Flexible Hydrophobic Paper-Based Microfluidic Field-Effect Biosensor Amplified by RNA-Cleaving DNAzyme-Based DNA Nanostructure for Mg2+ Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagent
2.2. Apparatus
2.3. Preparation of MFES/SWNTs-PCA
2.4. Modification of MFES/SWNTs-PCA/RCD-Hairpin
2.5. Sensing Protocol
3. Results
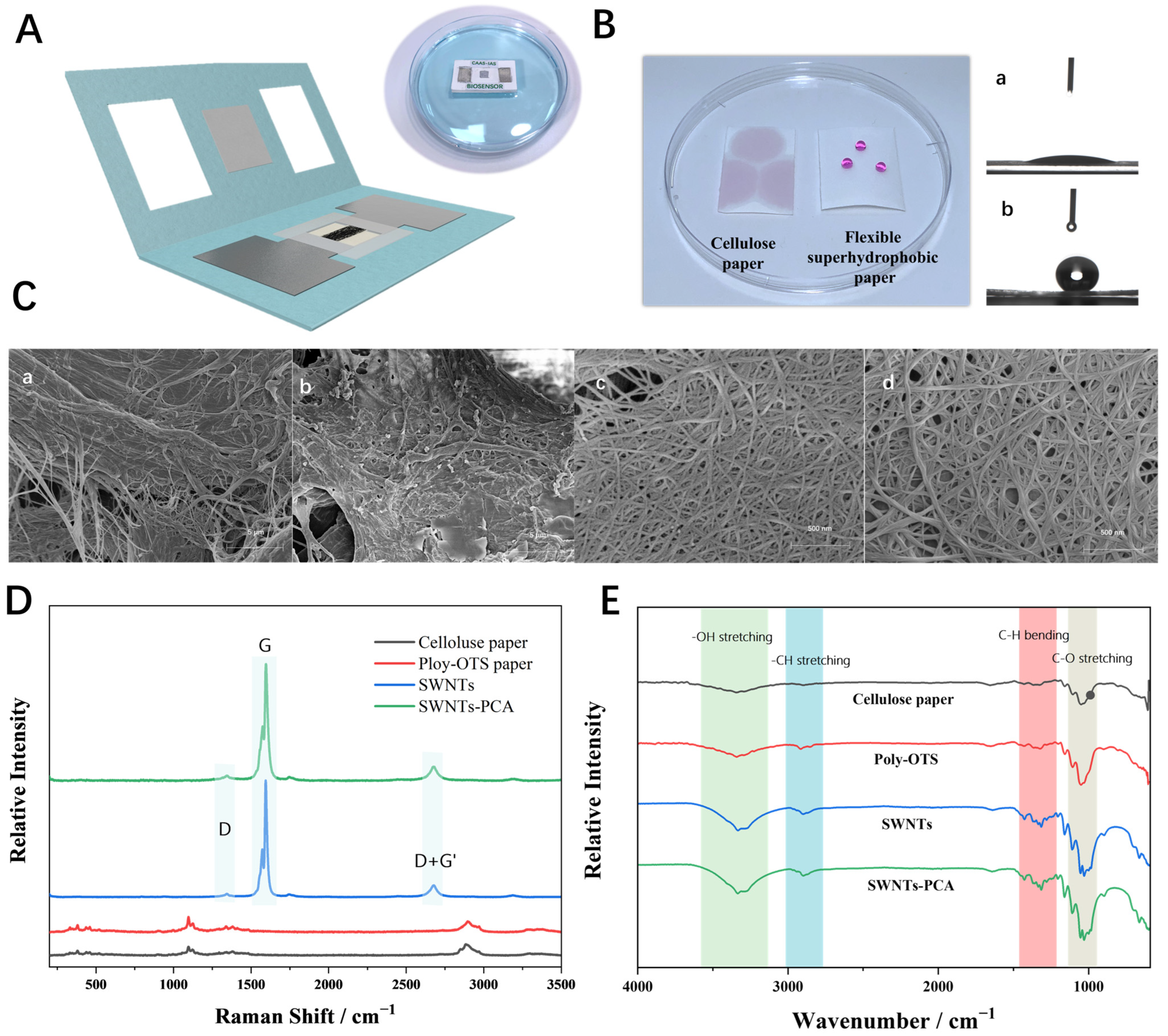
3.1. Characteristics of MFES/SWNTs-PCA
3.2. Characteristics of MFES/SWNTs-PCA/RCD-Hairpin
3.2.1. Electrochemical Characteristics
3.2.2. Optimization
3.2.3. Performance Evaluation
3.3. Sensitivity Amplified Using Similar DNA Origami Method
3.4. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gałęska, E.; Wrzecińska, M.; Kowalczyk, A.; Araujo, J.P. Reproductive consequences of electrolyte disturbances in domestic animals. Biology 2022, 11, 1006. [Google Scholar] [CrossRef]
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Regulation of magnesium balance: Lessons learned from human genetic disease. Clin. Kidney J. 2012, 5 (Suppl. S1), i15–i24. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Du, Y.; Cang, X.; Wang, J.; Chen, Z.; Yang, H.; Jiang, H. Binding competition to the POPG lipid bilayer of Ca2+, Mg2+, Na+, and K+ in different ion mixtures and biological implication. J. Phys. Chem. B 2013, 117, 850–858. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, K.; Zhao, Y.; Nan, X.; Yang, L.; Zhou, M.; Tang, X.; Xiong, B. Combined effects of vitamin B12 and fumarate on rumen propionate production and methanogenesis in dairy cow in vitro. Anim. Res. One Health 2023, 1, 204–218. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Hernandez, L.; Bruckmaier, R. Endocrine pathways to regulate calcium homeostasis around parturition and the prevention of hypocalcemia in periparturient dairy cows. Animal 2020, 14, 330–338. [Google Scholar] [CrossRef]
- Yu, X.; Wo, Y.; Ma, F.; Shan, Q.; Wei, J.; Sun, P. Zinc methionine improves the lipid metabolism that is associated with the alteration of intestine mucosal proteomes and microbiota of newborn Holstein dairy calves. Anim. Res. One Health 2024, 2, 71–85. [Google Scholar] [CrossRef]
- Gerzen, O.P.; Votinova, V.O.; Potoskueva, I.K.; Tzybina, A.E.; Nikitina, L.V. Direct Effects of Toxic Divalent Cations on Contractile Proteins with Implications for the Heart: Unraveling Mechanisms of Dysfunction. Int. J. Mol. Sci. 2023, 24, 10579. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Gotelli, C.; Wittwer, F.; Wagemann-Fluxá, C.; Sepúlveda-Varas, P. Association between prepartum subclinical magnesium imbalance and postpartum diseases in grazing dairy cows in Southern Chile. Trop. Anim. Health Prod. 2024, 56, 206. [Google Scholar] [CrossRef]
- Emam, M.H.; Shepley, E.; Mahmoud, M.M.; Ruch, M.; Elmaghawry, S.; Abdelrazik, W.; Abdelaal, A.M.; Crooker, B.A.; Caixeta, L.S. The association between prepartum rumination time, activity and dry matter intake and subclinical hypocalcemia and hypomagnesemia in the first 3 days postpartum in Holstein dairy cows. Animals 2023, 13, 1621. [Google Scholar] [CrossRef]
- Jarujamrus, P.; Malahom, N.; Puchum, S.; Meelapsom, R.; Amatatongchai, M.; Siripinyanond, A.; Chairam, S.; Kulsing, C. Complexometric and argentometric titrations using thread-based analytical devices. Talanta Int. J. Pure Appl. Anal. Chem. 2018, 183, 228–236. [Google Scholar] [CrossRef]
- Fellows, C.M.; Al Hamzah, A.A.; Ihm, S. Pathways to magnesium supplementation of drinking water: An overview of the saline water conversion corporation experience. Chem. Eng. J. Adv. 2023, 16, 100574. [Google Scholar] [CrossRef]
- Rehan, K.; Rehan, I.; Sultana, S.; Zubair, K.M.; Farooq, Z.; Mateen, A.; Humayun, M. Determination of Metals Present in Textile Dyes Using Laser-Induced Breakdown Spectroscopy and Cross-Validation Using Inductively Coupled Plasma/Atomic Emission Spectroscopy. Int. J. Spectrosc. 2017, 2017, 1614654. [Google Scholar]
- Pesaran, S.; Shojaeifard, Z.; Tashkhourian, J.; Hemmateenejad, B. A miniaturized all-in-one optical nose based on 3D printing technology and patterned paper substrate for discrimination of volatile organic compounds. Sens. Actuators B Chem. 2023, 375, 132889. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, Y.; Ma, T.; Li, L.; Li, X.; Zhu, P.; Zhou, S.; Yu, J.; Zhang, Y. Photoelectrochemical sensors based on paper and their emerging applications in point-of-care testing. Biosens. Bioelectron. 2023, 236, 115400. [Google Scholar] [CrossRef]
- Zheng, J.; Feng, C.; Qiu, S.; Xu, K.; Wang, C.; Liu, X.; Lv, J.; Yu, H.; Wu, S. Application and prospect of semiconductor biosensors in detection of viral zoonoses. J. Semicond. 2023, 44, 023102. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, A.G.; Sun, B.R.; Chen, K.S.; Yu, H.Z. Functional and versatile superhydrophobic coatings via stoichiometric silanization. Nat. Commun. 2021, 12, 982. [Google Scholar] [CrossRef]
- Wang, H.; Chen, R.; Zhang, F.; Yu, Z.; Wang, Y.; Tang, Z.; Yang, L.; Tang, X.; Xiong, B. Superhydrophobic paper-based microfluidic field-effect transistor biosensor functionalized with semiconducting single-walled carbon nanotube and DNAzyme for hypocalcemia diagnosis. Int. J. Mol. Sci. 2022, 23, 7799. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, H.; Yu, Z.; Tang, X.; Zhou, M.; Guo, Y.; Xiong, B. Hydrophobic paper used to construct a disposable chemiresistive immunosensor for the simultaneous detection of FB1 and AFB1. Adv. Mater. Interfaces 2024, 11, 2400307. [Google Scholar] [CrossRef]
- Han, L.; Li, Y.; Cao, L.; Gao, H.; Huang, E.; Zhao, S.; Li, W. One-step fabrication of OTS modified superhydrophobic cotton fabric and its efficient oil-water separation application. Sep. Purif. Technol. 2024, 340, 126760. [Google Scholar] [CrossRef]
- Wang, H.; Chen, R.; He, Y.; Zhu, X.; Yu, Z.; Feng, Z.; Pan, D.; Yang, L.; Tang, X.; Xiong, B. Controllable self-cleaning FET self-assembled RNA-cleaving DNAzyme based DNA nanotree for culture-free Staphylococcus aureus detection. J. Nanobiotechnol. 2024, 22, 414. [Google Scholar] [CrossRef]
- Pan, D.; Mijit, M.; Wang, H.; Sun, C.; Pingcuo, B.; Yu, Z.; Xiong, B.; Tang, X. Rapid Genotyping of FecB Mutation in Sheep Using CRISPR-Cas12a Integrated with DNA Nanotree Biosensing Platform. Biomolecules 2025, 15, 315. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Willner, I. Dynamic Reconfigurable DNA Nanostructures, Networks and Materials. Angew. Chem. Int. Ed. 2023, 62, e202215332. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Shang, Y.; Xie, Y.; Ding, B. DNA origami: From molecular folding art to drug delivery technology. Adv. Mater. 2024, 36, 2301035. [Google Scholar] [CrossRef]
- Spratt, J.; Dias, J.M.; Kolonelou, C.; Kiriako, G.; Engström, E.; Petrova, E.; Karampelias, C.; Cervenka, I.; Papanicolaou, N.; Lentini, A. Multivalent insulin receptor activation using insulin–DNA origami nanostructures. Nat. Nanotechnol. 2024, 19, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cañari-Chumpitaz, C.; Alexander, L.; Zhang, H.; Fan, C.; Bustamante, C. DNA origami–enhanced force spectroscopy and AlphaFold structural analyses reveal the folding landscape of calcium-binding proteins. Sci. Adv. 2025, 11, eadv1962. [Google Scholar] [CrossRef]
- Zhan, Z.; Lin, R.; Tran, V.T.; An, J.; Wei, Y.; Du, H.; Tran, T.; Lu, W. Paper/carbon nanotube-based wearable pressure sensor for physiological signal acquisition and soft robotic skin. ACS Appl. Mater. Interfaces 2017, 9, 37921–37928. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tran, T.T.; Modha, S.; Tsutsui, H.; Mulchandani, A. A paper-based chemiresistive biosensor employing single-walled carbon nanotubes for low-cost, point-of-care detection. Biosens. Bioelectron. 2019, 130, 367–373. [Google Scholar] [CrossRef]
- Bila, H.; Kurisinkal, E.E.; Bastings, M.M. Engineering a stable future for DNA-origami as a biomaterial. Biomater. Sci. 2019, 7, 532–541. [Google Scholar] [CrossRef]
- Mahmoudi-Moghaddam, H.; Tajik, S.; Beitollahi, H.J.M.J. A new electrochemical DNA biosensor based on modified carbon paste electrode using graphene quantum dots and ionic liquid for determination of topotecan. Microchem. J. 2019, 150, 104085. [Google Scholar] [CrossRef]
- Yan, X.-J.; Gao, Y.-Y.; Liu, H.-B.; Qiao, X.; Xie, C.-Z.; Li, Q.-Z.; Gao, W.-Z.; Sun, H.-B.; Xu, J.-Y. A novel double target fluorescence probe for Al3+/Mg2+ detection with distinctively different responses and its applications in cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 120067. [Google Scholar] [CrossRef]
- Sadhanala, H.K.; Aryal, S.; Sharma, K.; Orpaz, Z.; Michaeli, S.; Gedanken, A. Nitrogen-doped carbon dots as a highly selective and sensitive fluorescent probe for sensing Mg2+ ions in aqueous solution, and their application in the detection and imaging of intracellular Mg2+ ions. Sens. Actuators B Chem. 2022, 366, 131958. [Google Scholar] [CrossRef]
- Li, L.; Ding, Y.; Zhang, C.; Xian, H.; Chen, S.; Dai, G.; Wang, X.; Ye, C. Ratiometric Fluorescence Detection of Mg2+ Based on Regulating Crown-Ether Modified Annihilators for Triplet–Triplet Annihilation Upconversion. J. Phys. Chem. B 2022, 126, 3276–3282. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Suh, B.; Kim, C. A chalcone-based fluorescent chemosensor for detecting Mg2+ and Cd2+. Luminescence 2022, 37, 332–339. [Google Scholar] [CrossRef]
- Amirjani, A.; Salehi, K.; Sadrnezhaad, S. Simple SPR-based colorimetric sensor to differentiate Mg2+ and Ca2+ in aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 268, 120692. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Tang, H.; He, S.; Gao, Z.; Niu, R.; Zheng, Y.; Han, S. A ratiometric magnesium sensor using DNAzyme-templated CdTe quantum dots and Cy5. Sens. Actuators B Chem. 2018, 272, 146–150. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, Y.; Lei, J.; Zhang, L.; Ju, H. Design and biosensing of Mg2+-dependent DNAzyme-triggered ratiometric electrochemiluminescence. Anal. Chem. 2014, 86, 5158–5163. [Google Scholar] [CrossRef]
- Zhang, K.; Peng, L.; Tian, X.; Guang, S.; Xu, H. Based on theoretical calculations designed a novel dual-channel chemo-sensor for Mg2+ and Zn2+ detection and bioimaging applications. Microchem. J. 2023, 189, 108328. [Google Scholar] [CrossRef]
- Dev, K.; Singh, S.; Bhardwaj, S.; Kukreti, P.; Ramakanth, D.; Kumar, P.; Saini, S.; Roy, P.; Srivastava, V.C.; Ghosh, K. Solvent-Selective Fluorescence Sensing of Mg2+ and Al3+ Ions by Pincer-Type NNO Schiff Base Ligand: An Experimental and DFT Optimized Approach. Chem. Eur. J. 2024, 30, e202403256. [Google Scholar] [CrossRef]




| Name | Sequence and Modifications (from 5′-3′) |
|---|---|
| Substrate with rA | GAGAGAGAAGAGAGTTTACCGTCACTATT/rA/GCATTCAAT-(CH2)6-NH2 |
| Substrate without rA | GAGAGAGAAGAGAGTT TACCGTCACTATTAGCATTCAAT-(CH2)6-NH2 |
| DNAzyme | ATTGAATGAGCGATCCGGAACGGCACCCATGTATAGTGACGGTATTGAGAGAAGAGAGAG |
| T-shaped hairpin_1 | ATATGCGTAGGAATGGAGCTTTGCTCCATTCCTACTGGTAGAGTGCAGGTTTCCTGCACTCTACC GCATATCTCTCTTCTCTCTC |
| T-shaped hairpin_2 | CTCTCTTCTCTCTCATATGCGTAGGAATGGAGCTTTGCTCCATTCCTACTGGTAGAGTGCAGGTTTCCTGCACTCTACC GCATAT |
| Sensor | Method | Linear Range (μM) | Detection Limit (μM) | Reference |
|---|---|---|---|---|
| NHMI-Mg2+ | Fluorescence | 0.25~2.5 | 0.069 | [30] |
| NCDs-Mg2+ | Fluorescence | 80~720 | 60 | [31] |
| PtOEP/9-AEC | Fluorescence | 5~70 | 2.52 | [32] |
| SBOD | Fluorescent | 10~60 | 3.8 | [33] |
| L-tryptophan-AgNPs sensor | Colorimetric probe | 1~200 | 3 | [34] |
| DNAzyme-CdTe QDs) | Fluorescence | 0.001~0.020 | 0.0003 | [35] |
| ECL Biosensor | Electrochemi-luminescence | 10~10,000 | 2.8 | [36] |
| FHBS-Biosensor | Fluorescence | 0.4~70 | 0.4 | [37] |
| SB-Mg2+ Biosensor | Fluorescence | - | 7.1 | [38] |
| MFES/SWNTs-PCA/RCD-Nanostructure | IV | 1~1000 | 0.57 | This work |
| Sample | Added Mg2+ Concentration (mg/L) | The Proposed Biosensor (mg/L) | AAS (mg/L) | Recovery (%) |
|---|---|---|---|---|
| 1 | - | 15.76 | 14.78 | 106.63 |
| 50 | 69.78 | - | 107.72 | |
| 2 | - | 16.87 | 18.23 | 92.54 |
| 50 | 71.42 | - | 104.68 | |
| 3 | - | 14.12 | 13.34 | 105.58 |
| 50 | 67.31 | - | 106.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; He, Y.; Yu, Z.; Chen, R.; Feng, Z.; Chen, D.; Shakweer, W.M.E.-S.; Zhang, F.; Nan, X.; Mijit, M.; et al. Flexible Hydrophobic Paper-Based Microfluidic Field-Effect Biosensor Amplified by RNA-Cleaving DNAzyme-Based DNA Nanostructure for Mg2+ Detection. Biosensors 2025, 15, 405. https://doi.org/10.3390/bios15070405
Wang H, He Y, Yu Z, Chen R, Feng Z, Chen D, Shakweer WME-S, Zhang F, Nan X, Mijit M, et al. Flexible Hydrophobic Paper-Based Microfluidic Field-Effect Biosensor Amplified by RNA-Cleaving DNAzyme-Based DNA Nanostructure for Mg2+ Detection. Biosensors. 2025; 15(7):405. https://doi.org/10.3390/bios15070405
Chicago/Turabian StyleWang, Hui, Yue He, Zhixue Yu, Ruipeng Chen, Zemeng Feng, Dongfei Chen, Waleid Mohamed El-Sayed Shakweer, Fan Zhang, Xuemei Nan, Mukaddas Mijit, and et al. 2025. "Flexible Hydrophobic Paper-Based Microfluidic Field-Effect Biosensor Amplified by RNA-Cleaving DNAzyme-Based DNA Nanostructure for Mg2+ Detection" Biosensors 15, no. 7: 405. https://doi.org/10.3390/bios15070405
APA StyleWang, H., He, Y., Yu, Z., Chen, R., Feng, Z., Chen, D., Shakweer, W. M. E.-S., Zhang, F., Nan, X., Mijit, M., Xiong, B., Yang, L., & Tang, X. (2025). Flexible Hydrophobic Paper-Based Microfluidic Field-Effect Biosensor Amplified by RNA-Cleaving DNAzyme-Based DNA Nanostructure for Mg2+ Detection. Biosensors, 15(7), 405. https://doi.org/10.3390/bios15070405






