Nonlinear Imaging Detection of Organ Fibrosis in Minute Samples for Early Stage Utilizing Dual-Channel Two-Photon and Second-Harmonic Excitation
Abstract
1. Introduction
2. Materials and Methods
2.1. The EVG (Elastica Van Gieson) Staining
2.2. Identify Regions of Interest by Grid-Targeted Method
2.3. Preparation of Collagen and Elastin Fiber Samples
3. Results
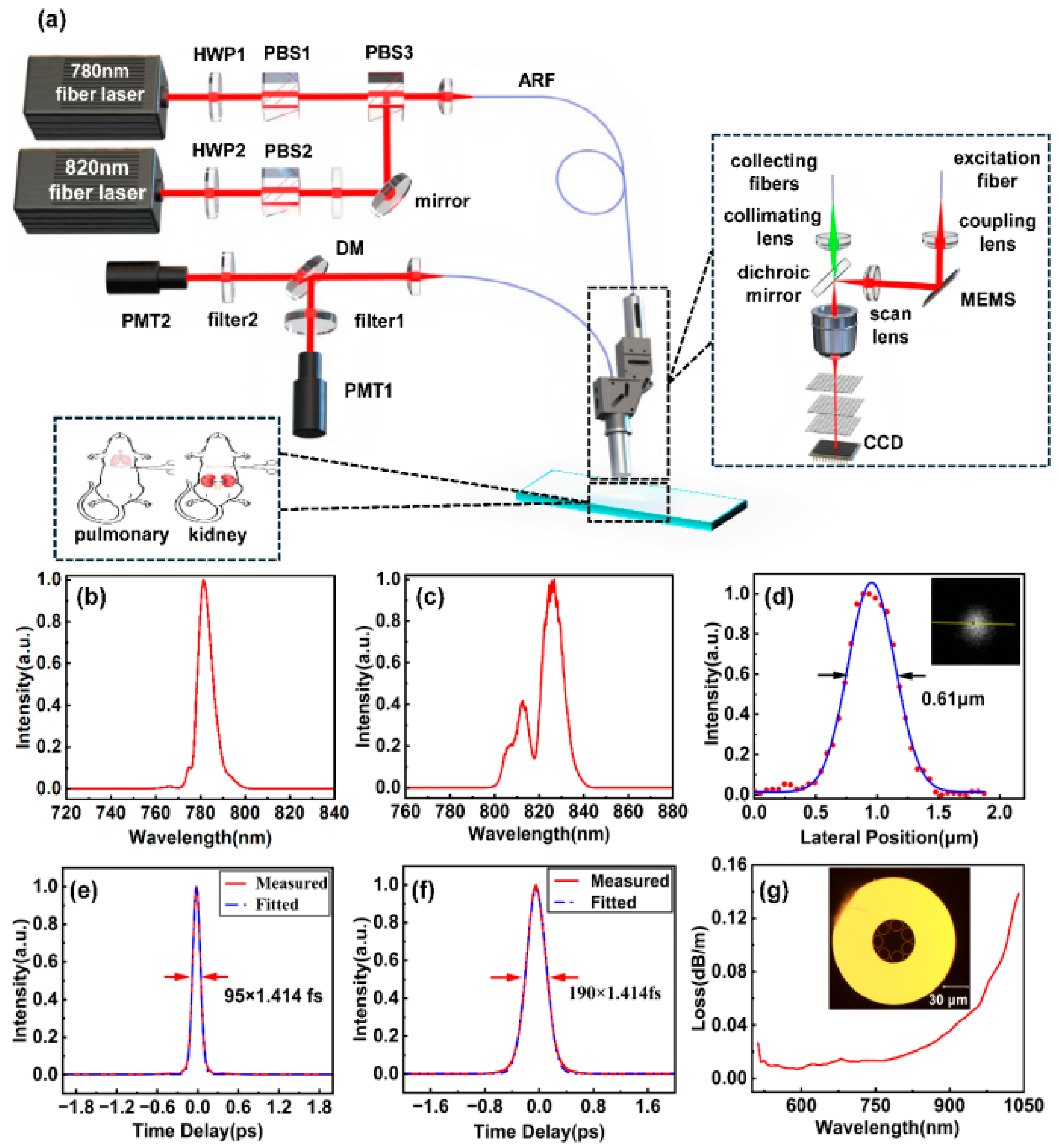
3.1. Imaging System Setup and Configuration
3.2. Label-Free Visualization of Collagen and Elastin Fibers Utilizing Multiphoton Microscopy
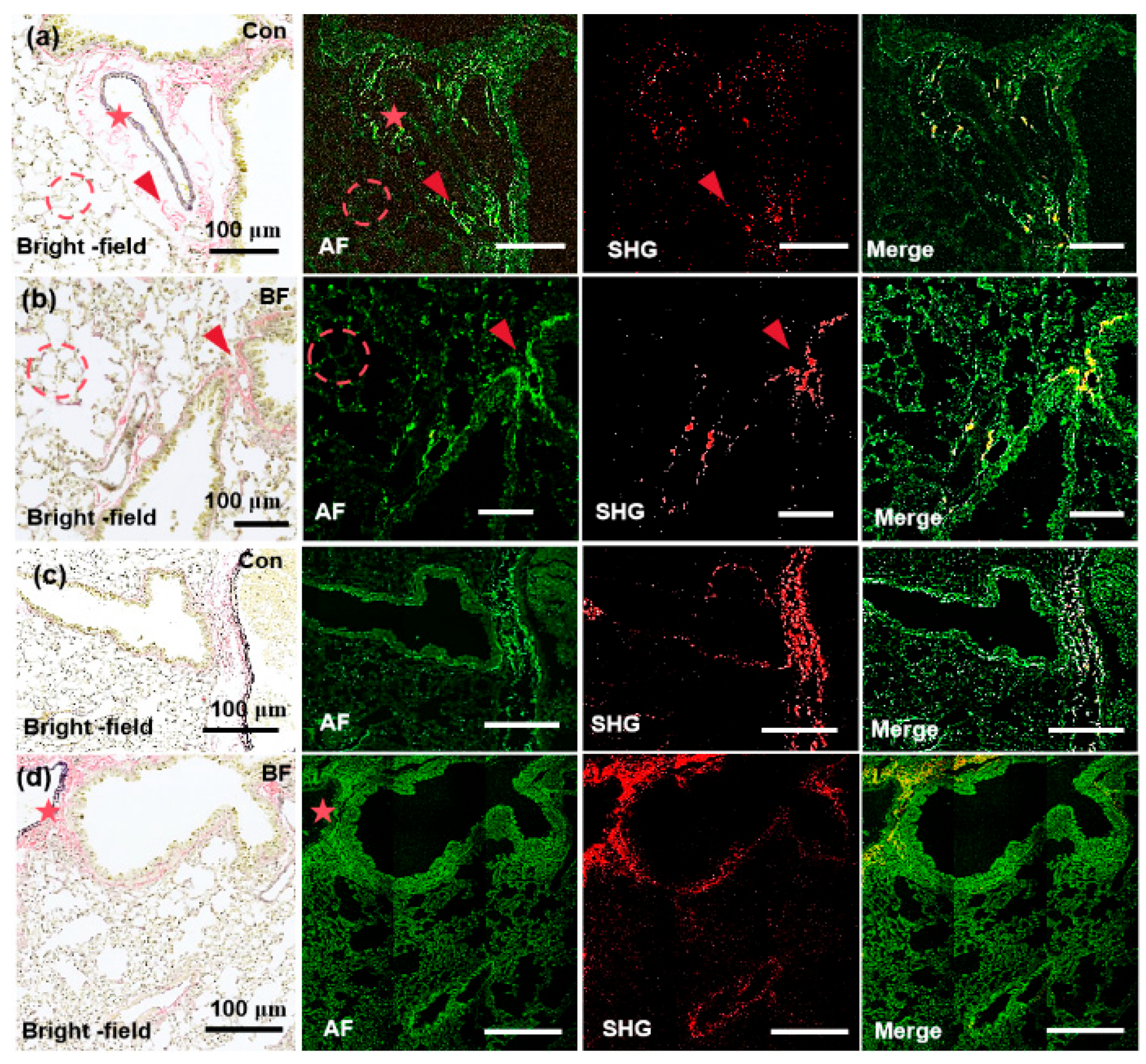
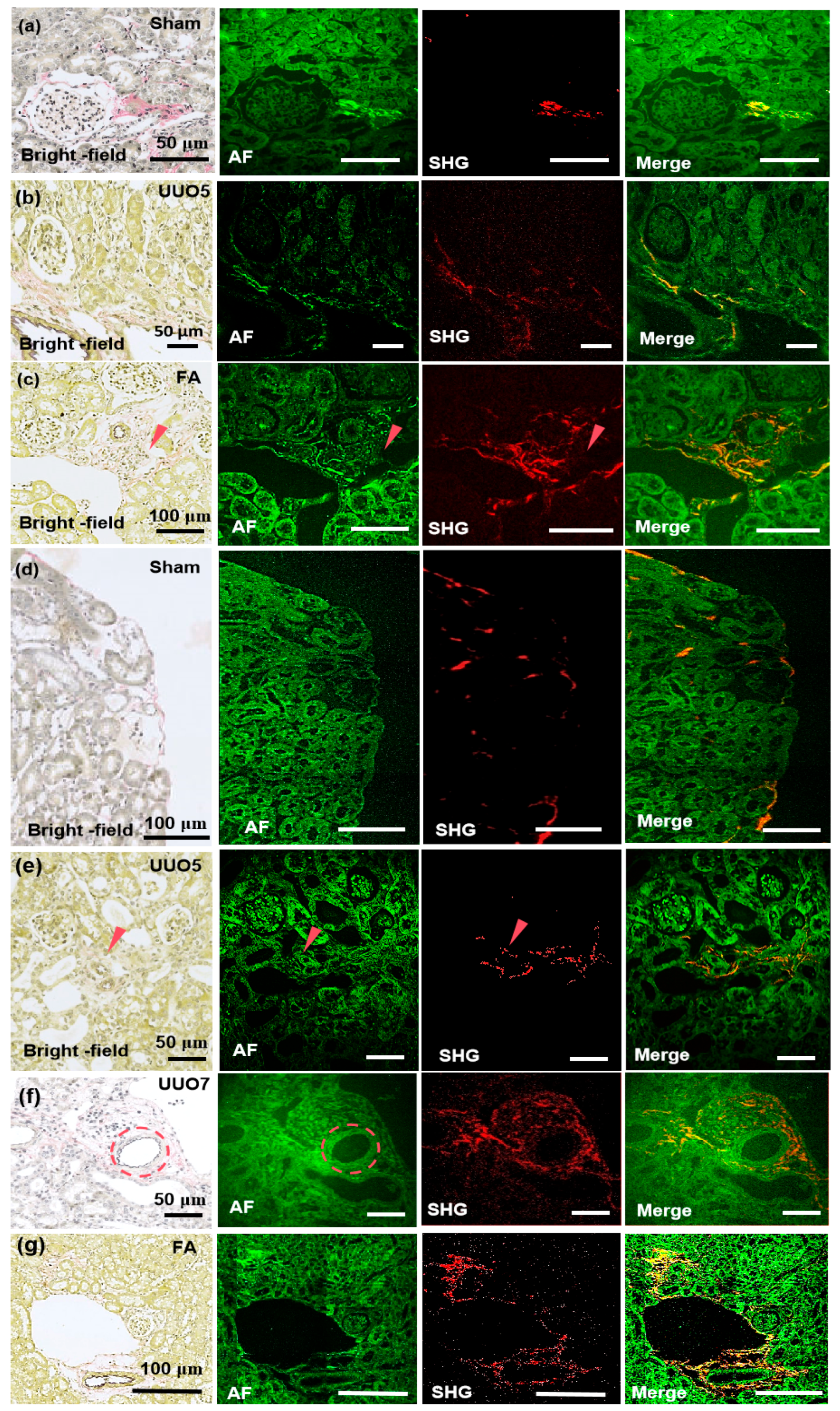
3.3. Detection of Renal Fibrosis and Pulmonary Fibrosis Utilizing the MPM System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Autofluorescence |
| ARF | Antiresonant Fiber |
| CCD | Charge Coupled Device |
| ECM | Extracellular Matrix |
| EVG | Elastica van Gieson |
| MEMS | Micro-Electromechanical Systems |
| MPM | Multiphoton microscopy |
| NA | Numerical Aperture |
| PBS | Polarization Beam Splitter |
| PMT | Photomultiplier Tube |
| ROI | Regions Of Interest |
| SHG | Second Harmonic Generation |
| THG | Third-Harmonic Generation |
| TIFF | Tagged Image File Format |
| TPEF | Two-Photon Excitation Fluorescence |
| UUO | Unilateral Ureteral Obstruction |
| UV | Ultraviolet |
References
- Jun, J.I.; Lau, L.F. Resolution of organ fibrosis. J. Clin. Investig. 2018, 128, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Lurje, I.; Gaisa, N.T.; Weiskirchen, R.; Tacke, F. Mechanisms of organ fibrosis: Emerging concepts and implications for novel treatment strategies. Mol. Aspects Med. 2023, 92, 101191. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Pardo, A. From pulmonary fibrosis to progressive pulmonary fibrosis: A lethal pathobiological jump. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 321, L600–L607. [Google Scholar] [CrossRef] [PubMed]
- Farris, A.B.; Vizcarra, J.; Amgad, M.; Cooper, L.A.D.; Gutman, D.; Hogan, J. Image Analysis pipeline for renal allograft evaluation and fibrosis quantification. Kidney Int. Rep. 2021, 6, 1878–1887. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Yamada, G.; Vuppalanchi, R.; Van Natta, M.; Loomba, R.; Guy, C.; Brandman, D.; Tonascia, J.; Chalasani, N.; Neuschwander-Tetri, B.; et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin. Gastroenterol. Hepatol. 2019, 17, 1877–1885. [Google Scholar] [CrossRef]
- Collins, B.F.; Luppi, F. Diagnosis and management of fibrotic interstitial lung diseases. Clin. Chest Med. 2021, 42, 321–335. [Google Scholar] [CrossRef]
- Farris, A.B.; Chan, S.; Climenhaga, J.; Adam, B.; Bellamy, C.O. Banff fibrosis study: Multicenter visual assessment and computerized analysis of interstitial fibrosis in kidney biopsies. Am. J. Transplant. 2014, 14, 897–907. [Google Scholar] [CrossRef]
- Chen, Y.; He, H.; Luo, L.; Liu, K.; Jiang, M.; Liu, Q. Studying pulmonary fibrosis due to microbial infection via automated microscopic image analysis. Front. Microbiol. 2023, 14, 1176339. [Google Scholar] [CrossRef]
- Agrawal, G.P. Nonlinear fiber optics: Its history and recent progress [Invited]. J. Opt. Soc. Am. B 2011, 28, A1–A10. [Google Scholar] [CrossRef]
- Baues, M.; Dasgupta, A.; Ehling, J.; Prakash, J.; Boor, P.; Lammers, T. Fibrosis imaging: Current concepts and future directions. Adv. Drug Deliv. Rev. 2017, 121, 9–26. [Google Scholar] [CrossRef]
- Hermsen, M.; Bel, T.D.; Boer, M.D.; Steenbergen, E.J.; van der Laak, J.A. Deep learning–Based histopathologic assessment of kidney tissue. J. Am. Soc. Nephrol. 2019, 30, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liu, F.; Fu, H.; Mao, J. Advances in imaging techniques to assess kidney fibrosis. Ren. Fail. 2023, 45, 2171887. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, S.; Dobrinskikh, E.; Montford, J.; Dvornikov, A.; Lehman, A.; Furgeson, S. Label-free fluorescence lifetime and second harmonic generation imaging microscopy improves quantification of experimental renal fibrosis. Kidney Int. 2016, 90, 1123–1128. [Google Scholar] [CrossRef]
- Bhuiyan, S.; Widdop, R.E.; Samuel, C.S. Determination of interstitial collagen deposition and related topography using the second harmonic generation-based histoIndex platform. Methods Mol. Biol. 2023, 2664, 173–184. [Google Scholar]
- Bhuiyan, S.; Shen, M.; Chelvaretnam, S.; Tan, A.Y.; Ho, G.; Samuel, C.S. Assessment of renal fibrosis and anti-fibrotic agents using a novel diagnostic and stain-free second-harmonic generation platform. FASEB. J. 2021, 35, e21595. [Google Scholar] [CrossRef]
- Saitou, T.; Takanezawa, S.; Ninomiya, H.; Watanabe, T.; Yamamoto, S.; Hiasa, Y.; Imamura, T. Tissue intrinsic fluorescence spectra-based digital pathology of liver fibrosis by marker-controlled segmentation. Front. Med. 2018, 5, 00350. [Google Scholar] [CrossRef]
- Durgam, S.; Singh, B.; Cole, S.L.; Brokken, M.T.; Stewart, M. Quantitative assessment of tendon hierarchical structure by combined second harmonic generation and immunofluorescence microscopy. Tissue Eng. Part C Methods 2020, 26, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Islam, M.N.; Boostanpour, K.; Aran, D.; Jin, G.; Christenson, S.; Matthay, M.A.; Eckalbar, W.L.; DePianto, D.J.; Arron, J.R.; et al. Molecular programs of fibrotic change in aging human lung. Nat. Commun. 2021, 12, 6309. [Google Scholar] [CrossRef]
- James, D.S.; Brereton, C.J.; Davies, D.E.; Jones, M.G.; Campagnola, P.J. Examining lysyl oxidase-like modulation of collagen architecture in 3D spheroid models of idiopathic pulmonary fibrosis via second-harmonic generation microscopy. J. Biomed. Opt. 2021, 26, 066501. [Google Scholar] [CrossRef]
- Fan, N.; Wu, C.; Zhou, Y.; Wang, X.; Li, P.; Liu, Z.; Tang, B. Rapid two-photon fluorescence imaging of monoamine oxidase b for diagnosis of early-stage liver fibrosis in mice. Anal. Chem. 2021, 93, 7110–7117. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, J.; Xu, J.; Zheng, C.; Niu, Y.; Wang, C.; Xu, P. A Smart Fluorescent Probe for NO Detection and Application in Myocardial Fibrosis Imaging. Analytical chemistry. Anal. Chem. 2020, 92, 5064–5072. [Google Scholar] [CrossRef] [PubMed]
- Pantazis, P.; Maloney, J.; Wu, D.; Fraser, S.E. Second harmonic generating (SHG) nanoprobes for in vivo imaging. Proc. Natl. Acad. Sci. USA 2010, 107, 14535–14540. [Google Scholar] [CrossRef] [PubMed]
- Scheinfeld, N. Nephrogenic fibrosing dermopathy: A comprehensive review for the dermatologist. Am. J. Clin. Dermatol. 2006, 7, 237–247. [Google Scholar] [CrossRef]
- Sun, Q.; Baues, M.; Klinkhammer, B.M.; Ehling, J.; Djudjaj, S.; Drude, N.I.; Boor, P. Elastin imaging enables noninvasive staging and treatment monitoring of kidney fibrosis. Sci. Transl. Med. 2019, 11, aat4865. [Google Scholar] [CrossRef]
- Chen, W.; Yan, X.; Xu, A.; Sun, Y.; Wang, B.; Huang, T. Dynamics of elastin in liver fibrosis: Accumulates late during progression and degrades slowly in regression. J. Cell. Physiol. 2019, 234, 22613–22622. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, D.A.; Lefort, C.; Steffenhagen, M.; Mansuryan, T.; Ducourthial, G. Development of a nonlinear fiber-optic spectrometer for human lung tissue exploration. Biomed. Opt. Express 2012, 3, 840–853. [Google Scholar] [CrossRef]
- Hall, G.; Tilbury, K.B.; Campbell, K.R.; Eliceiri, K.W.; Campagnola, P.J. Experimental and simulation study of the wavelength dependent second harmonic. Opt. Lett. 2014, 39, 1897–1900. [Google Scholar] [CrossRef]
- Thornhill, P.D. Separation of a series of chromophores and fluorophores present in elastin. Biochem. J. 1975, 147, 215–219. [Google Scholar] [CrossRef]
- Zipfel, W.R.; Williams, R.M.; Christie, R.; Nikitin, A.Y.; Hyman, B.T.; Webb, W.W. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl. Acad. Sci. USA 2003, 100, 7075–7080. [Google Scholar] [CrossRef]
- Cheng, Q.D.; Chung, H.Y.; Schubert, R.; Chia, S.H.; Falke, S.; Mudogo, C.N.; Betzel, C. Protein-crystal detection with a compact multimodal multiphoton microscope. Commun. Biol. 2021, 3, 569. [Google Scholar] [CrossRef]
- Mikami, H.; Gao, L.; Goda, K. Ultrafast optical imaging technology: Principles and applications of emerging methods. Nanophotonics 2016, 5, 497–507. [Google Scholar] [CrossRef]
- Aghigh, A.; Bancelin, S.; Rivard, M.; Pinsard, M.; Akutsu, A.; Ibrahim, H. Second harmonic generation microscopy: A powerful tool for bio-imaging. Biophys. Rev. 2023, 15, 43–70. [Google Scholar] [CrossRef] [PubMed]
- Zach, A.; Mohseni, M.; Polzer, C.; Nicholson, J.W.; Hellerer, T. All-fiber widely tunable ultrafast laser source for multimodal imaging in nonlinear microscopy. Opt. Lett. 2019, 44, 5218–5221. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Batjargal, O.; Cromey, B.; Kieu, K. All-fiber high-power 1700 nm femtosecond laser based on optical parametric chirped-pulse amplification. Opt. Express 2020, 28, 2317–2325. [Google Scholar] [CrossRef]
- Kiriyama, H.; Mori, M.; Nakai, Y.; Yamamoto, Y.; Tanoue, M.; Akutsu, A.; Miyanaga, N. High-energy, high-contrast, multiterawatt laser pulses by optical parametric chirped-pulse amplification. Opt. Lett. 2007, 32, 2315–2317. [Google Scholar] [CrossRef]
- Gade, P.S.; Robertson, A.M.; Chuang, C.Y. Multiphoton imaging of collagen, elastin, and calcification in intact soft-tissue samples. Curr. Protoc. Cytom. 2018, 128, e51. [Google Scholar] [CrossRef]
- Kazlouskaya, V.; Malhotra, S.; Lambe, J.; Idriss, M.H.; Elston, D.; Andres, C. The utility of elastic Verhoeff-Van Gieson staining in dermatopathology. Pathology 2012, 40, 211–225. [Google Scholar] [CrossRef]
- Park, C.Y.; Marando, C.M.; Liao, J.A.; Lee, J.K.; Kwon, J.; Chuck, R.S. Details of the collagen and elastin architecture in the human limbal conjunctiva, tenon’s capsule and sclera revealed by two-photon excited fluorescence microscopy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5602–5610. [Google Scholar] [CrossRef]
- Safaee, M.M.; Nishitani, S.; Mcfarlane, I.R.; Yang, S.J.; Sun, E.; Landry, M.P. Dual infrared 2-photon microscopy achieves minimal background deep tissue imaging in brain and plant tissues. Adv. Funct. Mater. 2024, 34, 202404709. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Niu, G.; Wu, F.; Yan, L.; Zhang, H.; Zhao, J. Lysosome-targetable polythiophene nanoparticles for two-photon excitation photodynamic therapy and deep tissue imaging. J. Mater. Chem. B 2017, 5, 3651–3657. [Google Scholar]
- Stringari, C.; Abdeladim, L.; Malkinson, G.; Mahou, P.; Beaurepaire, E. Multicolor two-photon imaging of endogenous fluorophores in living tissues by wavelength mixing. Sci. Rep. 2017, 7, 3792. [Google Scholar] [CrossRef] [PubMed]
- Alberini, R.; Spagnoli, A.; Sadeghinia, M.J.; Skallerud, B.; Terzano, M.; Holzapfel, G.A. Second harmonic generation microscopy, biaxial mechanical tests and fiber dispersion models in human skin biomechanics. Acta Biomater. 2024, 185, 266–280. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.-S.; Cheng, Q.-D.; Liu, Y.-Z.; Zhang, R.; Li, D.-W.; Wang, A.-M.; Feng, L.-S.; Jia, X. Nonlinear Imaging Detection of Organ Fibrosis in Minute Samples for Early Stage Utilizing Dual-Channel Two-Photon and Second-Harmonic Excitation. Biosensors 2025, 15, 357. https://doi.org/10.3390/bios15060357
Yu B-S, Cheng Q-D, Liu Y-Z, Zhang R, Li D-W, Wang A-M, Feng L-S, Jia X. Nonlinear Imaging Detection of Organ Fibrosis in Minute Samples for Early Stage Utilizing Dual-Channel Two-Photon and Second-Harmonic Excitation. Biosensors. 2025; 15(6):357. https://doi.org/10.3390/bios15060357
Chicago/Turabian StyleYu, Bo-Song, Qing-Di Cheng, Yi-Zhou Liu, Rui Zhang, Da-Wei Li, Ai-Min Wang, Li-Shuang Feng, and Xiao Jia. 2025. "Nonlinear Imaging Detection of Organ Fibrosis in Minute Samples for Early Stage Utilizing Dual-Channel Two-Photon and Second-Harmonic Excitation" Biosensors 15, no. 6: 357. https://doi.org/10.3390/bios15060357
APA StyleYu, B.-S., Cheng, Q.-D., Liu, Y.-Z., Zhang, R., Li, D.-W., Wang, A.-M., Feng, L.-S., & Jia, X. (2025). Nonlinear Imaging Detection of Organ Fibrosis in Minute Samples for Early Stage Utilizing Dual-Channel Two-Photon and Second-Harmonic Excitation. Biosensors, 15(6), 357. https://doi.org/10.3390/bios15060357




