Antifouling Modification of Gold Surfaces for Acoustic Wave Sensor Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Materials
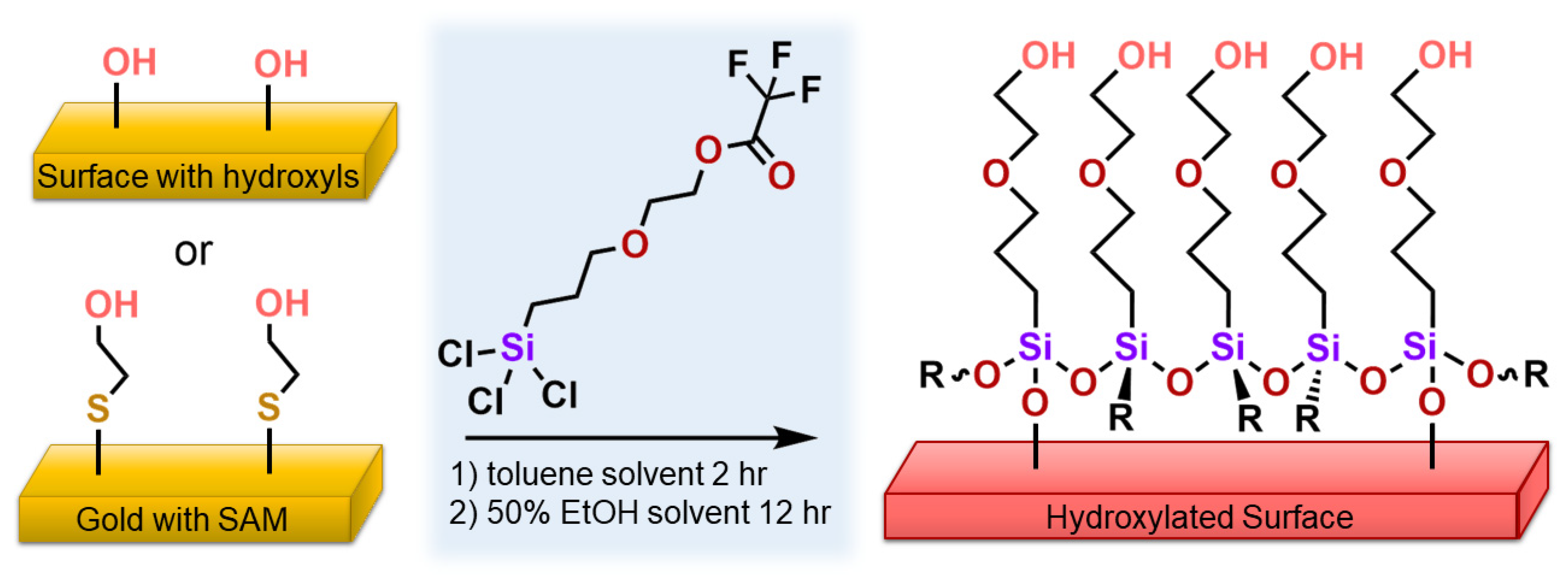
2.2. Synthesis and Characterization of 2-(3-Trichlorosilylpropyloxy)-ethyltrifluoroacetate
2.3. Cleaning and Coating Procedures
2.4. Characterization of the Modified Gold and Silicon-Based Substrates
2.4.1. Contact Angle Measurements
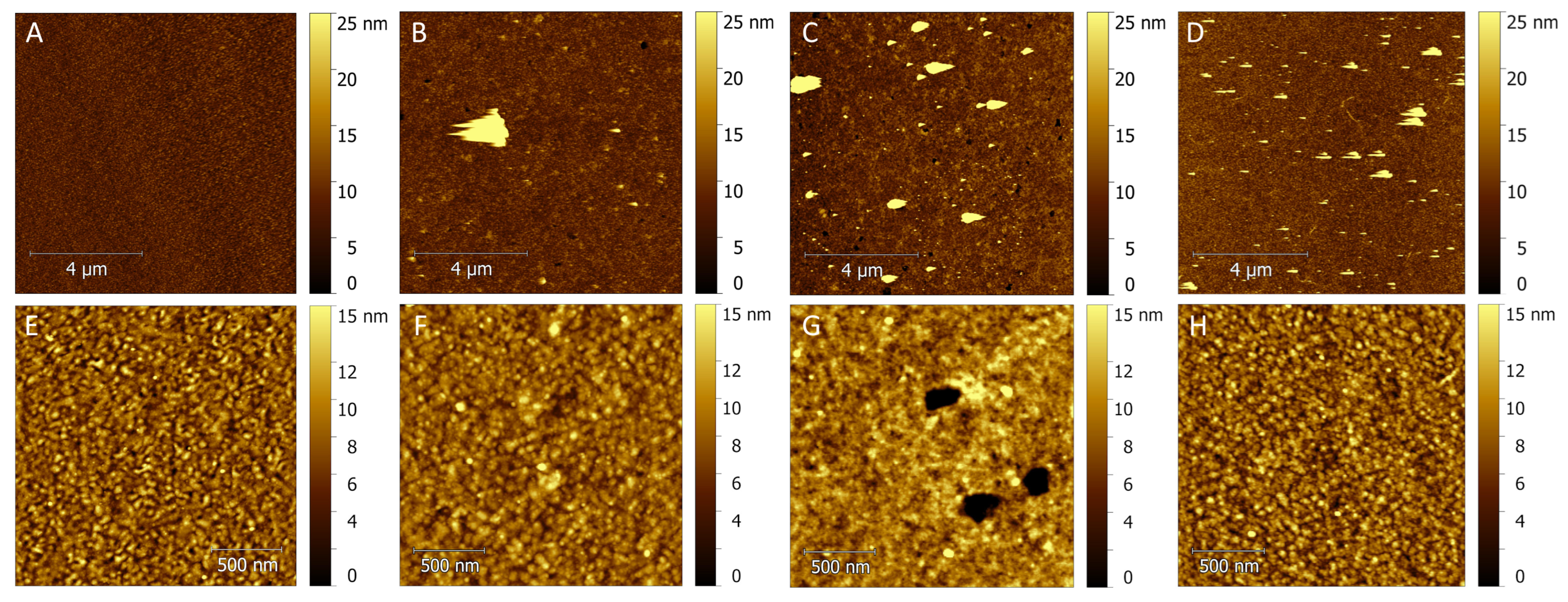
2.4.2. AFM Surface Characterization
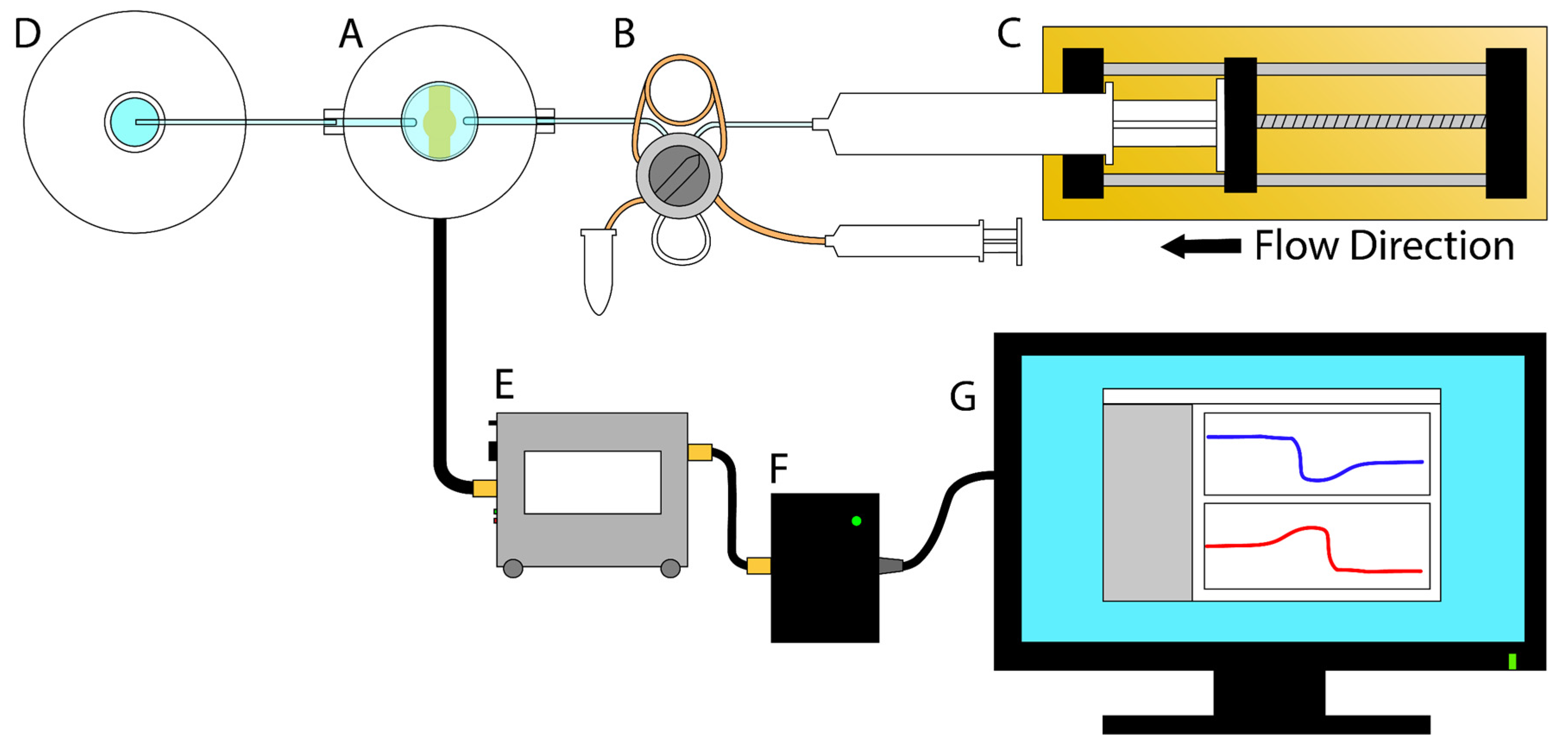
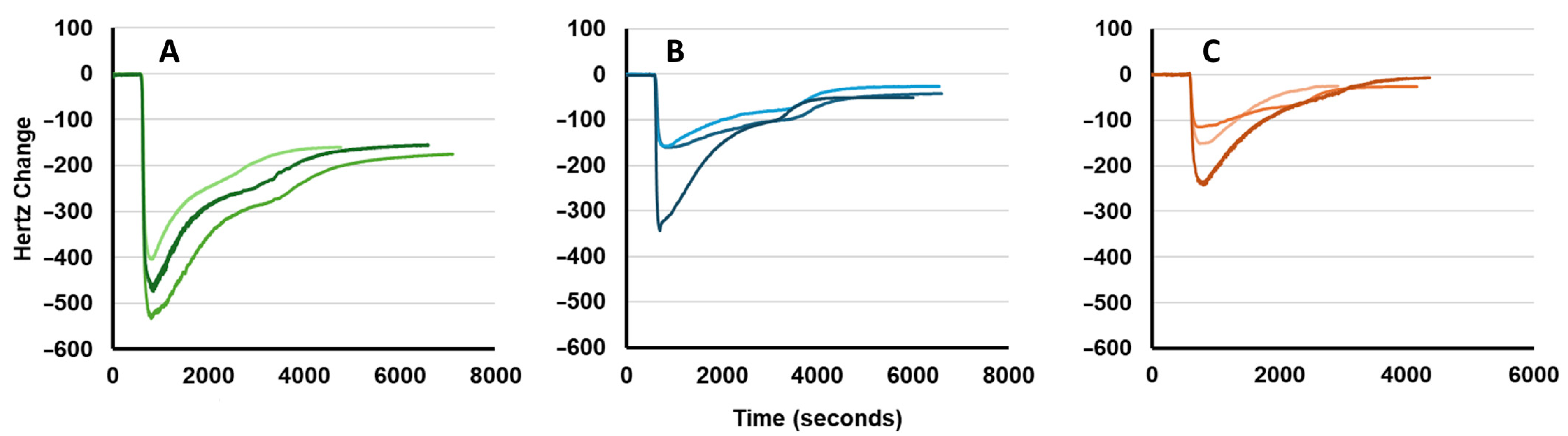
2.4.3. TSM Antifouling Measurements
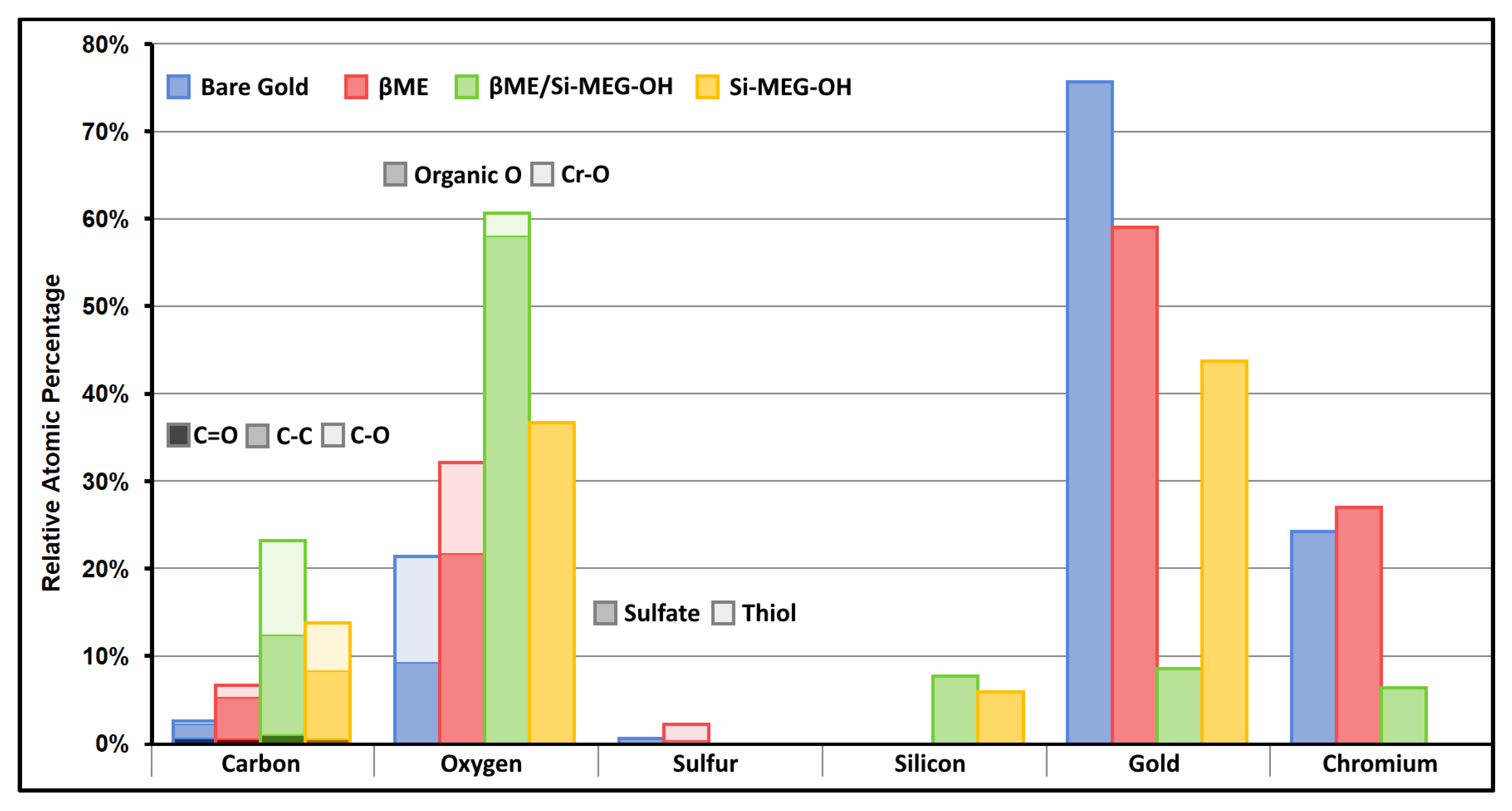
2.4.4. XPS Analysis of the TSM Crystals
3. Results and Discussion
3.1. NMR Characterization of Si-MEG-TFA
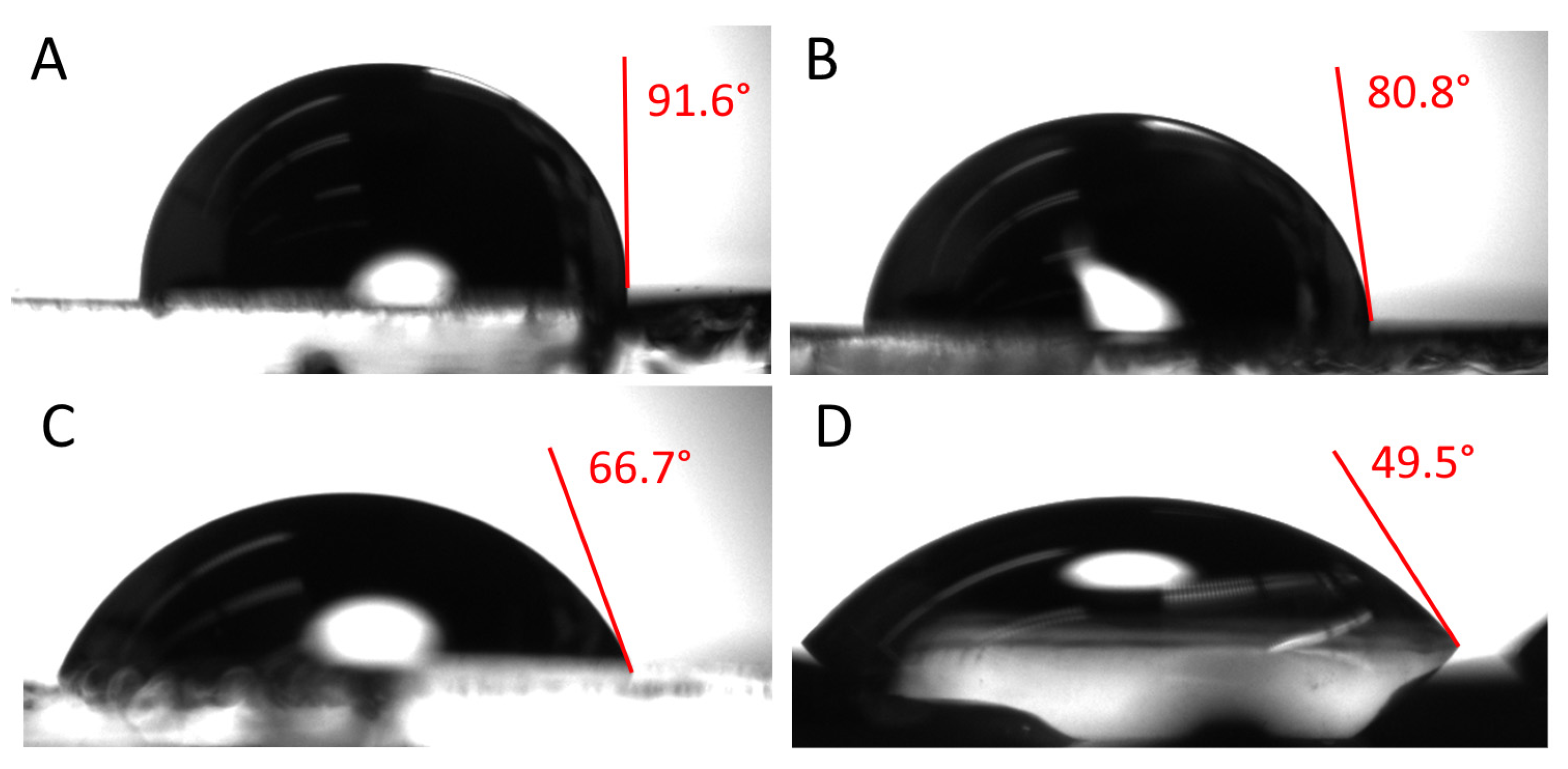
3.2. Contact Angle Measurement Results
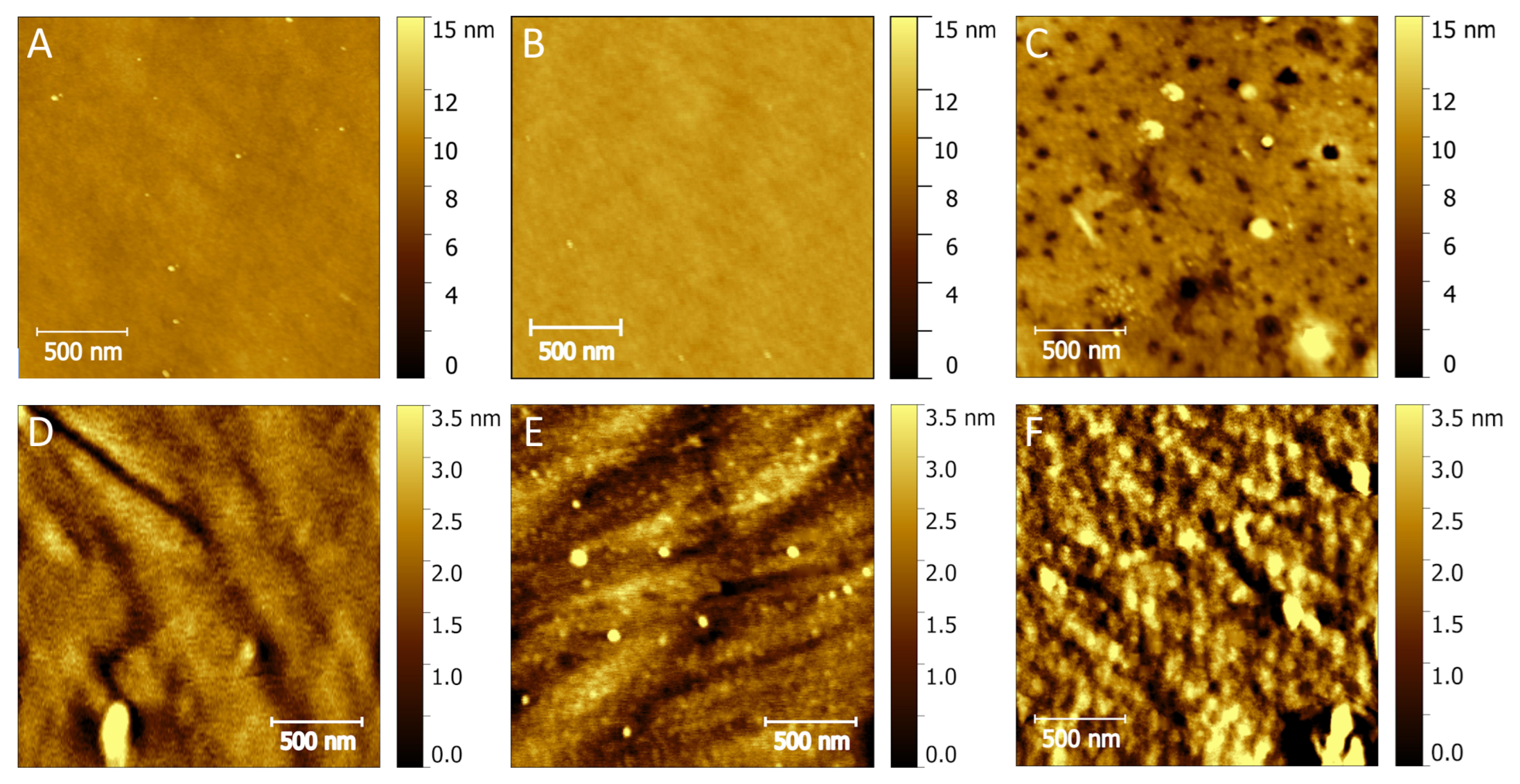
3.3. AFM Results of Modified Substrates
3.4. TSM Fouling Data with Undiluted Goat Serum
3.5. XPS Data for the TSM Gold Electrodes on Quartz Crystals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armero, L.; Plou, J.; Valera, P.S.; Serna, S.; García, I.; Liz-Marzán, L.M. Multiplex Determination of Glycan Profiles on Urinary Prostate-Specific Antigen by Quartz-Crystal Microbalance Combined with Surface-Enhanced Raman Scattering. ACS Sens. 2024, 9, 4811–4821. [Google Scholar] [CrossRef] [PubMed]
- Huertas, C.S.; Soler, M.; Estevez, M.-C.; Lechuga, L.M. One-Step Immobilization of Antibodies and DNA on Gold Sensor Surfaces via a Poly-Adenine Oligonucleotide Approach. Anal. Chem. 2020, 92, 12596–12604. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Devireddy, R.; Gartia, M.R. Surface Plasmon Resonance (SPR) Sensor for Cancer Biomarker Detection. Biosensors 2023, 13, 396. [Google Scholar] [CrossRef]
- Szunerits, S.; Pagneux, Q.; M’Barek, Y.B.; Vassal, S.; Boukherroub, R. Do Not Let Electrode Fouling Be the Enemy of Bioanalysis. Bioelectrochemistry 2023, 153, 108479. [Google Scholar] [CrossRef]
- Eng, Y.J.; Nguyen, T.M.; Luo, H.-K.; Chan, J.M.W. Antifouling Polymers for Nanomedicine and Surfaces: Recent Advances. Nanoscale 2023, 15, 15472–15512. [Google Scholar] [CrossRef]
- De La Franier, B.; Asker, D.; van den Berg, D.; Hatton, B.; Thompson, M. Reduction of Microbial Adhesion on Polyurethane by a Sub-Nanometer Covalently-Attached Surface Modifier. Colloids Surf. B Biointerfaces 2021, 200, 111579. [Google Scholar] [CrossRef]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and Antimicrobial Biomaterials: An Overview. Apmis 2017, 125, 392–417. [Google Scholar] [CrossRef]
- Yao, S.; Yan, H.; Tian, S.; Luo, R.; Zhao, Y.; Wang, J. Anti-Fouling Coatings for Blood-Contacting Devices. Smart Mater. Med. 2024, 5, 166–180. [Google Scholar] [CrossRef]
- Nilebäck, L.; Hedin, J.; Widhe, M.; Floderus, L.S.; Krona, A.; Bysell, H.; Hedhammar, M. Self-Assembly of Recombinant Silk as a Strategy for Chemical-Free Formation of Bioactive Coatings: A Real-Time Study. Biomacromolecules 2017, 18, 846–854. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Antifouling (Bio)Materials for Electrochemical (Bio)Sensing. Int. J. Mol. Sci. 2019, 20, 423. [Google Scholar] [CrossRef]
- Yang, T.; De La Franier, B.; Thompson, M. Anti-Thrombogenicity Study of a Covalently-Attached Monolayer on Stent-Grade Stainless Steel. Materials 2021, 14, 2342. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Tran, H.-V.; Lee, T.R. Self-Assembled Monolayer Coatings on Gold and Silica Surfaces for Antifouling Applications: A Review. Coatings 2022, 12, 1462. [Google Scholar] [CrossRef]
- Yılmazoğlu, E.; Karakuş, S. Synthesis and Specific Biomedical Applications of Polymer Brushes. Appl. Surf. Sci. Adv. 2023, 18, 100544. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, F. Polymer Brushes for Antibiofouling and Lubrication. Biosurf. Biotribol. 2017, 3, 97–114. [Google Scholar] [CrossRef]
- Sheikh, S.; Yang, D.Y.; Blaszykowski, C.; Thompson, M. Single Ether Group in a Glycol-Based Ultra-Thin Layer Prevents Surface Fouling from Undiluted Serum. Chem. Commun. 2012, 48, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Blaszykowski, C.; Sheikh, S.; Thompson, M. Surface Chemistry to Minimize Fouling from Blood-Based Fluids. Chem. Soc. Rev. 2012, 41, 5599–5612. [Google Scholar] [CrossRef]
- Onclin, S.; Ravoo, B.J.; Reinhoudt, D.N. Engineering Silicon Oxide Surfaces Using Self-Assembled Monolayers. Angew. Chem. Int. Ed. Engl. 2005, 44, 6282–6304. [Google Scholar] [CrossRef]
- De La Franier, B.; Jankowski, A.; Thompson, M. Functionalizable Self-Assembled Trichlorosilyl-Based Monolayer for Application in Biosensor Technology. Appl. Surf. Sci. 2017, 414, 435–441. [Google Scholar] [CrossRef]
- Fedorov, K.; Blaszykowski, C.; Sheikh, S.; Reheman, A.; Romaschin, A.; Ni, H.; Thompson, M. Prevention of Thrombogenesis from Whole Human Blood on Plastic Polymer by Ultrathin Monoethylene Glycol Silane Adlayer. Langmuir 2014, 30, 3217–3222. [Google Scholar] [CrossRef]
- Sheikh, S.; Blaszykowski, C.; Nolan, R.; Thompson, D.; Thompson, M. On the Hydration of Subnanometric Antifouling Organosilane Adlayers: A Molecular Dynamics Simulation. J. Colloid Interface Sci. 2015, 437, 197–204. [Google Scholar] [CrossRef]
- De La Franier, B.; Thompson, M. Surface Adsorption of the Cancer Biomarker Lysophosphatidic Acid in Serum Studied by Acoustic Wave Biosensor. Materials 2021, 14, 4158. [Google Scholar] [CrossRef] [PubMed]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-Assembled Monolayers of Thiols and Dithiols on Gold: New Challenges for a Well-Known System. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, S.; De La Franier, B.; Hianik, T.; Thompson, M. Surface Probe Linker with Tandem Anti-Fouling Properties for Application in Biosensor Technology. Biosensors 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Zina, F.; Nooredeen, N.M.; Azzouzi, S.; Ali, M.B.; Abbas, M.N.; Errachid, A. Novel Sensitive Impedimetric Microsensor for Phosphate Detection Based on a Novel Copper Phthalocyanine Derivative. Anal. Lett. 2018, 51, 371–386. [Google Scholar] [CrossRef]
- Mattiuzzi, A.; Troian-Gautier, L.; Mertens, J.; Reniers, F.; Bergamini, J.-F.; Lenne, Q.; Lagrost, C.; Jabin, I. Robust Hydrophobic Gold, Glass and Polypropylene Surfaces Obtained through a Nanometric Covalently Bound Organic Layer. RSC Adv. 2020, 10, 13553–13561. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Yu, B.; Browne, C.; Garnier, G. Reversible pH Responsive Bovine Serum Albumin Hydrogel Sponge Nanolayer. Front. Bioeng. Biotechnol. 2020, 8, 573. [Google Scholar] [CrossRef]
- Braiek, M.; Rokbani, K.B.; Chrouda, A.; Mrabet, B.; Bakhrouf, A.; Maaref, A.; Jaffrezic-Renault, N. An Electrochemical Immunosensor for Detection of Staphylococcus Aureus Bacteria Based on Immobilization of Antibodies on Self-Assembled Monolayers-Functionalized Gold Electrode. Biosensors 2012, 2, 417–426. [Google Scholar] [CrossRef]
- Kochana, J.; Starzec, K.; Wieczorek, M.; Knihnicki, P.; Góra, M.; Rokicińska, A.; Kościelniak, P.; Kuśtrowski, P. Study on Self-Assembled Monolayer of Functionalized Thiol on Gold Electrode Forming Capacitive Sensor for Chromium(VI) Determination. J. Solid State Electrochem. 2019, 23, 1463–1472. [Google Scholar] [CrossRef]
- Herrer, L.; Sebastian, V.; Martín, S.; González-Orive, A.; Pérez-Murano, F.; Low, P.J.; Luis Serrano, J.; Santamaría, J.; Cea, P. High Surface Coverage of a Self-Assembled Monolayer by in Situ Synthesis of Palladium Nanodeposits. Nanoscale 2017, 9, 13281–13290. [Google Scholar] [CrossRef]
- Kocábová, J.; Vavrek, F.; Nováková Lachmanová, Š.; Šebera, J.; Valášek, M.; Hromadová, M. Self-Assembled Monolayers of Molecular Conductors with Terpyridine-Metal Redox Switching Elements: A Combined AFM, STM and Electrochemical Study. Molecules 2022, 27, 8320. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Peter, Y.-A.; Nadeau, J.L. Facile Biosynthesis, Separation and Conjugation of Gold Nanoparticles to Doxorubicin. Nanotechnology 2008, 19, 495101. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Ivasyshyn, V.; Qiu, L.; Enache, M.; Dong, J.; Rousseva, S.; Portale, G.; Stöhr, M.; Hummelen, J.C.; Chiechi, R.C. Thiol-Free Self-Assembled Oligoethylene Glycols Enable Robust Air-Stable Molecular Electronics. Nat. Mater. 2020, 19, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Moores, B.; Simons, J.; Xu, S.; Leonenko, Z. AFM-Assisted Fabrication of Thiol SAM Pattern with Alternating Quantified Surface Potential. Nanoscale Res. Lett. 2011, 6, 185. [Google Scholar] [CrossRef]
- Tabata, K.; Hirano, Y.; Suzuki, E. XPS Studies on the Oxygen Species of LaMn1−xCuxO3+λ. Appl. Catal. A Gen. 1998, 170, 245–254. [Google Scholar] [CrossRef]
- Briggs, D.; Beamson, G. XPS Studies of the Oxygen 1s and 2s Levels in a Wide Range of Functional Polymers. Anal. Chem. 1993, 65, 1517–1523. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Bunin, I.Z.; Ryazantseva, M. XPS Study of Sulfide Minerals Surface Oxidation under High-Voltage Nanosecond Pulses. Miner. Eng. 2019, 143, 105939. [Google Scholar] [CrossRef]
- Baer, D.R.; Artyushkova, K.; Brundle, C.R.; Castle, J.E.; Engelhard, M.H.; Gaskell, K.J.; Grant, J.T.; Haasch, R.T.; Linford, M.R.; Powell, C.J.; et al. Practical Guides for X-Ray Photoelectron Spectroscopy (XPS): First Steps in Planning, Conducting and Reporting XPS Measurements. J. Vac. Sci. Technol. A 2019, 37, 031401. [Google Scholar] [CrossRef]


| Coating * | Frequency Change (Hz) | RSD (%) |
|---|---|---|
| Bare | 163.5 ± 10.7 | 6.54 |
| βME + Si-MEG-OH | 39.7 ± 12.6 | 31.6 |
| Si-MEG-OH | 19.8 ± 10.7 | 53.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delica, A.; Nazarov, M.A.; Franier, B.D.L.; Thompson, M. Antifouling Modification of Gold Surfaces for Acoustic Wave Sensor Applications. Biosensors 2025, 15, 343. https://doi.org/10.3390/bios15060343
Delica A, Nazarov MA, Franier BDL, Thompson M. Antifouling Modification of Gold Surfaces for Acoustic Wave Sensor Applications. Biosensors. 2025; 15(6):343. https://doi.org/10.3390/bios15060343
Chicago/Turabian StyleDelica, Aries, Mikhail A. Nazarov, Brian De La Franier, and Michael Thompson. 2025. "Antifouling Modification of Gold Surfaces for Acoustic Wave Sensor Applications" Biosensors 15, no. 6: 343. https://doi.org/10.3390/bios15060343
APA StyleDelica, A., Nazarov, M. A., Franier, B. D. L., & Thompson, M. (2025). Antifouling Modification of Gold Surfaces for Acoustic Wave Sensor Applications. Biosensors, 15(6), 343. https://doi.org/10.3390/bios15060343







