Biological Barrier Models-on-Chips: A Novel Tool for Disease Research and Drug Discovery
Abstract
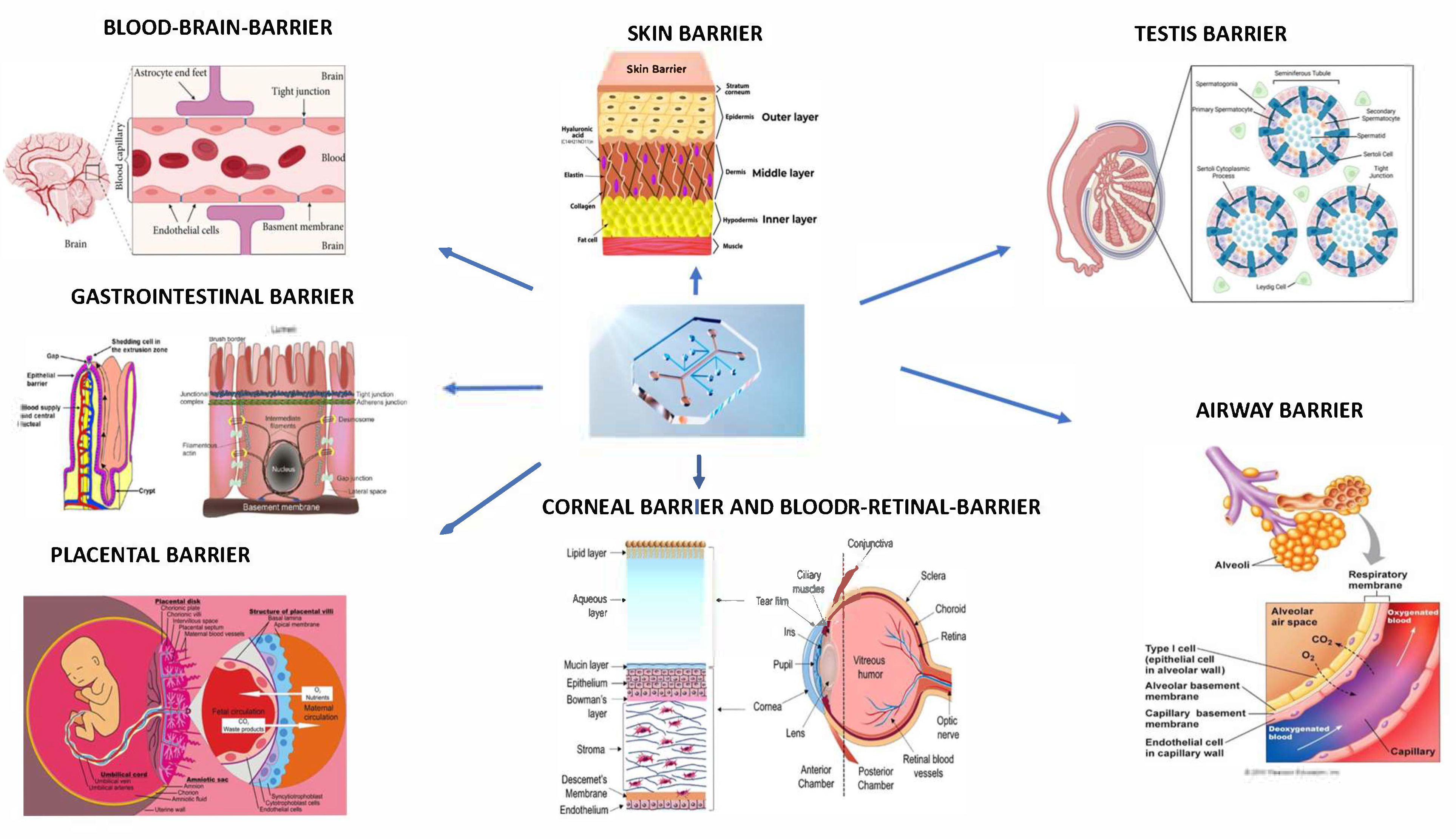
1. Introduction
2. Blood–Brain Barrier (BBB)-On-Chip
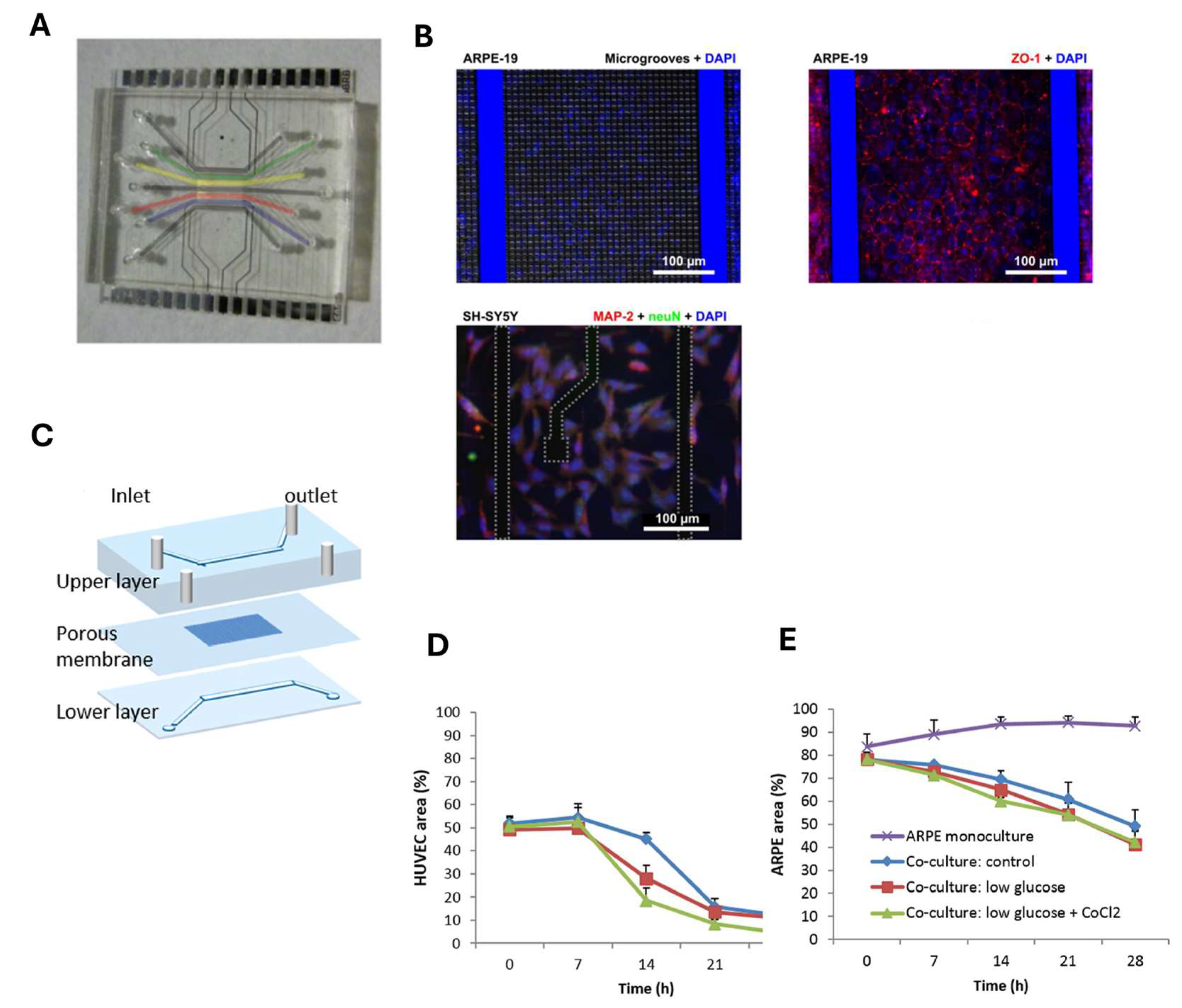
3. Blood–Retinal Barrier (BRB)-on-Chip
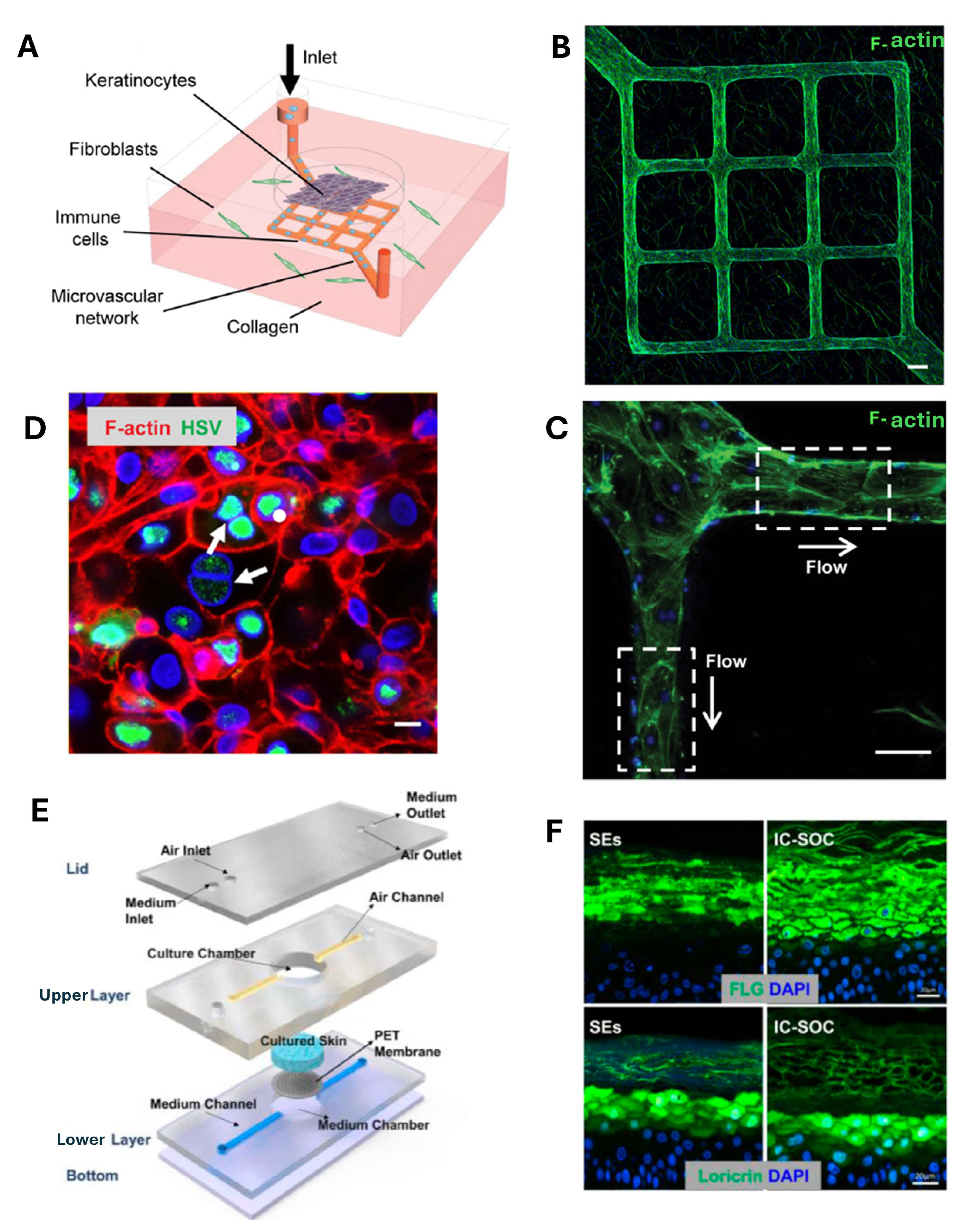
4. Skin-on-Chip
5. Cornea-on-Chip
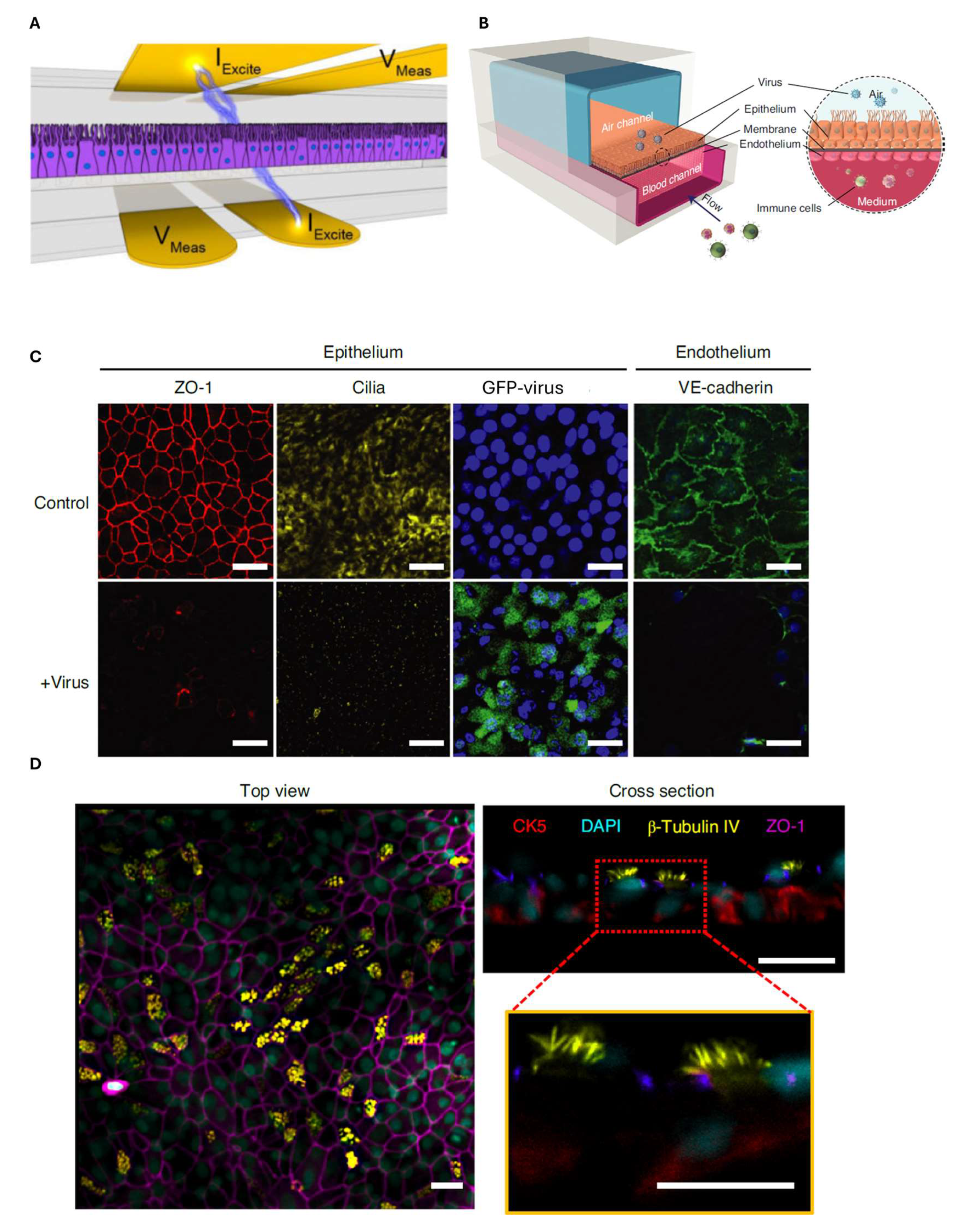
6. Airway-on-Chip
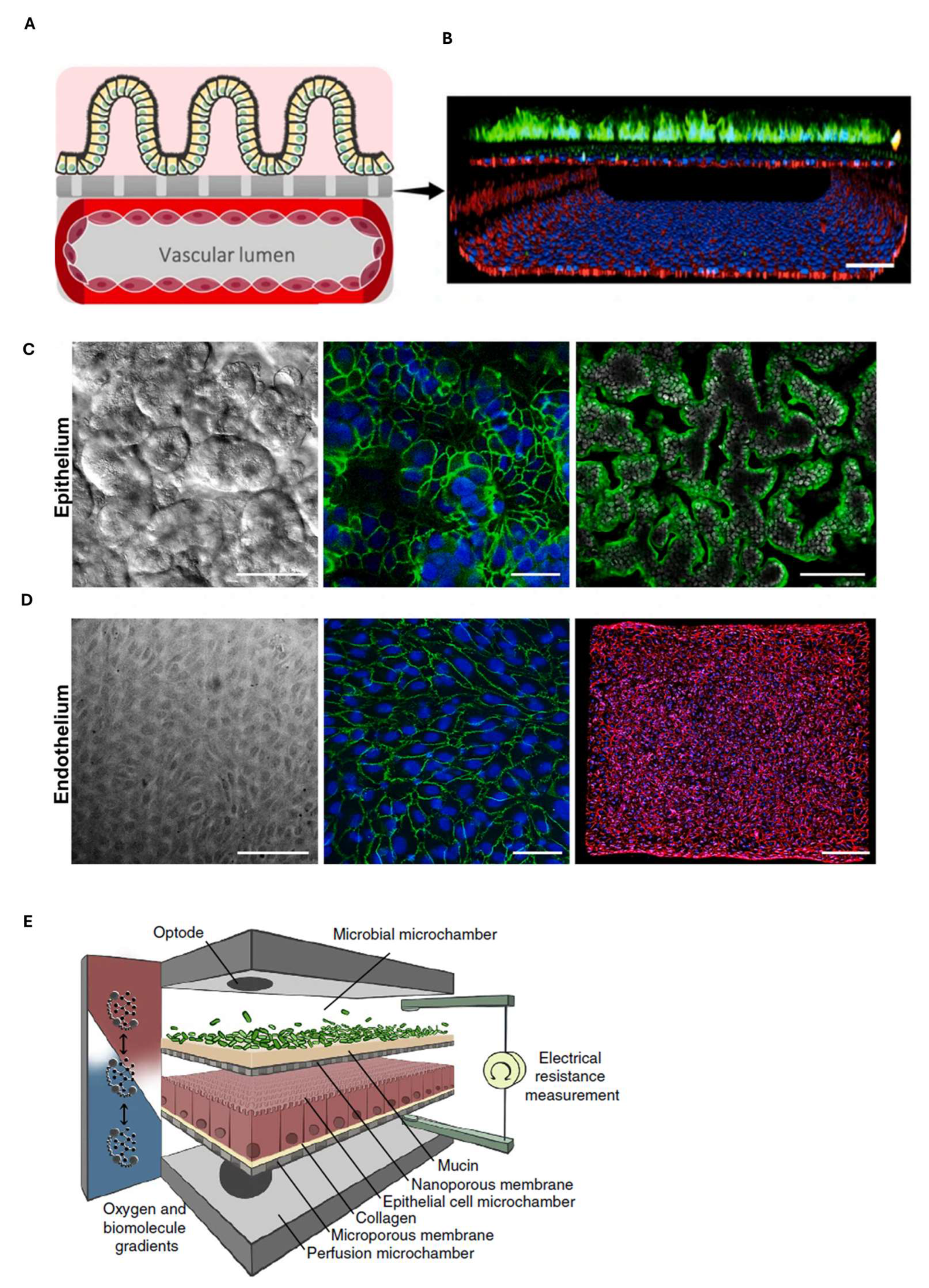
7. Gastrointestinal Barrier-on-Chip
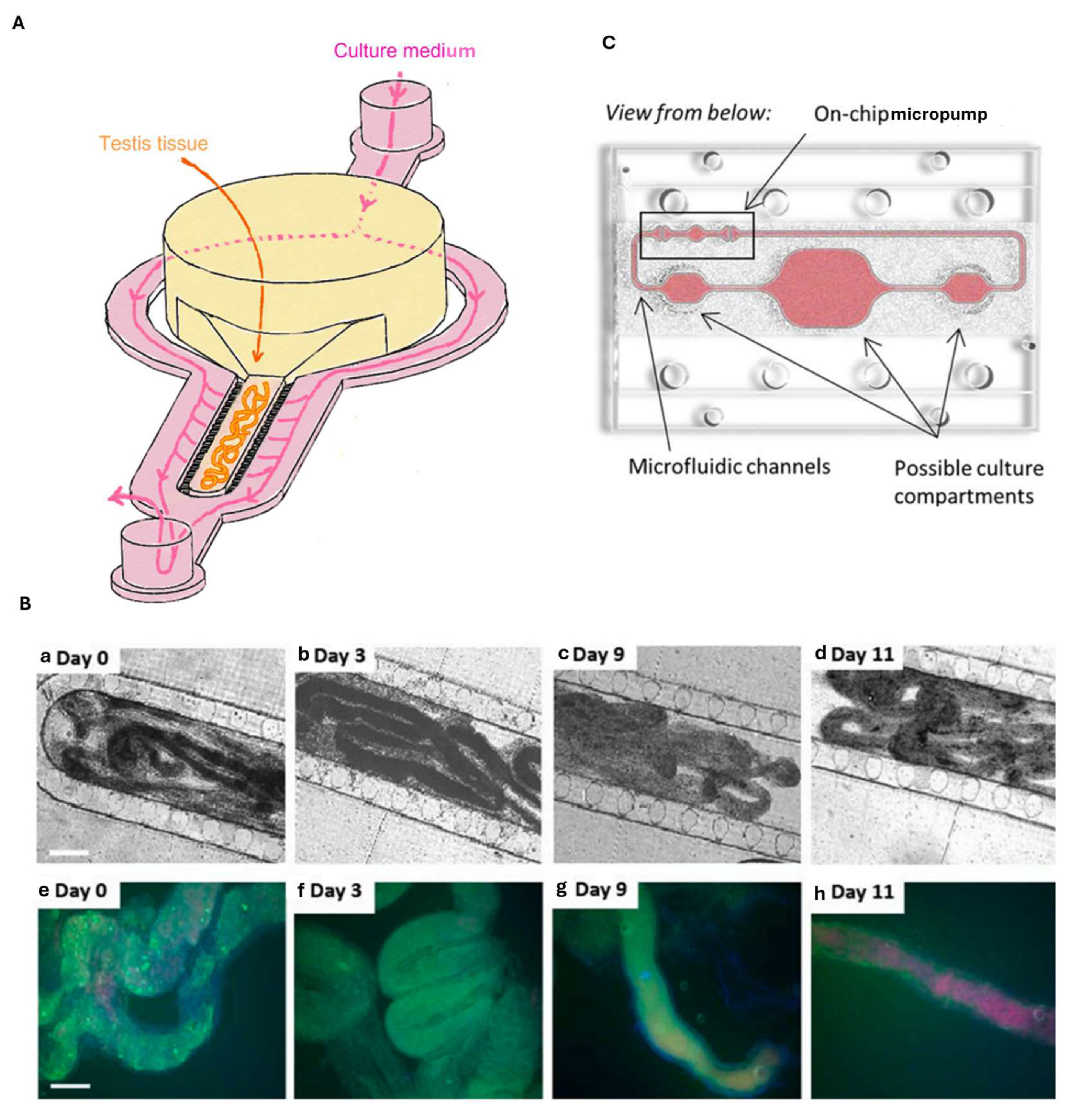
8. Testis-on-Chip
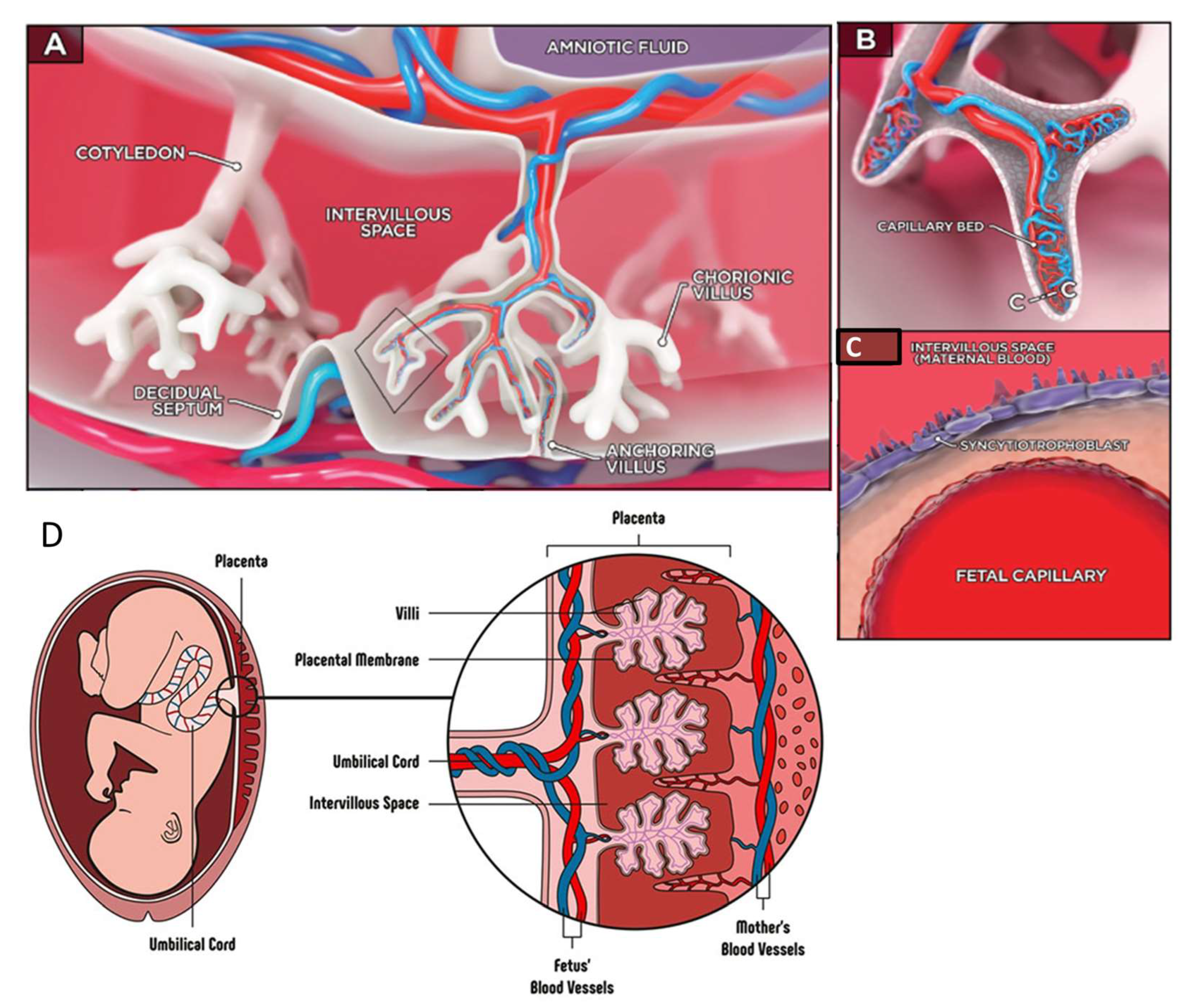
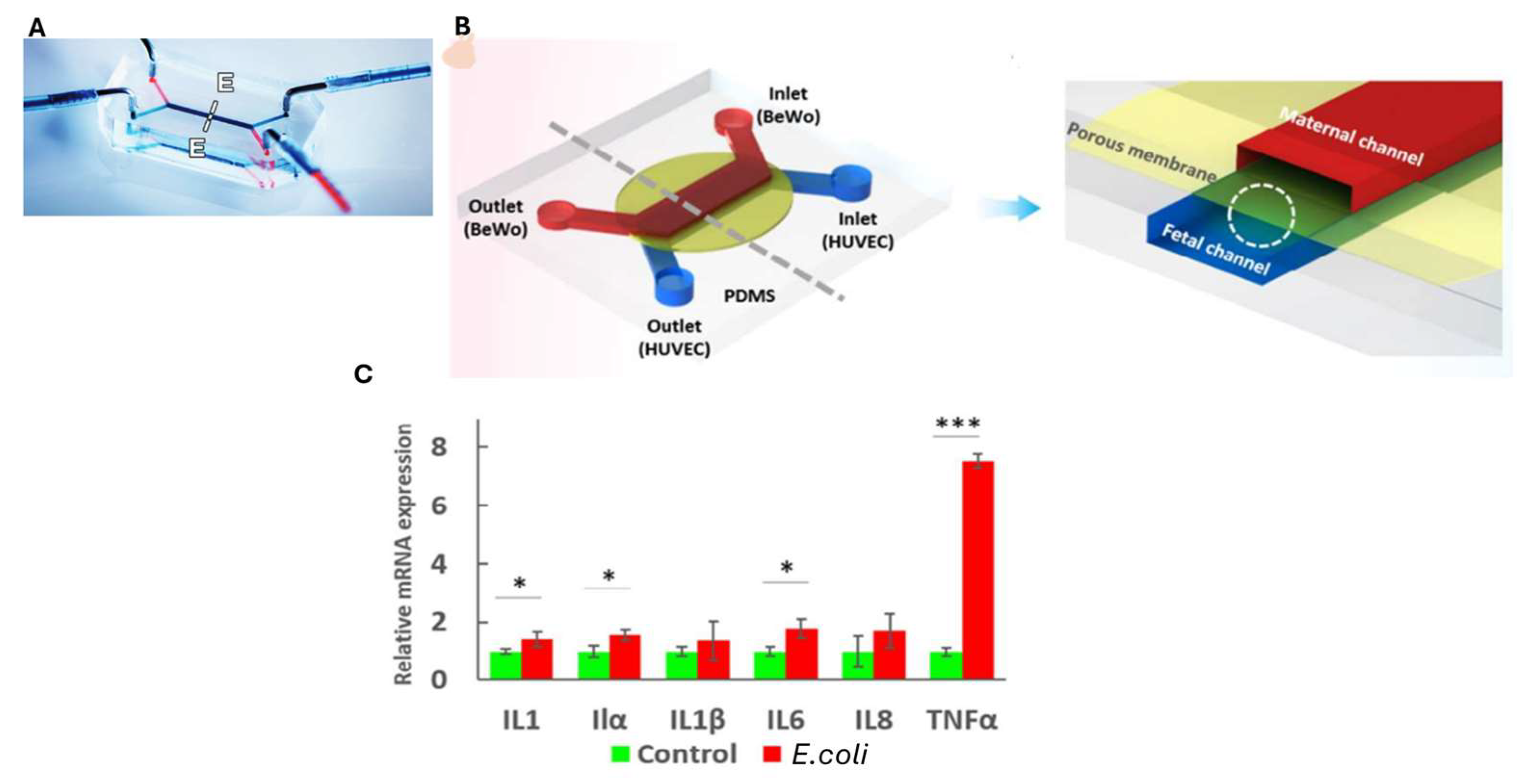
9. Placenta-on-Chip
| Barriers-on-Chips | Key Features | Critical Issues | Fabrication Method and Channel Size | Cell Lines | Preclinical Applications |
|---|---|---|---|---|---|
| Blood–brain barrier-on-chip |
| CHANNEL SIZE |
| ||
| Blood–retinal barrier-on-chip |
CHANNEL SIZE
| ||||
| Skin-on-chip |
|
|
CHANNEL SIZE | ||
| Cornea-on-chip |
| CHANNEL SIZE
| |||
| Airway-on-chip |
CHANNEL SIZE
| ||||
| Gastrointestinal barrier-on chip |
CHANNEL SIZE |
| |||
| Testis-on-chip |
|
|
CHANNEL SIZE
| ||
| Placenta-on-chip | CHANNEL SIZE |
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OoC | organ-on-chip |
| hiPSCs | human induced pluripotent stem cells |
| BBB | blood–brain barrier |
| BRB | blood–retinal barrier |
| C6-NCs | coumarin-6 nanocrystals |
| MEAs | multiple-electrode arrays |
| SNPs | single-nucleotide polymorphisms |
| ESCs | embryonic stem cells |
| iBMECs | induced brain microvascular endothelial cells |
| ZO-1 | zonula occludens-1 |
| CSF | cerebrospinal fluid |
| TEER | transepithelial/transendothelial electrical resistance |
| ECs | endothelial cells |
| COP | cyclo-olefin polymer |
| EIS | electrical impedance spectroscopy |
| hCMEC/D3 | human brain microvascular endothelial cells |
| iPS-BMVECs | human brain microvascular endothelium derived from induced pluripotent stem cells |
| VE-cadherin | vascular endothelial cadherin |
| iBRB | inner blood retinal barrier |
| oBRB | outer blood retinal barrier |
| RPE | retinal pigment epithelial |
| ILM | inner limiting barrier |
| GCL | ganglion cell layer |
| INL | outer nuclear layer |
| OS | outer segments |
| AMD | age-related macular degeneration |
| PDMS | polydimethylsiloxane |
| HUVECs | human umbilical vein endothelial cells |
| ARPE-19 | retinal pigment epithelium cells |
| CNV | choroidal neovascularization |
| HRECs | primary human retinal endothelial cells |
| SH-SY5Y | human neuroblastoma cell line |
| DRIE | deep reactive-ion etching |
| NeuN | neuronal nuclei |
| MAP2 | microtubule-associated protein 2 |
| MVN | microvascular networks |
| HRMVEC | human retinal microvascular endothelial cells |
| HRP | primary human retinal microvascular pericytes cells |
| HRA | primary human retinal astrocytes cells |
| VEGF | vascular endothelial growth factor |
| CoCl2 | cobalt chloride |
| ALI | air–liquid interface |
| SoC | skin-on-chip |
| HSV | herpes simplex virus |
| TRPV1 | transient receptor protein-villanoid-1 |
| IC-SoC | interface-controlled skin-on-chip |
| P. acnes | Propionibacterium acnes |
| PET | polyethylene terephthalate |
| HaCaT | human keratinocytes cells |
| HCEpi | human corneal epithelial cells |
| HCEnd | human corneal endothelial cells |
| EPI-ALI | (air–liquid interface)–(epithelial) |
| Papp | permeability coefficient |
| FITC | fluorescein 5(6)-isothiocyanate |
| MMP-2 | matrix metalloproteinase-2 |
| CK-3/12 | cytokeratin 3/12 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| COPD | chronic obstructive pulmonary disease |
| TNF-α | tumor necrosis factor α |
| ICAM-1 | intercellular adhesion molecule 1 |
| ITO | indium tin oxide |
| NCI-H1437 | human lung adenocarcinoma cells |
| CI | cellular index |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus |
| CK-5 | cytokeratin 5 |
| GI | gastrointestinal |
| IBD | inflammatory bowel disease |
| LGG | Lactobacillus rhamnosus GG |
| PECAM-1 | platelet endothelial cell adhesion molecule 1 |
| Caco-2 | human colorectal adenocarcinoma cells |
| CCL20 | C-C motif ligand 2 |
| hAOs | human antral organoids |
| gMSCs | primary gastric mesenchymal stromal cells |
| PBMCs | human peripheral blood mononuclear cells |
| NF-KB | nuclear factor KB |
| BTB | blood–testis barrier |
| CYP450 | cytochrome P450 |
| HepaRG | human liver spheroids |
| HPVECs | placental vascular endothelial cells |
| BeWo | human placental choriocarcinoma cells |
| FKBPL | binding protein FK506 |
| Gal-3 | galectin 3 |
| THP-1 | human macrophages |
| IL-1 α/β/8 | interleukin 1 α/β/8 |
References
- Arik, Y.B.; van der Helm, M.W.; Odijk, M.; Segerink, L.I.; Passier, R.; van den Berg, A.; van der Meer, A.D. Barriers-on-chips: Measurement of barrier function of tissues in organs-on-chips. Biomicrofluidics 2018, 12, 042218. [Google Scholar] [CrossRef] [PubMed]
- Zoio, P.; Lopes-Ventura, S.; Oliva, A. Barrier-on-a-Chip with a Modular Architecture and Integrated Sensors for Real-Time Measurement of Biological Barrier Function. Micromachines 2021, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Cary, C.; Stapleton, P. Determinants and mechanisms of inorganic nanoparticle translocation across mammalian biological barriers. Arch. Toxicol. 2023, 97, 2111–2131. [Google Scholar] [CrossRef]
- Nazari, H.; Shrestha, J.; Naei, V.Y.; Bazaz, S.R.; Sabbagh, M.; Thiery, J.P.; Warkiani, M.E. Advances in TEER measurements of biological barriers in microphysiological systems. Biosens. Bioelectron. 2023, 234, 115355. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Reed, M.S.; Jiang, S.D.; Cao, X.; Hasan, T.; Doyley, M.M.; Pogue, B.W. Prototype dual-channel fluorescence/transmission optical tomography system for quantification of capillary permeability and porphyrin production. In Proceedings of the SPIE BiOS, San Francisco, CA, USA, 12 March 2024; Volume 12825. [Google Scholar] [CrossRef]
- Chen, L.Y.; Lin, K.R.; Chen, Y.J.; Chiang, Y.J.; Ho, K.C.; Shen, L.F.; Song, I.W.; Liu, K.M.; Yang-Yen, H.F.; Chen, Y.J.; et al. Palmitoyl Acyltransferase Activity of ZDHHC13 Regulates Skin Barrier Development Partly by Controlling PADi3 and TGM1 Protein Stability. J. Investig. Dermatol. 2020, 140, 959–970.e953. [Google Scholar] [CrossRef]
- Li, Y.; Miao, X.; Chen, T.; Yi, X.; Wang, R.; Zhao, H.; Lee, S.M.; Wang, X.; Zheng, Y. Zebrafish as a visual and dynamic model to study the transport of nanosized drug delivery systems across the biological barriers. Colloids Surf. B Biointerfaces 2017, 156, 227–235. [Google Scholar] [CrossRef]
- Bhise, N.S.; Ribas, J.; Manoharan, V.; Zhang, Y.S.; Polini, A.; Massa, S.; Dokmeci, M.R.; Khademhosseini, A. Organ-on-a-chip platforms for studying drug delivery systems. J. Control. Release 2014, 190, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Nithin, R.; Aggarwal, A.; Sravani, A.B.; Mallya, P.; Lewis, S. Organ-On-A-Chip: An Emerging Research Platform. Organogenesis 2023, 19, 2278236. [Google Scholar] [CrossRef]
- Obeid, P.J.; Yammine, P.; El-Nakat, H.; Kassab, R.; Tannous, T.; Nasr, Z.; Maarawi, T.; Dahdah, N.; El Safadi, A.; Mansour, A.; et al. Organ-On-A-Chip Devices: Technology Progress and Challenges. Chembiochem 2024, 25, e202400580. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; Rangineni, D.P.; Abdelsaid, H.; Ma, Y.; Bhushan, A. Tiny Organs, Big Impact: How Microfluidic Organ-on-Chip Technology Is Revolutionizing Mucosal Tissues and Vasculature. Bioengineering 2024, 11, 476. [Google Scholar] [CrossRef]
- Walsh, D.I., 3rd; Dydek, E.V.; Lock, J.Y.; Carlson, T.L.; Carrier, R.L.; Kong, D.S.; Cabrera, C.R.; Thorsen, T. Emulation of Colonic Oxygen Gradients in a Microdevice. SLAS Technol. 2018, 23, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Monteduro, A.G.; Rizzato, S.; Caragnano, G.; Trapani, A.; Giannelli, G.; Maruccio, G. Organs-on-chips technologies—A guide from disease models to opportunities for drug development. Biosens. Bioelectron. 2023, 231, 115271. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, M.; Phung, N.; Grimm, J.; Andreou, C. Organ-on-chip systems as a model for nanomedicine. Nanoscale 2023, 15, 9927–9940. [Google Scholar] [CrossRef]
- Ahadian, S.; Civitarese, R.; Bannerman, D.; Mohammadi, M.H.; Lu, R.; Wang, E.; Davenport-Huyer, L.; Lai, B.; Zhang, B.; Zhao, Y.; et al. Organ-On-A-Chip Platforms: A Convergence of Advanced Materials, Cells, and Microscale Technologies. Adv. Healthc. Mater. 2018, 7, 1700506. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Albani, M.D.P.e.D.; Tomasoni, S.; Gatta, R. Cellule Staminali Pluripotenti Indotte e Organ on Chip: Cosa Sono e Come Funziona la Ricerca del Futuro. 2023. Available online: https://www.marionegri.it/magazine/cellule-staminali-pluripotenti-indotte-e-organ-on-chip (accessed on 10 May 2025).
- Inoue, H.; Yamanaka, S. The use of induced pluripotent stem cells in drug development. Clin. Pharmacol. Ther. 2011, 89, 655–661. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- Lermant, A.; Rabussier, G.; Lanz, H.L.; Davidson, L.; Porter, I.M.; Murdoch, C.E. Development of a human iPSC-derived placental barrier-on-chip model. iScience 2023, 26, 107240. [Google Scholar] [CrossRef]
- Jagadeesan, S.; Workman, M.J.; Herland, A.; Svendsen, C.N.; Vatine, G.D. Generation of a Human iPSC-Based Blood-Brain Barrier Chip. J. Vis. Exp. JoVE 2020, 159, e60925. [Google Scholar] [CrossRef]
- Chen, H.; Luo, Z.; Lin, X.; Zhu, Y.; Zhao, Y. Sensors-integrated organ-on-a-chip for biomedical applications. Nano Res. 2023, 16, 10072–10099. [Google Scholar] [CrossRef]
- Holzreuter, M.A.; Segerink, L.I. Innovative electrode and chip designs for transendothelial electrical resistance measurements in organs-on-chips. Lab A Chip 2024, 24, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, M.; Werr, G.; Johansson, S.; Barbe, L.; Grandmougin, L.; Wilmes, P.; Tenje, M. Integration of multiple flexible electrodes for real-time detection of barrier formation with spatial resolution in a gut-on-chip system. Microsyst. Nanoeng. 2024, 10, 18. [Google Scholar] [CrossRef]
- Benam, K.H.; Konigshoff, M.; Eickelberg, O. Breaking the In Vitro Barrier in Respiratory Medicine. Engineered Microphysiological Systems for Chronic Obstructive Pulmonary Disease and Beyond. Am. J. Respir. Crit. Care Med. 2018, 197, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhang, H.; Wang, X. Microfluidic-Chip-Integrated Biosensors for Lung Disease Models. Biosensors 2021, 11, 456. [Google Scholar] [CrossRef]
- Bennet, T.J.; Randhawa, A.; Hua, J.; Cheung, K.C. Airway-On-A-Chip: Designs and Applications for Lung Repair and Disease. Cells 2021, 10, 1602. [Google Scholar] [CrossRef]
- Li, K.; Yang, X.; Xue, C.; Zhao, L.; Zhang, Y.; Gao, X. Biomimetic human lung-on-a-chip for modeling disease investigation. Biomicrofluidics 2019, 13, 031501. [Google Scholar] [CrossRef]
- Cui, B.; Cho, S.W. Blood-brain barrier-on-a-chip for brain disease modeling and drug testing. BMB Rep. 2022, 55, 213–219. [Google Scholar] [CrossRef]
- Palasantzas, V.; Tamargo-Rubio, I.; Le, K.; Slager, J.; Wijmenga, C.; Jonkers, I.H.; Kumar, V.; Fu, J.; Withoff, S. iPSC-derived organ-on-a-chip models for personalized human genetics and pharmacogenomics studies. Trends Genet. TIG 2023, 39, 268–284. [Google Scholar] [CrossRef]
- Morelli, M.; Kurek, D.; Ng, C.P.; Queiroz, K. Gut-on-a-Chip Models: Current and Future Perspectives for Host-Microbial Interactions Research. Biomedicines 2023, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Taggi, V.; Riera Romo, M.; Piquette-Miller, M.; Meyer Zu Schwabedissen, H.E.; Neuhoff, S. Transporter Regulation in Critical Protective Barriers: Focus on Brain and Placenta. Pharmaceutics 2022, 14, 1376. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Bi, W.; Cai, S.; Lei, T.; Wang, L. Implementation of blood-brain barrier on microfluidic chip: Recent advance and future prospects. Ageing Res. Rev. 2023, 87, 101921. [Google Scholar] [CrossRef]
- Guarino, V.; Zizzari, A.; Bianco, M.; Gigli, G.; Moroni, L.; Arima, V. Advancements in modelling human blood brain-barrier on a chip. Biofabrication 2023, 15, 022003. [Google Scholar] [CrossRef]
- Arriera Ematoencefalica. Available online: https://www.my-personaltrainer.it/farmacologia/barriera-ematoencefalica-16.html (accessed on 10 May 2025).
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic Blood-Brain Barrier Model Provides In Vivo-Like Barrier Properties for Drug Permeability Screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef]
- Bang, S.; Lee, S.-R.; Ko, J.; Son, K.; Tahk, D.; Ahn, J.; Im, C.; Jeon, N.L. A Low Permeability Microfluidic Blood-Brain Barrier Platform with Direct Contact between Perfusable Vascular Network and Astrocytes. Sci. Rep. 2017, 7, 8083. [Google Scholar] [CrossRef]
- Mir, M.; Palma-Florez, S.; Lagunas, A.; Lopez-Martinez, M.J.; Samitier, J. Biosensors Integration in Blood-Brain Barrier-on-a-Chip: Emerging Platform for Monitoring Neurodegenerative Diseases. ACS Sens. 2022, 7, 1237–1247. [Google Scholar] [CrossRef]
- van der Helm, M.W.; van der Meer, A.D.; Eijkel, J.C.; van den Berg, A.; Segerink, L.I. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 2016, 4, e1142493. [Google Scholar] [CrossRef] [PubMed]
- Deli, M.A.; Porkolab, G.; Kincses, A.; Meszaros, M.; Szecsko, A.; Kocsis, A.E.; Vigh, J.P.; Valkai, S.; Veszelka, S.; Walter, F.R.; et al. Lab-on-a-chip models of the blood-brain barrier: Evolution, problems, perspectives. Lab A Chip 2024, 24, 1030–1063. [Google Scholar] [CrossRef] [PubMed]
- Kincses, A.; Vigh, J.P.; Petrovszki, D.; Valkai, S.; Kocsis, A.E.; Walter, F.R.; Lin, H.Y.; Jan, J.S.; Deli, M.A.; Der, A. The Use of Sensors in Blood-Brain Barrier-on-a-Chip Devices: Current Practice and Future Directions. Biosensors 2023, 13, 357. [Google Scholar] [CrossRef]
- Kawakita, S.; Mandal, K.; Mou, L.; Mecwan, M.M.; Zhu, Y.; Li, S.; Sharma, S.; Hernandez, A.L.; Nguyen, H.T.; Maity, S.; et al. Organ-On-A-Chip Models of the Blood-Brain Barrier: Recent Advances and Future Prospects. Small 2022, 18, e2201401. [Google Scholar] [CrossRef]
- Park, T.E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Badiola-Mateos, M.; Di Giuseppe, D.; Paoli, R.; Lopez-Martinez, M.J.; Mencattini, A.; Samitier, J.; Martinelli, E. A novel multi-frequency trans-endothelial electrical resistance (MTEER) sensor array to monitor blood-brain barrier integrity. Sens. Actuators B Chem. 2021, 334, 129599. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Yu, Y.; Sizdahkhani, S.; Ho, W.S.; Yin, F.; Wang, L.; Zhu, G.; Zhang, M.; Jiang, L.; et al. A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors. Sci. Rep. 2016, 6, 36670. [Google Scholar] [CrossRef]
- Partyka, P.P.; Godsey, G.A.; Galie, J.R.; Kosciuk, M.C.; Acharya, N.K.; Nagele, R.G.; Galie, P.A. Mechanical stress regulates transport in a compliant 3D model of the blood-brain barrier. Biomaterials 2017, 115, 30–39. [Google Scholar] [CrossRef]
- Ragelle, H.; Goncalves, A.; Kustermann, S.; Antonetti, D.A.; Jayagopal, A. Organ-On-A-Chip Technologies for Advanced Blood-Retinal Barrier Models. J. Ocul. Pharmacol. Ther. 2020, 36, 30–41. [Google Scholar] [CrossRef]
- Ragelle, H.; Dernick, K.; Khemais, S.; Keppler, C.; Cousin, L.; Farouz, Y.; Louche, C.; Fauser, S.; Kustermann, S.; Tibbitt, M.W.; et al. Human Retinal Microvasculature-on-a-Chip for Drug Discovery. Adv. Healthc. Mater. 2020, 9, e2001531. [Google Scholar] [CrossRef]
- Yeste, J.; Garcia-Ramirez, M.; Illa, X.; Guimera, A.; Hernandez, C.; Simo, R.; Villa, R. A compartmentalized microfluidic chip with crisscross microgrooves and electrophysiological electrodes for modeling the blood-retinal barrier. Lab A Chip 2017, 18, 95–105. [Google Scholar] [CrossRef]
- Maurissen, T.L.; Spielmann, A.J.; Schellenberg, G.; Bickle, M.; Vieira, J.R.; Lai, S.Y.; Pavlou, G.; Fauser, S.; Westenskow, P.D.; Kamm, R.D.; et al. Modeling early pathophysiological phenotypes of diabetic retinopathy in a human inner blood-retinal barrier-on-a-chip. Nat. Commun. 2024, 15, 1372. [Google Scholar] [CrossRef]
- Chen, L.J.; Ito, S.; Kai, H.; Nagamine, K.; Nagai, N.; Nishizawa, M.; Abe, T.; Kaji, H. Microfluidic co-cultures of retinal pigment epithelial cells and vascular endothelial cells to investigate choroidal angiogenesis. Sci. Rep. 2017, 7, 3538. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Kim, J.J. Recent advances in in vitro skin-on-a-chip models for drug testing. Expert Opin. Drug Metab. Toxicol. 2023, 19, 249–267. [Google Scholar] [CrossRef]
- Sun, W.; Liu, Z.; Xu, J.; Cheng, Y.; Yin, R.; Ma, L.; Li, H.; Qian, X.; Zhang, H. 3D skin models along with skin-on-a-chip systems: A critical review. Chin. Chem. Lett. 2023, 34, 107819. [Google Scholar] [CrossRef]
- Costa, S.; Vilas-Boas, V.; Lebre, F.; Granjeiro, J.M.; Catarino, C.M.; Moreira Teixeira, L.; Loskill, P.; Alfaro-Moreno, E.; Ribeiro, A.R. Microfluidic-based skin-on-chip systems for safety assessment of nanomaterials. Trends Biotechnol. 2023, 41, 1282–1298. [Google Scholar] [CrossRef] [PubMed]
- Michielon, E.; Boninsegna, M.; Waaijman, T.; Fassini, D.; Spiekstra, S.W.; Cramer, J.; Gaudriault, P.; Kodolanyi, J.; de Gruijl, T.D.; Homs-Corbera, A.; et al. Environmentally Controlled Microfluidic System Enabling Immune Cell Flow and Activation in an Endothelialised Skin-On-Chip. Adv. Healthc. Mater. 2024, 13, e2400750. [Google Scholar] [CrossRef]
- Ahn, J.; Ohk, K.; Won, J.; Choi, D.H.; Jung, Y.H.; Yang, J.H.; Jun, Y.; Kim, J.A.; Chung, S.; Lee, S.H. Modeling of three-dimensional innervated epidermal like-layer in a microfluidic chip-based coculture system. Nat. Commun. 2023, 14, 1488. [Google Scholar] [CrossRef]
- Sun, S.; Jin, L.; Zheng, Y.; Zhu, J. Modeling human HSV infection via a vascularized immune-competent skin-on-chip platform. Nat. Commun. 2022, 13, 5481. [Google Scholar] [CrossRef]
- Martorina, F.; Casale, C.; Urciuolo, F.; Netti, P.A.; Imparato, G. In vitro activation of the neuro-transduction mechanism in sensitive organotypic human skin model. Biomaterials 2017, 113, 217–229. [Google Scholar] [CrossRef]
- Smythe, P.; Wilkinson, H.N. The Skin Microbiome: Current Landscape and Future Opportunities. Int. J. Mol. Sci. 2023, 24, 3950. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.C.; Kalan, L.R. The dynamic balance of the skin microbiome across the lifespan. Biochem. Soc. Trans. 2023, 51, 71–86. [Google Scholar] [CrossRef]
- Fernandez-Carro, E.; Angenent, M.; Gracia-Cazana, T.; Gilaberte, Y.; Alcaine, C.; Ciriza, J. Modeling an Optimal 3D Skin-on-Chip within Microfluidic Devices for Pharmacological Studies. Pharmaceutics 2022, 14, 1417. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Weng, D.; Li, X.; An, Q.; Yang, Y.; Yu, B.; Ma, Y.; Wang, J. Analysis of drug efficacy for inflammatory skin on an organ-chip system. Front. Bioeng. Biotechnol. 2022, 10, 939629. [Google Scholar] [CrossRef]
- Van Meenen, J.; Ni Dhubhghaill, S.; Van den Bogerd, B.; Koppen, C. An Overview of Advanced In Vitro Corneal Models: Implications for Pharmacological Testing. Tissue Eng. Part B Rev. 2022, 28, 506–516. [Google Scholar] [CrossRef]
- Lieto, K.; Skopek, R.; Lewicka, A.; Stelmasiak, M.; Klimaszewska, E.; Zelent, A.; Szymanski, L.; Lewicki, S. Looking into the Eyes-In Vitro Models for Ocular Research. Int. J. Mol. Sci. 2022, 23, 9158. [Google Scholar] [CrossRef]
- Kravchenko, S.V.; Myasnikova, V.V.; Sakhnov, S.N. Application of the organ-on-a-chip technology in experimental ophthalmology. Vestn. Oftalmol. 2023, 139, 114–120. [Google Scholar] [CrossRef]
- Li, Q.; Wong, H.L.; Ip, Y.L.; Chu, W.Y.; Li, M.S.; Saha, C.; Shih, K.C.; Chan, Y.K. Current microfluidic platforms for reverse engineering of cornea. Mater. Today. Bio 2023, 20, 100634. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Hao, R.; Zhang, Y.; Yang, H. A Human Cornea-On-A-Chip. In Proceedings of the 2021 IEEE 34th International Conference on Micro Electro Mechanical Systems (MEMS), Gainesville, FL, USA, 25–29 January 2021; pp. 982–985. [Google Scholar] [CrossRef]
- Manafi, N.; Shokri, F.; Achberger, K.; Hirayama, M.; Mohammadi, M.H.; Noorizadeh, F.; Hong, J.; Liebau, S.; Tsuji, T.; Quinn, P.M.J.; et al. Organoids and organ chips in ophthalmology. Ocul. Surf. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Kim, M.; Choi, K.; Lin, A.; Kim, J. Current and Future Cornea Chip Models for Advancing Ophthalmic Research and Therapeutics. Adv. Biol. 2025, 9, e2400571. [Google Scholar] [CrossRef]
- Yu, Z.; Hao, R.; Du, J.; Wu, X.; Chen, X.; Zhang, Y.; Li, W.; Gu, Z.; Yang, H. A human cornea-on-a-chip for the study of epithelial wound healing by extracellular vesicles. iScience 2022, 25, 104200. [Google Scholar] [CrossRef]
- Seo, J.; Byun, W.Y.; Alisafaei, F.; Georgescu, A.; Yi, Y.S.; Massaro-Giordano, M.; Shenoy, V.B.; Lee, V.; Bunya, V.Y.; Huh, D. Multiscale reverse engineering of the human ocular surface. Nat. Med. 2019, 25, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, L.; Xu, J.; Yao, Y.; Ding, J.; Wang, L.; Luo, C.; Yang, W.; Li, L. A biomimetic human disease model of bacterial keratitis using a cornea-on-a-chip system. Biomater. Sci. 2024, 12, 5239–5252. [Google Scholar] [CrossRef] [PubMed]
- Dezube, R. Panoramica Sui Sintomi Delle Patologie Polmonari. 2023. Available online: https://www.msdmanuals.com/it/casa/disturbi-polmonari-e-delle-vie-respiratorie/sintomi-delle-patologie-polmonari/panoramica-sui-sintomi-delle-patologie-polmonari (accessed on 10 May 2025).
- Frey, A.; Lunding, L.P.; Ehlers, J.C.; Weckmann, M.; Zissler, U.M.; Wegmann, M. More Than Just a Barrier: The Immune Functions of the Airway Epithelium in Asthma Pathogenesis. Front. Immunol. 2020, 11, 761. [Google Scholar] [CrossRef]
- Sunil, A.A.; Skaria, T. Novel regulators of airway epithelial barrier function during inflammation: Potential targets for drug repurposing. Expert Opin. Ther. Targets 2022, 26, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Rae, B.; Vasse, G.F.; Mosayebi, J.; Berge, M.V.D.; Pouwels, S.D.; Heijink, I.H. Development of a Widely Accessible, Advanced Large-Scale Microfluidic Airway-on-Chip. Bioengineering 2025, 12, 182. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Benam, K.H.; Villenave, R.; Lucchesi, C.; Varone, A.; Hubeau, C.; Lee, H.H.; Alves, S.E.; Salmon, M.; Ferrante, T.C.; Weaver, J.C.; et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat. Methods 2016, 13, 151–157. [Google Scholar] [CrossRef]
- Henry, O.Y.F.; Villenave, R.; Cronce, M.J.; Leineweber, W.D.; Benz, M.A.; Ingber, D.E. Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab A Chip 2017, 17, 2264–2271. [Google Scholar] [CrossRef]
- Khalid, M.A.U.; Kim, Y.S.; Ali, M.; Lee, B.G.; Cho, Y.-J.; Choi, K.H. A lung cancer-on-chip platform with integrated biosensors for physiological monitoring and toxicity assessment. Biochem. Eng. J. 2020, 155, 107469. [Google Scholar] [CrossRef]
- Fisher, C.R.; Mba Medie, F.; Luu, R.J.; Gaibler, R.B.; Mulhern, T.J.; Miller, C.R.; Zhang, C.J.; Rubio, L.D.; Marr, E.E.; Vijayakumar, V.; et al. A High-Throughput, High-Containment Human Primary Epithelial Airway Organ-on-Chip Platform for SARS-CoV-2 Therapeutic Screening. Cells 2023, 12, 2639. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Bai, H.; Rodas, M.; Cao, W.; Oh, C.Y.; Jiang, A.; Moller, R.; Hoagland, D.; Oishi, K.; Horiuchi, S.; et al. A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nat. Biomed. Eng. 2021, 5, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wen, T.; Zhu, W.; Li, K.; Gong, X.; Li, Z. Microfluidic strategies for biomimetic lung chip establishment and SARS-CoV2 study. Mater. Today Bio 2024, 24, 100905. [Google Scholar] [CrossRef]
- Koceva, H.; Amiratashani, M.; Akbarimoghaddam, P.; Hoffmann, B.; Zhurgenbayeva, G.; Gresnigt, M.S.; Marcelino, V.R.; Eggeling, C.; Figge, M.T.; Amorim, M.J.; et al. Deciphering respiratory viral infections by harnessing organ-on-chip technology to explore the gut-lung axis. Open Biol. 2025, 15, 240231. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Beltran-Velasco, A.I.; Clemente-Suarez, V.J. Harnessing Gut Microbiota for Biomimetic Innovations in Health and Biotechnology. Biomimetics 2025, 10, 73. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2023, 19, 275–293. [Google Scholar] [CrossRef]
- Trujillo-de Santiago, G.; Lobo-Zegers, M.J.; Montes-Fonseca, S.L.; Zhang, Y.S.; Alvarez, M.M. Gut-microbiota-on-a-chip: An enabling field for physiological research. Microphysiol. Syst. 2018, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.S.; Choi, Y.Y.; Mo, S.J.; Ha, J.H.; Lee, Y.S.; Lee, H.U.; Park, S.D.; Shim, J.J.; Lee, J.L.; Chung, B.G. Contributions of the microbiome to intestinal inflammation in a gut-on-a-chip. Nano Converg. 2022, 9, 8. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Li, G.; Yao, W.; Yang, W. The Gut-Organ-Axis Concept: Advances the Application of Gut-on-Chip Technology. Int. J. Mol. Sci. 2023, 24, 4089. [Google Scholar] [CrossRef]
- Xian, C.; Zhang, J.; Zhao, S.; Li, X.G. Gut-on-a-chip for disease models. J. Tissue Eng. 2023, 14, 20417314221149882. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, B.; Liu, X.; Peng, L.; Liu, J.; Hu, Y.; Ji, X.; Lv, H.; Wang, S. Current gut-on-a-chip platforms for clarifying the interactions between diet, gut microbiota, and host health. Trends Food Sci. Technol. 2023, 134, 1–12. [Google Scholar] [CrossRef]
- Thomas, D.P.; Zhang, J.; Nguyen, N.T.; Ta, H.T. Microfluidic Gut-on-a-Chip: Fundamentals and Challenges. Biosensors 2023, 13, 136. [Google Scholar] [CrossRef]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab A Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef]
- Maurer, M.; Gresnigt, M.S.; Last, A.; Wollny, T.; Berlinghof, F.; Pospich, R.; Cseresnyes, Z.; Medyukhina, A.; Graf, K.; Groger, M.; et al. A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials 2019, 220, 119396. [Google Scholar] [CrossRef]
- Nikolaev, M.; Mitrofanova, O.; Broguiere, N.; Geraldo, S.; Dutta, D.; Tabata, Y.; Elci, B.; Brandenberg, N.; Kolotuev, I.; Gjorevski, N.; et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 2020, 585, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Jalili-Firoozinezhad, S.; Prantil-Baun, R.; Jiang, A.; Potla, R.; Mammoto, T.; Weaver, J.C.; Ferrante, T.C.; Kim, H.J.; Cabral, J.M.S.; Levy, O.; et al. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Cell Death Dis. 2018, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Signore, M.A.; De Pascali, C.; Giampetruzzi, L.; Siciliano, P.A.; Francioso, L. Gut-on-Chip microphysiological systems: Latest advances in the integration of sensing strategies and adoption of mature detection mechanisms. Sens. Bio-Sens. Res. 2021, 33, 100443. [Google Scholar] [CrossRef]
- Shah, P.; Fritz, J.V.; Glaab, E.; Desai, M.S.; Greenhalgh, K.; Frachet, A.; Niegowska, M.; Estes, M.; Jager, C.; Seguin-Devaux, C.; et al. A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat. Commun. 2016, 7, 11535. [Google Scholar] [CrossRef]
- Beaurivage, C.; Kanapeckaite, A.; Loomans, C.; Erdmann, K.S.; Stallen, J.; Janssen, R.A.J. Development of a human primary gut-on-a-chip to model inflammatory processes. Sci. Rep. 2020, 10, 21475. [Google Scholar] [CrossRef]
- Jeong, H.J.; Park, J.H.; Kang, J.H.; Sabate Del Rio, J.; Kong, S.H.; Park, T.E. Organoid-Based Human Stomach Micro-Physiological System to Recapitulate the Dynamic Mucosal Defense Mechanism. Adv. Sci. 2023, 10, e2300164. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Venzac, B.; Burgers, T.; Schlatt, S.; Le Gac, S. Testis-on-chip platform to study ex vivo primate spermatogenesis and endocrine dynamics. Organs-A-Chip 2022, 4, 100023. [Google Scholar] [CrossRef]
- Ercan, F.; Acikel Elmas, M. The alterations of blood-testis barrier in experimental testicular ınjury models. Marmara Med. J. 2022, 35, 249–253. [Google Scholar] [CrossRef]
- Su, J.; Yang, Y.; Zhao, F.; Zhang, Y.; Su, H.; Wang, D.; Li, K.; Song, Y.; Cao, G. Study of spermatogenic and Sertoli cells in the Hu sheep testes at different developmental stages. FASEB J. 2023, 37, e23084. [Google Scholar] [CrossRef]
- Shen, J.; Wang, X.; Yang, C.; Ren, G.; Wang, L.; Piao, S.; Zhang, B.; Sun, W.; Ge, X.; Jing, J.; et al. Development and evaluation of a microfluidic human testicular tissue chip: A novel in vitro platform for reproductive biology and pharmacology studies. Lab A Chip 2025, 25, 577–589. [Google Scholar] [CrossRef]
- Park, S.R.; Kook, M.G.; Kim, S.R.; Lee, C.M.; Lee, J.W.; Park, J.K.; Park, C.H.; Oh, B.C.; Jung, Y.; Hong, I.S. Development of a novel testis-on-a-chip that demonstrates reciprocal crosstalk between Sertoli and Leydig cells in testicular tissue. Exp. Mol. Med. 2024, 56, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Lopez, I.; Truskey, G.A. Multi-cellular engineered living systems to assess reproductive toxicology. Reprod. Toxicol. 2024, 127, 108609. [Google Scholar] [CrossRef]
- AbuMadighem, A.; Shuchat, S.; Lunenfeld, E.; Yossifon, G.; Huleihel, M. Testis on a chip-a microfluidic three-dimensional culture system for the development of spermatogenesisin-vitro. Biofabrication 2022, 14, 035004. [Google Scholar] [CrossRef]
- Baert, Y.; Ruetschle, I.; Cools, W.; Oehme, A.; Lorenz, A.; Marx, U.; Goossens, E.; Maschmeyer, I. A multi-organ-chip co-culture of liver and testis equivalents: A first step toward a systemic male reprotoxicity model. Hum. Reprod. 2020, 35, 1029–1044. [Google Scholar] [CrossRef]
- Arumugasaamy, N.; Rock, K.D.; Kuo, C.Y.; Bale, T.L.; Fisher, J.P. Microphysiological systems of the placental barrier. Adv. Drug Deliv. Rev. 2020, 161–162, 161–175. [Google Scholar] [CrossRef]
- Lee, J.S.; Romero, R.; Han, Y.M.; Kim, H.C.; Kim, C.J.; Hong, J.S.; Huh, D. Placenta-on-a-chip: A novel platform to study the biology of the human placenta. J. Matern.-Fetal Neonatal Med. 2016, 29, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Deval, G.; Boland, S.; Fournier, T.; Ferecatu, I. On Placental Toxicology Studies and Cerium Dioxide Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12266. [Google Scholar] [CrossRef]
- Liu, L.; Tang, L.; Chen, S.; Zheng, L.; Ma, X. Decoding the molecular pathways governing trophoblast migration and placental development; a literature review. Front. Endocrinol. 2024, 15, 1486608. [Google Scholar] [CrossRef]
- Blundell, C.; Yi, Y.S.; Ma, L.; Tess, E.R.; Farrell, M.J.; Georgescu, A.; Aleksunes, L.M.; Huh, D. Placental Drug Transport-on-a-Chip: A Microengineered In Vitro Model of Transporter-Mediated Drug Efflux in the Human Placental Barrier. Adv. Healthc. Mater. 2018, 7, 1700786. [Google Scholar] [CrossRef]
- Costa, J.; Mackay, R.; de Aguiar Greca, S.C.; Corti, A.; Silva, E.; Karteris, E.; Ahluwalia, A. The Role of the 3Rs for Understanding and Modeling the Human Placenta. J. Clin. Med. 2021, 10, 3444. [Google Scholar] [CrossRef]
- Elzinga, F.A.; Khalili, B.; Touw, D.J.; Prins, J.R.; Olinga, P.; Leuvenink, H.G.D.; van Goor, H.; Gordijn, S.J.; Nagelkerke, A.; Mian, P. Placenta-on-a-Chip as an In Vitro Approach to Evaluate the Physiological and Structural Characteristics of the Human Placental Barrier upon Drug Exposure: A Systematic Review. J. Clin. Med. 2023, 12, 4315. [Google Scholar] [CrossRef]
- Shojaei, S.; Ali, M.S.; Suresh, M.; Upreti, T.; Mogourian, V.; Helewa, M.; Labouta, H.I. Dynamic placenta-on-a-chip model for fetal risk assessment of nanoparticles intended to treat pregnancy-associated diseases. Biochim. Et Biophys. Acta. Mol. Basis Dis. 2021, 1867, 166131. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yan, J.; Nie, K.; Chen, Y.; Wu, Y.; Wang, S.; Zhang, J. Microfluidic chips in female reproduction: A systematic review of status, advances, and challenges. Theranostics 2024, 14, 4352–4374. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P. The solved and unsolved mysteries of human life. J. Appl. Cosmetol. 2023, 41, 67–74. [Google Scholar] [CrossRef]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; LaMarca, B. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 2023, 19, 257–270. [Google Scholar] [CrossRef]
- Ghorbanpour, S.M.; Richards, C.; Pienaar, D.; Sesperez, K.; Aboulkheyr Es, H.; Nikolic, V.N.; Karadzov Orlic, N.; Mikovic, Z.; Stefanovic, M.; Cakic, Z.; et al. A placenta-on-a-chip model to determine the regulation of FKBPL and galectin-3 in preeclampsia. Cell. Mol. Life Sci. 2023, 80, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yin, F.; Wang, H.; Wang, L.; Yuan, J.; Qin, J. Placental Barrier-on-a-Chip: Modeling Placental Inflammatory Responses to Bacterial Infection. ACS Biomater. Sci. Eng. 2018, 4, 3356–3363. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.H.; Heidary Araghi, B.; Beydaghi, V.; Geraili, A.; Moradi, F.; Jafari, P.; Janmaleki, M.; Valente, K.P.; Akbari, M.; Sanati-Nezhad, A. Skin Diseases Modeling using Combined Tissue Engineering and Microfluidic Technologies. Adv. Healthc. Mater. 2016, 5, 2459–2480. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, F.; Campbell, M. The blood-retina barrier in health and disease. FEBS J. 2023, 290, 878–891. [Google Scholar] [CrossRef]



 ), medium (
), medium ( ), and low (
), and low ( ) relevance/ability.
) relevance/ability.
 ), medium (
), medium ( ), and low (
), and low ( ) relevance/ability.
) relevance/ability.Animal Model | 2D Cell Culture | 3D Cell Culture | Organ-on-Chip | References | ||||
|---|---|---|---|---|---|---|---|---|
| Animal Model | 2D Cell Culture | 3D Cell Culture | Organ-on-Chip | |||||
| Translatability of results |  |  |  |  | [9,10,11,12,15,24,27,28,29,30,31] | [15,17] | [15,17] | [9,10,11,12,15] |
| Cell–cell interactions |  |  |  |  | [9,10,11,15] | [15,17] | [15,17] | [9,10,11,15] |
| Disease model recapitulation |  |  |  |  | [9,10,11,12,15,31] | [15,17] | [15,17] | [9,10,11,12,15] |
| Drug discovery |  |  |  |  | [9,10,11,15] | [15,17] | [15,17] | [9,10,11,15] |
| Biosensor integration |  |  |  |  | [9,10,11,15] | [15,17] | [15,17] | [9,10,11,15,24,26] |
| Ethical issues |  |  |  |  | [9,10,11,15,31] | [15,17] | [15,17] | [9,10,11,15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caragnano, G.; Monteduro, A.G.; Rizzato, S.; Giannelli, G.; Maruccio, G. Biological Barrier Models-on-Chips: A Novel Tool for Disease Research and Drug Discovery. Biosensors 2025, 15, 338. https://doi.org/10.3390/bios15060338
Caragnano G, Monteduro AG, Rizzato S, Giannelli G, Maruccio G. Biological Barrier Models-on-Chips: A Novel Tool for Disease Research and Drug Discovery. Biosensors. 2025; 15(6):338. https://doi.org/10.3390/bios15060338
Chicago/Turabian StyleCaragnano, Giusi, Anna Grazia Monteduro, Silvia Rizzato, Gianluigi Giannelli, and Giuseppe Maruccio. 2025. "Biological Barrier Models-on-Chips: A Novel Tool for Disease Research and Drug Discovery" Biosensors 15, no. 6: 338. https://doi.org/10.3390/bios15060338
APA StyleCaragnano, G., Monteduro, A. G., Rizzato, S., Giannelli, G., & Maruccio, G. (2025). Biological Barrier Models-on-Chips: A Novel Tool for Disease Research and Drug Discovery. Biosensors, 15(6), 338. https://doi.org/10.3390/bios15060338









