Silver Nanoparticles for Biosensing and Drug Delivery: A Mechanical Study on DNA Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Silver Nanoparticles
2.2.1. Chemical Reduction Approach
2.2.2. Green Synthesis with Gallic Acid
2.2.3. Seed-Mediated Growth of Triangular Nanoprisms
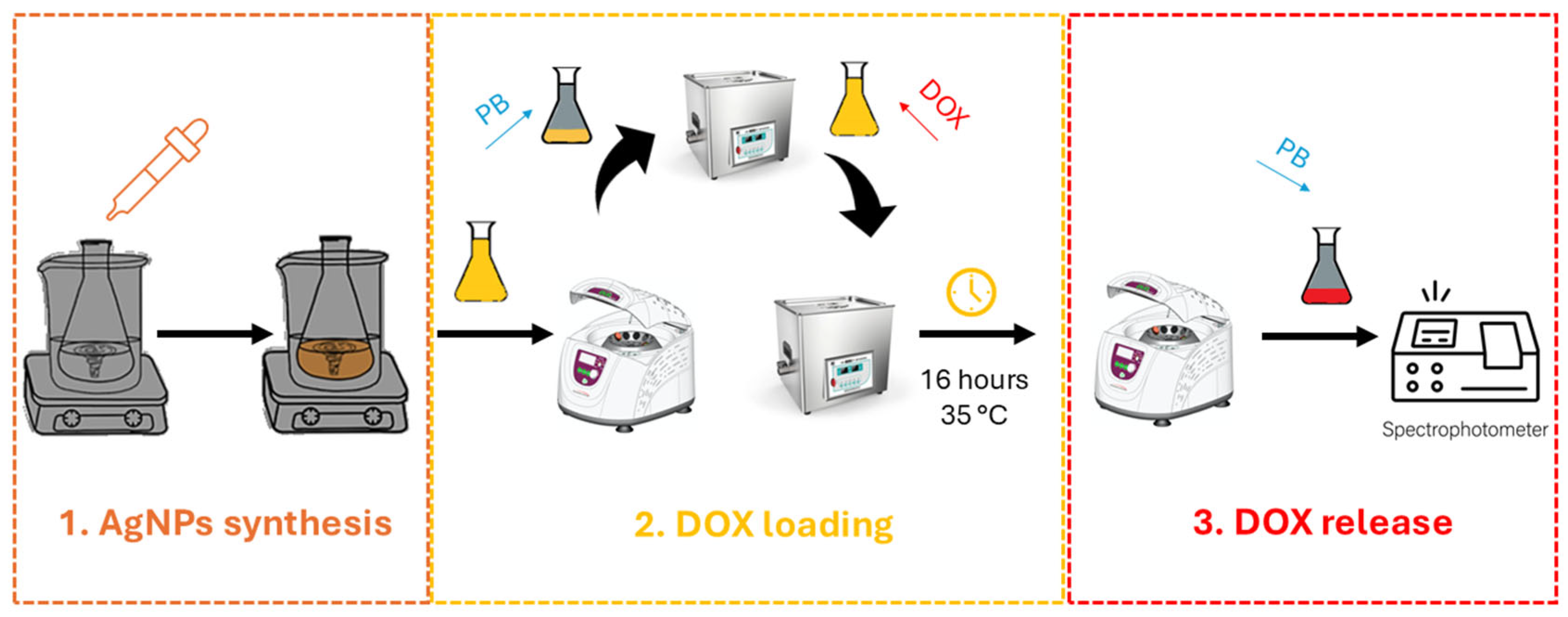
2.3. Functionalization with Doxorubicin (DOX)
2.4. Biosensor Fabrication and Electrochemical Measurements
3. Results
3.1. Characterization of AgNPs
3.1.1. UV-Vis Spectra and Particle Size
3.1.2. Stability and pH Dependence
3.2. Doxorubicin Loading and Release
3.2.1. DOX-Loading Efficiency
3.2.2. pH-Sensitive Release Profiles
3.2.3. ROS-Mediated Cytotoxic Enhancement
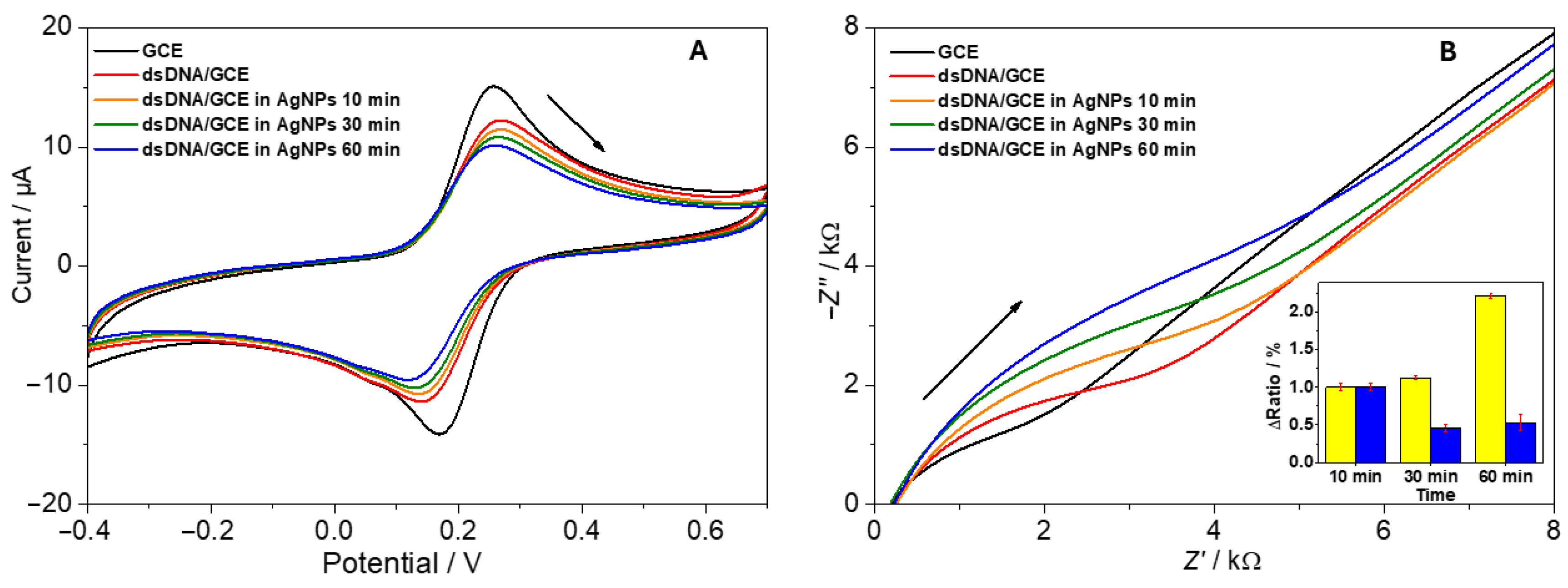
3.3. Biosensor Applications
- -
- Clean GCE: This exhibited well-defined redox peaks with symmetrical anodic and cathodic profiles.
- -
- dsDNA-modified GCE: This showed reduced peak currents due to electrostatic repulsion between the negatively charged phosphate backbone and negatively charged [Fe(CN)6].
- -
- dsDNA + AgNPs (spherical): Additional blocking was observed after extended incubation caused by dsDNA and AgNPs electrostatic interaction; however, some electrons could tunnel through conductive AgNP pathways [33].
- -
- dsDNA + AgNPs (nanoprisms): Notably, triangular nanoprism addition often displayed a slightly faster restoration of redox peak intensities, possibly owing to their larger, anisotropic surfaces that enhance electron bridging [47].
4. Discussion
4.1. Synthesis and Characterization Challenges
4.2. pH-Sensitive Drug Release and ROS Generation
4.3. Environmental and Health Considerations
4.4. Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzoubi, F.Y.; Ahmad, A.A.; Aljarrah, I.A.; Migdadi, A.B.; Al-Bataineh, Q.M. Localize Surface Plasmon Resonance of Silver Nanoparticles Using Mie Theory. J. Mater. Sci. Mater. Electron. 2023, 34, 2128. [Google Scholar] [CrossRef]
- Mahmudin, L.; Wulandani, R.; Riswan, M.; Sari, E.K.; Jayanti, P.D.; Ulum, M.S.; Arifin, M.; Suharyadi, E. Silver Nanoparticles-Based Localized Surface Plasmon Resonance Biosensor for Escherichia Coli Detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 311, 123985. [Google Scholar] [CrossRef] [PubMed]
- Meher, A.; Tandi, A.; Moharana, S.; Chakroborty, S.; Mohapatra, S.S.; Mondal, A.; Dey, S.; Chandra, P. Silver Nanoparticle for Biomedical Applications: A Review. Hybrid Adv. 2024, 6, 100184. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Gomes, H.I.O.; Martins, C.S.M.; Prior, J.A.V. Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells. Nanomaterials 2021, 11, 964. [Google Scholar] [CrossRef]
- Gupta, P.C.; Sharma, N.; Rai, S.; Mishra, P. Use of Smart Silver Nanoparticles in Drug Delivery System. In Metal and Metal-Oxide Based Nanomaterials: Synthesis, Agricultural, Biomedical and Environmental Interventions; Springer: New York, NY, USA, 2024; pp. 213–241. [Google Scholar]
- Park, E.-J.; Bae, E.; Yi, J.; Kim, Y.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C.; Park, K. Repeated-Dose Toxicity and Inflammatory Responses in Mice by Oral Administration of Silver Nanoparticles. Environ. Toxicol. Pharmacol. 2010, 30, 162–168. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green Synthesis of Silver Nanoparticles Using Plant Extracts and Their Antimicrobial Activities: A Review of Recent Literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef] [PubMed]
- Ahani, M.; Khatibzadeh, M. Green Synthesis of Silver Nanoparticles Using Gallic Acid as Reducing and Capping Agent: Effect of PH and Gallic Acid Concentration on Average Particle Size and Stability. Inorg. Nano-Metal. Chem. 2022, 52, 234–240. [Google Scholar] [CrossRef]
- Ahamed, M.; Khan, M.A.M.; Siddiqui, M.K.J.; AlSalhi, M.S.; Alrokayan, S.A. Green Synthesis, Characterization and Evaluation of Biocompatibility of Silver Nanoparticles. Phys. E Low Dimens. Syst. Nanostruct. 2011, 43, 1266–1271. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N. Progressing Nanotechnology to Improve Targeted Cancer Treatment: Overcoming Hurdles in Its Clinical Implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Sidhic, J.; Aswathi, M.K.; Prasad, A.; Tom, A.; Mohan, P.; Sarbadhikary, P.; Narayanankutty, A.; George, S.; Abrahamse, H.; George, B.P. Advancements in Metal and Metal Oxide Nanoparticles for Targeted Cancer Therapy and Imaging: Mechanisms, Applications, and Safety Concerns. J. Drug Deliv. Sci. Technol. 2025, 105, 106622. [Google Scholar] [CrossRef]
- Raja, G.; Jang, Y.-K.; Suh, J.-S.; Kim, H.-S.; Ahn, S.H.; Kim, T.-J. Microcellular Environmental Regulation of Silver Nanoparticles in Cancer Therapy: A Critical Review. Cancers 2020, 12, 664. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; De Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Ghadiri, M.; Vasheghani-Farahani, E.; Atyabi, F.; Kobarfard, F.; Mohamadyar-Toupkanlou, F.; Hosseinkhani, H. Transferrin-Conjugated Magnetic Dextran-Spermine Nanoparticles for Targeted Drug Transport across Blood-Brain Barrier. J. Biomed. Mater. Res. A 2017, 105, 2851–2864. [Google Scholar] [CrossRef] [PubMed]
- Alibolandi, M.; Abnous, K.; Sadeghi, F.; Hosseinkhani, H.; Ramezani, M.; Hadizadeh, F. Folate Receptor-Targeted Multimodal Polymersomes for Delivery of Quantum Dots and Doxorubicin to Breast Adenocarcinoma: In Vitro and In Vivo Evaluation. Int. J. Pharm. 2016, 500, 162–178. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Farokhi, M.; Shokrgozar, M.A.; Atyabi, F.; Hosseinkhani, H. Silk Fibroin Nanoparticle as a Novel Drug Delivery System. J. Control. Release 2015, 206, 161–176. [Google Scholar] [CrossRef]
- Alibolandi, M.; Abnous, K.; Ramezani, M.; Hosseinkhani, H.; Hadizadeh, F. Synthesis of AS1411-Aptamer-Conjugated CdTe Quantum Dots with High Fluorescence Strength for Probe Labeling Tumor Cells. J. Fluoresc. 2014, 24, 1519–1529. [Google Scholar] [CrossRef]
- He, W.; Hosseinkhani, H.; Mohammadinejad, R.; Roveimiab, Z.; Hueng, D.-Y.; Ou, K.-L.; Domb, A.J. Polymeric Nanoparticles for Therapy and Imaging. Polym. Adv. Technol. 2014, 25, 1216–1225. [Google Scholar] [CrossRef]
- Sarabi, R.; Sadeghi, E.; Hosseinkhani, H.; Mahmoudi, M.; Kalantari, M.-D.; Adeli, M. Polyrotaxane Capped Quantum Dots as New Candidates for Cancer Diagnosis and Therapy. J. Nanostruct. Polym. Nanocompos. 2011, 7, 18–31. [Google Scholar]
- Beck, F.; Loessl, M.; Baeumner, A.J. Signaling Strategies of Silver Nanoparticles in Optical and Electrochemical Biosensors: Considering Their Potential for the Point-of-Care. Microchim. Acta 2023, 190, 91. [Google Scholar] [CrossRef] [PubMed]
- Beck, F.; Horn, C.; Baeumner, A.J. Ag Nanoparticles Outperform Au Nanoparticles for the Use as Label in Electrochemical Point-of-Care Sensors. Anal. Bioanal. Chem. 2022, 414, 475–483. [Google Scholar] [CrossRef]
- Huynh, G.T.; Kesarwani, V.; Walker, J.A.; Frith, J.E.; Meagher, L.; Corrie, S.R. Nanomaterials for Reactive Oxygen Species Detection and Monitoring in Biological Environments. Front. Chem. 2021, 9, 728717. [Google Scholar] [CrossRef]
- Chen, L.-C.; Wang, E.; Tai, C.-S.; Chiu, Y.-C.; Li, C.-W.; Lin, Y.-R.; Lee, T.-H.; Huang, C.-W.; Chen, J.-C.; Chen, W.L. Improving the Reproducibility, Accuracy, and Stability of an Electrochemical Biosensor Platform for Point-of-Care Use. Biosens. Bioelectron. 2020, 155, 112111. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Ishaq, I.; Saleem, M.H.; Ali, B.; Muhammad Ali, S.; Iqbal, J. Electrochemical Biosensing in Oncology: A Review Advancements and Prospects for Cancer Diagnosis. Cancer Biol. Ther. 2025, 26, 2475581. [Google Scholar] [CrossRef]
- Zhao, S.; Zang, G.; Zhang, Y.; Liu, H.; Wang, N.; Cai, S.; Durkan, C.; Xie, G.; Wang, G. Recent Advances of Electrochemical Sensors for Detecting and Monitoring ROS/RNS. Biosens. Bioelectron. 2021, 179, 113052. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.T.T.; Dang, V.Q.; Bach, T.N.; Khuyen, B.X.; Ta, H.K.T.; Ju, H.; Phan, B.T.; Tran, N.H.T. Functionalized Silver Nanoparticles for SERS Amplification with Enhanced Reproducibility and for Ultrasensitive Optical Fiber Sensing in Environmental and Biochemical Assays. RSC Adv. 2022, 12, 31352–31362. [Google Scholar] [CrossRef]
- Rabbi, M.B.U.; Haque, S.; Bedoura, S. Advancements in Synthesis, Immobilization, Characterization, and Multifaceted Applications of Silver Nanoparticles: A Comprehensive Review. Heliyon 2024, 10, e40931. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef] [PubMed]
- Nemčeková, K.; Svitková, V.; Sochr, J.; Gemeiner, P.; Labuda, J. Gallic Acid-Coated Silver Nanoparticles as Perspective Drug Nanocarriers: Bioanalytical Study. Anal. Bioanal. Chem. 2022, 414, 5493–5505. [Google Scholar] [CrossRef] [PubMed]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and Study of Silver Nanoparticles. J. Chem. Educ. 2007, 84, 322. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Nino-Martinez, N.; Martinez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and Antibacterial Activity of Silver Nanoparticles with Different Sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Hirun, N.; Dokmaisrijan, S.; Tantishaiyakul, V. Experimental FTIR and Theoretical Studies of Gallic Acid–Acetonitrile Clusters. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 86, 93–100. [Google Scholar] [CrossRef]
- Métraux, G.S.; Mirkin, C.A. Rapid Thermal Synthesis of Silver Nanoprisms with Chemically Tailorable Thickness. Adv. Mater. 2005, 17, 412–415. [Google Scholar] [CrossRef]
- Imantay, A.; Mashurov, N.; Zhaisanbayeva, B.A.; Mun, E.A. Doxorubicin-Conjugated Nanoparticles for Potential Use as Drug Delivery Systems. Nanomaterials 2025, 15, 133. [Google Scholar] [CrossRef]
- Millour, M.; Gagne, J.-P.; Doiron, K.; Lemarchand, K.; Pelletier, É. Silver Nanoparticles Aggregative Behavior at Low Concentrations in Aqueous Solutions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125191. [Google Scholar] [CrossRef]
- Alarcon, R.; Walter, M.; Paez, M.; Azócar, M.I. Ostwald Ripening and Antibacterial Activity of Silver Nanoparticles Capped by Anti-Inflammatory Ligands. Nanomaterials 2023, 13, 428. [Google Scholar] [CrossRef]
- Fernando, I.; Zhou, Y. Impact of PH on the Stability, Dissolution and Aggregation Kinetics of Silver Nanoparticles. Chemosphere 2019, 216, 297–305. [Google Scholar] [CrossRef]
- Maleki, R.; Afrouzi, H.H.; Hosseini, M.; Toghraie, D.; Piranfar, A.; Rostami, S. PH-Sensitive Loading/Releasing of Doxorubicin Using Single-Walled Carbon Nanotube and Multi-Walled Carbon Nanotube: A Molecular Dynamics Study. Comput. Methods Programs Biomed. 2020, 186, 105210. [Google Scholar] [CrossRef] [PubMed]
- Le Thi, P.; Nguyen, D.T.; Huu, T.A.N.; Tran, Q.-H.; Truong, M.-D.; Hang, N.T.; Tran, N.Q.; Park, K.D. Hyaluronic Acid-Coated Silver Nanoparticles Releasing Doxorubicin for Combinatorial Antitumor Therapy. J. Ind. Eng. Chem. 2025, 142, 431–440. [Google Scholar] [CrossRef]
- Czupiel, P.; Delplace, V.; Shoichet, M. Nanoparticle Delivery of a PH-Sensitive Prodrug of Doxorubicin and a Mitochondrial Targeting VES-H8R8 Synergistically Kill Multi-Drug Resistant Breast Cancer Cells. Sci. Rep. 2020, 10, 8726. [Google Scholar] [CrossRef]
- Fallah, S.; Yusefi-Tanha, E.; Peralta-Videa, J.R. Interaction of Nanoparticles and Reactive Oxygen Species and Their Impact on Macromolecules and Plant Production. Plant Nano Biol. 2024, 10, 100105. [Google Scholar] [CrossRef]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver Nanoparticles: Electron Transfer, Reactive Oxygen Species, Oxidative Stress, Beneficial and Toxicological Effects. Mini Review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef]
- Gao, X.; Dong, S.; Fu, L.; Zhang, B.; Hsu, H.-Y.; Zou, G. Use of Triangular Silver Nanoplates as Low Potential Redox Mediators for Electrochemical Sensing. Anal. Chem. 2021, 93, 3295–3300. [Google Scholar] [CrossRef] [PubMed]
- Serec, K.; Šegedin, N.; Krajačić, M.; Dolanski Babić, S. Conformational Transitions of Double-Stranded DNA in Thin Films. Appl. Sci. 2021, 11, 2360. [Google Scholar] [CrossRef]
- Rodriguez Barroso, L.G.; Lanzagorta Garcia, E.; Mojicevic, M.; Huerta, M.; Pogue, R.; Devine, D.M.; Brennan-Fournet, M. Triangular Silver Nanoparticles Synthesis: Investigating Potential Application in Materials and Biosensing. Appl. Sci. 2023, 13, 8100. [Google Scholar] [CrossRef]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J. Drug-Induced Oxidative Stress in Cancer Treatments: Angel or Devil? Redox Biol. 2023, 63, 102754. [Google Scholar] [CrossRef]
- Nguyen, M.P.; Pham, D.P.; Kim, D. Oxidative Stress-Induced Silver Nano-Carriers for Chemotherapy. Pharmaceuticals 2022, 15, 1449. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Liu, X.; Xu, C.; You, Q.; Zhang, Y.; Zhu, L. Environmental Behavior of Silver Nanomaterials in Aquatic Environments: An Updated Review. Sci. Total Environ. 2024, 907, 167861. [Google Scholar] [CrossRef]
- Temizel-Sekeryan, S.; Hicks, A.L. Global Environmental Impacts of Silver Nanoparticle Production Methods Supported by Life Cycle Assessment. Resour. Conserv. Recycl. 2020, 156, 104676. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Demin, V.A.; Gmoshinsky, I.V.; Demin, V.F.; Anciferova, A.A.; Buzulukov, Y.P.; Khotimchenko, S.A.; Tutelyan, V.A. Modeling Interorgan Distribution and Bioaccumulation of Engineered Nanoparticles (Using the Example of Silver Nanoparticles). Nanotechnol. Russ. 2015, 10, 288–296. [Google Scholar] [CrossRef]
- Kogikoski, S.J.; Gonçalves Pontes, R.; Paschoalino, W.J.; Kubota, L.T. Opportunities for DNA Origami in Electrochemical Sensing Applications. ACS Electrochem. 2025, 1, 263–279. [Google Scholar] [CrossRef]
- Postigo, A.; Marcuello, C.; Verstraeten, W.; Sarasa, S.; Walther, T.; Lostao, A.; Göpfrich, K.; del Barrio, J.; Hernández-Ainsa, S. Folding and Functionalizing DNA Origami: A Versatile Approach Using a Reactive Polyamine. J. Am. Chem. Soc. 2025, 147, 3919–3924. [Google Scholar] [CrossRef]



| Synthesis Method | Shape | Size Range nm | SPR Peak nm |
|---|---|---|---|
| Chemical reduction | Spherical | 10–20 | 420 |
| Green synthesis | Spherical | 15 | 410 |
| Seed-mediated growth | Triangular prisms | 50–100 | 750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemčeková, K.; Dudoňová, P.; Holka, T.; Balážová, S.; Hornychová, M.; Szebellaiová, V.; Naumowicz, M.; Gemeiner, P.; Mackuľak, T.; Gál, M.; et al. Silver Nanoparticles for Biosensing and Drug Delivery: A Mechanical Study on DNA Interaction. Biosensors 2025, 15, 331. https://doi.org/10.3390/bios15050331
Nemčeková K, Dudoňová P, Holka T, Balážová S, Hornychová M, Szebellaiová V, Naumowicz M, Gemeiner P, Mackuľak T, Gál M, et al. Silver Nanoparticles for Biosensing and Drug Delivery: A Mechanical Study on DNA Interaction. Biosensors. 2025; 15(5):331. https://doi.org/10.3390/bios15050331
Chicago/Turabian StyleNemčeková, Katarína, Patrícia Dudoňová, Tomáš Holka, Sabína Balážová, Michaela Hornychová, Viktória Szebellaiová, Monika Naumowicz, Pavol Gemeiner, Tomáš Mackuľak, Miroslav Gál, and et al. 2025. "Silver Nanoparticles for Biosensing and Drug Delivery: A Mechanical Study on DNA Interaction" Biosensors 15, no. 5: 331. https://doi.org/10.3390/bios15050331
APA StyleNemčeková, K., Dudoňová, P., Holka, T., Balážová, S., Hornychová, M., Szebellaiová, V., Naumowicz, M., Gemeiner, P., Mackuľak, T., Gál, M., & Svitková, V. (2025). Silver Nanoparticles for Biosensing and Drug Delivery: A Mechanical Study on DNA Interaction. Biosensors, 15(5), 331. https://doi.org/10.3390/bios15050331