Betalain Pigments: Isolation and Application as Reagents for Colorimetric Methods and Biosensors
Abstract
1. Introduction
2. Characterization of Betalain
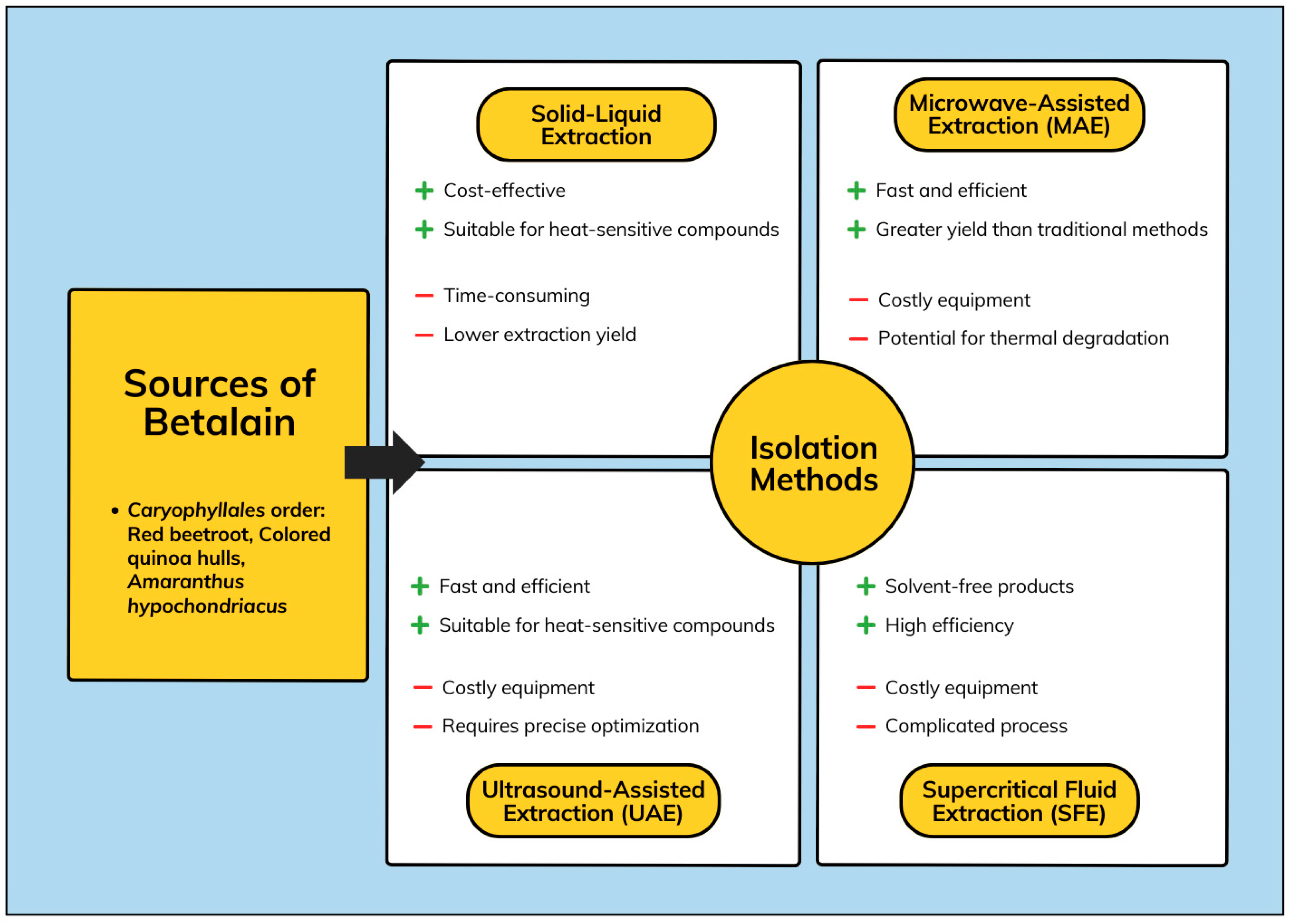
2.1. Source of Betalain
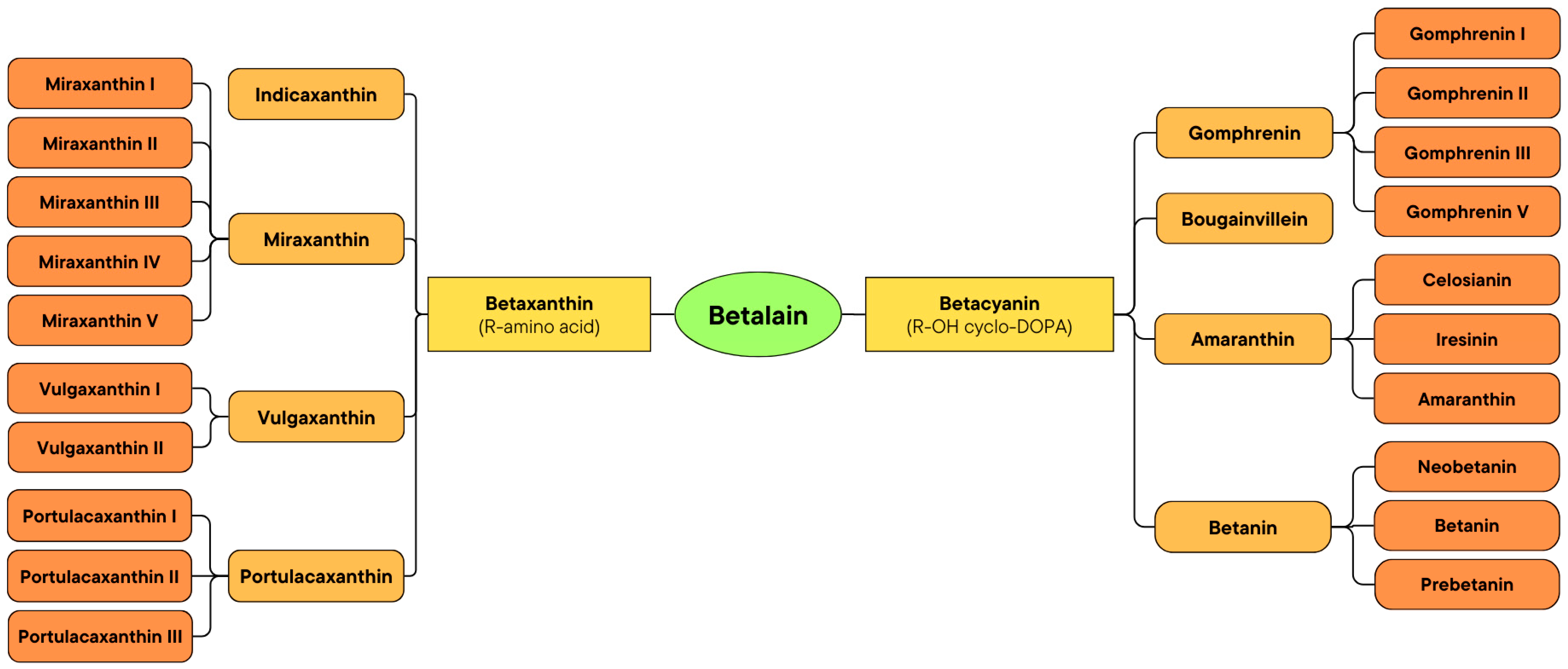
2.2. Classification of Betalain
2.3. Isolation of Betalain
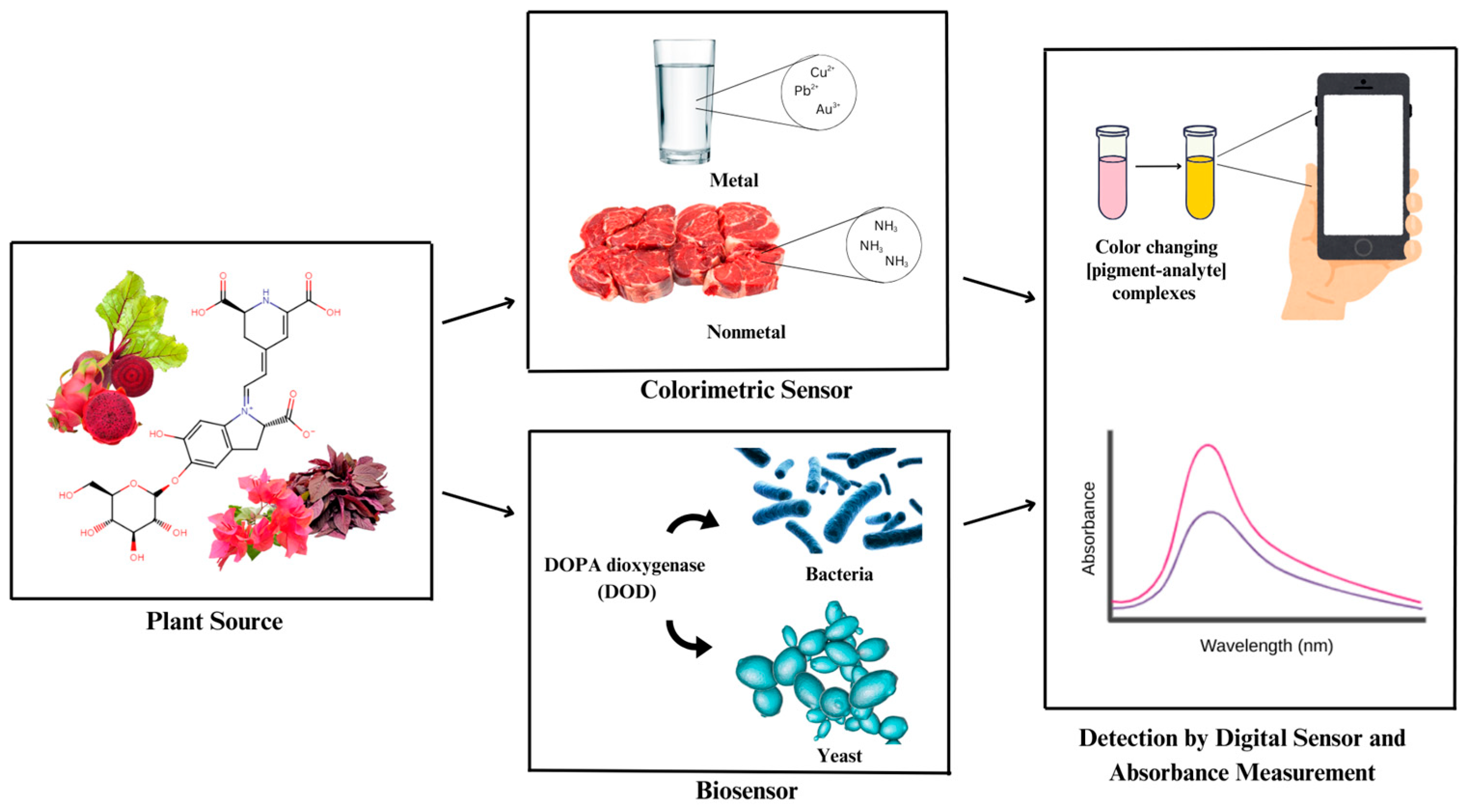
2.4. Application of Betalain as Colorimetric and Biosensor
3. Betalain as Colorimetric Sensor
3.1. Metal
3.2. Nonmetal
4. Betalain as Biosensor
4.1. Bacteria
4.2. Yeast
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garrigues, S.; de la Guardia, M. Challenges in Green Analytical Chemistry; Green Chemistry; Royal Society of Chemistry: London, UK, 2020. [Google Scholar]
- Koel, M.; Kaljurand, M. Green Analytical Chemistry; Royal Society of Chemistry: Cambridge, UK, 2019. [Google Scholar]
- Grumezescu, A.M.; Holban, A.M. Natural and Artificial Flavoring Agents and Food Dyes. In Handbook of Food Bioengineering; Elsevier Science: London, UK, 2017. [Google Scholar]
- Ferreira, I.C.F.R.; Barros, L. Functional Food Ingredients from Plants; Elsevier Science: London, UK, 2019. [Google Scholar]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Saeedi, M.; Nabavi, S.F.; Silva, A.S. Recent Advances in Natural Products Analysis; Elsevier Science: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Esteves, L.C.; Machado, C.O.; Gonçalves, L.C.P.; Cavalcante, V.F.; Obeid, G.; Correra, T.C.; Bastos, E.L. Structural Effects on the Antioxidant Properties of Amino Acid Betaxanthins. Antioxidants 2022, 11, 2259. [Google Scholar] [CrossRef]
- Coultate, T.P. Food: The Chemistry of Its Components; RSC paperbacks; Royal Society of Chemistry: London, UK, 2009. [Google Scholar]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta 2005, 222, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Kumorkiewicz-Jamro, A.; Świergosz, T.; Sutor, K.; Spórna-Kucab, A.; Wybraniec, S. Multi-Colored Shades of Betalains: Recent Advances in Betacyanin Chemistry. Nat. Prod. Rep. 2021, 38, 2315–2346. [Google Scholar] [CrossRef]
- Iraklii, E.; Nadia, L.; Jade, P.; Olena, Z. Colorimetric Sensors and Sensor Arrays. In Nanomaterials Design for Sensing Applications; Olena, Z., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 1; pp. 1–39. [Google Scholar]
- Skopińska, A.; Szot, D.; Starzak, K.; Wybraniec, S. Effect of Cu (II) Cations on 2-Decarboxy-betanin Stability in Aqueous-Organic Solutions. Chall. Mod. Technol. 2015, 6, 24–29. [Google Scholar]
- Awual, M.R.; Hasan, M.M.; Shahat, A.; Naushad, M.; Shiwaku, H.; Yaita, T. Investigation of ligand immobilized nano-composite adsorbent for efficient cerium(III) detection and recovery. Chem. Eng. J. 2015, 265, 210–218. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, J.; Hu, G.; Zhang, E.; Yu, H.H. Colorimetric Sensors for Chemical and Biological Sensing Applications. Sensors 2023, 23, 2749. [Google Scholar] [CrossRef] [PubMed]
- Shing, W.L. A Novel In Vivo Beta-Carotene Biosensor for Heavy Metals Detection. 2015. Available online: https://www.researchgate.net/publication/286883268 (accessed on 9 August 2024).
- Chen, P.-H.; Lin, C.; Guo, K.-H.; Yeh, Y.-C. Development of a pigment-based whole-cell biosensor for the analysis of environmental copper. RSC Adv. 2017, 7, 29302–29305. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Yeh, Y.-C. Dual-Signal Microbial Biosensor for the Detection of Dopamine without Inference from Other Catecholamine Neurotransmitters. Anal. Chem. 2017, 89, 11178–11182. [Google Scholar] [CrossRef]
- Chou, Y.-C.; Shih, C.-I.; Chiang, C.-C.; Hsu, C.-H.; Yeh, Y.-C. Reagent-free DOPA-dioxygenase colorimetric biosensor for selective detection of L-DOPA. Sens. Actuators B Chem. 2019, 297, 126717. [Google Scholar] [CrossRef]
- Deloache, W.C.; Russ, Z.N.; Narcross, L.; Gonzales, A.M.; Martin, V.J.J.; Dueber, J.E. An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose. Nat. Chem. Biol. 2015, 11, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Liu, Q.; Li, Y.; Yang, J.; Song, X.; Liu, X.; Xu, H.; Qiao, M. A high-throughput method for screening of L-tyrosine high-yield strains by Saccharomyces cerevisiae. J. Gen. Appl. Microbiol. 2018, 64, 198–201. [Google Scholar] [CrossRef]
- Miettinen, K.; Leelahakorn, N.; Almeida, A.; Zhao, Y.; Hansen, L.R.; Nikolajsen, I.E.; Andersen, J.B.; Givskov, M.; Staerk, D.; Bak, S.; et al. A GPCR-based yeast biosensor for biomedical, biotechnological, and point-of-use cannabinoid determination. Nat. Commun. 2022, 13, 3664. [Google Scholar] [CrossRef]
- Fan, C.; Zhang, D.; Mo, Q.; Yuan, J. Engineering Saccharomyces cerevisiae based biosensors for copper detection. Microb. Biotechnol. 2022, 15, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef]
- Maroušek, J.; Kolář, L.; Vochozka, M.; Stehel, V.; Maroušková, A. Novel method for cultivating beetroot reduces nitrate content. J. Clean. Prod. 2017, 168, 60–62. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Biological properties and applications of betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef]
- Carreón-Hidalgo, J.P.; Franco-Vásquez, D.C.; Gómez-Linton, D.R.; Pérez-Flores, L.J. Betalain plant sources, biosynthesis, extraction, stability enhancement methods, bioactivity, and applications. Food Res. Int. 2022, 151, 110821. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.F.H.; Husnain, M. Betalains: Potential drugs with versatile phytochemistry. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 169–189. [Google Scholar] [CrossRef]
- Kujala, T.; Loponen, J.; Pihlaja, K. Betalains and Phenolics in Red Beetroot (Beta Vulgaris) Peel Extracts: Extraction and Characterisation. 2001. Available online: www.znaturforsch.com (accessed on 1 August 2024).
- Rodrigues, A.C.B.; Mariz, I.d.F.A.; Maçoas, E.M.S.; Tonelli, R.R.; Martinho, J.M.G.; Quina, F.H.; Bastos, E.L. Bioinspired water-soluble two-photon fluorophores. Dye. Pigment. 2018, 150, 105–111. [Google Scholar] [CrossRef]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Rubio, M.A.; Hernández-García, S.; García-Carmona, F.; Gandía-Herrero, F. Extension of life-span using a RNAi model and in vivo antioxidant effect of Opuntia fruit extracts and pure betalains in Caenorhabditis elegans. Food Chem. 2019, 274, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Neelwarne, B. Red Beet Biotechnology: Food and Pharmaceutical Applications; Springer: New York, NY, USA, 2012. [Google Scholar]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.; Gabr, A.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Zin, M.M.; Márki, E.; Bánvölgyi, S. Conventional Extraction of Betalain Compounds from Beetroot Peels With Aqueous Ethanol Solvent. Acta Aliment. 2020, 49, 163–169. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E.; Ballard, T.; Liceaga, A.; Martín-González, M.F.S. Microwave-assisted extraction of betalains from red beet (Beta vulgaris). LWT Food Sci. Technol. 2014, 59, 276–282. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Flora, G.; Sevanan, M.; Sripriya, R.; Chen, W.; Park, J.-H.; Banu, J.R.; Kumar, G. Technological advances in the production of carotenoids and their applications—A critical review. Bioresour. Technol. 2023, 367, 128215. [Google Scholar] [CrossRef]
- da Silva, H.R.P.; da Silva, C.; Bolanho, B.C. Ultrasonic-assisted extraction of betalains from red beet (Beta vulgaris L.). J. Food Process. Eng. 2018, 41, e12833. [Google Scholar] [CrossRef]
- Alupului, A.; Calinescu, I.; Lavric, V. Ultrasonic vs. microwave extraction intensification of active principles from medicinal plants. AIDIC Conf. Ser. 2009, 9, 1–8. [Google Scholar]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Farooq, S.; Farooq, S.; Rather, S.A.; Ganaie, T.A. Supercritical CO2 extraction of natural products. In Extraction of Natural Products from Agro-Industrial Wastes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 79–90. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, A.; Manzoor, B.; Nayeem, M.; Jabeen, A.; Amin, Q.A. Extraction of essential oils. In Extraction Processes in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2024; pp. 279–298. [Google Scholar] [CrossRef]
- Kataoka, H. Pharmaceutical Analysis|Sample Preparation. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Laqui-Vilca, C.; Aguilar-Tuesta, S.; Mamani-Navarro, W.; Montaño-Bustamante, J.; Condezo-Hoyos, L. Ultrasound-assisted optimal extraction and thermal stability of betalains from colored quinoa (Chenopodium quinoa Willd) hulls. Ind. Crop. Prod. 2018, 111, 606–614. [Google Scholar] [CrossRef]
- Diamanti, A.C.; Igoumenidis, P.E.; Mourtzinos, I.; Yannakopoulou, K.; Karathanos, V.T. Green extraction of polyphenols from whole pomegranate fruit using cyclodextrins. Food Chem. 2017, 214, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Tutunchi, P.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Extraction of red beet extract with β-cyclodextrin-enhanced ultrasound assisted extraction: A strategy for enhancing the extraction efficacy of bioactive compounds and their stability in food models. Food Chem. 2019, 297, 124994. [Google Scholar] [CrossRef]
- Nutter, J.; Fernandez, M.V.; Jagus, R.J.; Agüero, M.V. Development of an aqueous ultrasound-assisted extraction process of bioactive compounds from beet leaves: A proposal for reducing losses and increasing biomass utilization. J. Sci. Food Agric. 2021, 101, 1989–1997. [Google Scholar] [CrossRef]
- Hernández-Aguirre, O.A.; Muro, C.; Hernández-Acosta, E.; Alvarado, Y.; Díaz-Nava, M.d.C. Extraction and Stabilization of Betalains from Beetroot (Beta vulgaris) Wastes Using Deep Eutectic Solvents. Molecules 2021, 26, 6342. [Google Scholar] [CrossRef]
- Tabio-García, D.; Paraguay-Delgado, F.; Sánchez-Madrigal, M.Á.; Quintero-Ramos, A.; Espinoza-Hicks, J.C.; Meléndez-Pizarro, C.O.; Ruiz-Gutiérrez, M.G.; Espitia-Rangel, E. Optimisation of the ultrasound-assisted extraction of betalains and polyphenols from Amaranthus hypochondriacus var. Nutrisol. Ultrason. Sonochem. 2021, 77, 105680. [Google Scholar] [CrossRef]
- Moghimi, M.; Honarvar, M.; Ghavami, M. Betalain Extraction from Beetroot Using Supercritical Carbon Dioxide and Microwave Pretreatment by Response Surface Method (RSM). Iran. J. Chem. Chem. Eng. 2023, 42, 925–938. [Google Scholar]
- Goswami, T.P.; Kadam, A.; Mashru, R. New Smartphone Based Colorimetric Method Development and Validation of Drugs Containing Nitrogen, Phosphorus and Sulphur. J. Drug Deliv. Ther. 2022, 12, 51–63. [Google Scholar] [CrossRef]
- Shrestha, Y.K.; Shrestha, S.K. Fundamentals of Colorimetry. In Advances in Colorimetry; IntechOpen: Hamilton, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Buratto, J.S.; Fernandes, C.H.D.S.; Rosa, J.C.G.; Vanzo, A.T.d.F.; Caviglione, J.H. Use of quantitative colorimetry and visual evaluation for color characterization of triticale seeds after phenol reaction. J. Seed Sci. 2021, 43, e202143008. [Google Scholar] [CrossRef]
- Gummadi, S.; Kommoju, M. Colorimetric Approaches To Drug Analysis And Applications—A Review. Am. J. PharmTech Res. 2019, 9, 14–37. [Google Scholar] [CrossRef]
- Kiwfo, K.; Woi, P.M.; Saenjum, C.; Sukkho, T.; Grudpan, K. Initiatives in Utilizing Natural Reagents and Natural Materials for Chemical Analysis: Talent and Challenge for ASEAN in New Normal Chemical Analysis. Malays. J. Anal. Sci. 2022, 26, 399–414. [Google Scholar]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Tetyana, P.; Shumbula, P.M.; Njengele-Tetyana, Z. Biosensors: Design, Development and Applications. In Nanopores; IntechOpen: Hamilton, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Kahn, K.; Plaxco, K.W. Principles of Biomolecular Recognition. In Recognition Receptors in Biosensors; Springer: New York, NY, USA, 2010; pp. 3–45. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation—Structural and chromatic aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Miguel, M.G. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Li, F.; Guo, S.; Shui, Y.; Xue, H.; Wang, L. Portable colorimetric detection of copper ion in drinking water via red beet pigment and smartphone. Microchem. J. 2019, 150, 104176. [Google Scholar] [CrossRef]
- Hashemi, N.; Mousazadeh, M.H. Green synthesis of photoluminescent carbon dots derived from red beetroot as a selective probe for Pd2+ detection. J. Photochem. Photobiol. A Chem. 2021, 421, 113534. [Google Scholar] [CrossRef]
- Guo, Y.; Li, T.; Xie, L.; Tong, X.; Tang, C.; Shi, S. Red pitaya peels-based carbon dots for real-time fluorometric and colorimetric assay of Au3+, cellular imaging, and antioxidant activity. Anal. Bioanal. Chem. 2021, 413, 935–943. [Google Scholar] [CrossRef]
- Liu, J.; Xie, F.; Li, R.; Li, T.; Jia, Z.; Wang, Y.; Wang, Y.; Zhang, X.; Fan, C. TiO2-x/Ag3PO4 photocatalyst: Oxygen vacancy dependent visible light photocatalytic performance and BPA degradative pathway. Mater. Sci. Semicond. Process. 2019, 97, 1–10. [Google Scholar] [CrossRef]
- Naghdi, S.; Rezaei, M.; Abdollahi, M. A starch-based pH-sensing and ammonia detector film containing betacyanin of paperflower for application in intelligent packaging of fish. Int. J. Biol. Macromol. 2021, 191, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Qin, Y.; Zhang, M.; Zhang, J.; Qian, C.; Liu, J. Development of active and smart packaging films based on starch, polyvinyl alcohol and betacyanins from different plant sources. Int. J. Biol. Macromol. 2021, 183, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, P.; Liu, L.; Li, S.; Zhao, Y.; Xie, J.; Xu, H. κ-carrageenan-based pH-sensing films incorporated with anthocyanins or/and betacyanins extracted from purple sweet potatoes and peels of dragon fruits. Process. Biochem. 2022, 121, 463–480. [Google Scholar] [CrossRef]
- Chaari, M.; Elhadef, K.; Akermi, S.; Tounsi, L.; Ben Hlima, H.; Ennouri, M.; Abdelkafi, S.; Agriopoulou, S.; Ali, D.S.; Mellouli, L.; et al. Development of a novel colorimetric pH-indicator film based on CMC/flaxseed gum/betacyanin from beetroot peels: A powerful tool to monitor the beef meat freshness. Sustain. Chem. Pharm. 2024, 39, 101543. [Google Scholar] [CrossRef]
| Source of Betalain | Extraction Method | Extraction Solvent | Extraction Conditions | Reference |
|---|---|---|---|---|
| Red beetroot (B. vulgaris) | MAE | Mixture of ethanol and water (1:1) | 0.1 to 25 ratio of solids to solvent, power 400 W and 100% duty cycle for 90–120 s (betanins) and 140–150 s (betaxanthins) | [36] |
| Red beetroot (B. vulgaris) | UAE | 25% of ethanol in water | Temperatures of 52 °C and 37 °C, extraction duration of 90 min | [38] |
| Colored quinoa (Chenopodium quinoa Willd) hulls | UAE | Water | Amplitude at 70%, cycle at 0.6, and extraction duration of 9.2 s for betacyanin and amplitude at 90%, cycle at 0.7, and extraction duration of 40 s for betaxanthin | [39] |
| Red beetroot (B. vulgaris) | UAE with β-cyclodextrin | Water, aqueous 5% β-cyclodextrin solution | Red beet powders mixed with extraction solvent (aqueous 5% β-cyclodextrin solution) at a 1:10 w/v ratio, extraction duration of 3 h, ultrasonic 28 kHz, 80 W | [48] |
| Beetroot (B. vulgaris L. var. conditiva) leaves | UAE | Water | Ultrasonic power of 90 W, 1:20 solid-to-liquid ratio, and extraction duration of 16 min | [49] |
| Beetroot (B. vulgaris L., Rhonda type) peels | Traditional extraction | Aqueous ethanol | Temperature of 20 °C, 0.8 w/v solvent mixture, and extraction duration of 1 h | [34] |
| Beetroot (B. vulgaris) waste | UAE using DES | Mixture of magnesium chloride hexahydrate [MgCl2·6H2O] and urea [U] (2:1) and pH 3 | Temperature of 25 °C for 3 h, followed by subsequent vortex agitation for 900 s | [50] |
| Amaranthus hypochondriacus var. Nutrisol. | UAE | Distilled water | Temperature 41.80 °C and UPD 188.84 mW/mL for 10 min | [51] |
| Beetroot (B. vulgaris) | SFE | Carbon dioxide | Temperature 45 °C, microwave power 300 W, pressure 27.5 MPa, and CO2 flow rate 2 mL/min | [52] |
| Pigment | Analyte | Sample | Color Change | Detection | Parameter Performance | Reference |
|---|---|---|---|---|---|---|
| 2-decarboxy-betanin pigment | Cu2+ | Aqueous-organic solutions (EtOH and MeOH) | Decreased pigment retention | UV-Vis Spectrophotometer | Not determined | [12] |
| Red beet extract | Cu2+ | Drinking water | Purple to orange-red | Naked eye | Linearity range of 4–20 μM, with a detection limit of 0.84 μM | [64] |
| Red beetroot | Pd2+ | Well water and mineral water | Quenched green-emitting CDs fluorescence | Fluorescent | Limit of detection of 33 nM in a linear range from 3 μM to 43 μM | [65] |
| Red pitaya peel (Hylocereus polyrhizus) extract | Au3+ | River water and laboratory tap | Quenched blue-emitting CDs fluorescence and yellow to purple | Fluorescent and naked eye | Limit of detection of 0.072 μM at 0.3–8.0 μM Au3+ and 2.2 μM at 3.3–60.0 μM. | [66] |
| Pigment | Analyte | Sample | Color Change | Detection | Parameter Performance | Reference |
|---|---|---|---|---|---|---|
| Paperflower (Bougainvillea glabra) extract | NH3 | Caspian sprat | Light pink to yellow | Naked eye | Liniearity range of 0.1–0.001 mg/ml | [68] |
| Red pitaya (H. polyrhizus) extract, prickly pear fruit extract, red beetroot (B. vulgaris L.) extract, globe amaranth flower extract, red amaranth (A. tricolor L.) extract | NH3 | Shrimp | Purple-red to yellow on red pitaya extract | Naked eye | Response time of SP-GAFE film is 20 min | [69] |
| Purple sweet potato and Dragon fruit peel extract | NH3 | Pork tenderloin | Red to green | Naked eye | Response time of 15 min | [70] |
| Beetroot peel (Beta vulgaris L.) extract | NH3 | Raw beef meat | Pink to yellow | Naked eye | Response time of 30 min | [71] |
| Analyte Sensed | Type of Sensor | Property of Betalain Exploited | Performance Parameters | Reference |
|---|---|---|---|---|
| Environmental copper (freshwater and tap water) | Whole-cell based | Betaxanthin fluorescence | Response time of 6 h and linearity range at 0–250 µM | [16] |
| Dopamine | Whole-cell based | Betaxanthin fluorescence | Detection limit of 11.1 µM and linearity range at 0–2000 µM | [17] |
| L-DOPA in physiological fluids | Enzyme-based and whole-cell-based | Betaxanthin fluorescence | Detection limit of 2.8 µM (enzyme-based), response time of 1 h (whole-cell based), and linearity range at 5–500 µM | [18] |
| Analyte Sensed | Type of Sensor | Property of Betalain Exploited | Performance Parameters | Reference |
|---|---|---|---|---|
| L-DOPA | Enzyme-coupled | Betaxanthin fluorescence | Linearity range at 2.5–2500 µM | [19] |
| L-tyrosine high-yield mutant cells | Whole-cell based | Betaxanthin fluorescence | - | [20] |
| Cannabinoids | Whole-cell based | Betanin, betaxanthin, and betacyanin fluorescence | Detection limit of 100 pM and response time of 15 min | [21] |
| Environmental copper | Whole-cell based | Betaxanthin fluorescence | Detection limit of 0.32 ppm | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratiwi, R.; Maharani, D.S.; Redjeki, S.G. Betalain Pigments: Isolation and Application as Reagents for Colorimetric Methods and Biosensors. Biosensors 2025, 15, 349. https://doi.org/10.3390/bios15060349
Pratiwi R, Maharani DS, Redjeki SG. Betalain Pigments: Isolation and Application as Reagents for Colorimetric Methods and Biosensors. Biosensors. 2025; 15(6):349. https://doi.org/10.3390/bios15060349
Chicago/Turabian StylePratiwi, Rimadani, Devita Salsa Maharani, and Sarah Gustia Redjeki. 2025. "Betalain Pigments: Isolation and Application as Reagents for Colorimetric Methods and Biosensors" Biosensors 15, no. 6: 349. https://doi.org/10.3390/bios15060349
APA StylePratiwi, R., Maharani, D. S., & Redjeki, S. G. (2025). Betalain Pigments: Isolation and Application as Reagents for Colorimetric Methods and Biosensors. Biosensors, 15(6), 349. https://doi.org/10.3390/bios15060349





