Electrochemical Microneedles for Real-Time Monitoring in Interstitial Fluid: Emerging Technologies and Future Directions
Abstract
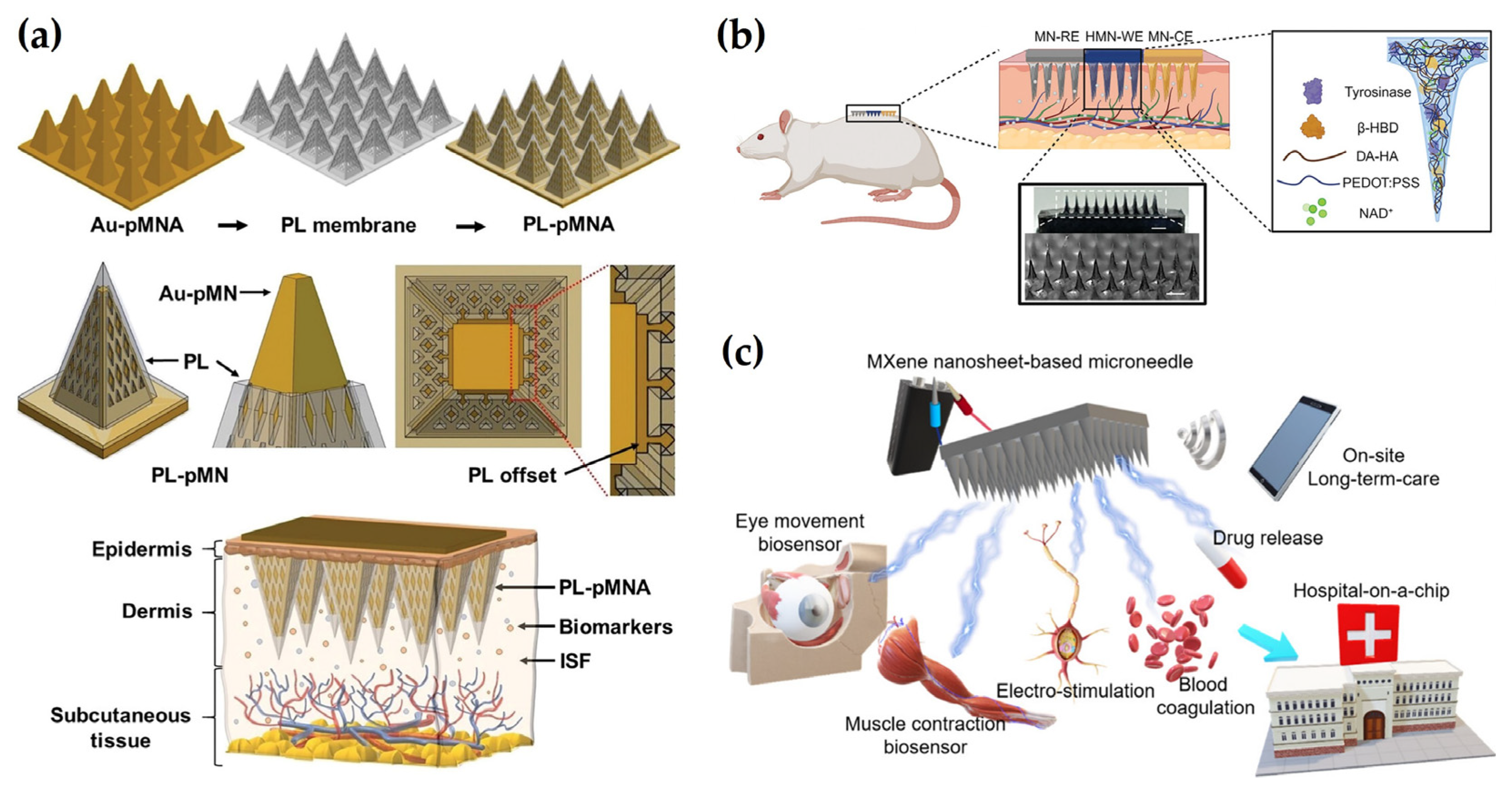
1. Introduction
2. Functional Materials for Electrochemical MNs
2.1. Conductive Metals
2.2. Conductive Polymers
2.3. 2D Materials
2.4. Tunable Materials
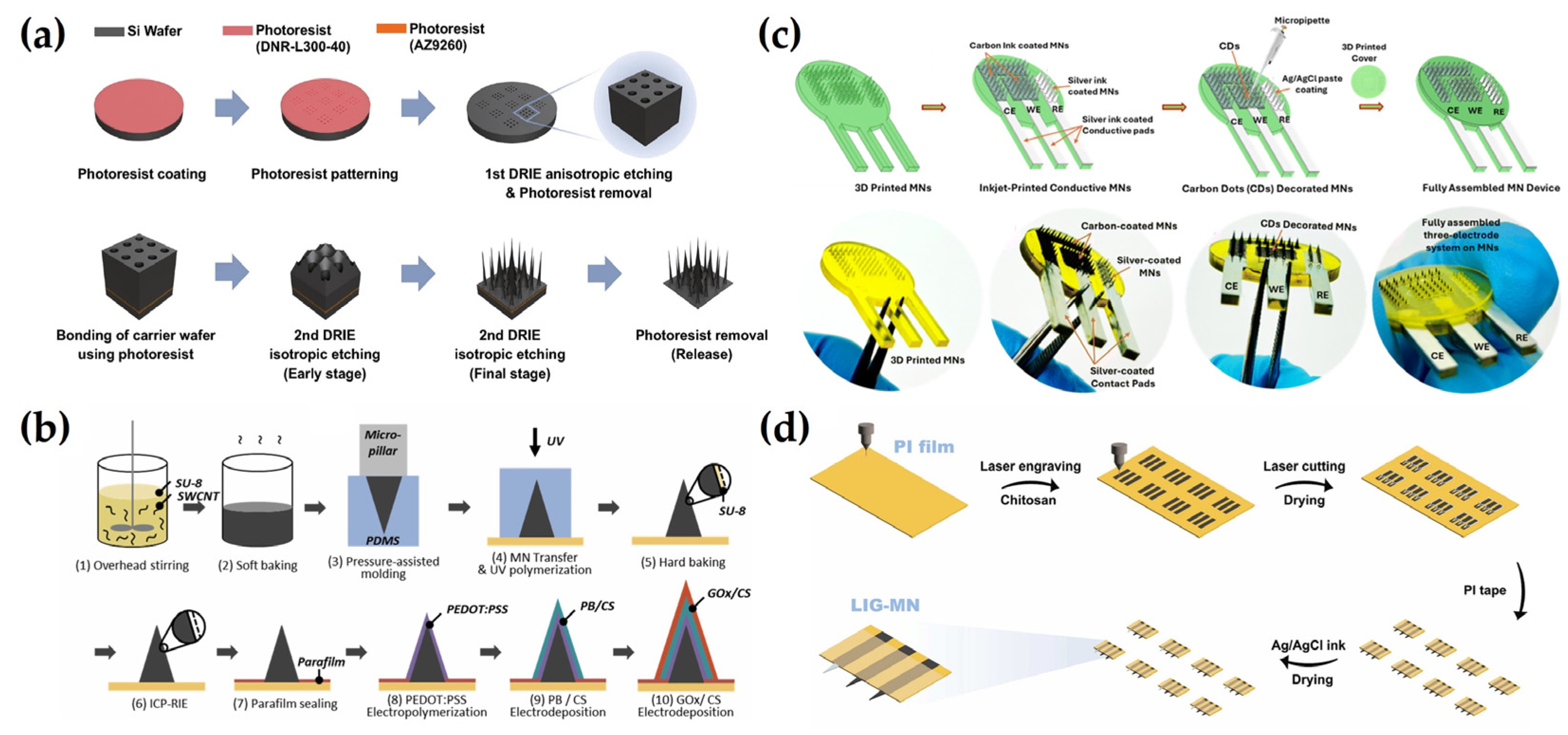
3. Fabrication Strategies for Electrochemical MNs
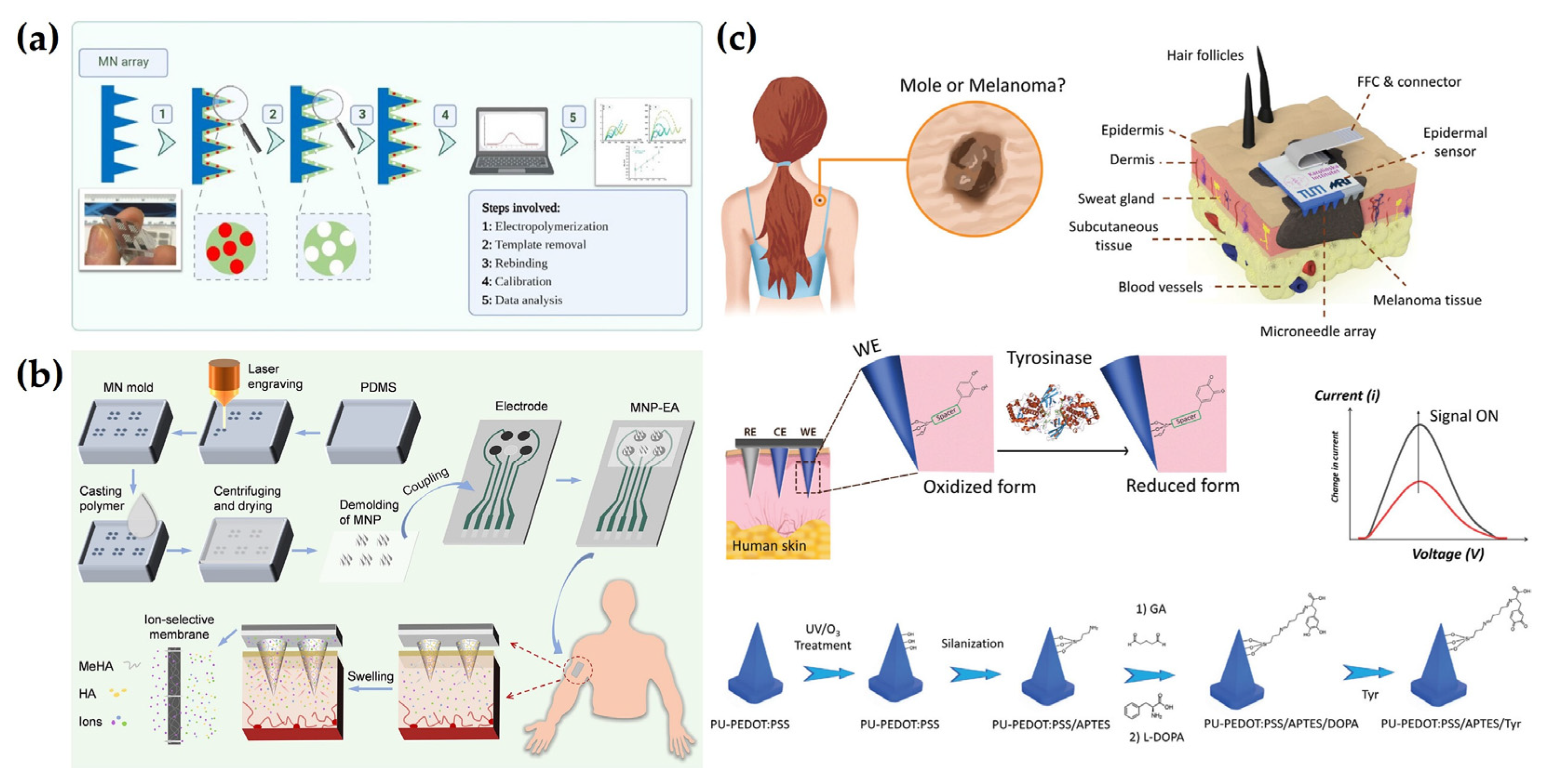
3.1. Photolithography
3.2. Casting and Molding
3.3. 3D Printing
3.4. Laser Cutting
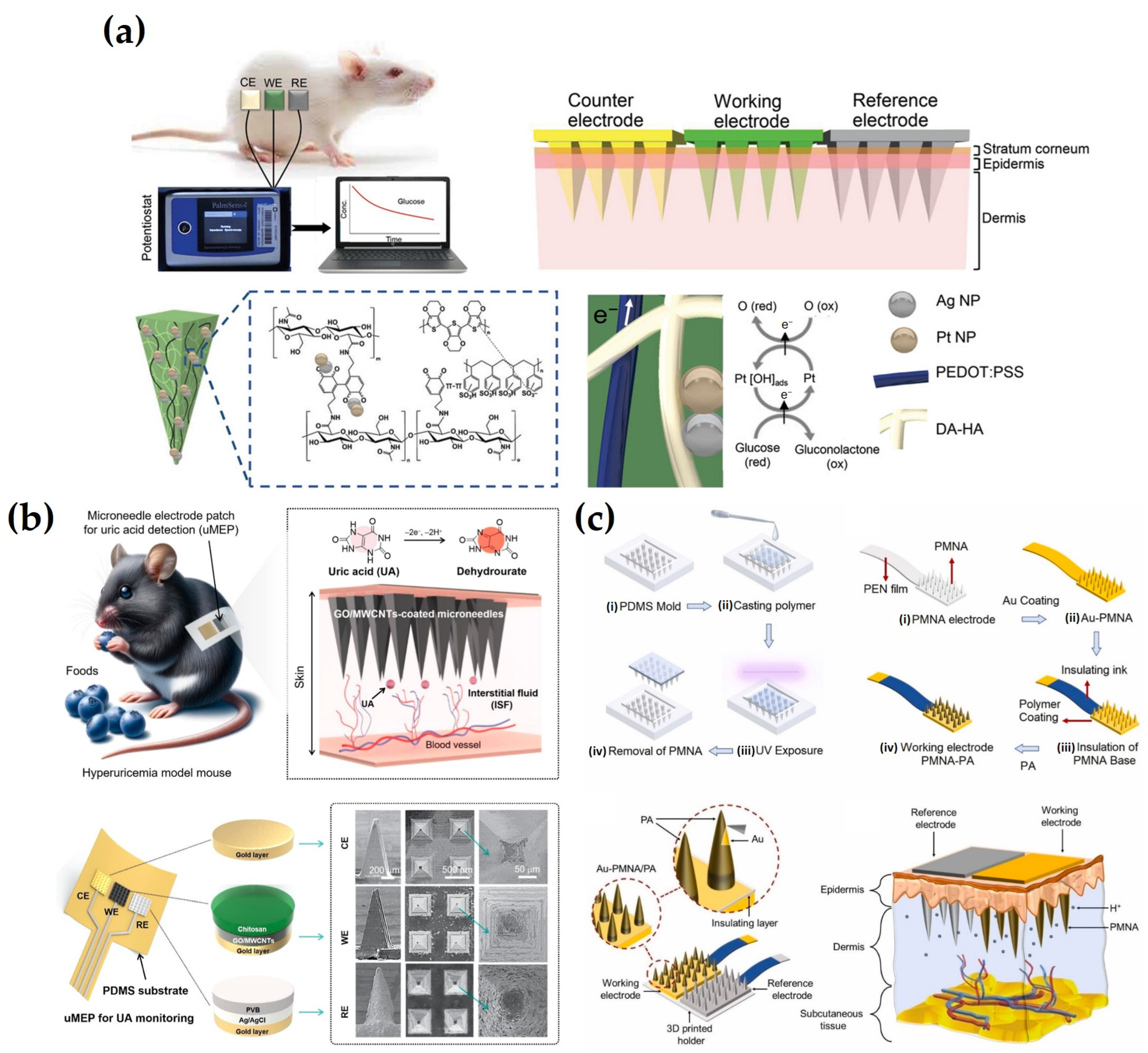
4. Electrochemical Sensing Strategies in ISF Analysis
4.1. Direct Label-Free Detection
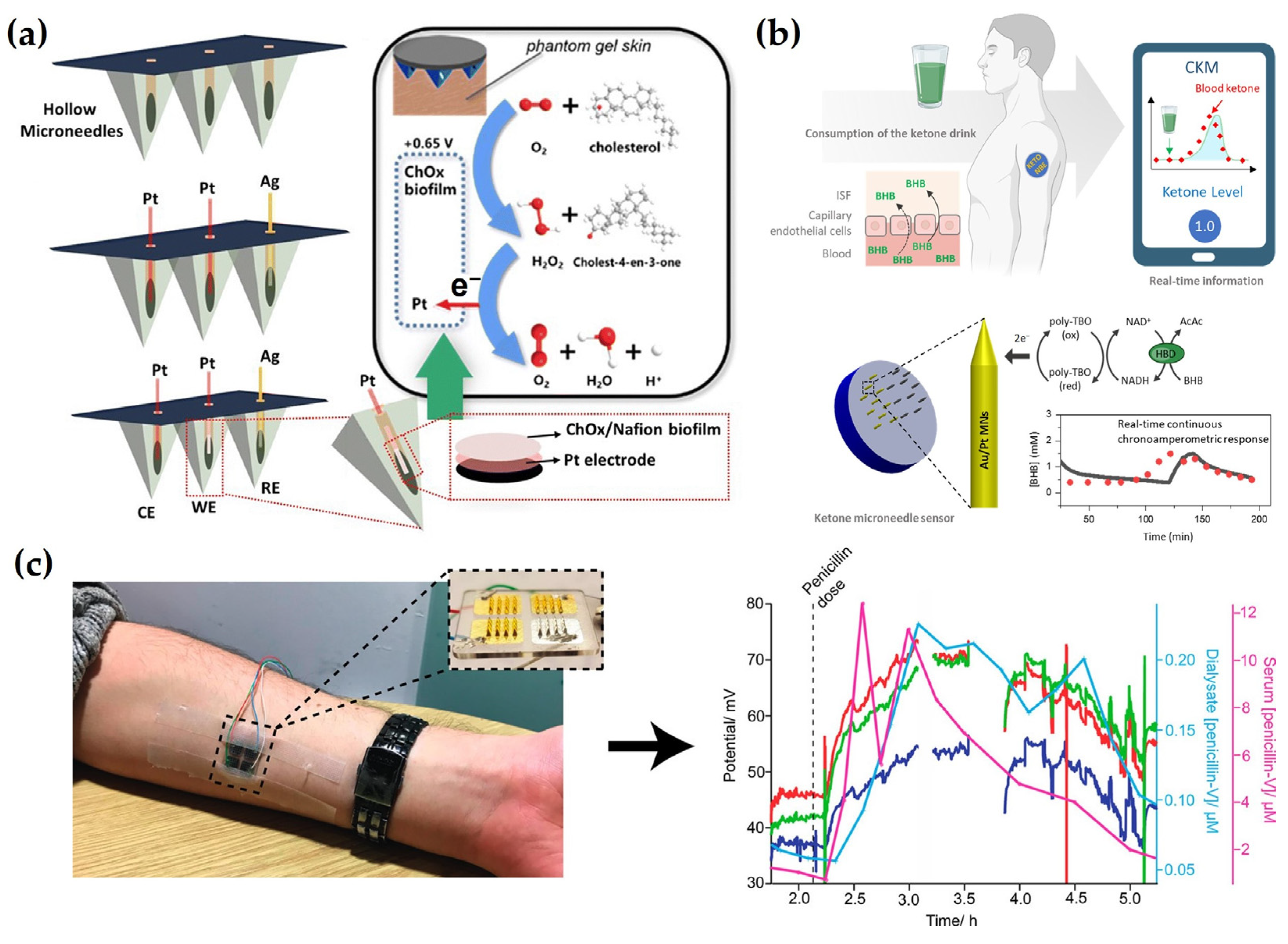
4.2. Enzyme-Based Detection
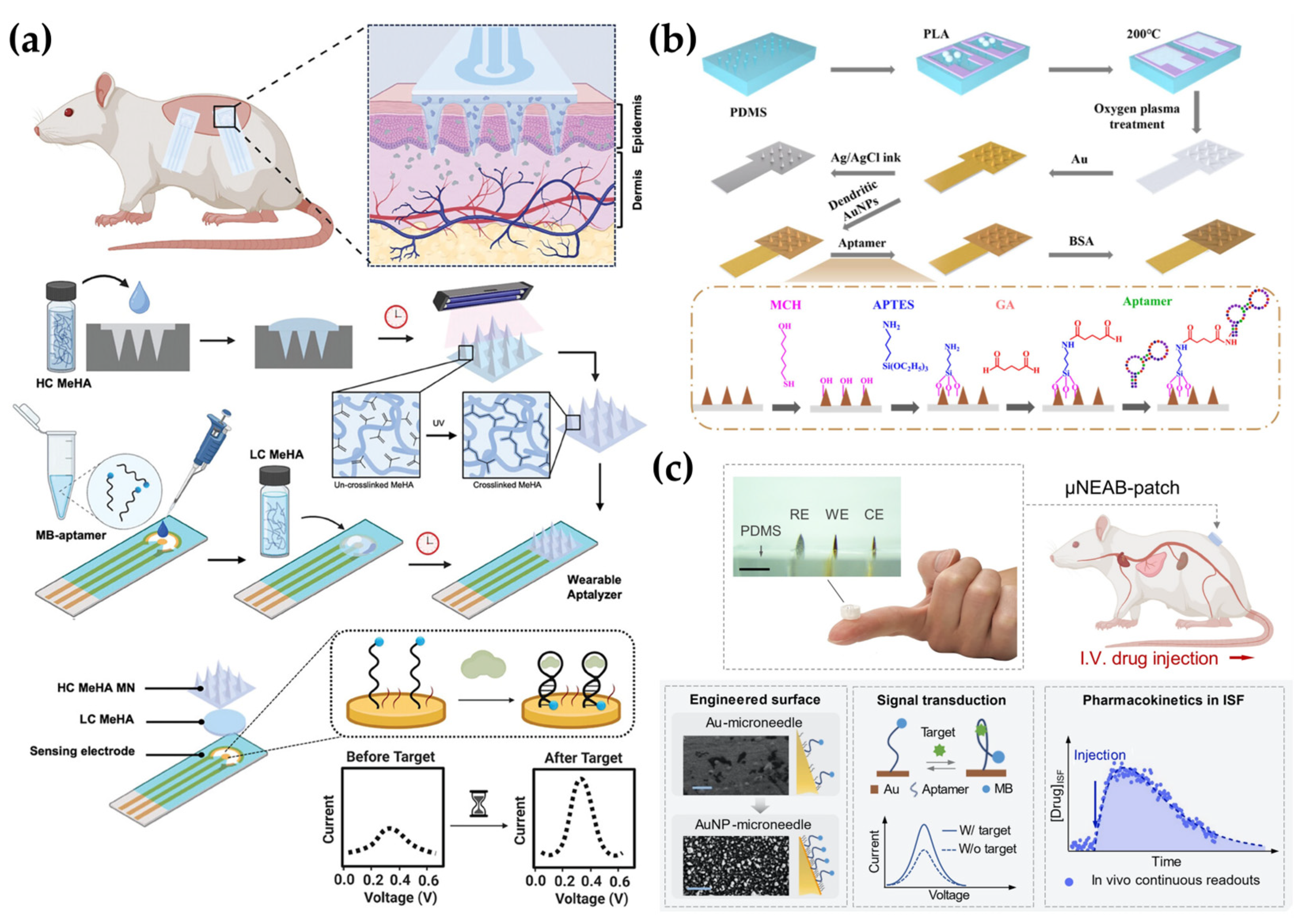
4.3. Aptamer-Based Detection
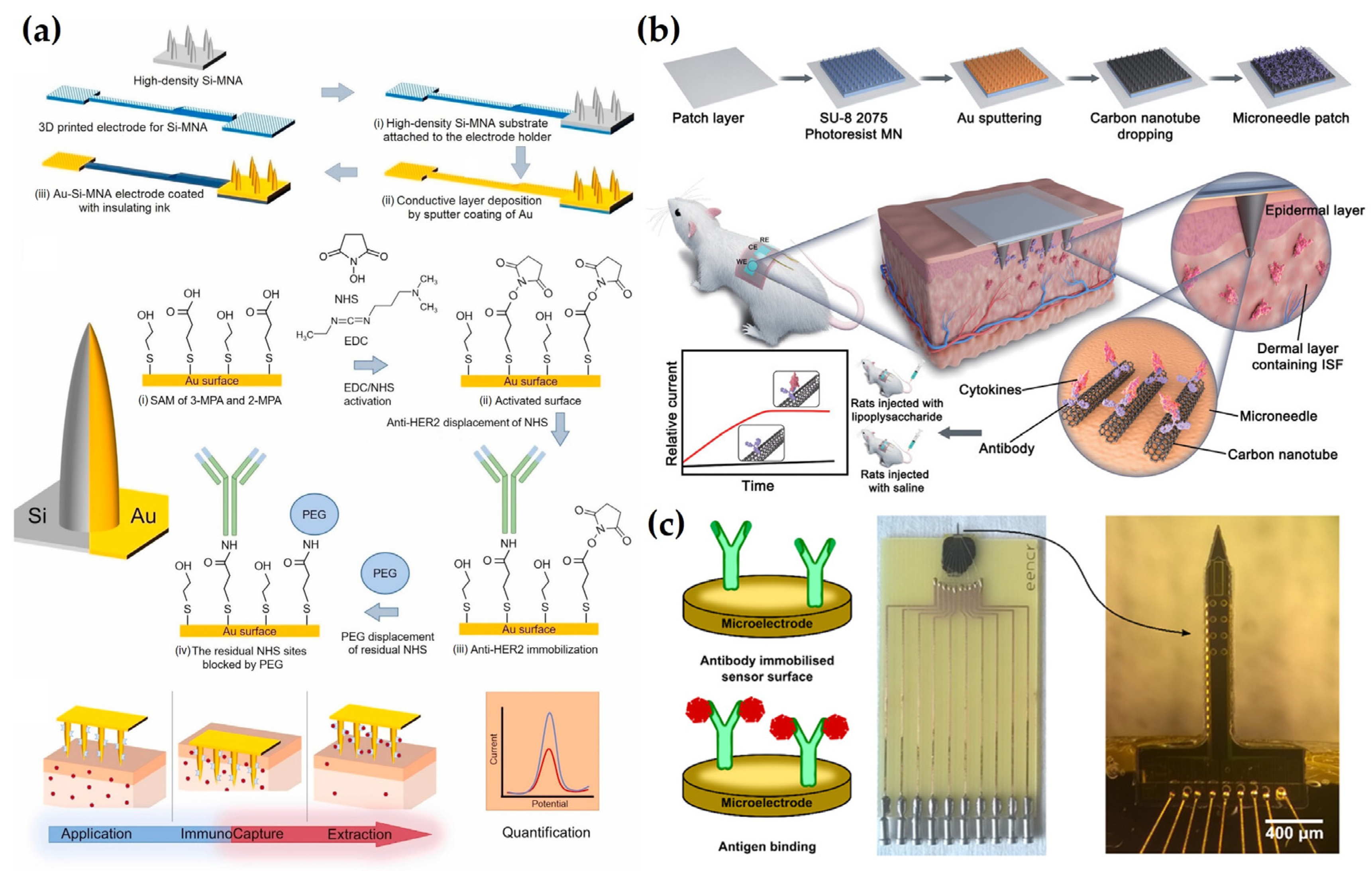
4.4. Antibody-Based Detection
4.5. Other Detection Methods
5. Smart Electrochemical MN Systems for Advanced Diagnostics
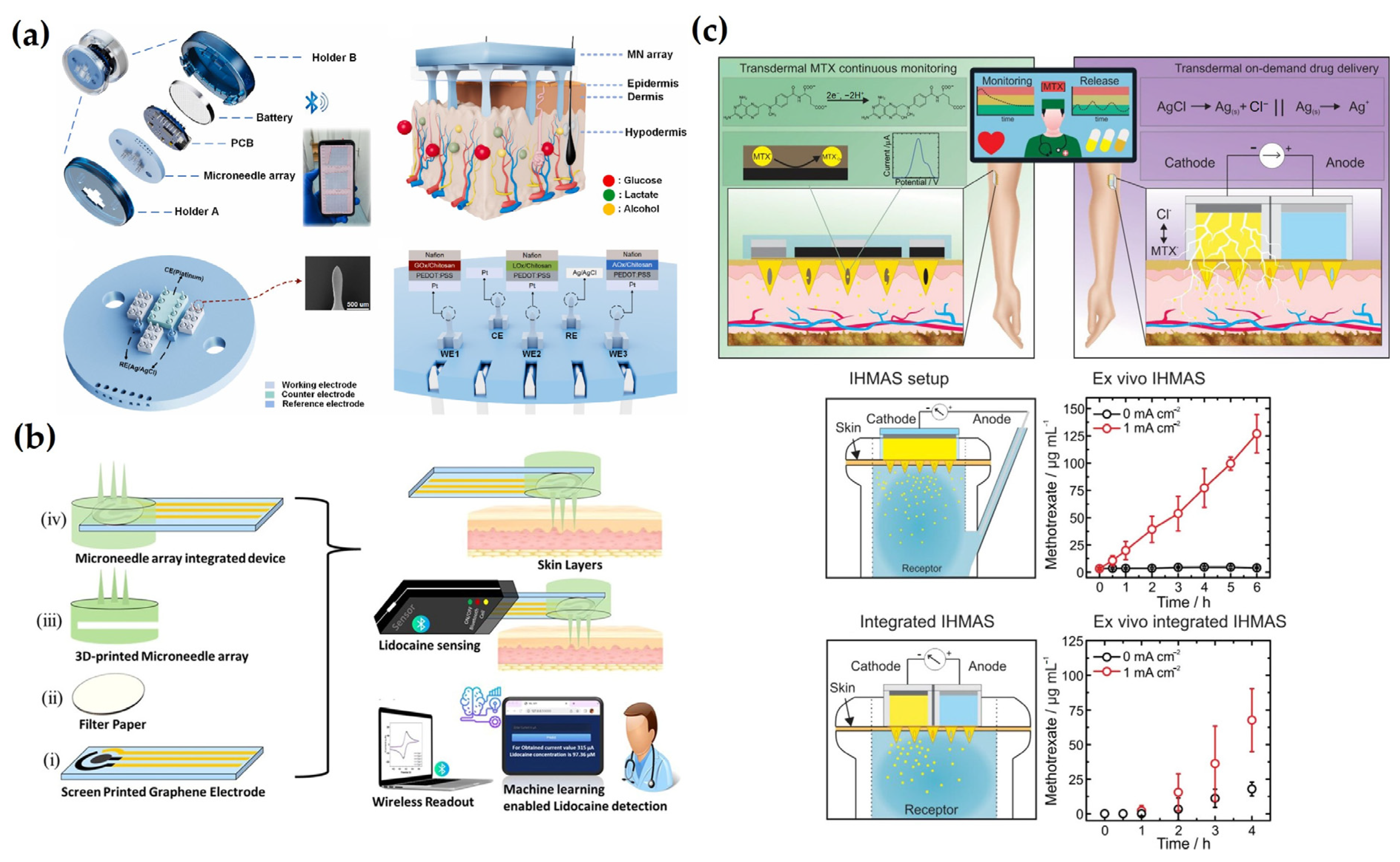
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Tehrani, F.; Teymourian, H.; Mack, J.; Shaver, A.; Reynoso, M.; Kavner, J.; Huang, N.; Furmidge, A.; Duvvuri, A.; et al. Microneedle Aptamer-Based Sensors for Continuous, Real-Time Therapeutic Drug Monitoring. Anal. Chem. 2022, 94, 8335–8345. [Google Scholar] [CrossRef]
- Saunders, J.; Thompson, I.A.P.; Soh, H.T. Generalizable Molecular Switch Designs for In Vivo Continuous Biosensing. Acc. Chem. Res. 2025, 58, 703–713. [Google Scholar] [CrossRef]
- Ahmad, A.; Imran, M.; Ahsan, H. Biomarkers as Biomedical Bioindicators: Approaches and Techniques for the Detection, Analysis, and Validation of Novel Biomarkers of Diseases. Pharmaceutics 2023, 15, 1630. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Yang, H.; Hou, Y.; Huang, H.; Qiu, J.; Wang, C.; Sang, Y.; Liu, H.; Han, L. Microfluidic Platforms for Real-Time In Situ Monitoring of Biomarkers for Cellular Processes. Adv. Mater. 2024, 36, 2307051. [Google Scholar] [CrossRef]
- Selleck, M.J.; Senthil, M.; Wall, N.R. Making Meaningful Clinical Use of Biomarkers. Biomark. Insights 2017, 12, 117727191771523. [Google Scholar] [CrossRef] [PubMed]
- Hanash, S.M.; Baik, C.S.; Kallioniemi, O. Emerging Molecular Biomarkers—Blood-Based Strategies to Detect and Monitor Cancer. Nat. Rev. Clin. Oncol. 2011, 8, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Bhawal, R.; Oberg, A.L.; Zhang, S.; Kohli, M. Challenges and Opportunities in Clinical Applications of Blood-Based Proteomics in Cancer. Cancers 2020, 12, 2428. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, X.; Zhu, J.; Wang, B.; Jelinek, D.; Zhao, Y.; Wu, T.-Y.; Horrillo, A.; Tan, J.; Yeung, J.; et al. Wearable Microneedle-Based Electrochemical Aptamer Biosensing for Precision Dosing of Drugs with Narrow Therapeutic Windows. Sci. Adv. 2022, 8, eabq4539. [Google Scholar] [CrossRef]
- Ma, G.; Wu, C. Microneedle, Bio-Microneedle and Bio-Inspired Microneedle: A Review. J. Control. Release 2017, 251, 11–23. [Google Scholar] [CrossRef]
- Wu, Z.; Qiao, Z.; Chen, S.; Fan, S.; Liu, Y.; Qi, J.; Lim, C.T. Interstitial Fluid-Based Wearable Biosensors for Minimally Invasive Healthcare and Biomedical Applications. Commun. Mater. 2024, 5, 33. [Google Scholar] [CrossRef]
- Hu, Y.; Chatzilakou, E.; Pan, Z.; Traverso, G.; Yetisen, A.K. Microneedle Sensors for Point-of-Care Diagnostics. Adv. Sci. 2024, 11, e2306560. [Google Scholar] [CrossRef] [PubMed]
- Samant, P.P.; Niedzwiecki, M.M.; Raviele, N.; Tran, V.; Mena-Lapaix, J.; Walker, D.I.; Felner, E.I.; Jones, D.P.; Miller, G.W.; Prausnitz, M.R. Sampling Interstitial Fluid from Human Skin Using a Microneedle Patch. Sci. Transl. Med. 2020, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.Q.; Miller, P.R.; Taylor, R.M.; Boyd, G.; Mach, P.M.; Rosenzweig, C.N.; Baca, J.T.; Polsky, R.; Glaros, T. Proteomic Characterization of Dermal Interstitial Fluid Extracted Using a Novel Microneedle-Assisted Technique. J. Proteome. Res. 2018, 17, 479–485. [Google Scholar] [CrossRef]
- Basu, A.; Dube, S.; Slama, M.; Errazuriz, I.; Amezcua, J.C.; Kudva, Y.C.; Peyser, T.; Carter, R.E.; Cobelli, C.; Basu, R. Time Lag of Glucose from Intravascular to Interstitial Compartment in Humans. Diabetes 2013, 62, 4083–4087. [Google Scholar] [CrossRef]
- Dai, Y.; Nolan, J.; Madsen, E.; Fratus, M.; Lee, J.; Zhang, J.; Lim, J.; Hong, S.; Alam, M.A.; Linnes, J.C.; et al. Wearable Sensor Patch with Hydrogel Microneedles for In Situ Analysis of Interstitial Fluid. ACS Appl. Mater. Interfaces 2023, 15, 56773. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle Arrays as Transdermal and Intradermal Drug Delivery Systems: Materials Science, Manufacture and Commercial Development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Larrañeta, E.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Microneedles: A New Frontier in Nanomedicine Delivery. Pharm. Res. 2016, 33, 1055–1073. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, J.; Seth, A.; Liu, L.; You, M.; Gupta, P.; Rathi, P.; Wang, Y.; Cao, S.; Jiang, Q.; et al. Microneedle Patch for the Ultrasensitive Quantification of Protein Biomarkers in Interstitial Fluid. Nat. Biomed. Eng. 2021, 5, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; Narayan, R.; Litvan, I.; Wang, J. Wearable Electrochemical Microneedle Sensor for Continuous Monitoring of Levodopa: Toward Parkinson Management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef]
- Teymourian, H.; Tehrani, F.; Mahato, K.; Wang, J. Lab under the Skin: Microneedle Based Wearable Devices. Adv. Healthc. Mater. 2021, 10, 2002255. [Google Scholar] [CrossRef]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Minimally-invasive Microneedle-based Biosensor Array for Simultaneous Lactate and Glucose Monitoring in Artificial Interstitial Fluid. Electroanalysis 2019, 31, 374–382. [Google Scholar] [CrossRef]
- Liu, N.; Xu, Z.; Morrin, A.; Luo, X. Low Fouling Strategies for Electrochemical Biosensors Targeting Disease Biomarkers. Anal. Methods 2019, 11, 702–711. [Google Scholar] [CrossRef]
- Dong, Y.; Mao, S.; Chen, S.; Ma, J.; Jaffrezic-Renault, N.; Guo, Z. Opportunities and Challenges of Microneedle Electrochemical Sensors for Interstitial Fluid Detection. TrAC Trends Anal. Chem. 2024, 180, 117891. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wu, X.; Wang, P.; Luo, X.; Lv, S. Advances in Microneedles for Transdermal Diagnostics and Sensing Applications. Microchim. Acta 2024, 191, 406. [Google Scholar] [CrossRef]
- Park, S. Recent Advances of Electrochemical Sensors on Microneedles. JMST Adv. 2024, 6, 141–148. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, J.; Zhang, X.; Zhao, J.; Ling, G.; Zhang, P. Innovative Nanozyme-Based Detection Methods for Biomarkers in Interstitial Fluid. TrAC Trends Anal. Chem. 2025, 189, 118248. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, L.; Fan, L.; Wang, L.; Bian, F.; Sun, W.; Zhao, Y. Electrical Microneedles for Wound Treatment. Advanced Sci. 2024, 2409519. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Qian, K. Nanomaterial-Based Electrochemical Sensors: Mechanism, Preparation, and Application in Biomedicine. Adv. Nanobiomed. Res. 2021, 1, 2000104. [Google Scholar] [CrossRef]
- Shao, Y.; Qi, X.; Wang, H.; Tang, B.; Cheng, Y.; Zhang, Z.; Zhang, X.; Zhu, M. Aptamer-Based Tri-Mode Sensing for Detecting Oxytetracycline Mediated by SYBR Green I and Functionalized Au Nanoparticles. Biosens. Bioelectron. 2025, 270, 116930. [Google Scholar] [CrossRef]
- Farshi, Z.S.; Bayat, F.; Chaghamirzaei, P.; Jawad, M.; Amani-Ghadim, A.R. Effect of Au Nanoparticles’ Arrangement on Melamine Detection Sensitivity in Localized Surface Plasmon Resonance Sensors. Microchem. J. 2025, 208, 112532. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.S.; Choi, Y.-S.; Yoo, K.; Kim, M.; Ham, K.-M.; Shin, M.; Kim, H.-M.; Pham, X.-H.; Yang, C.-H.; et al. Synthesis of Porous Au-Shell-Coated Silica Nanoparticles (SiO 2 @Au@AuPS) under Mild Conditions for Photothermal Therapy and Chemotherapy of Cancer Cells. ACS Appl. Nano Mater. 2025, 8, 2563–2573. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, L.; Miao, X.; Xie, Y.; Zhang, Z.; Wang, Y.; Ren, W.; Jiang, W.; Wang, X.; Wu, A.; et al. SERS/Fluorescence Dual-Modal Imaging Bioprobe for Accurate Diagnosis of Breast Cancer. Anal. Chem. 2025, 97, 5527–5537. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, G.; Alsadig, A.; Chiriacò, M.S.; Turco, A.; Foscarini, A.; Ferrara, F.; Gigli, G.; Primiceri, E. Beyond Traditional Biosensors: Recent Advances in Gold Nanoparticles Modified Electrodes for Biosensing Applications. Talanta 2024, 268, 125280. [Google Scholar] [CrossRef]
- Vu, Q.K.; Tran, Q.H.; Vu, N.P.; Anh, T.-L.; Le Dang, T.T.; Matteo, T.; Nguyen, T.H.H. A Label-Free Electrochemical Biosensor Based on Screen-Printed Electrodes Modified with Gold Nanoparticles for Quick Detection of Bacterial Pathogens. Mater. Today Commun. 2021, 26, 101726. [Google Scholar] [CrossRef]
- Pothipor, C.; Jakmunee, J.; Bamrungsap, S.; Ounnunkad, K. An Electrochemical Biosensor for Simultaneous Detection of Breast Cancer Clinically Related MicroRNAs Based on a Gold Nanoparticles/Graphene Quantum Dots/Graphene Oxide Film. Analyst 2021, 146, 4000–4009. [Google Scholar] [CrossRef]
- Jin, D.; Xu, Z.; Zhao, H.; Deng, S.; Qu, Z.; Dou, R.; Liu, W. A Minimally Invasive Sensing System Based on Hydrogel Microneedle Patches and Au/Cu2O Nanospheres Modified Screen-Printed Carbon Electrode for Glucose Monitoring in Interstitial Skin Fluid. Microchem. J. 2024, 205, 111367. [Google Scholar] [CrossRef]
- Dervisevic, M.; Harberts, J.; Sánchez-Salcedo, R.; Voelcker, N.H. 3D Polymeric Lattice Microstructure-Based Microneedle Array for Transdermal Electrochemical Biosensing. Adv. Mater. 2024, 36, 2412999. [Google Scholar] [CrossRef]
- Zhang, B.L.; Yang, Y.; Zhao, Z.Q.; Guo, X.D. A Gold Nanoparticles Deposited Polymer Microneedle Enzymatic Biosensor for Glucose Sensing. Electrochim. Acta 2020, 358, 136917. [Google Scholar] [CrossRef]
- Downs, A.M.; Bolotsky, A.; Weaver, B.M.; Bennett, H.; Wolff, N.; Polsky, R.; Miller, P.R. Microneedle Electrochemical Aptamer-Based Sensing: Real-Time Small Molecule Measurements Using Sensor-Embedded, Commercially-Available Stainless Steel Microneedles. Biosens. Bioelectron. 2023, 236, 115408. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, R.; Huang, L.; Ren, S.; Liu, B.; Gao, Z. CRISPR Analysis Based on Pt@MOF Dual-Modal Signal for Multichannel Fluorescence and Visual Detection of Norovirus. Biosens. Bioelectron. 2025, 273, 117153. [Google Scholar] [CrossRef]
- Akram, R.; Arshad, A.; Jakmunee, J. Controlled Synthesis of Bimetallic Au@Os Nanoparticles as Peroxidase Mimics: A Novel Approach to Cysteine Detection. Talanta 2025, 287, 127691. [Google Scholar] [CrossRef] [PubMed]
- Alsuhile, A.; Pein, P.S.; Barım, Ş.B.; Bozbağ, S.E.; Smirnova, I.; Erkey, C.; Schroeter, B. Synthesis of Pt Carbon Aerogel Electrocatalysts with Multiscale Porosity Derived from Cellulose and Chitosan Biopolymer Aerogels via Supercritical Deposition for Hydrogen Evolution Reaction. Adv. Energy Sustain. Res. 2025, 2400433. [Google Scholar] [CrossRef]
- Choi, J.; Lee, E.; Woo, S.; Whang, Y.; Kwon, Y.; Seo, M.; Cho, E.; Park, G.-G. Effect of Palladium Core Size on the Activity and Durability of Pt-Monolayer Electrocatalysts for Oxygen Reduction Reaction. Appl. Surf. Sci. 2025, 689, 162477. [Google Scholar] [CrossRef]
- Gutiérrez de la Rosa, S.Y.; Muñiz Diaz, R.; Villalobos Gutiérrez, P.T.; Patakfalvi, R.; Gutiérrez Coronado, Ó. Functionalized Platinum Nanoparticles with Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 9404. [Google Scholar] [CrossRef]
- Dong, L.; Ren, S.; Zhang, X.; Yang, Y.; Wu, Q.; Lei, T. In-Situ Synthesis of Pt Nanoparticles/Reduced Graphene Oxide/Cellulose Nanohybrid for Nonenzymatic Glucose Sensing. Carbohydr. Polym. 2023, 303, 120463. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, S.; Wang, H.; He, E.; Liu, J.; Dai, Y.; Xie, J.; Song, Y.; Wang, Y.; Wang, Y.; et al. PtNPt/MWCNT-PEDOT:PSS-Modified Microelectrode Arrays for the Synchronous Dopamine and Neural Spike Detection in Rat Models of Sleep Deprivation. ACS Appl. Bio Mater. 2021, 4, 4872–4884. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Song, Y.; Zhang, Y.; Xu, S.; Xing, Y.; Wang, M.; Cai, X. Platinum/Graphene Oxide Coated Microfabricated Arrays for Multinucleus Neural Activities Detection in the Rat Models of Parkinson’s Disease Treated by Apomorphine. ACS Appl. Bio Mater. 2019, 2, 4010–4019. [Google Scholar] [CrossRef] [PubMed]
- Siriviriyanun, A.; Imae, T. Advantages of Electrodes with Dendrimer-Protected Platinum Nanoparticles and Carbon Nanotubes for Electrochemical Methanol Oxidation. Phys. Chem. Chem. Phys. 2013, 15, 4921. [Google Scholar] [CrossRef]
- Abdel-Karim, R.; Reda, Y.; Abdel-Fattah, A. Review—Nanostructured Materials-Based Nanosensors. J. Electrochem. Soc. 2020, 167, 037554. [Google Scholar] [CrossRef]
- GhavamiNejad, P.; GhavamiNejad, A.; Zheng, H.; Dhingra, K.; Samarikhalaj, M.; Poudineh, M. A Conductive Hydrogel Microneedle-Based Assay Integrating PEDOT:PSS and Ag-Pt Nanoparticles for Real-Time, Enzyme-Less, and Electrochemical Sensing of Glucose. Adv. Healthc. Mater. 2023, 12, 2202362. [Google Scholar] [CrossRef]
- Ming, T.; Lan, T.; Yu, M.; Wang, H.; Deng, J.; Kong, D.; Yang, S.; Shen, Z. Platinum Black/Gold Nanoparticles/Polyaniline Modified Electrochemical Microneedle Sensors for Continuous In Vivo Monitoring of PH Value. Polymers 2023, 15, 2796. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, N.; Verma, A.; Singh, A.; Vandana; Kumar, T. Metal-Conducting Polymer Hybrid Composites: A Promising Platform for Electrochemical Sensing. Inorg. Chem. Commun. 2023, 157, 111334. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) Based Electrode Materials for Energy Storage and Conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Paneru, S.; Kumar, D. Ag-Doped-CuO Nanoparticles Supported Polyaniline (PANI) Based Novel Electrochemical Sensor for Sensitive Detection of Paraoxon-Ethyl in Three Real Samples. Sens. Actuators B Chem 2023, 379, 133270. [Google Scholar] [CrossRef]
- Mugo, S.M.; Robertson, S.V.; Lu, W. A Molecularly Imprinted Electrochemical Microneedle Sensor for Multiplexed Metabolites Detection in Human Sweat. Talanta 2023, 259, 124531. [Google Scholar] [CrossRef]
- Mugo, S.M.; Lu, W.; Wood, M.; Lemieux, S. Wearable Microneedle Dual Electrochemical Sensor for Simultaneous PH and Cortisol Detection in Sweat. Electrochem. Sci. Adv. 2022, 2, e2100039. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, X.; Liu, Z.; Zheng, S.; Yao, C.; Zhang, T.; Huang, S.; Zhang, J.; Wang, J.; Farah, S.; et al. Plug-In Design of the Microneedle Electrode Array for Multi-Parameter Biochemical Sensing in Gouty Arthritis. ACS Sens. 2025, 10, 159–174. [Google Scholar] [CrossRef]
- Ahmed, Y.M.; Eldin, M.A.; Galal, A.; Atta, N.F. Electrochemical Sensor Based on PEDOT/CNTs-Graphene Oxide for Simultaneous Determination of Hazardous Hydroquinone, Catechol, and Nitrite in Real Water Samples. Sci. Rep. 2024, 14, 5654. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.; Zhao, X.; Han, X.; Jia, Y.; Zong, M. Core-Shell N-Doped Carbon Nanofibers@poly(3,4-Ethylenedioxythiophene): Flexible Composite Fibers for Enhanced Electromagnetic Wave Absorption. Compos. Part A Appl. Sci. Manuf. 2022, 163, 107227. [Google Scholar] [CrossRef]
- Obeidat, A.M.; Rastogi, A.C. Co-Electrodeposited Poly (3, 4-Ethylenedioxythiophene) (PEDOT)-Multiwall Carbon Nanotubes (MWCNT) Hybrid Electrodes Based Solid-State Supercapacitors Using Ionic Liquid Gel Electrolyte for Energy Storage with Pulsed Power Capabilities. J. Energy Storage 2023, 67, 107563. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zuo, Q.; Li, B.; Li, Y.; Cai, W.; Qing, J.; Li, Y.; Liu, X.; Shi, J.; et al. Improved Efficiency of Organic Solar Cell Using MoS2 Doped Poly(3,4-Ethylenedioxythiophene)(PEDOT) as Hole Transport Layer. Appl. Surf. Sci. 2022, 590, 153042. [Google Scholar] [CrossRef]
- Cheng, Q.; Abdiryim, T.; Jamal, R.; Liu, X.; Xue, C.; Xie, S.; Tang, X.; Wei, J. A Novel Molecularly Imprinted Electrochemical Sensor from Poly (3, 4-Ethylenedioxythiophene)/Chitosan for Selective and Sensitive Detection of Levofloxacin. Int. J. Biol. Macromol. 2024, 267, 131321. [Google Scholar] [CrossRef] [PubMed]
- Nuh, S.; Numnuam, A.; Thavarungkul, P.; Phairatana, T. A Novel Microfluidic-Based OMC-PEDOT-PSS Composite Electrochemical Sensor for Continuous Dopamine Monitoring. Biosensors 2022, 13, 68. [Google Scholar] [CrossRef]
- Xu, Z.; Song, J.; Liu, B.; Lv, S.; Gao, F.; Luo, X.; Wang, P. A Conducting Polymer PEDOT:PSS Hydrogel Based Wearable Sensor for Accurate Uric Acid Detection in Human Sweat. Sens. Actuators B Chem. 2021, 348, 130674. [Google Scholar] [CrossRef]
- Ausri, I.R.; Sadeghzadeh, S.; Biswas, S.; Zheng, H.; GhavamiNejad, P.; Huynh, M.D.T.; Keyvani, F.; Shirzadi, E.; Rahman, F.A.; Quadrilatero, J.; et al. Multifunctional Dopamine-Based Hydrogel Microneedle Electrode for Continuous Ketone Sensing. Adv. Mater. 2024, 36, 2402009. [Google Scholar] [CrossRef]
- Odinotski, S.; Dhingra, K.; GhavamiNejad, A.; Zheng, H.; GhavamiNejad, P.; Gaouda, H.; Mohammadrezaei, D.; Poudineh, M. A Conductive Hydrogel-Based Microneedle Platform for Real-Time PH Measurement in Live Animals. Small 2022, 18, 2200201. [Google Scholar] [CrossRef]
- Hassan, J.Z.; Raza, A.; Din Babar, Z.U.; Qumar, U.; Kaner, N.T.; Cassinese, A. 2D Material-Based Sensing Devices: An Update. J. Mater. Chem. A 2023, 11, 6016–6063. [Google Scholar] [CrossRef]
- Su, S.; Chao, J.; Pan, D.; Wang, L.; Fan, C. Electrochemical Sensors Using Two-Dimensional Layered Nanomaterials. Electroanalysis 2015, 27, 1062–1072. [Google Scholar] [CrossRef]
- Pang, Y.; Yang, Z.; Yang, Y.; Ren, T. Wearable Electronics Based on 2D Materials for Human Physiological Information Detection. Small 2020, 16, 1901124. [Google Scholar] [CrossRef]
- Tabish, T.A.; Abbas, A.; Narayan, R.J. Graphene Nanocomposites for Transdermal Biosensing. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1699. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A Review on Graphene-Based Nanocomposites for Electrochemical and Fluorescent Biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef]
- Reza, M.S.; Seonu, S.; Abu Zahed, M.; Asaduzzaman, M.; Song, H.; Hoon Jeong, S.; Park, J.Y. Reduced Graphene Oxide-Functionalized Polymer Microneedle for Continuous and Wide-Range Monitoring of Lactate in Interstitial Fluid. Talanta 2024, 270, 125582. [Google Scholar] [CrossRef] [PubMed]
- Sharifuzzaman, M.; Do Shin, Y.; Yoo, J.; Reza, M.S.; kim, Y.-R.; Park, J.Y. An Oxygen-Insensitive and Minimally Invasive Polymeric Microneedle Sensor for Continuous and Wide-Range Transdermal Glucose Monitoring. Talanta 2023, 263, 124747. [Google Scholar] [CrossRef] [PubMed]
- Panicker, L.R.; Shamsheera, F.; Narayan, R.; Kotagiri, Y.G. Wearable Electrochemical Microneedle Sensors Based on the Graphene-Silver-Chitosan Nanocomposite for Real-Time Continuous Monitoring of the Depression Biomarker Serotonin. ACS Appl. Nano Mater. 2023, 6, 20601–20611. [Google Scholar] [CrossRef]
- Soomro, R.A.; Jawaid, S.; Zhu, Q.; Abbas, Z.; Xu, B. A Mini-Review on MXenes as Versatile Substrate for Advanced Sensors. Chin. Chem. Lett. 2020, 31, 922–930. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Lin, Y.-T.; Yu, J.; Chang, H.-T.; Lu, T.-Y.; Huang, T.-Y.; Preet, A.; Hsu, Y.-J.; Wang, L.; Lin, T.-E. MXene Nanosheet-Based Microneedles for Monitoring Muscle Contraction and Electrostimulation Treatment. ACS Appl. Nano Mater. 2021, 4, 7917–7924. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Sang, S.; Yin, L. Flexible Microneedle Sensor for Rapid and Highly Sensitive Detection of Glucose Inspired by the Ultrafast Water Absorption Property of Stalks. Microchem. J. 2025, 212, 113413. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; He, X.; Yang, Y.; Chen, X.; Li, J. Advances in Microneedle Technology for Biomedical Detection. Biomater. Sci. 2024, 12, 5134–5149. [Google Scholar] [CrossRef]
- Zou, H.; Wu, W.; Zhou, J.; Deng, C. SILAR Growth of ZnO NSs/CdS QDs on the Optical Fiber-Based Opto-Electrode with Guided In Situ Light and Its Application for the “Signal-On” Detection of Inflammatory Cytokine. Anal. Chem. 2024, 96, 5446–5454. [Google Scholar] [CrossRef]
- Meng, S.; Dong, N.; Liu, S.; Chen, Z.; Zhu, M.; Zhang, X.; Liu, D.; You, T. Referenced-Closed Bipolar Electrode to Enable the Photoelectrochemical-Electrochromic Synchronous Biosensing. Anal. Chem. 2025, 97, 4533–4541. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.; Wang, Y.; Yang, S.; Zhang, Y.; Wei, W.; Qiao, Y.; Dai, W.; Ge, R.; Dong, H. Interstitial Fluid Biomarkers’ Minimally Invasive Monitoring Using Microneedle Sensor Arrays. Anal Chem 2022, 94, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zeng, W.; Li, Y. Recent Progress in MOF-Based Electrochemical Sensors for Non-Enzymatic Glucose Detection. Molecules 2023, 28, 4891. [Google Scholar] [CrossRef]
- Liu, K.; Wang, H.; Zhu, F.; Chang, Z.; Du, R.; Deng, Y.; Qi, X. Lab on the Microneedles: A Wearable Metal–Organic Frameworks-Based Sensor for Visual Monitoring of Stress Hormone. ACS Nano 2024, 18, 14207–14217. [Google Scholar] [CrossRef]
- Sun, T.; Fan, R.; Xiao, R.; Xing, T.; Qin, M.; Liu, Y.; Hao, S.; Chen, W.; Yang, Y. Anionic Ln–MOF with Tunable Emission for Heavy Metal Ion Capture and L-Cysteine Sensing in Serum. J. Mater. Chem. A Mater. 2020, 8, 5587–5594. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, X.; Xu, H.; Zhao, B. Applications of MOFs as Luminescent Sensors for Environmental Pollutants. Small 2021, 17, 2005327. [Google Scholar] [CrossRef]
- Qi, X.; Liu, K.; Chang, Z. Beyond Powders: Monoliths on the Basis of Metal-Organic Frameworks (MOFs). Chem. Eng. J. 2022, 441, 135953. [Google Scholar] [CrossRef]
- Dardano, P.; Caliò, A.; Di Palma, V.; Bevilacqua, M.; Di Matteo, A.; De Stefano, L. A Photolithographic Approach to Polymeric Microneedles Array Fabrication. Materials 2015, 8, 8661–8673. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Kwon, N.; Lee, Y.; Kim, S.; Lee, T.; Choi, J.-W. Nanotechnology-Based Wearable Electrochemical Biosensor for Disease Diagnosis. ACS Sens. 2025, 10, 1675–1689. [Google Scholar] [CrossRef]
- Abdullah, A.C.; Ahmadinejad, E.; Tasoglu, S. Optimizing Solid Microneedle Design: A Comprehensive ML-Augmented DOE Approach. ACS Meas. Sci. Au. 2024, 4, 504–514. [Google Scholar] [CrossRef]
- Barrett, C.; Dawson, K.; O’Mahony, C.; O’Riordan, A. Development of Low Cost Rapid Fabrication of Sharp Polymer Microneedles for In Vivo Glucose Biosensing Applications. ECS J. Solid State Sci. Technol. 2015, 4, S3053–S3058. [Google Scholar] [CrossRef]
- Roh, H.; Yoon, Y.J.; Park, J.S.; Kang, D.-H.; Kwak, S.M.; Lee, B.C.; Im, M. Fabrication of High-Density Out-of-Plane Microneedle Arrays with Various Heights and Diverse Cross-Sectional Shapes. Nanomicro Lett 2022, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Dardano, P.; De Martino, S.; Battisti, M.; Miranda, B.; Rea, I.; De Stefano, L. One-Shot Fabrication of Polymeric Hollow Microneedles by Standard Photolithography. Polymers 2021, 13, 520. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Park, S.; Han, S.Y.; Hong, H.; Yang, D.S.; Kim, Y.J.; Lee, J.; Kim, J.; Cho, S.-W.; et al. Individually-Addressable Composite Microneedle Electrode Array by Mold-and-Place Method for Glucose Detection. Sens. Actuators B Chem. 2024, 401, 134884. [Google Scholar] [CrossRef]
- Zhao, Q.; Gribkova, E.; Shen, Y.; Cui, J.; Naughton, N.; Liu, L.; Seo, J.; Tong, B.; Gazzola, M.; Gillette, R.; et al. Highly Stretchable and Customizable Microneedle Electrode Arrays for Intramuscular Electromyography. Sci. Adv. 2024, 10, 7202. [Google Scholar] [CrossRef] [PubMed]
- Kadian, S.; Sahoo, S.S.; Shukla, S.; Narayan, R.J. Development of 3D-Printed Conducting Microneedle-Based Electrochemical Point-of-Care Device for Transdermal Sensing of Chlorpromazine. J. Mater. Chem. B 2025, 13, 2114–2123. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Q.; Luo, X.; Yang, L.; Cui, Y. Continuous Monitoring of Diabetes with an Integrated Microneedle Biosensing Device through 3D Printing. Microsyst. Nanoeng. 2021, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, S.; Wang, H.; Zeng, Q.; Wang, F.; Zheng, Y.; Haick, H.; Shi, G.; Zhang, M. Stick-and-Sensing Microneedle Patch for Personalized Nutrition Management. Sens. Actuators B Chem. 2024, 418, 136207. [Google Scholar] [CrossRef]
- Huang, X.-S.; Huang, S.; Zheng, S.-T.; Liang, B.-M.; Zhang, T.; Yue, W.; Liu, F.-M.; Shi, P.; Xie, X.; Chen, H.-J. Fabrication of Multiple-Channel Electrochemical Microneedle Electrode Array via Separated Functionalization and Assembly Method. Biosensors 2024, 14, 243. [Google Scholar] [CrossRef]
- Tran, K.T.M.; Nguyen, T.D. Lithography-Based Methods to Manufacture Biomaterials at Small Scales. J. Sci. Adv. Mater. Devices 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Eş, I.; Kafadenk, A.; Gormus, M.B.; Inci, F.; Eş, I.; Kafadenk, A.; Gormus, M.B.; Inci, F. Xenon Difluoride Dry Etching for the Microfabrication of Solid Microneedles as a Potential Strategy in Transdermal Drug Delivery. Small 2023, 19, 2206510. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Bhartia, B.; Mukhopadhyay, K.; Sharma, A. Long Term Biopotential Recording by Body Conformable Photolithography Fabricated Low Cost Polymeric Microneedle Arrays. Sens. Actuators A Phys. 2015, 236, 164–172. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Lai, L.; Li, Y. Preparation of Microneedle Array Mold Based on MEMS Lithography Technology. Micromachines 2020, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Seo, H.; Yoon, H. Fabrication Methods of Microneedles with Polymeric Materials. Korean J. Chem. Eng. 2025, 42, 1–11. [Google Scholar] [CrossRef]
- Faraji Rad, Z.; Prewett, P.D.; Davies, G.J. Rapid Prototyping and Customizable Microneedle Design: Ultra-Sharp Microneedle Fabrication Using Two-Photon Polymerization and Low-Cost Micromolding Techniques. Manuf. Lett. 2021, 30, 39–43. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef]
- Chang, K.T.; Shen, Y.K.; Fan, F.Y.; Lin, Y.; Kang, S.C. Optimal Design and Fabrication of a Microneedle Arrays Patch. J. Manuf. Process. 2020, 54, 274–285. [Google Scholar] [CrossRef]
- Juster, H.; van der Aar, B.; de Brouwer, H. A Review on Microfabrication of Thermoplastic Polymer-Based Microneedle Arrays. Polym. Eng. Sci. 2019, 59, 877–890. [Google Scholar] [CrossRef]
- Chiaranairungroj, M.; Pimpin, A.; Srituravanich, W. Fabrication of High-Density Microneedle Masters towards the Commercialisation of Dissolving Microneedles. Micro Nano Lett. 2018, 13, 284–288. [Google Scholar] [CrossRef]
- Sirbubalo, M.; Tucak, A.; Muhamedagić, K.; Rahić, O.; Čekić, A.; Vranić, E. Photopolymerization-Based Technologies for Microneedle Arrays Production. IFMBE Proc. 2021, 84, 670–678. [Google Scholar] [CrossRef]
- Olowe, M.; Parupelli, S.K.; Desai, S. A Review of 3D-Printing of Microneedles. Pharmaceutics 2022, 14, 2693. [Google Scholar] [CrossRef]
- Moussi, K.; Kavaldzhiev, M.; Perez, J.E.; Alsharif, N.; Merzaban, J.; Kosel, J. 3D Printed Microneedle Array for Electroporation. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; IEEE: New York, NY, USA, 2020; Volume 2020, pp. 2202–2205. [Google Scholar]
- Luo, X.; Yang, L.; Cui, Y. Microneedles: Materials, Fabrication, and Biomedical Applications. Biomed. Microdevices 2023, 25, 20. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Teixeira, J.A.; Oliveira, N.; Ferreira, S.; Botelho, C.M. Microneedles’ Device: Design, Fabrication, and Applications. Macromol 2024, 4, 320–355. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, Q.; Li, X.; Gao, B. Emerging Microelectronic Microneedles (EMN) for Biomedical Applications. J. Mater. Chem. C Mater. 2024, 12, 9868–9887. [Google Scholar] [CrossRef]
- Nejad, H.R.; Sadeqi, A.; Kiaee, G.; Sonkusale, S. Low-Cost and Cleanroom-Free Fabrication of Microneedles. Microsyst. Nanoeng. 2018, 4, 17073. [Google Scholar] [CrossRef]
- Ribet, F.; Stemme, G.; Roxhed, N. Real-Time Intradermal Continuous Glucose Monitoring Using a Minimally Invasive Microneedle-Based System. Biomed. Microdevices 2018, 20, 101. [Google Scholar] [CrossRef]
- Parrilla, M.; Detamornrat, U.; Domínguez-Robles, J.; Donnelly, R.F.; De Wael, K. Wearable Hollow Microneedle Sensing Patches for the Transdermal Electrochemical Monitoring of Glucose. Talanta 2022, 249, 123695. [Google Scholar] [CrossRef]
- Askari, V.R.; Tayebi-Khorrami, V.; Sabbaghzade, N.; Fadaei, M.R.; Baradaran Rahimi, V. Microneedle Used in Biosensing. In Materials and Components of Biosensors in Healthcare; Elsevier: Amsterdam, The Netherlands, 2025; pp. 483–512. [Google Scholar]
- Goud, K.Y.; Mahato, K.; Teymourian, H.; Longardner, K.; Litvan, I.; Wang, J. Wearable Electrochemical Microneedle Sensing Platform for Real-Time Continuous Interstitial Fluid Monitoring of Apomorphine: Toward Parkinson Management. Sens. Actuators B Chem. 2022, 354, 131234. [Google Scholar] [CrossRef]
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of Nanomaterials for Electrochemical Sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef]
- Ma, T.J. Remote Sensing Detection Enhancement. J. Big Data 2021, 8, 127. [Google Scholar] [CrossRef]
- Brinda, K.N.; Yhobu, Z.; Nagaraju, D.H.; Budagumpi, S. Working Principle and Sensing Mechanism of Electrochemical Sensors. In 2D Materials-Based Electrochemical Sensors; Elsevier: Amsterdam, The Netherlands, 2023; pp. 9–44. [Google Scholar]
- Hemdan, M.; Ali, M.A.; Doghish, A.S.; Mageed, S.S.A.; Elazab, I.M.; Khalil, M.M.; Mabrouk, M.; Das, D.B.; Amin, A.S. Innovations in Biosensor Technologies for Healthcare Diagnostics and Therapeutic Drug Monitoring: Applications, Recent Progress, and Future Research Challenges. Sensors 2024, 24, 5143. [Google Scholar] [CrossRef] [PubMed]
- Tortolini, C.; Cass, A.E.G.; Pofi, R.; Lenzi, A.; Antiochia, R. Microneedle-Based Nanoporous Gold Electrochemical Sensor for Real-Time Catecholamine Detection. Microchim. Acta 2022, 189, 180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhu, B.; Li, X.; Cao, J.; Qi, M.; Zhou, L.; Su, B. Microneedle Electrode Patch Modified with Graphene Oxide and Carbon Nanotubes for Continuous Uric Acid Monitoring and Diet Management in Hyperuricemia. ACS Appl. Bio Mater. 2024, 7, 8456–8464. [Google Scholar] [CrossRef]
- Ming, T.; Lan, T.; Yu, M.; Duan, X.; Cheng, S.; Wang, H.; Deng, J.; Kong, D.; Yang, S.; Shen, Z. A Novel Electrochemical Microneedle Sensor for Highly Sensitive Real Time Monitoring of Glucose. Microchem. J. 2024, 207, 112021. [Google Scholar] [CrossRef]
- Li, Z.; Kadian, S.; Mishra, R.K.; Huang, T.; Zhou, C.; Liu, S.; Wang, Z.; Narayan, R.; Zhu, Z. Electrochemical Detection of Cholesterol in Human Biofluid Using Microneedle Sensor. J. Mater. Chem. B 2023, 11, 6075–6081. [Google Scholar] [CrossRef]
- Gowers, S.A.N.; Freeman, D.M.E.; Rawson, T.M.; Rogers, M.L.; Wilson, R.C.; Holmes, A.H.; Cass, A.E.; O’Hare, D. Development of a Minimally Invasive Microneedle-Based Sensor for Continuous Monitoring of β-Lactam Antibiotic Concentrations in Vivo. ACS Sens. 2019, 4, 1072–1080. [Google Scholar] [CrossRef]
- Bakhshandeh, F.; Zheng, H.; Barra, N.G.; Sadeghzadeh, S.; Ausri, I.; Sen, P.; Keyvani, F.; Rahman, F.; Quadrilatero, J.; Liu, J.; et al. Wearable Aptalyzer Integrates Microneedle and Electrochemical Sensing for In Vivo Monitoring of Glucose and Lactate in Live Animals. Adv. Mater. 2024, 36, 2313743. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Fan, Y.; Chen, B.Z.; Li, D.; He, Y.T.; Zhang, G.L.; Liang, L.; Du, J.; Wang, Y.; Guo, X.D. An Aptamer-Integrated Conductive Microneedle Biosensor for Real-Time Transdermal Cortisol Monitoring. Chem. Eng. J. 2024, 502, 157488. [Google Scholar] [CrossRef]
- Dervisevic, M.; Alba, M.; Adams, T.E.; Prieto-Simon, B.; Voelcker, N.H. Electrochemical Immunosensor for Breast Cancer Biomarker Detection Using High-Density Silicon Microneedle Array. Biosens. Bioelectron. 2021, 192, 113496. [Google Scholar] [CrossRef]
- Xu, J.; Yang, B.; Kong, J.; Zhang, Y.; Fang, X. Real-Time Monitoring and Early Warning of a Cytokine Storm In Vivo Using a Wearable Noninvasive Skin Microneedle Patch. Adv. Heal. Mater. 2023, 12, 2203133. [Google Scholar] [CrossRef]
- Russell, C.; Ward, A.C.; Vezza, V.; Hoskisson, P.; Alcorn, D.; Steenson, D.P.; Corrigan, D.K. Development of a Needle Shaped Microelectrode for Electrochemical Detection of the Sepsis Biomarker Interleukin-6 (IL-6) in Real Time. Biosens. Bioelectron. 2019, 126, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.; Correia, B.P.; Sharma, S.; Moreira, F.T.C. Molecular Imprinted Polymers on Microneedle Arrays for Point of Care Transdermal Sampling and Sensing of Inflammatory Biomarkers. ACS Omega 2022, 7, 39039–39044. [Google Scholar] [CrossRef] [PubMed]
- Poursharifi, N.; Hassanpouramiri, M.; Zink, A.; Ucuncu, M.; Parlak, O. Transdermal Sensing of Enzyme Biomarker Enabled by Chemo-Responsive Probe-Modified Epidermal Microneedle Patch in Human Skin Tissue. Adv. Mater. 2024, 36, 2403758. [Google Scholar] [CrossRef]
- Ozcelikay, G.; Karadurmus, L.; Kaya, S.I.; Bakirhan, N.K.; Ozkan, S.A. A Review: New Trends in Electrode Systems for Sensitive Drug and Biomolecule Analysis. Crit. Rev. Anal. Chem. 2020, 50, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Ahn, H.; Chaj Ulloa, J.; Gao, W. Microneedle Sensors for Dermal Interstitial Fluid Analysis. Med-X 2024, 2, 1–23. [Google Scholar] [CrossRef]
- Rahman, F.; Ryan, A.; Bocchino, A.; Galvin, P.; Teixeira, S.R. Microneedle-Based Electrochemical Sensors for Real-Time PH and Sodium Monitoring in Physiological Environments. Sens. Biosensing Res. 2025, 48, 100777. [Google Scholar] [CrossRef]
- Moreno, M.; Arribas, A.S.; Bermejo, E.; Chicharro, M.; Zapardiel, A.; Rodríguez, M.C.; Jalit, Y.; Rivas, G.A. Selective Detection of Dopamine in the Presence of Ascorbic Acid Using Carbon Nanotube Modified Screen-Printed Electrodes. Talanta 2010, 80, 2149–2156. [Google Scholar] [CrossRef]
- Lv, M.; Wang, L.; Hou, Y.; Qiao, X.; Luo, X. A Wearable Antifouling Electrochemical Sensor Integrated with an Antimicrobial Microneedle Array for Uric Acid Detection in Interstitial Fluid. Anal. Chim. Acta 2025, 1339, 343610. [Google Scholar] [CrossRef]
- Dervisevic, M.; Dervisevic, E.; Esser, L.; Easton, C.D.; Cadarso, V.J.; Voelcker, N.H. Wearable Microneedle Array-Based Sensor for Transdermal Monitoring of PH Levels in Interstitial Fluid. Biosens. Bioelectron. 2023, 222, 114955. [Google Scholar] [CrossRef]
- Molinero-Fernandez, Á.; Wang, Q.; Xuan, X.; Konradsson-Geuken, Å.; Crespo, G.A.; Cuartero, M. Demonstrating the Analytical Potential of a Wearable Microneedle-Based Device for Intradermal CO 2 Detection. ACS Sens. 2024, 9, 361–370. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical Enzymatic Biosensors. Biosens. Bioelectron. 2017, 92, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Gao, F.; Zhang, J.; Wang, J.; Huang, Y. Overview on the Development of Electrochemical Immunosensors by the Signal Amplification of Enzyme- or Nanozyme-Based Catalysis Plus Redox Cycling. Molecules 2024, 29, 2796. [Google Scholar] [CrossRef] [PubMed]
- Cass, A.E.G.; Sharma, S. Microneedle Enzyme Sensor Arrays for Continuous In Vivo Monitoring. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2017; Volume 589, pp. 413–427. [Google Scholar]
- Caliò, A.; Dardano, P.; Di Palma, V.; Bevilacqua, M.F.; Di Matteo, A.; Iuele, H.; De Stefano, L. Polymeric Microneedles Based Enzymatic Electrodes for Electrochemical Biosensing of Glucose and Lactic Acid. Sens. Actuators B Chem. 2016, 236, 343–349. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device Integration of Electrochemical Biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Tariq, N.; Ashraf, M.W.; Tayyaba, S. A Review on Solid Microneedles for Biomedical Applications. J. Pharm. Innov. 2022, 17, 1464–1483. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of Bio-Electrochemical Sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Moonla, C.; Reynoso, M.; Casanova, A.; Chang, A.-Y.; Djassemi, O.; Balaje, A.; Abbas, A.; Li, Z.; Mahato, K.; Wang, J. Continuous Ketone Monitoring via Wearable Microneedle Patch Platform. ACS Sens. 2024, 9, 1004–1013. [Google Scholar] [CrossRef]
- Wang, J.; Guo, J.; Zhang, J.; Zhang, W.; Zhang, Y. RNA Aptamer-Based Electrochemical Aptasensor for C-Reactive Protein Detection Using Functionalized Silica Microspheres as Immunoprobes. Biosens. Bioelectron. 2017, 95, 100–105. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Ozalp, V.C.; Dincbal, U.; Arica, M.Y. Fast and Sensitive Detection of Salmonella in Milk Samples Using Aptamer-Functionalized Magnetic Silica Solid Phase and MCM-41-Aptamer Gate System. ACS Biomater. Sci. Eng. 2018, 4, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Cheng, Y.; Wang, J.; Lu, J.; Zhang, B.; Zhao, Y.; Gu, Z. Aptamer-Functionalized Barcode Particles for the Capture and Detection of Multiple Types of Circulating Tumor Cells. Adv. Mater. 2014, 26, 7333–7338. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Luan, C.; Liu, Y.; Chen, B.; Zhao, Y. Aptamer-Based Hydrogel Barcodes for the Capture and Detection of Multiple Types of Pathogenic Bacteria. Biosens. Bioelectron. 2018, 100, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Shaver, A.; Curtis, S.D.; Arroyo-Currás, N. Alkanethiol Monolayer End Groups Affect the Long-Term Operational Stability and Signaling of Electrochemical, Aptamer-Based Sensors in Biological Fluids. ACS Appl. Mater. Interfaces 2020, 12, 11214–11223. [Google Scholar] [CrossRef]
- Downs, A.M.; Plaxco, K.W. Real-Time, In Vivo Molecular Monitoring Using Electrochemical Aptamer Based Sensors: Opportunities and Challenges. ACS Sens. 2022, 7, 2823–2832. [Google Scholar] [CrossRef]
- Dauphin-Ducharme, P.; Yang, K.; Arroyo-Currás, N.; Ploense, K.L.; Zhang, Y.; Gerson, J.; Kurnik, M.; Kippin, T.E.; Stojanovic, M.N.; Plaxco, K.W. Electrochemical Aptamer-Based Sensors for Improved Therapeutic Drug Monitoring and High-Precision, Feedback-Controlled Drug Delivery. ACS Sens. 2019, 4, 2832–2837. [Google Scholar] [CrossRef]
- Friedel, M.; Werbovetz, B.; Drexelius, A.; Watkins, Z.; Bali, A.; Plaxco, K.W.; Heikenfeld, J. Continuous Molecular Monitoring of Human Dermal Interstitial Fluid with Microneedle-Enabled Electrochemical Aptamer Sensors. Lab Chip 2023, 23, 3289–3299. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Huang, R.; Wang, D.; Deng, D.; Zhang, Q.; Luo, L. The Applications of Electrochemical Immunosensors in the Detection of Disease Biomarkers: A Review. Molecules 2023, 28, 3605. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Xu, X.; Cui, X.; Chen, Y.; Yang, L. A Highly Sensitive Capillary-Based Immunosensor by Combining with Peroxidase Nanocomplex-Mediated Signal Amplification for Detection of Procalcitonin in Human Serum. ACS Omega 2019, 4, 6210–6217. [Google Scholar] [CrossRef]
- Zhu, G.; Yin, X.; Jin, D.; Zhang, B.; Gu, Y.; An, Y. Paper-Based Immunosensors: Current Trends in the Types and Applied Detection Techniques. TrAC Trends Anal. Chem. 2019, 111, 100–117. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Kausaite-Minkstimiene, A.; Ramanaviciene, A. Metal Nanoparticle and Quantum Dot Tags for Signal Amplification in Electrochemical Immunosensors for Biomarker Detection. Chemosensors 2021, 9, 85. [Google Scholar] [CrossRef]
- Gao, S.; Guisán, J.M.; Rocha-Martin, J. Oriented Immobilization of Antibodies onto Sensing Platforms—A Critical Review. Anal. Chim. Acta 2022, 1189, 338907. [Google Scholar] [CrossRef] [PubMed]
- Khumngern, S.; Jeerapan, I. Advances in Wearable Electrochemical Antibody-Based Sensors for Cortisol Sensing. Anal. Bioanal. Chem. 2023, 415, 3863–3877. [Google Scholar] [CrossRef]
- Beilen, J.B.; van Li, Z. Enzyme Technology: An Overview. Curr. Opin. Biotechnol. 2002, 13, 338–344. [Google Scholar] [CrossRef]
- Song, S.; Kim, Y.J.; Kang, H.-L.; Yoon, S.; Hong, D.-K.; Kim, W.-H.; Shin, I.-S.; Seong, W.K.; Lee, K.-N. Sensitivity Improvement in Electrochemical Immunoassays Using Antibody Immobilized Magnetic Nanoparticles with a Clean ITO Working Electrode. Biochip. J. 2020, 14, 308–316. [Google Scholar] [CrossRef]
- Zhu, D.D.; Tan, Y.R.; Zheng, L.W.; Lao, J.Z.; Liu, J.Y.; Yu, J.; Chen, P. Microneedle-Coupled Epidermal Sensors for In-Situ-Multiplexed Ion Detection in Interstitial Fluids. ACS Appl. Mater. Interfaces 2023, 15, 14146–14154. [Google Scholar] [CrossRef]
- Ye, S.; Feng, S.; Huang, L.; Bian, S. Recent Progress in Wearable Biosensors: From Healthcare Monitoring to Sports Analytics. Biosensors 2020, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; De Wael, K. Wearable Self-Powered Electrochemical Devices for Continuous Health Management. Adv. Funct. Mater. 2021, 31, 2107042. [Google Scholar] [CrossRef]
- Ben Halima, H.; Lakard, B.; Jaffrezic-Renault, N. Microneedle-Based Sensors for Wearable Diagnostics. Chemosensors 2025, 13, 68. [Google Scholar] [CrossRef]
- Yang, Y.; Sheng, C.; Dong, F.; Liu, S. An Integrated Wearable Differential Microneedle Array for Continuous Glucose Monitoring in Interstitial Fluids. Biosens. Bioelectron. 2024, 256, 116280. [Google Scholar] [CrossRef]
- Huang, L.; Tian, S.; Zhao, W.; Liu, K.; Ma, X.; Guo, J. Multiplexed Detection of Biomarkers in Lateral-Flow Immunoassays. Analyst 2020, 145, 2828–2840. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Liu, Q.; Wang, Q.; Qiu, H.; Li, H.; Xu, T. Fully Integrated Microneedle Biosensor Array for Wearable Multiplexed Fitness Biomarkers Monitoring. Biosens. Bioelectron. 2024, 265, 116697. [Google Scholar] [CrossRef]
- Gao, J.; Huang, W.; Chen, Z.; Yi, C.; Jiang, L. Simultaneous Detection of Glucose, Uric Acid and Cholesterol Using Flexible Microneedle Electrode Array-Based Biosensor and Multi-Channel Portable Electrochemical Analyzer. Sens. Actuators B Chem. 2019, 287, 102–110. [Google Scholar] [CrossRef]
- Ashraf, G.; Ahmed, K.; Aziz, A.; Asif, M.; Kong, J.; Fang, X. Microneedle Wearables in Advanced Microsystems: Unlocking next-Generation Biosensing with AI. TrAC Trends Anal. Chem. 2025, 187, 118208. [Google Scholar] [CrossRef]
- Kadian, S.; Sahoo, S.S.; Kumari, P.; Narayan, R.J. Machine Learning Enabled Onsite Electrochemical Detection of Lidocaine Using a Microneedle Array Integrated Screen Printed Electrode. Electrochim. Acta 2024, 475, 143664. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Gong, X.; Zheng, Y.; Yi, S.; Cheng, Y.; Li, Y.; Liu, B.; Xie, X.; Yi, C.; et al. Recent Progress in Microneedles-Mediated Diagnosis, Therapy, and Theranostic Systems. Adv. Heal. Mater. 2022, 11, 2102547. [Google Scholar] [CrossRef]
- Teymourian, H.; Tehrani, F.; Longardner, K.; Mahato, K.; Podhajny, T.; Moon, J.-M.; Kotagiri, Y.G.; Sempionatto, J.R.; Litvan, I.; Wang, J. Closing the loop for patients with Parkinson disease: Where are we? Nat. Rev. Neurol. 2022, 18, 497–507. [Google Scholar] [CrossRef]
- Parrilla, M.; Detamornrat, U.; Domínguez-Robles, J.; Tunca, S.; Donnelly, R.F.; De Wael, K. Wearable Microneedle-Based Array Patches for Continuous Electrochemical Monitoring and Drug Delivery: Toward a Closed-Loop System for Methotrexate Treatment. ACS Sens. 2023, 8, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, X.; Chen, G.; Che, J.; Zhang, D. Wearable Microneedle-Integrated Sensors for Household Health Monitoring. Eng. Regen. 2022, 3, 420–426. [Google Scholar] [CrossRef]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable Biosensors: An Alternative and Practical Approach in Healthcare and Disease Monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef]
- Duan, H.; Peng, S.; He, S.; Tang, S.; Goda, K.; Wang, C.H.; Li, M. Wearable Electrochemical Biosensors for Advanced Healthcare Monitoring. Adv. Sci. 2025, 12, e2411433. [Google Scholar] [CrossRef] [PubMed]
- Bequette, B.W. Continuous Glucose Monitoring: Real-Time Algorithms for Calibration, Filtering, and Alarms. J. Diabetes Sci. Technol. 2010, 4, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Maia, R.F.; Machado, P.; Rodrigues, R.O.; Faustino, V.; Schütte, H.; Gassmann, S.; Lima, R.A.; Minas, G. Recent Advances and Perspectives of MicroNeedles for Biomedical Applications. Biophys. Rev. 2025, 1–20. [Google Scholar] [CrossRef]
- Tucak, A.; Sirbubalo, M.; Hindija, L.; Rahić, O.; Hadžiabdić, J.; Muhamedagić, K.; Čekić, A.; Vranić, E. Microneedles: Characteristics, Materials, Production Methods and Commercial Development. Micromachines 2020, 11, 961. [Google Scholar] [CrossRef]
| Method | Resolution | Scalability | Material Compatibility | Reference |
|---|---|---|---|---|
| Photolithography | 3 μm | Pilot scale | Si wafer | [91] |
| PEGDA | [92] | |||
| Casting and molding | 3 μm | Industrial scale | SWCNT | [93] |
| liquid PI | [94] | |||
| 3D printing | 2 μm | Pilot scale | Bio resin | [95] |
| Clear light-sensitive resin | [96] | |||
| Laser cutting | 1.06 μm | Pilot scale | PI film | [97] |
| Stainless steel | [98] |
| Receptor Type | Target Analytes | Linear Range | Advantages | Disadvantages | Lab to Commercial Translation | Reference |
|---|---|---|---|---|---|---|
| Direct redox | DA, EP, NEP | 0.5–100 µM, 0.5–75 µM, 0.5–75 µM | Eco-friendly, low-cost, and highly reproducible electrode modification process | Difficulty in individual quantification of DA, EP, and NEP | Requires future work for wireless integration and in vivo deployment | [125] |
| Serotonin (5-HT) | 0–95 μM | Simple fabrication process | Difficulty in ensuring manufacturing consistency | Wearable potential demonstrated, but no in vivo validation yet | [74] | |
| UA | 0–500 μM | Anti-biofouling and reusable | Limited scalability and insufficient process automation | Despite limited commercialization, precision manufacturing via 3D printing and related techniques proposed | [126] | |
| Enzyme | Glucose | 0–20 mM | Low cost and good portability | Reduced sensitivity at high glucose and limited practicality due to electrode integration | Low-cost and validated in lab, yet electrode integration and fabrication hurdles limit clinical translation. | [127] |
| Cholesterol | 1–15 mM (in aISF) | High selectivity and long-term stability | Stabilizing materials required to maintain enzyme activity and prevent leaching | Lab performance validated, future wearable integration suggested | [128] | |
| β-lactam antibiotic | approximately 10–800 μM | High specificity, stability after sterilization, and good storage stability | Sensitivity reduction due to initial enzyme leaching | In vivo testing demonstrates feasibility, but further sensitivity optimization needed for clinical deployment | [129] | |
| Aptamer | Glucose, Lactate | 0–50 mM, 0–20 mM | High sensitivity and specificity in real skin environments | Multiplex detection via two sensors, and sensor fabrication complexity | Testing on human skin or subjects for in vivo monitoring | [130] |
| Cortisol | 1–1000 nM | High performance, stability, repeatability, and immunity to interference | Multi-step sensor fabrication | Long-term in vivo testing for continuous monitoring | [131] | |
| Vancomycin | 6–42 μM (clinical window) | Biocompatible, sterilizable and stable | Small redox currents due to small working electrode surface area | Translation into in vivo and enhancement of surface area for accuracy and multiplexing | [39] | |
| Antibody | HER2 | 10–250 ng/mL (in aISF) | Dual-function platform with high sensitivity and specificity | Structural instability of the SAM and high fabrication cost | Lab performance validated and in vivo application suggested | [132] |
| IL-6, IL-1β, TNF-α | 1–5000 pg/mL | High sensitivity, specificity, and stable performance | High cost and limited long-term storage | Difficult to scale up due to antibody immobilization and in vivo application limitations | [133] | |
| IL-6 | 0–60 pg/mL | High specificity without the need for complex surface modification | Structural instability of the SAM and high fabrication cost | Lab performance validated and in vivo application suggested | [134] | |
| MIP | IL-6 | 1 pg/mL–10 ng/mL | High reusability and low production cost | Lack of binding site precision | Scalable, low-cost platform suitable for POC, but lacks in vivo validation and multiplex capacity | [135] |
| Chemo-responsive probe | Tyr | 0.3–0.7 mg/mL | Reusability enabled via probe regeneration by CV | Manual fabrication limits scalability | Lab performance validated, but not yet ready for mass production | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, S.; Choi, M.Y.; Kim, M.J.; Sim, S.B.; Haizan, I.; Choi, J.-H. Electrochemical Microneedles for Real-Time Monitoring in Interstitial Fluid: Emerging Technologies and Future Directions. Biosensors 2025, 15, 380. https://doi.org/10.3390/bios15060380
Cha S, Choi MY, Kim MJ, Sim SB, Haizan I, Choi J-H. Electrochemical Microneedles for Real-Time Monitoring in Interstitial Fluid: Emerging Technologies and Future Directions. Biosensors. 2025; 15(6):380. https://doi.org/10.3390/bios15060380
Chicago/Turabian StyleCha, Suhyeon, Min Yu Choi, Min Jung Kim, Sang Baek Sim, Izzati Haizan, and Jin-Ha Choi. 2025. "Electrochemical Microneedles for Real-Time Monitoring in Interstitial Fluid: Emerging Technologies and Future Directions" Biosensors 15, no. 6: 380. https://doi.org/10.3390/bios15060380
APA StyleCha, S., Choi, M. Y., Kim, M. J., Sim, S. B., Haizan, I., & Choi, J.-H. (2025). Electrochemical Microneedles for Real-Time Monitoring in Interstitial Fluid: Emerging Technologies and Future Directions. Biosensors, 15(6), 380. https://doi.org/10.3390/bios15060380





