Machine-Learning-Assisted Nanozyme-Based Sensor Arrays: Construction, Empowerment, and Applications
Abstract
1. Introduction
2. Construction of Nanozyme-Based Sensor Arrays
2.1. Nanozyme Materials and Activities
2.2. Sensing Variables
2.3. Signal Outputs
3. Machine Learning Advances Nanozyme-Based Sensor Arrays
3.1. PCA, LDA, and HCA for Target Classification and Clustering
3.2. Other Machine Learning Algorithms for Target Analysis
4. Applications of Machine-Learning-Assisted Nanozyme-Based Sensor Arrays
4.1. Environmental Detection
4.2. Food Analysis
4.3. Biomedical Sensing
5. Conclusions
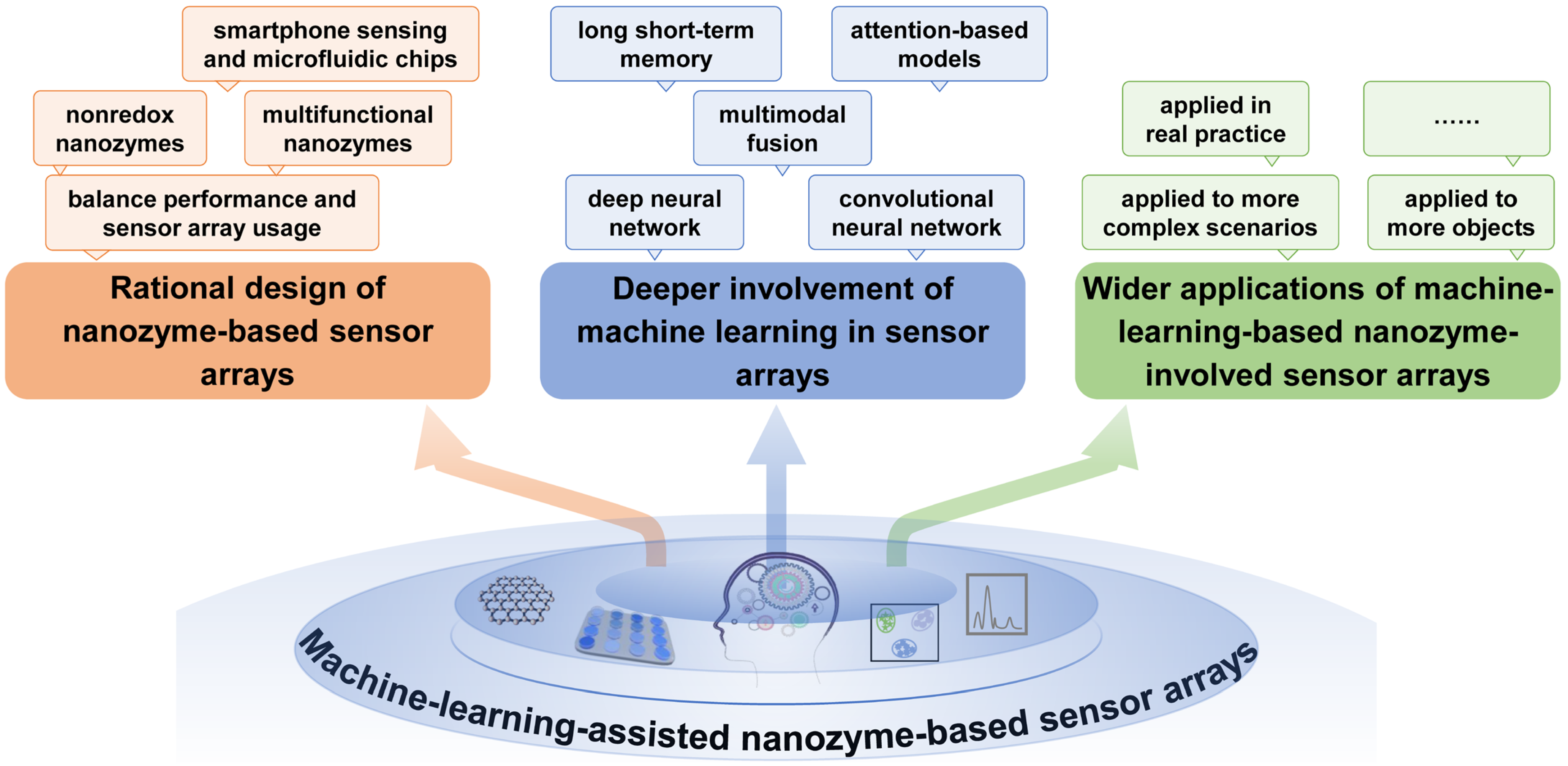
- (1)
- Rational design of nanozyme-based sensor arrays. The rational design of a sensor array is very important for its analytical performance, and more attention should be paid to it to advance sensor arrays. For example, nonredox nanozymes (such as hydrolase-mimicking activity) could be explored as sensing units to avoid the possible oxidation–reduction interference from sample matrices [48]. As nanozymes often possess some other interesting features apart from their catalytic function, developing multifunctional nanozymes (such as fluorescent nanozymes [60]) can provide multidimensional signals for more precise analysis. Of course, recognition performance should be ensured while simplifying the fabrication and use of sensor arrays as much as possible, thus reducing the material, operation, and data processing costs. Furthermore, combining a convenient and easy-to-use sensor array with smartphones and microfluidic chips may benefit fast and on-site data analysis and result presentation [34].
- (2)
- Deeper involvement of machine learning in sensor arrays. Currently, only a limited number of machine learning algorithms have been incorporated into the data processing and result presentation of sensor arrays [90]. In fact, there are some other procedures that require the involvement of advanced algorithms to better facilitate performance improvement and convenient application. For example, machine learning can guide the iterative design and optimization of nanozyme materials with better catalytic properties [91,92], and machine learning can participate in signal pretreatment and noise suppression, as well as the screening and removal of false data. The comparison of different machine learning algorithms in the same scenario is also necessary to present effective, optimized and undistorted results. In addition, it is necessary to explore more advanced deep learning technologies to maximize the functionality of sensor arrays. For instance, CNN can be used to process image-like data generated by sensor arrays to achieve more accurate and efficient feature extraction; long short-term memory networks (LSTMs) can be used to analyze the dynamics of time series, which is crucial for real-time monitoring and predictive modeling; and the integration of multimodal data fusion technology can enhance the overall performance of sensor arrays by combining the information of different types of sensors. Future research can further explore the application of deep learning methods in nanozyme-based sensor arrays to develop more efficient, accurate and interpretable sensor systems.
- (3)
- Wider applications of machine-learning-based nanozyme-involved sensor arrays. At present, nanozyme-based sensor arrays have found some laboratorial applications under the assistance of machine learning. Even so, their scope is expected to expand to more objects and more complex scenarios. For emerging analytes in specific fields, more types of receptors should be explored and combined with nanozymes to fabricate new types of sensor arrays. Furthermore, the use of machine-learning-assisted nanozyme-based sensor arrays in real practice is supposed. However, real-world deployment faces critical challenges. From a cost perspective, although nanozymes offer lower production costs than natural enzymes, scalable synthesis with consistent catalytic activity remains a bottleneck; for example, batch-to-batch variations in metal-doped carbon nanozymes can affect sensor reproducibility [69]. Device miniaturization, while advanced by smartphone and microfluidic integration [34,78], requires further innovation to compress multimodal sensing units (e.g., colorimetric–fluorescent–photothermal modules) into portable devices without compromising signal quality. Additionally, environmental factors (e.g., humidity, temperature) and matrix interference (e.g., proteins, salts in biological fluids) can alter nanozyme performance, necessitating robust calibration strategies. It is worth noting that regulatory approval is another hurdle: for biomedical applications, nanozyme-based sensors must meet strict biocompatibility criteria, but current guidelines on nanozyme characterization are fragmented, which complicates clinical validation. To address these, collaborative efforts across materials science, engineering, and regulatory science are urgent, including the establishment of nanozyme production standards and the development of self-calibrating sensor architectures.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| NPs | Nanoparticles |
| POD | Peroxidase |
| ML | Machine learning |
| OXD | Oxidase |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| LAC | Laccase |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
| PCA | Principal component analysis |
| LDA | Linear discriminant analysis |
| HCA | Hierarchical cluster analysis |
| SVM | Support vector machine |
| ANN | Artificial neural network |
| RF | Random forest |
| GOx | Glucose oxidase |
| OPD | o-Phenylenediamine |
| ABTS | 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) |
| SERS | Surface enhanced Raman scattering |
| DAB | Diaminobenzidine |
| CNN | Convolutional neural network |
| KNN | K-nearest neighbor |
| PPO | Polyphenol oxidase |
| TCs | Tetracyclines |
| AChE | Acetylcholinesterase |
| BChE | Butyrylcholinesterase |
| DT | Decision tree |
| UTI | Urinary tract infection |
| ROS | Reactive oxygen species |
| MOFs | Metal–organic frameworks |
| SER-DL | Segmentation–extraction–regression deep learning |
| ISFE-DL | Image segmentation–feature extraction deep learning |
| PLS-DA | Partial least squares discriminant analysis |
| PLS-LDA | Partial least squares–linear discriminant analysis |
| UMAP | Uniform manifold approximation and projection |
| HRP | Horseradish peroxidase |
| DNN | Deep neural network |
| TC | Tetracycline |
| SVR | Support vector regression |
| GDY | Graphdiyne |
References
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-Like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with Enzyme-Like Characteristics (Nanozymes): Next-Generation Artificial Enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with Enzyme-Like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar]
- Li, S.; Zhang, Y.; Wang, Q.; Lin, A.; Wei, H. Nanozyme-Enabled Analytical Chemistry. Anal. Chem. 2022, 94, 312–323. [Google Scholar] [CrossRef]
- Diao, Q.; Chen, X.; Tang, Z.; Li, S.; Tian, Q.; Bu, Z.; Liu, H.; Liu, J.; Niu, X. Nanozymes: Powerful Catalytic Materials for Environmental Pollutant Detection and Degradation. Environ. Sci. Nano 2024, 11, 766–796. [Google Scholar] [CrossRef]
- Zhang, Y.; Rui, X.; Simpson, B.K. Trends in Nanozymes Development versus Traditional Enzymes in Food Science. Curr. Opin. Food Sci. 2021, 37, 10–16. [Google Scholar] [CrossRef]
- Cao, C.; Yang, N.; Wang, X.; Shao, J.; Song, X.; Liang, C.; Wang, W.; Dong, X. Biomedicine Meets Nanozyme Catalytic Chemistry. Coord. Chem. Rev. 2023, 491, 215245. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New Horizons for Responsive Biomedical Applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Zhang, Y.; Ya, S.; Huang, J.; Ju, Y.; Fang, X.; Ouyang, X.; Zeng, Q.; Zhou, X.; Yan, X.; Nie, G.; et al. Spatial Isolation of Single Copper(I) Sites for Cascade Enzyme-Like Catalysis and Simultaneous Ferroptosis/Cuproptosis Boosted Immunotherapy. Exploration 2025, e20240275. [Google Scholar] [CrossRef]
- Niu, X.; Cheng, N.; Ruan, X.; Du, D.; Lin, Y. Review—Nanozyme-Based Immunosensors and Immunoassays: Recent Developments and Future Trends. J. Electrochem. Soc. 2020, 167, 037508. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An Emerging Alternative to Natural Enzyme for Biosensing and Immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Liang, H.; Chen, X.; Bu, Z.; Bai, Q.; Liu, J.; Tian, Q.; Tang, Z.; Li, S.; Diao, Q.; Niu, X. When Nanozymes Meet Deoxyribonucleic Acid: Understanding Their Interactions and Biomedical Diagnosis Applications. Interdiscip. Med. 2024, 2, e20230057. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Du, D.; Ni, L.; Pan, J.; Niu, X. Emerging Applications of Nanozymes in Environmental Analysis: Opportunities and Trends. TrAC Trends Anal. Chem. 2019, 120, 115653. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Zhou, X.; Yu, Y.; Liu, J.; Hu, N.; Wang, H.; Li, G.; Wu, Y. Recent Progress in the Construction of Nanozyme-Based Biosensors and Their Applications to Food Safety Assay. TrAC Trends Anal. Chem. 2019, 121, 115668. [Google Scholar] [CrossRef]
- Tian, Q.; Li, S.; Tang, Z.; Zhang, Z.; Du, D.; Zhang, X.; Niu, X.; Lin, Y. Nanozyme-Enabled Biomedical Diagnosis: Advances, Trends, and Challenges. Adv. Healthc. Mater. 2024, 14, 2401630. [Google Scholar] [CrossRef]
- Li, T.; Zhu, X.; Hai, X.; Bi, S.; Zhang, X. Recent Progress in Sensor Arrays: From Construction Principles of Sensing Elements to Applications. ACS Sens. 2023, 8, 994–1016. [Google Scholar] [CrossRef]
- Rakow, N.A.; Suslick, K.S. A Colorimetric Sensor Array for Odour Visualization. Nature 2000, 406, 710–713. [Google Scholar] [CrossRef]
- Askim, J.R.; Mahmoudi, M.; Suslick, K.S. Optical Sensor Arrays for Chemical Sensing: The Optoelectronic Nose. Chem. Soc. Rev. 2013, 42, 8649–8682. [Google Scholar] [CrossRef]
- Xia, J.; Li, Z.; Ding, Y.; Shah, L.A.; Zhao, H.; Ye, D.; Zhang, J. Construction and Application of Nanozyme Sensor Arrays. Anal. Chem. 2024, 96, 8221–8233. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, H.; Li, B.; Lu, N. Advances in the Application of Sensor Arrays Based on Nanozymes. Biosens. Bioelectron. X 2024, 21, 100542. [Google Scholar] [CrossRef]
- Masson, J.F. Roadmap for the Use of Machine Learning and Artificial Intelligence in Sensing. ACS Sens. 2024, 9, 3805–3807. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A Review on Metal- and Metal Oxide-Based Nanozymes: Properties, Mechanisms, and Applications. Nano-Micro Lett. 2021, 13, 154. [Google Scholar] [CrossRef]
- Cai, S.; Yang, R. Noble Metal-Based Nanozymes. In Nanozymology; Yan, X., Ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Lyu, Z.; Pan, J.; Ding, S.; Ruan, X.; Zhu, W.; Du, D.; Lin, Y. Metal–organic Framework Based Nanozymes: Promising Materials for Biochemical Analysis. Chem. Commun. 2020, 56, 11338–11353. [Google Scholar] [CrossRef]
- Fu, R.; Ma, Z.; Zhao, H.; Jin, H.; Tang, Y.; He, T.; Ding, Y.; Zhang, J.; Ye, D. Research Progress in Iron-Based Nanozymes: Catalytic Mechanisms, Classification, and Biomedical Applications. Anal. Chem. 2023, 95, 10844–10858. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, X.; Han, L.; Li, F. “Non-Naked” Gold with Glucose Oxidase-Like Activity: A Nanozyme for Tandem Catalysis. Small 2018, 14, 1803256. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, B.; Pan, X.; Wang, H.; Wu, Q.; Li, S.; Jiang, B.; Liu, H. Carbon-Based Nanozymes: Design, Catalytic Mechanism, and Bioapplication. Coord. Chem. Rev. 2023, 475, 214896. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Z.; Tie, Z.; Wang, B.; Ye, M.; Du, L.; Cui, R.; Liu, W.; Wan, C.; Liu, Q.; et al. Data-Informed Discovery of Hydrolytic Nanozymes. Nat. Commun. 2022, 13, 827. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Wang, C.; Xing, D. Overview of Nanozymes with Phosphatase-Like Activity. Biosens. Bioelectron. 2023, 237, 115470. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, L.; Prins, L.J.; Rastrelli, F.; Mancin, F.; Scrimin, P. Hydrolytic Nanozymes. Eur. J. Org. Chem. 2020, 2020, 5044–5055. [Google Scholar] [CrossRef]
- Qin, Y.; Zhong, X.; Liang, C.; Liang, Z.; Nong, Y.; Deng, L.; Guo, Y.; Li, J.; Zhang, M.; Tang, S.; et al. Nanozyme-Based Colorimetric Sensor Arrays Coupling with Smartphone for discrimination and “Segmentation-Extraction-Regression” Deep Learning Assisted Quantification of Flavonoids. Biosens. Bioelectron. 2024, 263, 116604. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Khan, M.A.; Wu, X.; Cai, J.; Lu, T.; Ning, T.; Liu, Z.; Lu, W.; Ye, D.; Zhao, H.; et al. Fe–N–C Single-Atom Nanozymes Based Sensor Array for Dual Signal Selective Determination of Antioxidants. Biosens. Bioelectron. 2022, 205, 114097. [Google Scholar] [CrossRef]
- Zhong, X.; Qin, Y.; Liang, C.; Liang, Z.; Nong, Y.; Luo, S.; Guo, Y.; Yang, Y.; Wei, L.; Li, J.; et al. Smartphone-Assisted Nanozyme Colorimetric Sensor Array Combined “Image Segmentation-Feature Extraction” Deep Learning for Detecting Unsaturated Fatty Acids. ACS Sens. 2024, 9, 5167–5178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Khan, M.A.; Yu, Z.; Yang, W.; Zhao, H.; Ye, D.; Chen, X.; Zhang, J. The Identification of Oral Cariogenic Bacteria through Colorimetric Sensor Array Based on Single-Atom Nanozymes. Small 2024, 20, 2403878. [Google Scholar] [CrossRef]
- Xia, J.; Fu, R.; Li, Z.; Ding, Y.; Zhao, H.; Ye, D. Graphyne-Supported Manganese Single-Atom Nanozyme Sensor Array for Bisphenol Identification. Talanta 2025, 285, 127326. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Han, L.; Wang, X.; Li, W.; Guo, H.; Wei, H. Nanozyme Sensor Arrays Based on Heteroatom-Doped Graphene for Detecting Pesticides. Anal. Chem. 2020, 92, 7444–7452. [Google Scholar] [CrossRef]
- Xiao, Y.; Gong, W.; Zhao, M.; Zhang, M.; Lu, N. Surface-Engineered Prussian Blue Nanozymes as Artificial Receptors for Universal Pattern Recognition of Metal Ions and Proteins. Sens. Actuators B Chem. 2023, 390, 134006. [Google Scholar] [CrossRef]
- Wang, X.; Qin, L.; Zhou, M.; Lou, Z.; Wei, H. Nanozyme Sensor Arrays for Detecting Versatile Analytes from Small Molecules to Proteins and Cells. Anal. Chem. 2018, 90, 11696–11702. [Google Scholar] [CrossRef]
- Wu, F.; Wang, H.; Lv, J.; Shi, X.; Wu, L.; Niu, X. Colorimetric Sensor Array Based on Au2Pt Nanozymes for Antioxidant Nutrition Quality Evaluation in Food. Biosens. Bioelectron. 2023, 236, 115417. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xue, Y.; Gao, Z.; Tang, K.; Wang, G.; Chen, Z.; Zuo, X. Antioxidant Identification Using a Colorimetric Sensor Array Based on Co-N-C Nanozyme. Colloids Surf. B 2021, 208, 112060. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, Y.; Dong, M.; Cao, L.; Jiang, K. A Simple Ag–MoS2 Hybrid Nanozyme-Based Sensor Array for Colorimetric Identification of Biothiols and Cancer Cells. RSC Adv. 2024, 14, 31560–31569. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, F.; Wu, L.; Guan, J.; Niu, X. Nanozyme Colorimetric Sensor Array Based on Monatomic Cobalt for the Discrimination of Sulfur-Containing Metal Salts. J. Hazard. Mater. 2023, 456, 131643. [Google Scholar] [CrossRef]
- Zhu, X.; Li, T.; Hai, X.; Bi, S. A nanozyme-based colorimetric sensor array as electronic tongue for Thiols Discrimination and Disease Identification. Biosens. Bioelectron. 2022, 213, 114438. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, G.; Chen, S.; Su, X.; Xu, D.; Zhai, Y.; Liu, Y.; Hu, G.; Guo, C.; Yang, H.B.; et al. Machine Learning-Assistant Colorimetric Sensor Arrays for Intelligent and Rapid Diagnosis of Urinary Tract Infection. ACS Sens. 2024, 9, 1945–1956. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Tian, Q.; Liu, J.; Tang, Z.; Niu, X. Kinetics Difference-Driven Organophosphorus Hydrolase-Like Nanozyme-Coded Pattern for Identifying p-Nitrophenyl Pesticides. Anal. Chem. 2025, 97, 2537–2545. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Zeng, Y.; Liu, J.; Niu, X. Single-Nanozyme Single-Readout Enabled Efficient Identification of Polyphenols for Chinese Tea Authentication and Brewing Evaluation. Food Chem. 2025, 467, 142328. [Google Scholar] [CrossRef]
- Bai, Y.; Nie, S.; Gao, W.; Li, N.; Zhu, P.; Zhang, L.; Yu, J. Enzyme-Nanozyme Cascade Flow Reactor Synergy with Deep Learning for Differentiation and Point-of-Care Testing of Multiple Organophosphorus Pesticides. Adv. Funct. Mater. 2024, 45, 2419499. [Google Scholar] [CrossRef]
- Li, F.; Jiang, J.; Peng, H.; Li, C.; Li, B.; He, J. Platinum Nanozyme Catalyzed Multichannel Colorimetric Sensor Array for Identification and Detection of Pesticides. Sens. Actuators B Chem. 2022, 369, 132334. [Google Scholar] [CrossRef]
- Zeng, K.; Wang, Y.; Tao, Y.; Huang, R.; Dong, Q.; Deng, C.; Wu, Q.; Niu, X.; Wei, D.; Zhang, Z. Single Sensing Element with Multiple Adsorption Peaks Assembled Nanozyme Sensor Array for Discrimination of Multiple Metal Ions. Microchem. J. 2025, 209, 112786. [Google Scholar] [CrossRef]
- Jing, W.; Qiang, S.; Jia, Z.; Shi, Q.H.; Meng, X.; Yu, M.; Ma, H.; Zhao, K.; Dai, Y. Smartphone-Integrated Nanozymes Sensor Array for High Throughput Recognition of Organophosphorus Pesticides. Sens. Actuators B Chem. 2023, 389, 133857. [Google Scholar] [CrossRef]
- Yue, N.; Lai, Y.; Wu, J.; Zhang, Q.; Qi, W.; Su, R. Optimization of Metal–Organic Framework Nanozyme Activity via Histidine Modification for Simultaneous Pesticide Detection. Chem. Eng. J. 2024, 493, 152630. [Google Scholar] [CrossRef]
- Huang, S.; Xiang, H.; Lv, J.; Zhu, D.; Yu, L.; Guo, Y.; Xu, L. Au Nanozyme-Based Colorimetric Sensor Array Integrates Machine Learning to Identify and Discriminate Monosaccharides. J. Colloid Interface Sci. 2024, 672, 200–208. [Google Scholar] [CrossRef]
- Niu, X.; Liu, B.; Hu, P.; Zhu, H.; Wang, M. Nanozymes with Multiple Activities: Prospects in Analytical Sensing. Biosensors 2022, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, S.; Guan, Y.; Wu, C.; Qian, Y.; Zhou, H.; Qian, Y.; Yue, Y.; Yue, W. Ultrasmall CuMn-His Nanozymes with Multienzyme Activity at Neutral pH: Construction of a Colorimetric Sensing Array for Biothiol Detection and Disease Identification. ACS Appl. Mater. Interfaces 2024, 16, 34538–34548. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Lei, L.; Tian, T.; Yang, X.; Wang, L.; Li, Y.; Huang, H. A Novel Strategy for Identification of Pesticides in Different Categories by Concentration-Independent Model Based on a Nanozyme with Multienzyme-Like Activities. Biosens. Bioelectron. 2023, 237, 115458. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Wei, H. Multifunctional Nanozymes: Enzyme-Like Catalytic Activity Combined with Magnetism and Surface Plasmon Resonance. Nanoscale Horiz. 2018, 3, 367–382. [Google Scholar] [CrossRef]
- Diao, Q.; Tang, Z.; Liu, J.; Niu, X. Fluorescent Nanozymes: Emerging Versatile Materials Advancing Analytical Chemistry. TrAC Trends Anal. Chem. 2025, 188, 118237. [Google Scholar] [CrossRef]
- Tian, T.; Song, D.; Zhen, L.; Bi, Z.; Zhang, L.; Huang, H.; Li, Y. Colorimetric–Fluorescence–Photothermal Tri-Mode Sensor Array Combining the Machine Learning Method for the Selective Identification of Sulfonylurea Pesticides. Biosens. Bioelectron. 2025, 277, 117286. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, L.; Xie, C.; Qin, W.; Wang, M.; Hu, L.; Xia, Z. Anchoring Biomimetic Zn Site in Metal–Organic Framework Nanozyme to Enhance Phosphatase-Like Catalytic Activity for Discrimination of Organophosphorus Pesticides. Chem. Eng. J. 2025, 506, 160046. [Google Scholar] [CrossRef]
- Jing, W.; Yang, Y.; Shi, Q.; Wang, Y.; Liu, F. Machine Learning-Based Nanozyme Sensor Array as an Electronic Tongue for the Discrimination of Endogenous Phenolic Compounds in Food. Anal. Chem. 2024, 96, 16027–16035. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Zou, Y.; Tian, T.; Ma, Y.; Huang, H.; Li, Y. Machine Learning-Assisted Melamine-Cu Nanozyme and Cholinesterase Integrated Array for Multi-Category Pesticide Intelligent Recognition. Biosens. Bioelectron. 2024, 266, 116747. [Google Scholar] [CrossRef]
- Hu, C.; Xie, W.; Liu, J.; Zhang, Y.; Sun, Y.; Cai, Z.; Lin, Z. Bioinspired Iron Porphyrin Covalent Organic Frameworks-Based Nanozymes Sensor Array: Machine Learning-Assisted Identification and Detection of Thiols. ACS Appl. Mater. Interfaces 2024, 16, 71048–71059. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Li, Z.; Liu, F.; Jing, W. Machine Learning Assisted Multi-Signal Nanozyme Sensor Array for the Antioxidant Phenolic Compounds Intelligent Recognition. Food Chem. 2025, 471, 142826. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zou, B.; Zhang, X.; Yang, J.; Bi, Z.; Huang, H.; Li, Y. A Sensor Array Based on a Nanozyme with Polyphenol Oxidase Activity for the Identification of Tea Polyphenols and Chinese Green Tea. Biosens. Bioelectron. 2024, 250, 116056. [Google Scholar] [CrossRef]
- Tian, T.; Song, D.; Zhang, L.; Huang, H.; Li, Y. Facile and Selective Recognition of Sulfonylurea Pesticides Based on the Multienzyme-Like Activities Enhancement of Nanozymes Combining Sensor Array. J. Hazard. Mater. 2024, 469, 133847. [Google Scholar] [CrossRef] [PubMed]
- Ren, E.; Qiu, H.; Yu, Z.; Cao, M.; Sohail, M.; Lu, G.P.; Zhang, X.; Lin, Y. Nanozyme Sensor Array Based on Fe, Se Co-Doped Carbon Material for the Discrimination of Sulfur-Containing Compounds. J. Hazard. Mater. 2024, 470, 134127. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Tang, H.; Xie, C.; Zhao, Y.; Wu, P. Non-Colorimetric Sensing with 3,3′,5,5′-Tetramethylbenzidine. Sens. Actuators B Chem. 2025, 422, 136643. [Google Scholar] [CrossRef]
- Yang, X.; Bi, Z.; Yin, C.; Huang, H.; Li, Y. A Novel Hybrid Sensor Array Based on the Polyphenol Oxidase and Its Nanozymes Combined with the Machine Learning Based Dual Output Model to Identify Tea Polyphenols and Chinese Teas. Talanta 2024, 272, 125842. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, H.; Long, F.; She, X.; Lan, W.; Long, W.; Wang, S.; Wei, L.; She, Y.; Fu, H. Cloud Machine Learning-Enhanced Peroxidase-Like Enzyme Visual Sensor for Rapid Detection of Sulfur-Containing Pesticides. Sens. Actuators B Chem. 2025, 431, 137448. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, C.; Yu, Y.; Yang, S.; Shi, F.; Xu, L.; Gao, X.; Liu, Y.; Huang, H.; Stewart, C.; et al. Machine Learning-Assisted Nanoenzyme/Bioenzyme Dual-Coupled Array for Rapid Detection of Amyloids. Anal. Chem. 2023, 95, 4605–4611. [Google Scholar] [CrossRef]
- Noreldeen, H.A.A.; He, S.B.; Wu, G.W.; Peng, H.P.; Deng, H.H.; Chen, W. Deep Convolutional Neural Network-Based 3D Fluorescence Sensor Array for Sugar Identification in Serum Based on the Oxidase-Mimicking Property of CuO Nanoparticles. Talanta 2024, 280, 126679. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, L.; Lin, M.; Xing, H.; Wei, H. Fluorescent Graphitic Carbon Nitride-Based Nanozymes with Peroxidase-Like Activities for Ratiometric Biosensing. Anal. Chem. 2019, 91, 10648–10656. [Google Scholar] [CrossRef]
- Lan, C.; Meng, L.; Xu, N. Dual-Channel Ratiometric Colorimetric Sensor Array for Quantifcation and Discrimination of o-, m-, and p-Phenols. Food Anal. Methods 2023, 16, 96–106. [Google Scholar] [CrossRef]
- Liu, M.X.; Zhang, H.; Zhang, X.W.; Chen, S.; Yu, Y.L.; Wang, J.H. Nanozyme Sensor Array Plus Solvent-Mediated Signal Amplification Strategy for Ultrasensitive Ratiometric Fluorescence Detection of Exosomal Proteins and Cancer Identification. Anal. Chem. 2021, 93, 9002–9010. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Ji, Y.; Wang, L.; Liu, X.; Wang, F.; Li, C. Nanozyme-Inhibited SERS Multichannel Paper-Based Sensor Array for the Quantification and Identification of Biothiols and Cancer Cells Based on Three Ag-Based Nanomaterials. Anal. Chem. 2024, 96, 11353–11365. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Tian, T.; Wang, L.; Zou, Y.; Zhao, L.; Xiao, J.; Huang, H.; Li, Y. Multi-Signal Sensor Array Based on a Fluorescent Nanozyme for Broad-Spectrum Screening of Pesticides. Chem. Eng. J. 2024, 482, 148784. [Google Scholar] [CrossRef]
- Qiu, X.; Yang, J.; Bai, R.; Zhao, M.; Shao, C.; Zhao, Q.; Gu, Y.; Guo, C.; Li, C.M. Dual-Site Peroxidase-Mimic Graphdiyne-Based Colorimetric Sensor Arrays with Machine Learning for Screening of Multiple Antibiotics. Sens. Actuators B Chem. 2025, 427, 137158. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Shao, C.; Liu, T.; Sun, M.; Wu, C.; Su, G.; Wang, Y.; Ye, J.; Hu, H.; et al. Nanozyme-Induced Deep Learning-Assisted Smartphone Integrated Colorimetric and Fluorometric Dual-Mode for Detection of Tetracycline Analogs. Anal. Chim. Acta 2024, 1297, 342373. [Google Scholar] [CrossRef]
- Jiang, S.; Su, G.; Wu, J.; Song, C.; Lu, Z.; Wu, C.; Wang, Y.; Wang, P.; He, M.; Zhao, Y.; et al. Co3O4/CoFe2O4 Hollow Nanocube Multifunctional Nanozyme with Oxygen Vacancies for Deep-Learning-Assisted Smartphone Biosensing and Organic Pollutant Degradation. ACS Appl. Mater. Interfaces 2023, 15, 11787–11801. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Liu, P.; Wang, M.; Niu, X.; Pan, J. A single-nanozyme colorimetric array based on Target-Induced Differential Surface Passivation for Quantification and Discrimination of Cl−, Br− and I− Ions. Anal. Chim. Acta 2021, 1160, 338451. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Guo, D.; Xu, X.; Pan, J.; Niu, X. Colorimetric Quantification and Discrimination of Phenolic Pollutants Based on Peroxidase-Like Fe3O4 Nanoparticles. Sens. Actuators B Chem. 2020, 303, 127225. [Google Scholar] [CrossRef]
- Xu, X.; Wu, S.; Guo, D.; Niu, X. Construction of a Recyclable Oxidase-Mimicking Fe3O4@MnOx-Based Colorimetric Sensor Array for Quantifying and Identifying Chlorophenols. Anal. Chim. Acta 2020, 1107, 203–212. [Google Scholar] [CrossRef]
- Zhou, X.; Li, L.; Wang, Y.; Kong, T.; Cao, Z.; Xie, H.; Liang, W.; Wang, Y.; Qian, S.; Chao, J.; et al. Nanozyme Inhibited Sensor Array for Biothiol Detection and Disease Discrimination Based on Metal Ion-Doped Carbon Dots. Anal. Chem. 2023, 95, 8906–8913. [Google Scholar] [CrossRef]
- Qiu, H.; Pu, F.; Ran, X.; Liu, C.; Ren, J.; Qu, X. Nanozyme as Artificial Receptor with Multiple Readouts for Pattern Recognition. Anal. Chem. 2018, 90, 11775–11779. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, Z.; Wu, S.; Pan, J.; Xu, X.; Niu, X. A Peroxidase-Mimicking Zr-Based MOF Colorimetric Sensing Array to Quantify and Discriminate Phosphorylated Proteins. Anal. Chim. Acta 2020, 1121, 26–34. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, N.; Xiao, Y.; Zhang, Y.; Tang, Z.; Zhang, M. Metal-Nanoparticle-Supported Nanozyme-Based Colorimetric Sensor Array for Precise Identification of Proteins and Oral Bacteria. ACS Appl. Mater. Interfaces 2022, 14, 11156–11166. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Tang, Z.; Liu, S.; Peng, J.; Liang, H.; Niu, X. Machine Learning-Enabled Time-Resolved Nanozyme-Encoded Recognition of Endogenous Mercaptans for Disease Diagnosis. Anal. Chem. 2025, 97, 10463–10473. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, J.; Wu, Y.; Liu, H.; Meng, F.; Liu, Q.; Midgley, A.C.; Zhang, X.; Qi, T.; Kang, H.; et al. Prediction and Design of Nanozymes using Explainable Machine Learning. Adv. Mater. 2022, 34, 2201736. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Z.; Chen, Z.; Guo, M.; Zhang, Y.; Wang, L.; Zhu, Z. Machine Learning in Nanozymes: From Design to Application. Biomater. Sci. 2024, 12, 2229–2243. [Google Scholar] [CrossRef] [PubMed]




| Title | Nanozyme Activity and Sensor Array Construction | Machine Learning | Sensor Array Application | Ref. |
|---|---|---|---|---|
| Construction and Application of Nanozyme Sensor Arrays | ① Types of nanozyme activities and their regulatory factors; ② Principles and methods of sensor array construction (colorimetric, ratio, fluorescence, multimodal) | Not mentioned | Small molecules, ions, pesticide residues, mycotoxin and bacteria, metal salts, phenolic compounds, antibiotics | [21] |
| Advances in the Application of Sensor Arrays Based on Nanozymes | ① Classification and activity regulation of nanozymes; ② Sensor array construction strategies (multiunit signal integration, signal amplification and regulation) | Not involved | Small molecules, proteins, pesticide residues, metal ions | [22] |
| Machine-Learning-Assisted Nanozyme-Based Sensor Arrays: Construction, Empowerment, and Applications | ① Nanozyme materials and catalytic activities; ② Core elements of sensor arrays, namely sensing variables (material difference, environmental regulation, time dependence, multiwavelength signal, multisubstrate response, multimicrosignal set) and signal outputs (single mode, multimode) | ① Classical machine learning algorithms, including dimension reduction and classification (PCA, LDA, HCA) as well as prediction (SVW, ANN, RF); ② Deep learning and emerging algorithms, including CNN, GNN, and AM | Environmental detection, food analysis, biomedical sensing | This work |
| Sensing Variable | Feature | Advantage | Disadvantage | Applicable Scene |
|---|---|---|---|---|
| A series of materials with the same catalytic type | Unique fingerprints are obtained by taking advantage of the differences in catalytic activity of various nanozyme materials and their interactions with target substances | Obtaining unique fingerprints for a variety of targets | Increased material costs | Suitable for scenarios that require high specificity in recognizing multiple target objects |
| Reaction regulators for nanozyme catalysis | Sensor arrays are constructed by taking advantage of the sensitivity of nanozyme catalytic reactions to environmental conditions (such as pH) | Reducing material costs and obtaining different signals by adjusting reaction conditions | The regulators available are limited | Applicable to scenarios that are cost-sensitive and have a small number of target species categories |
| Reaction-time-dependent kinetic signals | Capturing signals generated at different time points during nanozyme catalytic reactions to construct sensor arrays | Simple operation, cost-effectiveness | Requiring precise time control and signal recording | Suitable for scenarios where reaction kinetics are distinct and targets affect kinetics differently |
| Signals recorded at different wavelengths | Using the absorbance differences of the same material at various wavelengths to construct sensor arrays | Providing additional signal dimensions | Requiring precise spectrometric measurement equipment | Suitable for scenarios where the material has significant absorbance differences at different wavelengths |
| Various substrates used in nanozyme catalysis | Employing different substrates as electron donors to participate in nanozyme reactions, offering distinct responses to similar analytes for identification | Obtaining different signals for multiple substrates | Requiring multiple substrates | Suitable for scenarios with substrate-selective nanozymes and multiple target analytes |
| Multiple activity types provided by the same material | Constructing sensor arrays by utilizing different catalytic types presented in the same nanozyme | Providing multiple reaction signals | Complex operation and requiring the recording of multiple signals | Suitable for scenarios with nanozymes having multiple catalytic activities |
| Multiple-dimensional signals provided by a multifunctional nanozyme | Constructing sensor arrays by combining the catalytic features of nanozymes with their electrical, optical, magnetic, and thermal properties | Offering multidimensional signals to enhance identification accuracy | High equipment requirements and complex signal processing | Suitable for scenarios requiring high-precision identification and multidimensional signal recording |
| Cross-use of more than one variable | Combining the above-mentioned sensing variables to achieve more precise sensing results | Providing a rich combination of signals to improve identification capability | Complex experimental design and data analysis | Suitable for complex scenarios requiring high precision and specificity to identify multiple targets |
| Machine Learning Algorithm | Data Type | Applicable Objective | Main Feature |
|---|---|---|---|
| PCA | Numerical data (high-dimensional data) | Dimensionality reduction, feature extraction | Unsupervised learning, linear transformation to project data into lower-dimensional space while retaining maximum variance |
| LDA | Numerical data (labeled) | Classification | Supervised learning, linear transformation to maximize class separation while minimizing within-class variance |
| HCA | Numerical data | Clustering | Unsupervised learning, builds a hierarchical tree structure to group data, suitable for exploratory data analysis |
| SVM | Numerical data (linearly or nonlinearly separable) | Classification, regression (SVR) | Finds the optimal hyperplane to maximize class margins, suitable for high-dimensional data and small sample problems |
| ANN | Numerical data | Classification, regression | Multilayer neural network structure that learns nonlinear relationships in data, suitable for complex pattern recognition |
| RF | Numerical data, categorical data | Classification, regression | Ensemble learning method based on decision trees, improving generalization through random sampling and feature selection |
| DNN | Numerical data (large-scale data) | Classification, regression | Multilayer neural network structure that learns complex nonlinear relationships but requires large amounts of data for training |
| CNN | Image data, sequential data | Classification, regression | Utilizes convolutional layers to extract local features, suitable for data with spatial or temporal local correlations |
| GNN | Graph-structured data (nodes and edges) | Classification, regression, node embedding | Models relationships between nodes, suitable for data with complex interactions |
| AM | Numerical data, sequential data | Classification, regression | Focuses on key parts of the data through attention mechanisms, enhancing model interpretability and performance |
| LSTMs | Sequential data (time series) | Classification, regression | Suitable for processing sequential data with long-term dependencies, capable of remembering and forgetting information |
| Nanozyme Material | Activity | Sensing Variable | Signal Mode | Machine Learning | Application | Ref. |
|---|---|---|---|---|---|---|
| VA-Cu | POD and LAC | Enzymatic activities and reaction times | Colorimetric | LDA, HCA, and ANN | Discrimination of phenolic compounds | [63] |
| Au NPs | GOx | Enzymatic substrates | Colorimetric | LDA, HCA, and DNN | Identification and discrimination of monosaccharides | [55] |
| Cu-BTC | LAC and POD | Enzymatic activities and multidimensional signals | Colorimetric and photothermal | LDA, HCA, and ANN | Intelligent recognition of antioxidant phenolic compounds | [66] |
| Fe-COF-H and Fe-COF-OH | POD | Signals at different wavelengths | Colorimetric | LDA, DT, ANN, HCA, and RF | Identification and detection of thiols | [65] |
| GMP-Cu and ASP-Cu | PPO | Nanozyme materials | Colorimetric | PLS-LDA, HCA, and BPNN | Identification of tea polyphenols and Chinese tea | [71] |
| Au NPs | GOx | ss-DNA regulators | Fluorescence | LDA | Detection of amyloids | [73] |
| CeCo, CeMn, and CeFe | POD | Nanozyme materials | Colorimetric | LDA and SER-DL | Discrimination and quantification of flavonoids | [34] |
| TPA@GQD | POD | Metal ion regulators | Colorimetric | LDA and HCA | Thiol discrimination and disease identification | [46] |
| MnO2, Ag-MnO2, Pd-MnO2, and Pt-MnO2 | OXD | Nanozyme materials | Colorimetric | LDA and ISFE-DL | Detecting unsaturated fatty acids | [36] |
| Fe-N-C and Fe-N-C-Urea | OXD | Nanozyme materials | Colorimetric | LDA and HCA | Identification of oral cariogenic bacteria | [37] |
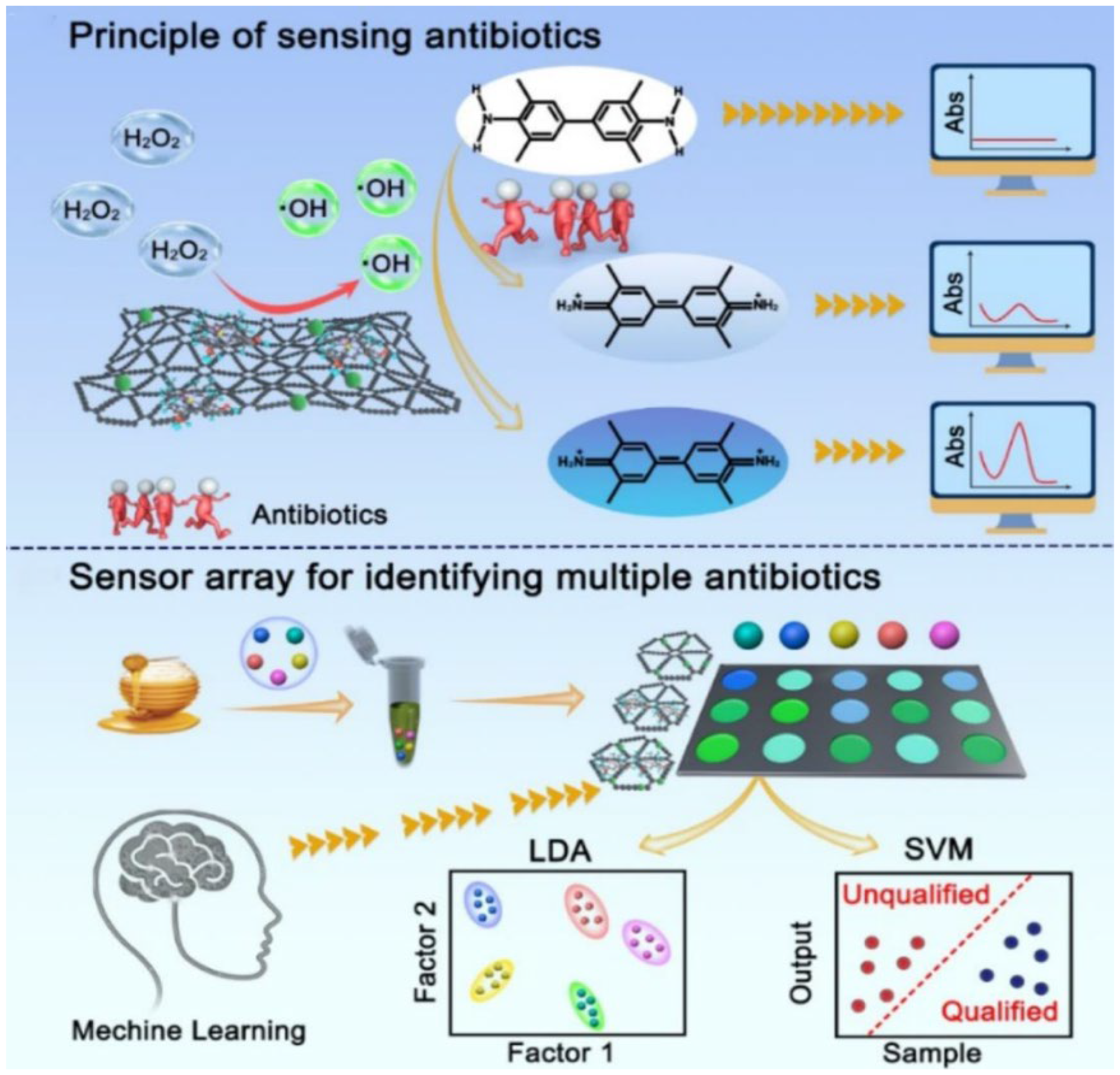
| GDY/Cu, GDY/Hemin, and GDY/Hemin/Cu | POD | Nanozyme materials | Colorimetric | LDA and SVM | Screening of multiple antibiotics | [80] |
| ASP-Cu | POD | Multidimensional signals | Colorimetric, fluorescence, and photothermal | LDA, HCA, and KNN | Identification of sulfonylurea pesticides | [61] |
| C3N4 NSs | POD | Aptamer regulators | Ratiometric fluorescence | LDA and HCA | Detection of exosomal proteins and cancer identification | [77] |
| CuO NPs | OXD | Signals at different wavelengths | Fluorescence | CNN-Max | Sugar identification | [74] |
| Mn-GY, Mn-GY-2N, and GY-2N | POD | Nanozyme materials | Colorimetric | HCA and LDA | Bisphenol identification | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Chen, X.; Diao, Q.; Tang, Z.; Niu, X. Machine-Learning-Assisted Nanozyme-Based Sensor Arrays: Construction, Empowerment, and Applications. Biosensors 2025, 15, 344. https://doi.org/10.3390/bios15060344
Liu J, Chen X, Diao Q, Tang Z, Niu X. Machine-Learning-Assisted Nanozyme-Based Sensor Arrays: Construction, Empowerment, and Applications. Biosensors. 2025; 15(6):344. https://doi.org/10.3390/bios15060344
Chicago/Turabian StyleLiu, Jinjin, Xinyu Chen, Qiaoqiao Diao, Zheng Tang, and Xiangheng Niu. 2025. "Machine-Learning-Assisted Nanozyme-Based Sensor Arrays: Construction, Empowerment, and Applications" Biosensors 15, no. 6: 344. https://doi.org/10.3390/bios15060344
APA StyleLiu, J., Chen, X., Diao, Q., Tang, Z., & Niu, X. (2025). Machine-Learning-Assisted Nanozyme-Based Sensor Arrays: Construction, Empowerment, and Applications. Biosensors, 15(6), 344. https://doi.org/10.3390/bios15060344






