Ultrasensitive and Real-Time Detection of Kanamycin Residues in Milk Using an Aptasensor Based on Microfluidic Capacitive Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Treatment
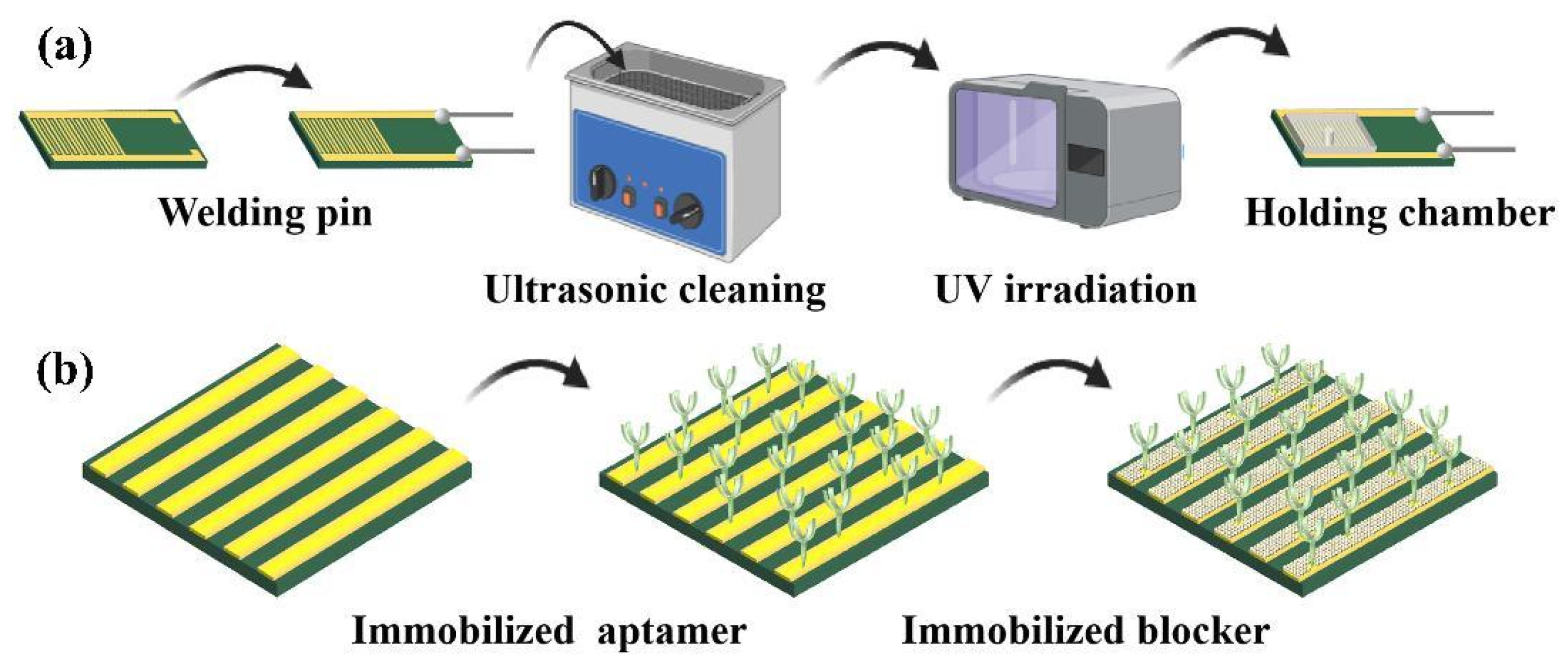
2.3. Aptasensor Preparation
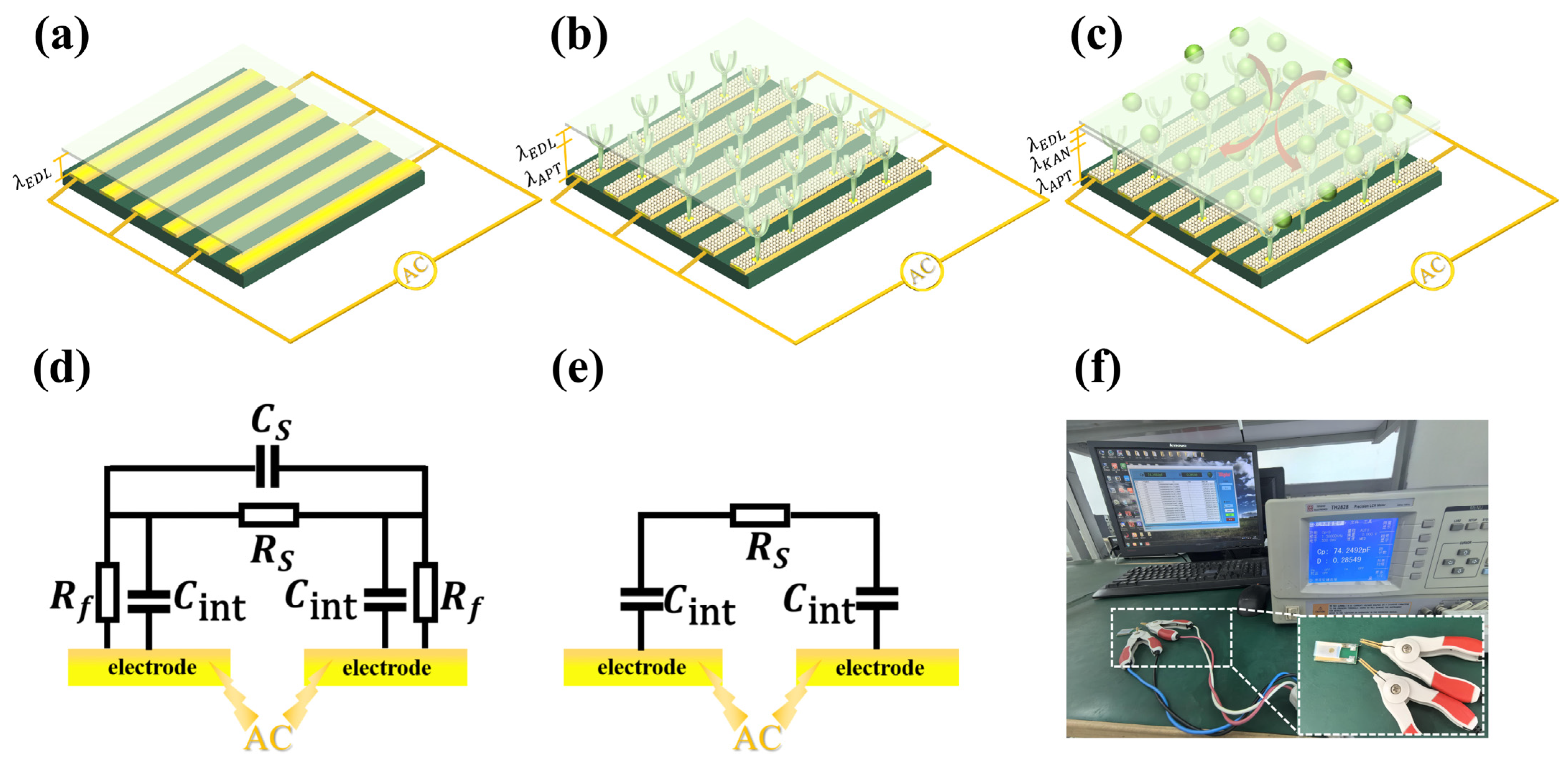
2.4. Enrichment Mechanism by ACET Effect
2.5. Interfacial Capacitive Sensing
3. Results and Discussion
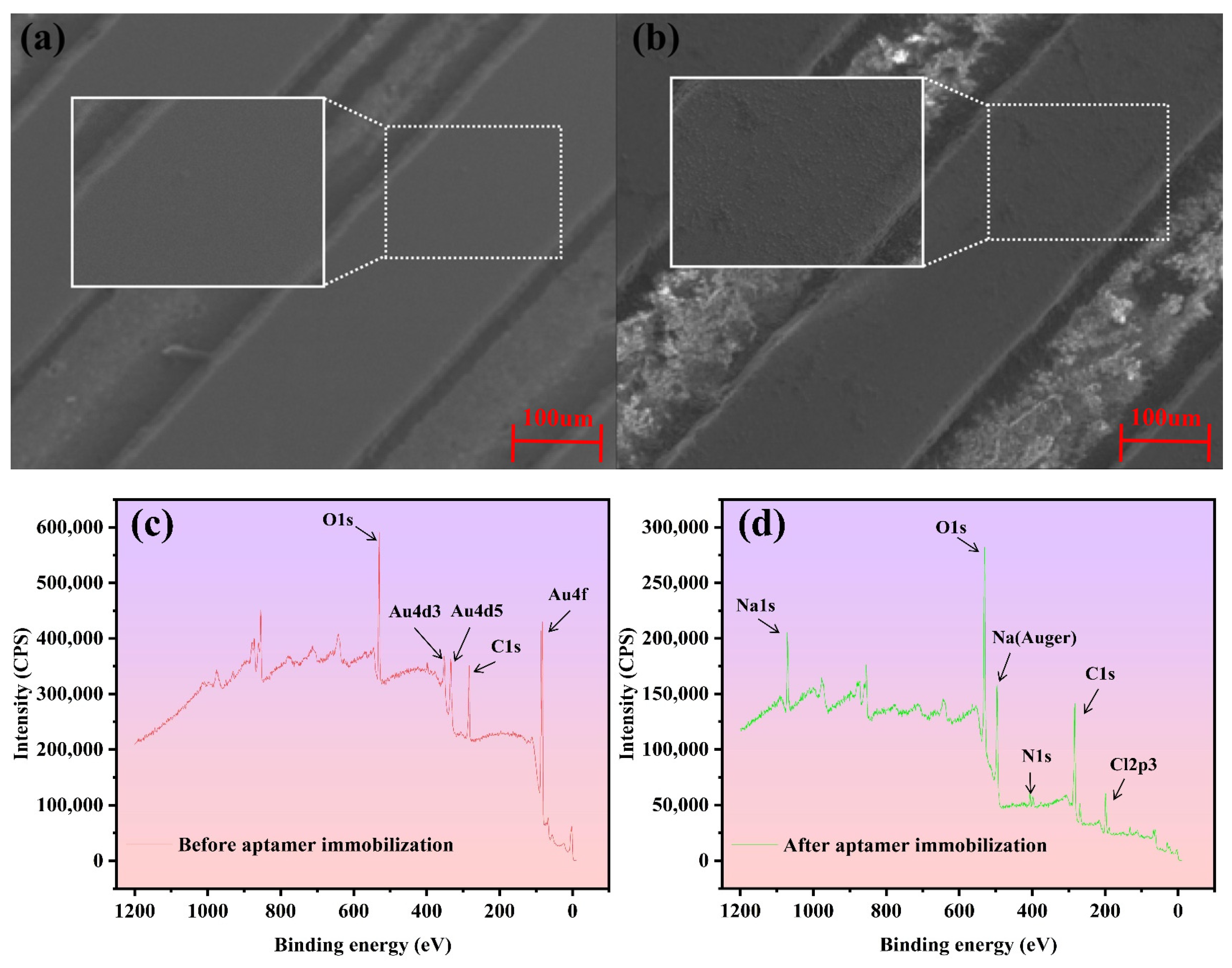
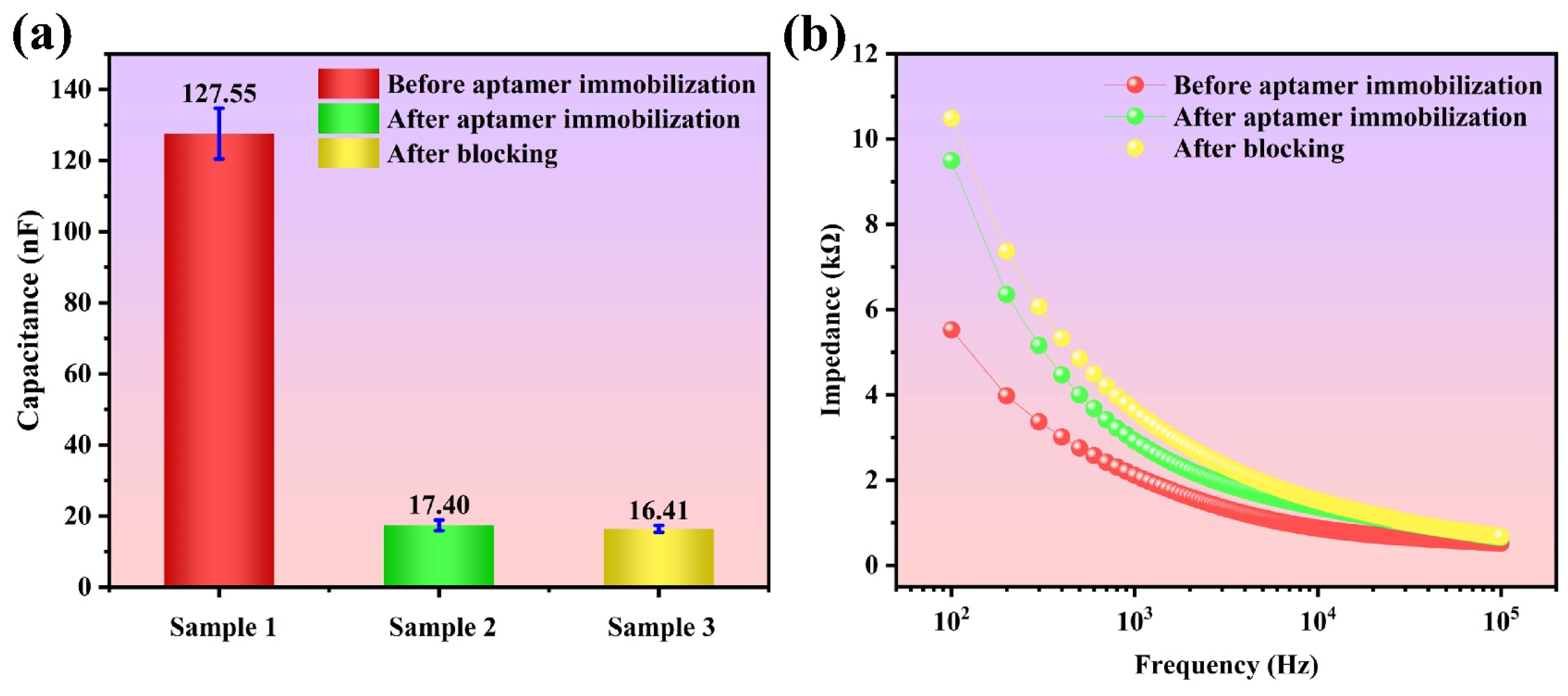
3.1. Sensor Characterization
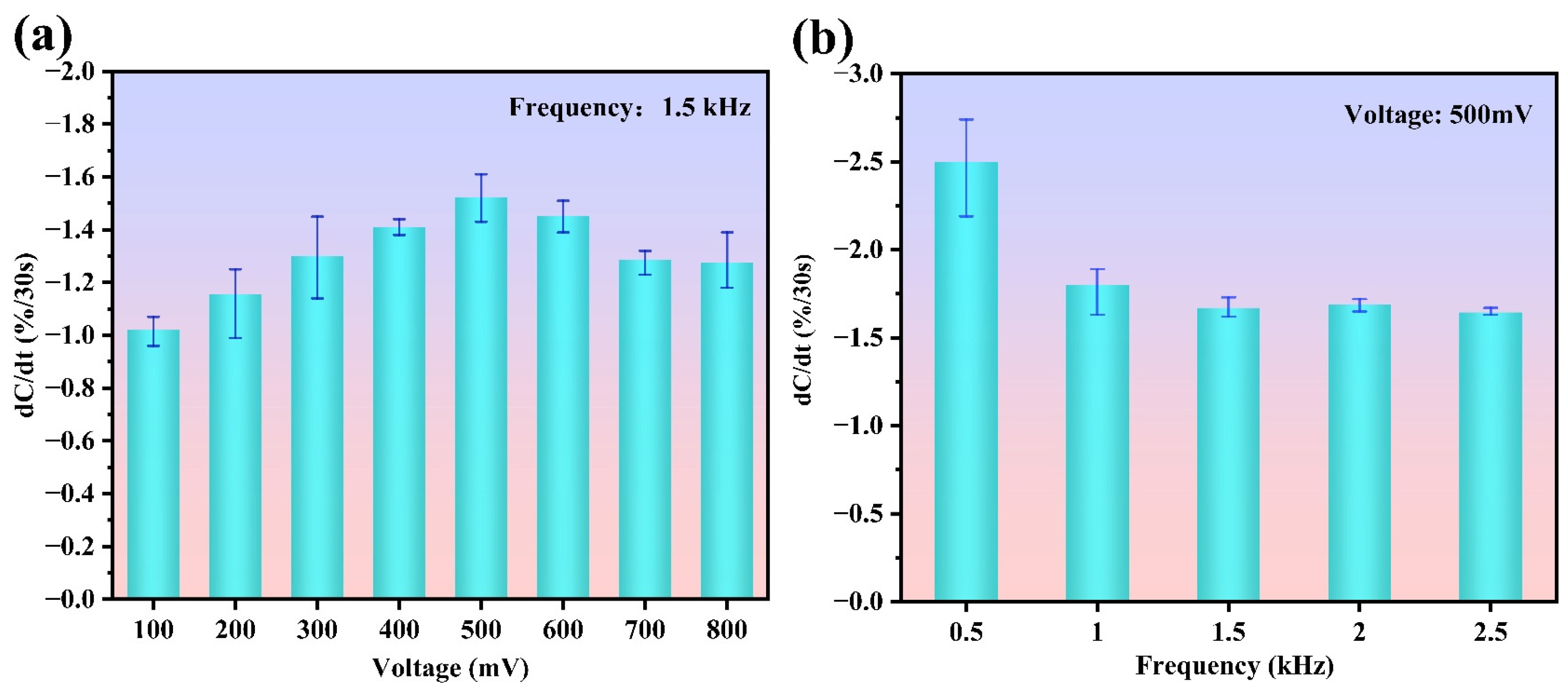
3.2. Optimization of Excitation Signal
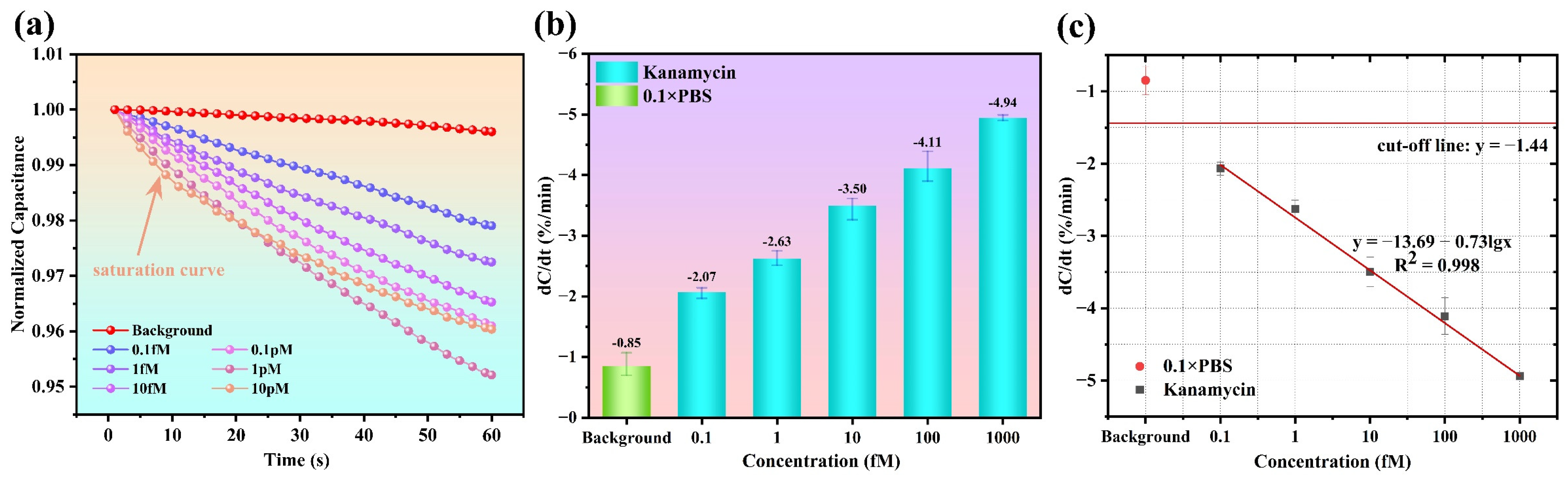
3.3. Dose Response and Sensor Calibration
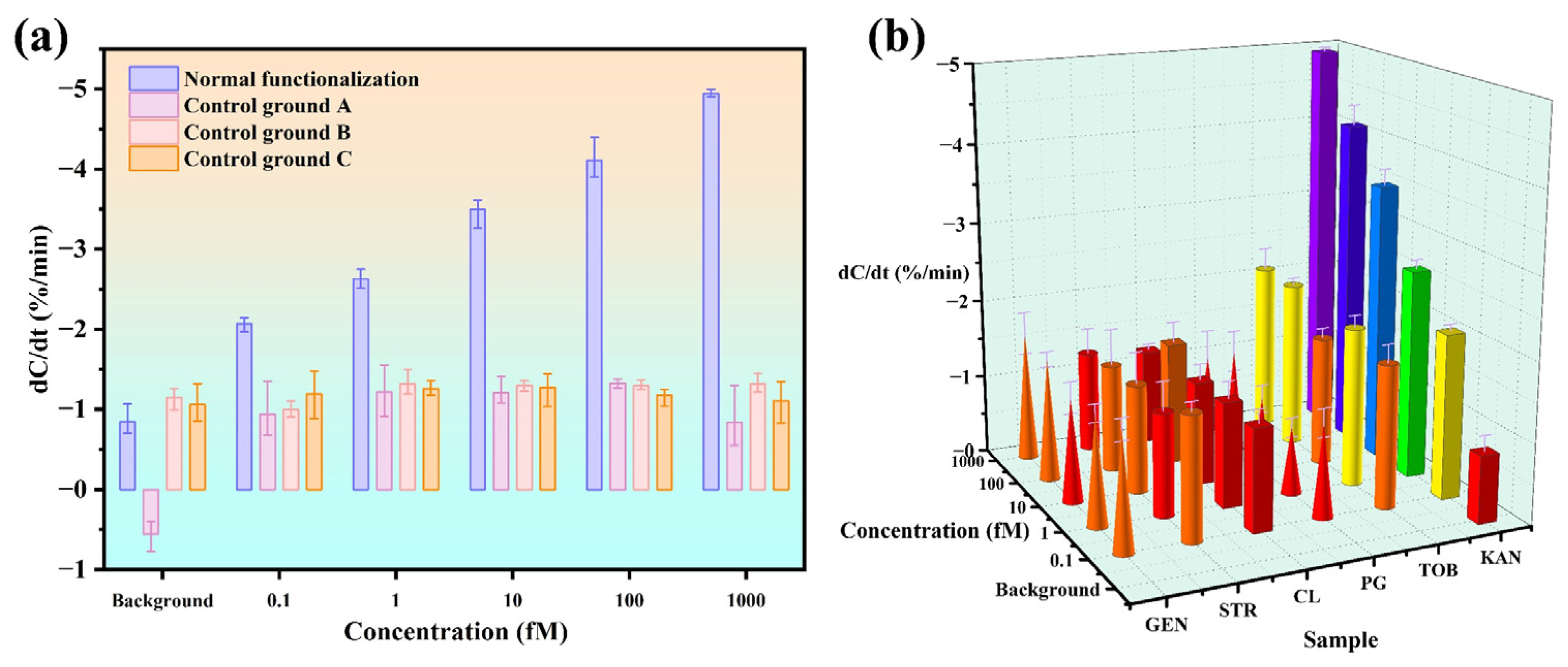
3.4. Sensor Specificity
3.5. Repeatability and Long-Term Stability of the Sensor
3.6. Application with Milk Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, C.; Ying, Y.; Ping, J. Colorimetric aggregation assay for kanamycin using gold nanoparticles modified with hairpin DNA probes and hybridization chain reaction-assisted amplification. Microchim. Acta 2019, 186, 448. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, Z.; Chen, Y.; Xu, L.; Xie, Q.; Deng, H.; Chen, W.; Peng, H. Regulating Valence States of Gold Nanocluster as a New Strategy for the Ultrasensitive Electrochemiluminescence Detection of Kanamycin. Anal. Chem. 2021, 93, 4635–4640. [Google Scholar] [CrossRef]
- Robati, R.Y.; Arab, A.; Ramezani, M.; Langroodi, F.A.; Abnous, K.; Taghdisi, S.M. Aptasensors for quantitative detection of kanamycin. Biosens. Bioelectron. 2016, 82, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; Bonomo, R.A. Overview: Global and Local Impact of Antibiotic Resistance. Infect. Dis. Clin. N. Am. 2016, 30, 313–322. [Google Scholar] [CrossRef]
- Redwan Haque, A.; Sarker, M.; Das, R.; Azad, M.A.K.; Hasan, M.M. A review on antibiotic residue in foodstuffs from animal source: Global health risk and alternatives. Int. J. Environ. Anal. Chem. 2021, 103, 3704–3721. [Google Scholar] [CrossRef]
- Cui, W.; Liu, J.; Zhao, W.; Zhang, J.; Wang, Y.; Li, Q.; Wang, R.; Qiao, M.; Xu, S. An enzyme-free and label-free fluorescent aptasensor for sensitive detection of kanamycin in milk samples based on hybridization chain reaction. J. Food Compos. Anal. 2023, 120, 105300. [Google Scholar] [CrossRef]
- Hui, X.; Wang, Y.; Zhang, H.; Fang, W.; Liu, H.; Dai, X. Overlooked abiotic humification for kanamycin removal in kanamycin fermentation waste by tea polyphenols: New, environmentally friendly and specific. Chem. Eng. J. 2023, 478, 147377. [Google Scholar] [CrossRef]
- Algethami, F.K.; Rabti, A.; Mastouri, M.; Ben Aoun, S.; Alqarni, L.S.; Elamin, M.R.; Raouafi, N. Sub-femtomolar capacitance-based biosensing of kanamycin using screen-printed electrodes coated with redox-active polymeric films. Microchim. Acta 2023, 190, 434. [Google Scholar] [CrossRef]
- McGlinchey, T.A.; Rafter, P.A.; Regan, F.; McMahon, G.P. A review of analytical methods for the determination of aminoglycoside and macrolide residues in food matrices. Anal. Chim. Acta 2008, 624, 1–15. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, D.-W.; Pu, H.; Wei, Q. Ultrasensitive analysis of kanamycin residue in milk by SERS-based aptasensor. Talanta 2019, 197, 151–158. [Google Scholar] [CrossRef]
- Pietschmann, J.; Dittmann, D.; Spiegel, H.; Krause, H.-J.; Schröper, F. A Novel Method for Antibiotic Detection in Milk Based on Competitive Magnetic Immunodetection. Foods 2020, 9, 1773. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Chen, X.; He, Z.; Yang, Y. Preparation of polyclonal antibody and development of a biotin-streptavidin-based ELISA method for detecting kanamycin in milk and honey. Chem. Res. Chin. Univ. 2017, 33, 876–881. [Google Scholar] [CrossRef]
- Galvidis, I.A.; Burkin, M.A. Monoclonal antibody-based enzyme-linked immunosorbent assay for the aminoglycoside antibiotic kanamycin in foodstuffs. Russ. J. Bioorganic Chem. 2010, 36, 722–729. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Wu, Q.; Li, L.; Wang, Y.; Yang, H. Determination of Kanamycin by High Performance Liquid Chromatography. Molecules 2019, 24, 1902. [Google Scholar] [CrossRef] [PubMed]
- Guironnet, A.; Sanchez-Cid, C.; Vogel, T.M.; Wiest, L.; Vulliet, E. Aminoglycosides analysis optimization using ion pairing liquid chromatography coupled to tandem mass spectrometry and application on wastewater samples. J. Chromatogr. A 2021, 1651, 462133. [Google Scholar] [CrossRef]
- El-Attug, M.N.; Adams, E.; Hoogmartens, J.; Van Schepdael, A. Capacitively coupled contactless conductivity detection as an alternative detection mode in CE for the analysis of kanamycin sulphate and its related substances. J. Sep. Sci. 2011, 34, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zou, M.; Xu, X.; Lei, R.; Li, K.; Li, N. Determination of human urinary kanamycin in one step using urea-enhanced surface plasmon resonance light-scattering of gold nanoparticles. Anal. Bioanal. Chem. 2009, 395, 2397–2403. [Google Scholar] [CrossRef]
- Sýkorová Goffová, Z.; Kožárová, I.; Máté, D.; Marcinčák, S.; Gondová, Z.; Sopková, D. Comparison of detection sensitivity of five microbial inhibition tests for the screening of aminoglycoside residues in fortified milk. Czech J. Food Sci. 2012, 30, 314–320. [Google Scholar] [CrossRef]
- Michelini, E.; Roda, A. Staying alive: New perspectives on cell immobilization for biosensing purposes. Anal. Bioanal. Chem. 2011, 402, 1785–1797. [Google Scholar] [CrossRef]
- Chen, T.; Cheng, G.; Ahmed, S.; Wang, Y.; Wang, X.; Hao, H.; Yuan, Z. New methodologies in screening of antibiotic residues in animal-derived foods: Biosensors. Talanta 2017, 175, 435–442. [Google Scholar] [CrossRef]
- Majdinasab, M.; Yaqub, M.; Rahim, A.; Catanante, G.; Hayat, A.; Marty, J. An Overview on Recent Progress in Electrochemical Biosensors for Antimicrobial Drug Residues in Animal-Derived Food. Sensors 2017, 17, 1947. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Kim, G.; Park, S.; Lim, J.; Mo, C. Comparison of Whole-Cell SELEX Methods for the Identification of Staphylococcus Aureus-Specific DNA Aptamers. Sensors 2015, 15, 8884–8897. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, X.; Li, W.; Liu, Z. Glycan-Imprinted Magnetic Nanoparticle-Based SELEX for Efficient Screening of Glycoprotein-Binding Aptamers. ACS Appl. Mater. Interfaces 2018, 10, 40918–40926. [Google Scholar] [CrossRef]
- Choi, J.-W.; Seo, M.; Kim, K.; Kim, A.R.; Lee, H.; Kim, H.-S.; Park, C.G.; Cho, S.W.; Kang, J.H.; Joo, J.; et al. Aptamer Nanoconstructs Crossing Human Blood–Brain Barrier Discovered via Microphysiological System-Based SELEX Technology. ACS Nano 2023, 17, 8153–8166. [Google Scholar] [CrossRef]
- Yazdian-Robati, R.; Bayat, P.; Oroojalian, F.; Zargari, M.; Ramezani, M.; Taghdisi, S.M.; Abnous, K. Therapeutic applications of AS1411 aptamer, an update review. Int. J. Biol. Macromol. 2020, 155, 1420–1431. [Google Scholar] [CrossRef]
- Dehghani, S.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Lavaee, P.; Nejabat, M.; Abnous, K.; Taghdisi, S.M. A label-free fluorescent aptasensor for detection of kanamycin based on dsDNA-capped mesoporous silica nanoparticles and Rhodamine B. Anal. Chim. Acta 2018, 1030, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Jesuraj, R.; Amalraj, A.; Vaidyanathan, V.K.; Perumal, P. Exceptional peroxidase-like activity of an iron and copper based organic framework nanosheet for consecutive colorimetric biosensing of glucose and kanamycin in real food samples. Analyst 2023, 148, 5157–5171. [Google Scholar] [CrossRef]
- Qi, X.; Zhao, Y.; Su, H.; Wang, L.; Li, L.; Ma, R.; Yan, X.; Sun, J.; Wang, S.; Mao, X. A label-free colorimetric aptasensor based on split aptamers-chitosan oligosaccharide-AuNPs nanocomposites for sensitive and selective detection of kanamycin. Talanta 2022, 238, 123032. [Google Scholar] [CrossRef]
- Cui, W.; Hu, C.; Zhu, R.; Qiu, D.; Gong, R.; Wang, R.; Li, Q.; Yan, T.; Li, C.; Qiao, M.; et al. Low-background fluorescent biosensor based on graphene oxide and aptamer biorecognition for sensitive detection of kanamycin. J. Food Compos. Anal. 2024, 131, 106261. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Gao, X.; Sun, Z.; Sun, X.; Guo, Y.; Li, F.; Boboriko, N.E. Fluorescent aptasensor based on DNA-AgNCs emitting in the visible red wavelength range for detection of kanamycin in milk. Sens. Actuators B Chem. 2022, 360, 131665. [Google Scholar] [CrossRef]
- Gao, X.; Sun, Z.; Wang, X.; Zhang, W.; Wang, Y.; Han, J.; Sun, X.; Guo, Y.; Li, F.; Xu, S. Construction of a ratiometric electrochemical aptasensor based on graphdiyne-methylene blue and Fc-labeled hairpin for cyclic signal amplification detection of kanamycin. Sens. Actuators B Chem. 2022, 373, 132706. [Google Scholar] [CrossRef]
- Li, X.; Jia, M.; Yu, L.; Li, Y.; He, X.; Chen, L.; Zhang, Y. An ultrasensitive label-free biosensor based on aptamer functionalized two-dimensional photonic crystal for kanamycin detection in milk. Food Chem. 2023, 402, 134239. [Google Scholar] [CrossRef] [PubMed]
- Song, K.-M.; Cho, M.; Jo, H.; Min, K.; Jeon, S.H.; Kim, T.; Han, M.S.; Ku, J.K.; Ban, C. Gold nanoparticle-based colorimetric detection of kanamycin using a DNA aptamer. Anal. Biochem. 2011, 415, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Islam, N.; Wu, J. AC electrokinetics based capture of yeast cells from ultra-fast through-flow for sensitive detection. Micro Nano Lett. 2017, 12, 901–906. [Google Scholar] [CrossRef]
- Wu, J. Interactions of electrical fields with fluids:laboratory-on-a-chip applications. IET Nanobiotechnol. 2008, 2, 14–27. [Google Scholar] [CrossRef]
- Qi, H.; Huang, X.; Wu, J.; Zhang, J.; Wang, F.; Qu, H.; Zheng, L. A disposable aptasensor based on a gold-plated coplanar electrode array for on-site and real-time determination of Cu2+. Anal. Chim. Acta 2021, 1183, 338991. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, S.; Wu, J.; Yu, Y.; Li, R.; Eda, S.; Chen, J.; Feng, G.; Lawrie, B.; Hu, A. Bisphenol A Sensors on Polyimide Fabricated by Laser Direct Writing for Onsite River Water Monitoring at Attomolar Concentration. ACS Appl. Mater. Interfaces 2016, 8, 17784–17792. [Google Scholar] [CrossRef]

| Methods | Turnaround Time | Linear Range | LOD | Sample | Reference |
|---|---|---|---|---|---|
| Colorimetry | – | 0.02–0.1 μM | 8 nM | Milk | [27] |
| Colorimetry | 55 min | 25–800 nM | 20.58 nM | Tap water, Milk | [28] |
| Fluorescence | – | 1–400 nM | 0.60 nM | Milk | [29] |
| Fluorescence | – | 100–1100 nM | 22.6 nM | Milk | [30] |
| Electrochemistry | 120 min | 50 nM–1 μM | 6.044 nM | Milk | [31] |
| Photonic crystal | – | 10.3 pM–10.3 μM | 2.3 pM | Milk | [32] |
| Capacitance | 1 min | 0.1 fM–1 pM | 16.56 aM | Milk | This work |
| Sample | Spiked Concentration (fM) | Theoretical Concentration (fM) | Detected Concentration (fM) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|---|
| Skimmed milk | 1.53 | 4.63 | 4.58 | 94.77 | 2.88 |
| 3.03 | 6.13 | 6.65 | 116.17 | 3.60 | |
| 6.03 | 9.13 | 8.37 | 86.90 | 2.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Chai, J.; Wu, J.; Zhang, J.; Qi, H. Ultrasensitive and Real-Time Detection of Kanamycin Residues in Milk Using an Aptasensor Based on Microfluidic Capacitive Strategy. Biosensors 2025, 15, 322. https://doi.org/10.3390/bios15050322
Zheng W, Chai J, Wu J, Zhang J, Qi H. Ultrasensitive and Real-Time Detection of Kanamycin Residues in Milk Using an Aptasensor Based on Microfluidic Capacitive Strategy. Biosensors. 2025; 15(5):322. https://doi.org/10.3390/bios15050322
Chicago/Turabian StyleZheng, Weidong, Jun Chai, Jayne Wu, Jian Zhang, and Haochen Qi. 2025. "Ultrasensitive and Real-Time Detection of Kanamycin Residues in Milk Using an Aptasensor Based on Microfluidic Capacitive Strategy" Biosensors 15, no. 5: 322. https://doi.org/10.3390/bios15050322
APA StyleZheng, W., Chai, J., Wu, J., Zhang, J., & Qi, H. (2025). Ultrasensitive and Real-Time Detection of Kanamycin Residues in Milk Using an Aptasensor Based on Microfluidic Capacitive Strategy. Biosensors, 15(5), 322. https://doi.org/10.3390/bios15050322






