Sensing of Volatile Organic Compounds by Haller’s Structure in Ixodidae Tick: Electroscutumography and Olfactometric Bioassay
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Culture of Ticks
2.2. Stimulants
2.3. Electrophysiological Assay by ESG Recording
2.4. Statistical Analysis
2.5. Microscopic Analysis
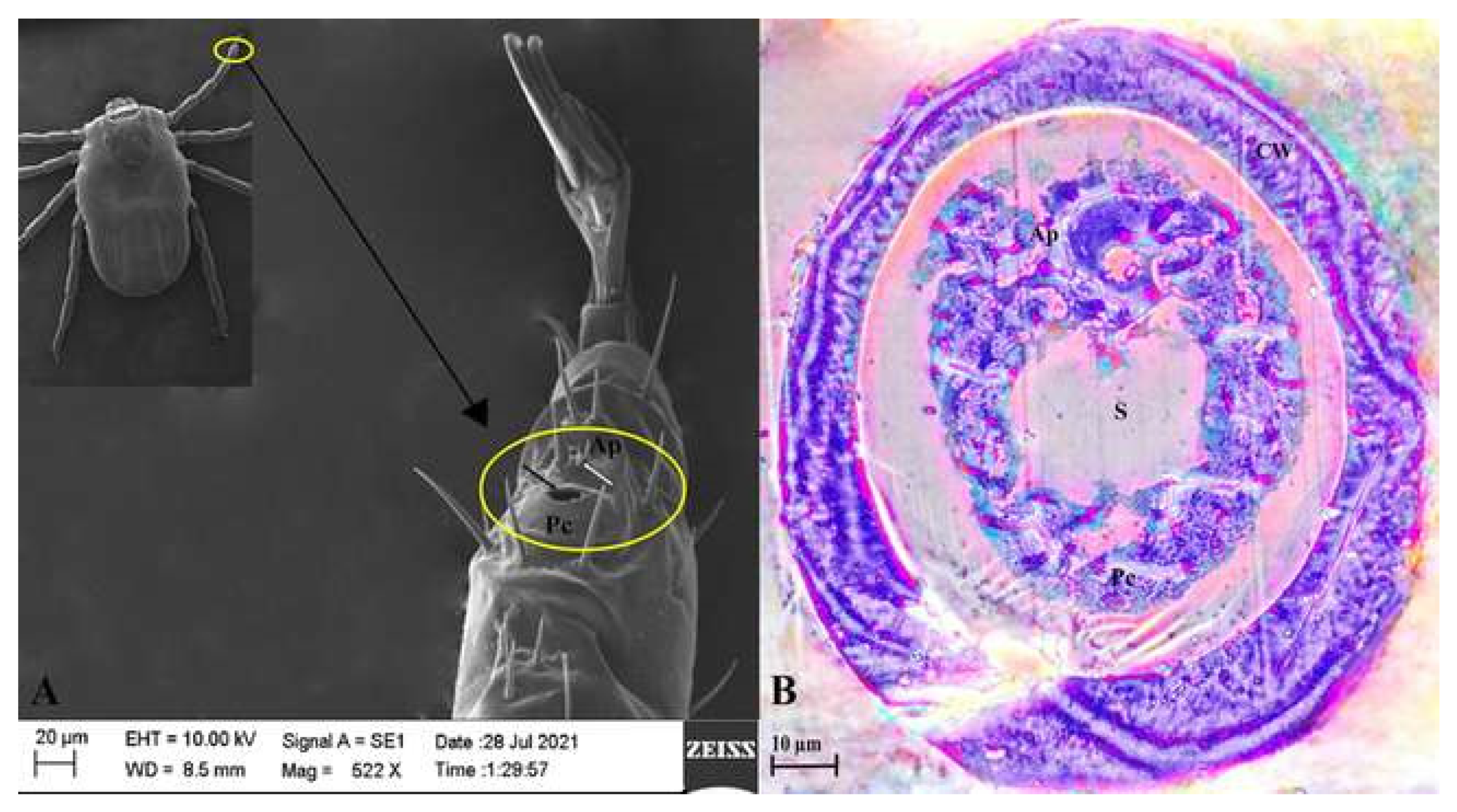
2.5.1. SEM
2.5.2. Light Microscope
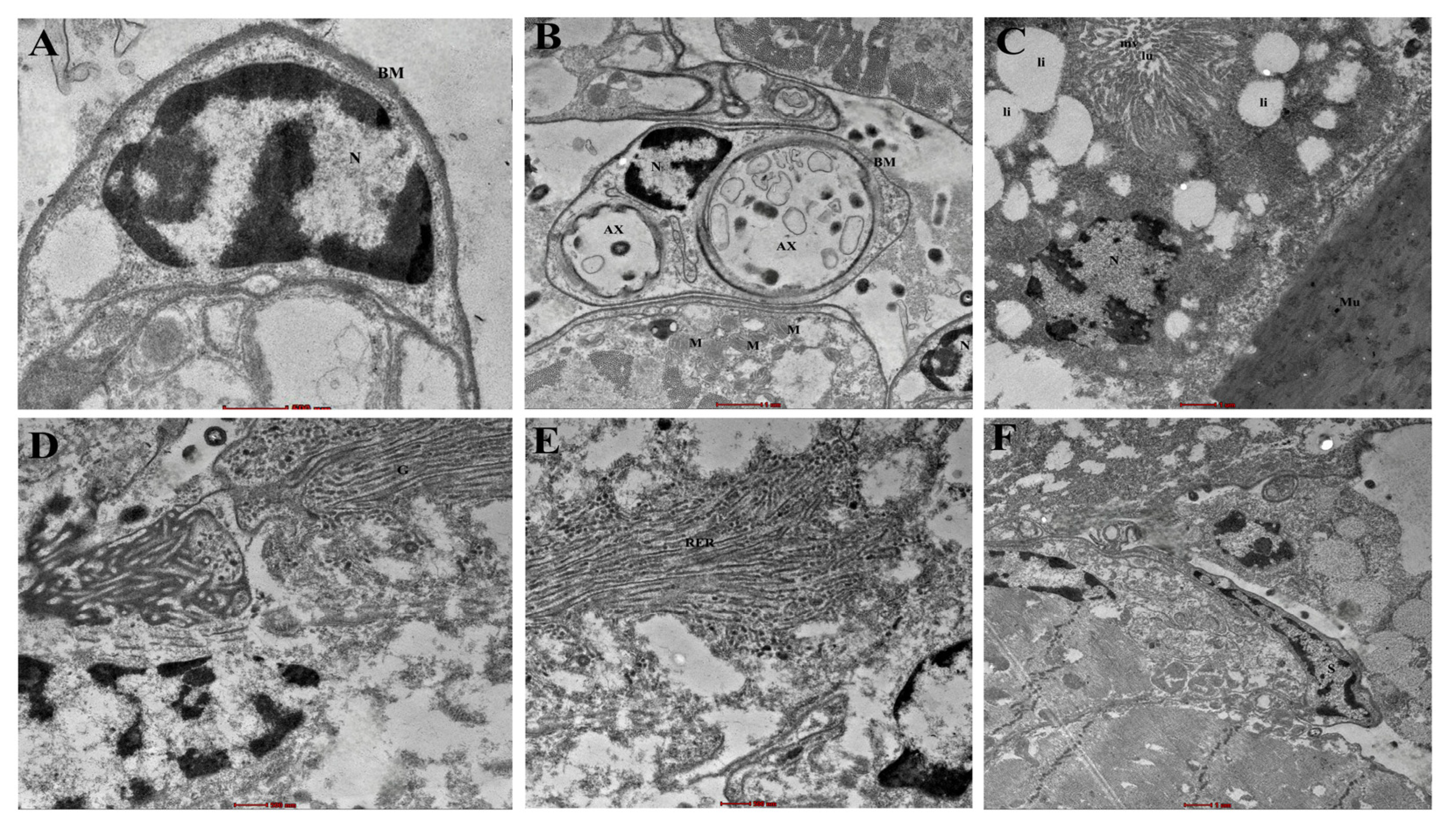
2.5.3. TEM
2.6. Olfactometry
2.6.1. Multichannel Olfactometer
2.6.2. Y-Tube Olfactometer
3. Results
3.1. Structural Anatomy of Haller’s Organ: SEM, Light Microscopy, and TEM Observations
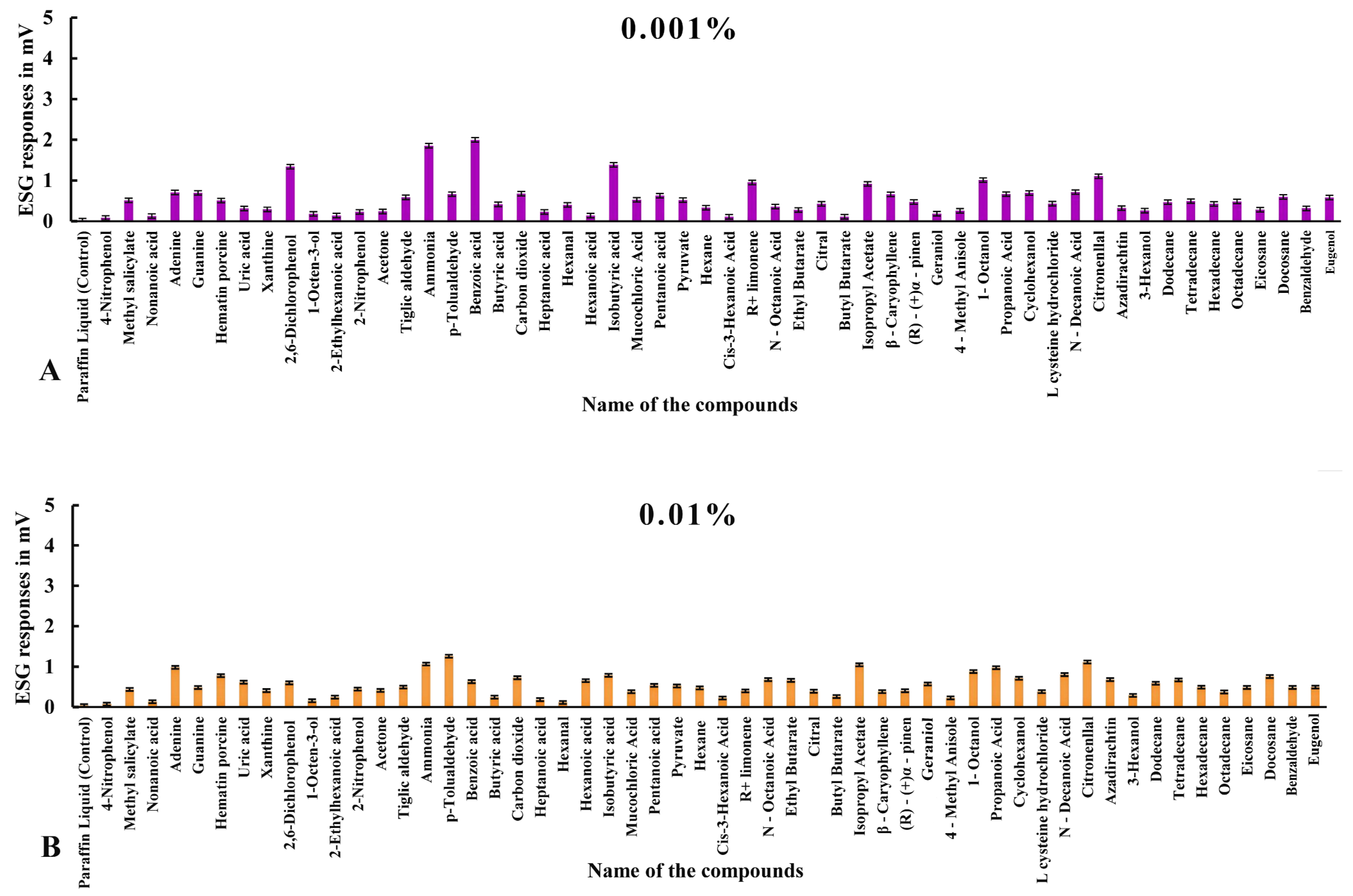
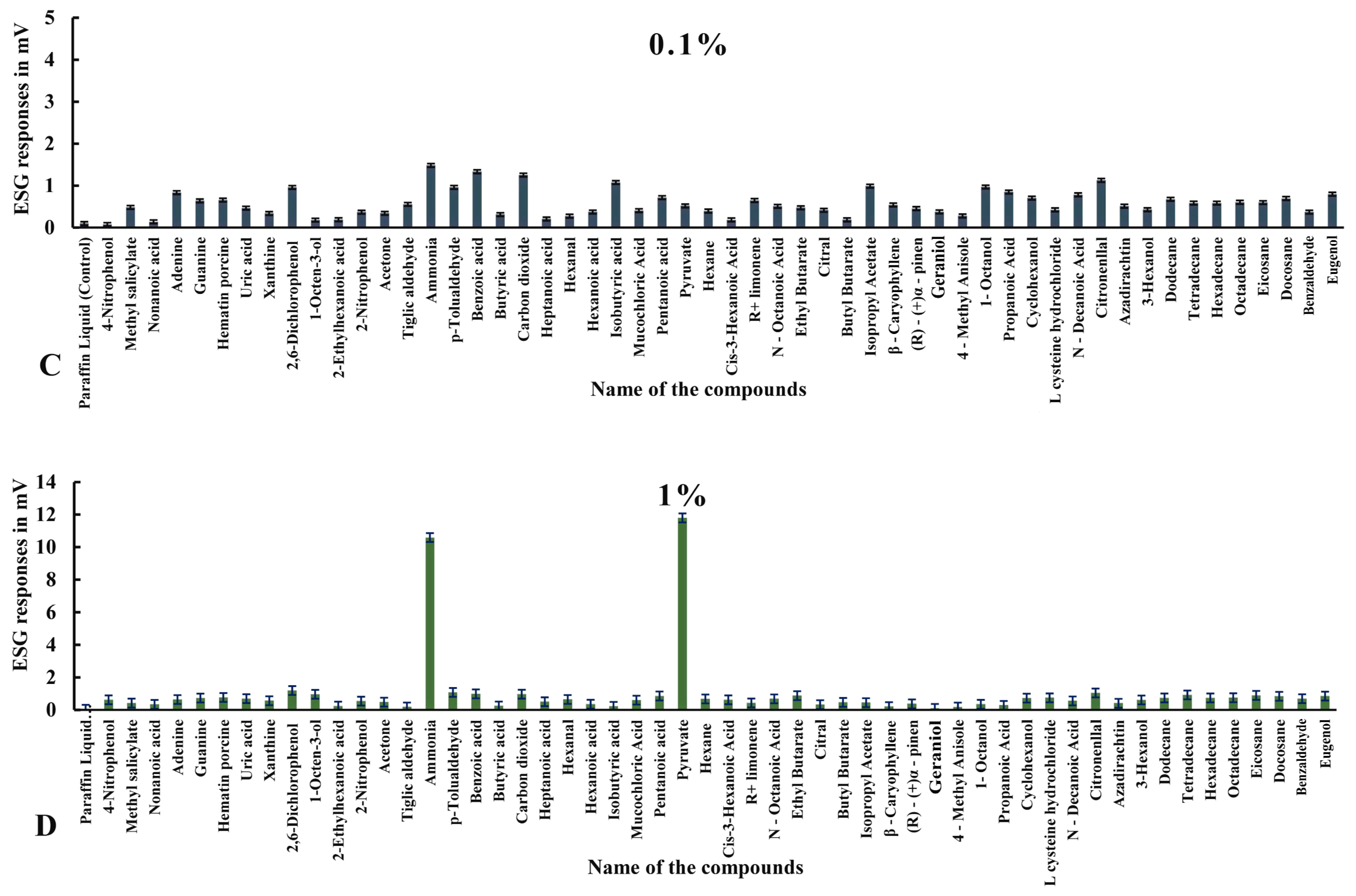
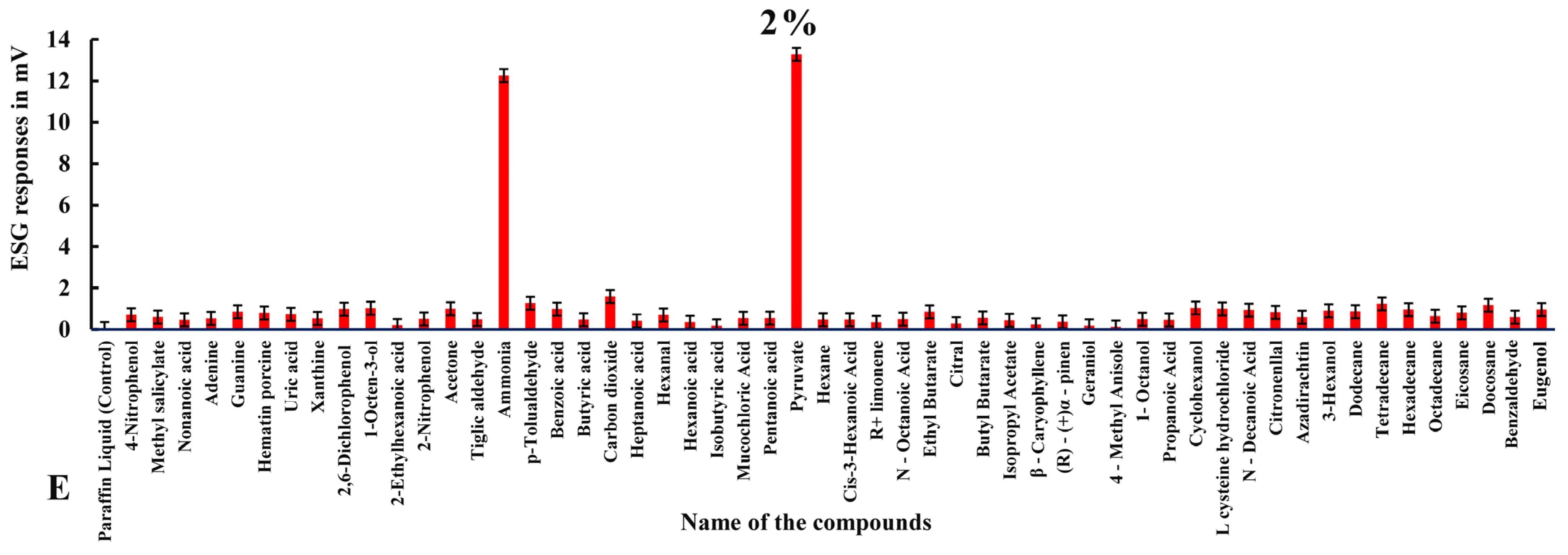
3.2. ESG Dose–Response Studies in Haemaphysalis darjeeling
3.2.1. ESG Responses to Compounds in Ticks with Intact Legs
3.2.2. ESG Responses to Compounds in Ticks with Surgically Removed Legs
3.3. One-Way ANOVA and Post Hoc Tukey Test in Ticks with Intact Legs
3.3.1. One-Way ANOVA Between Different Conditions
3.3.2. Determination of R-Squared Regression Values
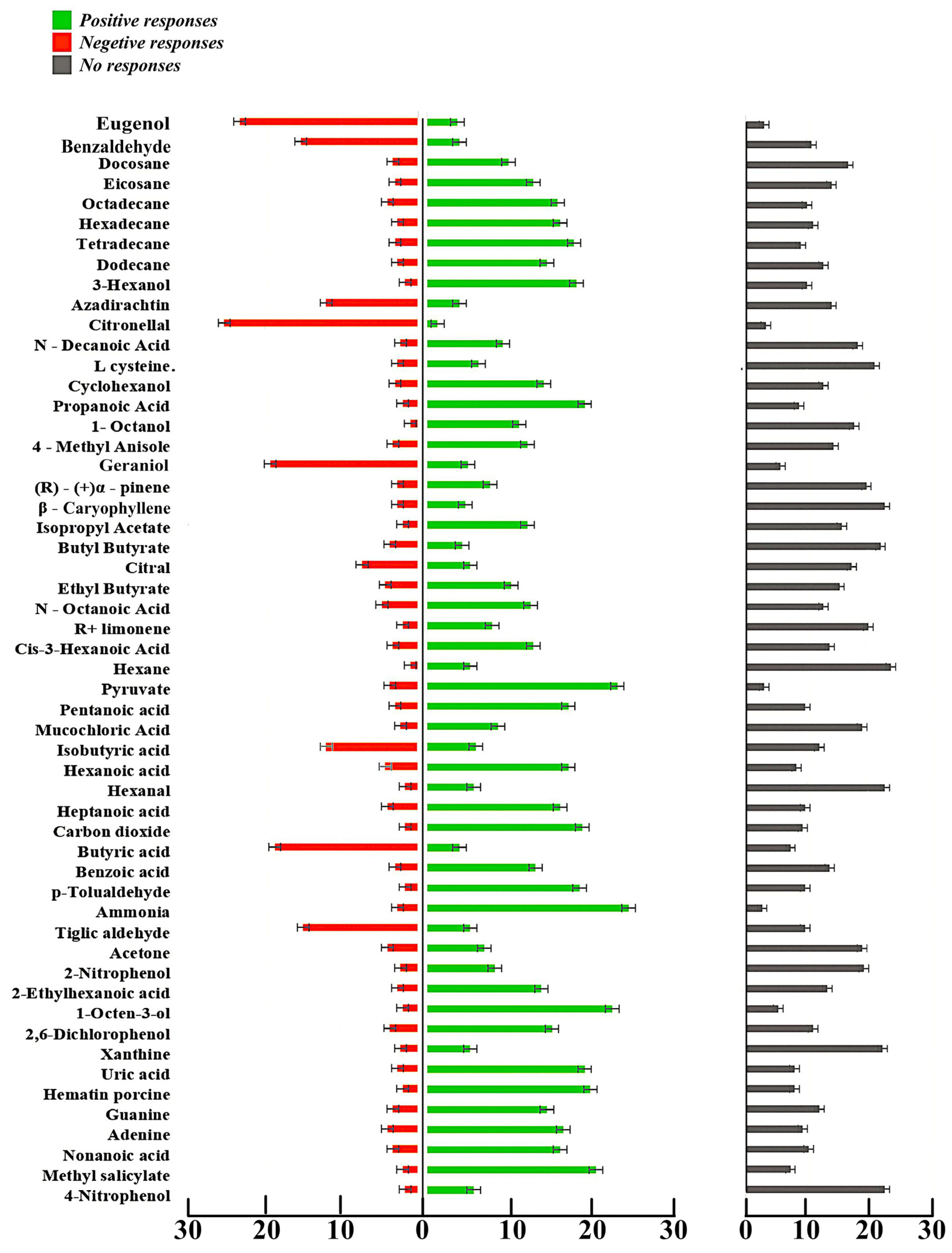
3.4. Olfactometric Bioassays
3.4.1. Multi-Arm Olfactometric Assay
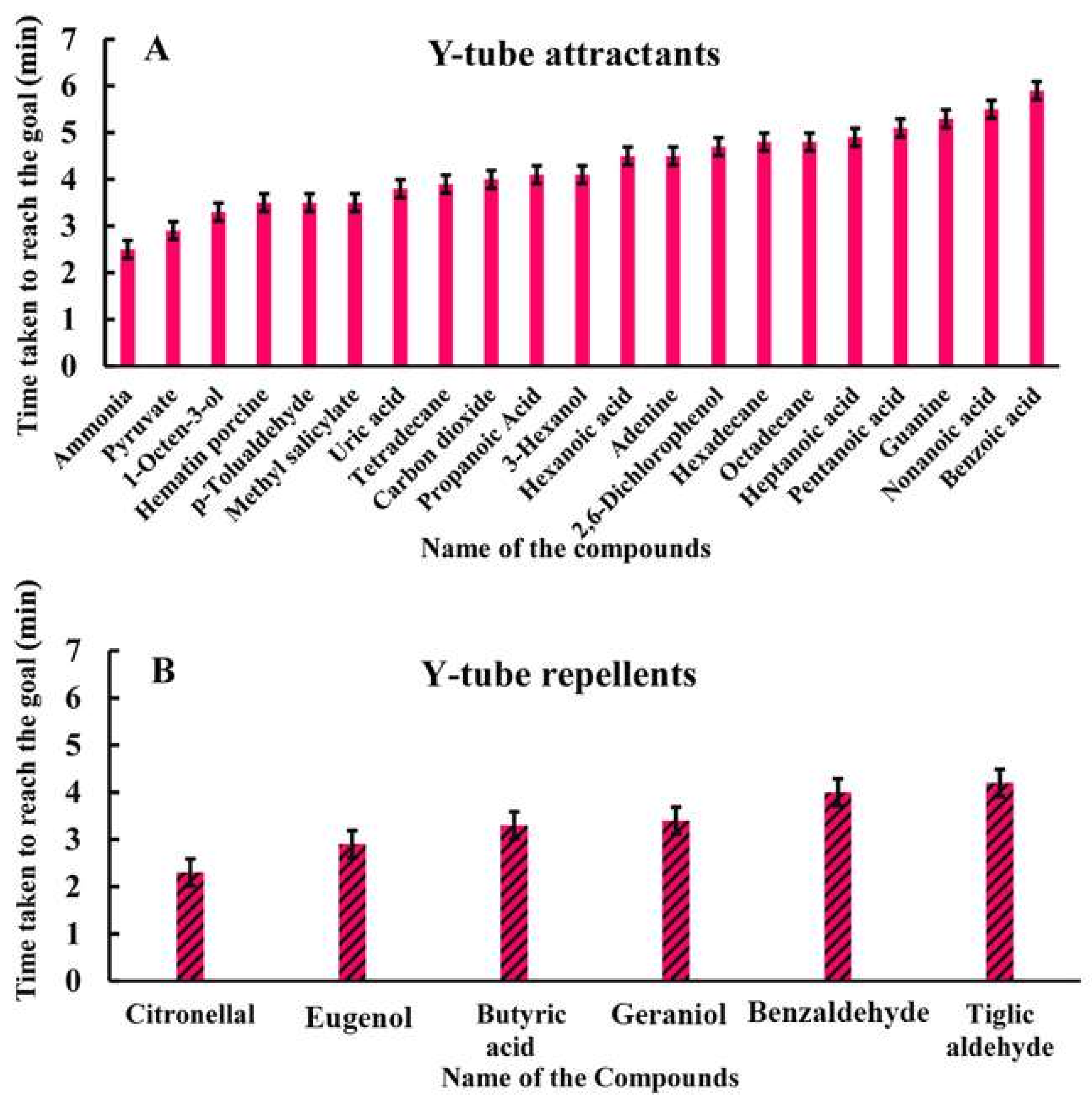
3.4.2. Y-Tube Olfactometric Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adenubi, O.T.; McGaw, L.J.; Eloff, J.N.; Naidoo, V. In vitro bioassays used in evaluating plant extracts for tick repellent and acaricidal properties: A critical review. Vet. Parasitol. 2018, 254, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Dzemo, W.D.; Thekisoe, O.; Vudriko, P. Development of acaricide resistance in tick populations of cattle: A systematic review and meta-analysis. Heliyon 2022, 8, e08718. [Google Scholar] [CrossRef] [PubMed]
- Obaid, M.K.; Islam, N.; Alouffi, A.; Khan, A.Z.; Vaz, I.d.S.; Tanaka, T.; Ali, A. Acaricides Resistance in Ticks: Selection, Diagnosis, Mechanisms, and Mitigation. Front. Cell. Infect. Microbiol. 2022, 12, 941831. [Google Scholar] [CrossRef] [PubMed]
- Novato, T.; Gomes, G.A.; Zeringóta, V.; Franco, C.T.; de Oliveira, D.R.; Melo, D.; de Carvalho, M.G.; Daemon, E.; de Oliveira Monteiro, C.M. In vitro assessment of the acaricidal activity of carvacrol, thymol, eugenol and their acetylated derivatives on Rhipicephalus microplus (Acari: Ixodidae). Vet. Parasitol. 2018, 260, 1–4. [Google Scholar] [CrossRef]
- Boito, J.P.; Santos, R.C.; Vaucher, R.A.; Raffin, R.; Machado, G.; Tonin, A.A.; Da Silva, A.S. Evaluation of tea tree oil for controlling Rhipicephalus microplus in dairy cows. Vet. Parasitol. 2016, 225, 70–72. [Google Scholar] [CrossRef]
- Ghosh, S.; Tiwari, S.S.; Srivastava, S.; Kumar, S.; Sharma, A.K.; Nagar, G.; Kumar, K.A.; Kumar, R.; Rawat, A. In vitro acaricidal properties of Semecarpus anacardium fruit and Datura stramonium leaf extracts against acaricide susceptible (IVRI-I line) and resistant (IVRI-V line) Rhipicephalus (Boophilus) microplus. Res. Vet. Sci. 2015, 101, 69–74. [Google Scholar] [CrossRef]
- Ghosh, S.; Tiwari, S.S.; Kumar, B.; Srivastava, S.; Sharma, A.K.; Kumar, S.; Bandyopadhyay, A.; Julliet, S.; Kumar, R.; Rawat, A.K.S. Identification of potential plant extracts for anti-tick activity against acaricide resistant cattle ticks, Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Exp. Appl. Acarol. 2015, 66, 159–171. [Google Scholar] [CrossRef]
- Mirzaeva, A.U.; Chakraborti, U.; Tukhtaev, K.R.; Akramova, F.D.; Azimov, D.A.; Musabaev, E.I.; Rabbimov, S.S.; Bhadra, K. Acaricidal efficacy of bitter almond against two tick species, Argas persicus and Haemaphysalis darjeeling—An in vitro study. Int. J. Acarol. 2022, 48, 450–456. [Google Scholar] [CrossRef]
- Sonenshine, D.E.; Roe, R.M. Biology of Ticks, 2nd ed.; Oxford University Press: Oxford, UK, 2014; Volume 1. [Google Scholar]
- Iovinella, I.; Ban, L.; Song, L.; Pelosi, P.; Dani, F.R. Proteomic analysis of castor bean tick Ixodes ricinus: A focus on chemosensory organs. Insect. Biochem. Mol. Biol. 2016, 78, 58–68. [Google Scholar] [CrossRef]
- Carr, A.L.; Mitchell, R.D., III; Dhammi, A.; Bissinger, B.W.; Sonenshine, D.E.; Roe, R.M. Tick Haller’s Organ, a New Paradigm for Arthropod Olfaction: How Ticks Differ from Insects. Int. J. Mol. Sci. 2017, 18, 1563. [Google Scholar] [CrossRef]
- Faraone, N.; Light, M.; Scott, C.; MacPherson, S.; Hillier, N.K. Chemosensory and Behavioural Responses of Ixodes scapularis to Natural Products: Role of Chemosensory Organs in Volatile Detection. Insects 2020, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Spach, D.H.; Liles, W.C.; Campbell, G.L.; Quick, R.E.; Anderson, D.E., Jr.; Fritsche, T.R. Tick-borne diseases in the United States. N. Engl. J. Med. 1993, 329, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Udayan, D.; Nair, S.N.; Juliet, S.; Ravindran, R.; Athalathil, S.; Adarshkrishna, T.P.; Ajithkumar, K.G.; Sreelekha, K.P.; Chandrashekar, L.; Ghosh, S. Acaricidal activity of Artemisia nilagirica Leaves Against Rhipicephalus (Boophilus) annulatus ticks. Planta Medica 2020, 86, 1335–1344. [Google Scholar] [CrossRef]
- Liu, X.Y.; Bonnet, S.I. Hard tick factors implicated in pathogen transmission. PLoS Negl. Trop. Dis. 2014, 8, e2566. [Google Scholar] [CrossRef]
- Brunner, J.L.; Cheney, L.; Keesing, F.; Killilea, M.; Logiudice, K.; Previtali, A.; Ostfeld, R.S. Molting success of Ixodes scapularis varies among individual blood meal hosts and species. J. Med. Entomol. 2011, 48, 860–866. [Google Scholar] [CrossRef]
- Debbarma, A.; Pandit, S.; Jas, R.; Baidya, S.; Mandal, S.C.; Kumar, A. Occurrence of tick and tick-born haemoparasitic diseases in different cattle breeds of West Bengal, India. Multilog. Sci. 2019, 8, 149–151. [Google Scholar]
- Josek, T.; Walden, K.K.; Allan, B.F.; Alleyne, M.; Robertson, H.M. A foreleg transcriptome for Ixodes scapularis ticks: Candidates for chemoreceptors and binding proteins that might be expressed in the sensory Haller’s organ. Ticks Tick-Borne Dis. 2018, 9, 1317–1327. [Google Scholar] [CrossRef]
- Borges, L.M.F.; Li, A.Y.; Olafson, P.U.; Renthal, R.; Bauchan, G.R.; Lohmeyer, K.H.; de León, A.A.P. Neuronal projections from the Haller’s organ and palp sensilla to the synganglion of Amblyomma americanum§. Rev. Bras. Parasitol. Veterinária 2016, 25, 217–224. [Google Scholar] [CrossRef]
- Šimo, L.; Slovák, M.; Park, Y.; Žitňan, D. Identification of a complex peptidergic neuroendocrine network in the hard tick, Rhipicephalus appendiculatus. Cell Tissue Res. 2009, 335, 639–655. [Google Scholar] [CrossRef]
- Carr, A.L.; Roe, R.M.; Arellano, C.; Sonenshine, D.E.; Schal, C.; Apperson, C.S. Responses of Amblyomma americanum and Dermacentor variabilis to odorants that attract haematophagous insects. Med. Vet. Entomol. 2013, 27, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.L.; Roe, M. Acarine attractants: Chemoreception, bioassay, chemistry and control. Pestic. Biochem. Physiol. 2016, 131, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Sonenshine, D.E. Pheromones and other semiochemicals of ticks and their use in tick control. Parasitology 2004, 129, 405–425. [Google Scholar] [CrossRef] [PubMed]
- VAN Duijvendijk, G.; Gort, G.; Sprong, H.; Takken, W. Behavioural responses of Ixodes ricinus nymphs to carbon dioxide and rodent odour. Med. Vet. Entomol. 2017, 31, 220–223. [Google Scholar] [CrossRef]
- Garner, K.D.; Payton, M.E.; Talley, J.L.; Noden, B.H. Olfactory responses of Amblyomma maculatum to rumen fluid and other odourants that attract blood-seeking arthropods. Med. Vet. Entomol. 2020, 34, 49–58. [Google Scholar] [CrossRef]
- Ferreira, L.L.; Filho, J.G.d.O.; Silva, F.d.O.; Ferraz, A.L.L.; Mascarin, G.M. Attract or repel Amblyomma sculptum ticks: Screening of semiochemicals. Vet. Parasitol. 2020, 278, 109036. [Google Scholar] [CrossRef]
- Faraone, N.; MacPherson, S.; Hillier, N.K. Behavioral responses of Ixodes scapularis tick to natural products: Development of novel repellents. Exp. Appl. Acarol. 2019, 79, 195–207. [Google Scholar] [CrossRef]
- Haggart, D.A.; Davis, E.E. Neurons sensitive to 2,6-dichlorophenol on the tarsi of the tick Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 1981, 18, 187–193. [Google Scholar] [CrossRef]
- Romashchenko, A.V.; Ratushnyak, A.S.; Zapara, T.A.; Tkachev, S.E.; Moshkin, M.P. The correlation between tick (Ixodes persulcatus Sch.) questing behaviour and synganglion neuronal responses to odors. J. Insect Physiol. 2012, 58, 903–910. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Canale, A.; Mehlhorn, H. Tick repellents and acaricides of botanical origin: A green roadmap to control tick-borne diseases? Parasitol. Res. 2016, 115, 2545–2560. [Google Scholar] [CrossRef]
- Dautel, H.; Dippel, C.; Werkhausen, A.; Diller, R. Efficacy testing of several Ixodes ricinus tick repellents: Different results with different assays. Ticks Tick-Borne Dis. 2013, 4, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Mazuecos, L.; Contreras, M.; Kasaija, P.D.; Manandhar, P.; Grąźlewska, W.; Guisantes-Batan, E.; Gomez-Alonso, S.; Deulofeu, K.; Fernandez-Moratalla, I.; Rajbhandari, R.M.; et al. Natural Clerodendrum-derived tick repellent: Learning from Nepali culture. Exp. Appl. Acarol. 2023, 90, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Quadros, D.G.; Johnson, T.L.; Whitney, T.R.; Oliver, J.D.; Chávez, A.S.O. Plant-Derived Natural Compounds for Tick Pest Control in Livestock and Wildlife: Pragmatism or Utopia? Insects 2020, 11, 490. [Google Scholar] [CrossRef]
- Ismail, M.S.M.; Tag, H.M.; Rizk, M.A. Acaricidal, ovicidal, and repellent effects of Tagetes patula leaf extract against Tetranychus urticae Koch (Acari: Tetranychidae). J. Plant Prot. Res. 2019, 59, 151–159. [Google Scholar] [CrossRef]
- Kemal, J.; Zerihun, T.; Alemu, S.; Sali, K.; Nasir, M.; Abraha, A.; Feyera, T. In Vitro Acaricidal Activity of Selected Medicinal Plants Traditionally Used against Ticks in Eastern Ethiopia. J. Parasitol. Res. 2020, 2020, 7834026. [Google Scholar] [CrossRef]
- Kumar, K.G.A.; Sharma, A.K.; Kumar, S.; Ray, D.D.; Rawat, A.K.S.; Srivastava, S.; Ghosh, S. Comparative in vitro anti-tick efficacy of commercially available products and newly developed phyto-formulations against field collected and resistant tick lines of Rhipicephalus (Boophilus) microplus. J. Parasit. Dis. 2016, 40, 1590–1596. [Google Scholar] [CrossRef]
- Deka, B.; Babu, A.; Baruah, C.; Sarkar, S. Plant Extracts as Potential Acaricides for the Management of Red Spider Mite, Oligonychus coffeae Nietner (Acarina: Tetranychidae), in the Tea Ecosystem: An Eco-Friendly Strategy. Front. Agron. 2022, 4, 685568. [Google Scholar] [CrossRef]
- Adenubi, O.; Fasina, F.; McGaw, L.; Eloff, J.; Naidoo, V. Plant extracts to control ticks of veterinary and medical importance: A review. S. Afr. J. Bot. 2016, 105, 178–193. [Google Scholar] [CrossRef]
- Gebremedhin, M.B.; Xu, Z.; Kuang, C.; Shumuye, N.A.; Cao, J.; Zhou, Y.; Zhang, H.; Zhou, J. Current Knowledge on Chemosensory-Related Candidate Molecules Potentially Involved in Tick Olfaction via Haller’s Organ. Insects 2023, 14, 294. [Google Scholar] [CrossRef]
- Koloski, C.W.; LeMoine, C.M.; Klonowski, A.R.; Smith, C.M.; Cassone, B.J. Molecular evidence for the inhibition of cytochrome p450s and cholinesterases in ticks by the repellent DEET. Ticks Tick-Borne Dis. 2019, 10, 515–522. [Google Scholar] [CrossRef]
- Mitchell, R.D.; Zhu, J.; Carr, A.L.; Dhammi, A.; Cave, G.; Sonenshine, D.E.; Roe, R.M. Infrared Light Detection by the Haller’s Organ of Adult American Dog Ticks, Dermacentor variabilis (Ixodida: Ixodidae). Ticks Tick-Borne Dis. 2017, 8, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.L.; Salgado, V.L. Ticks home in on body heat: A new understanding of Haller’s organ and repellent action. PLoS ONE 2019, 14, e0221659. [Google Scholar] [CrossRef] [PubMed]
- Foelix, R.F.; Axtell, R.C. Ultrastructure of Haller’s Organ in the Tick Amblyomma americanum (L.). Z. Zellforsch. Mikrosk. Anat. 1972, 124, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, H.S.; Roy, S.; Sanyal, A.K.; Misra, K.K. Microscopic anatomy of the Haller’s organ of snake ticks. Int. J. Acarol. 2016, 43, 1–9. [Google Scholar] [CrossRef]
- Leonovich, S.A. Structure of Haller’s organ and taxonomy of hard ticks of the subfamily Amblyomminae (Family Ixodidae). Entomol. Rev. 2021, 101, 709–724. [Google Scholar] [CrossRef]
- Klompen, J.S.H.; Oliver, J.H., Jr. Haller’s organ in the tick family argasidae (acari: Parasitiformes: Ixodid). J. Parasitol. 1993, 79, 591–603. [Google Scholar] [CrossRef]
- Waladde, S.M. The sensory nervous system of the adult cattle tick Boophilus microplus (canestrini) ixodidae part II. scanning electron microscopy. Aust. J. Entomol. 1977, 16, 73–79. [Google Scholar] [CrossRef]
- Soares, S.F.; Borges, L.M.F. Electrophysiological responses of the olfactory receptors of the tick Amblyomma cajennense (Acari: Ixodidae) to host-related and tick pheromone-related synthetic compounds. Acta Trop. 2012, 124, 192–198. [Google Scholar] [CrossRef]
- Steullet, P.; Guerin, P.M. Identification of vertebrate volatiles stimulating olfactory receptors on tarsus I of the tick Amblyomma variegatum Fabricius (Ixodidae). J. Comp. Physiol. A 1994, 174, 27–38. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Davison, A.C.; Tamò, C. A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol. Entomol. 2004, 29, 45–55. [Google Scholar] [CrossRef]
- Saïd, I.; de la Torre, R.A.; Morin, J.-P.; Rochat, D. Adaptation of a four-arm olfactometer for behavioural bioassays of large beetles. Chemoecology 2006, 16, 9–16. [Google Scholar] [CrossRef]
- Bhowmik, B.; Chakraborti, U.; Mandal, A.; Paul, B.; Bhadra, K. Attraction of Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae) to Host Plant Cucurbita maxima Duchesne (Cucurbitaceae) Volatiles. Agronomy 2022, 12, 2640. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Touhara, K. Insect olfaction: Receptors, signal transduction, and behavior. In Chemosensory Systems in Mammals, Fishes, and Insects, 1st ed.; Korsching, S., Meyerhof, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 203–220. [Google Scholar]
- Bhowmik, B.; Lakare, S.; Sen, A.; Bhadra, K. Olfactory stimulation of Apis cerana indica towards different doses of volatile constituents: SEM and EAG approaches. J. Asia-Pac. Entomol. 2016, 19, 847–859. [Google Scholar] [CrossRef]
- Bruce, W.A. Posterior capsule of Haller’s organ in the Lone star tick, Amblyomma americanum (Acari: Ixodidae). Fla. Entomol. 1971, 54, 65–75. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function, 5th ed.; Cambridge University Press: Cambrige, UK, 2013. [Google Scholar]
- Cruz, J.D.L.; Dusbabek, F. Haller’s organ and anterior pit setae in the genera Antricola and Parantricola (Ixodoidea: Argasidae). Folia Parasitol. 1989, 36, 275–279. [Google Scholar]
- Roma, G.C.; Nunes, P.H.; Remédio, R.N.; Camargo-Mathias, M.I. Synganglion histology in different stages of Rhipicephalus sanguineus ticks (Acari: Ixodidae). Parasitol. Res. 2012, 110, 2455–2463. [Google Scholar] [CrossRef]
- Carr, A.L.; Roe, R.M.; Sonenshine, D.E. Sensory systems of ticks and their role in tick-host interactions. In Ticks: Biology, Disease and Control; Nuttall, P., Bowman, S., Eds.; Cambridge University Press: Cambrige, UK, 2017. [Google Scholar]
- Guerenstein, P.G.; Hildebrand, J.G. Roles and effects of environmental carbon dioxide in insect life. Annu. Rev. Entomol. 2008, 53, 161–178. [Google Scholar] [CrossRef]
- Del Fabbro, S.D.; Nazzi, F. From Chemistry to Behavior. Molecular structure and bioactivity of repellents against Ixodes ricinus ticks. PLoS ONE 2013, 8, e67832. [Google Scholar] [CrossRef]
- Sonenshine, D.E.; Lane, R.S.; Nicholson, W.L.; Robert, S.; Lane, R.S. Ticks (Ixodida). In Medical and Veterinary Entomology; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar] [CrossRef]
- Buescher, M.D.; Rutledge, L.C.; Wirtz, R.A.; Nelson, J.H.; Inase, J.L. Repellent tests against Leptotrombidium (Leptotrombidium) fletcheri (Acari: Trombiculidae). J. Med. Entomol. 1984, 21, 278–282. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, N.; Barik, A. Alkanes in flower surface waxes of Momordica cochinchinensis influence attraction to Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae). Neotrop. Entomol. 2013, 42, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Sarkar, N.; Barik, A. Momordica cochinchinensis (Cucurbitaceae) leaf volatiles: Semiochemicals for host location by the insect pest, Aulacophora foveicollis (Coleoptera: Chrysomelidae). Chemoecology 2015, 25, 93–104. [Google Scholar] [CrossRef]
- Du, E.J.; Ahn, T.J.; Choi, M.S.; Kwon, I.; Kim, H.-W.; Kwon, J.Y.; Kang, K. The Mosquito Repellent Citronellal Directly Potentiates Drosophila TRPA1, Facilitating Feeding Suppression. Mol. Cells 2015, 38, 911–917. [Google Scholar] [CrossRef]
- Kathiravan, S.; Immadi, S.; Kurbet, P.S.; Alimudeen, S.; Kumar, K.G.A.; Varghese, A.; Deepa, C.K.; Juliet, S.; Ravindran, R. In vitro evaluation of acaricidal activity of eugenol and benzaldehyde against Rhipicephalus annulatus. Vet. Parasitol. 2024, 331, 110285. [Google Scholar] [CrossRef]
- Chen, Z.; van Mol, W.; Vanhecke, M.; Duchateau, L.; Claerebout, E. Acaricidal activity of plant-derived essential oil components against Psoroptes ovis in vitro and in vivo. Parasites Vectors 2019, 12, 425. [Google Scholar] [CrossRef]
- Muller, G.C.; Junnila, A.; Butler, J.; Kravchenko, V.D.; Revay, E.E.; Weiss, R.W.; Schlein, Y. Efficacy of the botanical repellents geraniol, linalool and citronella against mosquitoes. J. Vector Ecol. 2009, 34, 2–8. [Google Scholar] [CrossRef]
- Bohbot, J.D.; Dickens, J.C. Selectivity of odorant receptors in insects. Front. Cell. Neurosci. 2012, 6, 29. [Google Scholar] [CrossRef]
- Kajiya, K.; Inaki, K.; Tanaka, M.; Haga, T.; Kataoka, H.; Touhara, K. Molecular bases of odor discrimination: Reconstitution of olfactory receptors that recognize overlapping sets of odorants. J. Neurosci. 2001, 21, 6018–6025. [Google Scholar] [CrossRef]
- Gaillard, I.; Rouquier, S.; Giorgi, D. Olfactory receptors. Cell. Mol. Life Sci. 2004, 61, 456–469. [Google Scholar] [CrossRef]
- Benton, R. Molecular basis of odor detection in insects. Ann. N. Y. Acad. Sci. 2009, 1170, 478–481. [Google Scholar] [CrossRef]
- Koloski, C.W.; Cassone, B.J. Transcriptional profiling of Dermacentor variabilis (Acari: Ixodidae) provides insights into the role of the Haller’s organ in spatial DEET recognition. Ticks Tick-Borne Dis. 2022, 13, 101827. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, T.; Wang, Y.; Liu, S. G-Protein Coupled Receptors (GPCRs) in insects—A potential target for new insecticide development. Molecules 2021, 26, 2993. [Google Scholar] [CrossRef]
- Debboun, M.; Frances, S.P.; Strickman, D. (Eds.) Insect Repellents: Principles, Methods and Uses; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Oxford, UK, 2006. [Google Scholar]
| Sl No. | Name of the Compound | Purity | Supplier |
|---|---|---|---|
| 1 | 4-Nitrophenol | ≥99% | Sigma |
| 2 | Methyl salicylate | ≥99% | Sigma Aldrich |
| 3 | Nonanoic acid | ≥97% | Sigma Aldrich |
| 4 | Adenine | ≥99% | Merck |
| 5 | Guanine | ≥98% | Merck |
| 6 | Hematin porcine | - | Sigma Aldrich |
| 7 | Uric acid | ≥99% | Sigma Aldrich |
| 8 | Xanthine | ≥99% | Sigma |
| 9 | 2,6-Dichlorophenol | ≥99% | Sigma Aldrich |
| 10 | 1-Octen-3-ol | ≥98% | Aldrich |
| 11 | 2-Ethylhexanoic acid | - | Aldrich |
| 12 | 2-Nitrophenol | ≥98% | Aldrich |
| 13 | Acetone | ≥99.5% | Sigma-Aldrich |
| 14 | Tiglic aldehyde | ≥96% | Aldrich |
| 15 | Ammonia | ≥25% | CDH |
| 16 | p-Tolualdehyde | ≥97% | Sigma-Aldrich |
| 17 | Benzoic acid | ≥99.5% | Sigma-Aldrich |
| 18 | Butyric acid | ≥99% | Sigma-Aldrich |
| 19 | Carbon dioxide | ≥99.8% | Sigma-Aldrich |
| 20 | Heptanoic acid | ≥99% | Sigma-Aldrich |
| 21 | Hexanal | ≥98% | Aldrich |
| 22 | Hexanoic acid | ≥99% | Sigma-Aldrich |
| 23 | Isobutyric acid | ≥99% | Sigma-Aldrich |
| 24 | Mucochloric acid | ≥99% | Sigma-Aldrich |
| 25 | Pentanoic acid | ≥99% | Sigma-Aldrich |
| 26 | Pyruvate | ≥99% | Sigma-Aldrich |
| 27 | Hexane | ≥95% | Sigma |
| 28 | cis-3-Hexanoic acid | - | Sigma-Aldrich |
| 29 | (R)-(+) Limonene | ≥97% | Sigma-Aldrich |
| 30 | n-Octanoic acid | ≥97.5% | CDH |
| 31 | Ethyl butyrate | ≥99% | Sigma-Aldrich |
| 32 | Citral | ≥95% | Sigma-Aldrich |
| 33 | Butyl butyrate | ≥98% | Sigma-Aldrich |
| 34 | Isopropyl acetate | ≥99.6% | Sigma-Aldrich |
| 35 | β-Caryophyllene | ≥80% | Sigma-Aldrich |
| 36 | (R)-(+)α-pinene | - | Sigma-Aldrich |
| 37 | Geraniol | ≥98% | Sigma-Aldrich |
| 38 | 4-Methyl anisole | ≥99% | Sigma-Aldrich |
| 39 | 1-Octanol | ≥99% | CDH |
| 40 | Propanoic acid | ≥99.5% | Sigma-Aldrich |
| 41 | Cyclohexanol | ≥99% | Sigma-Aldrich |
| 42 | L-Cysteine hydrochloride | - | Sigma-Aldrich |
| 43 | n-Decanoic acid | ≥98% | Sigma-Aldrich |
| 44 | Citronellal | ≥98% | CDH |
| 45 | Azadirachtin | ≥95% | Sigma-Aldrich |
| 46 | 3-Hexanol | ≥97% | Sigma-Aldrich |
| 47 | Dodecane | ≥99% | Merck |
| 48 | Tetradecane | ≥99% | Sigma-Aldrich |
| 49 | Hexadecane | ≥99% | Sigma-Aldrich |
| 50 | Octadecane | ≥99% | Sigma-Aldrich |
| 51 | Eicosane | ≥99% | Sigma-Aldrich |
| 52 | Docosane | ≥99% | Sigma-Aldrich |
| 53 | Benzaldehyde | ≥99.5% | Sigma-Aldrich |
| 54 | Eugenol | ≥99% | Sigma-Aldrich |
| 55 | Paraffin liquid light (control, used as solvent) | ≥30% | Merck |
| SL No. | Name of the Compound | Concentration (w/v) | ||||
|---|---|---|---|---|---|---|
| 0.001% | 0.01% | 0.1% | 1% | 2% | ||
| 1 | 4-Nitrophenol | 0.082 ± 0.014 | 0.071 ± 0.009 | 0.074 ± 0.009 | 0.619 ± 0.015 | 0.707 ± 0.016 |
| 2 | Methyl salicylate | 0.512 ± 0.014 | 0.433 ± 0.017 | 0.480 ± 0.01 | 0.416 ± 0.01 | 0.598 ± 0.015 |
| 3 | Nonanoic acid | 0.129 ± 0.015 | 0.131 ± 0.011 | 0.137 ± 0.01 | 0.334 ± 0.017 | 0.463 ± 0.011 |
| 4 | Adenine | 0.705 ± 0.015 | 0.984 ± 0.010 | 0.833 ± 0.010 | 0.636 ± 0.016 | 0.531 ± 0.015 |
| 5 | Guanine | 0.697 ± 0.014 | 0.483 ± 0.015 | 0.637 ± 0.015 | 0.725 ± 0.017 | 0.852 ± 0.014 |
| 6 | Hematin porcine | 0.509 ± 0.014 | 0.779 ± 0.014 | 0.655 ± 0.011 | 0.761 ± 0.011 | 0.794 ± 0.017 |
| 7 | Uric acid | 0.311 ± 0.012 | 0.616 ± 0.013 | 0.463 ± 0.013 | 0.688 ± 0.018 | 0.730 ± 0.014 |
| 8 | Xanthine | 0.290 ± 0.016 | 0.408 ± 0.014 | 0.337 ± 0.013 | 0.561 ± 0.019 | 0.530 ± 0.013 |
| 9 | 2,6-Dichlorophenol | 1.340 ± 0.015 | 0.600 ± 0.015 | 0.956 ± 0.011 | 1.189 ± 0.013 | 0.977 ± 0.015 |
| 10 | 1-Octen-3-ol | 0.180 ± 0.014 | 0.157 ± 0.017 | 0.179 ± 0.012 | 0.958 ± 0.012 | 1.020 ± 0.012 |
| 11 | 2-Ethylhexanoic acid | 0.139 ± 0.008 | 0.244 ± 0.016 | 0.189 ± 0.009 | 0.235 ± 0.014 | 0.191 ± 0.012 |
| 12 | 2-Nitrophenol | 0.228 ± 0.014 | 0.444 ± 0.014 | 0.367 ± 0.012 | 0.536 ± 0.012 | 0.505 ± 0.016 |
| 13 | Acetone | 0.240 ± 0.013 | 0.412 ± 0.014 | 0.340 ± 0.013 | 0.477 ± 0.013 | 0.995 ± 0.01 |
| 14 | Tiglic aldehyde | 0.588 ± 0.013 | 0.495 ± 0.016 | 0.553 ± 0.009 | 0.174 ± 0.014 | 0.479 ± 0.014 |
| 15 | Ammonia | 1.854 ± 0.017 | 1.066 ± 0.014 | 1.481 ± 0.01 | 10.585 ± 0.018 | 12.256 ± 0.011 |
| 16 | p-Tolualdehyde | 0.666 ± 0.013 | 1.261 ± 0.015 | 0.958 ± 0.012 | 1.072 ± 0.015 | 1.264 ± 0.010 |
| 17 | Benzoic acid | 1.998 ± 0.013 | 0.630 ± 0.014 | 1.331 ± 0.07 | 0.986 ± 0.013 | 0.980 ± 0.012 |
| 18 | Butyric acid | 0.415 ± 0.013 | 0.245 ± 0.013 | 0.310 ± 0.011 | 0.240 ± 0.010 | 0.467 ± 0.012 |
| 19 | Carbon dioxide | 0.675 ± 0.013 | 0.726 ± 0.016 | 1.252 ± 0.010 | 0.966 ± 0.013 | 1.589 ± 0.017 |
| 20 | Heptanoic acid | 0.228 ± 0.018 | 0.182 ± 0.014 | 0.205 ± 0.013 | 0.501 ± 0.015 | 0.406 ± 0.013 |
| 21 | Hexanal | 0.401 ± 0.016 | 0.112 ± 0.011 | 0.273 ± 0.09 | 0.639 ± 0.012 | 0.697 ± 0.013 |
| 22 | Hexanoic acid | 0.137 ± 0.018 | 0.655 ± 0.013 | 0.371 ± 0.013 | 0.350 ± 0.016 | 0.351 ± 0.015 |
| 23 | Isobutyric acid | 1.385 ± 0.014 | 0.785 ± 0.015 | 1.074 ± 0.016 | 0.214 ± 0.017 | 0.175 ± 0.010 |
| 24 | Mucochloric acid | 0.526 ± 0.015 | 0.381 ± 0.012 | 0.403 ± 0.010 | 0.598 ± 0.014 | 0.537 ± 0.013 |
| 25 | Pentanoic acid | 0.626 ± 0.013 | 0.538 ± 0.012 | 0.714 ± 0.013 | 0.856 ± 0.010 | 0.544 ± 0.012 |
| 26 | Pyruvate | 0.520 ± 0.017 | 0.524 ± 0.012 | 0.517 ± 0.008 | 11.796 ± 0.011 | 13.284 ± 0.013 |
| 27 | Hexane | 0.333 ± 0.008 | 0.474 ± 0.013 | 0.394 ± 0.012 | 0.673 ± 0.012 | 0.466 ± 0.012 |
| 28 | cis-3-Hexanoic acid | 0.110 ± 0.013 | 0.223 ± 0.015 | 0.184 ± 0.013 | 0.620 ± 0.014 | 0.466 ± 0.009 |
| 29 | (R)-(+) Limonene | 0.951 ± 0.018 | 0.399 ± 0.013 | 0.646 ± 0.012 | 0.428 ± 0.016 | 0.345 ± 0.009 |
| 30 | n-Octanoic Acid | 0.360 ± 0.015 | 0.680 ± 0.013 | 0.507 ± 0.013 | 0.677 ± 0.009 | 0.496 ± 0.015 |
| 31 | Ethyl butyrate | 0.275 ± 0.017 | 0.661 ± 0.016 | 0.472 ± 0.012 | 0.874 ± 0.017 | 0.849 ± 0.015 |
| 32 | Citral | 0.428 ± 0.014 | 0.392 ± 0.014 | 0.413 ± 0.010 | 0.322 ± 0.015 | 0.281 ± 0.009 |
| 33 | Butyl butyrate | 0.111 ± 0.014 | 0.260 ± 0.012 | 0.185 ± 0.010 | 0.462 ± 0.013 | 0.554 ± 0.015 |
| 34 | Isopropyl acetate | 0.918 ± 0.014 | 1.047 ± 0.009 | 0.987 ± 0.014 | 0.444 ± 0.018 | 0.438 ± 0.013 |
| 35 | β-Caryophyllene | 0.659 ± 0.016 | 0.382 ± 0.014 | 0.539 ± 0.012 | 0.203 ± 0.014 | 0.227 ± 0.014 |
| 36 | (R)-(+)α-pinene | 0.472 ± 0.015 | 0.402 ± 0.012 | 0.452 ± 0.015 | 0.367 ± 0.009 | 0.365 ± 0.012 |
| 37 | Geraniol | 0.183 ± 0.015 | 0.572 ± 0.012 | 0.373 ± 0.014 | 0.890 ± 0.011 | 0.776 ± 0.013 |
| 38 | 4-Methyl anisole | 0.256 ± 0.008 | 0.225 ± 0.012 | 0.278 ± 0.014 | 0.172 ± 0.019 | 0.118 ± 0.017 |
| 39 | 1-Octanol | 1.012 ± 0.015 | 0.880 ± 0.011 | 0.967 ± 0.008 | 0.342 ± 0.011 | 0.492 ± 0.014 |
| 40 | Propanoic acid | 0.667 ± 0.014 | 0.974 ± 0.012 | 0.847 ± 0.012 | 0.277 ± 0.013 | 0.456 ± 0.015 |
| 41 | Cyclohexanol | 0.693 ± 0.011 | 0.714 ± 0.016 | 0.702 ± 0.012 | 0.721 ± 0.011 | 1.031 ± 0.018 |
| 42 | L-cysteine hydrochloride | 0.431 ± 0.014 | 0.382 ± 0.016 | 0.422 ± 0.011 | 0.741 ± 0.022 | 0.988 ± 0.012 |
| 43 | n-Decanoic acid | 0.715 ± 0.012 | 0.805 ± 0.017 | 0.781 ± 0.012 | 0.554 ± 0.021 | 0.927 ± 0.014 |
| 44 | Citronellal | 1.106 ± 0.011 | 1.117 ± 0.014 | 1.126 ± 0.009 | 1.04 ± 0.011 | 0.823 ± 0.013 |
| 45 | Azadirachtin | 0.322 ± 0.047 | 0.680 ± 0.038 | 0.510 ± 0.062 | 0.405 ± 0.042 | 0.589 ± 0.127 |
| 46 | 3-Hexanol | 0.259 ± 0.012 | 0.288 ± 0.015 | 0.426 ± 0.012 | 0.606 ± 0.014 | 0.899 ± 0.014 |
| 47 | Dodecane | 0.467 ± 0.014 | 0.589 ± 0.010 | 0.674 ± 0.010 | 0.737 ± 0.014 | 0.856 ± 0.001 |
| 48 | Tetradecane | 0.494 ± 0.017 | 0.672 ± 0.013 | 0.584 ± 0.014 | 0.916 ± 0.016 | 1.23 ± 0.013 |
| 49 | Hexadecane | 0.426 ± 0.018 | 0.491 ± 014 | 0.583 ± 0.014 | 0.738 ± 0.010 | 0.952 ± 0.015 |
| 50 | Octadecane | 0.485 ± 0.014 | 0.372 ± 0.008 | 0.603 ± 0.013 | 0.748 ± 0.014 | 0.637 ± 0.014 |
| 51 | Eicosane | 0.283 ± 0.016 | 0.485 ± 0.017 | 0.596 ± 0.014 | 0.894 ± 0.017 | 0.799 ± 0.02 |
| 52 | Docosane | 0.596 ± 0.013 | 0.754 ± 0.013 | 0.695 ± 0.011 | 0.834 ± 0.014 | 1.17 ± 0.021 |
| 53 | Benzaldehyde | 0.315 ± 0.010 | 0.485 ± 0.017 | 0.369 ± 0.017 | 0.684 ± 0.008 | 0.593 ± 0.013 |
| 54 | Eugenol | 0.583 ± 0.021 | 0.495 ± 0.013 | 0.795 ± 0.010 | 0.848 ± 0.010 | 0.957 ± 0.008 |
| SL No. | Name of the Compound | Concentration (w/v) | ||||
|---|---|---|---|---|---|---|
| 0.001% | 0.01% | 0.1% | 1% | 2% | ||
| 1 | 4-Nitrophenol | 0.024 ± 0.001 | 0.022 ± 0.002 | 0.196 ± 0.006 | 0.168 ± 0.009 | 0.156 ± 0.002 |
| 2 | Methyl salicylate | 0.120 ± 0.001 | 0.060 ± 0.009 | 0.117 ± 0.005 | 0.069 ± 0.014 | 0.062 ± 0.003 |
| 3 | Nonanoic acid | 0.029 ± 0.001 | 0.043 ± 0.003 | 0.114 ± 0.006 | 0.050 ± 0.001 | 0.150 ± 0.002 |
| 4 | Adenine | 0.172 ± 0.001 | 0.122 ± 0.003 | 0.145 ± 0.007 | 0.099 ± 0.013 | 0.126 ± 0.001 |
| 5 | Guanine | 0.035 ± 0.003 | 0.274 ± 0.007 | 0.153 ± 0.004 | 0.263 ± 0.002 | 0.089 ± 0.001 |
| 6 | Hematin porcine | 0.126 ± 0.001 | 0.221 ± 0.007 | 0.189 ± 0.007 | 0.098 ± 0.013 | 0.187 ± 0.001 |
| 7 | Uric acid | 0.045 ± 0.001 | 0.141 ± 0.003 | 0.218 ± 0.005 | 0.078 ± 0.008 | 0.115 ± 0.002 |
| 8 | Xanthine | 0.036 ± 0.001 | 0.052 ± 0.003 | 0.125 ± 0.006 | 0.304 ± 0.004 | 0.298 ± 0.001 |
| 9 | 2,6-Dichlorophenol | 0.213 ± 0.001 | 0.086 ± 0.006 | 0.322 ± 0.008 | 0.254 ± 0.007 | 0.210 ± 0.001 |
| 10 | 1-Octen-3-ol | 0.031 ± 0.009 | 0.055 ± 0.008 | 0.054 ± 0.005 | 0.210 ± 0.009 | 0.285 ± 0.002 |
| 11 | 2-Ethylhexanoic acid | 0.028 ± 0.001 | 0.045 ± 0.007 | 0.040 ± 0.002 | 0.022 ± 0.004 | 0.043 ± 0.001 |
| 12 | 2-Nitrophenol | 0.030 ± 0.001 | 0.113 ± 0.004 | 0.333 ± 0.009 | 0.122 ± 0.005 | 0.147 ± 0.002 |
| 13 | Acetone | 0.094 ± 0.001 | 0.105 ± 0.003 | 0.227 ± 0.002 | 0.281 ± 0.010 | 0.093 ± 0.001 |
| 14 | Tiglic aldehyde | 0.066 ± 0.001 | 0.070 ± 0.009 | 0.071 ± 0.009 | 0.069 ± 0.009 | 0.045 ± 0.002 |
| 15 | Ammonia | 0.687 ± 0.001 | 0.200 ± 0.002 | 0.392 ± 0.005 | 4.516 ± 0.013 | 4.199 ± 0.002 |
| 16 | p-Tolualdehyde | 0.110 ± 0.001 | 0.240 ± 0.007 | 0.324 ± 0.005 | 0.252 ± 0.012 | 0.276 ± 0.002 |
| 17 | Benzoic acid | 0.569 ± 0.001 | 0.095 ± 0.008 | 0.205 ± 0.006 | 0.208 ± 0.001 | 0.196 ± 0.002 |
| 18 | Butyric acid | 0.094 ± 0.001 | 0.052 ± 0.004 | 0.069 ± 0.007 | 0.055 ± 0.007 | 0.027 ± 0.002 |
| 19 | Carbon dioxide | 0.426 ± 0.008 | 0.544 ± 0.013 | 0.275 ± 0.018 | 0.174 ± 0.006 | 0.363 ± 0.003 |
| 20 | Heptanoic acid | 0.034 ± 0.000 | 0.066 ± 0.009 | 0.039 ± 0.003 | 0.063 ± 0.008 | 0.107 ± 0.002 |
| 21 | Hexanal | 0.075 ± 0.009 | 0.056 ± 0.004 | 0.118 ± 0.005 | 0.101 ± 0.006 | 0.140 ± 0.002 |
| 22 | Hexanoic acid | 0.047 ± 0.001 | 0.063 ± 0.004 | 0.041 ± 0.001 | 0.073 ± 0.013 | 0.114 ± 0.002 |
| 23 | Isobutyric acid | 0.269 ± 0.0008 | 0.135 ± 0.01 | 0.104 ± 0.009 | 0.055 ± 0.004 | 0.048 ± 0.002 |
| 24 | Mucochloric acid | 0.066 ± 0.001 | 0.287 ± 0.003 | 0.068 ± 0.006 | 0.121 ± 0.014 | 0.074 ± 0.002 |
| 25 | Pentanoic acid | 0.035 ± 0.005 | 0.157 ± 0.006 | 0.245 ± 0.025 | 0.042 ± 0.014 | 0.137 ± 0.003 |
| 26 | Pyruvate | 0.057 ± 0.001 | 0.050 ± 0.004 | 0.179 ± 0.008 | 0.469 ± 0.010 | 0.733 ± 0.003 |
| 27 | Hexane | 0.053 ± 0.001 | 0.071 ± 0.003 | 0.113 ± 0.002 | 0.104 ± 0.010 | 0.195 ± 0.001 |
| 28 | cis-3-Hexanoic acid | 0.046 ± 0.0009 | 0.049 ± 0.002 | 0.065 ± 0.006 | 0.127 ± 0.012 | 0.139 ± 0.002 |
| 29 | (R)-(+) Limonene | 0.174 ± 0.001 | 0.197 ± 0.006 | 0.115 ± 0.008 | 0.062 ± 0.008 | 0.057 ± 0.002 |
| 30 | N-Octanoic acid | 0.056 ± 0.001 | 0.151 ± 0.004 | 0.060 ± 0.004 | 0.088 ± 0.008 | 0.063 ± 0.002 |
| 31 | Ethyl butyrate | 0.049 ± 0.001 | 0.132 ± 0.002 | 0.091 ± 0.009 | 0.205 ± 0.009 | 0.130 ± 0.002 |
| 32 | Citral | 0.126 ± 0.001 | 0.067 ± 0.005 | 0.269 ± 0004 | 0.110 ± 0.007 | 0.067 ± 0.002 |
| 33 | Butyl butyrate | 0.032 ± 0.001 | 0.082 ± 0.007 | 0.063 ± 0.004 | 0.072 ± 0.004 | 0.060 ± 0.001 |
| 34 | Isopropyl acetate | 0.237 ± 0.001 | 0.186 ± 0.003 | 0.064 ± 0.008 | 0.040 ± 0.005 | 0.042 ± 0.002 |
| 35 | β-Caryophyllene | 0.161 ± 0.001 | 0.070 ± 0.004 | 0.066 ± 0.002 | 0.081 ± 0.010 | 0.047 ± 0.002 |
| 36 | (R)-(+)α-pinene | 0.063 ± 0.001 | 0.108 ± 0.002 | 0.062 ± 0.003 | 0.066 ± 0.007 | 0.119 ± 0.002 |
| 37 | Geraniol | 0.037 ± 0.009 | 0.135 ± 0.007 | 0.085 ± 0.006 | 0.033 ± 0.003 | 0.022 ± 0.001 |
| 38 | 4-Methyl anisole | 0.073 ± 0.001 | 0.070 ± 0.005 | 0.085 ± 0.007 | 0.049 ± 0.010 | 0.042 ± 0.002 |
| 39 | 1-Octanol | 0.247 ± 0.008 | 0.194 ± 0.007 | 0.100 ± 0.004 | 0.030 ± 0.002 | 0.031 ± 0.002 |
| 40 | Propanoic acid | 0.127 ± 0.001 | 0.042 ± 0.003 | 0.209 ± 0.002 | 0.055 ± 0.005 | 0.053 ± 0.001 |
| 41 | Cyclohexanol | 0.135 ± 0.001 | 0.125 ± 0.003 | 0.104 ± 0.008 | 0.161 ± 0.006 | 0.082 ± 0.002 |
| 42 | L-Cysteine hydrochloride | 0.095 ± 0.001 | 0.079 ± 0.005 | 0.091 ± 0.006 | 0.173 ± 0.008 | 0.205 ± 0.002 |
| 43 | n-Decanoic acid | 0.139 ± 0.001 | 0.071 ± 0.006 | 0.114 ± 0.005 | 0.083 ± 0.011 | 0.082 ± 0.002 |
| 44 | Citronellal | 0.317 ± 0.001 | 0.207 ± 0.007 | 0.156 ± 0.007 | 0.065 ± 0.005 | 0.037 ± 0.001 |
| 45 | Azadirachtin | 0.072 ± 0.044 | 0.070 ± 0.010 | 0.163 ± 0.019 | 0.193 ± 0.013 | 0.123 ± 0.027 |
| 46 | 3-Hexanol | 0.089 ± 0.018 | 0.104 ± 0.032 | 0.156 ± 0.014 | 0.222 ± 0.031 | 0.285 ± 0.030 |
| 47 | Dodecane | 0.099 ± 0.013 | 0.139 ± 0.027 | 0.181 ± 0.009 | 0.234 ± 0.031 | 0.195 ± 0.025 |
| 48 | Tetradecane | 0.189 ± 0.018 | 0.240 ± 0.013 | 0.163 ± 0.029 | 0.312 ± 0.029 | 0.224 ± 0.025 |
| 49 | Hexadecane | 0.167 ± 0.055 | 0.066 ± 0.014 | 0.108 ± 0.027 | 0.220 ± 0.023 | 0.344 ± 0.053 |
| 50 | Octadecane | 0.140 ± 0.066 | 0.091 ± 0.050 | 0.152 ± 0.036 | 0.180 ± 0.041 | 0.253 ± 0.033 |
| 51 | Eicosane | 0.116 ± 0.036 | 0.174 ± 0.035 | 0.268 ± 0.064 | 0.264 ± 0.012 | 0.324 ± 0.031 |
| 52 | Docosane | 0.132 ± 0.045 | 0.098 ± 0.021 | 0.253 ± 0.043 | 0.291 ± 0.037 | 0.255 ± 0.035 |
| 53 | Benzaldehyde | 0.105 ± 0.050 | 0.187 ± 0.035 | 0.173 ± 0.015 | 0.202 ± 0.031 | 0.218 ± 0.021 |
| 54 | Eugenol | 0.066 ± 0.006 | 0.162 ± 0.022 | 0.162 ± 0.051 | 0.139 ± 0.036 | 0.224 ± 0.024 |
| Sl No. | Name of Compound | Positive Responses | Negative Responses | No Response | Total Specimen Count | Binomial p Value at 0.05 Level of Significance | Significant/Non-Significant |
|---|---|---|---|---|---|---|---|
| 1 | 4-Nitrophenol | 5.7 ± 0.9 | 1.7 ± 0.9 | 22.7 ± 1.7 | 30 | 0.2266 | Non-significant |
| 2 | Methyl salicylate | 20.7 ± 0.9 | 2 ± 0.6 | 7.3 ± 1.2 | 30 | 0.0001 | Significant |
| 3 | Nonanoic acid | 16.3 ± 0.9 | 3.3 ± 0.9 | 10.3 ± 1.3 | 30 | 0.0022 | Significant |
| 4 | Adenine | 16.7 ± 1.5 | 4 ± 0.6 | 9.3 ± 1.3 | 30 | 0.0059 | Significant |
| 5 | Guanine | 14.7 ± 1.2 | 3.3 ± 0.7 | 12 ± 1.7 | 30 | 0.0154 | Significant |
| 6 | Hematin porcine | 20 ± 1.7 | 2 ± 0.6 | 8 ± 2.1 | 30 | 0.0001 | Significant |
| 7 | Uric acid | 19.3 ± 1.2 | 2.7 ± 0.3 | 8 ± 1.5 | 30 | 0.0004 | Significant |
| 8 | Xanthine | 5.3 ± 0.9 | 2.3 ± 1.5 | 22.3 ± 2.3 | 30 | 0.2266 | Non-significant |
| 9 | 2,6-Dichlorophenol | 15.3 ± 0.7 | 3.7 ± 1.2 | 11 ± 0.6 | 30 | 0.0096 | Significant |
| 10 | 1-Octen-3-ol | 22.7 ± 1.5 | 2 ± 1 | 5.3 ± 1.7 | 30 | 0.0000 | Significant |
| 11 | 2-Ethylhexanoic acid | 14 ± 0.6 | 2.7 ± 0.9 | 13.3 ± 0.7 | 30 | 0.0021 | Significant |
| 12 | 2-Nitrophenol | 8.3 ± 0.7 | 2.3 ± 0.7 | 19.3 ± 0.7 | 30 | 0.0547 | Non-significant |
| 13 | Acetone | 7 ± 1.2 | 4 ± 0.6 | 19 ± 1.5 | 30 | 0.2744 | Non-significant |
| 14 | Tiglic aldehyde | 5.3 ± 0.9 | 15 ± 1.2 | 9.7 ± 0.9 | 30 | 0.0207 | Significant |
| 15 | Ammonia | 24.7 ± 1.2 | 2.7 ± 0.9 | 2.7 ± 0.3 | 30 | 0.0000 | Significant |
| 16 | p-Tolualdehyde | 18.7 ± 0.9 | 1.7 ± 1.2 | 9.7 ± 1.5 | 30 | 0.0002 | Significant |
| 17 | Benzoic acid | 13.3 ± 0.3 | 3 ± 1 | 13.7 ± 1.3 | 30 | 0.0106 | Significant |
| 18 | Butyric acid | 4 ± 0.6 | 18.7 ± 1.8 | 7.3 ± 1.2 | 30 | 0.0022 | Significant |
| 19 | Carbon dioxide | 19 ± 1.2 | 1.7 ± 1.7 | 9.3 ± 2 | 30 | 0.0000 | Significant |
| 20 | Heptanoic acid | 16.3 ± 1.5 | 4 ± 1.5 | 9.7 ± 3 | 30 | 0.0059 | Significant |
| 21 | Hexanal | 5.7 ± 0.9 | 1.7 ± 0.9 | 22.7 ± 1.3 | 30 | 0.2266 | Non-significant |
| 22 | Hexanoic acid | 17.3 ± 0.9 | 4.3 ± 1.3 | 8.3 ± 1.5 | 30 | 0.0036 | Significant |
| 23 | Isobutyric acid | 6 ± 1.5 | 12 ± 1.5 | 12 ± 3.1 | 30 | 0.1189 | Non-significant |
| 24 | Mucochloric acid | 8.7 ± 0.9 | 2.3 ± 1.2 | 19 ± 1 | 30 | 0.1133 | Non-significant |
| 25 | Pentanoic acid | 17.3 ± 0.3 | 3 ± 0.6 | 9.7 ± 0.7 | 30 | 0.0013 | Significant |
| 26 | Pyruvate | 23.3 ± 0.9 | 3.7 ± 0.9 | 3 ± 1.5 | 30 | 0.0002 | Significant |
| 27 | Hexane | 5.3 ± 1.2 | 1 ± 0.6 | 23.7 ± 0.7 | 30 | 0.1094 | Non-significant |
| 28 | cis-3-Hexanoic acid | 13 ± 1.2 | 3.3 ± 1.3 | 13.7 ± 2.4 | 30 | 0.0106 | Significant |
| 29 | (R)-(+) Limonene | 8 ± 0.6 | 2 ± 1 | 20 ± 1.2 | 30 | 0.0547 | Non-significant |
| 30 | n-Octanoic acid | 12.7 ± 1.5 | 4.7 ± 0.3 | 12.7 ± 1.5 | 30 | 0.0717 | Non-significant |
| 31 | Ethyl Butyrate | 10.3 ± 1.8 | 4.3 ± 1.3 | 15.3 ± 0.7 | 30 | 0.0898 | Non-significant |
| 32 | Citral | 5.3 ± 0.9 | 7.3 ± 0.9 | 17.3 ± 0.9 | 30 | 0.3872 | Non-significant |
| 33 | Butyl butyrate | 4.3 ± 0.7 | 3.7 ± 1.2 | 22 ± 0.6 | 30 | 0.6367 | Non-significant |
| 34 | Isopropyl acetate | 12.3 ± 0.9 | 2 ± 1 | 15.7 ± 1.5 | 30 | 0.0065 | Significant |
| 35 | β-Caryophyllene | 4.7 ± 0.9 | 2.7 ± 1.5 | 22.7 ± 0.7 | 30 | 0.5000 | Non-significant |
| 36 | (R)-(+)α-pinene | 7.7 ± 1.8 | 2.7 ± 0.3 | 19.7 ± 1.5 | 30 | 0.1719 | Non-significant |
| 37 | Geraniol | 5 ± 1.2 | 19.3 ± 1.5 | 5.7 ± 1.2 | 30 | 0.0033 | Significant |
| 38 | 4-Methyl anisole | 12.3 ± 0.7 | 3.3 ± 0.9 | 14.3 ± 0.3 | 30 | 0.0176 | Significant |
| 39 | 1-Octanol | 11.3 ± 1.2 | 1 ± 0.6 | 17.7 ± 0.9 | 30 | 0.0032 | Significant |
| 40 | Propanoic acid | 19.3 ± 1.2 | 2 ± 0.6 | 8.7 ± 1.5 | 30 | 0.0001 | Significant |
| 41 | Cyclohexanol | 14.3 ± 0.9 | 3 ± 0.6 | 12.7 ± 1.2 | 30 | 0.0064 | Significant |
| 42 | L-cysteine hydrochloride | 6.3 ± 1.2 | 2.7 ± 0.7 | 21 ± 0.6 | 30 | 0.2539 | Non-significant |
| 43 | N-Decanoic acid | 9.3 ± 0.9 | 2.3 ± 1.5 | 18.3 ± 0.7 | 30 | 0.0327 | Significant |
| 44 | Citronellal | 1.3 ± 0.9 | 25.3 ± 1.2 | 3.3 ± 0.3 | 30 | 0.0000 | Significant |
| 45 | Azadirachtin | 14 ± 1.5 | 14 ± 1.5 | 2 ± 3.1 | 30 | 0.0384 | Significant |
| 46 | 3-Hexanol | 18.3 ± 0.9 | 1.7 ± 0.7 | 10 ± 1 | 30 | 0.0002 | Significant |
| 47 | Dodecane | 14.7 ± 0.7 | 2.7 ± 0.3 | 12.7 ± 0.9 | 30 | 0.0064 | Significant |
| 48 | Tetradecane | 18 ± 1.5 | 3 ± 1 | 9 ± 2.5 | 30 | 0.0007 | Significant |
| 49 | Hexadecane | 16.3 ± 1.5 | 2.7 ± 0.3 | 11 ± 1.2 | 30 | 0.0022 | Significant |
| 50 | Octadecane | 16 ± 1.5 | 4 ± 1.2 | 10 ± 1.7 | 30 | 0.0059 | Significant |
| 51 | Eicosane | 13 ± 0.6 | 3 ± 1.5 | 14 ± 1.5 | 30 | 0.0106 | Significant |
| 52 | Docosane | 10 ± 1 | 3.3 ± 0.9 | 16.7 ± 1.2 | 30 | 0.0461 | Significant |
| 53 | Benzaldehyde | 4 ± 0.6 | 17.3 ± 1.2 | 8.7 ± 1.2 | 30 | 0.0096 | Significant |
| 54 | Eugenol | 3.7 ± 0.9 | 23.3 ± 1.5 | 3 ± 2.1 | 30 | 0.0002 | Significant |
| SL No. | Name of Organic Compound | Time Taken by the Tick to Reach the Arm (in Minutes) | Type of Response | Number of Specimens |
| 1 | Ammonia | 2.5 | Positive | 30 |
| 2 | Pyruvate | 2.9 | Positive | 30 |
| 3 | 1-Octen-3-ol | 3.3 | Positive | 30 |
| 4 | Hematin porcine | 3.4 | Positive | 30 |
| 5 | p-Tolualdehyde | 3.5 | Positive | 30 |
| 6 | Methyl salicylate | 3.5 | Positive | 30 |
| 7 | Uric acid | 3.8 | Positive | 30 |
| 8 | Tetradecane | 3.9 | Positive | 30 |
| 9 | Carbon dioxide | 4.0 | Positive | 30 |
| 10 | Propanoic acid | 4.1 | Positive | 30 |
| 11 | 3-Hexanol | 4.4 | Positive | 30 |
| 12 | Hexanoic acid | 4.5 | Positive | 30 |
| 13 | Adenine | 4.5 | Positive | 30 |
| 14 | 2,6-Dichlorophenol | 4.7 | Positive | 30 |
| 15 | Hexadecane | 4.8 | Positive | 30 |
| 16 | Heptanoic acid | 4.9 | Positive | 30 |
| 17 | Pentanoic acid | 5.1 | Positive | 30 |
| 18 | Octadecane | 5.3 | positive | 30 |
| 19 | Guanine | 5.3 | Positive | 30 |
| 20 | Nonanoic acid | 5.5 | Positive | 30 |
| Sl. No. | Name of the Organic Compounds | Time Taken by the Tick to Reach the Vacant Arm (in Minutes) | Type of Responses | Number of Total Specimens |
| 1. | Citronellal | 2.3 | Negative | 30 |
| 2. | Eugenol | 2.9 | Negative | 30 |
| 3. | Butyric acid | 3.3 | Negative | 30 |
| 4. | Geraniol | 3.4 | Negative | 30 |
| 5. | Benzaldehyde | 4.0 | Negative | 30 |
| 6. | Tiglic aldehyde | 4.2 | Negative | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandal, A.; Paul, B.; Bhowmik, B.; Gundreddy, R.R.; Mirzaieva, A.U.; Bhadra, K. Sensing of Volatile Organic Compounds by Haller’s Structure in Ixodidae Tick: Electroscutumography and Olfactometric Bioassay. Biosensors 2025, 15, 358. https://doi.org/10.3390/bios15060358
Mandal A, Paul B, Bhowmik B, Gundreddy RR, Mirzaieva AU, Bhadra K. Sensing of Volatile Organic Compounds by Haller’s Structure in Ixodidae Tick: Electroscutumography and Olfactometric Bioassay. Biosensors. 2025; 15(6):358. https://doi.org/10.3390/bios15060358
Chicago/Turabian StyleMandal, Alivia, Bishwajeet Paul, Biswanath Bhowmik, Raja Reddy Gundreddy, Adolat U. Mirzaieva, and Kakali Bhadra. 2025. "Sensing of Volatile Organic Compounds by Haller’s Structure in Ixodidae Tick: Electroscutumography and Olfactometric Bioassay" Biosensors 15, no. 6: 358. https://doi.org/10.3390/bios15060358
APA StyleMandal, A., Paul, B., Bhowmik, B., Gundreddy, R. R., Mirzaieva, A. U., & Bhadra, K. (2025). Sensing of Volatile Organic Compounds by Haller’s Structure in Ixodidae Tick: Electroscutumography and Olfactometric Bioassay. Biosensors, 15(6), 358. https://doi.org/10.3390/bios15060358






