Review of Non-Invasive Imaging Technologies for Cutaneous Melanoma
Abstract
1. Introduction
2. Melanoma Characterization (Without Imaging)
2.1. Melanoma Formation and Subtypes
2.2. Pathological and Histopathological Analysis of Melanoma
3. Review Method
4. Imaging Modalities Currently Used in Medical Practice
4.1. Photography
4.2. Dermoscopy
4.3. Electrical Impedance Spectroscopy
4.4. Reflectance Confocal Microscopy
4.5. Optical Coherence Tomography
4.6. Multispectral Imaging
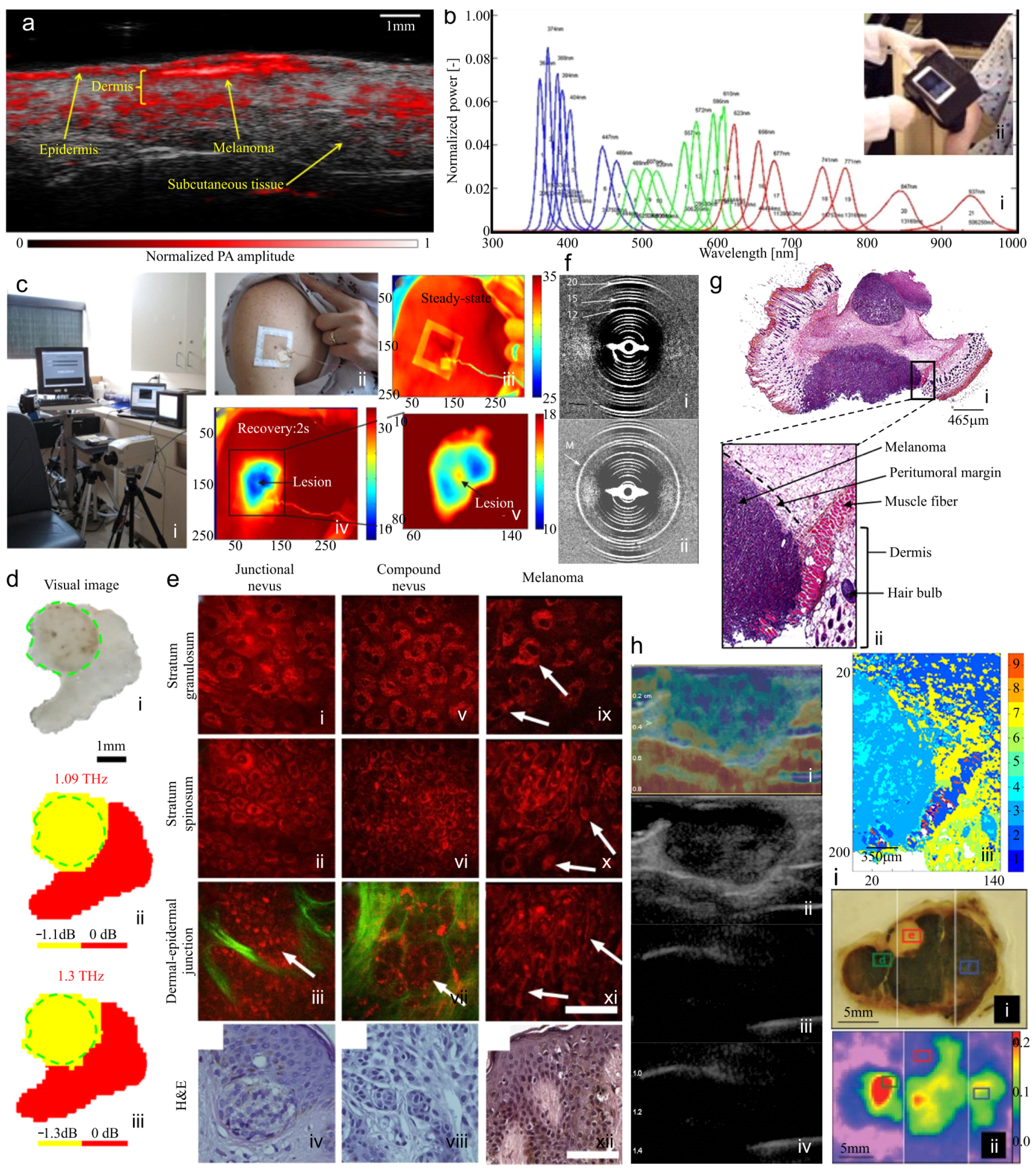
4.7. Ultrasound and High-Frequency Ultrasound
4.8. Magnetic Resonance Imaging
4.9. Raman Spectroscopy
4.10. Elastic Scattering Spectroscopy
4.11. PET/CT and SPECT/CT
4.12. Lymphoscintigraphy
4.13. Non-Imaging (Genetic) Melanoma Detection and Disease Management
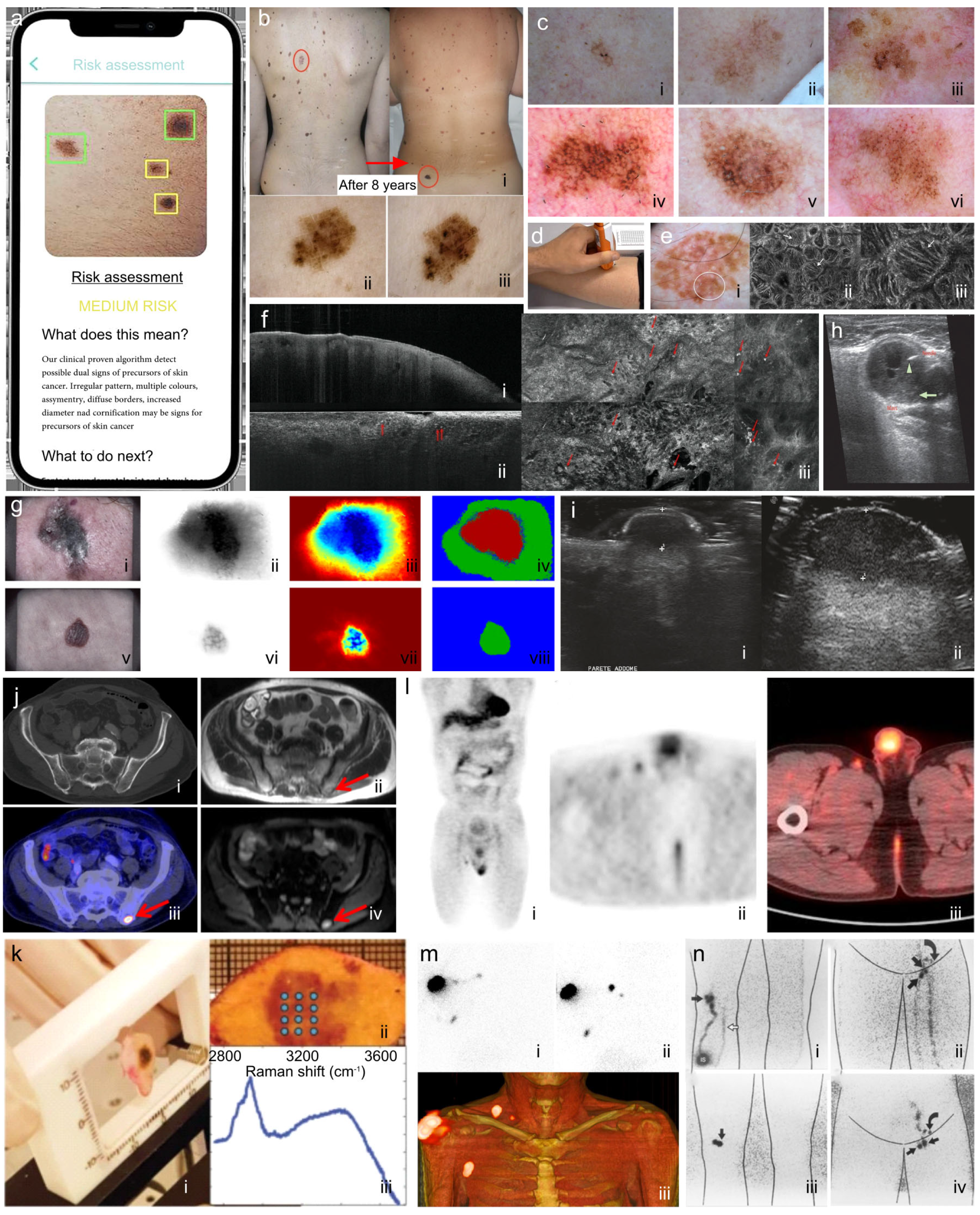
4.14. The Role of Artificial Intelligence in Melanoma Technologies
5. Technologies in Development for Non-Invasive Imaging of Cutaneous Melanoma
5.1. Photoacoustic Imaging (Optoacoustic Imaging)
5.2. Hyperspectral Imaging
5.3. Quantitative Dynamic Infrared Imaging (Thermographic Imaging)
5.4. Terahertz Pulsed Imaging
5.5. Multiphoton Imaging
5.6. Fiber Diffraction
5.7. Fourier Transform Infrared (FTIR) Spectroscopy and Microspectroscopy
5.8. Real-Time Elastography
5.9. Electron Paramagnetic Resonance Imaging
5.10. Multimodal Screening Technologies
6. Remarks on Barriers to Technological Adoption
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Linos, E.; Swetter, S.M.; Cockburn, M.G.; Colditz, G.A.; Clarke, C.A. Increasing Burden of Melanoma in the United States. J. Investig. Dermatol. 2009, 129, 1666–1674. (In English) [Google Scholar] [CrossRef]
- Erdmann, F.; Lortet-Tieulent, J.; Schüz, J.; Zeeb, H.; Greinert, R.; Breitbart, E.W.; Bray, F. International trends in the incidence of malignant melanoma 1953–2008—Are recent generations at higher or lower risk? Int. J. Cancer 2013, 132, 385–400. [Google Scholar] [CrossRef]
- Kosary, C.L.; Altekruse, S.F.; Ruhl, J.; Lee, R.; Dickie, L. Clinical and prognostic factors for melanoma of the skin using SEER registries: Collaborative stage data collection system, version 1 and version 2. Cancer 2014, 120, 3807–3814. [Google Scholar] [CrossRef]
- Guy, G.P., Jr.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C.; Centers for Disease Control and Prevention (CDC). Vital signs: Melanoma incidence and mortality trends and projections—United States, 1982–2030. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 591–596. (In English) [Google Scholar] [PubMed]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef]
- Street, W. Cancer Facts & Figures 2017. Cancer Facts and Statistics, American Cancer Society. 2017. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html (accessed on 12 January 2025).
- Coory, M.; Baade, P.; Aitken, J.; Smithers, M.; McLeod, G.R.C.; Ring, I. Trends for in situ and invasive melanoma in Queensland, Australia, 1982–2002. Cancer Causes Control 2006, 17, 21–27. (In English) [Google Scholar] [CrossRef] [PubMed]
- Matthews, N.H.; Li, W.-Q.; Qureshi, A.A.; Weinstock, M.A.; Cho, E. Epidemiology of Melanoma. In Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Ward, W.H., Farma, J.M., Eds.; Exon Publications: Brisbane, Australia, 2017. [Google Scholar]
- American_Cancer_Society. Key Statistics for Melanoma Skin Cancer. Available online: https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.html (accessed on 12 January 2025).
- Welch, H.G.; Woloshin, S.; Schwartz, L.M. Skin biopsy rates and incidence of melanoma: Population based ecological study. BMJ 2005, 331, 481. [Google Scholar] [CrossRef]
- Bharath, A.; Turner, R. Impact of climate change on skin cancer. J. R. Soc. Med. 2009, 102, 215–218. [Google Scholar] [CrossRef]
- Shellenberger, R.; Nabhan, M.; Kakaraparthi, S. Melanoma screening: A plan for improving early detection. Ann. Med. 2016, 48, 142–148. [Google Scholar] [CrossRef]
- Noone, A.M.; Howlander, N.; Kraphcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. Cancer Statistics Review, 1975–2015—SEER Statistics; National Cancer Institute: Rockville, MD, USA, 2018. [Google Scholar]
- Perlis, C.; Herlyn, M. Recent advances in melanoma biology. Oncologist 2004, 9, 182–187. (In English) [Google Scholar] [CrossRef]
- Aneja, S.; Aneja, S.; Bordeaux, J.S. Association of increased dermatologist density with lower melanoma mortality. Arch. Dermatol. 2012, 148, 174–178. [Google Scholar] [CrossRef]
- Cortez, J.L.; Vasquez, J.; Wei, M.L. The impact of demographics, socioeconomics, and health care access on melanoma outcomes. J. Am. Acad. Dermatol. 2021, 84, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Themstrup, L.; Jemec, G.B.E. Chapter 6—Optical Coherence Tomography for Skin Cancer and Actinic Keratosis. In Imaging in Dermatology; Hamblin, M.R., Avci, P., Gupta, G.K., Hamblin, M.R., Avci, P., Gupta, G.K., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 59–67. [Google Scholar]
- Garibyan, L.; Fisher, D.E. How sunlight causes melanoma. Curr. Oncol. Rep. 2010, 12, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.S. Prevalence of a history of skin cancer in 2007: Results of an incidence-based model. Arch. Dermatol. 2010, 146, 279–282. [Google Scholar] [CrossRef]
- Abbas, O.; Miller, D.D.; Bhawan, J. Cutaneous malignant melanoma: Update on diagnostic and prognostic biomarkers. Am. J. Dermatopathol. 2014, 36, 363–379. (In English) [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yu, H.; Zheng, X.; Wu, U.T.; Ming, W.-k.; Huang, H.; Song, J.; Zhang, X.; Lyu, J.; Deng, L. Analysis and prediction of 5-year survival in patients with cutaneous melanoma: A model-based period analysis. Front. Endocrinol. 2023, 14, 1238086. [Google Scholar] [CrossRef]
- Thomas, L.; Tranchand, P.; Berard, F.; Secchi, T.; Colin, C.; Moulin, G. Semiological value of ABCDE criteria in the diagnosis of cutaneous pigmented tumors. Dermatology 1998, 197, 11–17. [Google Scholar] [CrossRef]
- Harkemanne, E.; Baeck, M.; Tromme, I. Training general practitioners in melanoma diagnosis: A scoping review of the literature. BMJ Open 2021, 11, e043926. (In English) [Google Scholar] [CrossRef]
- Nelson, K.C.; Swetter, S.M.; Saboda, K.; Chen, S.C.; Curiel-Lewandrowski, C. Evaluation of the Number-Needed-to-Biopsy Metric for the Diagnosis of Cutaneous Melanoma: A Systematic Review and Meta-analysis. JAMA Dermatol. 2019, 155, 1167–1174. (In English) [Google Scholar] [CrossRef]
- Tes, D.; Aber, A.; Zafar, M.; Horton, L.; Fotouhi, A.; Xu, Q.; Moiin, A.; Thompson, A.D.; Moraes Pinto Blumetti, T.C.; Daveluy, S. Granular cell tumor imaging using optical coherence tomography. Biomed. Eng. Comput. Biol. 2018, 9, 1179597218790250. [Google Scholar] [CrossRef]
- Narayanamurthy, V.; Padmapriya, P.; Noorasafrin, A.; Pooja, B.; Hema, K.; Khan, A.a.Y.F.; Nithyakalyani, K.; Samsuri, F. Skin cancer detection using non-invasive techniques. RSC Adv. 2018, 8, 28095–28130. (In English) [Google Scholar] [CrossRef] [PubMed]
- Panchal, R.; Horton, L.; Poozesh, P.; Baqersad, J.; Nasiriavanaki, M. Vibration analysis of healthy skin: Toward a noninvasive skin diagnosis methodology. J. Biomed. Opt. 2019, 24, 015001. [Google Scholar] [CrossRef] [PubMed]
- State-of-the-Art 3D Imaging Can Spot Skin Cancer Early (Video); Baptist Health South Florida: Coral Gables, FL, USA, 2019.
- Freeman, T. Millimetre-Wave Imaging Delivers Non-Invasive Skin Cancer Diagnosis. Physics World. 2019. Available online: https://physicsworld.com/a/millimetre-wave-imaging-delivers-non-invasive-skin-cancer-diagnosis/ (accessed on 12 January 2025).
- Jalilian, E.; Xu, Q.; Horton, L.; Fotouhi, A.; Reddy, S.; Manwar, R.; Daveluy, S.; Mehregan, D.; Gelovani, J.; Avanaki, K. Contrast-enhanced optical coherence tomography for melanoma detection: An in vitro study. J. Biophotonics 2020, 13, e201960097. (In English) [Google Scholar] [CrossRef] [PubMed]
- Reed, J. Non-Invasive Imaging Techniques for Diagnosis. SkinCancer.net. 2020. Available online: https://skincancer.net/procedures-tests/imaging/non-invasive-techniques (accessed on 12 January 2025).
- Shapiro, L.; Basra, M.; Patel, H.; Payne, C.; Brazen, B.; Biglione, A. Utilization of Imaging Modalities in the Diagnosis of Melanoma: A Scoping Review. Cureus 2024, 16, 1–10. [Google Scholar] [CrossRef]
- Fakhoury, J.W.; Lara, J.B.; Manwar, R.; Zafar, M.; Xu, Q.; Engel, R.; Tsoukas, M.M.; Daveluy, S.; Mehregan, D.; Avanaki, K. Photoacoustic imaging for cutaneous melanoma assessment: A comprehensive review. J. Biomed. Opt. 2024, 29, S11518. [Google Scholar] [CrossRef]
- Atkins, M.B.; Curiel-Lewandrowski, C.; Fisher, D.E.; Swetter, S.M.; Tsao, H.; Aguirre-Ghiso, J.A.; Soengas, M.S.; Weeraratna, A.T.; Flaherty, K.T.; Herlyn, M.; et al. The State of Melanoma: Emergent Challenges and Opportunities. Clin. Cancer Res. 2021, 27, 2678–2697. [Google Scholar] [CrossRef]
- Bichakjian, C.K.; Halpern, A.C.; Johnson, T.M.; Foote Hood, A.; Grichnik, J.M.; Swetter, S.M.; Tsao, H.; Barbosa, V.H.; Chuang, T.-Y.; Duvic, M.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2011, 65, 1032–1047. (In English) [Google Scholar] [CrossRef]
- Reed, D.; Kudchadkar, R.; Zager, J.S.; Sondak, V.K.; Messina, J.L. Controversies in the Evaluation and Management of Atypical Melanocytic Proliferations in Children, Adolescents, and Young Adults. J. Natl. Compr. Cancer Netw. 2013, 11, 679–686. (In English) [Google Scholar] [CrossRef]
- Kauffmann, R.M.; Chen, S.L. Workup and staging of malignant melanoma. Surg. Clin. N. Am. 2014, 94, 963–972. (In English) [Google Scholar] [CrossRef]
- Sondak, V.K.; Taylor, J.M.G.; Sabel, M.S.; Wang, Y.; Lowe, L.; Grover, A.C.; Chang, A.E.; Yahanda, A.M.; Moon, J.; Johnson, T.M. Mitotic Rate and Younger Age Are Predictors of Sentinel Lymph Node Positivity: Lessons Learned From the Generation of a Probabilistic Model. Ann. Surg. Oncol. 2004, 11, 247–258. (In English) [Google Scholar] [CrossRef]
- Mathew, R.; Messina, J.L. Recent Advances in Pathologic Evaluation and Reporting of Melanoma. Semin. Oncol. 2012, 39, 184–191. (In English) [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. (In English) [Google Scholar] [CrossRef] [PubMed]
- Ross, M.I.; Gershenwald, J.E. Sentinel lymph node biopsy for melanoma: A critical update for dermatologists after two decades of experience. Clin. Dermatol. 2013, 31, 298–310. (In English) [Google Scholar] [CrossRef]
- Mandalà, M.; Galli, F.; Cattaneo, L.; Merelli, B.; Rulli, E.; Ribero, S.; Quaglino, P.; Giorgi, V.D.; Pigozzo, J.; Sileni, V.C.; et al. Mitotic rate correlates with sentinel lymph node status and outcome in cutaneous melanoma greater than 1 millimeter in thickness: A multi-institutional study of 1524 cases. J. Am. Acad. Dermatol. 2017, 76, 264–273.e262. (In English) [Google Scholar] [CrossRef] [PubMed]
- Azzola, M.F.; Shaw, H.M.; Thompson, J.F.; Soong, S.-j.; Scolyer, R.A.; Watson, G.F.; Colman, M.H.; Zhang, Y. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma. Cancer 2003, 97, 1488–1498. (In English) [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.-J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199–6206. (In English) [Google Scholar] [CrossRef]
- Ward, W.H.; Lambreton, F.; Goel, N.; Yu, J.Q.; Farma, J.M. Clinical Presentation and Staging of Melanoma. In Cutaneous Melanoma: Etiology and Therapy; Ward, W.H., Farma, J.M., Ward, W.H., Farma, J.M., Eds.; Exon Publications: Brisbane, Australia, 2017. [Google Scholar]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA A Cancer J. Clin. 2017, 67, 93–99. (In English) [Google Scholar] [CrossRef] [PubMed]
- Torbatian, Z.; Adamson, R.; Bance, M.; Brown, J. A split-aperture transmit beamforming technique with phase coherence grating lobe suppression. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2010, 57, 2588–2595. [Google Scholar] [CrossRef]
- Adamson, A.S.; Jarmul, J.A.; Pignone, M.P. Screening for melanoma in men: A cost-effectiveness analysis. J. Gen. Intern. Med. 2020, 35, 1175–1181. [Google Scholar] [CrossRef]
- Elder, D.E. Melanoma progression. Pathology 2016, 48, 147–154. [Google Scholar] [CrossRef]
- Smoller, B.R. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Mod. Pathol. 2006, 19, S34–S40. [Google Scholar] [CrossRef]
- Šitum, M.; Buljan, M.; Kolić, M.; Vučić, M. Melanoma–clinical, dermatoscopical, and histopathological morphological characteristics. Acta Dermatovenerol. Croat. 2014, 22, 1–12. [Google Scholar] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Elmore, J.G.; Barnhill, R.L.; Elder, D.E.; Longton, G.M.; Pepe, M.S.; Reisch, L.M.; Carney, P.A.; Titus, L.J.; Nelson, H.D.; Onega, T. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: Observer accuracy and reproducibility study. bmj 2017, 357, j2813. [Google Scholar] [CrossRef] [PubMed]
- Plaza, J.A.; Suster, D.; Perez-Montiel, D. Expression of immunohistochemical markers in primary and metastatic malignant melanoma: A comparative study in 70 patients using a tissue microarray technique. Appl. Immunohistochem. Mol. Morphol. 2007, 15, 421–425. [Google Scholar] [CrossRef]
- Nwafor, J.N.; Torere, B.E.; Agu, E.; Kadiku, L.; Ogunyemi, T.; Akinsanya, P.A.; Araromi, O.O.; Akahara, D.E.; Okobi, O.E. The Role of Biomarkers in the Diagnosis and Prognosis of Different Stages of Melanoma. Cureus 2023, 15, e38693. (In English) [Google Scholar] [CrossRef]
- Ladstein, R.G.; Bachmann, I.M.; Straume, O.; Akslen, L.A. Ki-67 expression is superior to mitotic count and novel proliferation markers PHH3, MCM4 and mitosin as a prognostic factor in thick cutaneous melanoma. BMC Cancer 2010, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Pizzichetta, M.A.; Stanganelli, I. Total Body Photography and Sequential Digital Dermoscopy for Melanoma Diagnosis. In Technology in Practical Dermatology: Non-Invasive Imaging, Lasers and Ulcer Management; Fimiani, M., Rubegni, P., Cinotti, E., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 121–126. [Google Scholar]
- Kränke, T.; Tripolt-Droschl, K.; Röd, L.; Hofmann-Wellenhof, R.; Koppitz, M.; Tripolt, M. New AI-algorithms on smartphones to detect skin cancer in a clinical setting-A validation study. PLoS ONE 2023, 18, e0280670. (In English) [Google Scholar] [CrossRef]
- Jahn, A.S.; Navarini, A.A.; Cerminara, S.E.; Kostner, L.; Huber, S.M.; Kunz, M.; Maul, J.-T.; Dummer, R.; Sommer, S.; Neuner, A.D.; et al. Over-Detection of Melanoma-Suspect Lesions by a CE-Certified Smartphone App: Performance in Comparison to Dermatologists, 2D and 3D Convolutional Neural Networks in a Prospective Data Set of 1204 Pigmented Skin Lesions Involving Patients’ Perception. Cancers 2022, 14, 3829. [Google Scholar] [CrossRef]
- Ji-Xu, A.; Dinnes, J.; Matin, R. Total body photography for the diagnosis of cutaneous melanoma in adults: A systematic review and meta-analysis. Br. J. Dermatol. 2021, 185, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Herman, C. Emerging technologies for the detection of melanoma: Achieving better outcomes. Clin. Cosmet. Investig. Dermatol. 2012, 5, 195. [Google Scholar] [CrossRef]
- Berk-Krauss, J.; Polsky, D.; Stein, J.A. Mole Mapping for Management of Pigmented Skin Lesions. Dermatol. Clin. 2017, 35, 439–445. (In English) [Google Scholar] [CrossRef]
- Dugonik, B.; Dugonik, A.; Marovt, M.; Golob, M. Image Quality Assessment of Digital Image Capturing Devices for Melanoma Detection. Appl. Sci. 2020, 10, 2876. [Google Scholar] [CrossRef]
- Risser, J.; Pressley, Z.; Veledar, E.; Washington, C.; Chen, S.C. The impact of total body photography on biopsy rate in patients from a pigmented lesion clinic. J. Am. Acad. Dermatol. 2007, 57, 428–434. (In English) [Google Scholar] [CrossRef]
- Drugge, E.D.; Volpicelli, E.R.; Sarac, R.M.; Strang, S.R.; Elston, D.M.; Drugge, R.J. Micromelanomas identified with time-lapse total body photography and dermoscopy. J. Am. Acad. Dermatol. 2018, 78, 182–183. (In English) [Google Scholar] [CrossRef] [PubMed]
- Green, W.H.; Wang, S.Q.; Cognetta, A.B. Total-body cutaneous examination, total-body photography, and dermoscopy in the care of a patient with xeroderma pigmentosum and multiple melanomas. Arch. Dermatol. 2009, 145, 910–915. (In English) [Google Scholar] [CrossRef] [PubMed]
- Salerni, G.; Carrera, C.; Lovatto, L.; Martí-Laborda, R.M.; Isern, G.; Palou, J.; Alós, L.; Puig, S.; Malvehy, J. Characterization of 1152 lesions excised over 10 years using total-body photography and digital dermatoscopy in the surveillance of patients at high risk for melanoma. J. Am. Acad. Dermatol. 2012, 67, 836–845. (In English) [Google Scholar] [CrossRef]
- Vestergaard, M.E.; Macaskill, P.; Holt, P.E.; Menzies, S.W. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: A meta-analysis of studies performed in a clinical setting. Br. J. Dermatol. 2008, 159, 669–676. (In English) [Google Scholar] [CrossRef]
- Primiero, C.A.; McInerney-Leo, A.M.; Betz-Stablein, B.; Whiteman, D.C.; Gordon, L.; Caffery, L.; Aitken, J.F.; Eakin, E.; Osborne, S.; Gray, L.; et al. Evaluation of the efficacy of 3D total-body photography with sequential digital dermoscopy in a high-risk melanoma cohort: Protocol for a randomised controlled trial. BMJ Open 2019, 9, e032969. (In English) [Google Scholar] [CrossRef]
- Banky, J.P.; Kelly, J.W.; English, D.R.; Yeatman, J.M.; Dowling, J.P. Incidence of new and changed nevi and melanomas detected using baseline images and dermoscopy in patients at high risk for melanoma. Arch. Dermatol. 2005, 141, 998–1006. (In English) [Google Scholar] [CrossRef] [PubMed]
- Ferreirinha, A.; Farricha, V.; João, A. Melanoma diagnosis with 3D total-body photography. Actas Dermo-Sifiliográficas 2025. (Accepted; In press). [Google Scholar] [CrossRef]
- Olsen, J.; Holmes, J.; Jemec, G.B. Advances in optical coherence tomography in dermatology—A review. J. Biomed. Opt. 2018, 23, 040901. [Google Scholar] [CrossRef]
- Longo, C.; Pampena, R.; Moscarella, E.; Chester, J.; Starace, M.; Cinotti, E.; Piraccini, B.M.; Argenziano, G.; Peris, K.; Pellacani, G. Dermoscopy of melanoma according to different body sites: Head and neck, trunk, limbs, nail, mucosal and acral. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1718–1730. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Marín, R.; Puig, S.; Malvehy, J. Dermoscopic criteria and melanocytic lesions. G. Ital. Dermatol. Venereol. 2012, 147, 149–159. [Google Scholar] [PubMed]
- Ulrich, M.; Stockfleth, E.; Roewert-Huber, J.; Astner, S. Noninvasive diagnostic tools for nonmelanoma skin cancer. Br. J. Dermatol. 2007, 157 (Suppl. S2), 56–58. (In English) [Google Scholar] [CrossRef]
- Farnetani, F.; Scope, A.; Mazzoni, L.; Mandel, V.D.; Manfredini, M.; Magi, S.; Vaschieri, C.; Kaleci, S.; Longo, C.; Ciardo, S.; et al. A comparative dermoscopic and reflectance confocal microscopy study of naevi and melanoma with negative pigment network. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2273–2282. (In English) [Google Scholar] [CrossRef]
- Thomas, L.; Puig, S. Dermoscopy, Digital Dermoscopy and Other Diagnostic Tools in the Early Detection of Melanoma and Follow-up of High-risk Skin Cancer Patients. Acta Derm. Venereol. 2017, 97, 14–21. (In English) [Google Scholar] [CrossRef]
- Benvenuto-Andrade, C.; Dusza, S.W.; Agero, A.L.C.; Scope, A.; Rajadhyaksha, M.; Halpern, A.C.; Marghoob, A.A. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch. Dermatol. 2007, 143, 329–338. [Google Scholar] [CrossRef]
- Harrison, K. The Accuracy of Skin Cancer Detection Rates with the Implementation of Dermoscopy Among Dermatology Clinicians: A Scoping Review. J. Clin. Aesthet. Dermatol. 2024, 17 (Suppl. S1), S18–S27. (In English) [Google Scholar]
- Ye, Z.; Zhang, D.; Zhao, Y.; Chen, M.; Wang, H.; Seery, S.; Qu, Y.; Xue, P.; Jiang, Y. Deep learning algorithms for melanoma detection using dermoscopic imaging: A systematic review and meta-analysis. Artif. Intell. Med. 2024, 155, 102934. [Google Scholar] [CrossRef]
- Jaimes, N.; Chen, L.; Dusza, S.W.; Carrera, C.; Puig, S.; Thomas, L.; Kelly, J.W.; Dang, L.; Zalaudek, I.; Braun, R.P.; et al. Clinical and Dermoscopic Characteristics of Desmoplastic Melanomas. JAMA Dermatol. 2013, 149, 413–421. (In English) [Google Scholar] [CrossRef]
- Stolz, W.; Schiffner, R.; Burgdorf, W.H.C. Dermatoscopy for facial pigmented skin lesions. Clin. Dermatol. 2002, 20, 276–278. (In English) [Google Scholar] [CrossRef] [PubMed]
- Pagnanelli, G.; Soyer, H.P.; Argenziano, G.; Talamini, R.; Barbati, R.; Bianchi, L.; Campione, E.; Carboni, I.; Carrozzo, A.M.; Chimenti, M.S.; et al. Diagnosis of pigmented skin lesions by dermoscopy: Web-based training improves diagnostic performance of non-experts. Br. J. Dermatol. 2003, 148, 698–702. (In English) [Google Scholar] [CrossRef]
- Argenziano, G.; Zalaudek, I.; Corona, R.; Sera, F.; Cicale, L.; Petrillo, G.; Ruocco, E.; Hofmann-Wellenhof, R.; Soyer, H.P. Vascular structures in skin tumors: A dermoscopy study. Arch. Dermatol. 2004, 140, 1485–1489. (In English) [Google Scholar] [CrossRef] [PubMed]
- Rubegni, P.; Sbano, P.; Burroni, M.; Cevenini, G.; Bocchi, C.; Severi, F.M.; Risulo, M.; Petraglia, F.; Dell’Eva, G.; Fimiani, M.; et al. Melanocytic skin lesions and pregnancy: Digital dermoscopy analysis. Skin Res. Technol. 2007, 13, 143–147. (In English) [Google Scholar] [CrossRef] [PubMed]
- Carli, P.; De Giorgi, V.; Crocetti, E.; Mannone, F.; Massi, D.; Chiarugi, A.; Giannotti, B. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: A retrospective study 1997–2001. Br. J. Dermatol. 2004, 150, 687–692. (In English) [Google Scholar] [CrossRef]
- Cinotti, E.; Tognetti, L.; Campoli, M.; Liso, F.; Cicigoi, A.; Cartocci, A.; Rossi, R.; Rubegni, P.; Perrot, J. Super-high magnification dermoscopy can aid the differential diagnosis between melanoma and atypical naevi. Clin. Exp. Dermatol. 2021, 46, 1216–1222. [Google Scholar] [CrossRef]
- Kardynal, A.; Olszewska, M. Modern non-invasive diagnostic techniques in the detection of early cutaneous melanoma. J. Dermatol. Case Rep. 2014, 8, 1–8. (In English) [Google Scholar] [CrossRef]
- Del Rosario, F.; Farahi, J.M.; Drendel, J.; Buntinx-Krieg, T.; Caravaglio, J.; Domozych, R.; Chapman, S.; Braunberger, T.; Dellavalle, R.P.; Norris, D.A.; et al. Performance of a computer-aided digital dermoscopic image analyzer for melanoma detection in 1,076 pigmented skin lesion biopsies. J. Am. Acad. Dermatol. 2018, 78, 927–934.e926. (In English) [Google Scholar] [CrossRef]
- Marchetti, M.A.; Cowen, E.A.; Kurtansky, N.R.; Weber, J.; Dauscher, M.; DeFazio, J.; Deng, L.; Dusza, S.W.; Haliasos, H.; Halpern, A.C.; et al. Prospective validation of dermoscopy-based open-source artificial intelligence for melanoma diagnosis (PROVE-AI study). npj Digit. Med. 2023, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Combalia, M.; Codella, N.; Rotemberg, V.; Carrera, C.; Dusza, S.; Gutman, D.; Helba, B.; Kittler, H.; Kurtansky, N.R.; Liopyris, K.; et al. Validation of artificial intelligence prediction models for skin cancer diagnosis using dermoscopy images: The 2019 International Skin Imaging Collaboration Grand Challenge. Lancet Digit. Health 2022, 4, e330–e339. [Google Scholar] [CrossRef]
- Regio Pereira, A.; Corral-Forteza, M.; Collgros, H.; El Sharouni, M.A.; Ferguson, P.M.; Scolyer, R.A.; Guitera, P. Dermoscopic features and screening strategies for the detection of small-diameter melanomas. Clin. Exp. Dermatol. 2022, 47, 932–941. (In English) [Google Scholar] [CrossRef]
- Kittler, H.; Guitera, P.; Riedl, E.; Avramidis, M.; Teban, L.; Fiebiger, M.; Weger, R.A.; Dawid, M.; Menzies, S. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch. Dermatol. 2006, 142, 1113–1119. (In English) [Google Scholar] [CrossRef] [PubMed]
- Altamura, D.; Avramidis, M.; Menzies, S.W. Assessment of the optimal interval for and sensitivity of short-term sequential digital dermoscopy monitoring for the diagnosis of melanoma. Arch. Dermatol. 2008, 144, 502–506. (In English) [Google Scholar] [CrossRef]
- Haenssle, H.A.; Korpas, B.; Hansen-Hagge, C.; Buhl, T.; Kaune, K.M.; Johnsen, S.; Rosenberger, A.; Schön, M.P.; Emmert, S. Selection of patients for long-term surveillance with digital dermoscopy by assessment of melanoma risk factors. Arch. Dermatol. 2010, 146, 257–264. (In English) [Google Scholar] [CrossRef] [PubMed]
- Tromme, I.; Devleesschauwer, B.; Beutels, P.; Richez, P.; Praet, N.; Sacré, L.; Marot, L.; Van Eeckhout, P.; Theate, I.; Baurain, J.-F.; et al. Selective Use of Sequential Digital Dermoscopy Imaging Allows a Cost Reduction in the Melanoma Detection Process: A Belgian Study of Patients with a Single or a Small Number of Atypical Nevi. PLoS ONE 2014, 9, e109339. [Google Scholar] [CrossRef] [PubMed]
- Fink, C.; Haenssle, H.A. Non-invasive tools for the diagnosis of cutaneous melanoma. Skin Res. Technol. 2017, 23, 261–271. [Google Scholar] [CrossRef]
- Welzel, J.; Schuh, S. Noninvasive diagnosis in dermatology. JDDG J. Der. Dtsch. Dermatol. Ges. 2017, 15, 999–1016. (In English) [Google Scholar] [CrossRef]
- Malvehy, J.; Hauschild, A.; Curiel-Lewandrowski, C.; Mohr, P.; Hofmann-Wellenhof, R.; Motley, R.; Berking, C.; Grossman, D.; Paoli, J.; Loquai, C.; et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: An international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br. J. Dermatol. 2014, 171, 1099–1107. (In English) [Google Scholar] [CrossRef]
- Braun, R.P.; Mangana, J.; Goldinger, S.; French, L.; Dummer, R.; Marghoob, A.A. Electrical Impedance Spectroscopy in Skin Cancer Diagnosis. Dermatol. Clin. 2017, 35, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Mohr, P.; Birgersson, U.; Berking, C.; Henderson, C.; Trefzer, U.; Kemeny, L.; Sunderkötter, C.; Dirschka, T.; Motley, R.; Frohm-Nilsson, M. Electrical impedance spectroscopy as a potential adjunct diagnostic tool for cutaneous melanoma. Skin Res. Technol. 2013, 19, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Anushree, U.; Shetty, S.; Kumar, R.; Bharati, S. Adjunctive Diagnostic Methods for Skin Cancer Detection: A Review of Electrical Impedance-Based Techniques. Bioelectromagnetics 2022, 43, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Bourgeois, M.; Ribero, S.; Barreiro, A.; Espinoza, N.; Carrera, C.; Garcia, A.; Alos, L.; Puig, S.; Malvehy, J. Reflectance Confocal Microscopy and Electrical Impedance Spectroscopy in the Early Detection of Melanoma in Changing Lesions during Long-term Follow-up of Very High-risk Patients. Acta Derm. Venereol. 2022, 102, adv00751. (In English) [Google Scholar] [CrossRef]
- Scibase. Press Release. Available online: https://scibase.com/us/scibase-receives-increased-us-medicare-fee-schedules-for-the-nevisense-melanoma-test/ (accessed on 12 January 2025).
- Smith, L.; MacNeil, S. State of the art in non-invasive imaging of cutaneous melanoma. Skin Res. Technol. 2011, 17, 257–269. (In English) [Google Scholar] [CrossRef]
- Ferris, L.K.; Harris, R.J. New Diagnostic Aides for Melanoma. Dermatol. Clin. 2012, 30, 535–545. [Google Scholar] [CrossRef]
- Rey-Barroso, L.; Burgos-Fernández, F.J.; Delpueyo, X.; Ares, M.; Royo, S.; Malvehy, J.; Puig, S.; Vilaseca, M. Visible and Extended Near-Infrared Multispectral Imaging for Skin Cancer Diagnosis. Sensors 2018, 18, 1441. (In English) [Google Scholar] [CrossRef]
- Braga, J.C.T.; Macedo, M.P.; Pinto, C.; Duprat, J.; Begnami, M.D.; Pellacani, G.; Rezze, G.G. Learning Reflectance Confocal Microscopy of Melanocytic Skin Lesions through Histopathologic Transversal Sections. PLoS ONE 2013, 8, e81205. [Google Scholar] [CrossRef]
- Marghoob, A.A.; Swindle, L.D.; Moricz, C.Z.M.; Negron, F.A.S.; Slue, B.; Halpern, A.C.; Kopf, A.W. Instruments and new technologies for the in vivo diagnosis of melanoma. J. Am. Acad. Dermatol. 2003, 49, 777–797. [Google Scholar] [CrossRef]
- Gareau, D.S.; Merlino, G.; Corless, C.; Kulesz-Martin, M.; Jacques, S.L. Noninvasive imaging of melanoma with reflectance mode confocal scanning laser microscopy in a murine model. J. Investig. Dermatol. 2007, 127, 2184–2190. (In English) [Google Scholar] [CrossRef]
- González, S.; Gilaberte-Calzada, Y. In vivo reflectance-mode confocal microscopy in clinical dermatology and cosmetology. Int. J. Cosmet. Sci. 2008, 30, 1–17. (In English) [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.; Collgros, H.; Scolyer, R.A.; Menzies, S.W.; Guitera, P. In Vivo Reflectance Confocal Microscopy for the Diagnosis of Melanoma and Melanotic Macules of the Lip. JAMA Dermatol. 2017, 153, 882–891. (In English) [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Longo, C.; Venturini, M.; Sala, R.; Pellacani, G. Reflectance confocal microscopy for in vivo skin imaging. Photochem. Photobiol. 2008, 84, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Dechent, C.; Cordova, M.; Postow, M.A.; Pulitzer, M.; Lezcano, C.; Halpern, A.C.; Rossi, A.M. Evaluation of the Response of Unresectable Primary Cutaneous Melanoma to Immunotherapy Visualized With Reflectance Confocal Microscopy: A Report of 2 Cases. JAMA Dermatol. 2019, 155, 347–352. (In English) [Google Scholar] [CrossRef]
- Ahlgrimm-Siess, V.; Laimer, M.; Rabinovitz, H.S.; Oliviero, M.; Hofmann-Wellenhof, R.; Marghoob, A.A.; Scope, A. Confocal Microscopy in Skin Cancer. Curr. Dermatol. Rep. 2018, 7, 105–118. [Google Scholar] [CrossRef]
- Soenen, A.; Vourc’h, M.; Khammari, A.; Nguyen, J.-M.; Bossard, C.; Musquer, M.D.; Vergier, B.; Dréno, B. Change in lentigo maligna score assessed by in vivo reflectance confocal microscopy after 1 month of imiquimod treatment for lentigo maligna management. J. Am. Acad. Dermatol. 2022, 86, 1042–1048. [Google Scholar] [CrossRef]
- Song, S.; Xu, J.; Wang, R.K. Long-range and wide field of view optical coherence tomography for in vivo 3D imaging of large volume object based on akinetic programmable swept source. Biomed. Opt. Express 2016, 7, 4734–4748. [Google Scholar] [CrossRef]
- Pezzini, C.; Kaleci, S.; Chester, J.; Farnetani, F.; Longo, C.; Pellacani, G. Reflectance confocal microscopy diagnostic accuracy for malignant melanoma in different clinical settings: Systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2268–2279. [Google Scholar] [CrossRef]
- Pellacani, G.; Guitera, P.; Longo, C.; Avramidis, M.; Seidenari, S.; Menzies, S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J. Investig. Dermatol. 2007, 127, 2759–2765. [Google Scholar] [CrossRef]
- Ge, X.; Chen, S.; Chen, S.; Liu, L. High Resolution Optical Coherence Tomography. J. Light. Technol. 2021, 39, 3824–3835. [Google Scholar] [CrossRef]
- Gambichler, T.; Jaedicke, V.; Terras, S. Optical coherence tomography in dermatology: Technical and clinical aspects. Arch. Dermatol. Res. 2011, 303, 457–473. (In English) [Google Scholar] [CrossRef] [PubMed]
- Avanaki, M.R.N.; Podoleanu, A.G.; Schofield, J.B.; Jones, C.; Sira, M.; Liu, Y.; Hojjat, A. Quantitative evaluation of scattering in optical coherence tomography skin images using the extended Huygens Fresnel theorem. Appl. Opt. 2013, 52, 1574–1580. (In English) [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Adabi, S.; Clayton, A.; Daveluy, S.; Mehregan, D.; Nasiriavanaki, M. Swept-Source Optical Coherence Tomography–Supervised Biopsy. Dermatol. Surg. 2018, 44, 768. (In English) [Google Scholar] [CrossRef] [PubMed]
- Adabi, S.; Turani, Z.; Fatemizadeh, E.; Clayton, A.; Nasiriavanaki, M. Optical coherence tomography technology and quality improvement methods for optical coherence tomography images of skin: A short review. Biomed. Eng. Comput. Biol. 2017, 8, 1179597217713475. [Google Scholar] [CrossRef]
- Avanaki, M.R.N.; Podoleanu, A. En-face time-domain optical coherence tomography with dynamic focus for high-resolution imaging. J. Biomed. Opt. 2017, 22, 056009. [Google Scholar] [CrossRef]
- Faiza, M.; Adabi, S.; Daoud, B.; Avanaki, M.R.N. High-resolution wavelet-fractal compressed optical coherence tomography images. Appl. Opt. 2017, 56, 1119–1123. (In English) [Google Scholar] [CrossRef]
- Adabi, S.; Fotouhi, A.; Xu, Q.; Daveluy, S.; Mehregan, D.; Podoleanu, A.; Nasiriavanaki, M. An overview of methods to mitigate artifacts in optical coherence tomography imaging of the skin. Skin Res. Technol. 2018, 24, 265–273. [Google Scholar] [CrossRef]
- Hojjatoleslami, A.; Avanaki, M.R. OCT skin image enhancement through attenuation compensation. Appl. Opt. 2012, 51, 4927–4935. [Google Scholar] [CrossRef]
- Nasiri-Avanaki, M.; Aber, A.; Hojjatoleslami, S.; Sira, M.; Schofield, J.B.; Jones, C.; Podoleanu, A.G. Dynamic focus optical coherence tomography: Feasibility for improved basal cell carcinoma investigation. In Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues X; International Society for Optics and Photonics: San Francisco, CA, USA, 2012; Volume 8225, p. 82252J. [Google Scholar]
- Avanaki, M.R.; Hojjatoleslami, A.; Sira, M.; Schofield, J.B.; Jones, C.; Podoleanu, A.G. Investigation of basal cell carcinoma using dynamic focus optical coherence tomography. Appl. Opt. 2013, 52, 2116–2124. [Google Scholar] [CrossRef]
- Herman, C.; Cetingul, M.P. Quantitative visualization and detection of skin cancer using dynamic thermal imaging. J. Vis. Exp. JoVE 2011, 51, 2679. (In English) [Google Scholar] [CrossRef]
- Turani, Z.; Fatemizadeh, E.; Blumetti, T.; Daveluy, S.; Moraes, A.F.; Chen, W.; Mehregan, D.; Andersen, P.E.; Nasiriavanaki, M. Optical Radiomic Signatures Derived from Optical Coherence Tomography Images Improve Identification of Melanoma. Cancer Res. 2019, 79, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Regeniter, P.; Bechara, F.G.; Orlikov, A.; Vasa, R.; Moussa, G.; Stücker, M.; Altmeyer, P.; Hoffmann, K.J. Characterization of benign and malignant melanocytic skin lesions using optical coherence tomography in vivo. J. Am. Acad. Dermatol. 2007, 57, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.-Y.; Shih, T.-Y.; Chang, Y.-H.; Chang, C.-H.; Kuo, W.-C. Deep Learning With Optical Coherence Tomography for Melanoma Identification and Risk Prediction. J. Biophotonics 2024, 18, e202400277. [Google Scholar] [CrossRef] [PubMed]
- Schuh, S.; Holmes, J.; Ulrich, M.; Themstrup, L.; Jemec, G.B.E.; Carvalho, N.D.; Pellacani, G.; Welzel, J. Imaging Blood Vessel Morphology in Skin: Dynamic Optical Coherence Tomography as a Novel Potential Diagnostic Tool in Dermatology. Dermatol. Ther. 2017, 7, 187–202. (In English) [Google Scholar] [CrossRef]
- Gambichler, T.; Schmid-Wendtner, M.H.; Plura, I.; Kampilafkos, P.; Stücker, M.; Berking, C.; Maier, T. A multicentre pilot study investigating high-definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 537–541. (In English) [Google Scholar] [CrossRef]
- Hinz, T.; Ehler, L.-K.; Voth, H.; Fortmeier, I.; Hoeller, T.; Hornung, T.; Schmid-Wendtner, M.-H. Assessment of Tumor Thickness in Melanocytic Skin Lesions: Comparison of Optical Coherence Tomography, 20-MHz Ultrasound and Histopathology. Dermatology 2011, 223, 161–168. (In English) [Google Scholar] [CrossRef]
- Latriglia, F.; Ogien, J.; Tavernier, C.; Fischman, S.; Suppa, M.; Perrot, J.L.; Dubois, A. Line-Field Confocal Optical Coherence Tomography (LC-OCT) for Skin Imaging in Dermatology. Life 2023, 13, 2268. (In English) [Google Scholar] [CrossRef]
- Chauvel-Picard, J.; Bérot, V.; Tognetti, L.; Orte Cano, C.; Fontaine, M.; Lenoir, C.; Pérez-Anker, J.; Puig, S.; Dubois, A.; Forestier, S.; et al. Line-field confocal optical coherence tomography as a tool for three-dimensional in vivo quantification of healthy epidermis: A pilot study. J. Biophotonics 2022, 15, e202100236. [Google Scholar] [CrossRef]
- Ogien, J.; Chauvel-Picard, J.; Bérot, V.; Tognetti, L.; Cano, C.O.; Fontaine, M.; Lenoir, C.; Pérez-Anker, J.; Puig, S.; Dubois, A. Three-dimensional microscopic quantification of in vivo healthy epidermis based on line-field confocal optical coherence tomography (LC-OCT) assisted by artificial intelligence. In Proceedings of the Photonics in Dermatology and Plastic Surgery, San Francisco, CA, USA, 22–23 January 2022; SPIE: San Francisco, CA, USA, 2022; Volume 11934, pp. 47–52. [Google Scholar]
- Ruini, C.; Schuh, S.; Sattler, E.; Welzel, J. Line-field confocal optical coherence tomography—Practical applications in dermatology and comparison with established imaging methods. Skin Res. Technol. 2021, 27, 340–352. [Google Scholar] [CrossRef]
- Perez-Anker, J.; Soglia, S.; Lenoir, C.; Albero, R.; Alos, L.; García, A.; Alejo, B.; Cinotti, E.; Orte Cano, C.; Habougit, C.; et al. Criteria for melanocytic lesions in LC-OCT. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 2005–2016. [Google Scholar] [CrossRef]
- Schuh, S.; Ruini, C.; Perwein, M.K.E.; Daxenberger, F.; Gust, C.; Sattler, E.C.; Welzel, J. Line-field confocal optical coherence tomography: A new tool for the differentiation between nevi and melanomas? Cancers 2022, 14, 1140. [Google Scholar] [CrossRef]
- Schmitt, J.; Yadlowsky, M.; Bonner, R. Subsurface imaging of living skin with optical coherence microscopy. Dermatology 1995, 191, 93–98. [Google Scholar] [CrossRef]
- Aguirre, A.D.; Fujimoto, J.G. Optical coherence microscopy. In Optical Coherence Tomography: Technology and Applications; Springer: Berlin/Heidelberg, Germany, 2008; pp. 505–542. [Google Scholar]
- Wan, Q.; Applegate, B.E. Multiphoton coherence domain molecular imaging with pump-probe optical coherence microscopy. Opt. Lett. 2010, 35, 532–534. [Google Scholar] [CrossRef]
- Levine, A.; Wang, K.; Markowitz, O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol. Clin. 2017, 35, 465–488. [Google Scholar] [CrossRef] [PubMed]
- Suppa, M.; Palmisano, G.; Tognetti, L.; Lenoir, C.; Cappilli, S.; Fontaine, M.; Cano, C.O.; Diet, G.; Perez-Anker, J.; Schuh, S. Line-field confocal optical coherence tomography in melanocytic and non-melanocytic skin tumors. Ital. J. Dermatol. Venereol. 2023, 158, 180–189. [Google Scholar] [CrossRef]
- Ni, G.; Ge, X.; Liu, L.; Zhang, J.; Wang, X.; Liu, J.; Liu, L.; Liu, Y. Towards indicating human skin state in vivo using geometry-dependent spectroscopic contrast imaging. IEEE Photonics Technol. Lett. 2020, 32, 697–700. [Google Scholar] [CrossRef]
- Ge, X.; Chen, S.; Lin, K.; Ni, G.; Bo, E.; Wang, L.; Liu, L. Deblurring, artifact-free optical coherence tomography with deconvolution-random phase modulation. Opto-Electron. Sci. 2024, 3, 230020. [Google Scholar] [CrossRef]
- Chen, S.; Ge, X.; Liu, X.; Ding, Q.; Wang, N.; Wang, X.; Chen, S.; Liang, H.; Deng, Y.; Xiong, Q. Understanding optical reflectance contrast for real-time characterization of epithelial precursor lesions. Bioeng. Transl. Med. 2019, 4, e10137. [Google Scholar] [CrossRef]
- Rey-Barroso, L.; Peña-Gutiérrez, S.; Yáñez, C.; Burgos-Fernández, F.J.; Vilaseca, M.; Royo, S. Optical technologies for the improvement of skin cancer diagnosis: A review. Sensors 2021, 21, 252. [Google Scholar] [CrossRef]
- Ge, X.; Tang, H.; Wang, X.; Liu, X.; Chen, S.; Wang, N.; Ni, G.; Yu, X.; Chen, S.; Liang, H. Geometry-dependent spectroscopic contrast in deep tissues. Iscience 2019, 19, 965–975. [Google Scholar] [CrossRef]
- Sakalauskienė, K.; Valiukevičienė, S.; Raišutis, R.; Linkevičiūtė, G. The significance of spectrophotometric image analysis for diagnosis of the melanocytic skin tumours in association with their thickness. Skin Res. Technol. 2018, 24, 692–698. [Google Scholar] [CrossRef]
- Tehrani, H.; Walls, J.; Cotton, S.; Sassoon, E.; Hall, P. Spectrophotometric intracutaneous analysis in the diagnosis of basal cell carcinoma: A pilot study. Int. J. Dermatol. 2007, 46, 371–375. (In English) [Google Scholar] [CrossRef] [PubMed]
- Dasgeb, B.; Kainerstorfer, J.; Mehregan, D.; Vreede, A.V.; Gandjbakhche, A. An introduction to primary skin imaging. Int. J. Dermatol. 2013, 52, 1319–1330. (In English) [Google Scholar] [CrossRef]
- Moncrieff, M.; Cotton, S.; Claridge, E.; Hall, P. Spectrophotometric intracutaneous analysis: A new technique for imaging pigmented skin lesions. Br. J. Dermatol. 2002, 146, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Matts, P.J.; Dykes, P.J.; Marks, R. The distribution of melanin in skin determined in vivo. Br. J. Dermatol. 2007, 156, 620–628. (In English) [Google Scholar] [CrossRef] [PubMed]
- Goessinger, E.V.; Dittrich, P.-G.; Nöcker, P.; Notni, G.; Weber, S.; Cerminara, S.; Mühleisen, B.; Navarini, A.A.; Maul, L.V. Classification of melanocytic lesions using direct illumination multispectral imaging. Sci. Rep. 2024, 14, 19036. [Google Scholar] [CrossRef]
- Bekina, A.; Diebele, I.; Rubins, U.; Zaharans, J.; Derjabo, A.; Spigulis, J. Multispectral assessment of skin malformations using a modified video-microscope. Latv. J. Phys. Tech. Sci. 2012, 49, 4–8. Available online: https://sciendo.com/article/10.2478/v10047-012-0024-2 (accessed on 12 January 2025). (In English).
- Monheit, G.; Cognetta, A.B.; Ferris, L.; Rabinovitz, H.; Gross, K.; Martini, M.; Grichnik, J.M.; Mihm, M.; Prieto, V.G.; Googe, P.; et al. The performance of MelaFind: A prospective multicenter study. Arch. Dermatol. 2011, 147, 188–194. (In English) [Google Scholar] [CrossRef]
- Fink, C.; Jaeger, C.; Jaeger, K.; Haenssle, H.A. Diagnostic performance of the MelaFind device in a real-life clinical setting. JDDG J. Der Dtsch. Dermatol. Ges. 2017, 15, 414–419. [Google Scholar] [CrossRef]
- Aloupogianni, E.; Ishikawa, M.; Kobayashi, N.; Obi, T. Hyperspectral and multispectral image processing for gross-level tumor detection in skin lesions: A systematic review. J. Biomed. Opt. 2022, 27, 060901. (In English) [Google Scholar] [CrossRef]
- GlobeNewswire News Room (Ed.) STRATA Skin Sciences Announces Licensing Agreement for Certain MelaFind Assets. STRATA Skin Sciences, Inc.: Horsham, PA, USA, 2018.
- Voit, C.; Van Akkooi, A.C.; Schäfer-Hesterberg, G.; Schoengen, A.; Kowalczyk, K.; Roewert, J.C.; Sterry, W.; Eggermont, A.M. Ultrasound morphology criteria predict metastatic disease of the sentinel nodes in patients with melanoma. J. Clin. Oncol. 2010, 28, 847–852. [Google Scholar] [CrossRef]
- Belfiore, M.P.; Reginelli, A.; Russo, A.; Russo, G.M.; Rocco, M.P.; Moscarella, E.; Ferrante, M.; Sica, A.; Grassi, R.; Cappabianca, S. Usefulness of high-frequency ultrasonography in the diagnosis of melanoma: Mini review. Front. Oncol. 2021, 11, 673026. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X. Ultrasound in Dermatology: Why, How, and When? Semin. Ultrasound CT MRI 2013, 34, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Jasaitiene, D.; Valiukeviciene, S.; Linkeviciute, G.; Raisutis, R.; Jasiuniene, E.; Kazys, R. Principles of high-frequency ultrasonography for investigation of skin pathology. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 375–382. (In English) [Google Scholar] [CrossRef]
- Polańska, A.; Dańczak-Pazdrowska, A.; Jałowska, M.; Żaba, R.; Adamski, Z. Current applications of high-frequency ultrasonography in dermatology. Postep. Dermatol. Alergol. 2017, 34, 535–542. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wortsman, X.C.; Holm, E.A.; Wulf, H.C.; Jemec, G.B.E. Real-time spatial compound ultrasound imaging of skin. Skin Res. Technol. 2004, 10, 23–31. (In English) [Google Scholar] [CrossRef]
- Wortsman, X. Chapter 25—Ultrasound Imaging in Dermatology. In Imaging in Dermatology; University of Chile: Santiago, Chile, 2016; pp. 357–374. [Google Scholar]
- Mlosek, R.K.; Malinowska, S. Ultrasound image of the skin, apparatus and imaging basics. J. Ultrason. 2013, 13, 212–221. [Google Scholar] [CrossRef]
- Catalano, O. Ultrasound of Cutaneous Melanoma: Primary Tumor Assessment and Locoregional Staging. In Textbook of Dermatologic Ultrasound; Wortsman, X., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 213–249. [Google Scholar]
- Kato, M.; Mabuchi, T.; Yamaoka, H.; Ikoma, N.; Tamiya, S.; Ozawa, A.; Taguchi, M.; Kuramochi, A.; Tsuchida, T. Diagnostic usefulness of findings in Doppler sonography for amelanotic melanoma. J. Dermatol. 2013, 40, 700–705. (In English) [Google Scholar] [CrossRef]
- Bessoud, B.; Lassau, N.; Koscielny, S.; Longvert, C.; Avril, M.-F.; Duvillard, P.; Rouffiac, V.; Leclère, J.; Roche, A. High-frequency sonography and color Doppler in the management of pigmented skin lesions. Ultrasound Med. Biol. 2003, 29, 875–879. (In English) [Google Scholar] [CrossRef]
- Wortsman, X. Common Applications of Dermatologic Sonography. J. Ultrasound Med. 2012, 31, 97–111. (In English) [Google Scholar] [CrossRef]
- Reginelli, A.; Belfiore, M.P.; Russo, A.; Turriziani, F.; Moscarella, E.; Troiani, T.; Brancaccio, G.; Ronchi, A.; Giunta, E.; Sica, A. A preliminary study for quantitative assessment with HFUS (High-Frequency Ultrasound) of nodular skin melanoma breslow thickness in adults before surgery: Interdisciplinary team experience. Curr. Radiopharm. 2020, 13, 48–55. [Google Scholar]
- Oranges, T.; Janowska, A.; Scatena, C.; Faita, F.; Lascio, N.D.; Izzetti, R.; Fidanzi, C.; Romanelli, M.; Dini, V. Ultra-High Frequency Ultrasound in Melanoma Management: A New Combined Ultrasonographic–Histopathological Approach. J. Ultrasound Med. 2023, 42, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Crisan, D.; Sannino, G.; Lupsor, M.; Badea, R.; Amzica, F. Ultrasonographic staging of cutaneous malignant tumors: An ultrasonographic depth index. Arch. Dermatol. Res. 2013, 305, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kunte, C.; Schuh, T.; Eberle, J.Y.; Baumert, J.; Konz, B.; Volkenandt, M.; Ruzicka, T.; Schmid-Wendtner, M.-H. The Use of High-Resolution Ultrasonography for Preoperative Detection of Metastases in Sentinel Lymph Nodes of Patients with Cutaneous Melanoma. Dermatol. Surg. 2009, 35, 1757–1765. (In English) [Google Scholar] [CrossRef]
- Xing, Y.; Bronstein, Y.; Ross, M.I.; Askew, R.L.; Lee, J.E.; Gershenwald, J.E.; Royal, R.; Cormier, J.N. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: A meta-analysis. J. Natl. Cancer Inst. 2011, 103, 129–142. (In English) [Google Scholar] [CrossRef]
- Ophuis, C.O.; Verhoef, C.; Grünhagen, D.J.; Siegel, P.; Schoengen, A.; Röwert-Huber, J.; Eggermont, A.M.; Voit, C.A.; van Akkooi, A. Long-term results of ultrasound guided fine needle aspiration cytology in conjunction with sentinel node biopsy support step-wise approach in melanoma. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 1509–1516. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.-H.; Dill-Müller, D. Ultrasound Technology in Dermatology. Semin. Cutan. Med. Surg. 2008, 1, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Badea, R.; Crisan, M.; Platon, M.L.; Fodor, L. Diagnosis and characterization of cutaneous tumors using combined ultrasonographic procedures (conventional and high resolution ultrasonography). Med. Ultrason. 2010, 12, 317–322. (In English) [Google Scholar]
- Reginelli, A.; Russo, A.; Berritto, D.; Patane, V.; Cantisani, C.; Grassi, R. Ultra-High-Frequency Ultrasound: A Modern Diagnostic Technique for Studying Melanoma. Ultraschall Med. 2023, 44, 360–378. (In English) [Google Scholar] [CrossRef]
- Wortsman, X.; Wortsman, J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J. Am. Acad. Dermatol. 2010, 62, 247–256. [Google Scholar] [CrossRef]
- Kleinerman, R.; Whang, T.B.; Bard, R.L.; Marmur, E.S. Ultrasound in dermatology: Principles and applications. J. Am. Acad. Dermatol. 2012, 67, 478–487. (In English) [Google Scholar] [CrossRef]
- Rajeswari, M.R.; Jain, A.; Sharma, A.; Singh, D.; Jagannathan, N.R.; Sharma, U.; Degaonkar, M.N. Evaluation of Skin Tumors by Magnetic Resonance Imaging. Lab. Investig. 2003, 83, 1279–1283. (In English) [Google Scholar] [CrossRef] [PubMed]
- Mirrashed, F.; Sharp, J.C. In vivo morphological characterisation of skin by MRI micro-imaging methods. Skin Res. Technol. 2004, 10, 149–160. (In English) [Google Scholar] [CrossRef] [PubMed]
- Foster, P.J.; Dunn, E.A.; Karl, K.E.; Snir, J.A.; Nycz, C.M.; Harvey, A.J.; Pettis, R.J. Cellular Magnetic Resonance Imaging: In Vivo Imaging of Melanoma Cells in Lymph Nodes of Mice. Neoplasia 2008, 10, 207–216. [Google Scholar] [CrossRef]
- Hatzoglou, V.; Tisnado, J.; Mehta, A.; Peck, K.K.; Daras, M.; Omuro, A.M.; Beal, K.; Holodny, A.I. Dynamic contrast-enhanced MRI perfusion for differentiating between melanoma and lung cancer brain metastases. Cancer Med. 2017, 6, 761–767. (In English) [Google Scholar] [CrossRef] [PubMed]
- Reinert, C.P.; Liang, C.; Weissinger, M.; Vogel, J.; Forschner, A.; Nikolaou, K.; la Fougère, C.; Seith, F. Whole-Body Magnetic Resonance Imaging (MRI) for Staging Melanoma Patients in Direct Comparison to Computed Tomography (CT): Results from a Prospective Positron Emission Tomography (PET)/CT and PET/MRI Study. Diagnostics 2023, 13, 1963. [Google Scholar] [CrossRef]
- Lanka, B.; Turner, M.; Orton, C.; Carrington, B.M. Cross-sectional imaging in non-melanoma skin cancer of the head and neck. Clin. Radiol. 2005, 60, 869–877. (In English) [Google Scholar] [CrossRef]
- Arnaiz, J.; Gallardo, E.; Piedra, T.; Sanz-Jimenez-Rico, J.R.; Trillo Bohajar, E.; Alonso Pena, D. Giant basal cell carcinoma on the lower leg: MRI findings. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 1167–1168. (In English) [Google Scholar] [CrossRef]
- Hong, H.; Sun, J.; Cai, W. Anatomical and molecular imaging of skin cancer. Clin. Cosmet. Investig. Dermatol. 2008, 1, 1–17. (In English) [Google Scholar]
- Jouvet, J.C.; Thomas, L.; Thomson, V.; Yanes, M.; Journe, C.; Morelec, I.; Bracoud, L.; Durupt, F.; Giammarile, F.; Berthezene, Y. Whole-body MRI with diffusion-weighted sequences compared with 18 FDG PET-CT, CT and superficial lymph node ultrasonography in the staging of advanced cutaneous melanoma: A prospective study. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 176–185. (In English) [Google Scholar] [CrossRef]
- Jansen, Y.J.L.; Willekens, I.; Seremet, T.; Awada, G.; Schwarze, J.K.; De Mey, J.; Brussaard, C.; Neyns, B. Whole-Body MRI for the Detection of Recurrence in Melanoma Patients at High Risk of Relapse. Cancers 2021, 13, 442. [Google Scholar] [CrossRef]
- McManus, D. Metastatic Melanoma; Radiopaedia: Victoria, Australia, 2023. [Google Scholar]
- Gniadecka, M.; Philipsen, P.A.; Wessel, S.; Gniadecki, R.; Wulf, H.C.; Sigurdsson, S.; Nielsen, O.F.; Christensen, D.H.; Hercogova, J.; Rossen, K. Melanoma diagnosis by Raman spectroscopy and neural networks: Structure alterations in proteins and lipids in intact cancer tissue. J. Investig. Dermatol. 2004, 122, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Colthup, N. Introduction to Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Li, Q.; Liu, C.; Wang, X.; Fan, S. Measuring the thermal conductivity of individual carbon nanotubes by the Raman shift method. Nanotechnology 2009, 20, 145702. (In English) [Google Scholar] [CrossRef] [PubMed]
- Haka, A.S.; Volynskaya, Z.I.; Gardecki, J.A.; Nazemi, J.; Shenk, R.; Wang, N.; Dasari, R.R.; Fitzmaurice, M.; Feld, M.S. Diagnosing breast cancer using Raman spectroscopy: Prospective analysis. J. Biomed. Opt. 2009, 14, 054023. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wu, X.; Fang, X.; Fu, Q.; Wang, P.; Wang, X.; Li, S.; Li, Y. Raman spectroscopy combined with deep learning for rapid detection of melanoma at the single cell level. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 122029. [Google Scholar] [CrossRef]
- Araújo, D.C.; Veloso, A.A.; de Oliveira Filho, R.S.; Giraud, M.-N.; Raniero, L.J.; Ferreira, L.M.; Bitar, R.A. Finding reduced Raman spectroscopy fingerprint of skin samples for melanoma diagnosis through machine learning. Artif. Intell. Med. 2021, 120, 102161. [Google Scholar] [CrossRef]
- Zhang, Y.; Moy, A.J.; Feng, X.; Nguyen, H.T.M.; Sebastian, K.R.; Reichenberg, J.S.; Wilke, C.O.; Markey, M.K.; Tunnell, J.W. Assessment of Raman Spectroscopy for Reducing Unnecessary Biopsies for Melanoma Screening. Molecules 2020, 25, 2852. [Google Scholar] [CrossRef]
- Lui, H.; Zhao, J.; McLean, D.; Zeng, H. Real-time Raman spectroscopy for in vivo skin cancer diagnosis. Cancer Res. 2012, 72, 2491–2500. (In English) [Google Scholar] [CrossRef]
- Franzen, L.; Windbergs, M. Applications of Raman spectroscopy in skin research—From skin physiology and diagnosis up to risk assessment and dermal drug delivery. Adv. Drug Deliv. Rev. 2015, 89, 91–104. [Google Scholar] [CrossRef]
- Santos, I.P.; van Doorn, R.; Caspers, P.J.; Bakker Schut, T.C.; Barroso, E.M.; Nijsten, T.E.C.; Noordhoek Hegt, V.; Koljenović, S.; Puppels, G.J. Improving clinical diagnosis of early-stage cutaneous melanoma based on Raman spectroscopy. Br. J. Cancer 2018, 119, 1339–1346. [Google Scholar] [CrossRef]
- Wang, H.; Osseiran, S.; Igras, V.; Nichols, A.J.; Roider, E.M.; Pruessner, J.; Tsao, H.; Fisher, D.E.; Evans, C.L. In vivo coherent Raman imaging of the melanomagenesis-associated pigment pheomelanin. Sci. Rep. 2016, 6, 37986. [Google Scholar] [CrossRef]
- Canpolat, M.; Akman-Karakas, A.; Gökhan-Ocak, G.A.; Bassorgun, I.C.; Çiftçioglu, A.M.; Alpsoy, E. Diagnosis and demarcation of skin malignancy using elastic light single-scattering spectroscopy: A pilot study. Dermatol. Surg. 2012, 38, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Canpolat, M.; Gökhan, A.G.; Çiftçioğlu, M.A.; Erin, N. Differentiation of melanoma from non-cancerous tissue in an animal model using elastic light single-scattering spectroscopy. Technol. Cancer Res. Treat. 2008, 7, 235–240. [Google Scholar] [CrossRef]
- Hartman, R.; Uwe Paasch, M.; Kelly Tepedino, M.; Max Fung, M.; McNiff, J.; Grant-Kels, J. Validation of a Handheld Elastic-Scattering Spectroscopy Device on Lesions Concerning for Melanoma. Available online: https://www.dermasensor.com/wp-content/uploads/80-0013.v1-DA3-v2.pdf (accessed on 12 January 2025).
- Hartman, R.I.; Trepanowski, N.; Chang, M.S.; Tepedino, K.; Gianacas, C.; McNiff, J.M.; Fung, M.; Braghiroli, N.F.; Grant-Kels, J.M. Multicenter prospective blinded melanoma detection study with a handheld elastic scattering spectroscopy device. JAAD Int. 2024, 15, 24–31. [Google Scholar] [CrossRef]
- Shurrab, K.; Kochaji, N.; Bachir, W. Elastic scattering spectroscopy for monitoring skin cancer transformation and therapy in the near infrared window. Lasers Med. Sci. 2020, 35, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, A.C.; Pasciak, A.S.; Bradley, Y.C. The Role of Positron Emission Tomography/Computed Tomography in Cutaneous Melanoma. Imaging Dermatol. 2016, 455–466. (In English) [Google Scholar] [CrossRef]
- Holtkamp, L.H.; Chakera, A.H.; Fung, S.; Stretch, J.R.; Saw, R.P.; Lee, K.; Ch’ng, S.; Gonzalez, M.; Thompson, J.F.; Emmett, L. Staging 18F-FDG PET/CT influences the treatment plan in melanoma patients with satellite or in-transit metastases. Melanoma Res. 2020, 30, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Sachpekidis, C.; Hassel, J.C.; Kopp-Schneider, A.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Quantitative Dynamic 18F-FDG PET/CT in Survival Prediction of Metastatic Melanoma under PD-1 Inhibitors. Cancers 2021, 13, 1019. [Google Scholar] [CrossRef]
- Ayati, N.; Sadeghi, R.; Kiamanesh, Z.; Lee, S.T.; Zakavi, S.R.; Scott, A.M. The value of 18 F-FDG PET/CT for predicting or monitoring immunotherapy response in patients with metastatic melanoma: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 428–448. [Google Scholar] [CrossRef]
- Annovazzi, A.; Vari, S.; Giannarelli, D.; Pasqualoni, R.; Sciuto, R.; Carpano, S.; Cognetti, F.; Ferraresi, V. Comparison of 18F-FDG PET/CT Criteria for the Prediction of Therapy Response and Clinical Outcome in Patients with Metastatic Melanoma Treated With Ipilimumab and PD-1 Inhibitors. Clin. Nucl. Med. 2020, 45, 187–194. [Google Scholar] [CrossRef]
- Moncrieff, M.; Pywell, S.; Snelling, A.; Gray, M.; Newman, D.; Beadsmoore, C.; Pawaroo, D.; Heaton, M. Effectiveness of SPECT/CT Imaging for Sentinel Node Biopsy Staging of Primary Cutaneous Melanoma and Patient Outcomes. Ann. Surg. Oncol. 2022, 29, 767–775. [Google Scholar] [CrossRef]
- Quartuccio, N.; Siracusa, M.; Pappalardo, M.; Arnone, A.; Arnone, G. Sentinel node identification in melanoma: Current clinical impact, new emerging spect radiotracers and technological advancements. An update of the last decade. Curr. Radiopharm. 2020, 13, 32–41. [Google Scholar] [CrossRef]
- Rietbergen, D.; Arias-Bouda, L.P.; van der Hage, J.; Olmos, R.V. Does 99mTc-tilmanocept, as next generation radiotracer, meet with the requirements for improved sentinel node imaging? Rev. Española Med. Nucl. E Imagen Mol. 2021, 40, 275–280. [Google Scholar] [CrossRef]
- Stathaki, M.I.; Kapsoritakis, N.; Michelakis, D.; Anagnostopoulou, E.; Bourogianni, O.; Tsaroucha, A.; Papadaki, E.; de Bree, E.; Koukouraki, S. The impact of sentinel lymph node mapping with hybrid single photon emission computed tomography/computed tomography in patients with melanoma. Comparison to planar radioisotopic lymphoscintigraphy. Melanoma Res. 2023, 33, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Quartuccio, N.; Garau, L.M.; Arnone, A.; Pappalardo, M.; Rubello, D.; Arnone, G.; Manca, G. Comparison of 99mTc-labeled colloid SPECT/CT and planar lymphoscintigraphy in sentinel lymph node detection in patients with melanoma: A meta-analysis. J. Clin. Med. 2020, 9, 1680. [Google Scholar] [CrossRef] [PubMed]
- Uren, R.F.; Nieweg, O.E.; Thompson, J.F. Lymphoscintigraphy in Patients with Melanoma. In Cutaneous Melanoma; Balch, C., Gershenwald, J., Thompson, J., Atkins, M., Kirkwood, J., Kefford, R., Sober, A., Halpern, A., Garbe, C., Scolyer, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–33. [Google Scholar]
- Uren, R.; Howman-Giles, R.; Thompson, J.; Shaw, H.; Quinn, M.; O’Brien, C.; McCarthy, W. Lymphoscintigraphy to identify sentinel lymph nodes in patients with melanoma. Melanoma Res. 1994, 4, 395–399. [Google Scholar] [CrossRef]
- Moncrieff, M.D.; Thompson, J.F. Evaluating and embracing modern imaging technology to guide sentinel node biopsy for melanoma. Ann. Surg. Oncol. 2022, 29, 5350–5352. [Google Scholar] [CrossRef]
- March, J.; Hand, M.; Truong, A.; Grossman, D. Practical application of new technologies for melanoma diagnosis: Part II. Mol. approaches. J. Am. Acad. Dermatol. 2015, 72, 943–958. (In English) [Google Scholar] [CrossRef]
- Thomsen, I.M.N.; Heerfordt, I.M.; Karmisholt, K.E.; Mogensen, M. Detection of cutaneous malignant melanoma by tape stripping of pigmented skin lesions—A systematic review. Skin Res. Technol. 2023, 29, e13286. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.K.; Gerami, P.; Skelsey, M.K.; Peck, G.; Hren, C.; Gorman, C.; Frumento, T.; Siegel, D.M. Real-world performance and utility of a noninvasive gene expression assay to evaluate melanoma risk in pigmented lesions. Melanoma Res. 2018, 28, 478. (In English) [Google Scholar] [CrossRef]
- Lee, N.; Scope, A.; Rabinovitz, H. Assessing Skin Cancer Using Epidermal Genetic Information Retrieved by Adhesive Patch Skin Surface Sampling. Dermatol. Clin. 2017, 35, 521–524. (In English) [Google Scholar] [CrossRef]
- Gerami, P.; Yao, Z.; Polsky, D.; Jansen, B.; Busam, K.; Ho, J.; Martini, M.; Ferris, L.K. Development and validation of a noninvasive 2-gene molecular assay for cutaneous melanoma. J. Am. Acad. Dermatol. 2017, 76, 114–120.e112. [Google Scholar] [CrossRef] [PubMed]
- Gerami, P.; Cook, R.W.; Russell, M.C.; Wilkinson, J.; Amaria, R.N.; Gonzalez, R.; Lyle, S.; Jackson, G.L.; Greisinger, A.J.; Johnson, C.E.; et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients undergoing sentinel lymph node biopsy. J. Am. Acad. Dermatol. 2015, 72, 780–785.e783. (In English) [Google Scholar] [CrossRef]
- Ferris, L.K.; Farberg, A.S.; Middlebrook, B.; Johnson, C.E.; Lassen, N.; Oelschlager, K.M.; Maetzold, D.J.; Cook, R.W.; Rigel, D.S.; Gerami, P. Identification of high-risk cutaneous melanoma tumors is improved when combining the online American Joint Committee on Cancer Individualized Melanoma Patient Outcome Prediction Tool with a 31-gene expression profile–based classification. J. Am. Acad. Dermatol. 2017, 76, 818–825.e813. [Google Scholar] [CrossRef] [PubMed]
- Scaini, M.C.; Catoni, C.; Poggiana, C.; Pigozzo, J.; Piccin, L.; Leone, K.; Scarabello, I.; Facchinetti, A.; Menin, C.; Elefanti, L.; et al. A multiparameter liquid biopsy approach allows to track melanoma dynamics and identify early treatment resistance. npj Precis. Oncol. 2024, 8, 78. [Google Scholar] [CrossRef]
- Gerami, P.; Alsobrook, J.P.; Palmer, T.J.; Robin, H.S. Development of a novel noninvasive adhesive patch test for the evaluation of pigmented lesions of the skin. J. Am. Acad. Dermatol. 2014, 71, 237–244. (In English) [Google Scholar] [CrossRef]
- Kashani-Sabet, M.; Leachman, S.A.; Stein, J.A.; Arbiser, J.L.; Berry, E.G.; Celebi, J.T.; Curiel-Lewandrowski, C.; Ferris, L.K.; Grant-Kels, J.M.; Grossman, D. Early detection and prognostic assessment of cutaneous melanoma: Consensus on optimal practice and the role of gene expression profile testing. JAMA Dermatol. 2023, 159, 545–553. [Google Scholar] [CrossRef]
- Gastman, B.R.; Gerami, P.; Kurley, S.J.; Cook, R.W.; Leachman, S.; Vetto, J.T. Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J. Am. Acad. Dermatol. 2019, 80, 149–157.e144. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Eroglu, Z.; Infante, J.; Patel, S.; Daud, A.; Johnson, D.B.; Gonzalez, R.; Kefford, R.; Hamid, O.; Schuchter, L. Long-term outcomes in patients with BRAF V600–mutant metastatic melanoma who received dabrafenib combined with trametinib. J. Clin. Oncol. 2018, 36, 667–673. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; del Marmol, V.; Dréno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer 2022, 170, 236–255. [Google Scholar] [CrossRef]
- The_Skincare_Network. Electrical Impedance Spectroscopy. Available online: https://www.skincarenetwork.co.uk/skin-cancer-treatment/electrical-impedance-spectroscopy/ (accessed on 12 January 2025).
- Murali, R.; Thompson, J.F.; Uren, R.F.; Scolyer, R.A. Fine-needle biopsy of metastatic melanoma: Clinical use and new applications. Lancet Oncol. 2010, 11, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Kim, S.; Wang, J.; Yun, M.; Cho, A. Evaluation of (18)F-FDG PET/CT Parameters for Detection of Lymph Node Metastasis in Cutaneous Melanoma. Nucl. Med. Mol. Imaging 2018, 52, 39–45. (In English) [Google Scholar] [CrossRef]
- Veeramani, N.; Jayaraman, P. A promising AI based super resolution image reconstruction technique for early diagnosis of skin cancer. Sci. Rep. 2025, 15, 5084. [Google Scholar] [CrossRef]
- Waqar, S.; George, S.; Jean-Baptiste, W.; Yusuf Ali, A.; Inyang, B.; Koshy, F.S.; George, K.; Poudel, P.; Chalasani, R.; Goonathilake, M.R.; et al. Recognizing Histopathological Simulators of Melanoma to Avoid Misdiagnosis. Cureus 2022, 14, e26127. (In English) [Google Scholar] [CrossRef]
- Rudie, J.D.; Gleason, T.; Barkovich, M.J.; Wilson, D.M.; Shankaranarayanan, A.; Zhang, T.; Wang, L.; Gong, E.; Zaharchuk, G.; Villanueva-Meyer, J.E. Clinical assessment of deep learning–based super-resolution for 3D volumetric brain MRI. Radiol. Artif. Intell. 2022, 4, e210059. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R. Redefining Radiology: A Review of Artificial Intelligence Integration in Medical Imaging. Diagnostics 2023, 13, 2760. (In English) [Google Scholar] [CrossRef] [PubMed]
- Popescu, D.; El-Khatib, M.; El-Khatib, H.; Ichim, L. New trends in melanoma detection using neural networks: A systematic review. Sensors 2022, 22, 496. [Google Scholar] [CrossRef]
- Gajera, H.K.; Nayak, D.R.; Zaveri, M.A. A comprehensive analysis of dermoscopy images for melanoma detection via deep CNN features. Biomed. Signal Process. Control 2023, 79, 104186. [Google Scholar] [CrossRef]
- Cirrincione, G.; Cannata, S.; Cicceri, G.; Prinzi, F.; Currieri, T.; Lovino, M.; Militello, C.; Pasero, E.; Vitabile, S. Transformer-based approach to melanoma detection. Sensors 2023, 23, 5677. [Google Scholar] [CrossRef]
- Hameed, M.; Zameer, A.; Raja, M.A.Z. A Comprehensive Systematic Review: Advancements in Skin Cancer Classification and Segmentation Using the ISIC Dataset. CMES-Comput. Model. Eng. Sci. 2024, 140, 1–35. [Google Scholar] [CrossRef]
- Magalhaes, C.; Mendes, J.; Vardasca, R. Systematic Review of Deep Learning Techniques in Skin Cancer Detection. BioMedInformatics 2024, 4, 2251–2270. [Google Scholar] [CrossRef]
- Dildar, M.; Akram, S.; Irfan, M.; Khan, H.U.; Ramzan, M.; Mahmood, A.R.; Alsaiari, S.A.; Saeed, A.H.M.; Alraddadi, M.O.; Mahnashi, M.H. Skin Cancer Detection: A Review Using Deep Learning Techniques. Int. J. Environ. Res. Public Health 2021, 18, 5479. [Google Scholar] [CrossRef]
- Grant, S.R.; Andrew, T.W.; Alvarez, E.V.; Huss, W.J.; Paragh, G. Diagnostic and Prognostic Deep Learning Applications for Histological Assessment of Cutaneous Melanoma. Cancers 2022, 14, 6231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Primiero, C.A.; Kulkarni, V.; Soyer, H.P.; Betz-Stablein, B. Artificial intelligence for the classification of pigmented skin lesions in populations with skin of color: A systematic review. Dermatology 2023, 239, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Durot, C.; Mulé, S.; Soyer, P.; Marchal, A.; Grange, F.; Hoeffel, C. Metastatic melanoma: Pretreatment contrast-enhanced CT texture parameters as predictive biomarkers of survival in patients treated with pembrolizumab. Eur. Radiol. 2019, 29, 3183–3191. [Google Scholar] [CrossRef]
- Guerrisi, A.; Russillo, M.; Loi, E.; Ganeshan, B.; Ungania, S.; Desiderio, F.; Bruzzaniti, V.; Falcone, I.; Renna, D.; Ferraresi, V. Exploring CT texture parameters as predictive and response imaging biomarkers of survival in patients with metastatic melanoma treated with PD-1 inhibitor nivolumab: A pilot study using a delta-radiomics approach. Front. Oncol. 2021, 11, 704607. [Google Scholar] [CrossRef]
- Gill, A.B.; Rundo, L.; Wan, J.C.M.; Lau, D.; Zawaideh, J.P.; Woitek, R.; Zaccagna, F.; Beer, L.; Gale, D.; Sala, E.; et al. Correlating Radiomic Features of Heterogeneity on CT with Circulating Tumor DNA in Metastatic Melanoma. Cancers 2020, 12, 3493. [Google Scholar] [CrossRef]
- Tang, P.; Yan, X.; Nan, Y.; Hu, X.; Menze, B.H.; Krammer, S.; Lasser, T. Joint-individual fusion structure with fusion attention module for multi-modal skin cancer classification. Pattern Recognit. 2024, 154, 110604. [Google Scholar] [CrossRef]
- Chen, Q.; Li, M.; Chen, C.; Zhou, P.; Lv, X.; Chen, C. MDFNet: Application of multimodal fusion method based on skin image and clinical data to skin cancer classification. J. Cancer Res. Clin. Oncol. 2023, 149, 3287–3299. [Google Scholar] [CrossRef]
- Rey-Barroso, L.; Vilaseca, M.; Royo, S.; Díaz-Doutón, F.; Lihacova, I.; Bondarenko, A.; Burgos-Fernández, F.J. Training State-of-the-Art Deep Learning Algorithms with Visible and Extended Near-Infrared Multispectral Images of Skin Lesions for the Improvement of Skin Cancer Diagnosis. Diagnostics 2025, 15, 355. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Hsiao, Y.-P.; Karmakar, R.; Mukundan, A.; Chaudhary, P.; Hsieh, S.-C.; Wang, H.-C. A Review of Recent Advances in Computer-Aided Detection Methods Using Hyperspectral Imaging Engineering to Detect Skin Cancer. Cancers 2023, 15, 5634. [Google Scholar] [CrossRef] [PubMed]
- Bsharat, S.M.; Abouelnour, S.; Ahmed, R.; Elkhatib, M.; Gaber, S.; Shehieb, W.; Arshad, K.; Assaleh, K. RGB-to-hyperspectral conversion for accessible melanoma detection: A CNN-based approach. J. Intell. Syst. 2024, 33, 20230271. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.V. Chapter 24—Photoacoustic Tomography in the Diagnosis of Melanoma. In Imaging in Dermatology; Hamblin, M.R., Avci, P., Gupta, G.K., Hamblin, M.R., Avci, P., Gupta, G.K., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 341–356. [Google Scholar]
- Ku, G.; Wang, L.V. Deeply penetrating photoacoustic tomography in biological tissues enhanced with an optical contrast agent. Opt. Lett. 2005, 30, 507–509. (In English) [Google Scholar] [CrossRef]
- Song, K.H.; Stein, E.W.; Margenthaler, J.A.; Wang, L.V. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. J. Biomed. Opt. 2008, 13, 054033. [Google Scholar] [CrossRef]
- Yao, J.; Maslov, K.I.; Hu, S.; Wang, L.V. Evans blue dye-enhanced capillary-resolution photoacoustic microscopy in vivo. J. Biomed. Opt. 2009, 14, 054049. [Google Scholar] [CrossRef]
- Kim, C.; Favazza, C.; Wang, L.V. In Vivo Photoacoustic Tomography of Chemicals: High-Resolution Functional and Molecular Optical Imaging at New Depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, E.C.; Chen, J.; Song, K.H.; Au, L.; Favazza, C.; Zhang, Q.; Cobley, C.M.; Gao, F.; Xia, Y. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano 2010, 4, 4559–4564. [Google Scholar] [CrossRef]
- Cai, X.; Li, W.; Kim, C.-H.; Yuan, Y.; Wang, L.V.; Xia, Y. In Vivo Quantitative Evaluation of the Transport Kinetics of Gold Nanocages in a Lymphatic System by Noninvasive Photoacoustic Tomography. ACS Nano 2011, 5, 9658–9667. [Google Scholar] [CrossRef]
- Wang, X.; Pang, Y.; Ku, G.; Xie, X.; Stoica, G.; Wang, L.V. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003, 21, 803–806. (In English) [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848. [Google Scholar] [CrossRef]
- Oh, J.-T.; Li, M.-L.; Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Three-dimensional imaging of skin melanoma in vivo by dual-wavelength photoacoustic microscopy. J. Biomed. Opt. 2006, 11, 034032. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Weight, R.M.; Viator, J.A.; Dale, P.S.; Caldwell, C.W.; Lisle, A.E. Photoacoustic detection of metastatic melanoma cells in the human circulatory system. Opt. Lett. 2006, 31, 2998–3000. [Google Scholar] [CrossRef]
- Zharov, V.P.; Galanzha, E.I.; Shashkov, E.V.; Khlebtsov, N.G.; Tuchin, V.V. In vivo photoacoustic flow cytometry for monitoring of circulating single cancer cells and contrast agents. Opt. Lett. 2006, 31, 3623–3625. [Google Scholar] [CrossRef]
- Holan, S.H.; Viator, J.A. Automated wavelet denoising of photoacoustic signals for circulating melanoma cell detection and burn image reconstruction. Phys. Med. Biol. 2008, 53, N227. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, M.; Song, K.H.; Swierczewska, M.; Green, D.; Sitharaman, B.; Wang, L.V. In vivo carbon nanotube-enhanced non-invasive photoacoustic mapping of the sentinel lymph node. Phys. Med. Biol. 2009, 54, 3291. (In English) [Google Scholar] [CrossRef]
- Song, K.H.; Kim, C.; Cobley, C.M.; Xia, Y.; Wang, L.V. Near-Infrared Gold Nanocages as a New Class of Tracers for Photoacoustic Sentinel Lymph Node Mapping on a Rat Model. Nano Lett. 2009, 9, 183–188. [Google Scholar] [CrossRef]
- Kim, C.; Erpelding, T.N.; Maslov, K.I.; Jankovic, L.; Akers, W.J.; Song, L.; Achilefu, S.; Margenthaler, J.A.; Pashley, M.D.; Wang, L.V.J.J.o.b.o. Handheld array-based photoacoustic probe for guiding needle biopsy of sentinel lymph nodes. J. Biomed. Opt. 2010, 15, 046010. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Cai, X.; Yalaz, C.; Senpan, A.; Omanakuttan, K.; Wickline, S.A.; Wang, L.V.; Lanza, G.M. Photoacoustic Sentinel Lymph Node Imaging with Self-Assembled Copper Neodecanoate Nanoparticles. ACS Nano 2012, 6, 1260–1267. [Google Scholar] [CrossRef]
- Zhou, Y.; Tripathi, S.V.; Rosman, I.; Ma, J.; Hai, P.; Linette, G.P.; Council, M.L.; Fields, R.C.; Wang, L.V.; Cornelius, L.A. Noninvasive determination of melanoma depth using a handheld photoacoustic probe. J. Investig. Dermatol. 2017, 137, 1370–1372. [Google Scholar] [CrossRef]
- Stoffels, I.; Jansen, P.; Petri, M.; Goerdt, L.; Brinker, T.J.; Griewank, K.G.; Poeppel, T.D.; Schadendorf, D.; Klode, J. Assessment of nonradioactive multispectral optoacoustic tomographic imaging with conventional lymphoscintigraphic imaging for sentinel lymph node biopsy in melanoma. JAMA Netw. Open 2019, 2, e199020. [Google Scholar] [CrossRef]
- Vonk, J.; Kukačka, J.; Steinkamp, P.; De Wit, J.; Voskuil, F.; Hooghiemstra, W.; Bader, M.; Jüstel, D.; Ntziachristos, V.; Van Dam, G. Multispectral optoacoustic tomography for in vivo detection of lymph node metastases in oral cancer patients using an EGFR-targeted contrast agent and intrinsic tissue contrast: A proof-of-concept study. Photoacoustics 2022, 26, 100362. [Google Scholar] [CrossRef] [PubMed]
- Grootendorst, D.J.; Jose, J.; Wouters, M.W.; van Boven, H.; Van der Hage, J.; Van Leeuwen, T.G.; Steenbergen, W.; Manohar, S.; Ruers, T.J. First experiences of photoacoustic imaging for detection of melanoma metastases in resected human lymph nodes. Lasers Surg. Med. 2012, 44, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Langhout, G.C.; Grootendorst, D.J.; Nieweg, O.E.; Wouters, M.W.; van der Hage, J.A.; Jose, J.; van Boven, H.; Steenbergen, W.; Manohar, S.; Ruers, T.J. Detection of melanoma metastases in resected human lymph nodes by noninvasive multispectral photoacoustic imaging. Int. J. Biomed. Imaging 2014, 2014, 163652. [Google Scholar] [CrossRef]
- Martell, M.T.; Haven, N.J.M.; Cikaluk, B.D.; Restall, B.S.; McAlister, E.A.; Mittal, R.; Adam, B.A.; Giannakopoulos, N.; Peiris, L.; Silverman, S.; et al. Deep learning-enabled realistic virtual histology with ultraviolet photoacoustic remote sensing microscopy. Nat. Commun. 2023, 14, 5967. [Google Scholar] [CrossRef]
- Lassau, N.; Lamuraglia, M.; Koscielny, S.; Spatz, A.; Roche, A.; Leclere, J.; Avril, M.-F. Prognostic value of angiogenesis evaluated with high-frequency and colour Doppler sonography for preoperative assessment of primary cutaneous melanomas: Correlation with recurrence after a 5 year follow-up period. Cancer Imaging 2006, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.I.; Menyaev, Y.A.; Yadem, A.C.; Sarimollaoglu, M.; Juratli, M.A.; Nedosekin, D.A.; Foster, S.R.; Jamshidi-Parsian, A.; Siegel, E.R.; Makhoul, I. In vivo liquid biopsy using Cytophone platform for photoacoustic detection of circulating tumor cells in patients with melanoma. Sci. Transl. Med. 2019, 11, eaat5857. [Google Scholar] [CrossRef]
- Hai, P.; Qu, Y.; Li, Y.; Zhu, L.; Shmuylovich, L.; Cornelius, L.A.; Wang, L.V. Label-free high-throughput photoacoustic tomography of suspected circulating melanoma tumor cells in patients in vivo. J. Biomed. Opt. 2020, 25, 036002. [Google Scholar] [CrossRef]
- Viator, J.A.; Hazur, M.; Sajewski, A.; Tarhini, A.; Sanders, M.E.; Edgar, R.H. Photoacoustic detection of circulating melanoma cells in late stage patients. J. Innov. Opt. Health Sci. 2020, 13, 2050023. [Google Scholar] [CrossRef]
- Gutierrez-Juarez, G.; Gupta, S.; Weight, R.M.; Polo-Parada, L.; Papagiorgio, C.; Bunch, J.; Viator, J. Optical photoacoustic detection of circulating melanoma cells in vitro. Int. J. Thermophys. 2010, 31, 784–792. [Google Scholar] [CrossRef]
- Benjamin, R.E.; James, T.; Krystal, L.; Hager, G.; Kevan, B.; Deepak, D.; Muba, T.; John, M.; Parsin Haji, R. Exploration of photoacoustic remote sensing virtual histology in skin malignancies. In Proceedings of the Photonics in Dermatology and Plastic Surgery, San Francisco, CA, USA, 28–29 January 2023; SPIE: Bellingham, WA, USA, 2023; Volume PC12352, p. PC1235201. [Google Scholar]
- Ecclestone, B.; Bell, K.; Boktor, M.; Pekar, V.; Taher, M.; Dinakaran, D.; Mackey, J.; Haji Reza, P. Label-free histological imaging of skin cancers with photoacoustic remote sensing virtual histology. In Proceedings of the Photonics in Dermatology and Plastic Surgery, San Francisco, CA, USA, 22–23 January 2022; SPIE: Bellingham, WA, USA, 2022. [Google Scholar]
- Kratkiewicz, K.; Manwar, R.; Rajabi-Estarabadi, A.; Fakhoury, J.; Meiliute, J.; Daveluy, S.; Mehregan, D.; Avanaki, K.M. Photoacoustic/Ultrasound/Optical Coherence Tomography Evaluation of Melanoma Lesion and Healthy Skin in a Swine Model. Sensors 2019, 19, 2815. [Google Scholar] [CrossRef]
- Abbasi, S.; Le, M.; Sonier, B.; Dinakaran, D.; Bigras, G.; Bell, K.; Mackey, J.R.; Reza, P.H. All-optical reflection-mode microscopic histology of unstained human tissues. Sci. Rep. 2019, 9, 13392. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, A.; Concannon, E.; Dorairaj, J.J.; Shaharan, S.; McGrath, J.; Jose, J.; Kelly, J.L.; Leahy, M.J. Preoperative measurement of cutaneous melanoma and nevi thickness with photoacoustic imaging. J. Med. Imaging 2018, 5, 015004. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, G.; Zhu, L.; Li, C.; Cornelius, L.A.; Wang, L.V. Handheld photoacoustic probe to detect both melanoma depth and volume at high speed in vivo. J. Biophotonics 2015, 8, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Haji Reza, P.; Shi, W.; Bell, K.; Paproski, R.J.; Zemp, R.J. Non-interferometric photoacoustic remote sensing microscopy. Light. Sci. Appl. 2017, 6, e16278. [Google Scholar] [CrossRef]
- Bell, K.; Abbasi, S.; Dinakaran, D.; Taher, M.; Bigras, G.; van Landeghem, F.K.; Mackey, J.R.; Haji Reza, P. Reflection-mode virtual histology using photoacoustic remote sensing microscopy. Sci. Rep. 2020, 10, 19121. [Google Scholar] [CrossRef]
- Reza, P.H.; Bell, K.; Shi, W.; Shapiro, J.; Zemp, R.J. Deep non-contact photoacoustic initial pressure imaging. Optica 2018, 5, 814–820. [Google Scholar] [CrossRef]
- Hosking, A.M.; Coakley, B.J.; Chang, D.; Talebi-Liasi, F.; Lish, S.; Lee, S.W.; Zong, A.M.; Moore, I.; Browning, J.; Jacques, S.L. Hyperspectral imaging in automated digital dermoscopy screening for melanoma. Lasers Surg. Med. 2019, 51, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Vasefi, F.; MacKinnon, N.; Farkas, D.L. Chapter 16—Hyperspectral and Multispectral Imaging in Dermatology. In Imaging in Dermatology; Hamblin, M.R., Avci, P., Gupta, G.K., Hamblin, M.R., Avci, P., Gupta, G.K., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 187–201. [Google Scholar]
- Vasefi, F.; MacKinnon, N.; Farkas, D.L. Toward in vivo diagnosis of skin cancer using multimode imaging dermoscopy: (II) molecular mapping of highly pigmented lesions. In Proceedings of the Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues XII, San Francisco, CA, USA, 3–6 February 2014; Volume 8947, p. 89470J. [Google Scholar] [CrossRef]
- Vasefi, F.; MacKinnon, N.; Saager, R.; Kelly, K.M.; Maly, T.; Booth, N.; Durkin, A.J.; Farkas, D.L. Separating melanin from hemodynamics in nevi using multimode hyperspectral dermoscopy and spatial frequency domain spectroscopy. J. Biomed. Opt. 2016, 21, 114001. (In English) [Google Scholar] [CrossRef]
- Paoli, J.; Pölönen, I.; Salmivuori, M.; Räsänen, J.; Zaar, O.; Polesie, S.; Koskenmies, S.; Pitkänen, S.; Övermark, M.; Isoherranen, K.; et al. Hyperspectral Imaging for Non-invasive Diagnostics of Melanocytic Lesions. Acta Derm. Venereol. 2022, 102, adv00815. (In English) [Google Scholar] [CrossRef]
- Tian, C.; Xu, Y.; Zhang, Y.; Zhang, Z.; An, H.; Liu, Y.; Chen, Y.; Zhao, H.; Zhang, Z.; Zhao, Q. Combining hyperspectral imaging techniques with deep learning to aid in early pathological diagnosis of melanoma. Photodiagnosis Photodyn. Ther. 2023, 43, 103708. [Google Scholar] [CrossRef]
- Ng, P.C.; Chi, Z.; Verdie, Y.; Lu, J.; Plataniotis, K.N. Hyper-Skin: A Hyperspectral Dataset for Reconstructing Facial Skin-Spectra from RGB Images. Adv. Neural Inf. Process. Syst. 2024, 36, 24158–24170. [Google Scholar]
- Lin, T.-L.; Lu, C.-T.; Karmakar, R.; Nampalley, K.; Mukundan, A.; Hsiao, Y.-P.; Hsieh, S.-C.; Wang, H.-C. Assessing the efficacy of the spectrum-aided vision enhancer (SAVE) to detect acral lentiginous melanoma, melanoma in situ, nodular melanoma, and superficial spreading melanoma. Diagnostics 2024, 14, 1672. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, N.; Vasefi, F.; Booth, N.; Farkas, D.L. Melanoma detection using smartphone and multimode hyperspectral imaging. In Proceedings of the Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues IX, San Francisco, CA, USA, 15–17 February 2016; pp. 143–150. [Google Scholar]
- Anbar, M. Clinical thermal imaging today. IEEE Eng. Med. Biol. Mag. 1998, 17, 25–33. [Google Scholar] [CrossRef]
- Jones, B.F. A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Trans. Med. Imaging 1998, 17, 1019–1027. [Google Scholar] [CrossRef]
- Çetingül, M.P.; Herman, C. A heat transfer model of skin tissue for the detection of lesions: Sensitivity analysis. Phys. Med. Biol. 2010, 55, 5933. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Manza, R.; Shaikh, S.; Gawali, B.; Yannawar, P.; Shaikh, S. Diagnosis of Melanoma Using Thermography: A Review. In Proceedings of the International Conference on Applications of Machine Intelligence and Data Analytics (ICAMIDA 2022), Aurangabad, India, 22–24 December 2022; p. 466. [Google Scholar]
- Shada, A.L.; Dengel, L.T.; Petroni, G.R.; Smolkin, M.E.; Acton, S.; Slingluff, C.L. Infrared thermography of cutaneous melanoma metastases. J. Surg. Res. 2013, 182, e9–e14. (In English) [Google Scholar] [CrossRef]
- Godoy, S.E.; Hayat, M.M.; Ramirez, D.A.; Myers, S.A.; Padilla, R.S.; Krishna, S. Detection theory for accurate and non-invasive skin cancer diagnosis using dynamic thermal imaging. Biomed. Opt. Express 2017, 8, 2301–2323. (In English) [Google Scholar] [CrossRef]
- Díaz, S.; Krohmer, T.; Moreira, A.; Godoy, S.E.; Figueroa, M. An instrument for accurate and non-invasive screening of skin cancer based on multimodal imaging. IEEE Access 2019, 7, 176646–176657. [Google Scholar] [CrossRef]
- Okabe, T.; Fujimura, T.; Okajima, J.; Kambayashi, Y.; Aiba, S.; Maruyama, S. First-in-human clinical study of novel technique to diagnose malignant melanoma via thermal conductivity measurements. Sci. Rep. 2019, 9, 3853. (In English) [Google Scholar] [CrossRef]
- Woodward, R.M.; Cole, B.E.; Wallace, V.P.; Pye, R.J.; Arnone, D.D.; Linfield, E.H.; Pepper, M. Terahertz pulse imaging in reflection geometry of human skin cancer and skin tissue. Phys. Med. Biol. 2002, 47, 3853–3863. (In English) [Google Scholar] [CrossRef] [PubMed]
- Vilagosh, Z.; Lajevardipour, A.; Wood, A.W. Computational phantom study of frozen melanoma imaging at 0.45 terahertz. Bioelectromagnetics 2019, 40, 118–127. (In English) [Google Scholar] [CrossRef] [PubMed]
- Sim, Y.C.; Ahn, K.-M.; Park, J.Y.; Park, C.-S.; Son, J.-H. Temperature-dependent terahertz imaging of excised oral malignant melanoma. IEEE J. Biomed. Health Info. 2013, 17, 779–784. (In English) [Google Scholar] [CrossRef]
- Wallace, V.P.; Fitzgerald, A.J.; Shankar, S.; Flanagan, N.; Pye, R.; Cluff, J.; Arnone, D.D. Terahertz pulsed imaging of basal cell carcinoma ex vivo and in vivo. Br. J. Dermatol. 2004, 151, 424–432. (In English) [Google Scholar] [CrossRef]
- Zaytsev, K.I.; Kudrin, K.G.; Karasik, V.E.; Reshetov, I.V.; Yurchenko, S.O. In vivo terahertz spectroscopy of pigmentary skin nevi: Pilot study of non-invasive early diagnosis of dysplasia. Appl. Phys. Lett. 2015, 106, 053702. [Google Scholar] [CrossRef]
- Li, D.; Yang, Z.; Fu, A.; Chen, T.; Chen, L.; Tang, M.; Zhang, H.; Mu, N.; Wang, S.; Liang, G. Detecting melanoma with a terahertz spectroscopy imaging technique. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 234, 118229. [Google Scholar] [CrossRef]
- Qi, X.; Bertling, K.; Torniainen, J.; Kong, F.; Gillespie, T.; Primiero, C.; Stark, M.S.; Dean, P.; Indjin, D.; Li, L.H.; et al. Terahertz in vivo imaging of human skin: Toward detection of abnormal skin pathologies. APL Bioeng. 2024, 8, 016117. [Google Scholar] [CrossRef]
- Zipfel, W.R.; Williams, R.M.; Christie, R.; Nikitin, A.Y.; Hyman, B.T.; Webb, W.W. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl. Acad. Sci. USA 2003, 100, 7075–7080. (In English) [Google Scholar] [CrossRef]
- Dimitrow, E.; Ziemer, M.; Koehler, M.J.; Norgauer, J.; König, K.; Elsner, P.; Kaatz, M. Sensitivity and specificity of multiphoton laser tomography for in vivo and ex vivo diagnosis of malignant melanoma. J. Investig. Dermatol. 2009, 129, 1752–1758. (In English) [Google Scholar] [CrossRef]
- Seidenari, S.; Arginelli, F.; Dunsby, C.; French, P.M.W.; König, K.; Magnoni, C.; Talbot, C.; Ponti, G. Multiphoton Laser Tomography and Fluorescence Lifetime Imaging of Melanoma: Morphologic Features and Quantitative Data for Sensitive and Specific Non-Invasive Diagnostics. PLoS ONE 2013, 8, e70682. (In English) [Google Scholar] [CrossRef]
- MPTflex-CARS; Multiphoton Tomography: Berlin, Germany, 2016.
- Chernyavskiy, O.; Vannucci, L.; Bianchini, P.; Difato, F.; Saieh, M.; Kubínová, L. Imaging of mouse experimental melanoma in vivo and ex vivo by combination of confocal and nonlinear microscopy. Microsc. Res. Tech. 2009, 72, 411–423. (In English) [Google Scholar] [CrossRef] [PubMed]
- Elagin, V.; Gubarkova, E.; Garanina, O.; Davydova, D.; Orlinskaya, N.; Matveev, L.; Klemenova, I.; Shlivko, I.; Shirmanova, M.; Zagaynova, E. In vivo multimodal optical imaging of dermoscopic equivocal melanocytic skin lesions. Sci. Rep. 2021, 11, 1405. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Imaging Technique Detects Skin Cancer Without Invasive Biopsy. Tech. Briefs 2019. Available online: https://www.techbriefs.com/component/content/article/33583-imaging-technique-detects-skin-cancer-without-invasive-biopsy (accessed on 12 January 2025).
- Heaton, A.R.; Burkard, N.J.; Sondel, P.M.; Skala, M.C. Quantifying in vivo collagen reorganization during immunotherapy in murine melanoma with second harmonic generation imaging. Biophotonics Discov. 2024, 1, 015004. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, H.; Zhai, S.; Zhang, H.; Shan, G.; Kwok, R.T.; Ma, C.; Sung, H.H.; Williams, I.D.; Lam, J.W. Highly efficient singlet oxygen generation, two-photon photodynamic therapy and melanoma ablation by rationally designed mitochondria-specific near-infrared AIEgens. Chem. Sci. 2020, 11, 2494–2503. [Google Scholar] [CrossRef]
- James, V.J. Fiber diffraction of skin and nails provides an accurate diagnosis of malignancies. Int. J. Cancer 2009, 125, 133–138. (In English) [Google Scholar] [CrossRef] [PubMed]
- James, V.J.; Kirby, N. The Connection between the Presence of Melanoma and Changes in Fibre Diffraction Patterns. Cancers 2010, 2, 1155–1165. (In English) [Google Scholar] [CrossRef]
- James, V.J.; O’Malley Ford, J.M. Fibre Diffraction Analysis of Skin Offers a Very Early and Extremely Accurate Diagnostic Test for Prostate Cancer. J. Cancer Res. 2014, 2014, 179608. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. (In English) [Google Scholar] [CrossRef]
- Ghimire, H.; Venkataramani, M.; Bian, Z.; Liu, Y.; Perera, A.G.U. ATR-FTIR spectral discrimination between normal and tumorous mouse models of lymphoma and melanoma from serum samples. Sci. Rep. 2017, 7, 16993. (In English) [Google Scholar] [CrossRef]
- Ollesch, J.; Heinze, M.; Heise, H.M.; Behrens, T.; Brüning, T.; Gerwert, K. It’s in your blood: Spectral biomarker candidates for urinary bladder cancer from automated FTIR spectroscopy. J. Biophotonics 2014, 7, 210–221. (In English) [Google Scholar] [CrossRef]
- Tosi, G.; Conti, C.; Giorgini, E.; Ferraris, P.; Garavaglia, M.G.; Sabbatini, S.; Staibano, S.; Rubini, C. FTIR microspectroscopy of melanocytic skin lesions: A preliminary study. Analyst 2010, 135, 3213–3219. [Google Scholar] [CrossRef]
- Wald, N.; Le Corre, Y.; Martin, L.; Mathieu, V.; Goormaghtigh, E. Infrared spectra of primary melanomas can predict response to chemotherapy: The example of dacarbazine. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 174–181. [Google Scholar] [CrossRef]
- Brézillon, S.; Untereiner, V.; Mohamed, H.T.; Ahallal, E.; Proult, I.; Nizet, P.; Boulagnon-Rombi, C.; Sockalingum, G.D. Label-free infrared spectral histology of skin tissue part II: Impact of a lumican-derived peptide on melanoma growth. Front. Cell Dev. Biol. 2020, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Abuh, S.O.; Barbora, A.; Minnes, R. Metastasis diagnosis using attenuated total reflection-Fourier transform infra-red (ATR-FTIR) spectroscopy. PLoS ONE 2024, 19, e0304071. [Google Scholar] [CrossRef] [PubMed]
- Hinz, T.; Wenzel, J.; Schmid-Wendtner, M.-H. Real-time tissue elastography: A helpful tool in the diagnosis of cutaneous melanoma? J. Am. Acad. Dermatol. 2011, 65, 424–426. (In English) [Google Scholar] [CrossRef] [PubMed]
- Botar-Jid, C.M.; Cosgarea, R.; Bolboacă, S.D.; Şenilă, S.C.; Lenghel, L.M.; Rogojan, L.; Dudea, S.M. Assessment of Cutaneous Melanoma by Use of Very- High-Frequency Ultrasound and Real-Time Elastography. Am. J. Roentgenol. 2016, 206, 699–704. (In English) [Google Scholar] [CrossRef]
- Ogata, D.; Uematsu, T.; Yoshikawa, S.; Kiyohara, Y. Accuracy of real-time ultrasound elastography in the differential diagnosis of lymph nodes in cutaneous malignant melanoma (CMM): A pilot study. Int. J. Clin. Oncol. 2014, 19, 716–721. (In English) [Google Scholar] [CrossRef]
- Ying, L.; Hou, Y.; Zheng, H.-M.; Lin, X.; Xie, Z.-L.; Hu, Y.-P. Real-time elastography for the differentiation of benign and malignant superficial lymph nodes: A meta-analysis. Eur. J. Radiol. 2012, 81, 2576–2584. (In English) [Google Scholar] [CrossRef]
- Dasgeb, B.; Morris, M.A.; Mehregan, D.; Siegel, E.L. Quantified ultrasound elastography in the assessment of cutaneous carcinoma. Br. J. Radiol. 2015, 88, 20150344. [Google Scholar] [CrossRef]
- Blois, M.S.; Zahlan, A.B.; Maling, J.E. Electron spin resonance studies on melanin. Biophys. J. 1964, 4, 471–490. (In English) [Google Scholar] [CrossRef]
- Katsuda, H.; Kobayashi, T.; Saito, H.; Matsunaga, T.; Ikeya, M. Electron spin resonance imaging of mouse B16 melanoma. Chem. Pharm. Bull. 1990, 38, 2838–2840. [Google Scholar] [CrossRef]
- Wehbi, M.; Harkemanne, E.; Mignion, L.; Joudiou, N.; Tromme, I.; Baurain, J.F.; Gallez, B. Towards Characterization of Skin Melanoma in the Clinic by Electron Paramagnetic Resonance (EPR) Spectroscopy and Imaging of Melanin. Mol. Imaging Biol. 2023, 26, 382–390. (In English) [Google Scholar] [CrossRef]
- Godechal, Q.; Ghanem, G.E.; Cook, M.G.; Gallez, B. Electron Paramagnetic Resonance Spectrometry and Imaging in Melanomas: Comparison between Pigmented and Nonpigmented Human Malignant Melanomas. Mol. Imaging 2013, 12, 7290.2012.00037. [Google Scholar] [CrossRef]
- Mignion, L.; Desmet, C.M.; Harkemanne, E.; Tromme, I.; Joudiou, N.; Wehbi, M.; Baurain, J.-F.; Gallez, B. Noninvasive detection of the endogenous free radical melanin in human skin melanomas using electron paramagnetic resonance (EPR). Free Radic. Biol. Med. 2022, 190, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Lin, K.; Hsieh, C.-M.; Liu, L.; Ge, X.; Liu, Q. Optical coherence tomography-guided confocal Raman microspectroscopy for rapid measurements in tissues. Biomed. Opt. Express 2021, 13, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Mazurenka, M.; Behrendt, L.; Meinhardt-Wollweber, M.; Morgner, U.; Roth, B. Development of a combined OCT-Raman probe for the prospective in vivo clinical melanoma skin cancer screening. Rev. Sci. Instrum. 2017, 88, 105103. [Google Scholar] [CrossRef]
- Varkentin, A.; Mazurenka, M.; Blumenröther, E.; Behrendt, L.; Emmert, S.; Morgner, U.; Meinhardt-Wollweber, M.; Rahlves, M.; Roth, B. Trimodal system for in vivo skin cancer screening with combined optical coherence tomography-Raman and colocalized optoacoustic measurements. J. Biophotonics 2018, 11, e201700288. [Google Scholar] [CrossRef]
- Kukk, A.F.; Wu, D.; Gaffal, E.; Panzer, R.; Emmert, S.; Roth, B. Multimodal imaging system with ultrasound, photoacoustics, and optical coherence tomography for optical biopsy of melanoma. In Proceedings of the Multimodal Biomedical Imaging XVIII, San Francisco, CA, USA, 28 January 2023; pp. 16–22. [Google Scholar]
- Roth, B.; Kukk, A.F.; Wu, D.; Panzer, R.; Emmert, S. Four-modal device comprising optical coherence tomography, photoacoustic tomography, ultrasound, and Raman spectroscopy developed for in vivo skin lesion assessment. Biomed. Opt. Express 2025. [CrossRef]
- Wang, C.; Guo, L.; Wang, G.; Ye, T.; Wang, B.; Xiao, J.; Liu, X.J.A.O. In-vivo imaging of melanoma with simultaneous dual-wavelength acoustic-resolution-based photoacoustic/ultrasound microscopy. Appl. Opt. 2021, 60, 3772–3778. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, D.; Yang, S.; Xing, D. Toward in vivo biopsy of melanoma based on photoacoustic and ultrasound dual imaging with an integrated detector. Biomed. Opt. Express 2016, 7, 279–286. [Google Scholar] [CrossRef]
- Kukk, A.F.; Scheling, F.; Panzer, R.; Emmert, S.; Roth, B. Non-invasive 3D imaging of human melanocytic lesions by combined ultrasound and photoacoustic tomography: A pilot study. Sci. Rep. 2024, 14, 2768. [Google Scholar] [CrossRef] [PubMed]
- Kukk, A.F.; Scheling, F.; Panzer, R.; Emmert, S.; Roth, B. Combined ultrasound and photoacoustic C-mode imaging system for skin lesion assessment. Sci. Rep. 2023, 13, 17947. [Google Scholar] [CrossRef] [PubMed]
- Wongvibulsin, S.; Yan, M.J.; Pahalyants, V.; Murphy, W.; Daneshjou, R.; Rotemberg, V. Current state of dermatology mobile applications with artificial intelligence features: A scoping review. JAMA Dermatol. 2024, 160, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Jain, A.; Araveeti, S.R.; Adhikari, S.; Garg, H.; Bhandari, M. FDA-Approved Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices: An Updated Landscape. Electronics 2024, 13, 498. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence-Enabled Device Software Functions. 2024. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/marketing-submission-recommendations-predetermined-change-control-plan-artificial-intelligence (accessed on 12 January 2025).
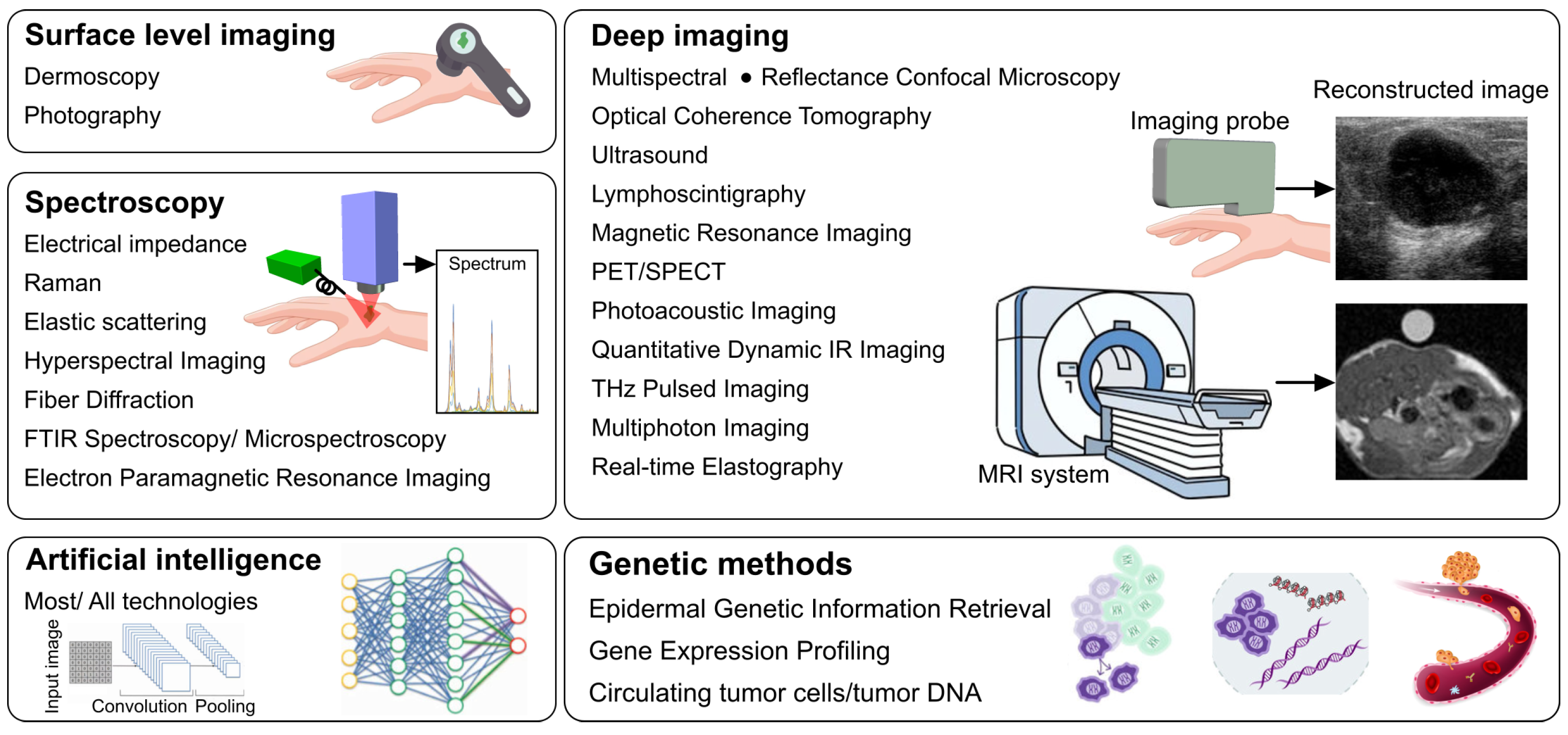
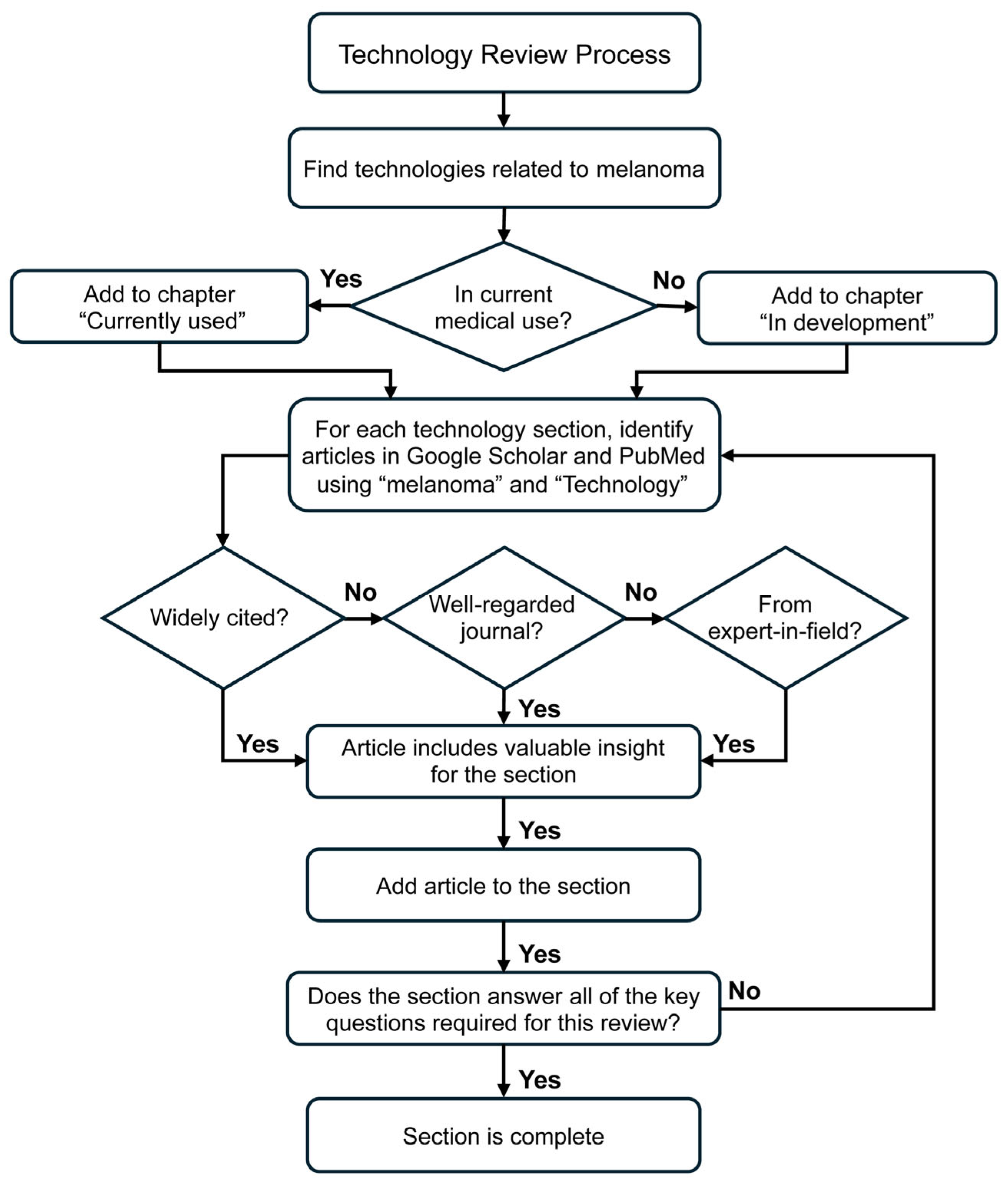
| Imaging Modalities Currently Used in Medical Practice | |||
|---|---|---|---|
| Technology | Biomarker | Main Applications | Main Limitation(s) |
| Smartphone/digital photography | ABCDE criteria. | Prescreening | Variable lighting, automated edge enhancement, user focusing decreases accuracy. |
| Total body photography | ABCDE criteria. | Prescreening | Detects changes over time, hence misses skin regions in genital, acral, scalp, within body folds. Large image files. |
| Dermoscopy | Irregular pigment network, asymmetrical structures, abrupt peripheral streaks, uneven color distribution within a lesion, and vascular features. | Prescreening/screening | Superficial assessment. Poor sensitivity for small melanomas, haphazard monitoring over time. |
| Electrical Impedance Spectroscopy | Melanomas show lower impedance at some frequencies due to higher water content and disrupted extracellular matrix and disrupted cell membranes. Melanomas have more pronounced change in impedance with change in frequency. | Prescreening/screening | Low specificity for melanoma, can be fooled by inflammation, ulceration, scar tissue. Requires trained physician to assess results. |
| Reflectance Confocal Microscopy (RCM) | Contrast is based on reflectance of different skin components. Melanin has a high refractive index and appears bright. RCM also detects irregularly shaped cells and disorganized arrangements of cells and structures. | Screening | Small field of view leads to lengthy imaging sessions for large lesions. Shallow imaging depth. |
| Optical coherence tomography (OCT) | Atypical cell structures and disorganized tissue architecture including honeycombed patterns, pagetoid spread, absence of dermal nests, and atypical melanocytes in the dermis. Also, optical features extracted and analyzed via machine learning. D-OCT: changes in microvascular structures in the skin including increased vascular density (angiogenesis), chaotic vessel architecture, irregular and dilated blood vessels, and irregular blood flow. | Screening | HD-OCT, OCM and LC-OCT have cellular resolution however do not have sufficient imaging depth for staging. Specificity and sensitivity have not been fully studied. D-OCT: focuses on blood flow changes caused by melanoma. |
| Multispectral Imaging | Differential absorption and reflectance of light at multiple wavelengths, capturing variations in melanin, hemoglobin, and oxygenation levels that distinguish malignant melanomas from benign lesions. | Prescreening/screening | Low specificity, cannot be used for rare melanomas. |
| Ultrasound (3.5–14 MHz) | Berlin morphology criteria for finding sentinel node metastases: peripheral perfusion, loss of central echoes and balloon shapes. | Staging, surgical/treatment guidance | May not provide reliable preoperative nodal staging. |
| High-Frequency Ultrasound (>15 MHz) | Hypoechoic lesions infiltrating the dermis from the epidermis. Anechoic content in suspicious lesions. Margins are usually sharp. Doppler shows increased and anarchic vascularization. | Staging | Allows deep penetration (1.5–8 mm) beneficial for estimating tumor thickness. Does not rely on melanin, thus useful for amelanotic melanoma. Poor sensitivity. |
| Raman Spectroscopy | Distinct spectral signature resulting from altered molecular compositions, such as elevated nucleic acids, proteins, lipids and melanin, reflecting the biochemical changes characteristic of malignant melanocytes compared to normal skin tissue. | Screening | Moderate specificity for in vivo screening. |
| Elastic scattering spectroscopy | Variation in light scattering patterns caused by differences in cellular and subcellular structures, such as nuclear size and density, which distinguish malignant melanoma from benign skin lesions. | Screening | Limited depth detection, high sensitivity but modest specificity. |
| Magnetic Resonance Imaging | Lesions are hyperintense by T1 due to reduced T1 relaxation time associated with melanin. T2 signal is reduced. T1 with contrast shows a heterogeneous peripheral rim enhancement. | Staging, surgical/treatment guidance | Low sensitivity, has long scanning times, and requires exogenous contrast agents. |
| Positron emission tomography/computed tomography (PET/CT) | 18F-FDG (glucose analog that accumulates in cells with high metabolic activity). | Staging, surgical treatment/guidance, post-surgical/treatment monitoring | Ionizing radiation, requiring tracers, expensive. |
| Single Photon Emission Computed Tomography (SPECT/CT) | 99mTc-labeled colloids/99mTc-Tilmanocept. | Staging, surgical/treatment guidance | Ionizing, requiring tracers, limited availability and cost, low spatial resolution. |
| Lymphoscintigraphy | 99mTc-labeled colloids. | Surgical guidance | This nuclear medicine imaging technique, low spatial resolution, limited sensitivity for early-stage metastases, and lack of real-time imaging. |
| Imaging Modalities Currently in Development | |||
| Technology | Biomarkers | Proposed Main Utility | Strengths and shortcomings |
| Photoacoustic Imaging (PAI) | Melanin is a strong endogenous chromophore, multispectral imaging enables visualization of melanoma-related angiogenesis, and other changes. Elevated optical absorption contrast of melanin at specific wavelengths, combined with increased vascularization and oxygenation heterogeneity that reflect the tumor’s metabolic and structural abnormalities. | Screening, staging, surgical/treatment guidance, post-surgical/treatment monitoring | Good depth penetration. Functional imaging (melanoma and hemoglobin). Versatile technique that can be implemented with either good depth penetration and/or high spatial resolution. |
| Hyperspectral Imaging | Unique spectral reflectance and absorption patterns across visible and near-infrared wavelengths, driven by variations in melanin concentration, hemoglobin content, and tissue morphology. | Screening | Portable imaging device. Can distinguish hemoglobin from melanin. Moderate specificity. |
| Quantitative Dynamic Infrared Imaging | Abnormal thermal signature and delayed heat dissipation patterns caused by increased metabolic activity and vascularization. | Screening | Can image large areas of skin rapidly. Low specificity, difficulty detecting small melanomas. |
| Terahertz Pulse Imaging | Altered terahertz refractive index and absorption coefficient, reflecting differences in water content, cellular density, and tissue composition between malignant melanomas and benign skin lesions. | Screening/possibly staging | Can define rough tumor margins but very limited depth detection. Poor sensitivity. |
| Multiphoton Imaging—Two Photon Excitation (2PE) Microscopy and Second Harmonic Generation (SHG) microscopy | Altered autofluorescence and reduced second-harmonic generation signals, changes in melanin distribution, collagen organization, and metabolic activity in malignant melanomas compared to normal or benign skin tissues. Reduced SHG signal intensity reflects structural abnormalities in the extracellular matrix associated with malignant melanoma progression. | Screening | Limited penetration (a few hundred µm) in depth penetration at subcellular (0.5 µm lateral and 1–2 µm axial) resolution. |
| Fiber Diffraction | Altered diffraction patterns of collagen and other fibrous proteins, indicating changes in molecular packing and structural organization associated with the tumor microenvironment in malignant melanoma. | Screening | Changes in fiber organization may occur in early-stage tumors. Poor sensitivity to cellular-level changes. Requires ordered molecular structures: fiber diffraction is best suited for analyzing highly ordered, periodic structures like collagen fibrils or keratin networks. |
| Fourier Transform Infrared Spectroscopy | Distinct absorption peaks corresponding to altered lipid, protein, and nucleic acid compositions, reflecting biochemical changes in malignant melanoma cells compared to normal or benign tissues. | Pathological staging | Rapid detection of melanoma in tissue samples through spectral changes. Not for in vivo use. |
| Real-time Elastography | Increased stiffness and altered elastic properties of malignant tissues, reflecting changes in extracellular matrix composition and tumor-induced mechanical heterogeneity. | Possibly screening, staging | Real-time imaging, excellent imaging depth up to 10 mm. Technique is time-consuming and labor-intensive. Poor specificity. |
| Electron paramagnetic resonance spectroscopy | The elevated levels of melanin-associated free radicals and altered paramagnetic properties, reflecting oxidative stress and metabolic changes characteristic of malignant melanoma. | Screening, staging | High quality 3-dimensional images, excellent penetration depth of 7 mm or more. Struggles with resolution and small melanomas. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horton, L.; Fakhoury, J.W.; Manwar, R.; Rajabi-Estarabadi, A.; Turk, D.; O’Leary, S.; Fotouhi, A.; Daveluy, S.; Jain, M.; Nouri, K.; et al. Review of Non-Invasive Imaging Technologies for Cutaneous Melanoma. Biosensors 2025, 15, 297. https://doi.org/10.3390/bios15050297
Horton L, Fakhoury JW, Manwar R, Rajabi-Estarabadi A, Turk D, O’Leary S, Fotouhi A, Daveluy S, Jain M, Nouri K, et al. Review of Non-Invasive Imaging Technologies for Cutaneous Melanoma. Biosensors. 2025; 15(5):297. https://doi.org/10.3390/bios15050297
Chicago/Turabian StyleHorton, Luke, Joseph W. Fakhoury, Rayyan Manwar, Ali Rajabi-Estarabadi, Dilara Turk, Sean O’Leary, Audrey Fotouhi, Steven Daveluy, Manu Jain, Keyvan Nouri, and et al. 2025. "Review of Non-Invasive Imaging Technologies for Cutaneous Melanoma" Biosensors 15, no. 5: 297. https://doi.org/10.3390/bios15050297
APA StyleHorton, L., Fakhoury, J. W., Manwar, R., Rajabi-Estarabadi, A., Turk, D., O’Leary, S., Fotouhi, A., Daveluy, S., Jain, M., Nouri, K., Mehregan, D., & Avanaki, K. (2025). Review of Non-Invasive Imaging Technologies for Cutaneous Melanoma. Biosensors, 15(5), 297. https://doi.org/10.3390/bios15050297





