A Gait Sub-Phase Switching-Based Active Training Control Strategy and Its Application in a Novel Rehabilitation Robot
Abstract
1. Introduction
2. Materials and Methods
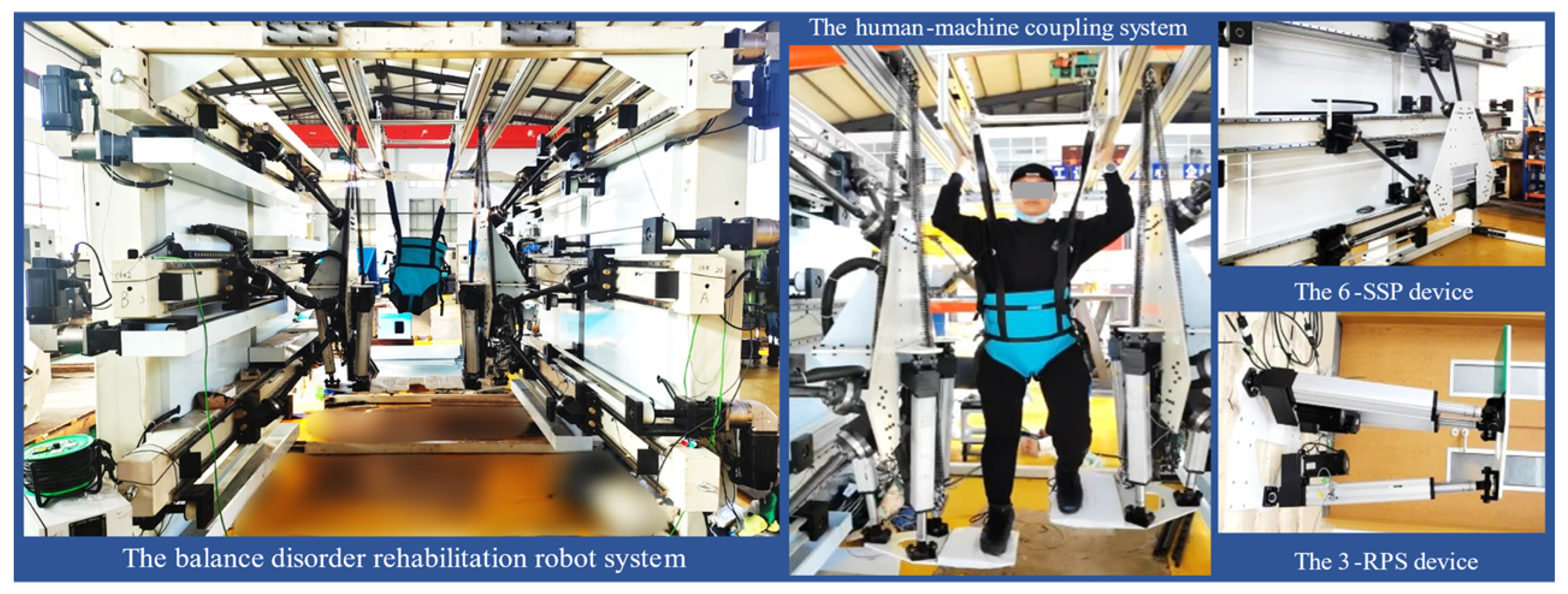
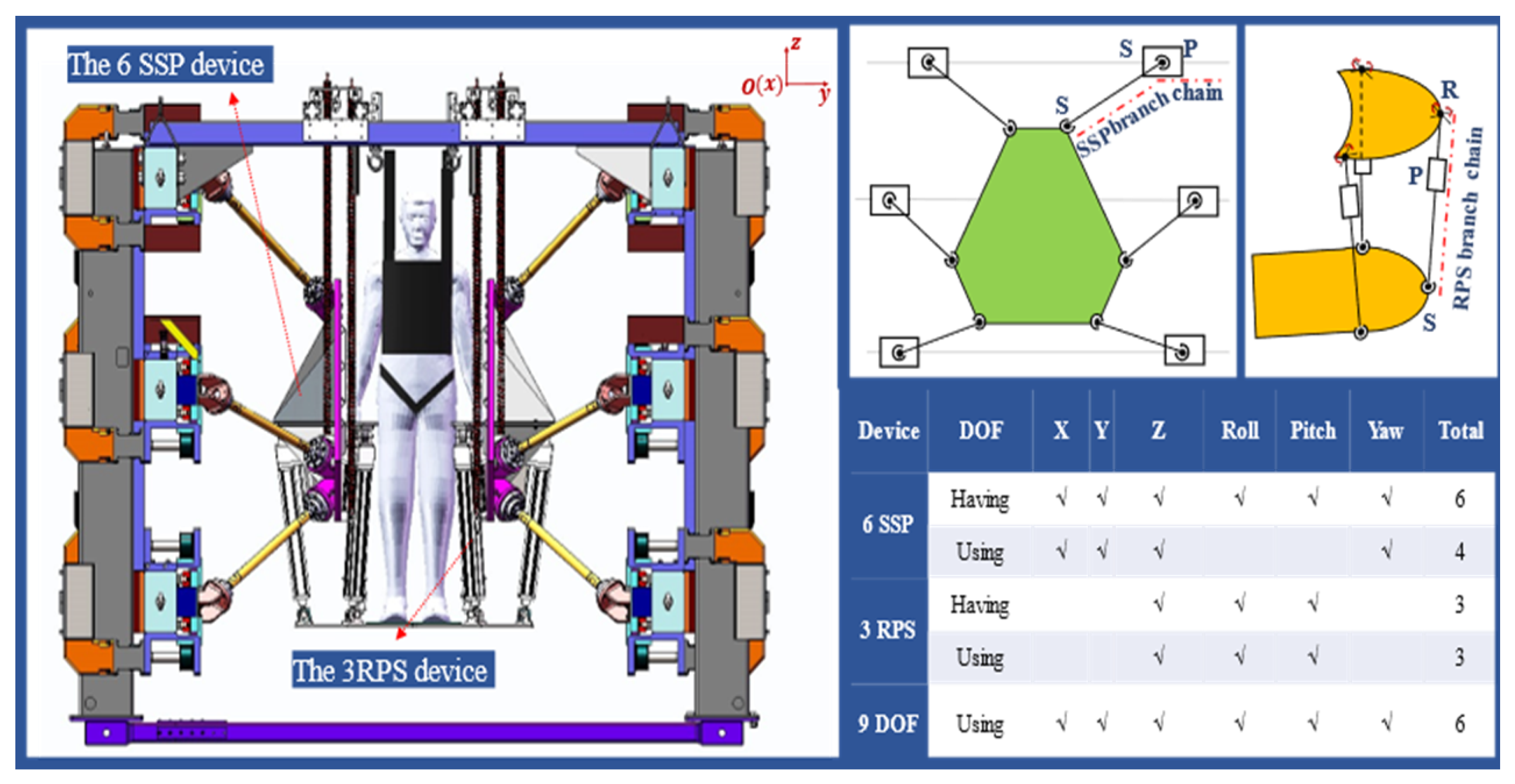
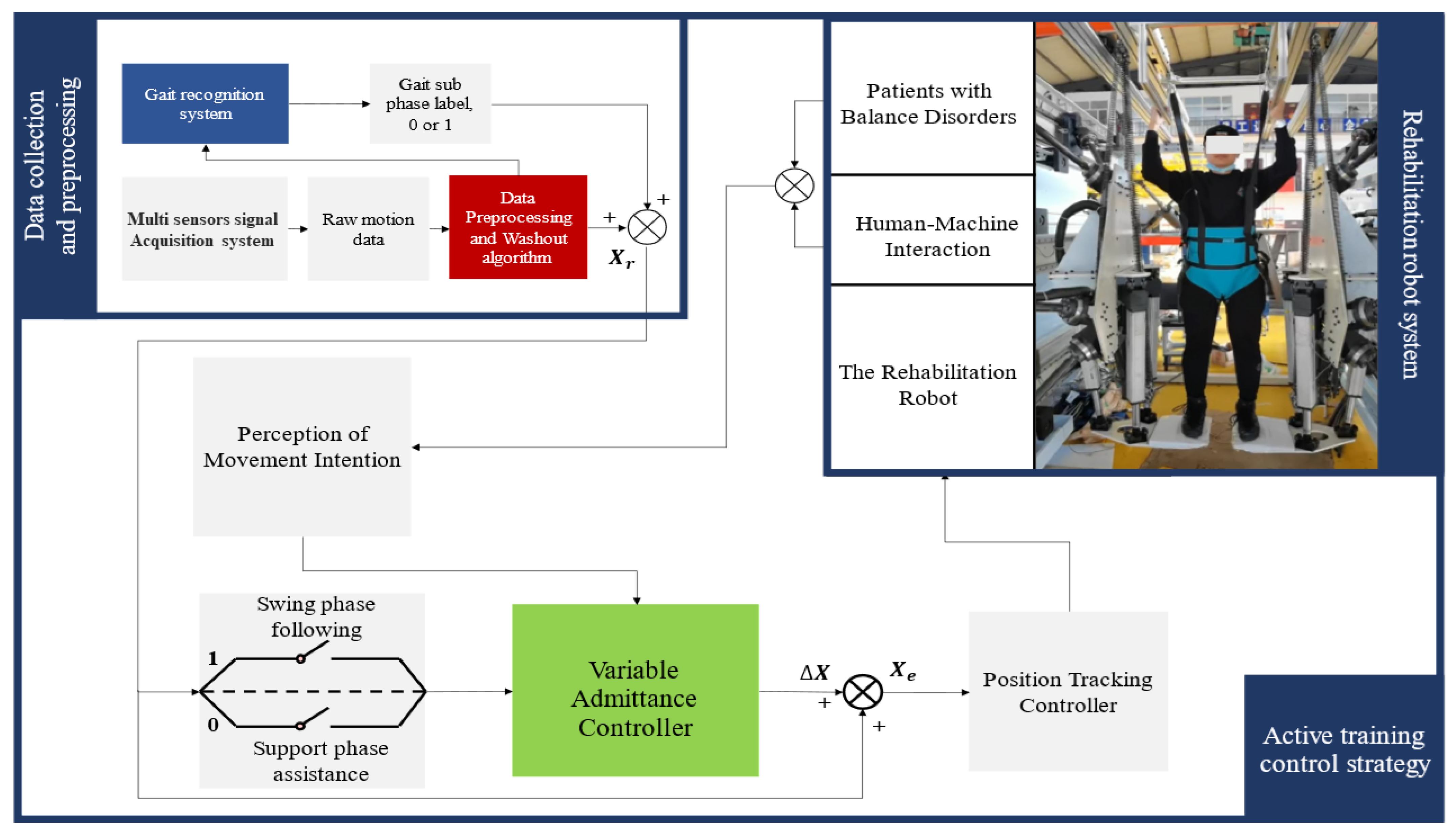
2.1. A Novel Rehabilitation Robot System
- Software Limit Mechanism: In the upper computer software application, the motor position commands are limited based on the calculated working space of the robot, ensuring that the robot operates within an appropriate range at the software control level.
- Hardware Limit Mechanism: Blocks are placed at appropriate positions at both ends of each guide rail slider to restrict the slider’s movement within a limited stroke.
- Emergency Stop Device: During patient training, medical staff are required to supervise the process. In the event of an emergency, pressing the Emergency Stop Device will immediately activate the brakes on all motors, applying braking force to stop the motors. The robot will immediately stop working and remain stationary.
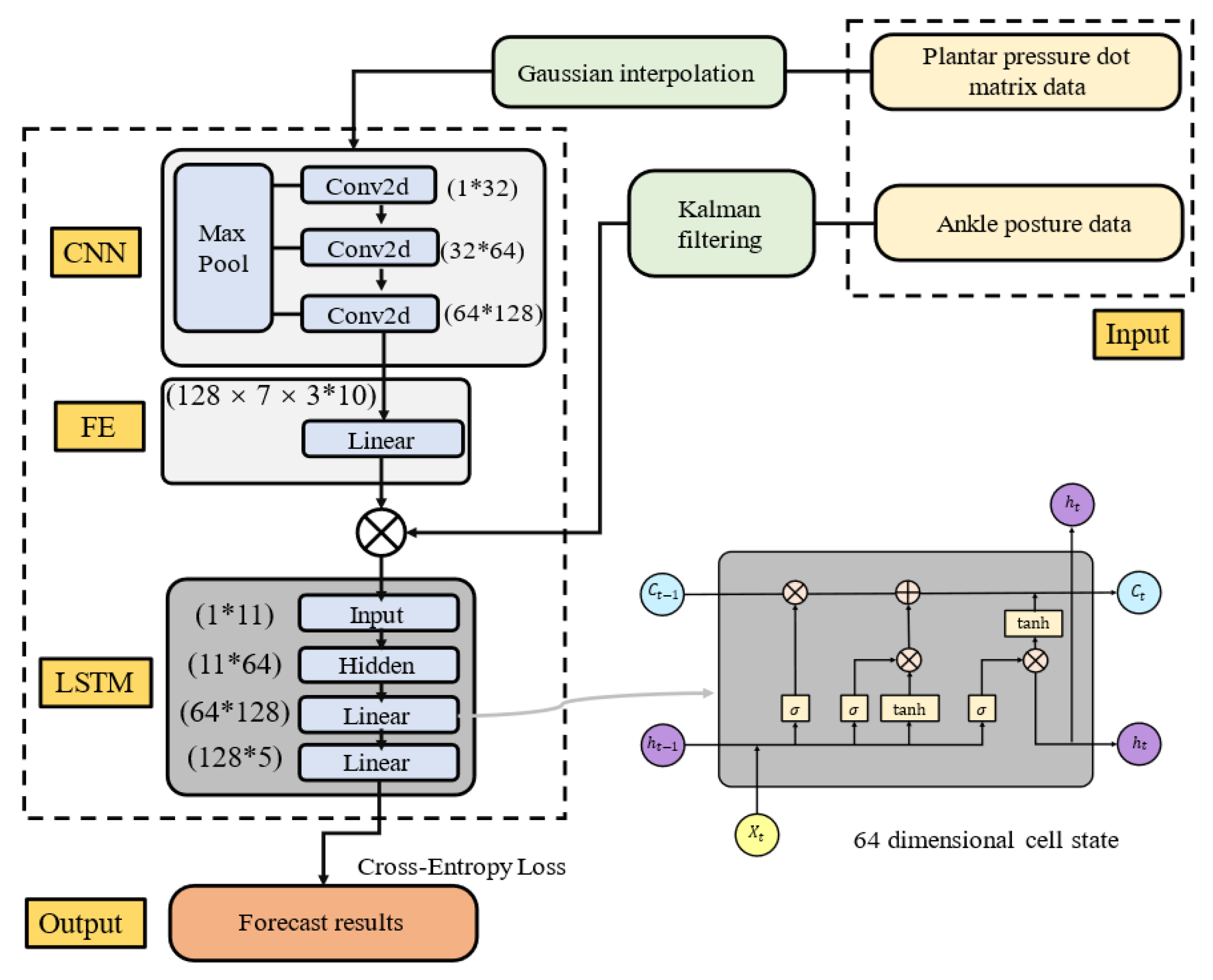
2.2. Gait Phase Identification System
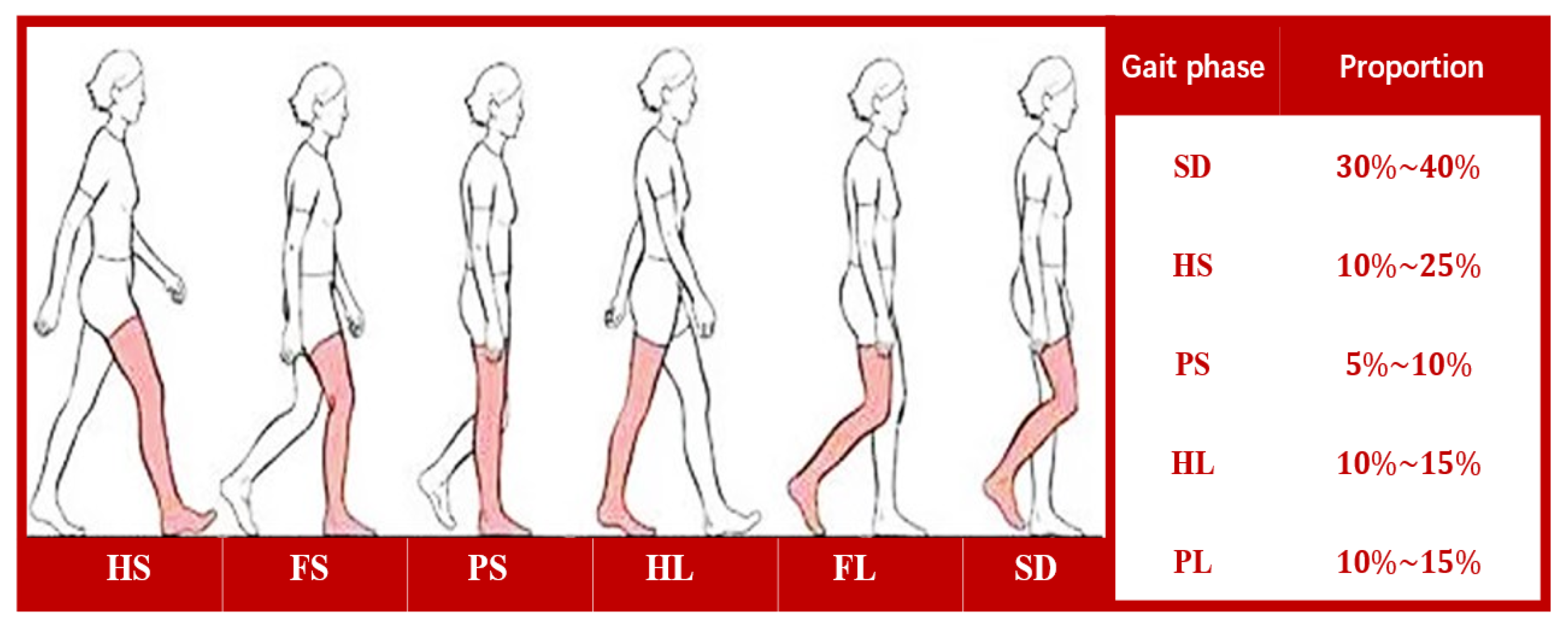
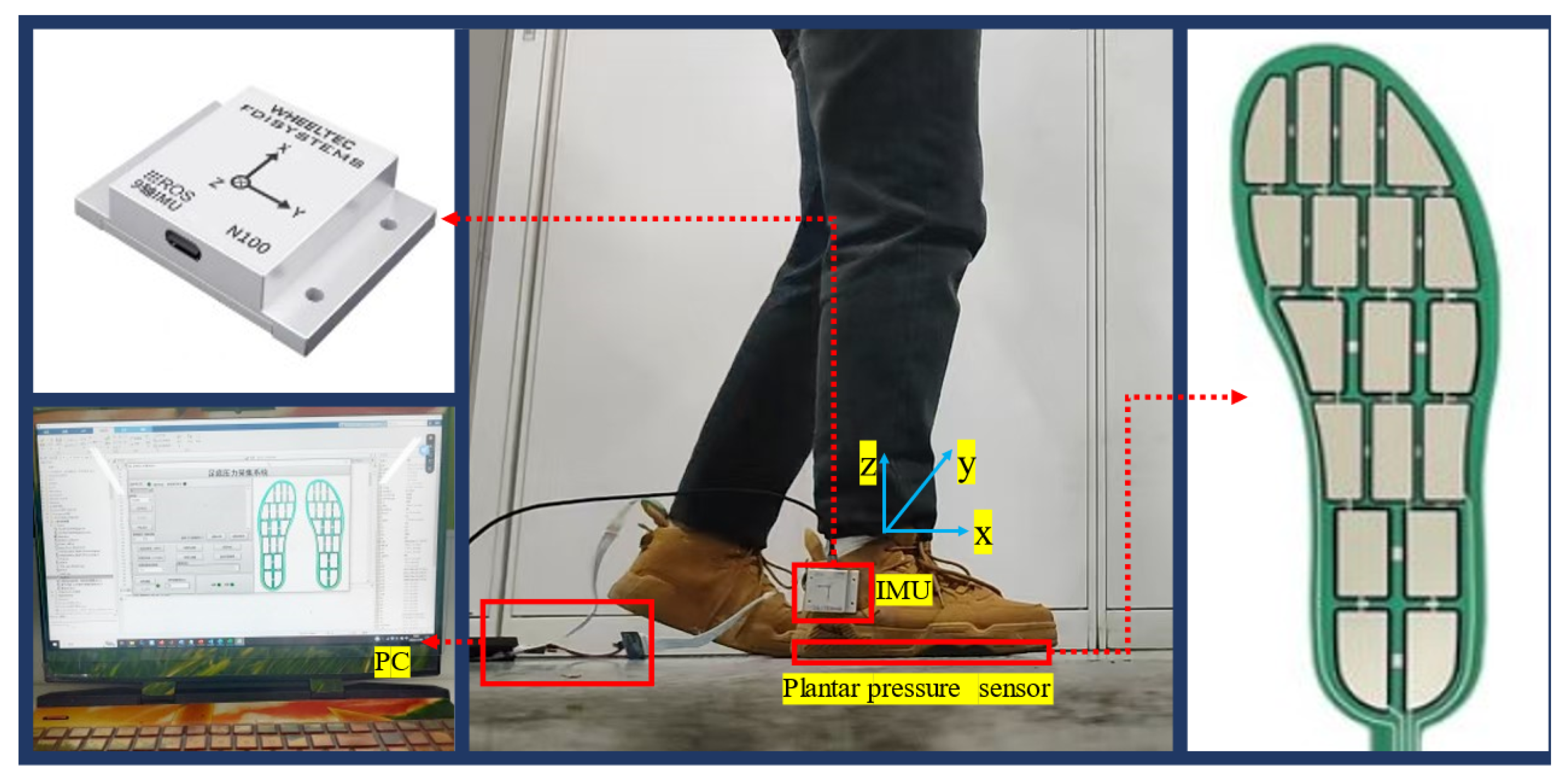
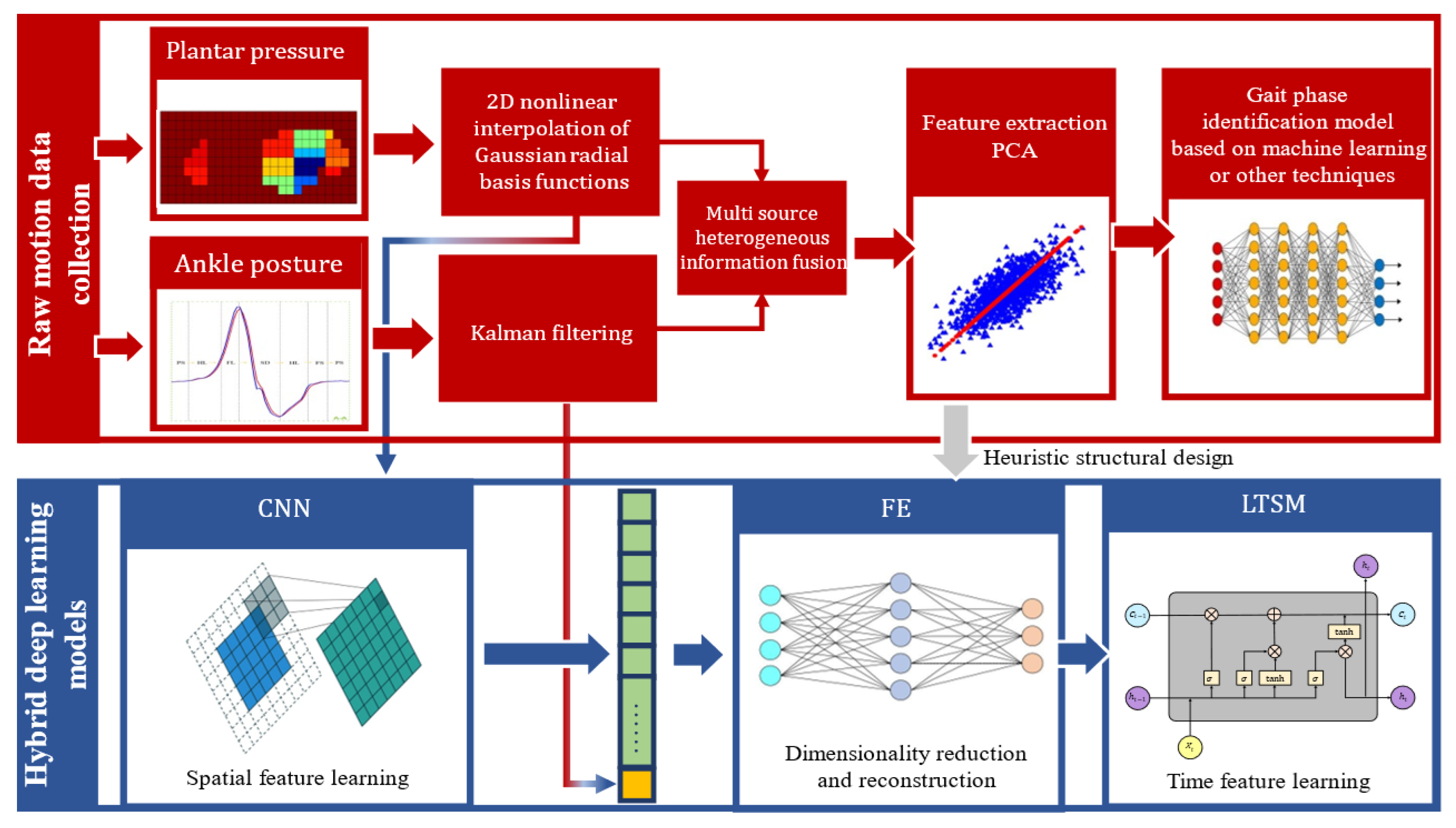
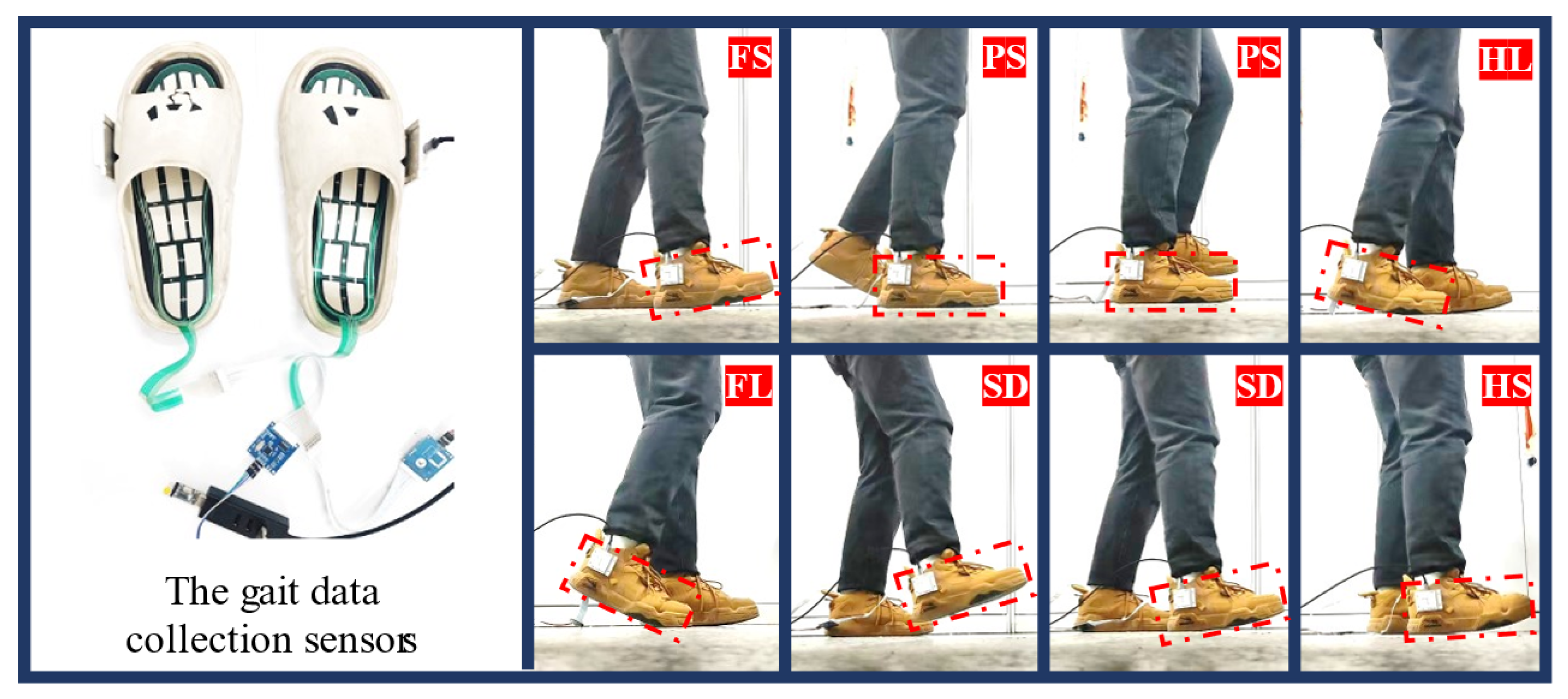
2.2.1. Gait Phase Division and Multi-Sensor System Composition
2.2.2. Data Preprocessing
- (1)
- Two-dimensional nonlinear interpolation based on Gaussian RBF
- (2)
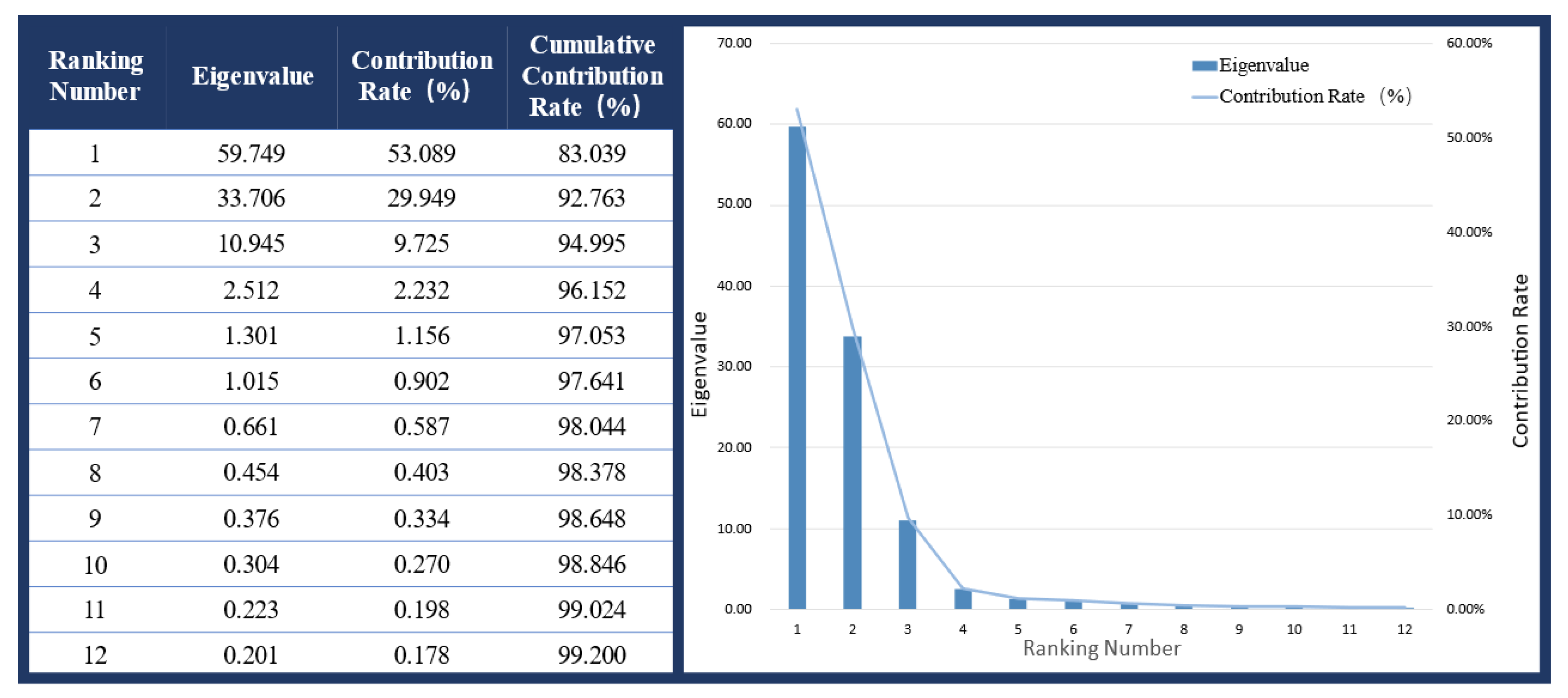
- Principal Component Analysis
- —the raw value of the i-th sample for the j-th feature.
- , —the mean and standard deviation of the j-th feature.
- Z—the matrix of standardized data.
- m—the number of samples.
- C—the covariance matrix.
- v—the eigenvector.
- —the corresponding eigenvalue.
- (3)
- Gait phase identification system
2.3. Active Training Control Strategy
2.3.1. Admittance Control
- q—the joint position vector of the robot.
- —the joint velocity vector.
- —the mass matrix.
- —the Coriolis and centrifugal force matrix.
- —the gravitational torque.
- —the control torque applied by the robot.
2.3.2. Active Training Control Strategy Based on Gait Phase Switching
3. Experiments and Results
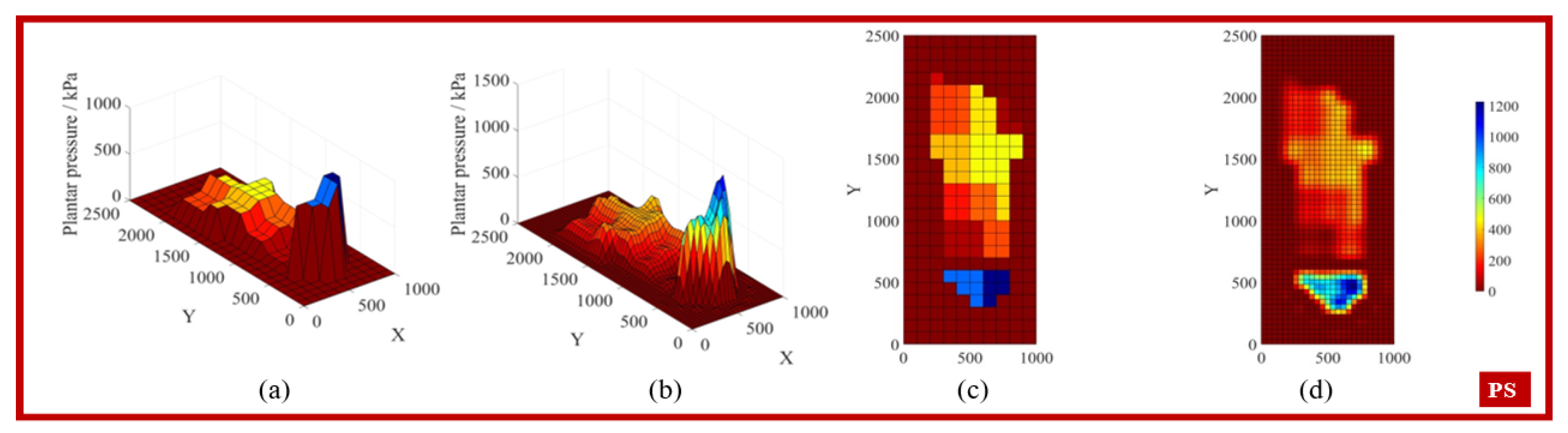
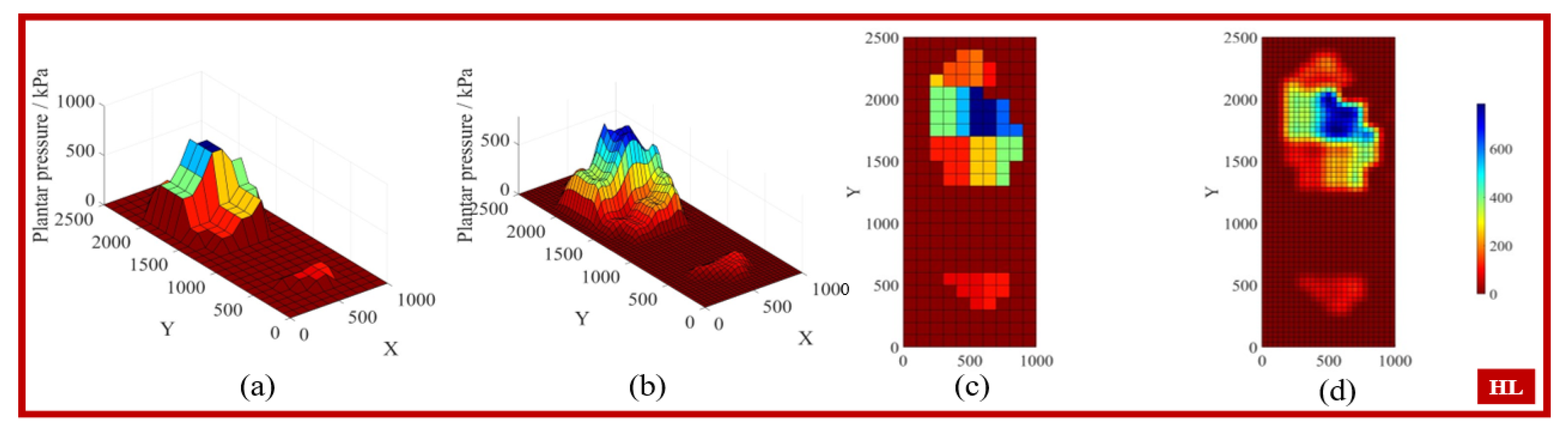
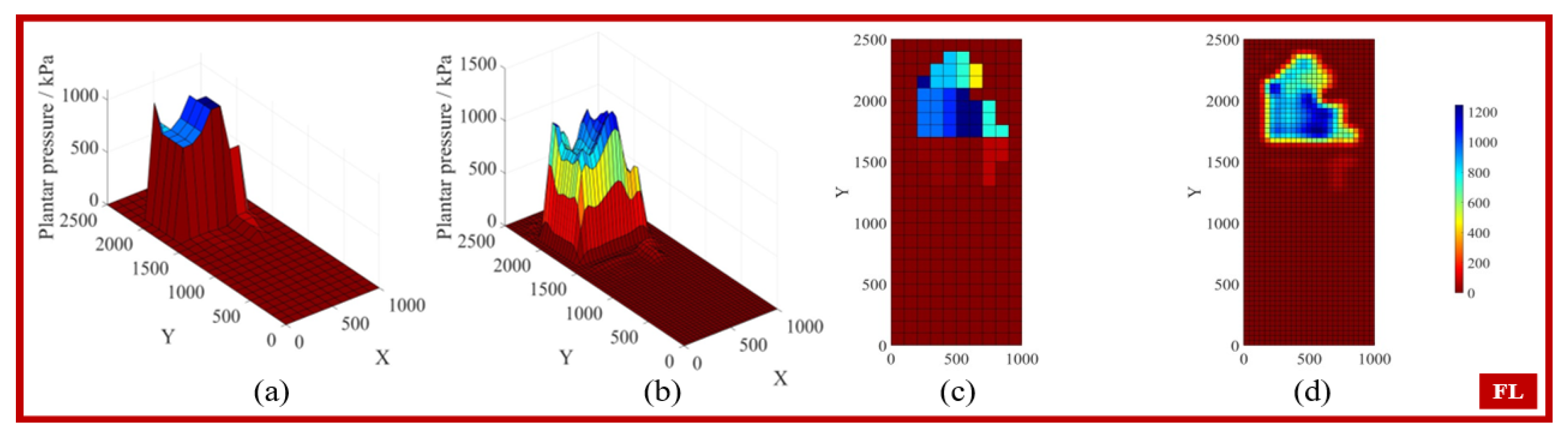
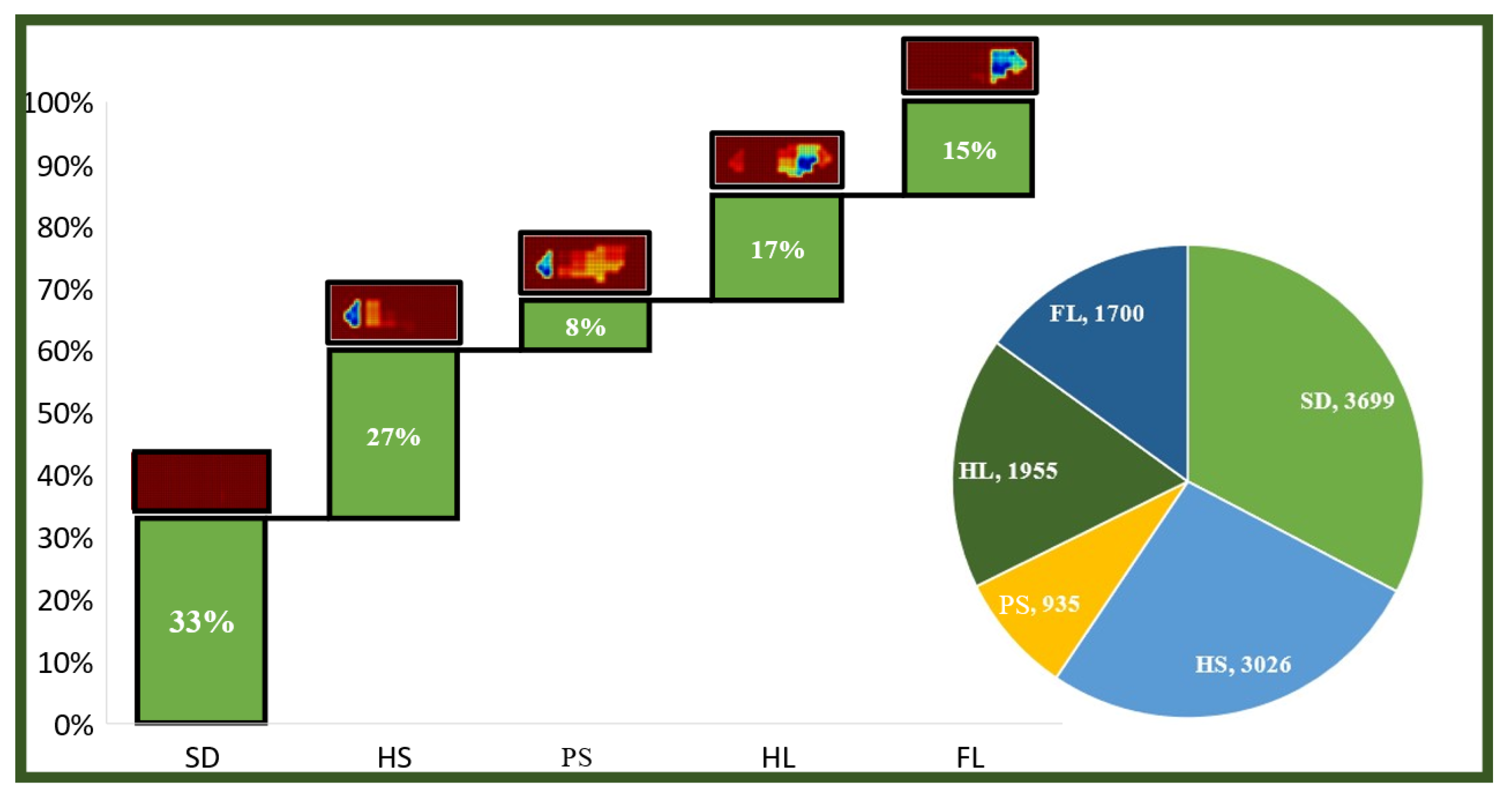
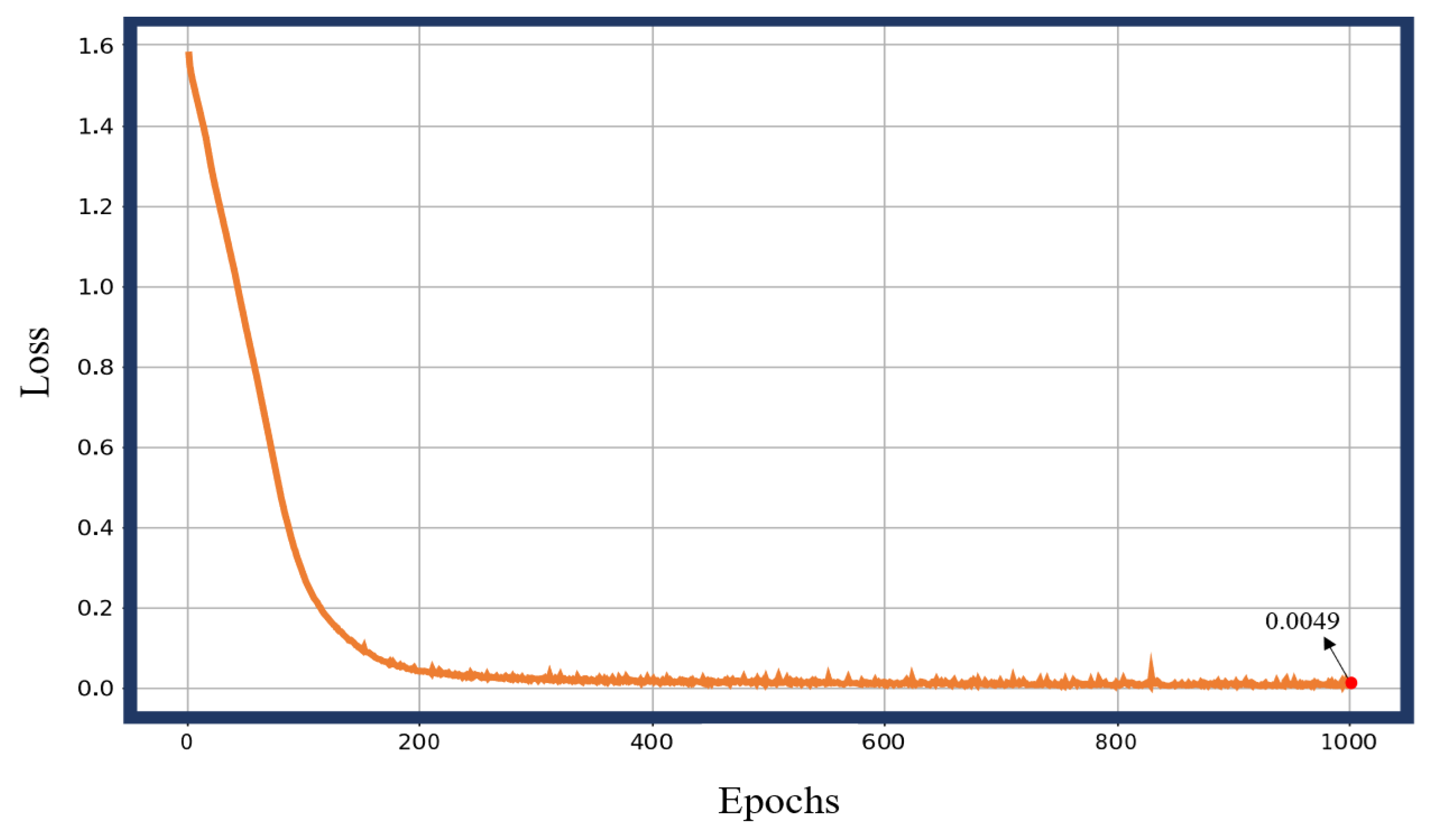
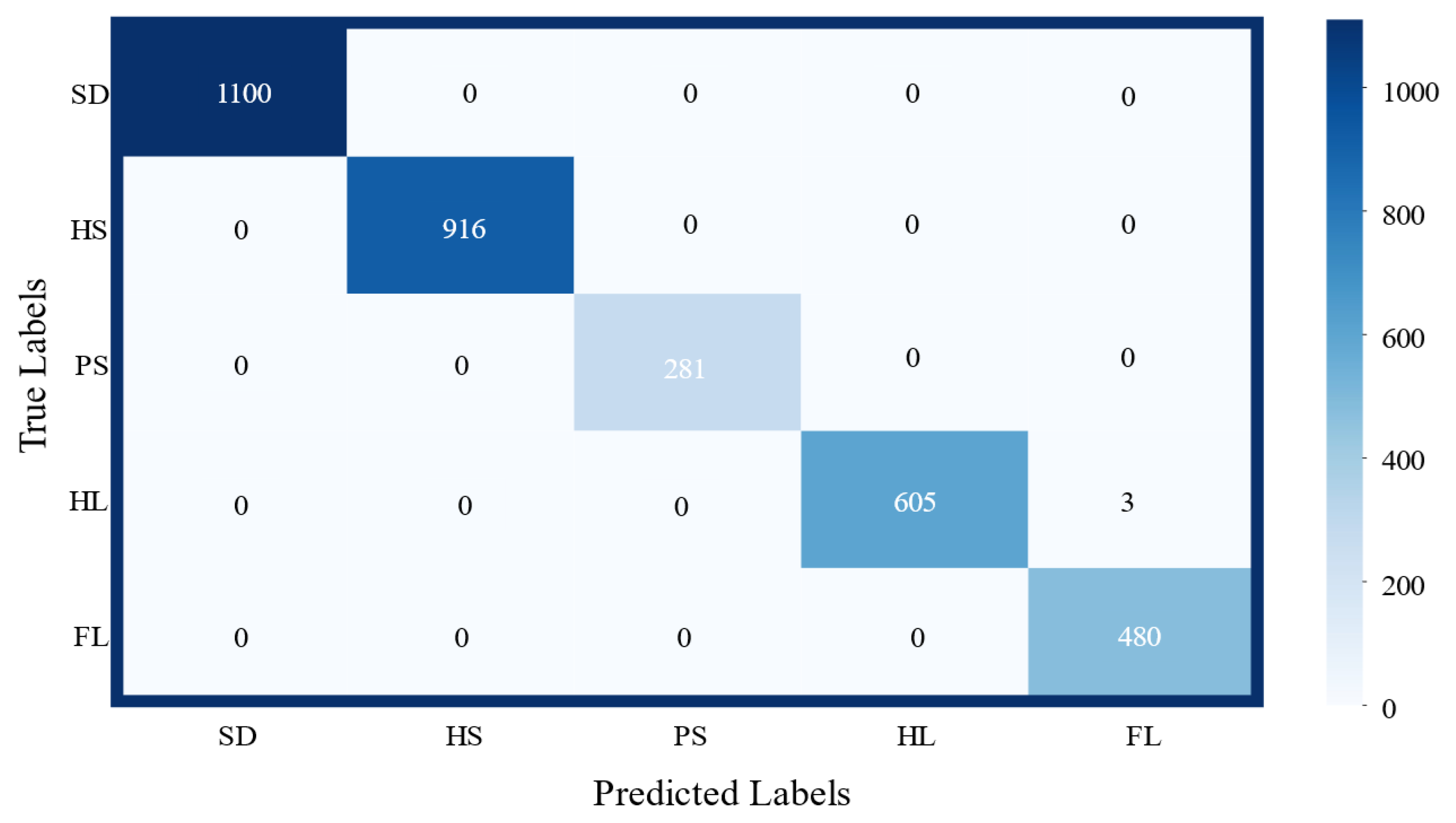
3.1. Gait Phase Identification and Result Analysis
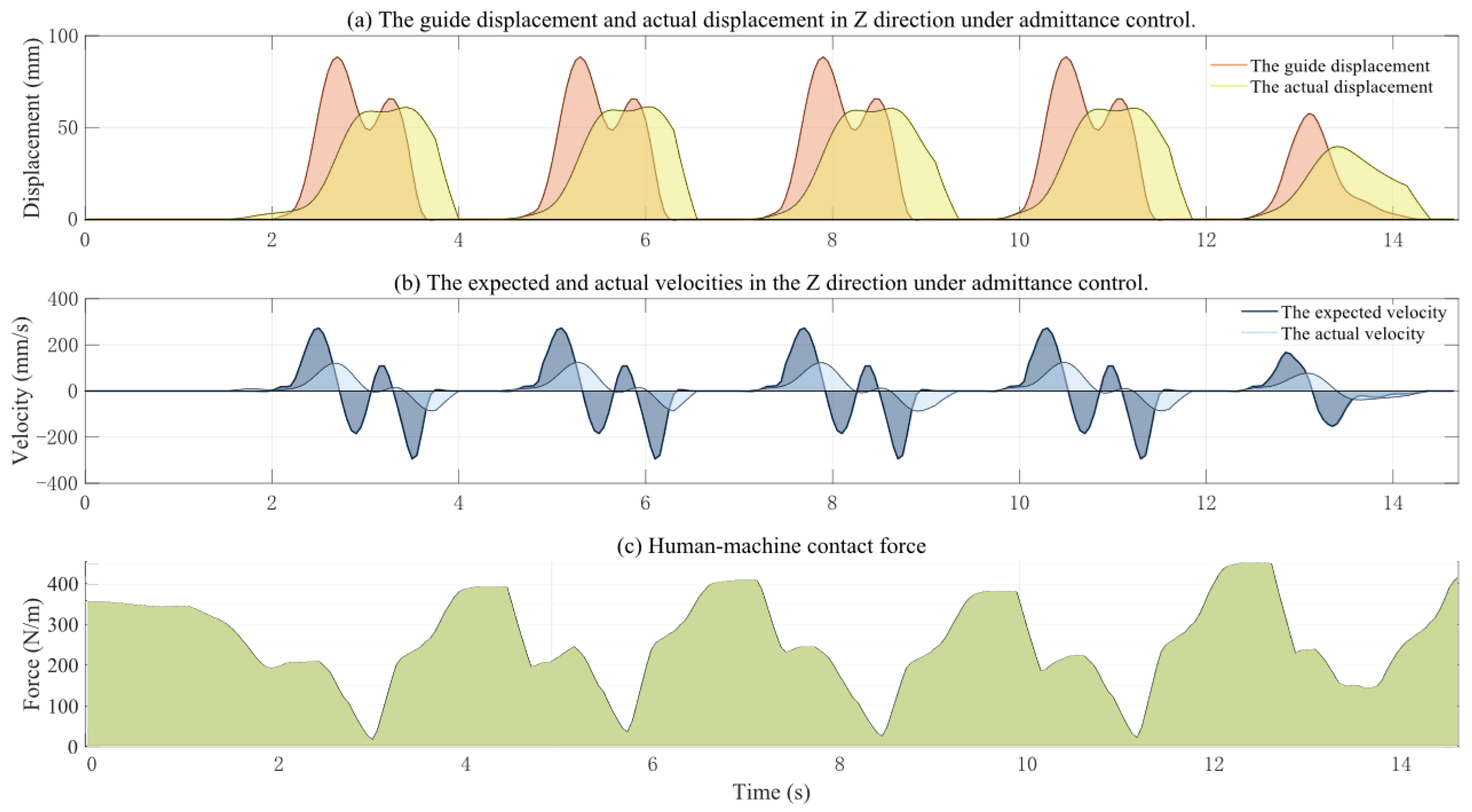
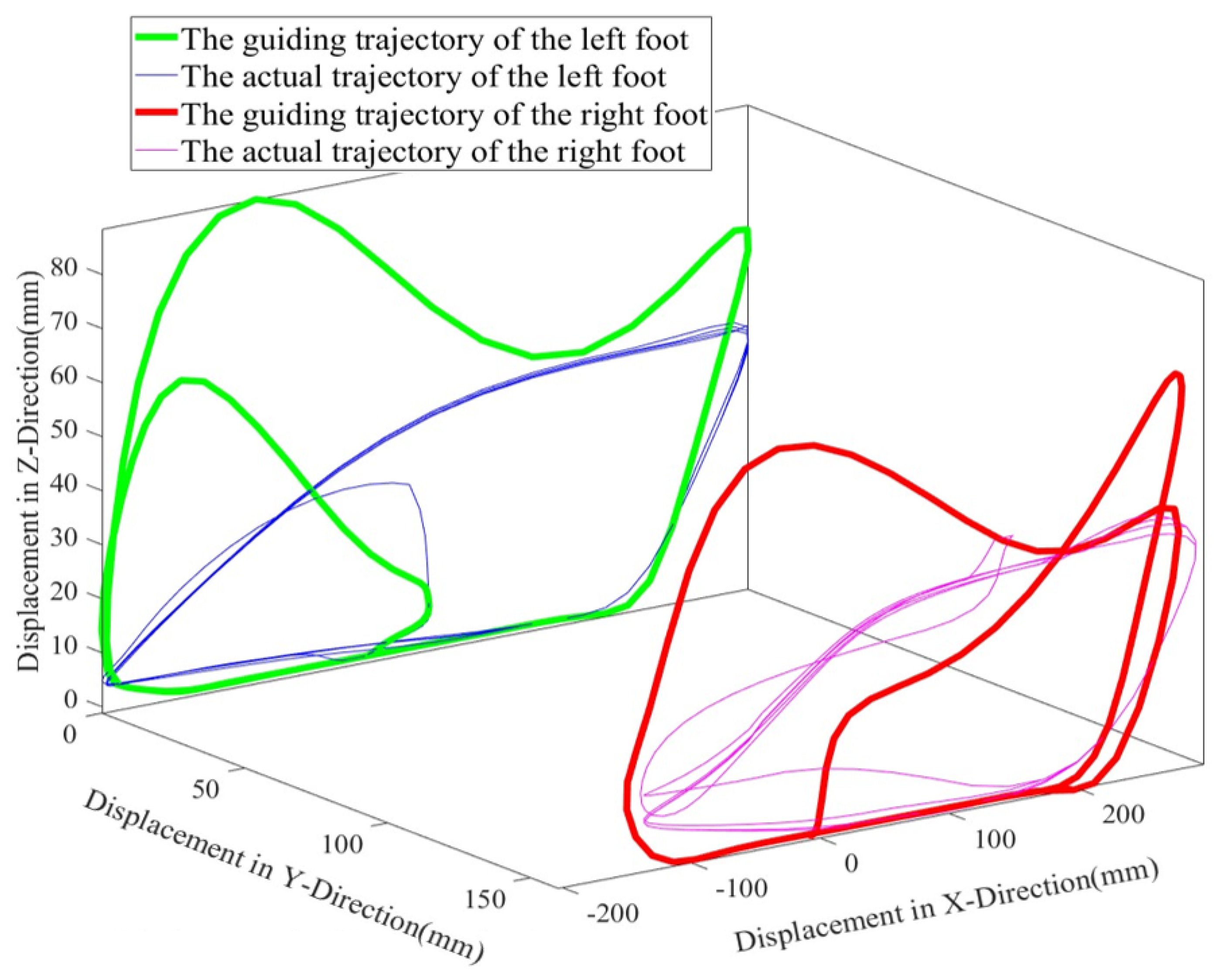
3.2. Active Training Control Strategy
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyeong, S. Gait Phase Recognition by Surface Electromyography Using a Recurrent Neural Network. Trans. Korean Soc. Mech. Eng. A 2024, 48, 727–734. [Google Scholar] [CrossRef]
- Guerra, B.M.V.; Schmid, M.; Sozzi, S.; Pizzocaro, S.; De Nunzio, A.M.; Ramat, S. Adopting BiLSTM for Gait Phase Recognition in Exoskeleton Control via sEMG Signals. In Proceedings of the IEEE International Symposium on Medical Measurements and Applications (MeMeA), Eindhoven, The Netherlands, 26–28 June 2024. [Google Scholar] [CrossRef]
- Horst, F.; Slijepcevic, D.; Simak, M.; Schoellhorn, I.W. Gutenberg Gait Database, a ground reaction force database of level overground walking in healthy individuals. Sci. Data 2021, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Smith, C.; Farewik, E.G. Gait Phase Recognition Using Deep Convolutional Neural Network with Inertial Measurement Units. Biosensors 2020, 10, 109. [Google Scholar] [CrossRef]
- Weidong, G.; Zhenwei, Z. Gait Phase Recognition Using Fuzzy Logic Regulation with Multisensor Data Fusion. J. Sens. 2021, 2021, 8776059. [Google Scholar] [CrossRef]
- Lee, J.; Hong, W.; Hur, P. Continuous Gait Phase Estimation Using LSTM for Robotic Transfemoral Prosthesis Across Walking Speeds. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1470–1477. [Google Scholar] [CrossRef]
- Luu, T.P.; Brantley, J.A.; Zhu, F.; Contreras-Vidal, J.L. Cortical Features of Locomotion-Mode Transitions via Non-invasive EEG. In Proceedings of the 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Banff, AB, Canada, 5–8 October 2017; pp. 2437–2441. [Google Scholar]
- Cui, Y.; Kang, Y. Multi-modal Gait Recognition via Effective Spatial-Temporal Feature Fusion. In Proceedings of the 2023 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Vancouver, BC, Canada, 17–24 June 2023. [Google Scholar] [CrossRef]
- Mogan, J.N.; Lee, C.P.; Lim, K.M. Ensemble CNN-ViT Using Feature-Level Fusion for Gait Recognition. IEEE Access 2024, 12, 108573–108583. [Google Scholar] [CrossRef]
- Hanif, C.A.; Mughal, M.A.; Khan, M.A.; Almujally, N.A.; Kim, T.; Cha, J.H. Human Gait Recognition for Biometrics Application Based on Deep Learning Fusion Assisted Framework. CMC-Comput. Mater. Contin. 2024, 78, 357–374. [Google Scholar] [CrossRef]
- Di Nardo, F.; Morbidoni, C.; Ventura, F.; Cucchiarelli, A.; Fioretti, S. Evaluating Bilateral Surface EMG Features for Automatic Identification of Gait Phase Transitions in Ground Walking Conditions. In Proceedings of the Mediterranean Conference on Medical and Biological Engineering and Computing (MEDICON) and International Conference on Medical and Biological Engineering (CMBEBIH), Sarajevo, Bosnia and Herzegovina, 14–16 September 2023; Volume 1, pp. 517–525. [Google Scholar] [CrossRef]
- Park, K.W.; Choi, J.; Kong, K. Data-Driven Modeling for Gait Phase Recognition in a Wearable Exoskeleton Using Estimated Forces. IEEE Trans. Robot. 2023, 39, 3072–3086. [Google Scholar] [CrossRef]
- Song, W.K. Upper Extremity Rehabilitation Robot Platform: From Unilateral Toward Bimanual. In Proceedings of the 18th International Conference on Intelligent Autonomous Systems (IAS), Suwon, Republic of Korea, 4–7 July 2023; Lee, S., An, J., Chong, N., Strand, M., Kim, J., Eds.; Lecture Notes in Networks and Systems. Springer Nature: Cham, Switzerland, 2024; pp. 419–427. [Google Scholar] [CrossRef]
- Ling, W.; Yu, G.; Li, Z. Lower Limb Exercise Rehabilitation Assessment Based on Artificial Intelligence and Medical Big Data. IEEE Access 2019, 7, 126787–126798. [Google Scholar] [CrossRef]
- Zuccon, G.; Doria, A.; Rosati, G.; Johnson, C.A.; McEligot, L.; Hertz, K.; Fernan, K.; Khan, I.; Edgerton, V.R.; Reinkensmeyer, D.J. Design of a Cable-Suspended Robot for Early Stage Gait Rehabilitation. IEEE Trans. Med. Robot. Bionics 2024, 6, 1616–1626. [Google Scholar] [CrossRef]
- Meng, W.; Tian, Z.; Zhu, C.; Ai, Q.; Liu, Q. Optimized Impedance Control of a Lightweight Gait Rehabilitation Exoskeleton Based on Accurate Knee Joint Torque Estimation. IEEE Trans. Med. Robot. Bionics 2024, 6, 1648–1657. [Google Scholar] [CrossRef]
- Penna, M.F.; Giordano, L.; Tortora, S.; Astarita, D.; Amato, L.; Dell’Agnello, F.; Menegatti, E.; Gruppioni, E.; Vitiello, N.; Crea, S.; et al. A muscle synergies-based controller to drive a powered upper-limb exoskeleton in reaching tasks. Wearable Technol. 2024, 5, e14. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Pi, M.; Jin, L.; Song, R.; Li, Z. Human Collaborative Control of Lower-Limb Prosthesis Based on Game Theory and Fuzzy Approximation. IEEE Trans. Cybern. 2025, 55, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Khoshdel, V.; Akbarzadeh, A.; Naghavi, N.; Sharifnezhad, A.; Souzanchi-Kashani, M. sEMG-based impedance control for lower-limb rehabilitation robot. Intell. Serv. Robot. 2018, 11, 97–108. [Google Scholar] [CrossRef]
- Adeola-Bello, Z.A.; Azlan, N.Z.; Abu Hassan, S.A. Control Strategy for Power Assist Upper Limb Rehabilitation Robot with the Therapist’s Motion Intention Prediction. IIUM Eng. J. 2023, 24, 285–300. [Google Scholar] [CrossRef]
- Mortezanejad, M.; Daryabor, A.; Ebrahimabadi, Z.; Rahimi, A.; Yousefi, M.; Ehsani, F.; Maleki, A. Kinetic changes of gait initiation in individuals with chronic ankle instability: A systematic review. Health Sci. Rep. 2024, 7, e70143. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Yi, P.; Zeng, K.; He, Z.; Zou, Q. Gait Pyramid Attention Network: Toward Silhouette Semantic Relation Learning for Gait Recognition. IEEE Trans. Biom. Behav. Identity Sci. 2022, 4, 582–595. [Google Scholar] [CrossRef]
- Tian, Y.; Sukthankar, R.; Shah, M. Spatiotemporal Deformable Part Models for Action Detection. In Proceedings of the 26th IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Portland, OR, USA, 23–28 June 2013; pp. 2642–2649. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Chu, G.; Cai, H.; Li, Y.; Zhao, J. Research on the configuration of a balanced disability rehabilitation robot based on redundant degrees of freedom. Adv. Mech. Eng. 2024, 16, 16878132241272231. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Zhao, J.; Jia, Z. Research on a New Rehabilitation Robot for Balance Disorders. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 3927–3936. [Google Scholar] [CrossRef]
- Yang, Z.X.; Wang, X.B.; Si, W.C. Application of Kalman Filtering Algorithm Aided by RBF Neural Network to Transfer Alignment of INS. In Proceedings of the International Conference on Mechanics and Materials Science (MMS), Guangzhou, China, 15–16 October 2016; pp. 502–509. [Google Scholar]
- Gryvill, H.; Tjelmeland, H. Ensemble Kalman filter with precision localization. Comput. Stat. 2025, 40, 2781–2805. [Google Scholar] [CrossRef]
- Tanaka, T.; Zinage, V.; Ugrinovskii, V.; Skoglund, M. Continuous-Time Channel Gain Control for Minimum-Information Kalman-Bucy Filtering. IEEE Trans. Autom. Control 2024, 69, 7255–7262. [Google Scholar] [CrossRef]
- Kumar, D.P.; Amgoth, T.; Annavarapu, C.S.R. Machine learning algorithms for wireless sensor networks: A survey. Inf. Fusion 2019, 49, 1–25. [Google Scholar] [CrossRef]
- Qi, L.; Zhiwei, Z. A Survey on Principal Component Analysis in Data Preprocessing and Its Applications. J. Comput. Sci. 2020, 47. [Google Scholar]
- Xiaoqiang, L.; Yue, M. Principal Component Analysis as a Preprocessing Step in Deep Learning for Classification Tasks. Neural Comput. Appl. 2021, 33, 4267–4277. [Google Scholar]
- Jia, Z.; Guangchang, D.; Feng, C.; Xiaodan, X.; Chengming, Q.; Lin, L. A Deep Learning Fusion Recognition Method Based On SAR Image Data. Procedia Comput. Sci. 2019, 147, 533–541. [Google Scholar] [CrossRef]
- Alexiou, E.; Zhou, X.; Viola, I.; Cesar, P. PointPCA: Point cloud objective quality assessment using PCA-based descriptors. Eurasip J. Image Video Process. 2024, 2024, 20. [Google Scholar] [CrossRef]
- Sidiropoulos, A.; Dimeas, F.; Papageorgiou, D.; Semetzidis, T.P.; Doulgeri, Z.; Zanella, A.; Grella, F.; Sagar, K.; Jilich, M.; Albini, A.; et al. Safe and Effective Collaboration with a High-Payload Robot: A Framework Integrating Novel Hardware and Software Modules. IEEE Robot. Autom. Mag. 2024, 31, 38–47. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Li, H.; Jiao, R.; Dong, M. Nonlinear Observer-Based Sliding Mode Control for Robot-Aided Bilateral Human-Compliant Rehabilitation Training of Upper Limb. IEEE Trans. Autom. Sci. Eng. 2025, 22, 9130–9139. [Google Scholar] [CrossRef]
- Hoffmann, A.; Ghiassi, M.; Kecskemethy, A. Use of Force-Controlled Compliance-Eigenvector Power-Iterations for Finding an Instantaneous Knee Axis: Mockup Study for a Fixed Hinge. In Proceedings of the International symposium on Advances in Robot Kinematics (ARK), Ljubljana, Slovenia, 30 June–4 July 2024; Volume 31, pp. 239–248. [Google Scholar] [CrossRef]
- Farajtabar, M.; Charbonneau, M. The path towards contact-based physical human-robot interaction. Robot. Auton. Syst. 2024, 182. [Google Scholar] [CrossRef]
- Abbas, M.; Narayan, J.; Dwivedy, S.K. Event-triggered adaptive control for upper-extremity therapeutic robot in active-assist mode: A simulation study. Proc. Inst. Mech. Eng. Part C-J. Mech. Eng. Sci. 2024, 238, 4628–4643. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Wang, Y.; Yang, B.; Ma, T.; Leng, Y.; Fu, C. A Piecewise Monotonic Gait Phase Estimation Model for Controlling a Powered Transfemoral Prosthesis in Various Locomotion Modes. IEEE Robot. Autom. Lett. 2022, 7, 9549–9556. [Google Scholar] [CrossRef]



| Performance Parameter | Value |
|---|---|
| Static Resistance | >1 MΩ |
| Working Voltage | 3.3–5 V |
| Response Time | <20 ms |
| Hysteresis | <6% |
| Drift | <8% |
| Electromagnetic Interference | Nil |
| Parameter | Accelerometer | Gyroscope |
|---|---|---|
| Range | ±16 g | ±2000°/S |
| Linearity | <0.1% FS | <0.1% FS |
| Bandwidth | 260 Hz | 256 Hz |
| Orthogonality Error | ±0.05° | ±0.05° |
| Resolving Power | <0.5 mg | <0.02°/S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wang, R.; Man, Z.; Liu, Y.; Zhao, J.; Cai, H. A Gait Sub-Phase Switching-Based Active Training Control Strategy and Its Application in a Novel Rehabilitation Robot. Biosensors 2025, 15, 356. https://doi.org/10.3390/bios15060356
Wu J, Wang R, Man Z, Liu Y, Zhao J, Cai H. A Gait Sub-Phase Switching-Based Active Training Control Strategy and Its Application in a Novel Rehabilitation Robot. Biosensors. 2025; 15(6):356. https://doi.org/10.3390/bios15060356
Chicago/Turabian StyleWu, Junyu, Ran Wang, Zhuoqi Man, Yubin Liu, Jie Zhao, and Hegao Cai. 2025. "A Gait Sub-Phase Switching-Based Active Training Control Strategy and Its Application in a Novel Rehabilitation Robot" Biosensors 15, no. 6: 356. https://doi.org/10.3390/bios15060356
APA StyleWu, J., Wang, R., Man, Z., Liu, Y., Zhao, J., & Cai, H. (2025). A Gait Sub-Phase Switching-Based Active Training Control Strategy and Its Application in a Novel Rehabilitation Robot. Biosensors, 15(6), 356. https://doi.org/10.3390/bios15060356







