Centrifugation-Induced Stable Colloidal Silver Nanoparticle Aggregates for Reproducible Surface-Enhanced Raman Scattering Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instruments
2.3. Synthesis of β-CD@AgNPs
2.4. Preparation of Centrifuge-Induced Aggregates
2.5. SERS Measurements
2.6. FDTD Simulation
3. Results
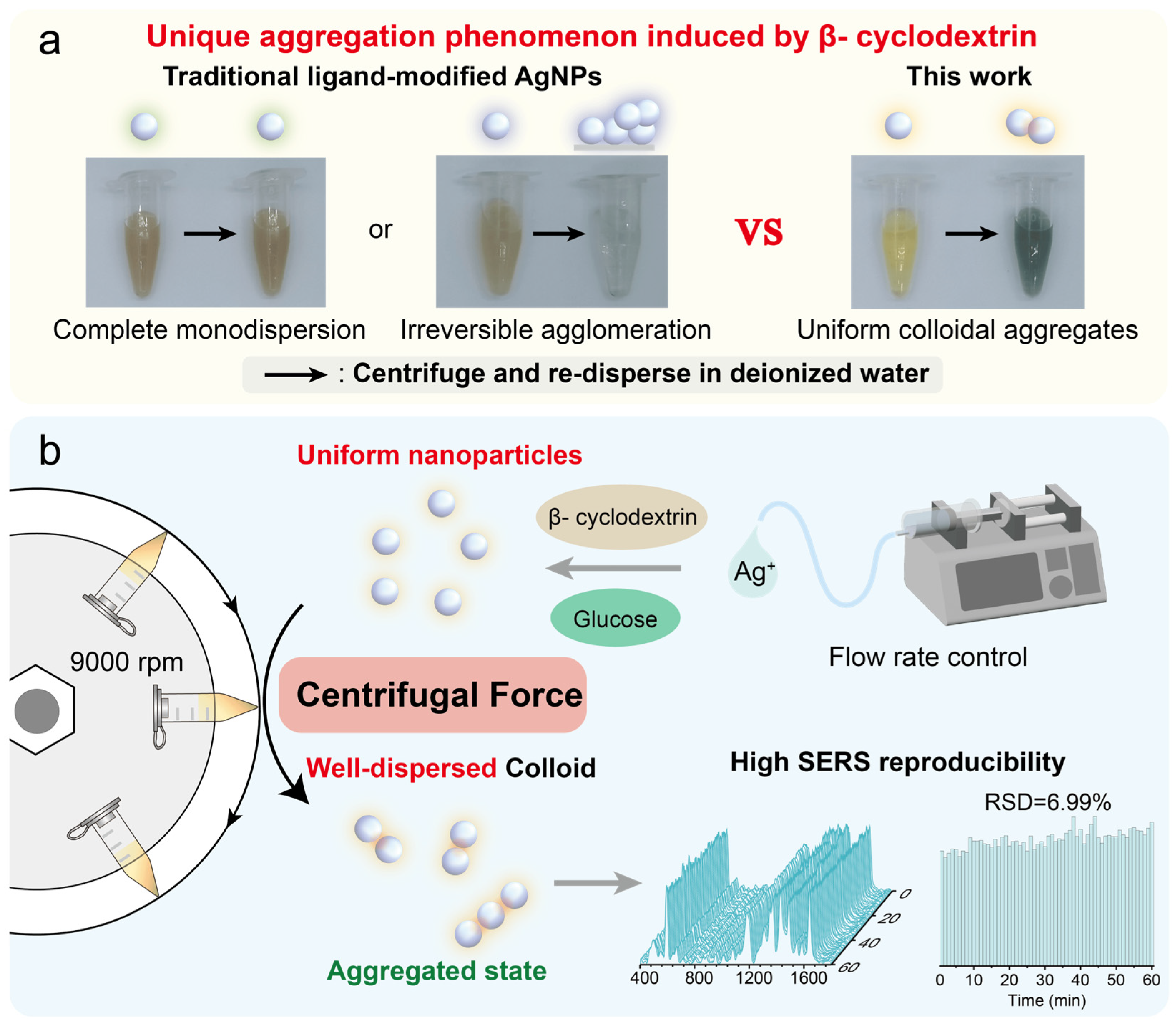
3.1. Synthesis of Uniform β-CD Stabilized AgNPs
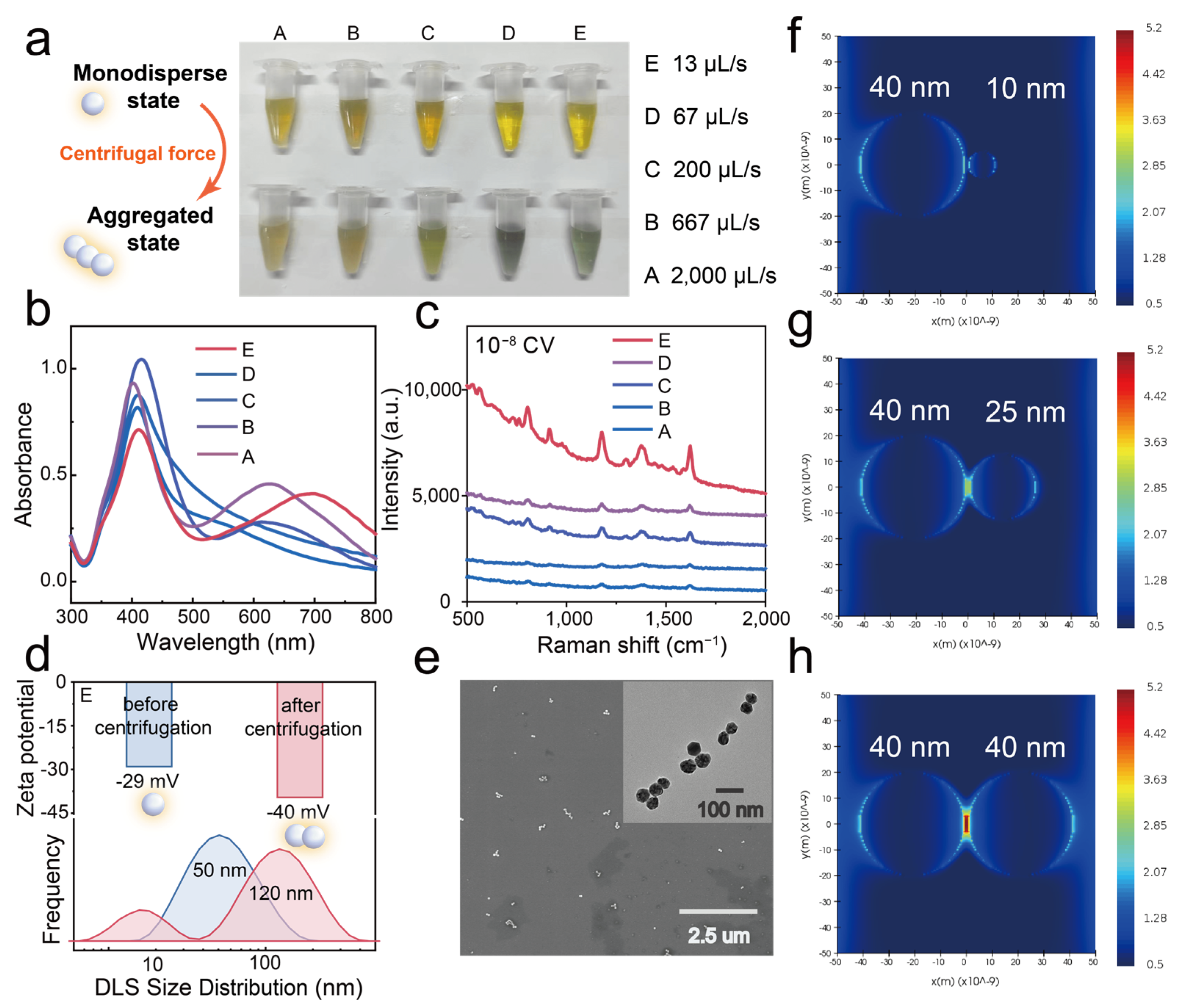
3.2. Centrifugation-Induced Aggregates of β-CD Stabilized AgNPs
3.3. High SERS Enhancement of Centrifugation-Induced Aggregates
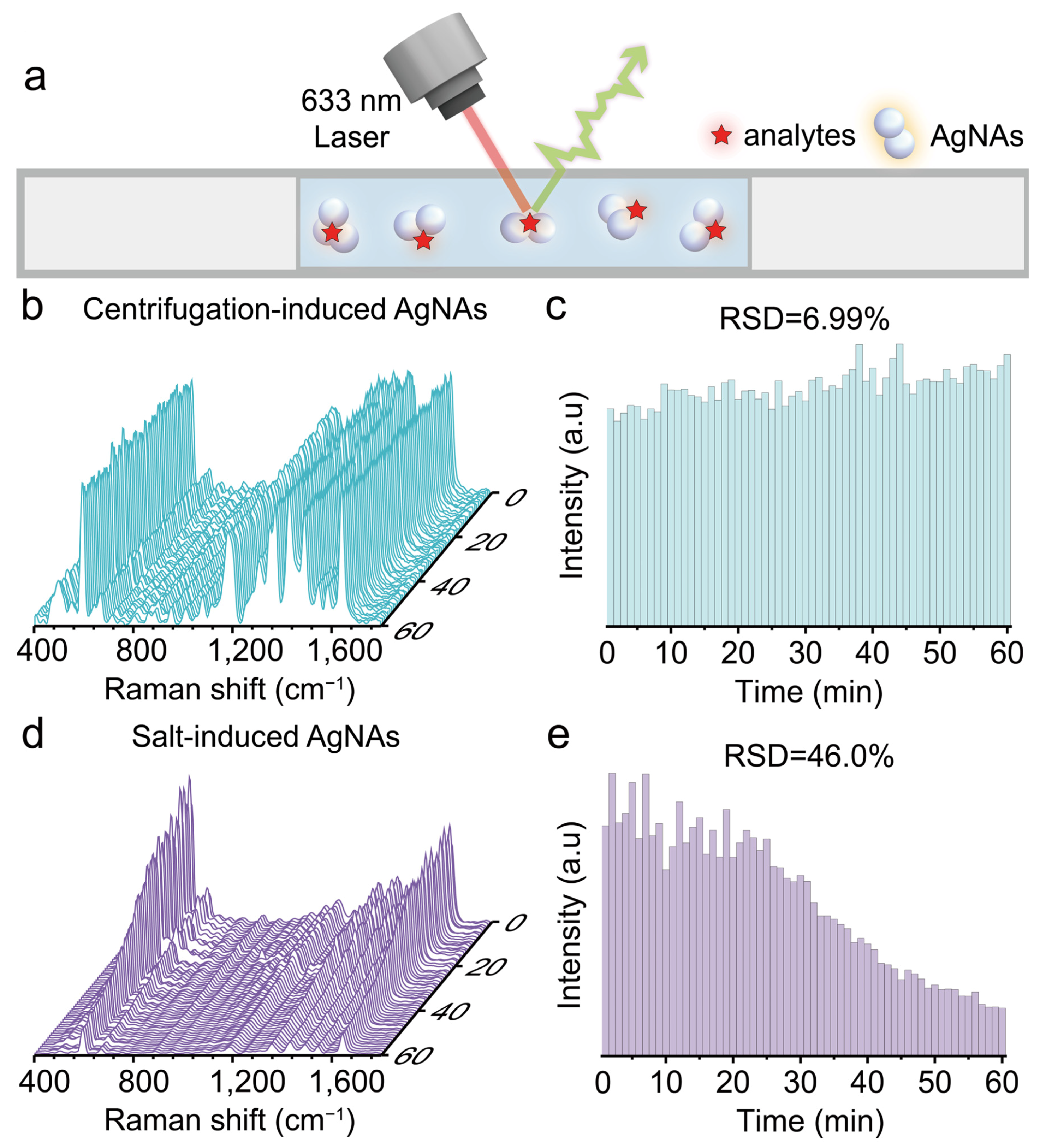
3.4. High SERS Reproducibility
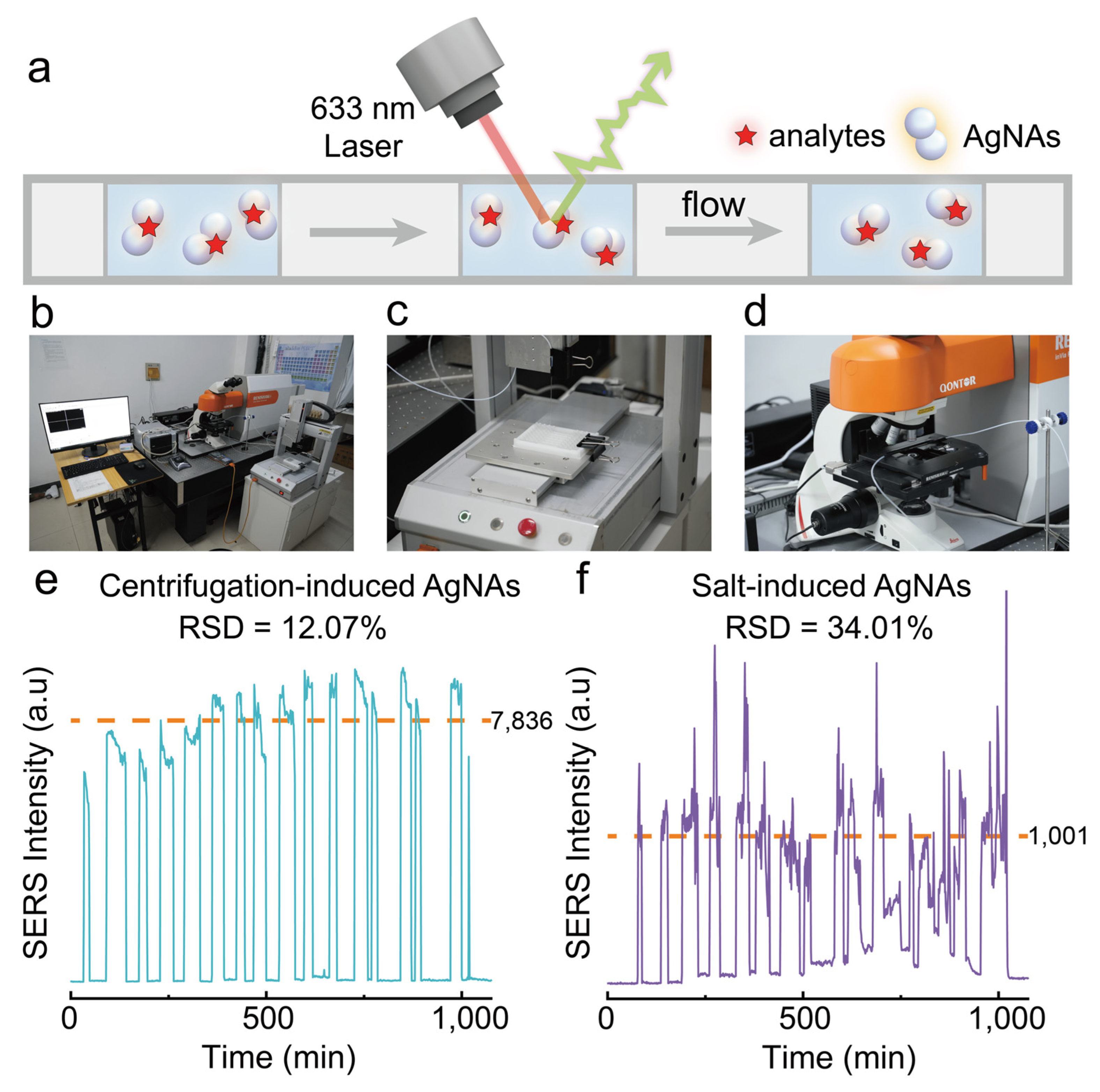
3.5. Potential of Continuous High-Throughput SERS Detection
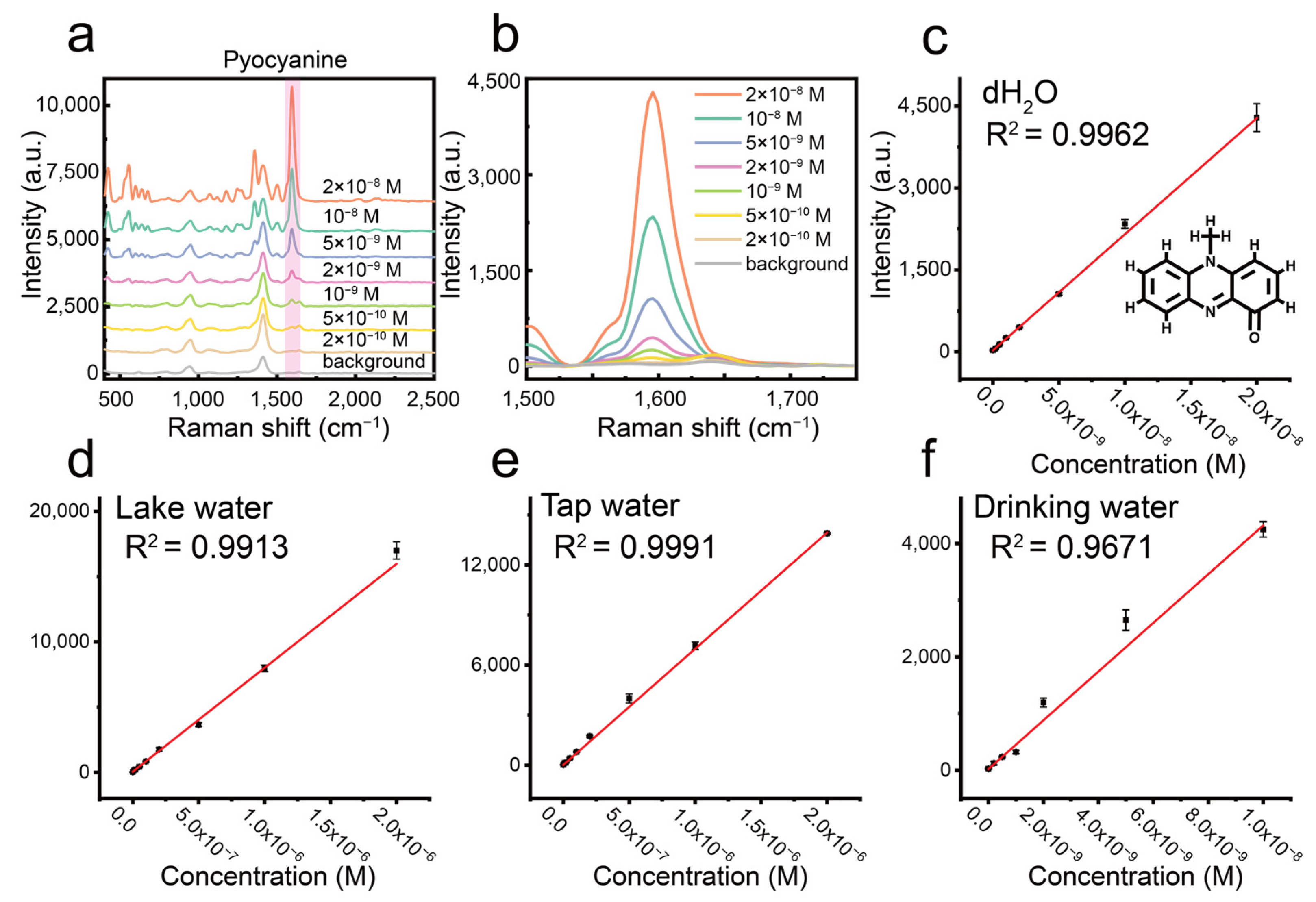
3.6. Detection of Pyocyanin in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bi, X.; Czajkowsky, D.M.; Shao, Z.; Ye, J. Digital colloid-enhanced Raman spectroscopy by single-molecule counting. Nature 2024, 628, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.L.; Alvarez-Puebla, R.; Liz-Marzán, L.M.; Trau, M.; Wang, J.; Fabris, L.; Wang, X.; Liu, G.; Xu, S.; Han, X.X.; et al. Surface-Enhanced Raman Spectroscopy for Biomedical Applications: Recent Advances and Future Challenges. ACS Appl. Mater. Interfaces 2025, 17, 16287–16379. [Google Scholar] [CrossRef]
- Langer, J.; Aberasturi, D.; Aizpurua, J.; Álvarez-Puebla, R.; Auguié, B.; Auguié, B.; Baumberg, J.; Bazan, G.; Bell, S.; Boisen, A.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2019, 14, 28–117. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.-J.; Wei, T.; Ma, X.; Zheng, X.-S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef] [PubMed]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Luo, K.; Chen, A.; Liu, Y.; Yang, J.; Cai, N.; Li, J. Constructing a highly sensitive SERS sensor based on necklace-like CNC/ZIF-8/Ag to detect and photo-degrade diquat in green tea leaves. Ind. Crops Prod. 2025, 225, 120453. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.H.; Koh, C.S.L.; Phan-Quang, G.C.; Han, X.; Lay, C.; Sim, H.Y.F.; Kao, Y.C.; An, Q.; Ling, X. Designing surface-enhanced Raman scattering (SERS) platforms beyond hotspot engineering: Emerging opportunities in analyte manipulations and hybrid materials. Chem. Soc. Rev. 2019, 48, 731–756. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Li, H.; Wang, X.; Xu, K.; Li, Q.; Zheng, T.; Li, J. Highly Sensitive Microarray Immunoassay for Multiple Mycotoxins on Engineered 3D Porous Silicon SERS Substrate with Silver Nanoparticle Magnetron Sputtering. Anal. Chem. 2024, 96, 2425–2434. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, F.; Su, M.; Du, S.; Liu, H. Halide-assisted activation of atomic hydrogen for photoreduction on two-liquid interfacial plasmonic arrays. Chem. Commun. 2019, 55, 1422–1425. [Google Scholar] [CrossRef]
- Cai, J.; Liu, R.; Jia, S.; Feng, Z.-H.; Lin, L.; Zheng, Z.-Q.; Wu, S.; Wang, Z. SERS hotspots distribution of the highly ordered noble metal arrays on flexible substrates. Opt. Mater. 2021, 122, 111779. [Google Scholar] [CrossRef]
- Pan, X.; Dong, J.; Li, Y.; Sun, X.; Yuan, C.; Qian, W. The strategy of two-scale interface enrichment for constructing ultrasensitive SERS substrates based on the coffee ring effect of AgNP@β-CD. RSC Adv. 2016, 6, 29586–29591. [Google Scholar] [CrossRef]
- Guselnikova, O.; Lim, H.; Kim, H.-J.; Kim, S.H.; Gorbunova, A.; Eguchi, M.; Postnikov, P.; Nakanishi, T.; Asahi, T.; Na, J.; et al. New Trends in Nanoarchitectured SERS Substrates: Nanospaces, 2D Materials, and Organic Heterostructures. Small 2022, 18, 2107182. [Google Scholar] [CrossRef]
- Phan-Quang, G.C.; Wee, E.H.Z.; Yang, F.; Lee, H.K.; Phang, I.Y.; Feng, X.; Alvarez-Puebla, R.A.; Ling, X.Y. Online Flowing Colloidosomes for Sequential Multi-analyte High-Throughput SERS Analysis. Angew. Chem. Int. Ed. 2017, 56, 5565–5569. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, L.; Zhang, X.; Wang, X.; Ji, W.; Zhao, J.; Ozaki, Y. CTAB-triggered Ag aggregates for reproducible SERS analysis of urinary polycyclic aromatic hydrocarbon metabolites. Chem. Commun. 2019, 55, 2146–2149. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Song, Y.; Wang, L.; Guo, C.; Sun, Y.; Liu, Z.; Li, Z. Ethanol-Induced Formation of Silver Nanoparticle Aggregates for Highly Active SERS Substrates and Application in DNA Detection. J. Phys. Chem. C 2008, 112, 1415–1422. [Google Scholar] [CrossRef]
- Michaels, A.M.; Nirmal, M.; Brus, L.E. Surface Enhanced Raman Spectroscopy of Individual Rhodamine 6G Molecules on Large Ag Nanocrystals. J. Am. Chem. Soc. 1999, 121, 9932–9939. [Google Scholar] [CrossRef]
- Amin, M.U.; Zhang, R.; Li, L.; You, H.; Fang, J. Solution-Based SERS Detection of Weak Surficial Affinity Molecules Using Cysteamine-Modified Au Bipyramids. Anal. Chem. 2021, 93, 7657–7664. [Google Scholar] [CrossRef]
- Otto, A.; Bruckbauer, A.; Chen, Y.X. On the chloride activation in SERS and single molecule SERS. J. Mol. Struct. 2003, 661–662, 501–514. [Google Scholar] [CrossRef]
- Bélteky, P.; Rónavári, A.; Igaz, N.; Szerencsés, B.; Tóth, I.; Pfeiffer, I.; Kiricsi, M.; Kónya, Z. Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity. Int. J. Nanomed. 2019, 14, 667–687. [Google Scholar] [CrossRef]
- Bell, S.E.J.; Sirimuthu, N.M.S. Surface-Enhanced Raman Spectroscopy (SERS) for Sub-Micromolar Detection of DNA/RNA Mononucleotides. J. Am. Chem. Soc. 2006, 128, 15580–15581. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Li, C.; Xu, Y.; Bell, S.E.J. Exploiting the chemical differences between Ag and Au colloids allows dramatically improved SERS detection of “non-adsorbing” molecules. Analyst 2019, 144, 448–453. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Miller, J.B.; Harris, J.M.; Hobbie, E.K. Purifying Colloidal Nanoparticles Through Ultracentrifugation with Implications for Interfaces and Materials. Langmuir 2014, 30, 7936–7946. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.M.A.; Valente, A.J.M.; Cova, T.F.; Abreu, P.; Marques, J.; Pais, A. Aggregation of Cyclodextrins: Fundamental Issues and Applications. In Cyclodextrin-A Versatile Ingredient; Arora, P., Dhingra, N., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Messner, M.; Kurkov, S.V.; Palazón, M.M.; Fernández, B.Á.; Brewster, M.E.; Loftsson, T. Self-assembly of cyclodextrin complexes: Effect of temperature, agitation and media composition on aggregation. Int. J. Pharm. 2011, 419, 322–328. [Google Scholar] [CrossRef]
- González-Gaitano, G.; Rodríguez, P.; Isasi, J.R.; Fuentes, M.; Tardajos, G.; Sánchez, M. The Aggregation of Cyclodextrins as Studied by Photon Correlation Spectroscopy. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 101–105. [Google Scholar] [CrossRef]
- Puskás, I.; Schrott, M.; Malanga, M.; Szente, L. Characterization and control of the aggregation behavior of cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 269–276. [Google Scholar] [CrossRef]
- Loftsson, T.; Másson, M.; Brewster, M.E. Self-Association of Cyclodextrins and Cyclodextrin Complexes. J. Pharm. Sci. 2004, 93, 1091–1099. [Google Scholar] [CrossRef]
- Bonini, M.; Rossi, S.; Karlsson, G.; Almgren, M.; Lo Nostro, P.; Baglioni, P. Self-Assembly of β-Cyclodextrin in Water. Part 1: Cryo-TEM and Dynamic and Static Light Scattering. Langmuir 2006, 22, 1478–1484. [Google Scholar] [CrossRef]
- He, Y.; Fu, P.; Shen, X.; Gao, H. Cyclodextrin-based aggregates and characterization by microscopy. Micron 2008, 39, 495–516. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, Z.; Zhang, L.; Miao, J.; Chen, Y.; Zhao, R.; Liu, M.; Chen, L.; Wang, X. Size-controllable colloidal Ag nano-aggregates with long-time SERS detection window for on-line high-throughput detection. Talanta 2023, 257, 124358. [Google Scholar] [CrossRef]
- Wu, X.; Chen, J.; Li, X.; Zhao, Y.; Zughaier, S.M. Culture-free diagnostics of Pseudomonas aeruginosa infection by silver nanorod array based SERS from clinical sputum samples. Nanomedicine: Nanotechnology, Biology and Medicine 2014, 10, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Žukovskaja, O.; Jahn, I.J.; Weber, K.; Cialla-May, D.; Popp, J. Detection of Pseudomonas aeruginosa Metabolite Pyocyanin in Water and Saliva by Employing the SERS Technique. Sensors 2017, 17, 1704. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.Q.; Thrift, W.J.; Bhattacharjee, A.; Ranjbar, S.; Gallagher, T.; Darvishzadeh-Varcheie, M.; Sanderson, R.N.; Capolino, F.; Whiteson, K.; Baldi, P.; et al. Longitudinal Monitoring of Biofilm Formation via Robust Surface-Enhanced Raman Scattering Quantification of Pseudomonas aeruginosa-Produced Metabolites. ACS Appl. Mater. Interfaces 2018, 10, 12364–12373. [Google Scholar] [CrossRef]
- Jia, F.; Barber, E.; Turasan, H.; Seo, S.; Dai, R.; Liu, L.; Li, X.; Bhunia, A.K.; Kokini, J.L. Detection of Pyocyanin Using a New Biodegradable SERS Biosensor Fabricated Using Gold Coated Zein Nanostructures Further Decorated with Gold Nanoparticles. J. Agric. Food Chem. 2019, 67, 4603–4610. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Khoo, E.H.; Salleh, N.A.b.M.; Teo, S.L.; Ow, S.Y.; Sutarlie, L.; Su, X. A portable SERS sensor for pyocyanin detection in simulated wound fluid and through swab sampling. Analyst 2021, 146, 6924–6934. [Google Scholar] [CrossRef]
- Kim, S.; Ansah, I.B.; Park, J.S.; Dang, H.; Choi, N.; Lee, W.-C.; Lee, S.H.; Jung, H.S.; Kim, D.-H.; Yoo, S.M.; et al. Early and direct detection of bacterial signaling molecules through one-pot Au electrodeposition onto paper-based 3D SERS substrates. Sens. Actuators B Chem. 2022, 358, 131504. [Google Scholar] [CrossRef]
- Atta, S.; Vo-Dinh, T. Solution-Based Ultra-Sensitive Surface-Enhanced Raman Scattering Detection of the Toxin Bacterial Biomarker Pyocyanin in Biological Fluids Using Sharp-Branched Gold Nanostars. Anal. Chem. 2023, 95, 2690–2697. [Google Scholar] [CrossRef]
- El-Said, W.A.; Saleh, T.S.; Al-Bogami, A.S.; Wani, M.Y.; Choi, J.-W. Development of Novel Surface-Enhanced Raman Spectroscopy-Based Biosensors by Controlling the Roughness of Gold/Alumina Platforms for Highly Sensitive Detection of Pyocyanin Secreted from Pseudomonas aeruginosa. Biosensors 2024, 14, 399. [Google Scholar] [CrossRef]


| Samples | Pretreatment | Spiked Concentration (nM) | Detected | Recovery (%) |
|---|---|---|---|---|
| Drinking water | 5.0 | 5.8 | 117% | |
| 1.0 | 8.2 | 82% | ||
| Tap water | Dilute 50 times | 100.0 | 111.4 | 111% |
| Dilute 50 times | 50.0 | 58.6 | 117% | |
| Lake water | Dilute 50 times | 100.0 | 111.1 | 111% |
| Dilute 50 times | 50.0 | 45.5 | 91% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Zhang, Z. Centrifugation-Induced Stable Colloidal Silver Nanoparticle Aggregates for Reproducible Surface-Enhanced Raman Scattering Detection. Biosensors 2025, 15, 298. https://doi.org/10.3390/bios15050298
Zhou T, Zhang Z. Centrifugation-Induced Stable Colloidal Silver Nanoparticle Aggregates for Reproducible Surface-Enhanced Raman Scattering Detection. Biosensors. 2025; 15(5):298. https://doi.org/10.3390/bios15050298
Chicago/Turabian StyleZhou, Tianyu, and Zhiyang Zhang. 2025. "Centrifugation-Induced Stable Colloidal Silver Nanoparticle Aggregates for Reproducible Surface-Enhanced Raman Scattering Detection" Biosensors 15, no. 5: 298. https://doi.org/10.3390/bios15050298
APA StyleZhou, T., & Zhang, Z. (2025). Centrifugation-Induced Stable Colloidal Silver Nanoparticle Aggregates for Reproducible Surface-Enhanced Raman Scattering Detection. Biosensors, 15(5), 298. https://doi.org/10.3390/bios15050298






