Advances in Antibody Discovery Using Ultrahigh-Throughput Droplet Microfluidics: Challenges and Future Perspectives" Biosensors 15, no. 7: 409. https://doi.org/10.3390/bios15070409
APA StyleDas, D., McGrath, J. S., Moore, J. H., Gardner, J., & Blom, D. (2025). Recent Advances in Antibody Discovery Using Ultrahigh-Throughput Droplet Microfluidics: Challenges and Future Perspectives. Biosensors, 15(7), 409. https://doi.org/10.3390/bios15070409





