Small Toxic Molecule Detection and Elimination Using Molecularly Imprinted Polymers (MIPs)
Abstract
1. Introduction
2. Rational Design Strategies for MIPs in the Detection and Elimination of Toxic Molecules
3. Detection Strategies for Small Toxic Molecules Using MIPs
3.1. Electrochemical Methods
3.2. Optical Methods
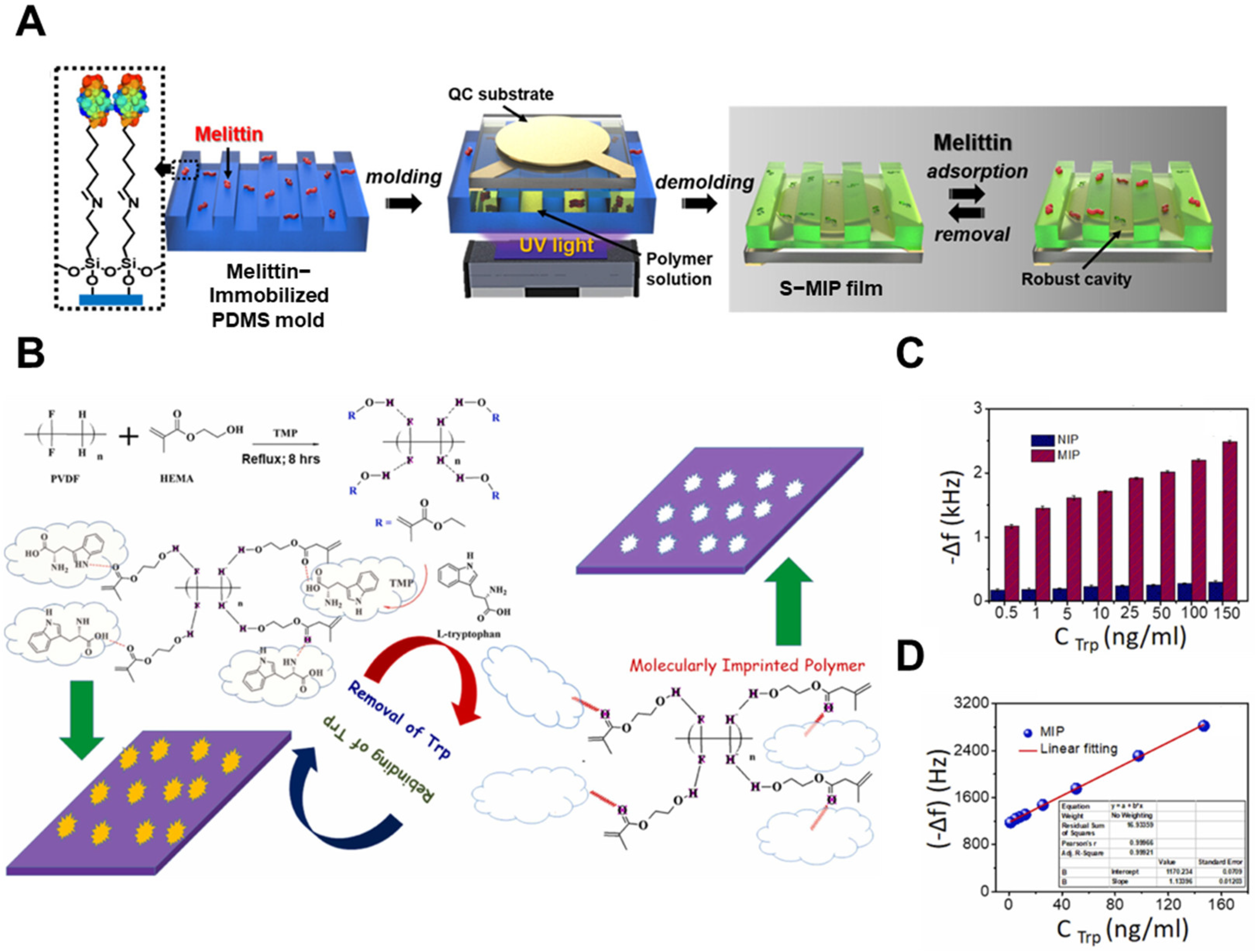
3.3. Gravimetric Methods
3.4. Hybrid Methods
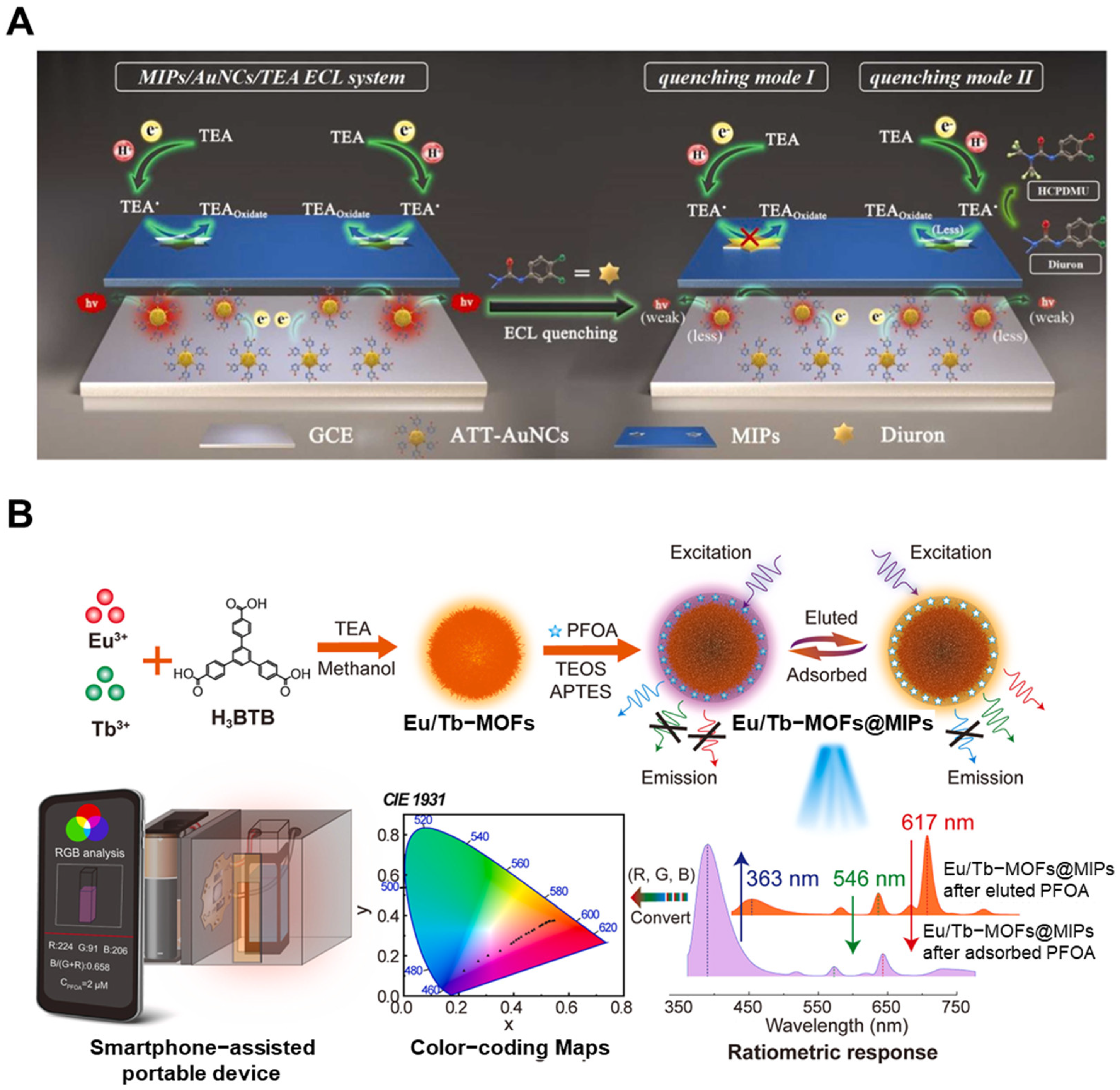
4. MIP-Based Elimination Strategies for Toxic Molecules
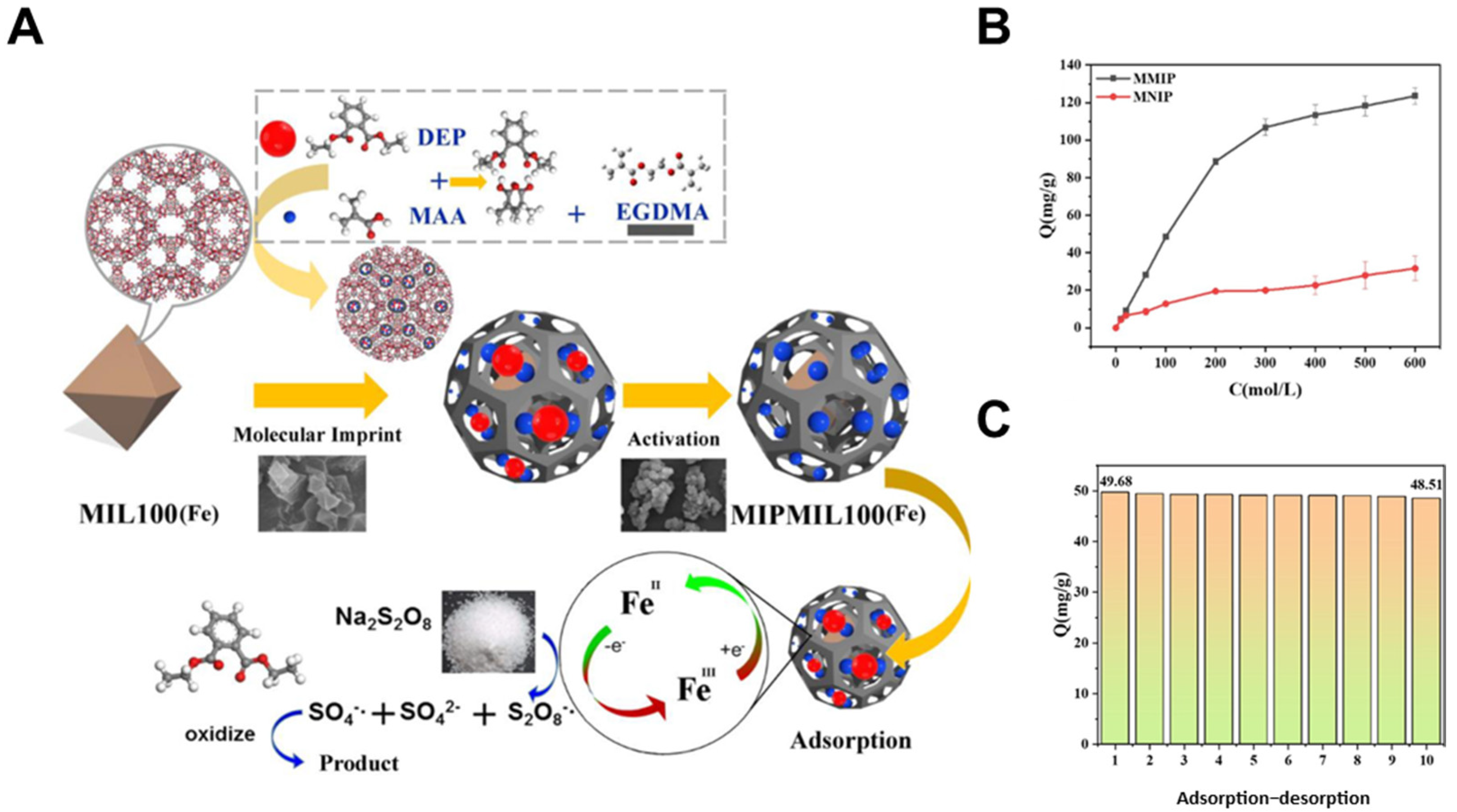
4.1. Selective Adsorption
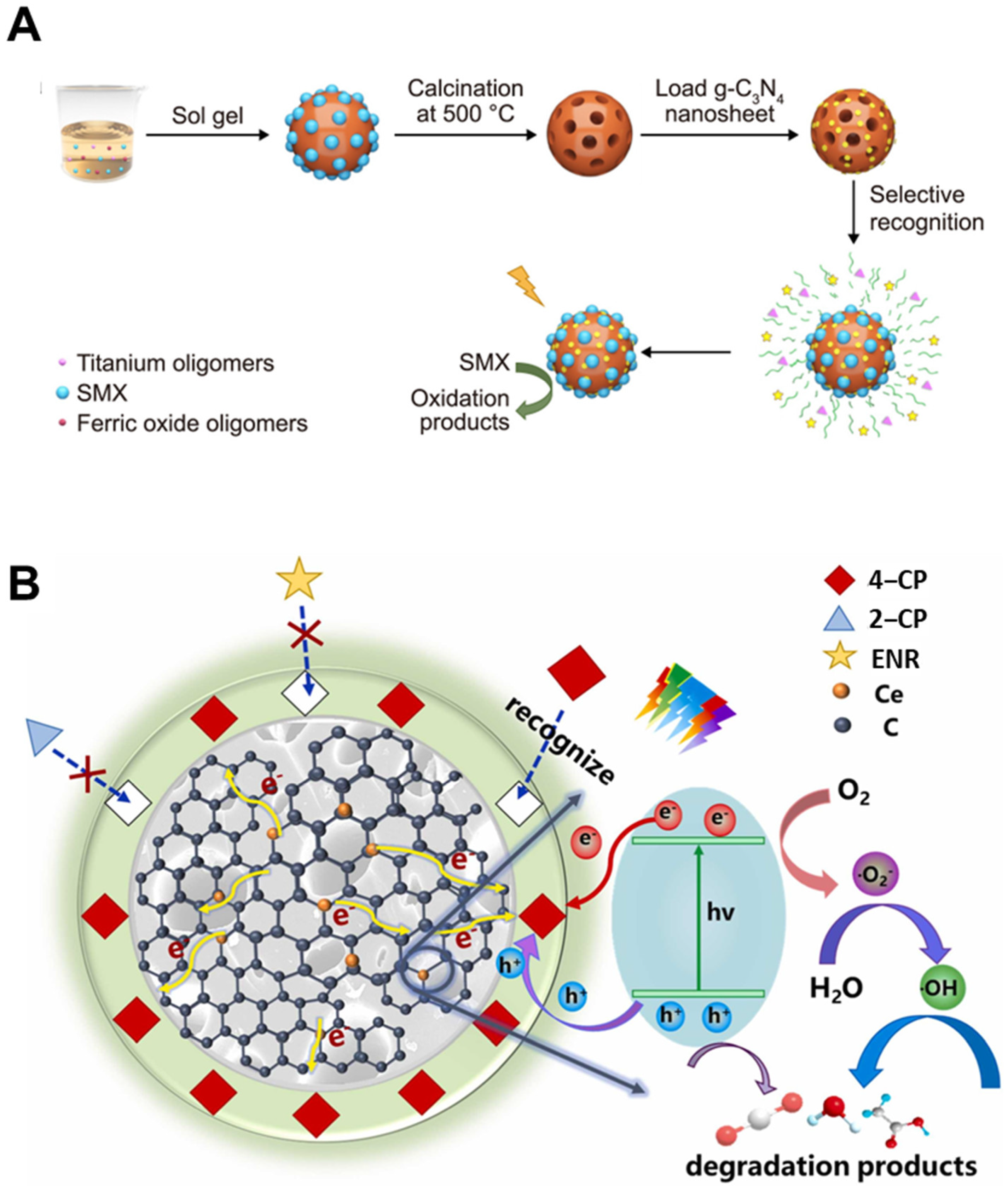
4.2. Photocatalytic Degradation
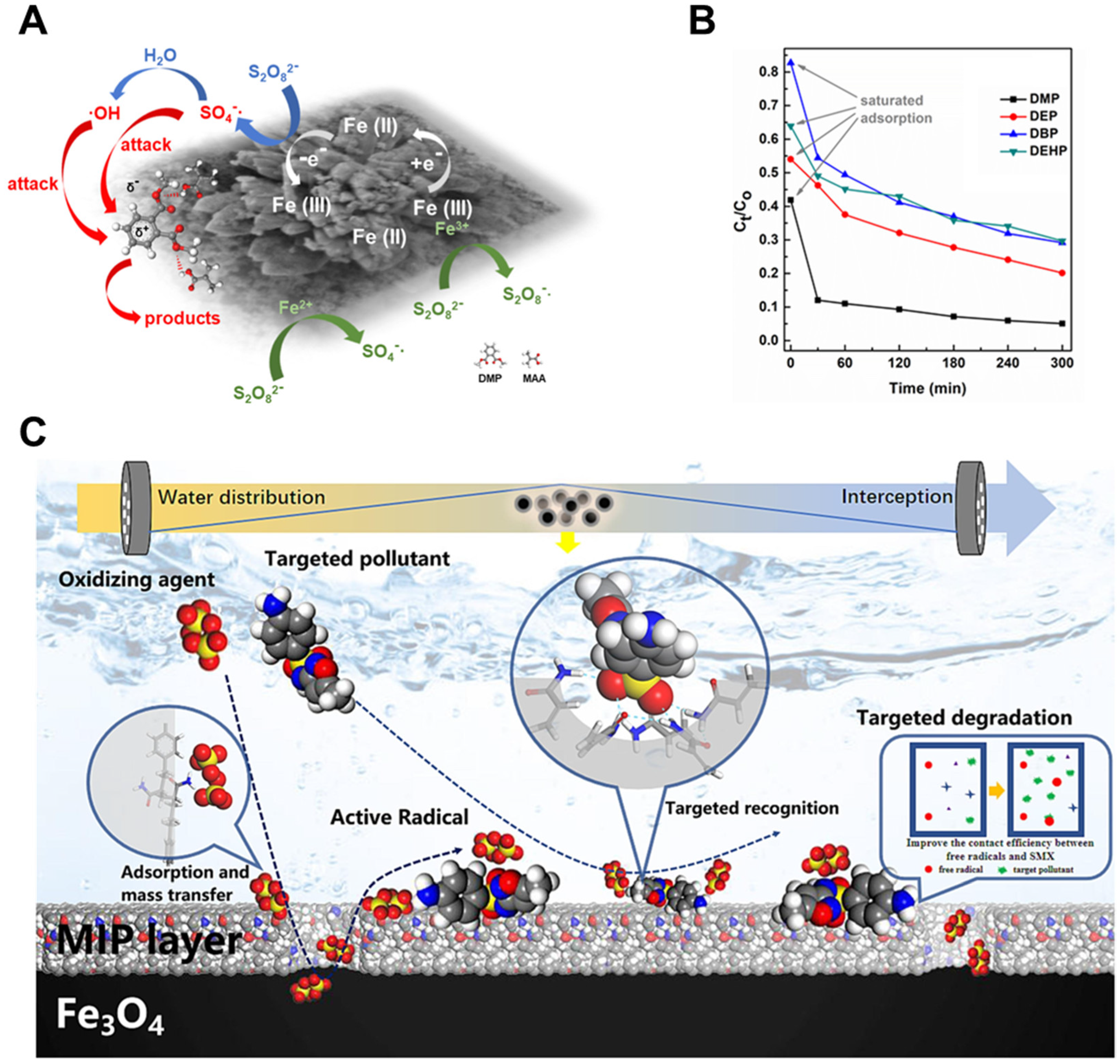
4.3. Advanced Oxidation Activation
4.4. Coupled Systems and Multifunctional Platforms
5. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, A.; Adhikary, S.; Bhattacharya, S.; Dutta, S.; Chatterjee, S.; Banerjee, D.; Ganguly, A.; Rajak, P. Pharmaceuticals and personal care products as emerging environmental contaminants: Prevalence, toxicity, and remedial approaches. ACS Chem. Health Saf. 2023, 30, 362–388. [Google Scholar] [CrossRef]
- Maqsood, Q.; Sumrin, A.; Waseem, R.; Hussain, M.; Imtiaz, M.; Hussain, N. Bioengineered microbial strains for detoxification of toxic environmental pollutants. Environ. Res. 2023, 227, 115665. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ.-Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumar, U.; Kumar, I.; Dwivedi, A.; Singh, P.; Mishra, S.; Seth, C.S.; Sharma, R.K. Critical review on toxic contaminants in surface water ecosystem: Sources, monitoring, and its impact on human health. Environ. Sci. Pollut. Res. Int. 2024, 31, 56428–56462. [Google Scholar] [CrossRef]
- Wu, Y.S.; Osman, A.I.; Hosny, M.; Elgarahy, A.M.; Eltaweil, A.S.; Rooney, D.W.; Chen, Z.; Rahim, N.S.; Sekar, M.; Gopinath, S.C.B.; et al. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega 2024, 9, 5100–5126. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef]
- Jermsittiparsert, K. Linkage between energy consumption, natural environment pollution, and public health dynamics in ASEAN. Int. J. Econ. Financ. Stud. 2021, 13, 1–21. [Google Scholar]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, E.Y.; Kambal, N.; et al. Pesticides impacts on human health and the environment with their mechanisms of action and possible countermeasures. Heliyon 2024, 10, e29128. [Google Scholar] [CrossRef]
- Kadam, U.S.; Hong, J.C. Advances in aptameric biosensors designed to detect toxic contaminants from food, water, human fluids, and the environment. Trends Environ. Anal. Chem. 2022, 36, e00184. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Song, G.; Huang, K.; Luo, Y.; Liu, Q.; He, X.; Cheng, N. Intelligent biosensing strategies for rapid detection in food safety: A review. Biosens. Bioelectron. 2022, 202, 114003. [Google Scholar] [CrossRef]
- Bristow, R.L.; Haworth-Duff, A.; Young, I.S.; Myers, P.; Hampson, M.R.; Williams, J.; Maher, S. An automated micro solid phase extraction gas chromatography-mass spectrometry (muSPE-GC-MS) detection method for geosmin and 2-methylisoborneol in drinking water. Sci. Rep. 2023, 13, 1768. [Google Scholar] [CrossRef]
- Nobile, M.; Chiesa, L.M.; Villa, R.E.; Danesi, L.; Arioli, F.; Panseri, S. Occurrence of perfluoroalkyl substances in canned tuna and their impact on food safety. Food Control 2024, 159, 110301. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Z.; Lai, P.; Luo, J.; He, X.; Yan, Y.; Huang, S.-X. Natural Trichothecene Mycotoxins from a Rotten Moldy Apple Core-Derived Trichothecium roseum: Occurrence, Identification, and Potential Transfer to Commercial Apple Juice. ACS Food Sci. Technol. 2023, 4, 118–125. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive review of emerging contaminants: Detection technologies, environmental impact, and management strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef]
- Gutierrez, M.; Grillini, V.; Mutavdzic Pavlovic, D.; Verlicchi, P. Activated carbon coupled with advanced biological wastewater treatment: A review of the enhancement in micropollutant removal. Sci. Total Environ. 2021, 790, 148050. [Google Scholar] [CrossRef]
- Godiya, C.B.; Park, B.J. Removal of bisphenol A from wastewater by physical, chemical and biological remediation techniques. A review. Environ. Chem. Lett. 2022, 20, 1801–1837. [Google Scholar] [CrossRef]
- Singh, D.; Singh, D.; Mishra, V.; Kushwaha, J.; Sengar, M.; Sinha, S.; Singh, S.; Giri, B.S. Strategies for biological treatment of waste water: A critical review. J. Clean. Prod. 2024, 454, 142266. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Geng, L.; Huang, J.; Fang, M.; Wang, H.; Liu, J.; Wang, G.; Hu, M.; Sun, J.; Guo, Y.; Sun, X. Recent progress of the research of metal-organic frameworks-molecularly imprinted polymers (MOFs-MIPs) in food safety detection field. Food Chem. 2024, 458, 140330. [Google Scholar] [CrossRef]
- Carballido, L.; Karbowiak, T.; Cayot, P.; Gerometta, M.; Sok, N.; Bou-Maroun, E. Applications of molecularly imprinted polymers and perspectives for their use as food quality trackers. Chem 2022, 8, 2330–2341. [Google Scholar] [CrossRef]
- Lamaoui, A.; Lahcen, A.A.; Amine, A. Unlocking the Potential of Molecularly Imprinted Polydopamine in Sensing Applications. Polymers 2023, 15, 3712. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, Y.; Hu, X.; Yang, Z. Emerging Combination of Hydrogel and Electrochemical Biosensors. Small 2025, 21, e2409711. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Pamme, N. Strategies to remove templates from molecularly imprinted polymer (MIP) for biosensors. TrAC Trends Anal. Chem. 2024, 170, 117437. [Google Scholar] [CrossRef]
- Parisi, O.I.; Francomano, F.; Dattilo, M.; Patitucci, F.; Prete, S.; Amone, F.; Puoci, F. The evolution of molecular recognition: From antibodies to molecularly imprinted polymers (MIPs) as artificial counterpart. J. Funct. Biomater. 2022, 13, 12. [Google Scholar] [CrossRef]
- Kadhem, A.J.; Gentile, G.J.; Fidalgo de Cortalezzi, M.M. Molecularly Imprinted Polymers (MIPs) in Sensors for Environmental and Biomedical Applications: A Review. Molecules 2021, 26, 6233. [Google Scholar] [CrossRef]
- Gkika, D.A.; Tolkou, A.K.; Lambropoulou, D.A.; Bikiaris, D.N.; Kokkinos, P.; Kalavrouziotis, I.K.; Kyzas, G.Z. Application of molecularly imprinted polymers (MIPs) as environmental separation tools. RSC Appl. Polym. 2024, 2, 127–148. [Google Scholar] [CrossRef]
- Li, Y.; Guan, C.; Liu, C.; Li, Z.; Han, G. Disease diagnosis and application analysis of molecularly imprinted polymers (MIPs) in saliva detection. Talanta 2024, 269, 125394. [Google Scholar] [CrossRef]
- Elugoke, S.E.; Adekunle, A.S.; Fayemi, O.E.; Akpan, E.D.; Mamba, B.B.; Sherif, E.S.M.; Ebenso, E.E. Molecularly imprinted polymers (MIPs) based electrochemical sensors for the determination of catecholamine neurotransmitters—Review. Electrochem. Sci. Adv. 2021, 1, e2000026. [Google Scholar] [CrossRef]
- Kang, M.S.; Cho, E.; Choi, H.E.; Amri, C.; Lee, J.H.; Kim, K.S. Molecularly imprinted polymers (MIPs): Emerging biomaterials for cancer theragnostic applications. Biomater. Res. 2023, 27, 45. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Wei, S.; Jakusch, M.; Mizaikoff, B. Capturing molecules with templated materials—Analysis and rational design of molecularly imprinted polymers. Anal. Chim. Acta 2006, 578, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, S. Engineering responsive polymer building blocks with host-guest molecular recognition for functional applications. Acc. Chem. Res. 2014, 47, 2084–2095. [Google Scholar] [CrossRef] [PubMed]
- Bedwell, T.S.; Anjum, N.; Ma, Y.; Czulak, J.; Poma, A.; Piletska, E.; Whitcombe, M.J.; Piletsky, S.A. New protocol for optimisation of polymer composition for imprinting of peptides and proteins. RSC Adv. 2019, 9, 27849–27855. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.F.; Menezes, L.F.; Pereira, A.C.; Nascimento, C.S., Jr. Molecularly Imprinted Polymer (MIP) for thiamethoxam: A theoretical and experimental study. J. Mol. Struct. 2021, 1231, 129980. [Google Scholar] [CrossRef]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef]
- Kupai, J.; Razali, M.; Buyuktiryaki, S.; Kecili, R.; Szekely, G. Long-term stability and reusability of molecularly imprinted polymers. Polym. Chem. 2017, 8, 666–673. [Google Scholar] [CrossRef]
- Rahman, S.; Bozal-Palabiyik, B.; Unal, D.N.; Erkmen, C.; Siddiq, M.; Shah, A.; Uslu, B. Molecularly imprinted polymers (MIPs) combined with nanomaterials as electrochemical sensing applications for environmental pollutants. Trends Environ. Anal. Chem. 2022, 36, e00176. [Google Scholar] [CrossRef]
- Cai, Y.; Cao, L.; Cai, H.; Yang, W.; Lu, H.; Adila, A.; Zhang, B.; Cao, Y.; Huang, W.; Xu, W. A rapid microfluidic paper-based chip sensor using ratiometric fluorescence and molecularly imprinted polymers for visual detection of sulfadiazine in actual samples. J. Food Compos. Anal. 2025, 139, 107108. [Google Scholar] [CrossRef]
- Ait Lahcen, A.; Rauf, S.; Aljedaibi, A.; de Oliveira Filho, J.I.; Beduk, T.; Mani, V.; Alshareef, H.N.; Salama, K.N. Laser-scribed graphene sensor based on gold nanostructures and molecularly imprinted polymers: Application for Her-2 cancer biomarker detection. Sens. Actuators B Chem. 2021, 347, 130556. [Google Scholar] [CrossRef]
- Le, Q.-H.; Nguyen, T.-L.; Dinh, V.-T.; Nguyen, H.-N.; Pham, H.-N.; Nguyen, T.-A.; Nguyen, L.-L.; Dinh, T.-M.-T.; Nguyen, V.-Q. Electrosynthesized nanostructured molecularly imprinted polymer for detecting diclofenac molecule. J. Electroanal. Chem. 2022, 921, 116709. [Google Scholar]
- Silva, A.T.; Figueiredo, R.; Azenha, M.; Jorge, P.A.S.; Pereira, C.M.; Ribeiro, J.A. Imprinted Hydrogel Nanoparticles for Protein Biosensing: A Review. ACS Sens. 2023, 8, 2898–2920. [Google Scholar] [CrossRef] [PubMed]
- Sanati, A.; Siavash Moakhar, R.; Hosseini, I.I.; Raeissi, K.; Karimzadeh, F.; Jalali, M.; Kharaziha, M.; Sheibani, S.; Shariati, L.; Presley, J.F.; et al. Gold Nano/Micro-Islands Overcome the Molecularly Imprinted Polymer Limitations to Achieve Ultrasensitive Protein Detection. ACS Sens. 2021, 6, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, C.; Zhang, B.; Wu, C.; Cao, Y.; Huang, W.; Xu, W. Construction of a Nitrogen-Doped Carbon Quantum Dot Fluorescent Molecularly Imprinted Sensor for Ultra-Sensitive Detection of Sulfadiazine in Pork Samples. Food Anal. Methods 2024, 17, 1689–1701. [Google Scholar] [CrossRef]
- Keçili, R.; Hussain, C.G.; Hussain, C.M. Fluorescent nanosensors based on green carbon dots (CDs) and molecularly imprinted polymers (MIPs) for environmental pollutants: Emerging trends and future prospects. Trends Environ. Anal. Chem. 2023, 40, e00213. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.; Wang, X.; Peng, H.; Xiong, H.; Chen, L. Strategies of molecular imprinting-based fluorescence sensors for chemical and biological analysis. Biosens. Bioelectron. 2018, 112, 54–71. [Google Scholar] [CrossRef]
- Bahrani, S.; Sadati Behbahani, E.; Ghaedi, M.; Amrollahi Miandeh, Y.; Asfaram, A. Molecular imprinted technology using in biosensing: A review. Microchem. J. 2024, 203, 110888. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Li, J.; Wang, X.; Zhang, Z.; Choo, J.; Chen, L. Molecular Imprinting: Green Perspectives and Strategies. Adv. Mater. 2021, 33, 2100543. [Google Scholar] [CrossRef]
- Ansari, S.; Masoum, S. Molecularly imprinted polymers for capturing and sensing proteins: Current progress and future implications. TrAC Trends Anal. Chem. 2019, 114, 29–47. [Google Scholar] [CrossRef]
- Kamaruzaman, S.; Nasir, N.M.; Mohd Faudzi, S.M.; Yahaya, N.; Mohamad Hanapi, N.S.; Wan Ibrahim, W.N. Solid-Phase Extraction of Active Compounds from Natural Products by Molecularly Imprinted Polymers: Synthesis and Extraction Parameters. Polymers 2021, 13, 3780. [Google Scholar] [CrossRef]
- Siegwardt, L.; Glößner, V.; Boehm, A.; Schneider, M.; Gallei, M. Poly(4-vinylpyridine) and Poly(methacrylic acid) Particle Architectures for pH-Responsive and Mechanochromic Opal Films. ACS Appl. Mater. Interfaces 2024, 16, 10722–10735. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Safitri, N.; Zulfa, A.; Neli, N.; Rahayu, D. Factors Affecting Preparation of Molecularly Imprinted Polymer and Methods on Finding Template-Monomer Interaction as the Key of Selective Properties of the Materials. Molecules 2021, 26, 5612. [Google Scholar] [CrossRef] [PubMed]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays—An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef] [PubMed]
- Bedair, A.; Hamed, M.; Hassab, M.E.; Kannouma, R.E.; Abdelhameed, R.M.; Mansour, F.R. Dummy template molecularly imprinted polymer-based sensors in analytical and bioanalytical applications. Talanta Open 2025, 11, 100431. [Google Scholar] [CrossRef]
- Herrera-Chacón, A.; Cetó, X.; del Valle, M. Molecularly imprinted polymers—Towards electrochemical sensors and electronic tongues. Anal. Bioanal. Chem. 2021, 413, 6117–6140. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef]
- Zuo, J.; Ma, P.; Li, Z.; Zhang, Y.; Xiao, D.; Wu, H.; Dong, A. Application of Molecularly Imprinted Polymers in Plant Natural Products: Current Progress and Future Perspectives. Macromol. Mater. Eng. 2023, 308, 2200499. [Google Scholar] [CrossRef]
- Nazim, T.; Lusina, A.; Cegłowski, M. Recent Developments in the Detection of Organic Contaminants Using Molecularly Imprinted Polymers Combined with Various Analytical Techniques. Polymers 2023, 15, 3868. [Google Scholar] [CrossRef]
- Zarejousheghani, M.; Rahimi, P.; Borsdorf, H.; Zimmermann, S.; Joseph, Y. Molecularly Imprinted Polymer-Based Sensors for Priority Pollutants. Sensors 2021, 21, 2406. [Google Scholar] [CrossRef]
- Sadeghi Googheri, M.S.; Campagnol, D.; Ugo, P.; Hozhabr Araghi, S.; Karimian, N. Dummy Template Molecularly Imprinted Polymers for Electrochemical Detection of Cardiac Troponin I: A Combined Computational and Experimental Approach. Chemosensors 2025, 13, 26. [Google Scholar] [CrossRef]
- Guan, G.; Pan, J.H.; Li, Z. Innovative utilization of molecular imprinting technology for selective adsorption and (photo)catalytic eradication of organic pollutants. Chemosphere 2021, 265, 129077. [Google Scholar] [CrossRef]
- Foroughirad, S.; Haddadi-Asl, V.; Khosravi, A.; Salami-Kalajahi, M. Effect of porogenic solvent in synthesis of mesoporous and microporous molecularly imprinted polymer based on magnetic halloysite nanotubes. Mater. Today Commun. 2021, 26, 101780. [Google Scholar] [CrossRef]
- van Wissen, G.; Lowdon, J.W.; Cleij, T.J.; Eersels, K.; van Grinsven, B. Porogenic Solvents in Molecularly Imprinted Polymer Synthesis: A Comprehensive Review of Current Practices and Emerging Trends. Polymers 2025, 17, 1057. [Google Scholar] [CrossRef] [PubMed]
- Martínez Saavedra, L.N.; Penido, R.G.; de Azevedo Santos, L.; Ramalho, T.C.; Lobo Baeta, B.E.; Pereira, M.C.; Candido da Silva, A. Molecularly imprinted polymers for selective adsorption of quinoline: Theoretical and experimental studies. RSC Adv. 2018, 8, 28775–28786. [Google Scholar] [CrossRef] [PubMed]
- Ostovan, A.; Arabi, M.; Wang, Y.; Li, J.; Li, B.; Wang, X.; Chen, L. Greenificated Molecularly Imprinted Materials for Advanced Applications. Adv. Mater. 2022, 34, 2203154. [Google Scholar] [CrossRef]
- Beyazit, S.; Tse Sum Bui, B.; Haupt, K.; Gonzato, C. Molecularly imprinted polymer nanomaterials and nanocomposites by controlled/living radical polymerization. Prog. Polym. Sci. 2016, 62, 1–21. [Google Scholar] [CrossRef]
- Ahadi, H.M.; Fardhan, F.M.; Rahayu, D.; Pratiwi, R.; Hasanah, A.N. Molecularly Imprinted Microspheres in Active Compound Separation from Natural Product. Molecules 2024, 29, 4043. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Zhang, W.; Ma, X.; Zhu, Y.; Zhao, X.; Fu, Y. Synthesis and characterization of molecularly imprinted polymer microspheres functionalized with POSS. Appl. Surf. Sci. 2020, 511, 145506. [Google Scholar] [CrossRef]
- Lim, K.F.; Holdsworth, C.I. Effect of Formulation on the Binding Efficiency and Selectivity of Precipitation Molecularly Imprinted Polymers. Molecules 2018, 23, 2996. [Google Scholar] [CrossRef]
- Pardeshi, S.; Singh, S.K. Precipitation polymerization: A versatile tool for preparing molecularly imprinted polymer beads for chromatography applications. RSC Adv. 2016, 6, 23525–23536. [Google Scholar] [CrossRef]
- Orowitz, T.E.; Ana Sombo, P.P.A.A.; Rahayu, D.; Hasanah, A.N. Microsphere Polymers in Molecular Imprinting: Current and Future Perspectives. Molecules 2020, 25, 3256. [Google Scholar] [CrossRef]
- Bravo-Yagüe, J.C.; Paniagua-González, G.; Garcinuño, R.M.; García-Mayor, A.; Fernández-Hernando, P. Colorimetric Molecularly Imprinted Polymer-Based Sensors for Rapid Detection of Organic Compounds: A Review. Chemosensors 2025, 13, 163. [Google Scholar] [CrossRef]
- Han, D.; Zhao, N.; Wang, S.; Cui, Y.; Yan, H. Bimetallic metal-organic framework-based molecularly imprinted sensors for selective on-site detection of 2,4-dichlorophenoxyacetic acid. Microchem. J. 2024, 205, 111318. [Google Scholar] [CrossRef]
- Frasco, M.F.; Truta, L.A.A.N.A.; Sales, M.G.F.; Moreira, F.T.C. Imprinting Technology in Electrochemical Biomimetic Sensors. Sensors 2017, 17, 523. [Google Scholar] [CrossRef]
- Han, E.; Pan, Y.; Li, L.; Cai, J. Bisphenol A detection based on nano gold-doped molecular imprinting electrochemical sensor with enhanced sensitivity. Food Chem. 2023, 426, 136608. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Athira, V.S.; Nair, S.S. Detection of chlorpyrifos based on molecular imprinting with a conducting polythiophene copolymer loaded on multi-walled carbon nanotubes. Food Chem. 2022, 381, 132010. [Google Scholar] [CrossRef]
- Cheng, Q.; Xue, C.; Abdiryim, T.; Jamal, R. Molecular imprinting electrochemical sensor based on hollow spherical PProDOT-2CH2OH and chitosan-derived carbon materials for highly sensitive detection of chloramphenicol. J. Hazard. Mater. 2024, 478, 135615. [Google Scholar] [CrossRef]
- Mani, A.; Rajeev, M.R.; Anirudhan, T.S. Silver decorated silanized graphene oxide based molecularly surface imprinted electrochemical sensor for the trace level detection of L-Tryptophan. Mater. Chem. Phys. 2023, 299, 127445. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Zhang, M.; Zhao, F.; Zeng, B. Ionic liquid assisted molecular self-assemble and molecular imprinting on gold nanoparticles decorated boron-doped ordered mesoporous carbon for the detection of zearalenone. Talanta 2020, 217, 121032. [Google Scholar] [CrossRef]
- Wang, K.; Tan, L.; Zhang, Y.; Zhang, D.; Wang, N.; Wang, J. A molecular imprinted fluorescence sensor based on carbon quantum dots for selective detection of 4-nitrophenol in aqueous environments. Mar. Pollut. Bull. 2023, 187, 114587. [Google Scholar] [CrossRef]
- Bhogal, S.; Mohiuddin, I.; Kumar, S.; Malik, A.K.; Kim, K.-H.; Kaur, K. Self-polymerized polydopamine-imprinted layer-coated carbon dots as a fluorescent sensor for selective and sensitive detection of 17β-oestradiol. Sci. Total Environ. 2022, 847, 157356. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, Y.; Huang, J.; Huang, M.; Zhang, S.; Li, K. A novel molecular imprinted probe with hybrid ratio fluorescent for visual detection of imazapyr. Microchem. J. 2024, 198, 110116. [Google Scholar] [CrossRef]
- Liu, L.; Grillo, F.; Canfarotta, F.; Whitcombe, M.; Morgan, S.P.; Piletsky, S.; Correia, R.; He, C.; Norris, A.; Korposh, S. Carboxyl-fentanyl detection using optical fibre grating-based sensors functionalised with molecularly imprinted nanoparticles. Biosens. Bioelectron. 2021, 177, 113002. [Google Scholar] [CrossRef] [PubMed]
- Gorai, P.; Mizuno, Y.; Kumar, M.; Jha, R. Molecular Imprinting Polymer Nanoparticles Coupled with an Optical Sensor for Sensitive and Label-Free Detection of p-Cresol. ACS Appl. Nano Mater. 2023, 6, 12946–12956. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Shen, Q.; Shen, D.; Kang, Q. A smartphone-based long optical path colorimetric turntable for selective determination of malachite green and investigation the specific adsorption behavior of the imprinted cavities within molecularly imprinted polymers. Microchem. J. 2023, 190, 108629. [Google Scholar] [CrossRef]
- Aydoğan, N.; Aylaz, G.; Bakhshpour, M.; Tugsuz, T.; Andaç, M. Molecularly Designed Ion-Imprinted Nanoparticles for Real-Time Sensing of Cu(II) Ions Using Quartz Crystal Microbalance. Biomimetics 2022, 7, 191. [Google Scholar] [CrossRef]
- Liu, J.; Cai, X.; Liu, J.; Liang, D.; Chen, K.; Tang, S.; Xu, B. Study on the Preparation of Estrone Molecularly Imprinted Polymers and Their Application in a Quartz Crystal Microbalance Sensor via a Computer-Assisted Design. Int. J. Mol. Sci. 2022, 23, 5758. [Google Scholar] [CrossRef]
- Yang, J.C.; Lee, J.; Lim, S.J.; Kwak, G.; Park, J. Molecularly Imprinted Chalcone-Branched Polyimide-Based Chemosensors with Stripe Nanopatterns for the Detection of Melittin. ACS Sens. 2023, 8, 2298–2308. [Google Scholar] [CrossRef]
- Prabakaran, K.; Jandas, P.J.; Luo, J.; Fu, C. A highly sensitive surface acoustic wave sensor modified with molecularly imprinted hydrophilic PVDF for the selective amino acid detection. Sens. Actuators A Phys. 2022, 341, 113525. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Wu, Q.; Yuan, Y.; Liu, W.; Han, C.; Wang, X. Detection of Dimethyl Methyl Phosphonate by Silica Molecularly Imprinted Materials. Nanomaterials 2023, 13, 2871. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Zhang, M.; Zeng, B.; Zhao, F. Molecularly imprinted photoelectrochemical sensor for aflatoxin B1 detection based on organic/inorganic hybrid nanorod arrays. Sens. Actuators B Chem. 2021, 339, 129900. [Google Scholar] [CrossRef]
- Kuang, K.; Li, Y.; Ji, Y.; Liu, Y.; Jia, N. Molecular imprinting-electrochemiluminescence sensor based on Ru(bpy)32+@ZnO-Au composite for sensitive detection of acrylamide. Microchem. J. 2024, 196, 109558. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Yi, Y.; He, Y.; Zhen, M.; Niu, Q.; Wu, X.; Li, L.; You, T. Dual-quenching molecular imprinting polymers electrochemiluminescence sensor for diuron detection: Triggered by “blocking effect” and interaction of diuron with Triethylamine. Sens. Actuators B Chem. 2024, 417, 136191. [Google Scholar] [CrossRef]
- Sulthana, S.F.; Iqbal, U.M.; Suseela, S.B.; Anbazhagan, R.; Chinthaginjala, R.; Chitathuru, D.; Ahmad, I.; Kim, T.-h. Electrochemical Sensors for Heavy Metal Ion Detection in Aqueous Medium: A Systematic Review. ACS Omega 2024, 9, 25493–25512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, C.; Chen, S.; Li, J. Miniaturized Electrochemical Sensing Platforms for Quantitative Monitoring of Glutamate Dynamics in the Central Nervous System. Angew. Chem. Int. Ed. 2024, 63, e202406867. [Google Scholar] [CrossRef]
- Shah, N.; Shah, M.; Rehan, T.; Khan, A.; Majeed, N.; Hameed, A.; Bououdina, M.; Abumousa, R.A.; Humayun, M. Molecularly imprinted polymer composite membranes: From synthesis to diverse applications. Heliyon 2024, 10, e36189. [Google Scholar] [CrossRef]
- Zheng, X.; Khaoulani, S.; Ktari, N.; Lo, M.; Khalil, A.M.; Zerrouki, C.; Fourati, N.; Chehimi, M.M. Towards Clean and Safe Water: A Review on the Emerging Role of Imprinted Polymer-Based Electrochemical Sensors. Sensors 2021, 21, 4300. [Google Scholar] [CrossRef]
- Mwanza, C.; Zhang, W.-Z.; Mulenga, K.; Ding, S.-N. Advancing green chemistry in environmental monitoring: The role of electropolymerized molecularly imprinted polymer-based electrochemical sensors. Green Chem. 2024, 26, 11490–11517. [Google Scholar] [CrossRef]
- Pan, M.; Yin, Z.; Liu, K.; Du, X.; Liu, H.; Wang, S. Carbon-Based Nanomaterials in Sensors for Food Safety. Nanomaterials 2019, 9, 1330. [Google Scholar] [CrossRef]
- Crevillen, A.G.; Escarpa, A.; García, C.D. Carbon-based Nanomaterials in Analytical Chemistry. In Carbon-Based Nanomaterials in Analytical Chemistry; Garcia, C.D., Crevillén, A.G., Escarpa, A., Eds.; The Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Prasad, B.B.; Kumar, A.; Singh, R. Molecularly imprinted polymer-based electrochemical sensor using functionalized fullerene as a nanomediator for ultratrace analysis of primaquine. Carbon 2016, 109, 196–207. [Google Scholar] [CrossRef]
- Malik, S.; Fujita, N.; Mukhopadhyay, P.; Goto, Y.; Kaneko, K.; Ikeda, T.; Shinkai, S. Creation of 1D [60]fullerene superstructures and its polymerization by γ-ray irradiation. J. Mater. Chem. 2007, 17, 2454–2458. [Google Scholar] [CrossRef]
- Ertuğrul Uygun, H.D.; Uygun, Z.O.; Canbay, E.; Girgin Sağın, F.; Sezer, E. Non-invasive cortisol detection in saliva by using molecularly cortisol imprinted fullerene-acrylamide modified screen printed electrodes. Talanta 2020, 206, 120225. [Google Scholar] [CrossRef] [PubMed]
- Lepore, M.; Delfino, I. Optical Sensors Technology and Applications. Sensors 2022, 22, 7905. [Google Scholar] [CrossRef] [PubMed]
- Barth, I.; Lee, H. Nanophotonic sensing and label-free imaging of extracellular vesicles. Light Sci. Appl. 2025, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Shaked, N.T.; Boppart, S.A.; Wang, L.V.; Popp, J. Label-free biomedical optical imaging. Nat. Photonics 2023, 17, 1031–1041. [Google Scholar] [CrossRef]
- Moreno-Bondi, M.C.; Benito-Peña, E.; Carrasco, S.; Urraca, J.L. Molecularly Imprinted Polymer-based Optical Chemosensors for Selective Chemical Determinations. In Molecularly Imprinted Polymers for Analytical Chemistry Applications; Kutner, W., Sharma, P.S., Eds.; The Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Li, R.; Feng, Y.; Pan, G.; Liu, L. Advances in Molecularly Imprinting Technology for Bioanalytical Applications. Sensors 2019, 19, 177. [Google Scholar] [CrossRef]
- Park, S.Y.; Tan, J.K.S.; Mo, X.; Song, Y.; Lim, J.; Liew, X.R.; Chung, H.; Kim, S. Carbon Quantum Dots with Tunable Size and Fluorescence Intensity for Development of a Nano-biosensor. Small 2025, 21, 2404524. [Google Scholar] [CrossRef]
- Feng, H.; Qian, Z. Functional Carbon Quantum Dots: A Versatile Platform for Chemosensing and Biosensing. Chem. Rec. 2018, 18, 491–505. [Google Scholar] [CrossRef]
- Miao, X.; Wu, C.; Xia, Y.; Yu, S.; Li, F.; Zhang, M. Ratiometric fluorescence probes for visible detection and accurate identification of MPEA vapor. Nat. Commun. 2024, 15, 10641. [Google Scholar] [CrossRef]
- Mazur, F.; Han, Z.; Tjandra, A.D.; Chandrawati, R. Digitalization of Colorimetric Sensor Technologies for Food Safety. Adv. Mater. 2024, 36, 2404274. [Google Scholar] [CrossRef]
- Oh, D.K.; Yang, J.C.; Hong, S.W.; Park, J. Molecular imprinting of polymer films on 2D silica inverse opal via thermal graft copolymerization for bisphenol A detection. Sens. Actuators B Chem. 2020, 323, 128670. [Google Scholar] [CrossRef]
- Lu, Z.; Qin, J.; Wu, C.; Yin, J.; Sun, M.; Su, G.; Wang, X.; Wang, Y.; Ye, J.; Liu, T.; et al. Dual-channel MIRECL portable devices with impedance effect coupled smartphone and machine learning system for tyramine identification and quantification. Food Chem. 2023, 429, 136920. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, H.; Xue, X.; Tan, F.; Ye, L. Signal amplification in molecular sensing by imprinted polymers. Microchim. Acta 2024, 191, 574. [Google Scholar] [CrossRef] [PubMed]
- Coe, B.J. Switchable Nonlinear Optical Metallochromophores with Pyridinium Electron Acceptor Groups. Acc. Chem. Res. 2006, 39, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Halim, S.A.; Ibrahim, M.A. Synthesis, DFT calculations, electronic structure, electronic absorption spectra, natural bond orbital (NBO) and nonlinear optical (NLO) analysis of the novel 5-methyl-8H-benzo[h]chromeno [2,3-b][1,6] naphthyridine-6(5H),8-dione (MBCND). J. Mol. Struct. 2017, 1130, 543–558. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, X.; Han, L.; Liu, H.; Sun, B. A smartphone-integrated optosensing platform based on red-emission carbon dots for real-time detection of pyrethroids. Biosens. Bioelectron. 2021, 191, 113460. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Mu, B.; Meng, S.; Mao, S.; Tao, W.; Li, Z. Lanthanide metal-organic framework-based surface molecularly imprinted polymers ratiometric fluorescence probe for visual detection of perfluorooctanoic acid with a smartphone-assisted portable device. Biosens. Bioelectron. 2024, 257, 116330. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Syritski, V. Electrochemically Synthesized MIP Sensors: Applications in Healthcare Diagnostics. Biosensors 2024, 14, 71. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kassanos, P.; Keshavarz, M.; Duru, P.; Kayalan, C.I.; Kale, İ.; Bayazit, M.K. Wearable Nano-Based Gas Sensors for Environmental Monitoring and Encountered Challenges in Optimization. Sensors 2023, 23, 8648. [Google Scholar] [CrossRef]
- Aoulad El Hadj Ali, Y.; Azzouz, A.; Ahrouch, M.; Lamaoui, A.; Raza, N.; Ait Lahcen, A. Molecular imprinting technology for next-generation water treatment via photocatalysis and selective pollutant adsorption. J. Environ. Chem. Eng. 2024, 12, 112768. [Google Scholar] [CrossRef]
- Wang, Y.; Ruan, H.; Zhang, J.; Huang, Y.; Guo, M.; Kong, D.; Luo, J.; Yang, M. Recyclable and selective PVDF-based molecularly imprinted membrane combining mussel-inspired biomimetic strategy for dimethomorph elimination. Chem. Eng. J. 2023, 478, 147322. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Y.; Liu, Y.; Zhao, F.; Zeng, B. Kill two birds with one stone: Selective and fast removal and sensitive determination of oxytetracycline using surface molecularly imprinted polymer based on ionic liquid and ATRP polymerization. J. Hazard. Mater. 2022, 434, 128907. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wan, J.; Wang, Y.; Chi, H.; Yan, Z.; Ding, S. Selective removal and persulfate catalytic decomposition of diethyl phthalate from contaminated water on modified MIL100 through surface molecular imprinting. Chemosphere 2020, 240, 124875. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sheng, T.; Yang, Z.; Huang, D.; Sheng, L. Synthesis of molecular-imprinting polymer coated magnetic nanocomposites for selective capture and fast removal of environmental tricyclic analogs. Chem. Eng. J. 2021, 426, 128678. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Ding, J.; Liu, L.-M.; Wu, R.; Ding, L.; Jiang, J.-Q.; Pang, J.-W.; Li, Y.; Ren, N.-Q.; Yang, S.-S. Selective removal of sulfamethoxazole by a novel double Z-scheme photocatalyst: Preferential recognition and degradation mechanism. Environ. Sci. Ecotechnol. 2024, 17, 100308. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, B.; Zhao, G. Selective photoelectrocatalytic removal of dimethyl phthalate on high-quality expressed molecular imprints decorated specific facet of single crystalline TiO2 photoanode. Appl. Catal. B Environ. 2020, 279, 119364. [Google Scholar] [CrossRef]
- Lyu, H.; Cheng, Z.; Wang, X.; Shen, B.; Tang, J.; Zhao, D. Target recognition and selective photocatalytic degradation of trace disinfection By-products by innovative molecular imprinting strategy. J. Hazard. Mater. 2025, 490, 137773. [Google Scholar] [CrossRef]
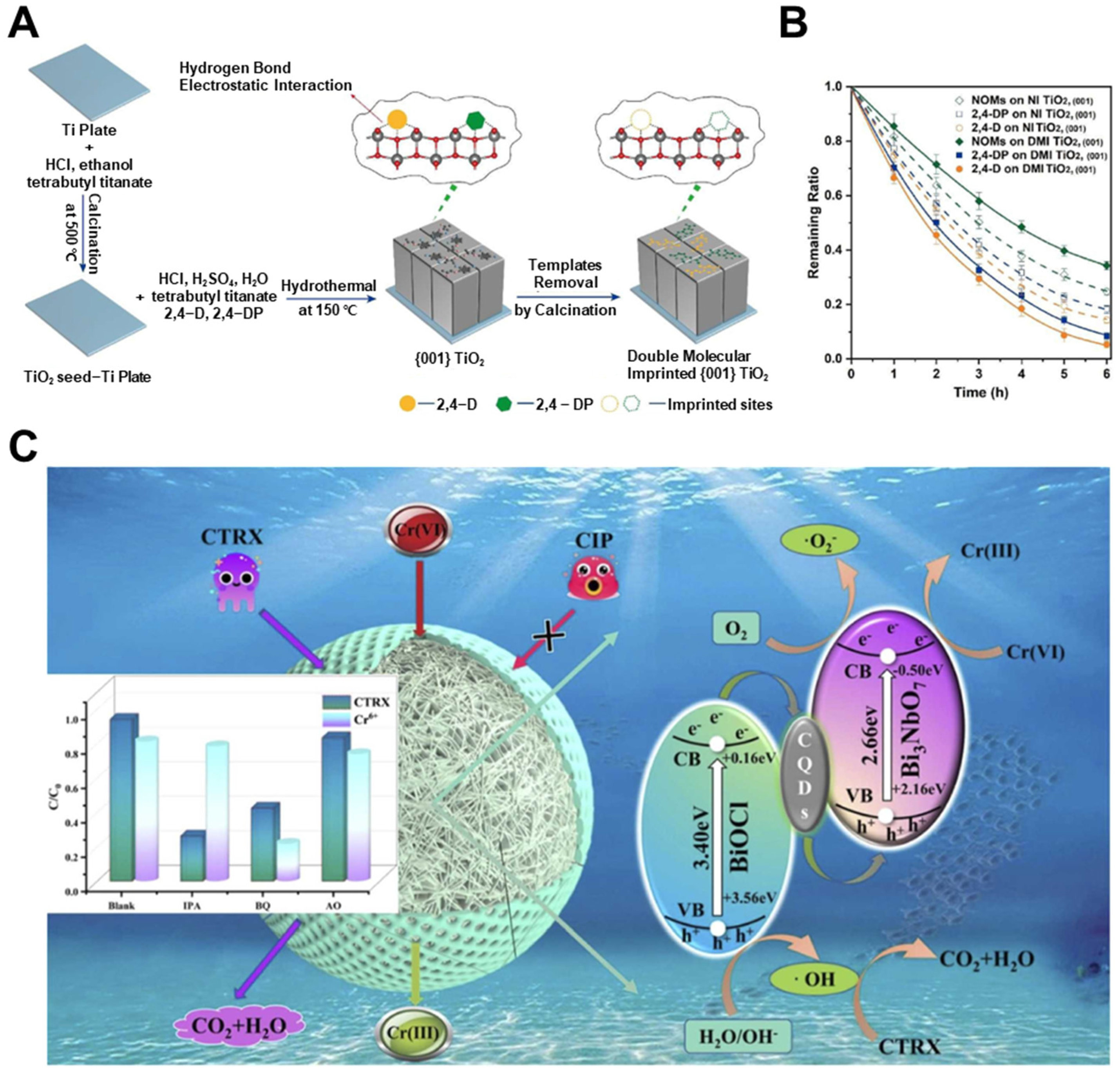
- Zhang, H.; Xiao, Y.; Zhang, Z.; Han, J.; Wu, Z.; Wei, Z.; Zhu, Y.; Guo, Q.; Tian, L.; Tang, Y. Simultaneous removal of CTRX and Cr(VI) by CQDs-doped bifunctional molecular imprinted BiOCl/Bi3NbO7 photocatalyst: The enhanced selective performance and charge separation abilities. J. Environ. Chem. Eng. 2024, 12, 113597. [Google Scholar] [CrossRef]
- Ding, S.; Wan, J.; Ma, Y.; Wang, Y.; Li, X.; Sun, J.; Pu, M. Targeted degradation of dimethyl phthalate by activating persulfate using molecularly imprinted Fe-MOF-74. Chemosphere 2021, 270, 128620. [Google Scholar] [CrossRef]
- Xie, Y.; Wan, J.; Yan, Z.; Wang, Y.; Xiao, T.; Hou, J.; Chen, H. Targeted degradation of sulfamethoxazole in wastewater by molecularly imprinted MOFs in advanced oxidation processes: Degradation pathways and mechanism. Chem. Eng. J. 2022, 429, 132237. [Google Scholar] [CrossRef]
- Tang, M.; Wan, J.; Wang, Y.; Ye, G.; Yan, Z.; Ma, Y.; Sun, J. Insights into molecular imprinting polydopamine in-situ activating peroxydisulfate for targeted removal of refractory organic pollutants: Overlooked N site. Appl. Catal. B Environ. 2023, 334, 122852. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Wang, L.; Xiao, K.; Zhao, H. Molecularly imprinted catalytic membrane reactor for improved advanced oxidation degradation efficiency of low concentration pollutants. Chem. Eng. J. 2023, 470, 144203. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, B.; Yang, R.; Yang, Z.; Zhang, D.; Duan, J.; Wang, J.; Zhang, A.; Liu, Y. Advances in molecularly imprinted materials for selective adsorption of phenolic pollutants from the water environment: Synthesis, applications, and improvement. Sci. Total Environ. 2024, 927, 172309. [Google Scholar] [CrossRef]
- Sharma, G.; Kandasubramanian, B. Molecularly Imprinted Polymers for Selective Recognition and Extraction of Heavy Metal Ions and Toxic Dyes. J. Chem. Eng. Data 2020, 65, 396–418. [Google Scholar] [CrossRef]
- Karrat, A.; Amine, A. Innovative approaches to suppress non-specific adsorption in molecularly imprinted polymers for sensing applications. Biosens. Bioelectron. 2024, 250, 116053. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Wu, T.; Zheng, H.; Kadasala, N.R.; Yang, S.; Guo, C.; Chen, L.; Liu, Y.; Yang, J. Recyclable Magnetic MIP-Based SERS Sensors for Selective, Sensitive, and Reliable Detection of Paclobutrazol Residues in Complex Environments. ACS Sustain. Chem. Eng. 2020, 8, 14549–14556. [Google Scholar] [CrossRef]
- Poonia, K.; Raizada, P.; Singh, A.; Verma, N.; Ahamad, T.; Alshehri, S.M.; Khan, A.A.P.; Singh, P.; Hussain, C.M. Magnetic molecularly imprinted polymer photocatalysts: Synthesis, applications and future perspective. J. Ind. Eng. Chem. 2022, 113, 1–14. [Google Scholar] [CrossRef]
- Li, D.; Wen, Z.; Lin, J.; Zeng, J.; Li, Z.; Ke, F.; Gao, D.; Wang, D. Molecularly imprinted polymer based on magnetic porous cellulose for specific adsorption of tetracyclines: Preparation, characterization, property evaluation, and application. Mater. Today Chem. 2023, 33, 101742. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Surya, S.G.; Beduk, T.; Vijjapu, M.T.; Lamaoui, A.; Durmus, C.; Timur, S.; Shekhah, O.; Mani, V.; Amine, A.; et al. Metal–Organic Frameworks Meet Molecularly Imprinted Polymers: Insights and Prospects for Sensor Applications. ACS Appl. Mater. Interfaces 2022, 14, 49399–49424. [Google Scholar] [CrossRef]
- Ogulewe, F.E.; Oladipo, A.A.; Gazi, M. Molecularly imprinted polymers and metal-organic framework-based nanomaterial sensors for food and beverage analysis and safety—A review. Talanta Open 2025, 11, 100448. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.-N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Yue, C.; He, L.; Li, H.; Zhang, H.; Yang, S.; Ma, T. Photocatalytic degradation by TiO2-conjugated/coordination polymer heterojunction: Preparation, mechanisms, and prospects. Appl. Catal. B Environ. Energy 2024, 344, 123605. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Amani, A.M. Photocatalytic systems: Reactions, mechanism, and applications. RSC Adv. 2024, 14, 20609–20645. [Google Scholar] [CrossRef] [PubMed]
- Chauke, N.M.; Mohlala, R.L.; Ngqoloda, S.; Raphulu, M.C. Harnessing visible light: Enhancing TiO2 photocatalysis with photosensitizers for sustainable and efficient environmental solutions. Front. Chem. Eng. 2024, 6, 1356021. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Iaria, C.; Scalisi, E.M.; Brundo, M.V.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Dattilo, S.; et al. Preferential removal of pesticides from water by molecular imprinting on TiO2 photocatalysts. Chem. Eng. J. 2020, 379, 122309. [Google Scholar] [CrossRef]
- Ding, S.; Wan, J.; Wang, Y.; Yan, Z.; Ma, Y. Activation of persulfate by molecularly imprinted Fe-MOF-74@SiO2 for the targeted degradation of dimethyl phthalate: Effects of operating parameters and chlorine. Chem. Eng. J. 2021, 422, 130406. [Google Scholar] [CrossRef]
- Li, C.-Y.; Yang, L.; Lu, Y.; Wang, M.-M.; Liu, J.-M.; Wang, S. Coupled adsorption and photocatalysis: A green synthesized g-C3N4@Mn–FeOOH@molecularly imprinted photocatalysis system for targeted removal of various heterocyclic amines. Food Front. 2023, 4, 1913–1926. [Google Scholar] [CrossRef]
- Silva, J.A. Advanced Oxidation Process in the Sustainable Treatment of Refractory Wastewater: A Systematic Literature Review. Sustainability 2025, 17, 3439. [Google Scholar] [CrossRef]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.V.; Kumar, M.S. Detoxification of water and wastewater by advanced oxidation processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- von Sonntag, C. Advanced oxidation processes: Mechanistic aspects. Water Sci. Technol. 2008, 58, 1015–1021. [Google Scholar] [CrossRef]
- Wilsey, M.K.; Taseska, T.; Meng, Z.; Yu, W.; Müller, A.M. Advanced electrocatalytic redox processes for environmental remediation of halogenated organic water pollutants. Chem. Commun. 2023, 59, 11895–11922. [Google Scholar] [CrossRef] [PubMed]
- Jaimes-López, R.; Jiménez-Vázquez, A.; Pérez-Rodríguez, S.; Estudillo-Wong, L.A.; Alonso-Vante, N. Catalyst for the Generation of OH Radicals in Advanced Electrochemical Oxidation Processes: Present and Future Perspectives. Catalysts 2024, 14, 703. [Google Scholar] [CrossRef]
- Yi, J.; Wan, J.; Wang, Y.; Ma, Y.; Yan, Z.; Zuo, S.; Zeng, C.; Yuan, W. Development of a novel polydopamine-imprinted MOFs targeted for removal of refractory organic pollutants: Synergistic mechanisms of adsorption and degradation. J. Clean. Prod. 2024, 460, 142601. [Google Scholar] [CrossRef]
- Tang, B.; Wang, Z.; Zhao, G. Preferential and simultaneous removal of chlorophenoxy herbicide pollutants via double molecular imprinted TiO2 single crystalline surface. Chem. Eng. J. 2022, 446, 137142. [Google Scholar] [CrossRef]
- Lou, Z.; Wen, X.; Song, L.; Yan, C.; Chen, H.; Lu, T.; Yu, J.; Xu, X.; Li, J. Oxygen vacancy engineered molecular imprinted TiO2 for preferential florfenicol remediation by electro-reductive approach: Enhanced dehalogenation performance and elimination of antibiotic resistance genes. Appl. Catal. B Environ. 2023, 336, 122923. [Google Scholar] [CrossRef]
- Hu, C.; Li, J.; Ke, J.; Liang, J.; Liu, Q.; Wang, Q.; Huang, W. The preparation and removal performance of carbamazepine/oxcarbazepine double template magnetic molecularly imprinted polymers. Sep. Purif. Technol. 2023, 306, 122556. [Google Scholar] [CrossRef]
- Bo Zhang, J.; Dai, C.; Li, J.; You, X.; Hu, J.; Duan, Y.; Guo, J.; Zhao, Y.; Han, Y.; Zhou, L.; et al. In-situ targeted removal of naphthalene from groundwater by peroxymonosulfate activation using molecularly imprinted activated carbon: Efficacy, mechanism and applicability. Sep. Purif. Technol. 2024, 348, 127730. [Google Scholar] [CrossRef]
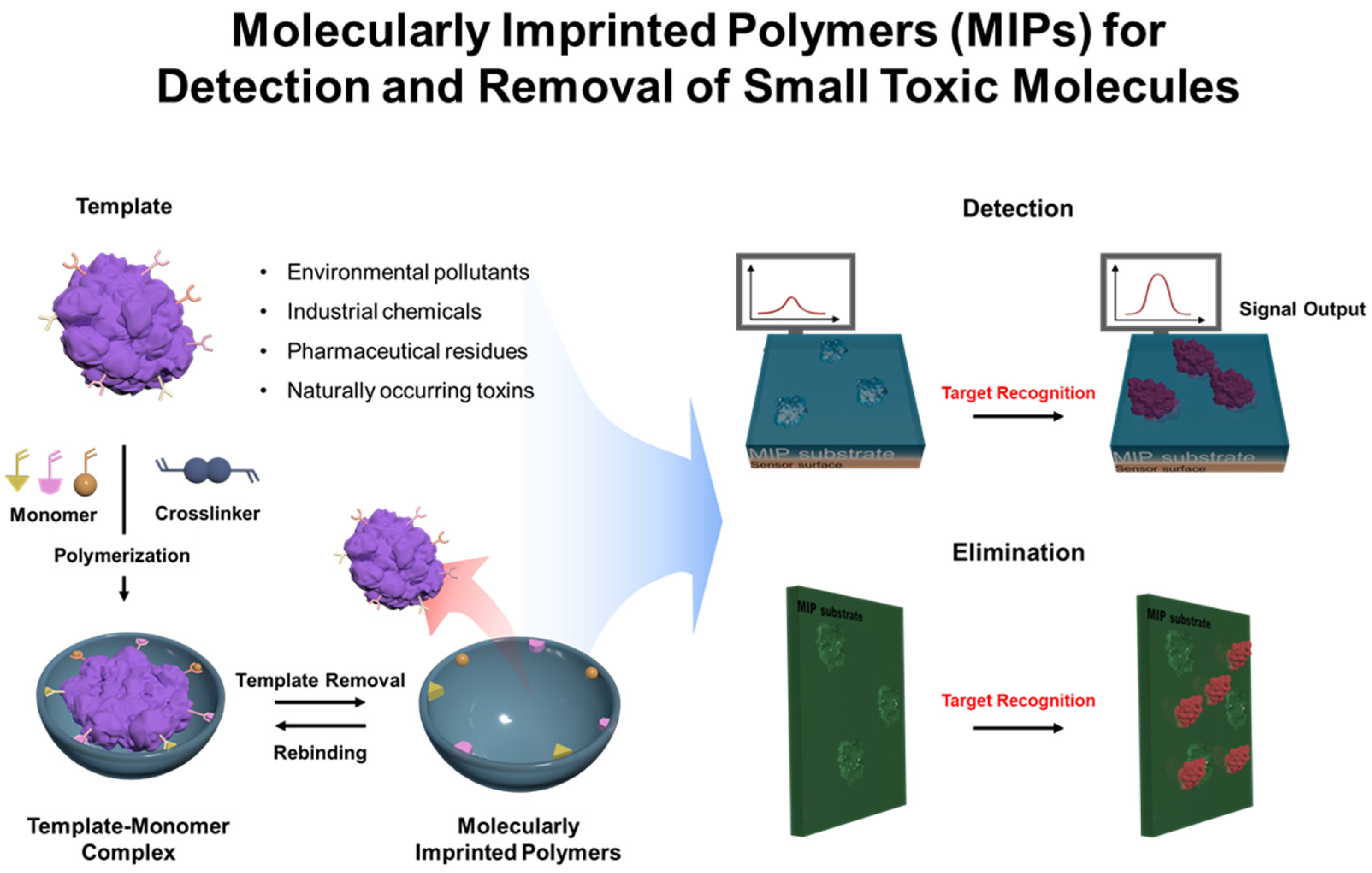
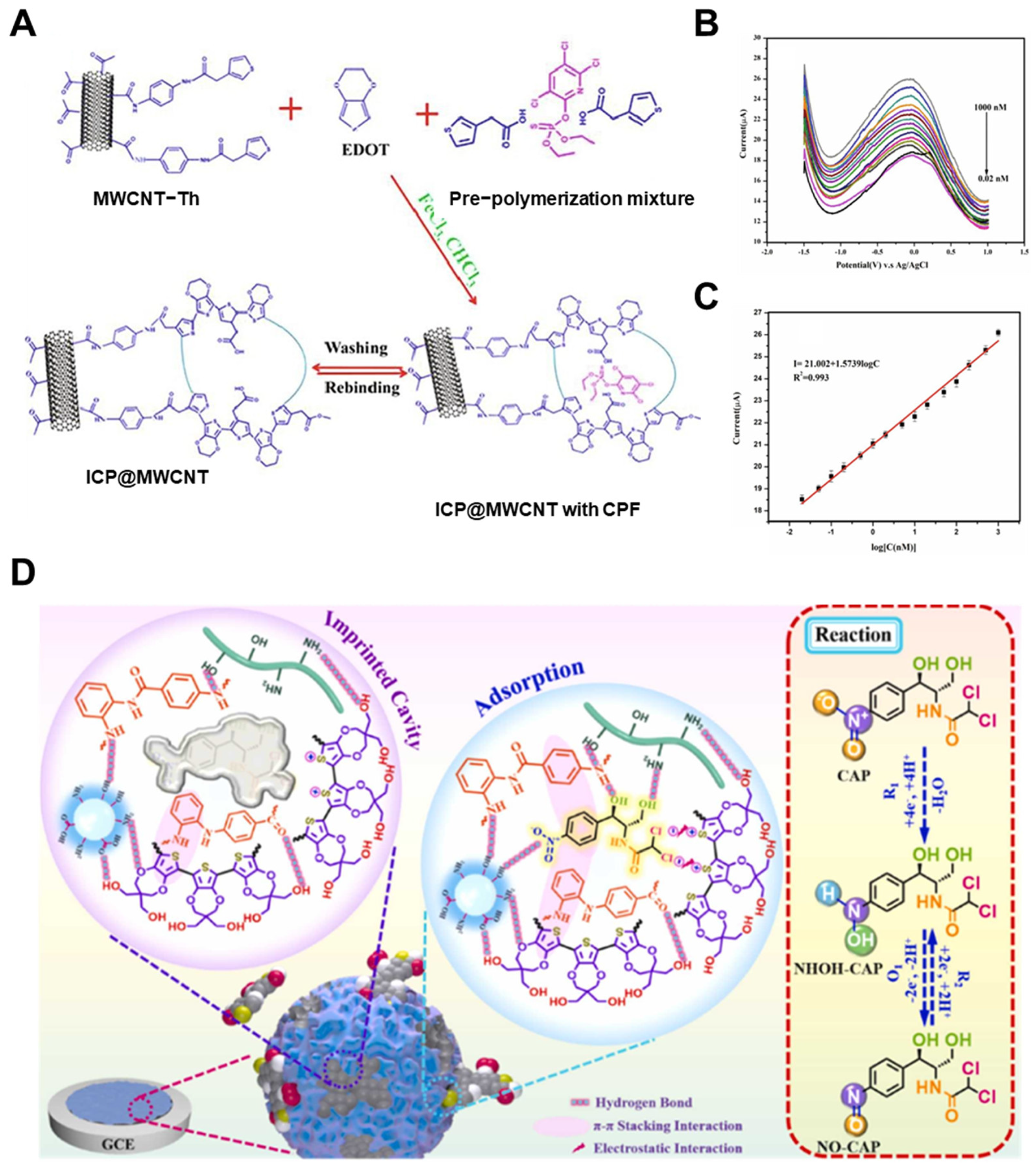
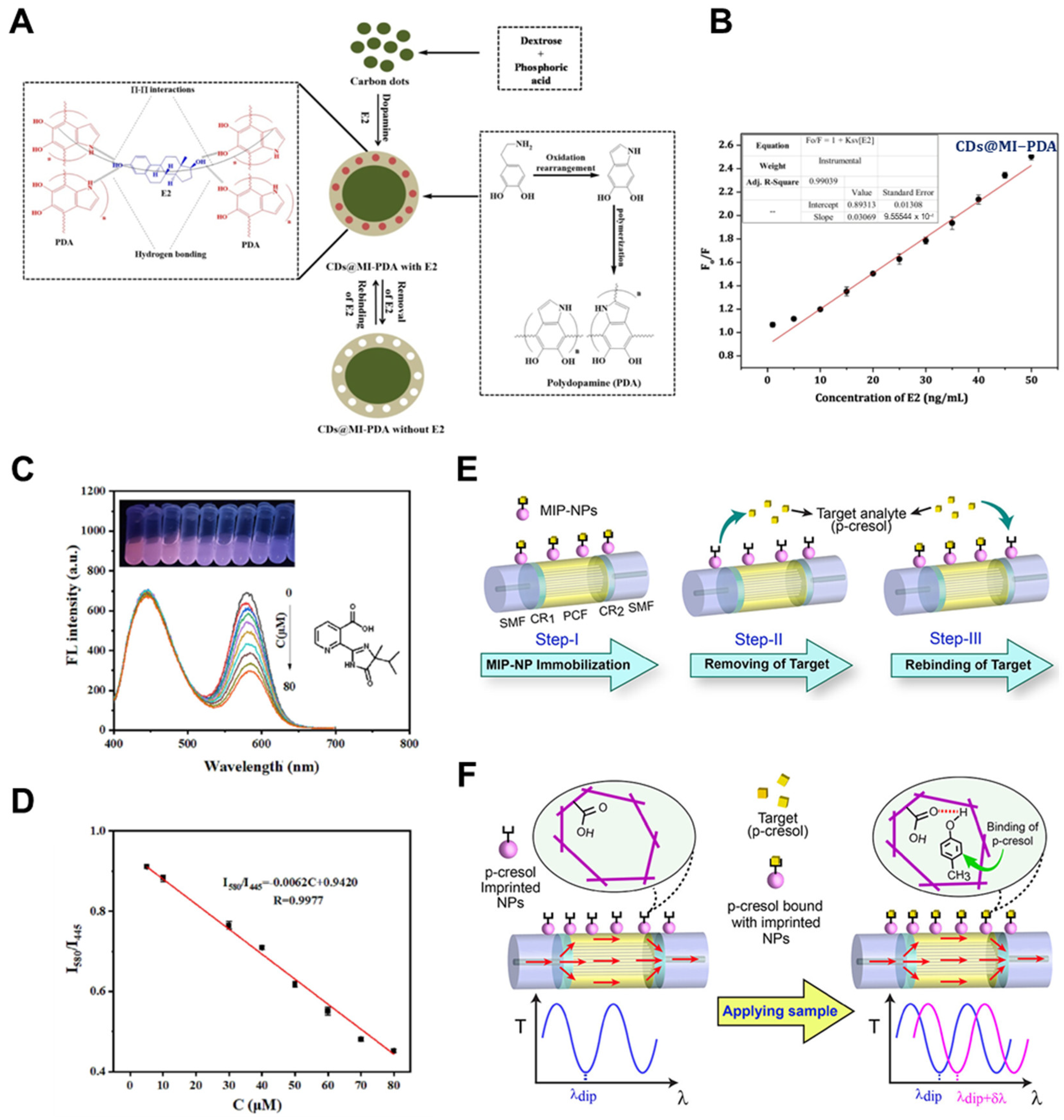
| Detection Method | Templates | Imprinting Technique | Polymerization Methods | MIP Format | Linear Range | LOD | Real Sample | Recovery/% | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Electrochemical Sensing | Bisphenol A | NR | Electropolymerization | Film | 0.5–100 μmol/L | 52 nmol/L | Tap water, milk, orange juice, bottle | 96.7–107.6 | [74] |
| Chlorpyrifos | Surface imprinting | in-situ polymerization | Film | 0.02–1000 nmol/L | 4.0 nmol/L | Tap water and cucumber | NR | [75] | |
| Chloramphenicol | NR | Electro polymerization | Film | 0.0001–125 μmol/L | 6.6 pmol/L | Eye drops, honey, tap water, aquaculture wastewater | 99.2–102.8 | [76] | |
| L-Tryptophan | Surface imprinting | NR | Film | NR | 3.23 × 10−10 mol/L | Blood | 98.0–102.0 | [77] | |
| Zearalenone | Dummy template imprinting | Electro polymerization | Film | 5 × 10−4–1 ng/mL | 1 × 10−4 ng/mL | Corn, rice, beer | 98.6–112.9 | [78] | |
| Optical Sensing | P-nitrophenol | Sol-gel imprinting | NR | Nanoparticle | 0–144 μmol/L | 0.41 μmol/L | Tap water, wastewater, seawater | 95.1–107.8 | [79] |
| 17β-oestradiol | Surface imprinting | Oxidative polymerization | Nanoparticle | 1–50 ng/mL | 0.34 ng/mL | Tap and river water | 96.4–102.2 | [80] | |
| Imazapyr | Sol-gel imprinting | NR | Nanoparticle | 5.0–80.0 μmol/L | 1.4 μmol/L | Soil and puerariae lobatae radix | 85.5–98.0 | [81] | |
| Butyrylfentanyl | Solid-phase imprinting | NR | Nanoparticle | 0–1000 ng/mL | 50 ng/mL | Human serum | NR | [82] | |
| p-cresol | NR | Thermal polymerization | Nanoparticle | 1.55 × 10−9–10−3 mol/L | 1.55 nmol/L | Tap water | 93–105 | [83] | |
| Malachite green | Surface imprinting | Thermal polymerization | Nanoparticle | 0–60 μg/L | 1.1 μg/L | Water | 94.1–105 | [84] | |
| Gravimetric Sensing | Cu(II) ions | Surface imprinting | Emulsion polymerization | Nanoparticle | 0.15–1.57 μmol/L | 40.7 nmol/L | Water | NR | [85] |
| Estrone | Surface imprinting | NR | Nanoparticle | NR | 16.00 μg/L | NR | NR | [86] | |
| Melittin | Microcontact imprinting | Photo polymerization | Film | 1–30 μg/mL | 0.3 μg/mL | NR | 100.3–107.9 | [87] | |
| L-Tryptophan | Surface imprinting | in-situ polymerization | Film | 0.5–150 ng/mL | 0.2 ng/mL | NR | NR | [88] | |
| Dimethyl methyl phosphonate | NR | Hydrolysis polymerization | Nanoparticle | NR | 80 ppb | NR | NR | [89] | |
| Photoelectrochemical Sensing | Aflatoxin B1 | Surface imprinting | Electrochemical polymerization | Film | 0.10–10 ng/mL | 0.058 ng/mL | Water | 93.5–112.8 | [90] |
| Acrylamide | Sol-gel imprinting | Electro polymerization | Film | 1–108 nmol/L | 0.123 nmol/L | Potato chips and cookies | 99.8–104.7 93.3–102.3 | [91] | |
| Diuron | NR | Electro polymerization | Nanoparticle | NR | 2.16 × 10−12 g/mL | Water | 94.6–103 | [92] |
| Elimination Method | Templates | Imprinting Technique | Polymerization Method | Adsorption Capacity | Removal Efficiency | Ref. |
|---|---|---|---|---|---|---|
| Selective Adsorption | Dimethomorph | Surface imprinting | Oxidative polymerization | 314 μg/cm−2 | 95.67% | [122] |
| Oxytetracycline | Surface imprinting | ATRP | 56.7 mg/g | NR | [123] | |
| Diethyl phthalate | Surface imprinting | NR | 13.6 mg/g | NR | [124] | |
| Tricyclic analogues | Surface imprinting | Bulk polymerization | 123.5 mg/g | NR | [125] | |
| Photocatalytic Degradation | Sulfamethoxazole | NR | EISA method | 20 mg/g | 96.8% | [126] |
| Phthalic acid esters | Surface imprinting | Thermal polymerization | NR | 91.3% | [127] | |
| p-chlorophenol | Surface imprinting | Thermal polymerization | 32.3 μg/L | 94% | [128] | |
| Ceftriaxone Sodium Cr(VI) | Surface imprinting | NR | NR | 94% and 80% | [129] | |
| Advanced Oxidation | Dimethyl phthalate | NR | Bulk Polymerization | NR | 90% | [130] |
| Sulfamethoxazole | Surface imprinting | NR | 38.04 mg/g | NR | [131] | |
| Sulfamethoxazole | Surface imprinting | Self-assembly | 11.04 mg/g | 95% | [132] | |
| Sulfamethoxazole | Surface imprinting | NR | 948.1 mg/L | 90% | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, M.S.; Lee, J.-H.; Kim, K.S. Small Toxic Molecule Detection and Elimination Using Molecularly Imprinted Polymers (MIPs). Biosensors 2025, 15, 393. https://doi.org/10.3390/bios15060393
Kang MS, Lee J-H, Kim KS. Small Toxic Molecule Detection and Elimination Using Molecularly Imprinted Polymers (MIPs). Biosensors. 2025; 15(6):393. https://doi.org/10.3390/bios15060393
Chicago/Turabian StyleKang, Min Seok, Jin-Ho Lee, and Ki Su Kim. 2025. "Small Toxic Molecule Detection and Elimination Using Molecularly Imprinted Polymers (MIPs)" Biosensors 15, no. 6: 393. https://doi.org/10.3390/bios15060393
APA StyleKang, M. S., Lee, J.-H., & Kim, K. S. (2025). Small Toxic Molecule Detection and Elimination Using Molecularly Imprinted Polymers (MIPs). Biosensors, 15(6), 393. https://doi.org/10.3390/bios15060393







