A Smart Nanoprobe for Visually Investigating the Activation Effect of Cyclical DOX Release on the p53 Pathway and Pathway-Related Molecules
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of PDA NPs
2.2. Fluorescence Quenching Assay
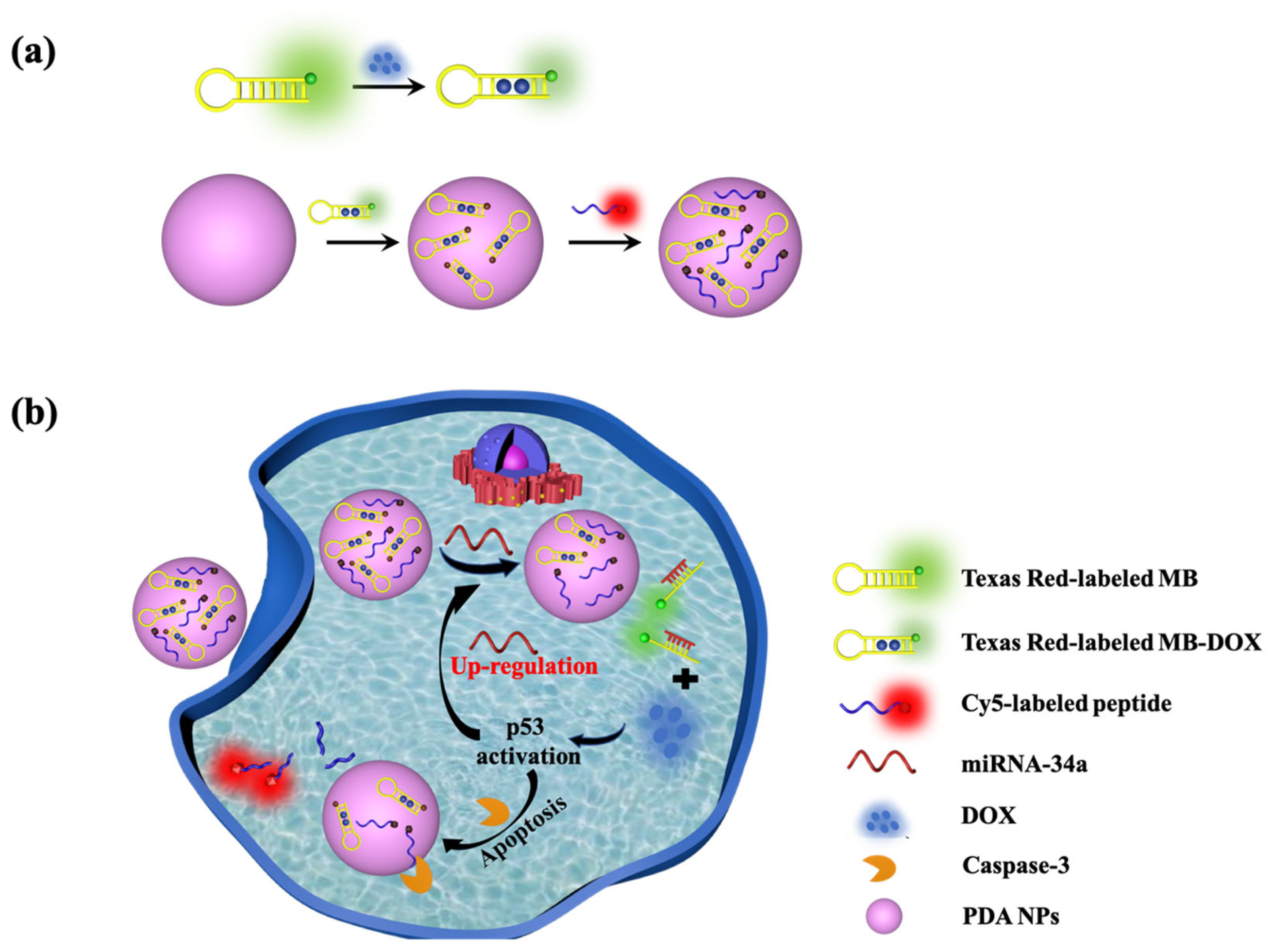
2.3. Preparation of the PDA-MB (DOX)-Pep Nanoprobe
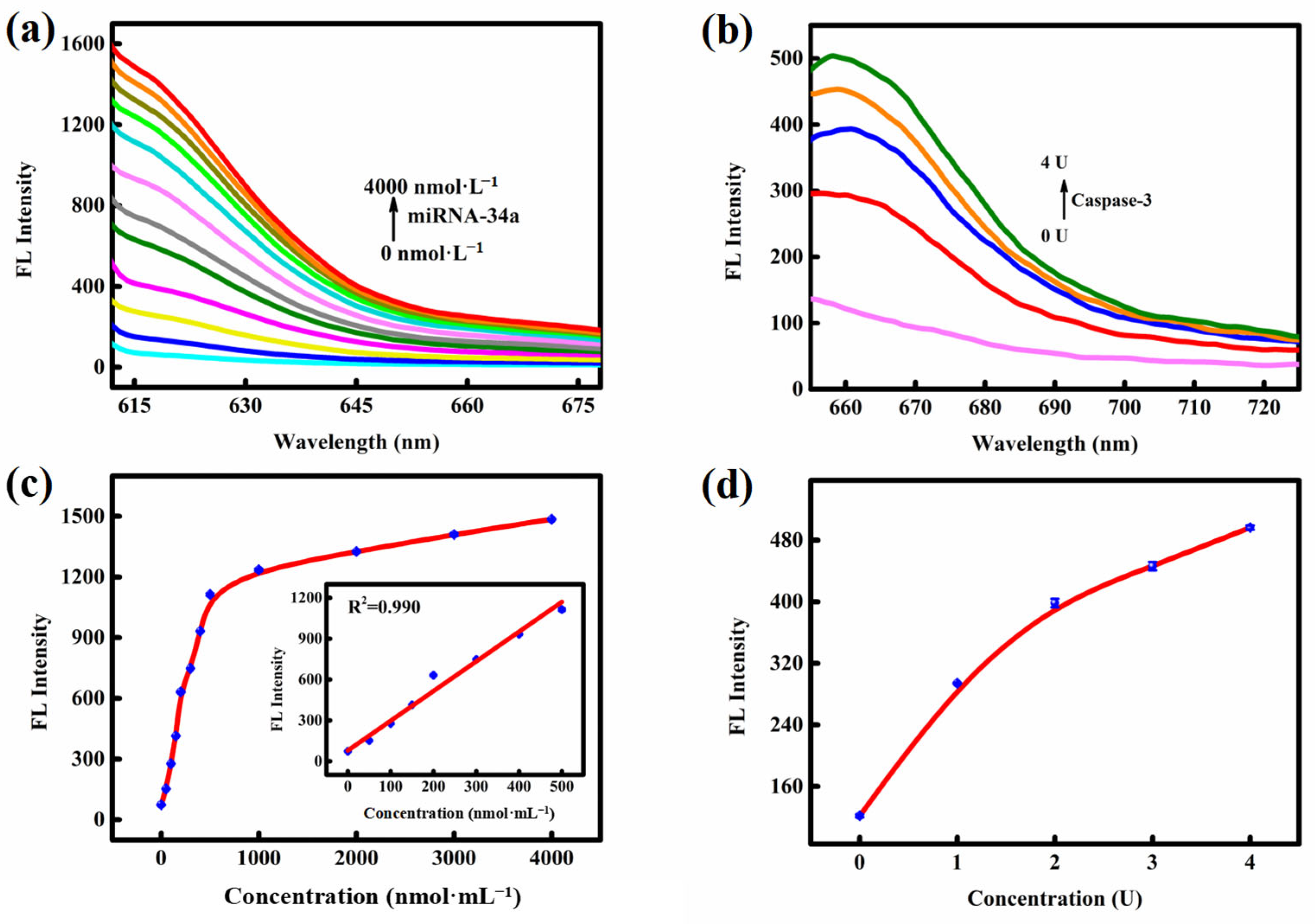
2.4. Targeted Regulation of DOX Release by miRNA-34a
2.5. Fluorescence Response of PDA-MB (DOX)-Pep Nanoprobe
2.6. Confocal Fluorescence Imaging
2.7. Apoptosis Detection
3. Results and Discussion
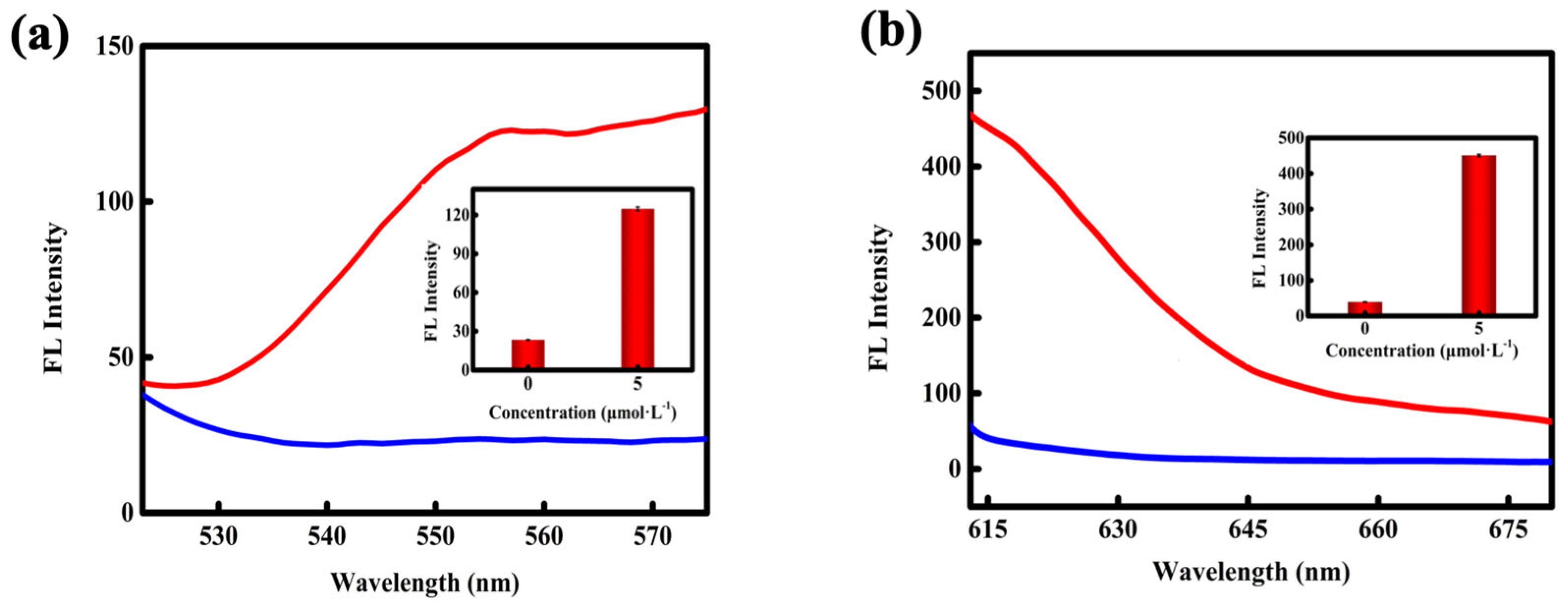
3.1. Interaction Between MB and DOX
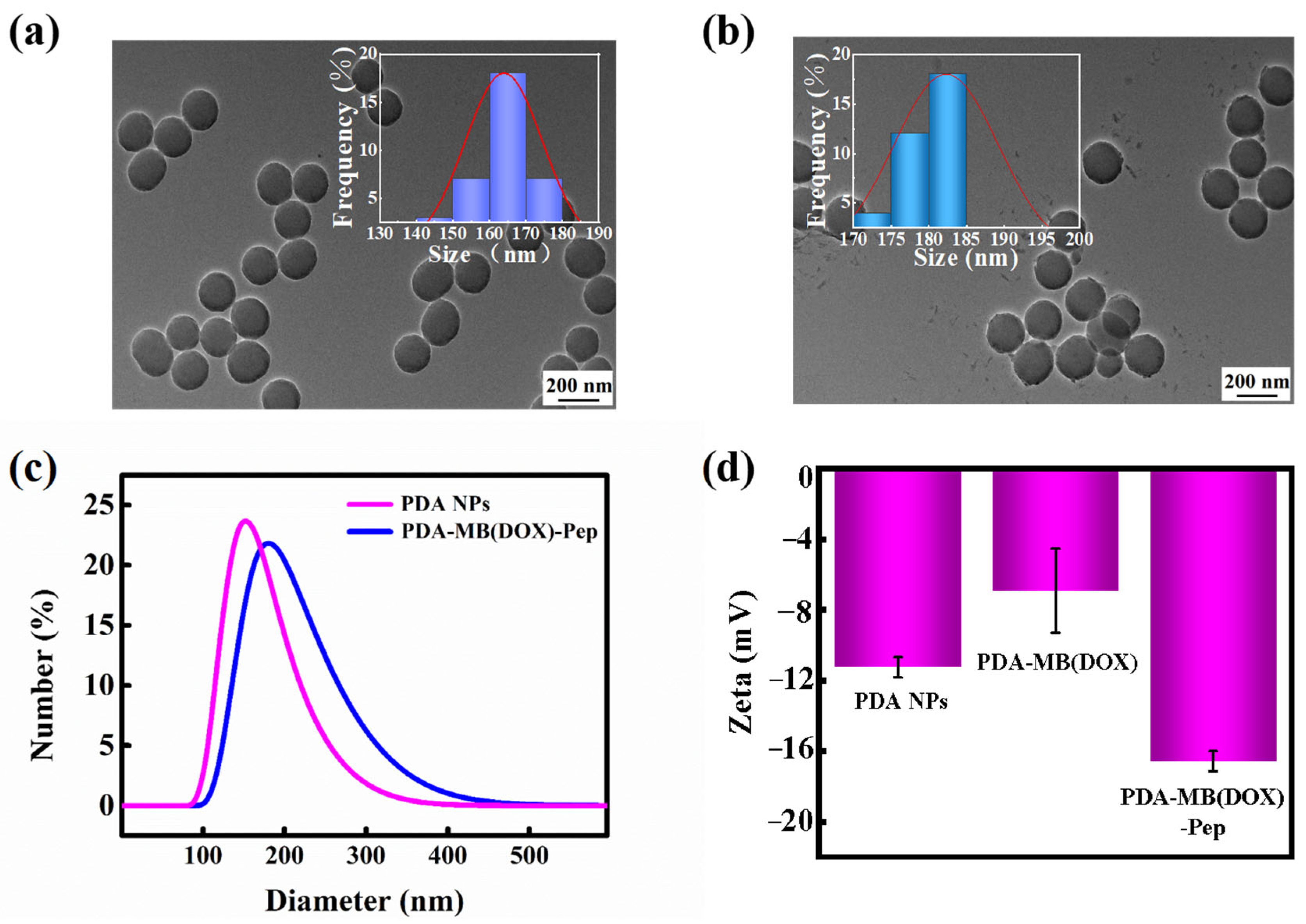
3.2. Synthesis and Characterization
3.3. In Vitro Investigations of the PDA-MB (DOX)-Pep Nanoprobe
3.4. MTT Assay
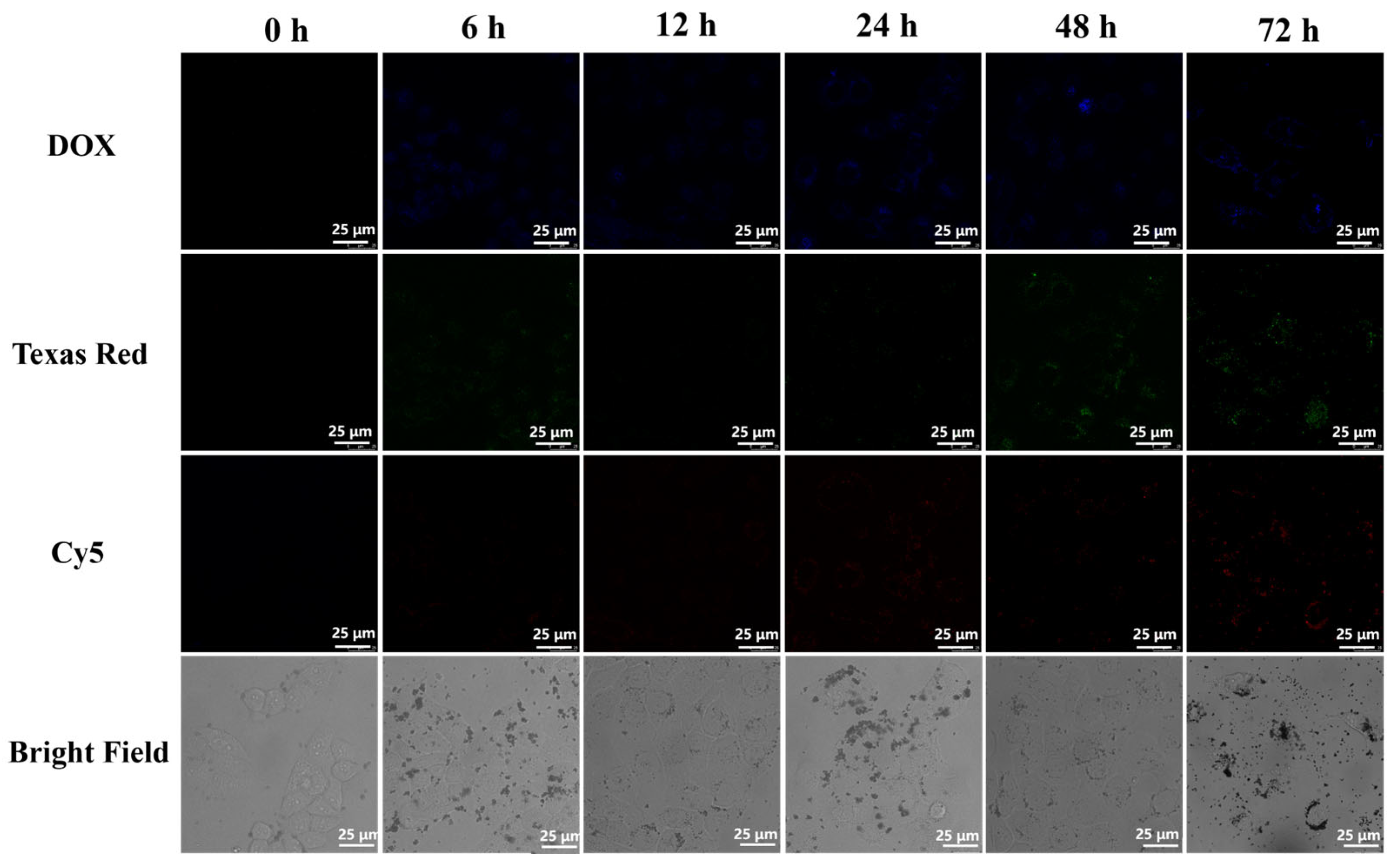
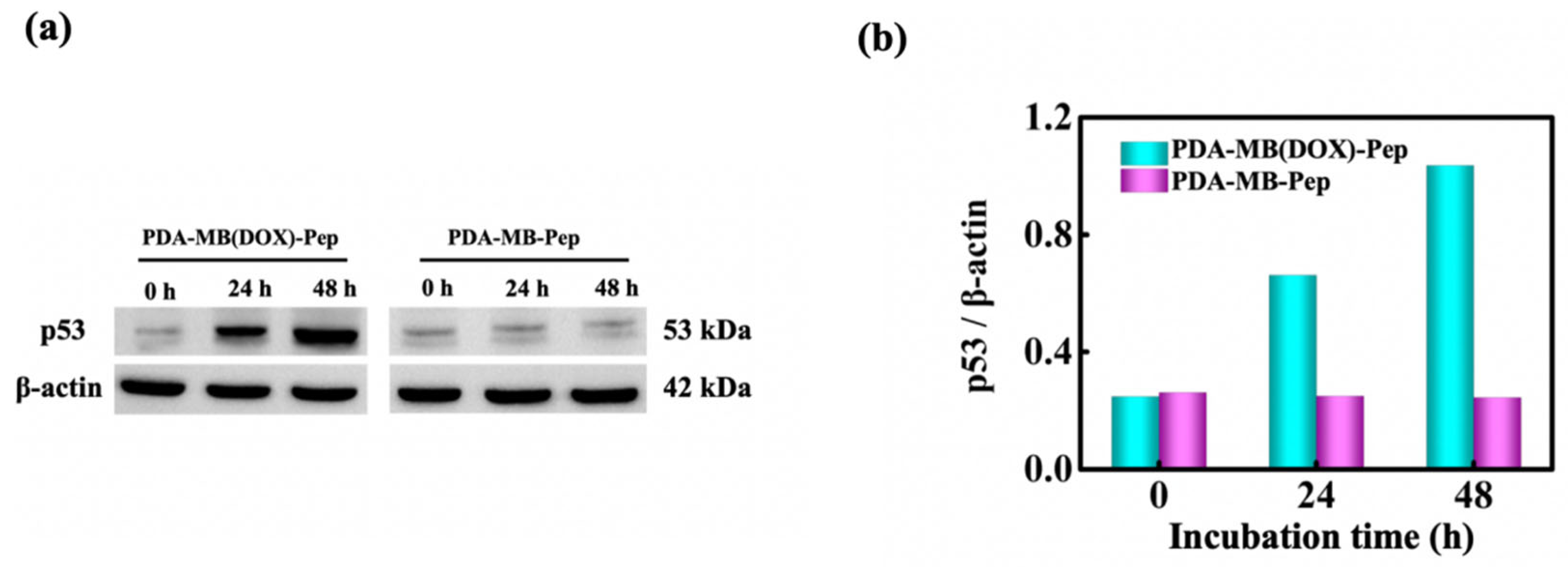
3.5. Intracellular Study of PDA-MB (DOX)-Pep
3.6. Cellular Uptake of the Nanoprobe
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Sritharan, S.; Sivalingam, S. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xiang, K.; Xu, M.; Li, Y.; Cui, J.; Zhang, L.; Tang, X. Prodrug nanomedicine inhibits chemotherapy-induced proliferative burst by altering the deleterious intercellular communication. ACS Nano. 2021, 15, 781–796. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, Y.; Ru, Y.; Yan, F.; Liu, F. Silica nanochannels as nanoreactors for the confined synthesis of Ag NPs to boost electrochemical stripping chemiluminescence of the luminol-O2 system for the sensitive aptasensor. Anal. Chem. 2024, 96, 10264–10273. [Google Scholar] [CrossRef]
- Hu, L.; Chen, M.; Chen, X.; Zhao, X.; Fang, Z.; Wang, H.; Dai, H. Chemotherapy-induced pyroptosis is mediated by BAK/BAX-caspase-3-GSDME pathway and inhibited by 2-bromopalmitate. Cell Death Discov. 2020, 11, 281. [Google Scholar] [CrossRef]
- Jiang, M.; Qi, M.; Li, L.; Li, Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020, 6, 112. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, H.; Zhao, J.; Wang, J.; Zhao, G.; Zhang, Y.; Liu, X.; Wang, Y. Advances in the application of metal oxide nanozymes in tumor detection and treatment. Colloids Surf. B Biointerfaces 2024, 235, 113767. [Google Scholar] [CrossRef]
- Luan, M.; Chang, J.; Pan, W.; Chen, Y.; Li, N.; Tang, B. Simultaneous fluorescence visualization of epithelial-mesenchymal transition and apoptosis processes in tumor cells for evaluating the impact of epithelial-mesenchymal transition on drug efficacy. Anal. Chem. 2018, 90, 10951–10957. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.; Zhang, J.; Hou, T.; Gao, T.; Liu, X.; Li, F. CRISPR-based homogeneous electrochemical strategy for near-Zero background detection of breast cancer extracellular vesicles via fluidity-enhanced magnetic capture nanoprobe. Anal. Chem. 2025, 97, 2176–2185. [Google Scholar] [CrossRef]
- Liu, M.; Wu, H.; Xu, C. Targeting cancer stem cell pathways for lung cancer therapy. Curr. Opin. Oncol. 2023, 35, 78–85. [Google Scholar] [CrossRef]
- Cheng, C.; Han, F.; Zhou, H.; Wang, H.; Zhao, J.; Zhao, Y.; Zhang, G.; Zhang, G.; Wang, Y.; Luan, M.; et al. Construction of electrochemical immunosensors based on Au@MXene and Au@CuS nanocomposites for sensitive detection of carcinoembryonic antigen. Talanta 2025, 283, 127147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Han, F.; Cheng, C.; Wang, H.; Zhao, G.; Jia, P.; Zhang, P.; Wang, Y.; Zhang, Y.; Wei, Q. Recent progress in noble metal-based single-atom nanozymes for biomedical applications. Microchem. J. 2024, 207, 111731. [Google Scholar] [CrossRef]
- Han, F.; Cheng, C.; Zhao, J.; Wang, J.; Zhao, G.; Zhang, Y.; Zhang, N.; Wang, N. Single-atom nanozymes: Emerging talent for sensitive detection of heavy metals. Colloids Surf. B Biointerfaces 2024, 242, 114093. [Google Scholar] [CrossRef]
- Yue, Y.; Lu, B.; Ni, G. Circ-0001495 influences the development of endometriosis through the miRNA-34c-5p/E2F3 axis. Reprod. Biol. 2024, 24, 100876. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.; Liu, R.; Kasinski, A.L.; Shen, H.; Slack, F.J. MicroRNA-34a: Potent tumor suppressor, cancer stem cell inhibitor and potential anticancer therapeutic. Front. Cell Dev. Biol. 2021, 9, 640587. [Google Scholar] [CrossRef]
- Kato, R.; Mizuno, R.; Kadowaki, M.; Shiozaki, M.; Akai, M.; Nakagawa, K. Sirt1 expression is associated with CD31 expression in blood cells from patients with chronic obstructive pulmonary disease. Respir. Res. 2016, 17, 139. [Google Scholar] [CrossRef]
- Raver-Shapira, N.; Marciano, E.; Meiri, E.; Spector, E.; Rosenfeld, N.; Moskovits, N.; Bentwich, Z.; Oren, M. Transcriptional activation of miR-34a Contributes to p53-mediated apoptosis. Mol. Cell 2007, 26, 731–743. [Google Scholar] [CrossRef]
- Bouamar, H.; Jiang, D.; Wang, L.; Lin, A.; Ortega, M.; Aguiar, R.C. MicroRNA 155 control of p53 activity is context dependent and mediated by aicda and socs1. Mol. Cell. Biol. 2015, 35, 1329–1340. [Google Scholar] [CrossRef]
- Dickins, R.A.; Hemann, M.T.; Zilfou, J.T.; Simpson, D.R.; Ibarra, I.; Hannon, G.J.; Lowe, S.W. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat. Genet. 2005, 37, 1289–1295. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zhao, Y.; Feng, Z. MicroRNA control of p53. J. Cell. Biochem. 2017, 118, 7–14. [Google Scholar] [CrossRef]
- Rokhlin, O.W.; Scheinker, V.S.; Taghiyev, A.F.; Bumcrot, D.; Glover, R.A.; Cohen, M.B. MicroRNA-34 mediates AR-dependent p53-induced apoptosis in prostate cancer. Cancer Biol. Ther. 2008, 7, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.M.; Al-Dalain, S.M. Betaine downregulates microRNA 34a expression via a p53-dependent manner in cisplatin-induced nephrotoxicity in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22856. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, V.; Jung, P.; Verdoodt, B.; Lodygin, D.; Epanchintsev, A.; Menssen, A.; Meister, G.; Hermeking, H. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle 2007, 6, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Chen, Z.; Sun, H.; Sun, P.; Zhao, J.; Wu, Y.; Xu, J.; Zhou, M.; Luan, M. Visually evaluating drug efficacy in living cells using COF-based fluorescent nanoprobe via CHA amplified detection of miRNA and simultaneous apoptosis imaging. Anal. Chim. Acta 2024, 1302, 342502. [Google Scholar] [CrossRef]
- Mai, H.; Vanness, B.C.; Linz, T.H. Reverse transcription-free digital-quantitative-PCR for microRNA analysis. Analyst 2023, 148, 3019–3027. [Google Scholar] [CrossRef]
- Parvin, R.; Zhang, L.; Zu, Y.; Ye, F. Photothermal responsive digital polymerase chain reaction resolving exosomal microRNAs expression in liver cancer. Small 2023, 19, 2207672. [Google Scholar] [CrossRef]
- Sule, R.; Rivera, G.; Gomes, A.V. Western blotting (immunoblotting): History, theory, uses, protocol and problems. Biotechniques 2023, 75, 99–114. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Pan, X.; Lu, W.; Zhang, J. Development and challenge of fluorescent probes for bioimaging applications: From visualization to diagnosis. Top. Curr. Chem. 2022, 380, 22. [Google Scholar] [CrossRef]
- Yang, L.; Guo, H.; Gao, Q.; Hou, T.; Zhang, J.; Liu, X.; Li, F. Integrating reliable Pt-S bond-mediated 3D DNA nanomachine with magnetic separation in a homogeneous electrochemical strategy for exosomal microRNA detection with low background and high sensitivity. Anal. Chem. 2023, 95, 17834–17842. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pillay, K. Nanomaterials for latent fingerprint detection: A review. J. Mater. Res. Technol. 2021, 12, 1856–1885. [Google Scholar] [CrossRef]
- Kim, J.S.; Zhang, H.; Shi, L.; Li, K.; Liu, X.; Won, M.; Liu, Y.Z.; Choe, Y.; Liu, X.Y.; Chen, S.; et al. Discovery of an ultra-rapid and sensitive lysosomal ffuorescence lipophagy process. Angew. Chem. Int. Ed. 2022, 61, e202116439. [Google Scholar] [CrossRef]
- Xiang, F.; Zhang, H.; Wu, Y.; Chen, Y.; Liu, Y.; Chen, S.; Guo, Y.; Yu, Y.; Li, K. Machine learning-assisted rational design of si-rhodamine as cathepsin-pH activated probe for accurate fluorescence navigation. Adv. Mater. 2024, 36, 2404828. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sun, Y.; Cai, M.; Zhao, Y.; Cao, W.; Liu, Z.; Cui, G.; Tang, B. Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis. Nat. Commun. 2018, 9, 231–240. [Google Scholar] [CrossRef]
- Gao, W.; Cao, W.; Sun, Y.; Wei, X.; Xu, K.; Zhang, H.; Tang, B. AuNP flares-capped mesoporous silica nanoplatform for MTH1 detection and inhibition. Biomaterials 2015, 69, 212–221. [Google Scholar] [CrossRef]
- Gao, W.; Cao, W.; Zhang, H.; Li, P.; Xu, K.; Tang, B. Targeting lysosomal membrane permeabilization to induce and image apoptosis in cancer cells by multifunctional Au–ZnO hybrid nanoparticles. Chem. Commun. 2014, 50, 8117–8120. [Google Scholar] [CrossRef]
- Fan, P.; Suri, A.K.; Fiala, R.; Live, D.; Patel, D.J. Molecular recognition in the FMN–RNA aptamer complex. J. Mol. Biol. 1996, 258, 480–500. [Google Scholar] [CrossRef]
- Qiao, G.; Zhuo, G.; Gao, Y.; Yu, L.; Li, N.; Tang, B. A tumor mRNA-dependent gold nanoparticle-molecular beacon carrier for controlled drug release and intracellular imaging. Chem. Commun. 2011, 47, 7458–7460. [Google Scholar] [CrossRef]
- Chena, B.; Mei, B.; Fan, R.; Chuan, D.; Ren, Y.; Mua, M.; Chen, H.; Zou, B.; Guo, G. Polydopamine-coated i-motif DNA/Gold nanoplatforms for synergistic photothermal- chemotherapy. Asian J. Pharm. Sci. 2023, 18, 100781. [Google Scholar] [CrossRef]
- Dong, Y.; Guo, Y.; Song, W.; Nie, G.; Li, F. Functional integration of DNA and peptide-based supramolecular nanoassemblies for cancer therapy. Acc. Mater. Res. 2023, 4, 892–905. [Google Scholar] [CrossRef]
- Su, H.; Zhao, F. Recent advances in intrinsically fluorescent polydopamine materials. Appl. Sci. 2022, 12, 4560. [Google Scholar] [CrossRef]
- Yu, W.; Lin, W.; Duan, N.; Wang, N.; Wu, S. A fluorescence and surface-enhanced raman scattering dual-mode aptasensor for sensitive detection of deoxynivalenol based on gold nanoclusters and silver nanoparticles modified metal-polydopamine framework. Anal. Chim. Acta 2023, 1244, 340846. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Loh, K.; Chu, Y.; Feng, R.; Satyavolu, N.S.; Xiong, N.S.; Huynh, S.M.; Huang, K. Optical control of metal ion probes in cells and zebrafish using highly selective DNAzymes conjugated to upconversion nanoparticles. J. Am. Chem. Soc. 2018, 140, 17656–17665. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Cheng, C.; Zhao, J.; Li, C.; Wang, Y.; Cao, X.; Wang, Y.; Zhang, Y.; Zhao, G. Multi-element transition metal electrochemiluminescence system based on efficient quenching strategy for sensitive detection of glial fibrillary acidic protein. Sens. Actuators B Chem. 2025, 438, 137767. [Google Scholar] [CrossRef]
- Yan, J.; Yang, L.; Lin, M.; Ma, M.; Lu, X.; Lee, P.S. Polydopamine spheres as active templates for convenient synthesis of various nanostructures. Small 2013, 9, 596–603. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Zhang, M.; Han, M.; Chen, D.; Zhu, Q.; Gao, W.; Qian, W.; Gu, Y. A telomerase-specific doxorubicin-releasing molecular beacon for cancer theranostics. Angew. Chem. Int. Ed. 2016, 55, 3304–3308. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, Y.; Xu, Z.; Chen, J. Dual-responsive dual-drug-loaded bioinspired polydopamine nanospheres as an efficient therapeutic nanoplatform against drug-resistant cancer cells. ACS Appl. Bio Mater. 2020, 3, 5730–5740. [Google Scholar] [CrossRef]
- Wu, D.; Duan, X.; Guan, Q.; Liu, J.; Yang, X.; Zhang, X.; Huang, P.; Shen, P.; Shuai, X.; Cao, X. Mesoporous polydopamine carrying manganese carbonyl responds to tumor microenvironment for multimodal imaging-guided cancer therapy. Adv. Funct. Mater. 2019, 29, 1900095. [Google Scholar] [CrossRef]
- Shumbula, N.P.; Nkabinde, S.S.; Ndala, Z.B.; Mpelane, S.; Shumbula, M.P.; Mdluli, P.S.; Njengele-Tetyana, Z. Evaluating the antimicrobial activity and cytotoxicity of polydopamine capped silver and silver/polydopamine core-shell nanocomposites. Arab. J. Chem. 2022, 15, 103798. [Google Scholar] [CrossRef]
- Ding, L.; Zhu, X.; Wang, Y.; Shi, B.; Ling, X.; Chen, H.; Nan, W.; Barrett, A.; Guo, Z.; Tao, Z.; et al. Intracellular fate of nanoparticles with polydopamine surface engineering and a novel strategy for exocytosis-inhibiting, lysosome impairment-based cancer therapy. Nano Lett. 2017, 17, 6790–6801. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Li, M.; Ma, K.; Wang, D.; Su, L.; Zhang, L.; Tang, B.-Z. Nanolab in a cell: Crystallization-induced in situ self-assembly for cancer theranostic amplification. J. Am. Chem. Soc. 2022, 144, 14388–14395. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Gao, C.; Chen, Z.; Wang, S.; Li, G.; Luan, M.; Wang, Y. A Smart Nanoprobe for Visually Investigating the Activation Effect of Cyclical DOX Release on the p53 Pathway and Pathway-Related Molecules. Biosensors 2025, 15, 383. https://doi.org/10.3390/bios15060383
Sun P, Gao C, Chen Z, Wang S, Li G, Luan M, Wang Y. A Smart Nanoprobe for Visually Investigating the Activation Effect of Cyclical DOX Release on the p53 Pathway and Pathway-Related Molecules. Biosensors. 2025; 15(6):383. https://doi.org/10.3390/bios15060383
Chicago/Turabian StyleSun, Ping, Chunlei Gao, Zhe Chen, Siyu Wang, Gang Li, Mingming Luan, and Yaoguang Wang. 2025. "A Smart Nanoprobe for Visually Investigating the Activation Effect of Cyclical DOX Release on the p53 Pathway and Pathway-Related Molecules" Biosensors 15, no. 6: 383. https://doi.org/10.3390/bios15060383
APA StyleSun, P., Gao, C., Chen, Z., Wang, S., Li, G., Luan, M., & Wang, Y. (2025). A Smart Nanoprobe for Visually Investigating the Activation Effect of Cyclical DOX Release on the p53 Pathway and Pathway-Related Molecules. Biosensors, 15(6), 383. https://doi.org/10.3390/bios15060383





