A Dual-Signal Electrochemiluminescence Sensor for Kanamycin Detection Based on a Self-Enhanced Zr MOF and Single Co-Reactant Competition Mechanism
Abstract
1. Introduction
2. Experimental Section
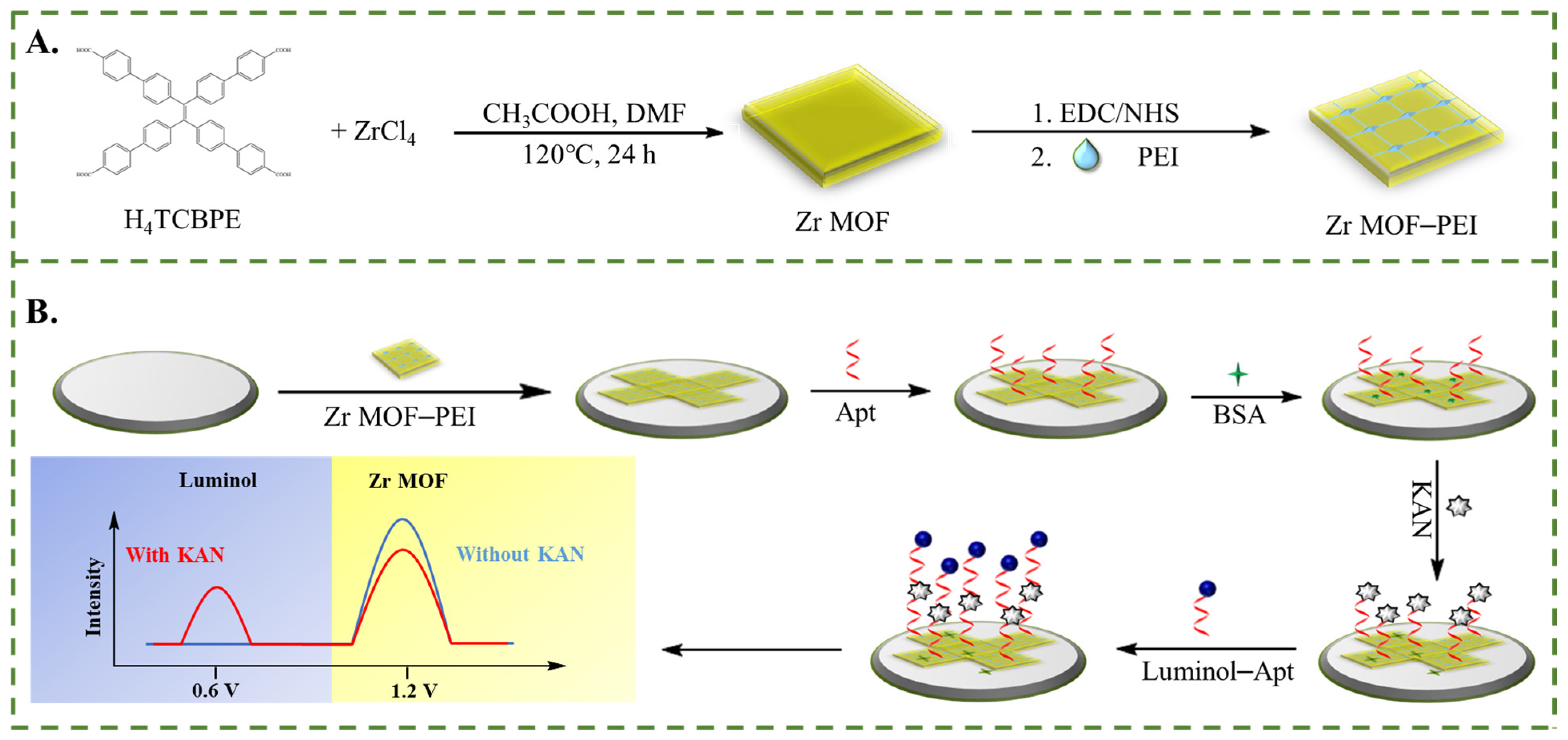
2.1. Preparation of Zr MOF and Zr MOF-PEI Composite
2.2. Preparation of Luminol–Apt
2.3. Preparation of ECL Aptamer Sensors
3. Results and Discussion
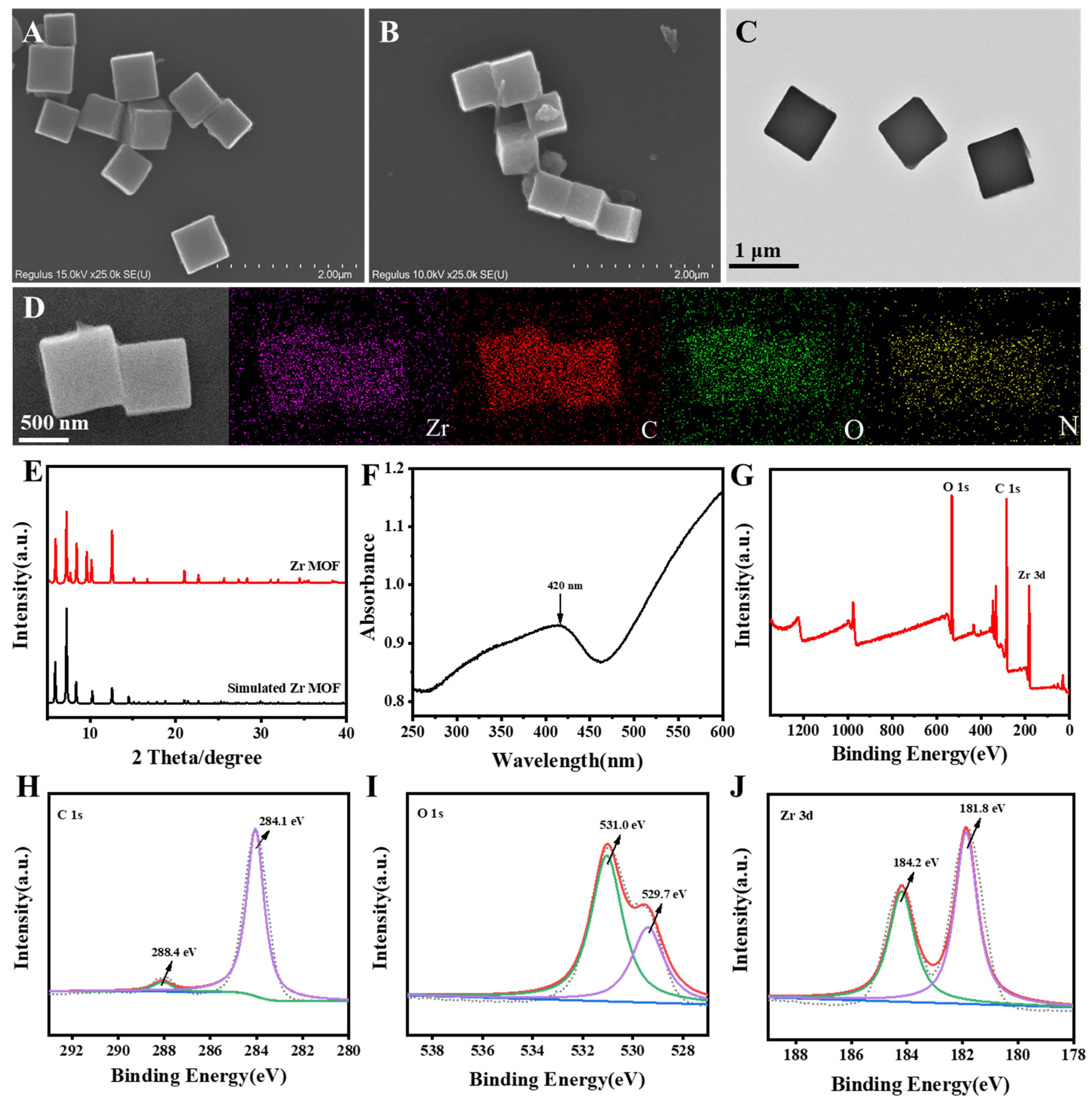
3.1. Characterization of Zr MOF and Zr MOF-PEI
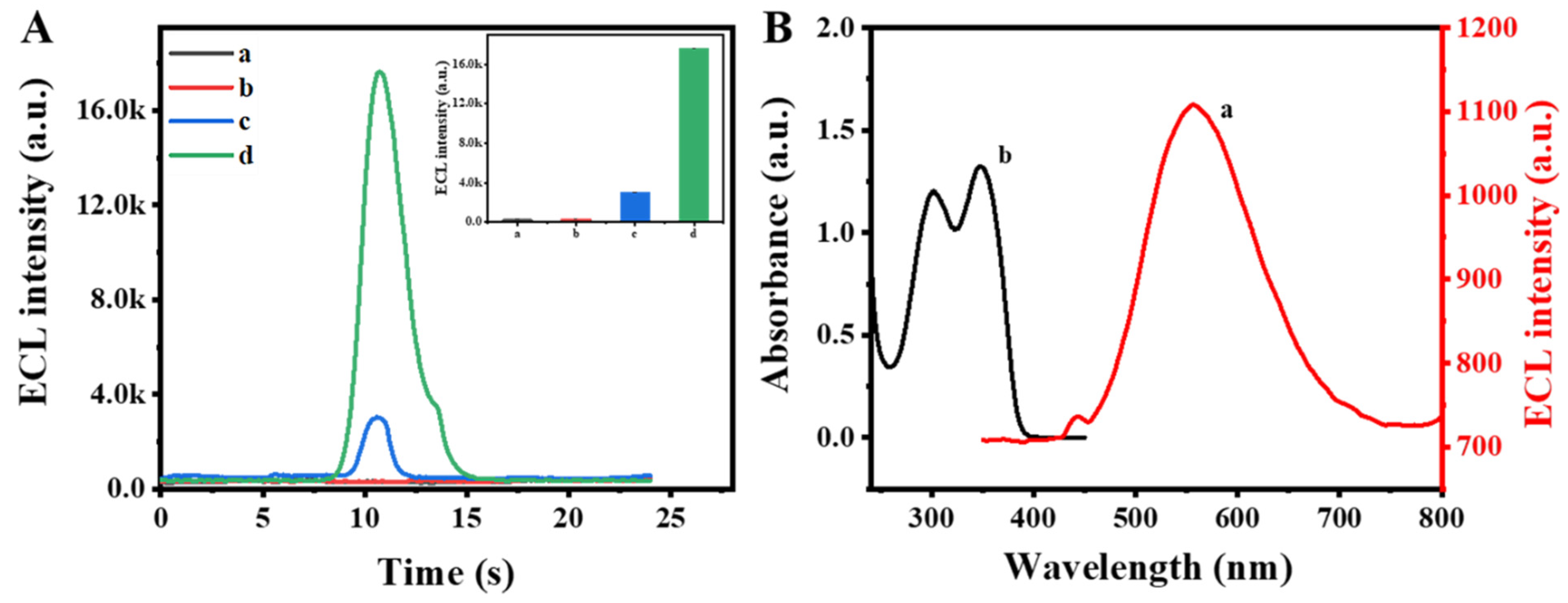
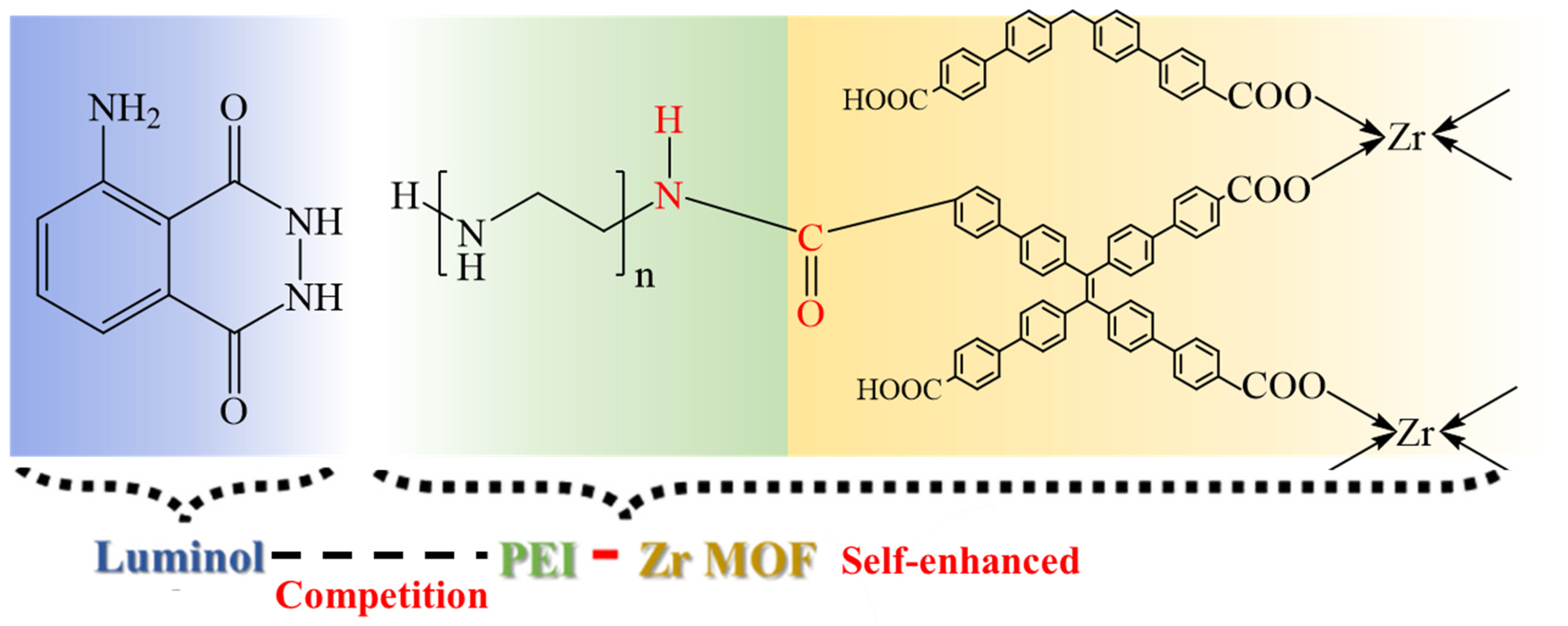
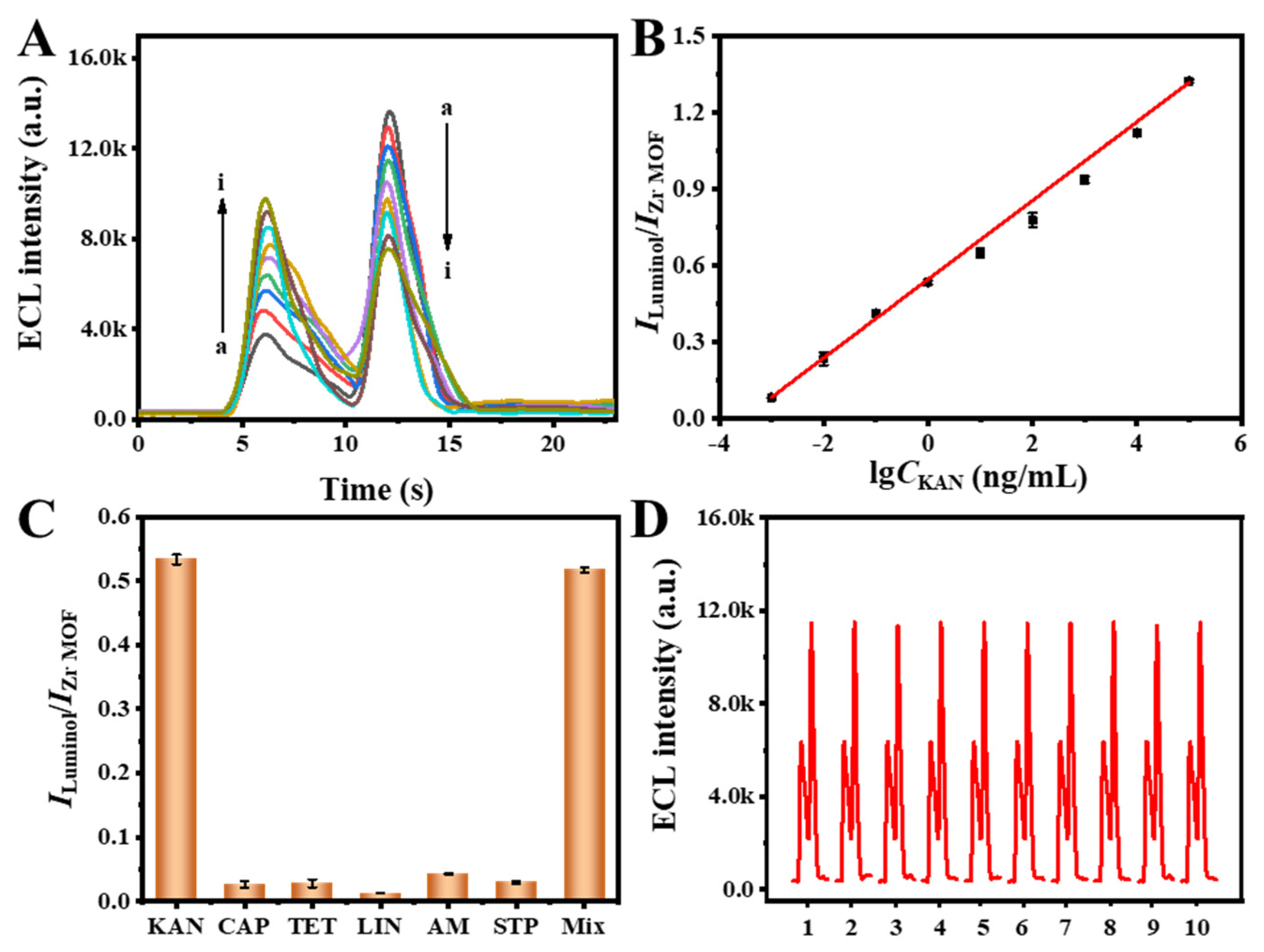
3.2. Performance of the ECL Self-Enhancement and Competition Mechanism
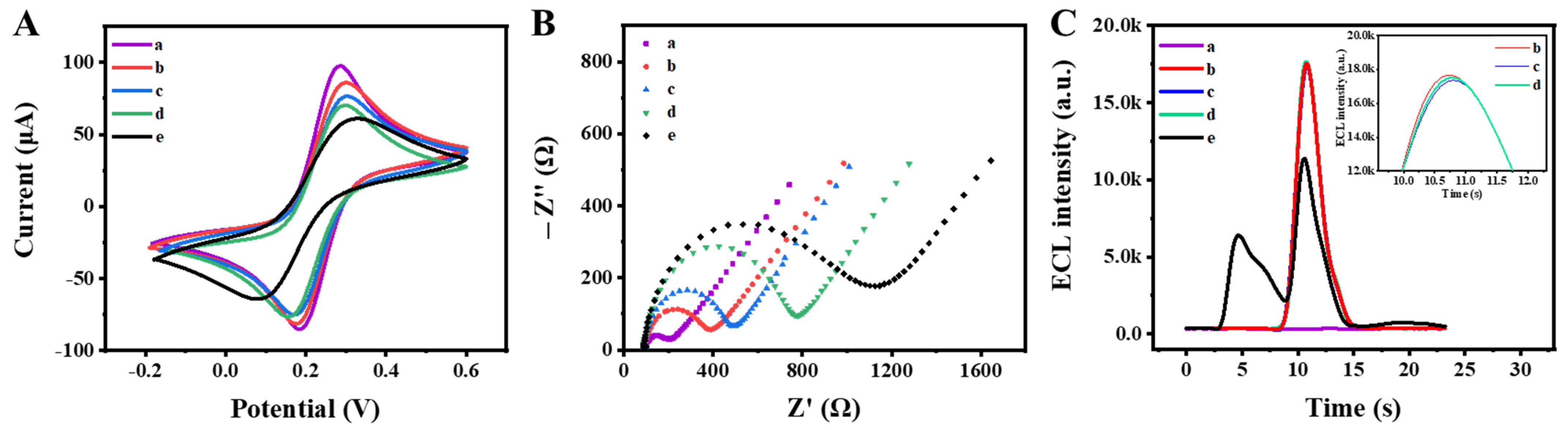
3.3. Electrochemical Characterization and ECL Behavior
3.4. Optimization of the Detection Conditions
3.5. Analytical Performance
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heysell, S.K.; Ahmed, S.; Rahman, M.T. Hearing loss with kanamycin treatment for multidrug-resistant tuberculosis in Bangladesh. Eur. Respir. J. 2018, 51, 1701778. [Google Scholar] [CrossRef] [PubMed]
- Brezden, A.; Mohamed, M.F.; Nepal, M.; Harwood, J.S.; Kuriakose, J.; Seleem, M.N.; Chmielewski, J. Correction to “dual targeting of intracellular pathogenic bacteria with a cleavable conjugate of kanamycin and an antibacterial cell-penetrating peptide”. J. Am. Chem. Soc. 2016, 138, 10945–10949. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.J.; Zhu, Y.B.; Zhou, X.H.; Lin, Q.; He, M. High-κ solid-gate transistor configured graphene biosensor with fully integrated structure and enhanced sensitivity. Adv. Funct. Mater. 2016, 26, 7668–7678. [Google Scholar] [CrossRef]
- Habeeb Rahman, A.P.; Pranjal; Behera, S.K.; Mishra, A.; Lundborg, C.S.; Tripathy, S.K. Transcriptomic regulation of Salmonella Typhimurium during sonophotocatalysis and the effect of stress adaptation on the antibiotic resistance and tolerance post-treatment. Chem. Eng. J. 2022, 446, 137442. [Google Scholar] [CrossRef]
- Chen, Z.H.; Liu, X.; Chen, L.W.; Han, Y.; Shen, Y.M.; Chen, B.L.; Wang, M.Z. Deglycosylation inactivation initiated by a novel periplasmic dehydrogenase complex provides a novel strategy for eliminating the recalcitrant antibiotic kanamycin. Environ. Sci. Technol. 2023, 57, 4298–4307. [Google Scholar] [CrossRef]
- Guo, Z.; He, J.X.; Mahadevegowda, S.H.; Kho, S.H.; Chan-Park, M.B.; Liu, X.W. Multifunctional glyco-nanosheets to eradicate drug-resistant bacteria on wounds. Adv. Healthc. Mater. 2020, 9, 2000265. [Google Scholar] [CrossRef]
- Wang, C.S.; Liu, C.; Luo, J.B.; Tian, Y.P.; Zhou, N.D. Direct electrochemical detection of kanamycin based on peroxidase-like activity of gold nanoparticles. Anal. Chim. Acta 2016, 936, 75–82. [Google Scholar] [CrossRef]
- Wang, W.Z.; Gunasekaran, S. Oxygen-terminated few-layered Ti3C2Tx MXene nanosheets as peroxidase-mimic nanozyme for colorimetric detection of kanamycin. Biosens. Bioelectron. 2022, 218, 114774. [Google Scholar] [CrossRef]
- Zhao, T.T.; Chen, Q.; Wen, Y.L.; Bian, X.J.; Tao, Q.; Liu, G.; Yan, J. A competitive colorimetric aptasensor for simple and sensitive detection of kanamycin based on terminal deoxynucleotidyl transferase-mediated signal amplification strategy. Food Chem. 2022, 377, 132072. [Google Scholar] [CrossRef]
- He, Y.H.; Wen, X.Y.; Zhang, B.Y.; Fan, Z.F. Novel aptasensor for the ultrasensitive detection of kanamycin based on graphene oxide quantum-dot-linked single-stranded DNA-binding protein. Sens. Actuators B Chem. 2018, 265, 20–26. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, W.Q.; Gao, X.L.; Sun, Z.C.; Sun, X.; Guo, Y.M.; Li, F.L.; Boboriko, N.E. Fluorescent aptasensor based on DNA-AgNCs emitting in the visible red wavelength range for detection of kanamycin in milk. Sens. Actuators B Chem. 2022, 360, 131665. [Google Scholar] [CrossRef]
- Wang, C.S.; Liu, J.; Han, X.Y.; Liu, C.; Tian, Y.P.; Zhou, N.D. UV-visible spectroscopic detection of kanamycin based on target-induced growth of gold nanoparticles. Anal. Methods 2017, 9, 4843. [Google Scholar] [CrossRef]
- Zhong, C.Z.; Zhang, C.Z.; Yang, Y.; Liang, X.X.; Pang, Q.; Zhou, L.Y.; Chen, P.C. Synergistic effect of photoelectrochemical aptasensor based on staggered gap ZnO/BiFeO3 heterojunction coupled with cDNA-CdS sensitizer enabling ultrasensitive assay of kanamycin. Food Chem. 2024, 437, 137877. [Google Scholar] [CrossRef]
- Gupta, V.; Dick, J. Real-time intracellular analysis of kanamycin using microaptasensors. ACS Sens. 2023, 8, 1143–1150. [Google Scholar] [CrossRef]
- Gao, X.L.; Sun, Z.C.; Wang, X.Y.; Zhang, W.Q.; Xu, D.Y.; Sun, X.; Guo, Y.M.; Xu, S.C.; Li, F.L. Construction of a dual-model aptasensor based on G-quadruplexes generated via rolling circle amplification for visual/sensitive detection of kanamycin. Sci. Total Environ. 2022, 839, 156276. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Q.; Deng, X.K.; Guo, Q.F.; Liu, D.D.; Nie, G.M.J. A novel electrochemiluminescence biosensor based on Ru (bpy)32+-functionalized MOF composites and cycle amplification technology of DNAzyme walker for ultrasensitive detection of kanamycin. Colloid Interface Sci. 2024, 659, 859–867. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.L.; Nie, Y.X.; Ma, Q. Recent development of organic nanoemitter-based ECL sensing application. TrAC 2021, 143, 116410. [Google Scholar] [CrossRef]
- Liao, Y.H.; Fan, Z.J.; Deng, H.P.; Yang, Y.; Li, J.Y.; Zhao, Z.Y.; Tan, Q.Q.; Li, B.; Huang, X. Zika virus liquid biopsy: A dendritic Ru (bpy)32+-polymer-amplified ECL diagnosis strategy using a drop of blood. ACS Cent. Sci. 2018, 4, 1403–1411. [Google Scholar] [CrossRef]
- Du, L.; Zhang, H.X.; Wang, Z.Y.; Zhuang, T.T.; Wang, Z.H. Boosting the electrochemiluminescence of luminol by high-intensity focused ultrasound pretreatment combined with 1T/2H MoS2 catalysis to construct a sensitive sensing platform. Ultrason. Sonochem. 2023, 92, 106264. [Google Scholar] [CrossRef]
- He, Y.; Hu, F.X.; Zhao, J.W.; Yang, G.M.; Zhang, Y.Y.; Chen, S.H.; Yuan, R. Bifunctional moderator-powered ratiometric electrochemiluminescence enzymatic biosensors for detecting organophosphorus pesticides based on dual-signal combined nanoprobes. Anal. Chem. 2021, 93, 8783–8790. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Tan, H.S.; Liu, M.J.; Li, S.S. Electrochemical ratiometric dual-signal immunoassay for accurate detection of carcinoembryonic antigen in clinical serum based on rGO-Pd@Au-Thi and Chi-Fc-Au. Sens. Actuators B Chem. 2023, 380, 133340. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Li, Y.R.; Zhang, Y.Q.; Xiang, Y.M.; Bai, R.R.; Liu, Y.; Li, M.L.; Meng, G.R.; Pan, S.L.; Zhang, F.; et al. Dual-signal ratiometric electrochemiluminescence biosensor based on Au NPs-induced low-potential emission of PFO Pdots and LSPR-ECL mechanism for ultra-sensitive detection of microRNA-141. Biosens. Bioelectron. 2024, 261, 116495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cui, X.M.; Li, F.H.; Yan, W.; Wang, Y.Y.; Shang, L.; Ma, R.N.; Jia, L.P.; Li, C.; Wang, H.S. Perylene diimide and g-C3N4 nanosheet as potential-resolved cathode luminophores for ultrasensitive ratiometric electrochemiluminescence immunosensor. Sens. Actuators B Chem. 2022, 371, 132492. [Google Scholar] [CrossRef]
- Dai, W.J.; Chen, G.X.; Wang, X.Y.; Zhen, S.J.; Huang, C.Z.; Zhan, L.; Li, Y.F. Facile synthesis of dual-ligand europium-metal organic gels for ratiometric electrochemiluminescence detecting I27L gene. Biosens. Bioelectron. 2024, 246, 115863. [Google Scholar] [CrossRef]
- Chen, G.X.; Hu, C.Y.; Dai, W.J.; Luo, Z.L.; Zang, H.; Sun, S.Y.; Zhen, S.J.; Zhan, L.; Huang, C.Z.; Li, Y.F. Coreactant-free zirconium metal-organic framework with dual emission for ratiometric electrochemiluminescence detection of HIV DNA. Anal. Chem. 2024, 96, 10102–10110. [Google Scholar] [CrossRef]
- Wang, X.M.; Hou, C.T.; Zhang, F.F.; Xia, J.F.; Wang, Z.H. Highly sensitive electrochemiluminescence biosensor based on novel TiO2-porphyrin organic framework with self-enhanced luminescent property. Sens. Actuators B Chem. 2023, 379, 133229. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhao, A.J.; Wang, Z.Z.; Xu, Y.H.; Feng, Y.J.; Lan, Y.B.; Han, Z.G.; Lu, X.Q. Enhancing the electrochemiluminescence of porphyrin via crystalline networks of metal-organic frameworks for sensitive detection of cardiac troponin I. Anal. Chem. 2023, 95, 11687–11694. [Google Scholar] [CrossRef]
- Guan, J.P.; Xiong, Y.; Wang, M.; Liu, Q.; Chen, X.Q. A novel functionalized CdTe@MOFs based fluorometric and colorimetric biosensor for dual-readout assay of creatinine. Sens. Actuators B Chem. 2024, 399, 134842. [Google Scholar] [CrossRef]
- Gumilar, G.; Chowdhury, S.; Shukri, G.; Patah, A.; Nugraha, N.; Henzie, J.; Anshori, I.; Kanetid, Y.V.; Yuliarto, B. The revelation of glucose adsorption mechanisms on hierarchical metal–organic frameworks using a surface plasmon resonance sensor. J. Mater. Chem. B 2023, 11, 4428. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Zhao, H.Q.; Shang, L.; Shen, G.D.; Ma, R.N.; Wang, H.S. A robust ECL-enhanced system based on UiO-66-COOH promoting perylene diimide derivative electrochemiluminescence for tumor biomarker detection. Sens. Actuators B Chem. 2025, 423, 136824. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.; Wu, T.T.; Zhang, X.Y.; Ren, X.; Feng, R.; Du, Y.; Lee, J.Y.; Liu, X.T.; Wei, Q. Zirconium based metal-organic frameworks with aggregation-induced electrochemiluminescence for sensitive analysis of aflatoxin B1 by signal dual-amplification strategy. Chem. Eng. J. 2024, 500, 157308. [Google Scholar] [CrossRef]
- Tian, S.Y.; Wang, J.H.; Jie, Y.; Ding, Z.; Wang, X.; Wang, J.J.; Hou, X.Y. MnO2 nanoparticles enhance the activity of the Zr-MOF matrix electrochemical sensor for efficiently identifying ultra-trace tetracycline residues in food. Microchim. Acta 2025, 192, 12. [Google Scholar] [CrossRef] [PubMed]
- Bushira, F.A.; Hussain, A.; Wang, P.; Li, H.J.; Zheng, L.R.; Gao, Z.Q.; Dong, H.F.; Jin, Y.D. Boosting electrochemiluminescence performance of a dual-active site iron single-atom catalyst-based luminol–dissolved oxygen system via plasmon-induced hot holes. Anal. Chem. 2024, 96, 9704–9712. [Google Scholar] [CrossRef]
- Chen, X.D.; Xv, H.J.; Li, C.; Kong, L.H.; Li, C.X.; Li, F. Fe-single-atom catalysts boosting electrochemiluminescence via bipolar electrode integrated with its peroxidase-like activity for bioanalysis. Biosens. Bioelectron. 2024, 258, 116351. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zou, Y.Q.; Ru, H.J.; Yan, F.; Liu, J.Y. Silica nanochannels as nanoreactors for the confined synthesis of Ag NPs to boost electrochemical stripping chemiluminescence of the luminol–O2 system for the sensitive aptasensor. Anal. Chem. 2024, 96, 10264–10273. [Google Scholar] [CrossRef]
- Wei, Z.W.; Gu, Z.Y.; Arvapally, R.K.; Chen, Y.P.; McDougald, R.M., Jr.; Ivy, J.F.; Yakovenko, A.A.; Feng, D.W.; Omary, M.A.; Zhou, H.C. Rigidifying fluorescent linkers by metal-organic framework formation for fluorescence blue shift and quantum yield enhancement. J. Am. Chem. Soc. 2014, 136, 8269–8276. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, K.Q.; Zhang, H.; Li, C.X.; Wang, J.L.; Huang, H.W.; Yi, Q.F.; Zhou, H. Dual-mode photoelectrochemical/electrochemical sensor based on Z-scheme AgBr/AgI-Ag-CNTs and aptamer structure switch for the determination of kanamycin. Microchim. Acta 2022, 417, 189. [Google Scholar] [CrossRef]
- Liu, X.Q.; Liu, P.P.; Tang, Y.F.; Yang, L.W.; Li, L.L.; Qi, Z.C.; Li, D.L.; Wong, D.K.Y. A photoelectrochemical aptasensor based on a 3D flower-like TiO2-MoS2-gold nanoparticle heterostructure for detection of kanamycin. Biosens. Bioelectron. 2018, 112, 193–201. [Google Scholar] [CrossRef]
- Chen, S.X.; Liang, Y.Q.; Zhou, Y.W. Analysis of kanamycin A in human plasma and in oral dosage form by derivatization with 1-naphthyl isothiocyanate and high-performance liquid chromatography. J. Sep. Sci. 2006, 29, 607–612. [Google Scholar] [CrossRef]
- Perez, J.J.; Chen, C.Y. Detection of acetyltransferase modification of kanamycin, an aminoglycoside antibiotic, in bacteria using ultrahigh-performance liquid chromatography tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2018, 32, 1549–1556. [Google Scholar] [CrossRef]
- Li, M.N.; Xie, Y.F.; Zhang, J.B.; Lie, L.L.; Su, X.G. Construction of a laccase mimic enzyme with fluorescence properties for kanamycin multi-mode analysis. Chem. Eng. J. 2023, 471, 144184. [Google Scholar] [CrossRef]
- Ramezani, M.; Danesh, N.M.; Abnous, P.; Lavaee, K.; Taghdisi, S.M. A selective and sensitive fluorescent aptasensor for detection of kanamycin based on catalytic recycling activity of exonuclease III and gold nanoparticles. Sen. Actuators B Chem. 2016, 222, 1–7. [Google Scholar] [CrossRef]
- Xu, R.R.; Cheng, Y.Q.; Qi, X.X.; Li, X.T.; Zhang, Z.W.; Chen, L.Y.; Sun, T.; Gao, Z.H.; Zhu, M.J. Target-induced gold nanoparticles colorimetric sensing coupled with aptamer for rapid and high-sensitivity detecting kanamycin. Anal. Chem. Acta 2022, 1230, 340377. [Google Scholar] [CrossRef]
- Ha, N.R.; Jung, I.P.; Kim, S.H.; Kim, A.R.; Yoon, M.Y. Paper chip-based colorimetric sensing assay for ultra-sensitive detection of residual kanamycin. Process Biochem. 2017, 62, 161–168. [Google Scholar] [CrossRef]
- Cheng, S.T.; Liu, H.M.; Zhang, H.; Chu, G.L.; Guo, Y.M.; Sun, X. Ultrasensitive electrochemiluminescence aptasensor for kanamycin detection based on silver nanoparticle-catalyzed chemiluminescent reaction between luminol and hydrogen peroxide. Sen. Actuators B Chem. 2020, 304, 127367. [Google Scholar] [CrossRef]
- Li, H.K.; Cai, Q.Q.; Bai, M.H.; Jie, G.F. Novel dual-potential color-resolved luminophore Ru(bpy)32+-doped CdSe QDs for bipolar electrode electrochemiluminescence biosensing. Anal. Chem. 2025, 97, 953–961. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, Y.W.; Cai, L.; Liu, C.; Zhang, B.; Fang, G.Z.; Wang, S. Polythionine-mediated AgNWs-AuNPs aggregation conductive network: Fabrication of molecularly imprinted electrochemiluminescence sensors for selective capture of kanamycin. J. Hazard. Mater. 2022, 434, 128882. [Google Scholar] [CrossRef]
| Sample | Spiked (ng/mL) | Found (ng/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Milk | 0 | No Found | - | - |
| 0.01 | 0.01 | 100 | 0.4 | |
| 1.00 | 1.00 | 100 | 1.1 | |
| 100.0 | 102.6 | 103 | 1.1 | |
| River water | 0 | No Found | - | - |
| 0.01 | 0.01 | 100 | 2.9 | |
| 1.00 | 1.03 | 103 | 2.2 | |
| 100.0 | 97.11 | 97.1 | 1.0 | |
| Honey | 0 | No Found | - | - |
| 0.01 | 0.01 | 100 | 1.2 | |
| 1.00 | 1.04 | 104 | 1.5 | |
| 100.0 | 98.78 | 98.8 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wang, X.; Yan, Z.; Zhang, F.; Xia, J.; Lv, L.; Wang, Z. A Dual-Signal Electrochemiluminescence Sensor for Kanamycin Detection Based on a Self-Enhanced Zr MOF and Single Co-Reactant Competition Mechanism. Biosensors 2025, 15, 291. https://doi.org/10.3390/bios15050291
Zhu Y, Wang X, Yan Z, Zhang F, Xia J, Lv L, Wang Z. A Dual-Signal Electrochemiluminescence Sensor for Kanamycin Detection Based on a Self-Enhanced Zr MOF and Single Co-Reactant Competition Mechanism. Biosensors. 2025; 15(5):291. https://doi.org/10.3390/bios15050291
Chicago/Turabian StyleZhu, Yawen, Xuemei Wang, Zhiyong Yan, Feifei Zhang, Jianfei Xia, Lili Lv, and Zonghua Wang. 2025. "A Dual-Signal Electrochemiluminescence Sensor for Kanamycin Detection Based on a Self-Enhanced Zr MOF and Single Co-Reactant Competition Mechanism" Biosensors 15, no. 5: 291. https://doi.org/10.3390/bios15050291
APA StyleZhu, Y., Wang, X., Yan, Z., Zhang, F., Xia, J., Lv, L., & Wang, Z. (2025). A Dual-Signal Electrochemiluminescence Sensor for Kanamycin Detection Based on a Self-Enhanced Zr MOF and Single Co-Reactant Competition Mechanism. Biosensors, 15(5), 291. https://doi.org/10.3390/bios15050291







