Abstract
Ecosystem pollution by mercury ions (Hg2+) is a major health concern, yet classical analytical methods for mercury analysis are limited. This paper reviews the advances in Hg2+ detection using DNA as recognition elements in the sensors. DNA as a recognition molecule is inexpensive, simple, and appropriate for real-time detection of Hg2+. This paper discusses the DNA-based sensors that were used for the detection of Hg2+. These can be carried out by electrochemistry, field effect transistors (FET), Raman spectroscopy, colorimetry, and fluorescence resonance energy transfer (FRET). The detection principles and the advantages of DNA in these sensors are also revealed. Finally, the paper provides an overview of prospects and potential challenges in the field.
1. Introduction
Humans and ecosystems face environmental pollution caused by heavy metals, as evidenced by incidents such as the Rhine pollution [1] and the Japanese Minamata disease [2], which resulted in severe health issues such as bone pain. The Rhine pollution incident comprised a 70-km-long pollution belt, killing all fish flowing through the river. The standard safe concentration of mercury in human blood is 1 µg/10 mL (5 × 10−7 M), and poisoning symptoms appear at 5~10 µg/10 mL (2.5 × 10−6 to 5 × 10−6 M). Lead, cadmium, mercury, and arsenic are refractory and accumulate in the biological chain, finally reaching toxic concentrations in the human body, causing damage to human health [3,4]. Mercury, arsenic, cadmium, copper, lead, tin, and manganese can cause irreversible damage to the human body if they exceed the standard value [5,6].
Hg2+ is a major threat to food safety, with potential contamination of food via multiple pathways, thus endangering human health through people’s dietary intake [7,8,9,10]. Hg2+ is easy to accumulate in the central nervous system of humans [11,12,13]. Ingestion of Hg2+ can cause lung injury, vomiting, diarrhea, nausea, movement disorders, as well as language and hearing impairment. Moreover, Hg2+ damages nerves and other organs and causes severe dysfunctions such as kidney and muscle issues, deformed limbs, paralysis, degeneration and necrosis of brain cells, difficulty in swallowing, and death [14,15,16,17,18]. The half-life of Hg2+ in the human body is rather long, approximately 70–80 days. Usually, Hg2+ is detected in blood or hair, which primarily reflects only organic mercury exposure [19]. Aggregation of Hg2+ concentration usually occurs in the liver, brain, kidney, and placenta, especially in the peripheral nerves, fetal brain, and bone marrow [20]. The presence of Hg2+ in even trace amounts can cause serious and irreversible harm to the human body, leading to a range of symptoms, including low-grade fever, fatigue, dizziness, headaches, sleep disturbances, adrenaline fluctuations, neurodevelopmental issues, and neurodegenerative conditions [21]. Given these risks, it is crucial to develop reliable methods for detecting Hg2+ to safeguard both human health and the environment [22,23,24,25,26].
Traditional detection methods, such as mass spectrometry [27,28,29,30,31], have made significant progress in the detection of Hg2+. However, these often require complex equipment, are costly, and can be time-consuming [32]. A significant breakthrough appeared in 2004 when Ono et al. [33] revealed that Hg2+ could selectively bind to DNA sequences containing thymine bases, forming a stable T-Hg2+-T structure [34]. This unique interaction opened the door to a new class of detection methods that leverage the stability and selectivity of DNA for quick and cost-effective detection [35,36]. Hg2+ replaces the hydrogen bonds typically found in Watson-Crick T-A pairing, thereby stabilizing the DNA duplex or structural motifs. Density functional theory (DFT) calculations have elucidated the electronic properties and bonding nature of the T-Hg2+-T complex [37,38]. The specific interaction of mercury ions (Hg2+) with DNA is determined by several key physicochemical factors, distinguishing it from other metal ions. One crucial factor is the soft acid-soft base principle (HSAB theory), which explains why Hg2+, a soft Lewis acid, preferentially interacts with thymine’s nitrogen (N3) and oxygen (O4) atoms, which are soft Lewis bases. This results in the formation of a highly stable T-Hg2+-T base pair, where Hg2+ bridges two thymine residues, replacing conventional hydrogen bonds. The unique coordination chemistry of Hg2+ enables selective and strong binding, stabilizing DNA structures and making it an excellent target for DNA-based mercury sensors. Compared to other metal ions, Hg2+ exhibits distinct electronic and coordination properties. While silver ions (Ag+) also form metal-mediated base pairs (e.g., C-Ag+-C), their interaction mechanism and stability differ from Hg2+ [39]. Monovalent cations such as lithium (Li+) [40], sodium (Na+) [41], and potassium (K+) [42] primarily interact with the phosphate backbone of DNA, influencing its structure and stability rather than forming base pair-mediated complexes. Bivalent metals like copper (Cu2+) [43], magnesium (Mg2+) [44], and lead (Pb2+) [45] interact differently; for example, Cu2+ can intercalate into DNA, Mg2+ stabilizes DNA duplexes via electrostatic interactions, and Pb2+ exhibits toxic binding effects that distort DNA structure. Ding et al. used isothermal titration calorimetry experiments to find that DNA tended to make the link ring of the hairpin a segment of four or five bases in the process of hairpin folding from a random coil induced by the external environment [46,47]. However, most researchers have not optimized DNA sequences for Hg2+ detection.
DNA is usually stable, low-cost, easy to fix and regenerate, which has been widely used in the sensor field. Various sensors have been developed based on the DNA recognition principle, including electrochemical sensors, FET, Raman, colorimetry, and FRET. This article reviews DNA-based approaches for Hg2+ detection, focusing on mechanisms, applications, and real-time monitoring. Compared to published reviews on related topics, such as fluorescence-based techniques, nanozymes, and surface-enhanced Raman spectroscopy, our manuscript focuses on DNA-based sensors (Table 1). DNA-based sensors show significant advantages, including high specificity, low cost, and high sensitivity. Therefore, DNA-based sensors have a wide range of applications in Hg2+ detection.

Table 1.
Recently published reviews on similar topics.
2. Electrochemistry
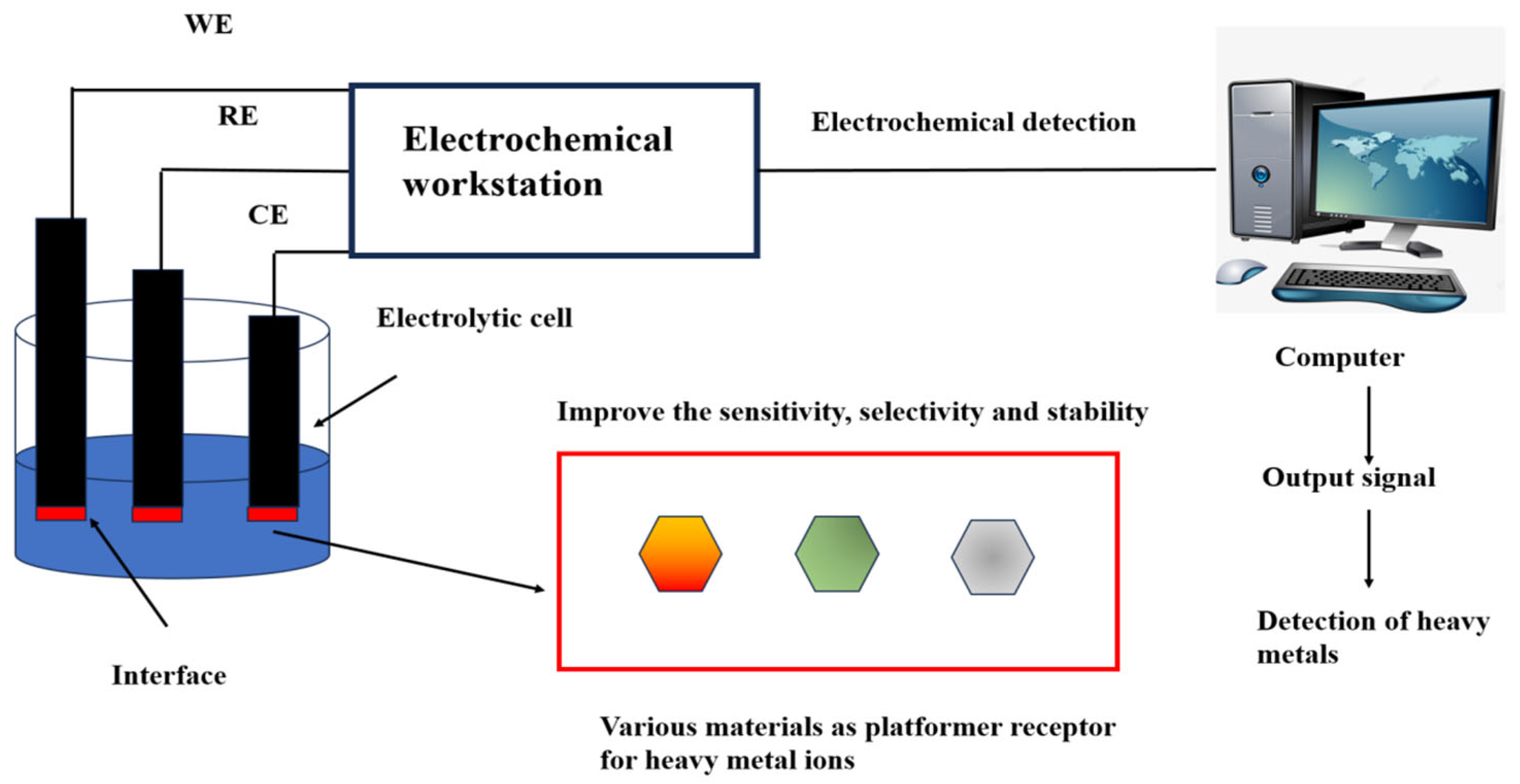
Electrochemical analysis is a pivotal branch of sensor technology, renowned for its accurate detection results and highly adaptable in in-situ measurements [62]. The US Environmental Protection Agency recommends using electrochemical technology to detect Hg2+ [63]. Electrochemical sensors were first used in oxygen detection in the 1950s and have been continuously improved by researchers to detect various analytes. The combination of DNA and electrochemistry for Hg2+ detection not only saves time but also improves the sensitivity of detection and simplifies the complexity of the experiment [64,65,66]. The reference electrode, working electrode, and counter electrode construct a three-electrode system in the electrochemical sensor [67] (Figure 1). The working electrode can be modified with different materials to specifically identify metal ion concentrations [68]. The presence of Hg2+ induces changes in current, potential, electrochemical impedance, capacitance, or electrochemiluminescence, enabling quantitative Hg2+ detection [69].
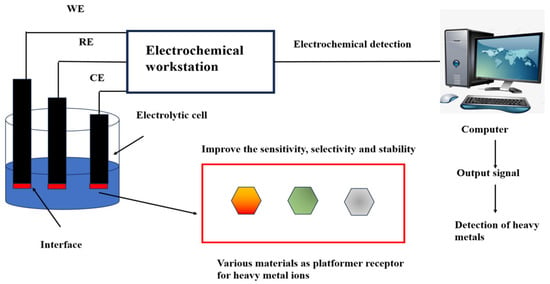
Figure 1.
Heavy metal ions detection using electrochemical sensing. The working electrode, reference electrode, and counter electrode are inserted into an electrolytic cell containing a heavy metal. The heavy metal ion platform or receptor at the interface of the working electrode reacts with the heavy metal to produce a changing current signal. The electrochemistry workstation, consisting of three electrodes, receives the signal and transmits it to the computer for analysis and processing. The output signal materializes the data to detect heavy metals directly or indirectly. Working electrode (WE): The interface contains a variety of materials that act as platforms or receptors for heavy metal ions. This improves the sensitivity, selectivity, and stability in heavy metal detection. The reference electrode (RE) is an electrode used as a reference when measuring the potential of various electrodes. The counter electrode (CE) balances the current to ensure that the electrochemical reaction can continue. Reproduced from Ju et al. (2015) with permission of Elsevier [67].
Many researchers often use DNA combined with graphene oxide (GO) to design a new electrochemical sensor for detecting metal ions (Table 2). This improves the sensitivity, selectivity, and even multi-path detection capabilities of the device [70]. GO-modified electrodes possess numerous advantages, including a large surface area, small volume, excellent electron transfer ability, and ease of surface modification [71,72]. Li et al. [73] used graphene in Nafion-G solution combined with in situ rhodium-plated electrodes to create an electrochemical sensor platform that enhanced signal detection. The study revealed that the composite membrane of Nafion-G showed highly sensitive detection for metal ions. This can interfere with the synergistic effect between graphene and Nafion. Noga Ratner et al. [74] developed a method for Hg2+ detection using indium tin oxide electrodes modified with gold nanoparticles based on glassy carbon, and the limit of detection (LOD) was 1 ng/L (5 × 10−12 M). Zhang et al. [75] combined graphene with DNA to design a faster and more sensitive electrochemical sensor to detect Hg2+. After the addition of Hg2+, the DNA sequence containing four thymine-thymidine (T-T) mismatches hybridized to the probe on the surface of the electrode with a T-Hg2+-T structure, increasing the peak current of [Ru(NH3)6]3+. The LOD in this method was 5.0 nM.

Table 2.
Comparison of electrochemistry-based Hg2+ detection biosensors.
To improve the sensitivity of detection, researchers gradually used GO composite nanomaterials with DNA to detect Hg2+. Fang et al. [76] synthesized the microspheres in one step on reduced GO (Cu2OMS-rGO) and then placed them in an electrochemical sensor to detect Hg2+ in water, which would be rich in thymine (T). The single-stranded oligonucleotide was immobilized on the electrode modified with Cu2OMS-rGO. Hg2+ can cause thymine to mismatch with itself. This makes a “T-Hg2+-T” structure form. Then, the analyte Hg2+ and the complementary single strand were introduced. DNA, which caused the double-stranded DNA carrying Hg2+ to be immobilized on the composite electrode. The detection sensitivity of the Cu2OMS-rGO complex to Hg2+ was high, and the LOD was 8.62 pM.
Overall, our observations indicate that the combination of DNA and GO or GO complex has become a leading approach for Hg2+ detection. The unique properties of GO contribute to enhanced sensitivity and selectivity, thereby improving the overall effectiveness of detection methods.
3. Field Effect Transistors
FET is a biosensing platform that has attracted high attention in disease detection, such as urinary infections, human immunodeficiency virus (HIV) infections, and hepatitis B [77,78,79,80,81]. The system possesses the following advantages: high sensitivity, fast response, and inexpensive manufacturing process. FET sensor provides a sensitive detection for the reaction taking place on the surface of the gate electrode without a redox label [82]. In 2014, Knopfmacher et al. [83] reported a DNA probe-modified organic field effect transistor (OFET) sensor for detecting Hg2+ using the spin coating of gold nanoparticles (AuNPs) onto polymer OFETs. The thiolated probe was attached to the surface. After combining with Hg2+, the DNA formed a change in the charge density of the hairpin structure and indirectly detected Hg2+. This method truly opened the gate of the OFET-binding DNA sensor for detecting Hg2+, and the LOD was obtained at 10 μM. It is believed that under continuous optimization, it can quickly achieve extremely high detection sensitivity and become a biosensor with environmental benefits.
Recently, graphene field-effect transistors (GFETs) have attracted high interest in the field of sensors [84,85,86,87,88,89,90,91,92,93]. Tu et al. [94] prepared a liquid-controlled graphene field effect transistor (GFET) array biosensor (a 6 × 6 GFET on a chip) that can quantitatively detect Hg2+ using the aptamers with a LOD of 40 pM and a fast response time of less than one second. Wang et al. [95] developed a single-strand DNA containing four phosphorothioate-modified RNAs (Hg-DPR) to improve the yield of the cleavage reaction. The FET using single-walled carbon nanotubes (FETs/SWNTs) was made based on Hg-DPR. After exposure to Hg2+, Hg-DPR could be effectively cleaved, which further changed the SWNTs conductivity. The Hg-DPR/SWNTs/FET successfully detected Hg2+ with a LOD of 10 pM.
Overall, FET sensors can achieve extremely high detection sensitivity for Hg2+ with DNA. Two-dimensional materials can cause changes in electrical signals and enable the detection. This can be further used to improve the excellent sensing performance.
4. Raman Spectroscopy
Raman spectroscopy is essentially a scattering spectrum discovered by CV Raman from India. The molecular vibration and molecular structure can be obtained from the spectrum. After the information, the researchers discovered that their high sensitivity, non-invasive, non-marking, and fingerprinting advantages are widely used in daily life [96,97,98]. Raman plays an indispensable role in detecting Hg2+ with highly sensitive, quick, and efficient DNA. Uchiyama et al. [99] and Benda et al. [33] performed detailed Raman characterization of the T-Hg(II)-T base, revealing the effect of Hg2+ binding to DNA.
Surface-enhanced Raman scattering (SERS) is used by some researchers as a detection method (Table 3). Compared with ordinary Raman, SERS is an optimized detection method based on surface plasmon resonance. There is electron resonance between the surface plasmon and the light field [100]. Raman signal can be significantly enhanced by 1014 [101]. SERS is a promising method for detecting Hg2+ [102,103,104,105,106]. Some researchers have focused on the discovery of the “T-Hg2+-T method” and the interpretation of the SERS phenomenon. This method depends on T-Hg2+-T and has high selectivity and sensitivity [107,108,109,110,111,112,113]. Han et al. [113] double-labeled the 5′ and 3′ ends of DNA with thiol and tetramethylrhodamine (TAMRA), respectively. DNA could be connected to the surface of gold microshells spontaneously. Hg2+ detection was carried out with a LOD of 50 nM.
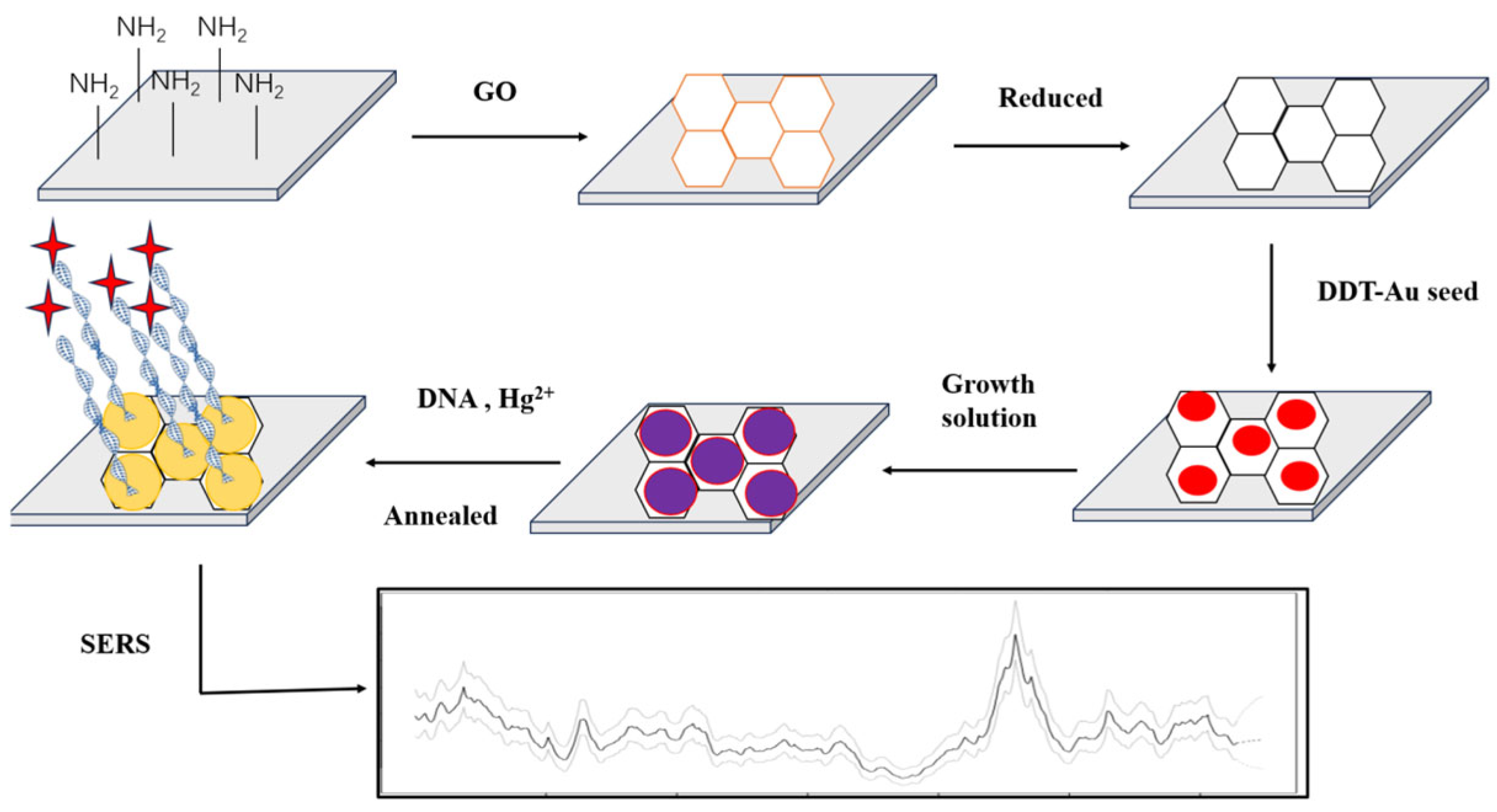
Wu et al. [114] reported a SERS sensor for Hg2+ sensing. Most importantly, this SERS sensing strategy was not affected by external factors and showed good SERS sensing performance and repeatability. The aptamer sensor reached 0.4 pM for LOD. Wang et al. [115] modified Ag nanoparticles (AgNPs) with TAMRA functionalized aptamers. T-Hg2+-T resulted in the hairpin structure of the aptamer. AgNPs aggregated, and the SERS intensity increased significantly. To facilitate the fixation of the probe DNA on the surface of the noble metal, the probe was further modified with a thiol group. The LOD was 5 nM. Despite the high selectivity of these SERS sensors, most of the NPs in these sensors were prepared in the liquid phase; therefore, they were easily aggregated in an uncontrollable manner, which affected the repeatability of SERS detection [103]. Many noble metals, such as Au, Ag, Cu, etc., can produce strong electromagnetic enhancement effects, which are often used to develop substrates [116]. For example, Ding et al. [107] grew AuNPs on the surface of rGO to form a SERS substrate and fixed the DNA probe with a thiol group on the surface of Au. The traces of Hg2+ were realized with the LOD of 0.1 nM (Figure 2).
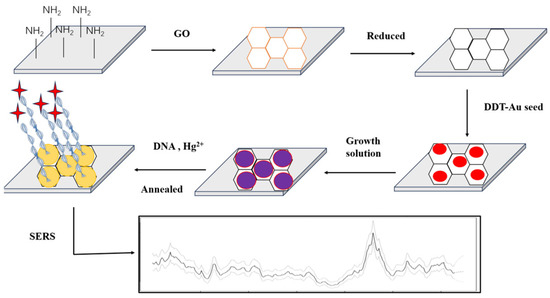
Figure 2.
Preparation of gold nanoparticles (AuNPs)/reduced graphene oxide (rGO). SERS: surface-enhanced Raman scattering; DTT: DL-Dithiothreitol. The active substrates of heterojunction SERS and sensing for Hg2+. AuNPs were grown on the surface of rGO to form a SERS substrate, and the DNA probe was fixed with a thiol group using an Au-S bond. The detected trace of Hg2+ by T-Hg2+-T coordination reached the detection limit of 0.1 nM. Reproduced from Liu et al. (2013) with permission from the American Chemical Society [107].
Liu et al. [117] developed a detection method using SERS. The method used a DNA molecular switch combined with a fragile silver film (Ag-film) to detect Hg2+ with high specificity. Single-stranded DNA was attached by the signal from a Raman probe with Hg2+. Due to the interaction between DNA bases and Hg2+, the specific structure of the DNA strand changed, and the signal was significantly enhanced. The SERS sensor achieved an ultra-low LOD (1.35 fM) of Hg2+ detection. Mohamed Shaban [118] invented a new type of SERS sensor, which functionalized the brass spiral nails through CoFe2O4 and grew carbon nanotubes (CNTs) on it. The Raman characteristic peak was enhanced 4 times, and the detection of Hg2+ with high sensitivity was realized through the adsorption of heavy metals with a range from 1 to 1000 ppb.
Cheng Tian et al. [119] reported a novel SERS sensing for trace Hg2+ using dual recycling amplification. During cyclic amplification, SERS is combined with a DNA amplification strategy to increase the Raman signal. When Hg2+ was added, the aptamer in the detection system formed a hairpin structure. Subsequently, through the role of polymerase, deoxy-ribonucleoside triphosphate (dNTPs) and Nicking endonuclease products from Bacillus brevis (Nt.BbvCI) in the reaction system, the trigger DNA could start the subsequent amplification reaction as a primer, and most SERS probes were stimulated to be fixed on the magnetic beads, which enhanced the Raman signal and improved the detection sensitivity with the LOD of 0.11 fM. Zhang et al. [120] developed a method for determining Hg2+. It is based on the principles of SERS and hybrid chain reaction (HCR). DNA is self-assembled on AuNPs to form a new signaling nanoprobe. The structure of T-Hg2+-T was fixed on magnetic beads. The HCR was initiated by the trigger DNA, which provided the binding sites to connect signaling nanoprobes. The sandwich structure was separated by using a magnetic field. The LOD was 0.08 pM.
Raman spectroscopy based on SPR provides a way to detect Hg2+ using the incident light frequency. Nanoparticles and other materials, such as carbon nanotubes, can improve the detection signals in Raman spectroscopy. DNA can also be amplified for higher signals for Hg2+. These two sides combine to further improve the detection sensitivity.

Table 3.
Comparison of Raman spectroscopy-based Hg2+ detection biosensors.
Table 3.
Comparison of Raman spectroscopy-based Hg2+ detection biosensors.
| DNA Probe | Nanomaterial or Other Auxiliary Material | Detection Linear Range | LOD | References |
|---|---|---|---|---|
| ssDNA | AuNPs/rGO/SiO2/Si heterojunction | 0.1–6000 nM | 0.1 nM | [107] |
| ssDNA | single gold micro-shell | 0–10 μM | 50 nM | [113] |
| dsDNA | Au@Ag NPs | 0–200 nM | 0.4 pM | [114] |
| ssDNA | AgNPs | 0–25 nM | 5 nM | [115] |
| ssDNA | Ag-film | 0.1 pM–10 μM | 1.35 fM | [117] |
| dsDNA | AuNPs | 0.1 pM–10 nM | 0.08 pM | [118] |
AuNPs: Gold nanoparticles. rGO: Reduced graphene oxide. AgNPs: Silver nanoparticles.
5. Colorimetry
Colorimetric detection is a traditional detection method based on color reaction. The content and composition of the substance to be tested are determined by comparing and measuring the color depth of the solution in which the analyte is to be measured [121,122]. The advantages and disadvantages of colorimetric detection methods are obvious. On the one hand, it can allow real-time qualitative or semi-quantitative detection without complex instruments. On the one hand, due to the limited recognition of our naked eyes, the relative error of the results obtained is relatively large. Compared to methods for detecting heavy metal ions, the development of colorimetric detection methods is limited. However, with the development of biology and nanotechnology, new methods for designing colorimetric biosensors are emerging [123,124]. In the process, researchers have discovered that gold nanoparticles have a high extinction coefficient. The ideal color material for colorimetric sensor design, and more importantly, when the gold nanoparticles are close to each other and aggregate, the color of the nanoparticles can change, and nanomolar concentrations can be observed with the naked eye [125,126,127]. Thus, sensitive detection is performed with minimal material consumption [128].
Knecht et al. [129] used the surface plasmon resonance coupling effect of AuNPs combined with a DNA sensor to detect Hg2+. First, AuNPs were prepared using citrate [130], and then the principle of DNA hybridization and T-Hg2+-T was used to promote the aggregation of AuNPs with a detection limit of 100 nM. Wang et al. [131] proposed a biosensor for detecting Hg2+ using the bovine serum albumin-protected silver clusters (BSA-Ag NCs). BSA-Ag NCs were activated by Hg2+, and the surrounding dissolved oxygen was used as an oxidant. BSA-Ag NCs could be “activated” by Hg2+. The LOD was 25 nM. Tan et al. [132] reported a universal sensing for Hg2+ using the target-mediated AuNPs growth. First, 15T bases were used to detect Hg2+ using T-Hg2+-T coordination. The aptamer was desorbed from the surface of AuNPs after binding to Hg2+, and the rest of the aptamers underwent a morphological change, which caused the structure of AuNPs to change to form different colored solutions. In this case, the LOD was 9.6 nM. Both 25-mer and 59-mer aptamers had LODs of 4.05 nM and 3 nM.
Zhu et al. [133] proposed a new strategy to detect Hg2+ using a colorimetric method: aggregation of cationic polymer-driven AuNPs. In this three-component system, DNA was electrostatically bound to diethylene glycol diethyl diacrylate (PDDA) in an AuNPs solution. The hairpin structure induced by T-Hg2+-T was formed in the DNA strand and then no longer interacted with PDDA, so free PDDA promoted the aggregation of AuNPs. Therefore, a colorimetric sensor was established, and AuNPs were aggregated to detect Hg2+. The LOD for Hg2+ based on the naked eye was 5 nM, which was used for rapid monitoring of Hg2+. The LOD by UV-Vis spectroscopy was as low as 0.15 nM. The DNA/AuNPs-based colorimetric method is more expensive than using DNA. Febrina et al. [134] developed a lower-cost CA-AuNPs filter paper sensor to detect Hg2+ in water. Cyanuric acid (CA) is a compound that is similar to thymine in structure, which could form a CA-Hg2+-CA structure with Hg2+. Meanwhile, CA could stabilize AuNPs to prevent aggregation and then immobilize them on the surface of filter paper. When the CA-Hg2+-CA complexes were formed by Hg2+, the stability of CA-AuNPs was reduced, and AuNPs aggregation occurred subsequently. In addition to the simple T-Hg2+-T principle, there are other color change and detection principles successively applied to detect Hg2+ in colorimetric reactions. Shao et al. [135] reported a gold nanoparticle colorimetric probe (AuNPs) with treated N-methylpyridone and chloroauric acid (HAuCl4) as precursors that directly respond to Hg2+. When Hg2+ is present, AuNPs will gather, and the absorbance at 700 nm will increase. The color changes with the LOD of 0.3 μM.
Wang et al. [136] used single-stranded (ssDNA) paired valence thiol groups. Inhibition of the oxidase-like activity of the metal-organic framework (expressed as MVC-MOF), synthesis of MVC-MOF by partial oxidation of cerium (III) can produce Ce(IV) ions, which typically confers MVC-MOF is typically oxidase-like. ssDNA binds MVC-MOF to mask its active site, which inhibits catalytic activity. A colorimetric detection for Hg (II) was reported using thymidine-rich ssDNA (T-ssDNA) as the model DNA. Hg2+ binds to T-ssDNA to form T-dsDNA, which causes MVC-MOF to convert 3, 3′, 5, 5′-tetramethylbenzidine to a blue product with oxygen. The LOD was 10.5 nM.
Overall, colorimetric detection is a traditional detection method using color reaction. It is based on the change of light signals. These simple and effective colorimetric assays have gradually expanded the range of applications, such as molecular diagnostics, drug delivery, and environmental detection, which are increasingly important and have high application potential (Table 4).

Table 4.
Comparison of colorimetry-based Hg2+ detection biosensors.
6. Fluorescence Resonance Energy Transfer Detection
FRET detection is a method based on the principle of FRET, which is sensitive and efficient in detecting the object by the change of fluorescence intensity. It is the most widely used and most sophisticated method for detecting heavy metal ions [42,137,138,139]. The method not only has low cost, low consumables, high sensitivity, and high accuracy, but it also has a sufficient theoretical basis, and the prepared sensor has strong stability and high practical application efficiency [42] (Table 5). Hg2+ was detected by Ono and Togashi [33] using FRET. Zhan et al. [140] used FAM dye and ethynyl modification to detect Hg2+ at both ends of multiple T-base DNA strands. After the addition of Hg2+, the structure of the DNA sequence was changed, which resulted in a fluorescence energy transfer between FAM and ethynyl with a LOD of 16.15 nM. Buranacha et al. [141] used a new type of biosensor aptamer. This used two kinds of DNA, a FRET donor and an acceptor, to carry out fluorescence hybridization. This resulted in the hybridization of the two DNA strands after Hg2+ was added. The LOD was 7.037 ± 0.18 nM. Ren et al. [142] inserted two DNA intercalators, YOYO-1 and TOTO-1, into the DNA strand. After the addition of Hg2+, FRET occurred between the conjugated polymer (PFP) with a positive charge and the dye in the DNA strand with a negative charge. Therefore, the fluorescence intensity was increased by 37 times, and the LOD was 6 nM.

Table 5.
Fluorescence resonance energy transfer sensors for Hg2+ detection.
Most researchers designed the biosensor using the unique T-Hg2+-T structure to detect Hg2+ and obtained a good response. For example, the DNA sensor described above relates more or less to the specific structure of T-Hg2+-T. Zhou et al. [143] used 2-aminopurine (2AP) as a fluorescent marker in the middle of 10-mer DNA homopolymers. The 2-AP-labeled DNA provides an ultra-low background without the need for external quenching. The addition of Hg2+ reduces the accumulation of 2AP and its adjacent thymine, enhancing the fluorescence signal. Zhu et al. [144] released the Hg2+ according to the principle that the T-Hg2+-T structure was cleaved by the enzyme Exo III. The DNA sequence was reconstructed into a G4 structure to detect Hg2+, which contained specific binding of iridium (III). Sun et al. [145] designed a FAM-ssDNA probe using GO as a fluorescence quencher and exonuclease I to hydrolyze ssDNA. When GO is close enough to the fluorescent dye, the fluorescent dye can be quenched by FRET. Under the condition of adding Exo I, the ssDNA was hydrolyzed so that the fluorescent dye of FAM was released, and the fluorescence was restored. However, ssDNA could form double strands through the T-Hg2+-T construct after Hg2+ was added. This inhibited the activity of Exo I; fluorescence did not recover. The LOD was 3.93 nM.
Wu et al. [146] proposed a novel dual FRET system that can be used to detect both Pb2+ and Hg2+. The system used AuNPs as acceptors and two-color up-conversion nanoparticles (UCNPs) as donors. Therefore, the donor-acceptor pairs can be formed. Due to the good overlap between the spectrum of UCNPs and AuNPs, the green and red up-conversion fluorescence could be quenched. After adding Hg2+ and Pb2+, the aptamer preferentially selected the corresponding analyte binding and formed a hairpin-like structure of Hg2+. The FRET was disrupted, and the up-conversion fluorescence was restored. The fluorescence intensity increased with increasing metal ion concentration under optimized experimental conditions, and both Hg2+ and Pb2+ could be quantified. The LODs of the two were 150 pM and 50 pM. Li et al. [147] used a naphthalimide derivative (AHN) as a fluorescent label for thymidine (T)-rich ssDNA and Fe3O4 nanoparticle as a quencher. The labeled ssDNA was not separated from the magnetized GO without Hg2+, which resulted in the complete quenching of the fluorescence. The fluorescence recovery with the addition of Hg2+ was achieved away from GO fluorescence, with the LOD of 0.65 nM.
Hg2+ has a strong sulfophilicity, and a DNA sensor can be designed in a new direction according to this property. In 2015, Liu et al. [148] reported a mechanism of Hg2+-induced phosphorothioate (PS)-modified RNA cleavage in which a non-bridged oxygen atom in a DNA strand deoxynucleotide is replaced by a sulfur atom (S). Because Hg2+ is extremely sulfophilic, its binding to S leads to cleavage of the phosphate bond, causing the end with FAM to be far from the quencher, and the fluorescence intensity is enhanced. The study emphasizes that even if there is only a single bottom, the substance (without DNase) can also be cleaved by Hg2+. Zhao et al. [149] developed a ratio-metric fluorescent probe (namely APS-NA) with acridone as an energy donor and 1, 8-naphthalimide as an energy acceptor. When Hg2+ was present, the dithioacetal bond between acridone and 1, 8-naphthalimide, was destroyed. Then, the bright blue fluorescence was emitted, which achieved the detection of Hg2+. In addition, Liu et al. [150] developed a sensor using fluorescence quenching with PS-RNA chemistry and tested three DNA probes, each containing 1, 3, and 5 PS-RNA Hg2+ cleavage sites, demonstrating that increased cleavage sites will increase the Hg2+ cleavage rate relatively. Fluorescent-labeled Poly-A DNA was adsorbed on the surface of NGOs, and after the addition of Hg2+, the release of the fluorophore from GO resulted in enhanced fluorescence. The fluorophore-labeled DNA was used for detection, which has a slower response to Hg2+ and is susceptible to various external factors, while the PS-RNA probe is stable and has strong anti-interference ability. It is less affected by temperature and pH.
Overall, FRET can be carried out to detect the object. The effect of quenching is higher, and the sensitivity of detection is usually higher. Therefore, more quenchers with low cost have been used to improve the effect of quenching.
7. Conclusions
Given the wide range of techniques available for Hg2+ sensing, it is crucial to thoroughly understand their respective strengths and weaknesses to select the most appropriate detection strategy. Therefore, in addition to a detailed discussion of the key features of each technique, a comparative table is presented to provide a clearer evaluation of their advantages and limitations (Table 6).

Table 6.
Advantages and limitations of Hg2+ detection techniques.
If the sensitivity of outdoor detection is not high, the electrochemical sensors are more suitable for point-of-care diagnosis because of the relatively low detection cost and easy-to-obtain direct detection results. Raman spectroscopy and electrochemical sensors are limited in the number of samples they can detect or are unable to detect multiple samples simultaneously. Given these issues, there is a continuing need to explore more stable, sensitive, low-cost, efficient, and time-efficient methods for detecting Hg2+. Advancements in this area are crucial not only for environmental protection but also for ensuring human health.
Funding
This work is supported by National Natural Science Foundation of China (NSAF, No. U2230132), the open fund of Information Materials and Intelligent Sensing Laboratory of Anhui Province (Grant No. IMIS202209), Guiding Science and Technology Plan Project for Social Development in Zhenjiang City (FZ2022052), and Zhenjiang Science and Technology Plan (Social development, SH2024098), and the National Foreign Experts Program Project of China (H20240553).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Timm, C.; Luther, S.; Jurzik, L.; Hamza, A.; Kistemann, T. Applying QMRA and DALY to assess health risks from river bathing. Int. J. Hyg. Environ. Health 2016, 219, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K. The discovery of the causal agent of Minamata disease. Am. J. Ind. Med. 1992, 21, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Y.; Gong, L.; Gan, S.; Gu, M.; Wang, S. Association between cadmium exposure and urolithiasis risk: A systematic review and meta-analysis. Medicine 2018, 97, e9460. [Google Scholar] [CrossRef]
- Lin, X.; Gu, Y.; Zhou, Q.; Mao, G.; Zou, B.; Zhao, J. Combined toxicity of heavy metal mixtures in liver cells. J. Appl. Toxicol. 2016, 36, 1163–1172. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.; Beeregowda, N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Seo, R. An overview of carcinogenic heavy metal: Molecular toxicity mechanism and prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef]
- Blaise, E. Global Mercury Assessment, 2002. Available online: www.unep.org/globalmercurypartnership/resources/report/global-mercury-assessment-2002 (accessed on 18 February 2025).
- Wang, G.; Zhao, Q.; Kang, X.; Guan, X. Probing mercury(II)-DNA interactions by nanopore stochastic sensing. J. Phys. Chem. B 2013, 117, 4763–4769. [Google Scholar] [CrossRef]
- Huang, D.; Niu, C.; Zeng, G.; Wang, X.; Lv, X. A highly sensitive protocol for the determination of Hg(2+) in environmental water using time-gated mode. Talanta 2015, 132, 606–612. [Google Scholar] [CrossRef]
- Huang, J.; Gao, X.; Jia, J.; Kim, J.K.; Li, Z. Graphene oxide-based amplified fluorescent biosensor for Hg(2+) detection through hybridization chain reactions. Anal. Chem. 2014, 86, 3209–3215. [Google Scholar] [CrossRef]
- Harris, H.; Pickering, J.; George, N. The chemical form of mercury in fish. Science 2003, 301, 1203. [Google Scholar] [CrossRef]
- Vo, T.; Bank, S.; Shine, P.; Edwards, V. Temporal increase in organic mercury in an endangered pelagic seabird assessed by century-old museum specimens. Proc. Natl. Acad. Sci. USA 2011, 108, 7466–7471. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lin, H.; Tseng, L. Oligonucleotide-functionalized silver nanoparticle extraction and laser-induced fluorescence for ultrasensitive detection of mercury(II) ion. Biosens. Bioelectron. 2012, 34, 185–190. [Google Scholar] [CrossRef]
- Huang, C.; Chang, T. Selective gold-nanoparticle-based “turn-on” fluorescent sensors for detection of mercury(II) in aqueous solution. Anal. Chem. 2006, 78, 8332–8338. [Google Scholar] [CrossRef]
- Alina, M.; Azrina, A.; Mohd Yunus, S.; Mohd Zakiuddin, S.; Mohd, H.; Muhammad Rizal, R. Heavy metals (mercury, arsenic, cadmium, plumbum) in selected marine fish and shellfish along the straits of malacca. Int. Food Res. J. 2012, 19, 135–140. [Google Scholar]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef]
- Park, D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef]
- Lewerenz, J. Methylmercury (environmental health criteria no. 101). 144 seiten, 5 abb. 11 tab. world health organization, geneva 1990. preis: 16,—sw.fr.; 12,80 us $. Mol. Nutr. Food Res. 2010, 35, 326–327. [Google Scholar] [CrossRef]
- Björkman, L.; Lundekvam, F.; Laegreid, T.; Bertelsen, I.; Morild, I.; Lilleng, P.; Lind, B.; Palm, B.; Vahter, M. Mercury in human brain, blood, muscle and toenails in relation to exposure: An autopsy study. Environ. Health 2007, 6, 30. [Google Scholar] [CrossRef]
- Garza-Lombó, C.; Posadas, Y.; Quintanar, L.; Gonsebatt, E.; Franco, R. Neurotoxicity linked to dysfunctional metal ion homeostasis and xenobiotic metal exposure: Redox signaling and oxidative stress. Antioxid. Redox Signal. 2018, 28, 1669–1703. [Google Scholar] [CrossRef]
- Qi, Y.; Xiu, F.R.; Yu, G.; Huang, L.; Li, B. Simple and rapid chemiluminescence aptasensor for Hg2+ in contaminated samples: A new signal amplification mechanism. Biosens. Bioelectron. 2017, 87, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, X.; Shi, H.; Luo, Y. T-T mismatch-driven biosensor using triple functional DNA-protein conjugates for facile detection of Hg2+. Biosens. Bioelectron. 2016, 78, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, J.; Jiang, B.; Yuan, R.; Xiang, Y. Metallo-toehold-activated catalytic hairpin assembly formation of three-way DNAzyme junctions for amplified fluorescent detection of Hg2+. ACS Appl. Mater. Inter. 2017, 9, 5733–5738. [Google Scholar] [CrossRef]
- Lehel, J.; Zwillinger, D.; Bartha, A.; Lányi, K.; Laczay, P. Food safety aspects of primary environmental contaminants in the edible tissues of roe deer (Capreolus capreolus). Environ. Sci. Pollut. 2017, 24, 25372–25382. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B. Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian Gulf. Environ. Pollut. 2018, 232, 154–163. [Google Scholar] [CrossRef]
- Wang, M.; Feng, W.; Shi, J.; Zhang, F.; Wang, B.; Zhu, M.; Li, B.; Zhao, Y.; Chai, Z. Development of a mild mercaptoethanol extraction method for determination of mercury species in biological samples by HPLC-ICP-MS. Talanta 2007, 71, 2034–2039. [Google Scholar] [CrossRef]
- Bloom, N.; Fitzgerald, W.F. Determination of volatile mercury species at the picogram level by low-temperature gas chromatography with cold-vapour atomic fluorescence detection. Anal. Chim. Acta 1988, 208, 151–161. [Google Scholar] [CrossRef]
- Fong, M.; Siu, S.; Lee, S.; Tam, S. Determination of mercury in whole blood and urine by inductively coupled plasma mass spectrometry. J. Anal. Toxicol. 2007, 31, 281–287. [Google Scholar] [CrossRef]
- Leopold, K.; Foulkes, M.; Worsfold, P. Methods for the determination and speciation of mercury in natural waters—A review. Anal. Chim. Acta 2010, 663, 127–138. [Google Scholar] [CrossRef]
- Karunasagar, D.; Arunachalam, J.; Gangadharan, S. Development of a ‘collect and punch’ cold vapour inductively coupled plasma mass spectrometric method for the direct determination of mercury at nanograms per litre levels. J. Anal. At. Spectrom. 1998, 13, 679–682. [Google Scholar] [CrossRef]
- Miller, L.; Watson, B.; Lester, P.; Lowe, A.; Pierce, M.; Liang, L. Characterization of soils from an industrial complex contaminated with elemental mercury. Environ. Res. 2013, 125, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Togashi, H. Highly selective oligonucleotide-based sensor for mercury(II) in aqueous solutions. Angew. Chem. Int. Edit 2004, 43, 4300–4302. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Togashi, H.; Tashiro, M.; Yamaguchi, H.; Oda, S.; Kudo, M.; Tanaka, Y.; Kondo, Y.; Sawa, R.; Fujimoto, T.; et al. MercuryII-mediated formation of thymine-HgII-thymine base pairs in DNA duplexes. J. Am. Chem. Soc. 2006, 128, 2172–2173. [Google Scholar] [CrossRef]
- Srinivasan, K.; Subramanian, K.; Murugan, K.; Benelli, G.; Dinakaran, K. Fluorescence quenching of MoS2 nanosheets/DNA/silicon dot nanoassembly: Effective and rapid detection of Hg2+ ions in aqueous solution. Environ. Sci. Pollut. 2018, 25, 10567–10576. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; An, F.; Xu, H.; Yin, Z.; Tang, S.; Yang, H.; Song, H. Graphitic carbon nitride supported platinum nanocomposites for rapid and sensitive colorimetric detection of mercury ions. Anal. Chim. Acta 2017, 980, 72–78. [Google Scholar] [CrossRef]
- Benda, L.; Straka, M.; Sychrovsky, V.; Bour, P. Detection of mercury-TpT dinucleotide binding by Raman Spectra: Acomputational study. J. Phys. Chem. A 2012, 116, 8313–8320. [Google Scholar] [CrossRef]
- Bhai, S.; Ganguly, B. Role of the backbone of nucleic acids in the stability of Hg2+-mediated canonical base pairs and thymine–thymine mispair: A DFT study. RSC Adv. 2020, 10, 40969–40982. [Google Scholar] [CrossRef]
- Reveguk, Z.; Khoroshilov, E.; Sharkov, A.; Pomogaev, V.; Buglak, A.; Kononov, A. Excited states in single-stranded and i-motif DNA with silver ions. J. Phys. Chem. B 2024, 128, 4377–4384. [Google Scholar] [CrossRef]
- Pakiari, A.; Farrokhnia, M. Nature of lithium interactions with DNA nucleobases: Theoretical study. Phys. Chem. Res. 2014, 2, 229–243. [Google Scholar]
- Auffinger, P.; Ascenzo, L.; Ennifar, E. Sodium and potassium interactions with nucleic acids. Met. Ions Life Sci. 2016, 16, 167–201. [Google Scholar]
- Song, Y.; Xie, X.; Liu, Y.; Zhu, Z.; Sun, L. Nanoscale study of DNA–Cu2+ interactions by liquid-cell electron microscopy. ACS Omega 2023, 8, 26325–26331. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Timsina, R.; Dewage, S.; Qiu, X.; Kirmizialtin, S. Sequence effects on Mg+2 ion mediated DNA—DNA interactions. Biophys. J. 2019, 116, 360a. [Google Scholar] [CrossRef]
- Wang, K.; Clough, P.; Zhao, P.; Anthony, E. Synthesis of highly effective stabilized CaO sorbents via a sacrificial N-doped carbon nanosheet template. J. Mater. Chem. A 2019, 7, 9173–9182. [Google Scholar] [CrossRef]
- Liang, Y.; Deng, B.; Shen, C.; Qin, X.; Liang, S. Determination of the binding sites and binding constants between Pb(ii) and DNA using capillary electrophoresis combined with electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 2015, 30, 903–908. [Google Scholar] [CrossRef]
- Ding, W.; Xu, M.; Zhu, H.; Liang, H. Mechanism of hairpin folding transformation of thymine-cytosine-rich oligonucleotides induced by Hg(II) and Ag(I) Ion. Eur. Phys. J. E 2013, 36, 9917–9924. [Google Scholar] [CrossRef]
- Ding, W.; Deng, W.; Zhu, H.; Liang, H. Metallo—Toeholds controlling DNA strand displacement driven by Hg(II) ions. Chem. Commun. 2013, 49, 9953–9955. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, C.; Chang, H. Gold nanoparticle probes for the detection of mercury, lead and copper ions. Analyst 2011, 136, 863–871. [Google Scholar] [CrossRef]
- Du, J.; Jiang, L.; Shao, Q.; Liu, X.; Marks, R.S.; Ma, J.; Chen, X. Colorimetric detection of mercury ions based on plasmonic nanoparticles. Small 2013, 9, 1467–1481. [Google Scholar] [CrossRef]
- Wan, J.; Ma, X.L.; Xing, L. Highly sensitive fluorescence detection of mercury (II) ions based on DNA machine amplification. Sens. Actuat. B Chem. 2013, 178, 615–620. [Google Scholar] [CrossRef]
- Song, C.; Yang, B.; Yang, Y.; Wang, L. SERS-based mercury ion detections: Principles, strategies and recent advances. Sci. China Chem. 2016, 59, 16–29. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Housaindokht, M.; Verdian, A.; Bozorgmehr, M. Simultaneous detection and determination of mercury (II) and lead (II) ions through the achievement of novel functional nucleic acid-based biosensors. Biosens. Bioelectron. 2018, 116, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Feng, F.; Liu, C.; Li, R.; Xiang, W.; Shi, H.; Gao, L. The detection of mercury ion using DNA as sensors based on fluorescence resonance energy transfer. Talanta 2019, 192, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Nanakali, N.; Salihi, A.; Rasti, B.; Sharifi, M.; Attar, F.; Derakhshankhah, H.; Mustafa, I.; Abdulqadir, S.; Falahati, M. Nanozyme-based sensing platforms for detection of toxic mercury ions: An alternative approach to conventional methods. Talanta 2020, 215, 120939. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Yang, J.; Wang, Y.; Yang, Y. Recent progress in functional materials for selective detection and removal of mercury(II) ions. Adv. Funct. Mater. 2021, 31, 1. [Google Scholar] [CrossRef]
- Gul, Z.; Ullah, S.; Khan, S.; Ullah, H.; Khan, M.U.; Ullah, M.; Ali, S.; Altaf, A. Recent progress in nanoparticles based sensors for the detection of mercury (II) ions in environmental and biological samples. Crit. Rev. Anal. Chem. 2024, 54, 44–60. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Li, K.; Yu, X. Small molecular fluorescent probes for the detection of lead, cadmium and mercury ions. Coord. Chem. Rev. 2021, 429, 213691. [Google Scholar] [CrossRef]
- Wang, S. Construction of DNA biosensors for mercury (II) ion detection based on enzyme-driven signal amplification strategy. Biomolecules 2021, 11, 399. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef]
- Udhayakumari, D. Review on fluorescent sensors-based environmentally related toxic mercury ion detection. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 451–476. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y. Recent advances in fluorescent materials for mercury(ii) ion detection. RSC Adv. 2023, 13, 19429–19446. [Google Scholar] [CrossRef]
- Wong, E.; Chow, E.; Gooding, J. The electrochemical detection of cadmium using surface-immobilized DNA. Electrochem. Commun. 2007, 9, 845–849. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; González-García, M.; Costa-García, A. Electrochemical determination of mercury: A review. Talanta 2013, 116, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Li, Y.; Meng, S.; Li, W.; Liu, D.; You, T. Electric field-enabled aptasensing interfacial engineering to simultaneously enhance specific preconcentration and electrochemical detection of mercury and lead ions. SCI Total Environ. 2023, 900, 166407. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lu, B.; Cheng, L.; Xu, X.; Wang, M.; Xu, W.; Cui, F. A double signal amplification-based homogeneous electrochemical sensor built on catalytic hairpin assembly and bisferrocene markers. Anal. Biochem. 2021, 632, 114140. [Google Scholar] [CrossRef]
- Chang, Y.; Tang, X.; Huang, J.; Chai, Y.; Zhuo, Y.; Li, H.; Yuan, R. Programming a “Crab Claw”-like DNA nanomachine as a super signal amplifier for ultrasensitive electrochemical assay of Hg2+. Anal. Chem. 2021, 93, 12075–12080. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Ju, H. Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens. Bioelectron. 2015, 63, 276–286. [Google Scholar] [CrossRef]
- Bontidean, I.; Berggren, C.; Johansson, G.; Csöregi, E.; Mattiasson, B.; Lloyd, R.; Jakeman, J.; Brown, L. Detection of heavy metal ions at femtomolar levels using protein-based biosensors. Anal. Chem. 1998, 70, 4162–4169. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, B.; Farias, A.; Ogorevc, B. Bismuth-coated carbon electrodes for anodic stripping voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef]
- Li, M.; Gou, L.; Al-Ogaidi, I.; Wu, N. Nanostructured sensors for detection of heavy metals: A review. ACS Sustain. Chem. Eng. 2013, 1, 713–723. [Google Scholar] [CrossRef]
- Wanekaya, K. Applications of nanoscale carbon-based materials in heavy metal sensing and detection. Analyst 2011, 136, 4383–4391. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Li, J.; Guo, S.; Zhai, Y.; Wang, E. High-sensitivity determination of lead and cadmium based on the nafion-graphene composite film. Anal. Chim. Acta 2009, 649, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Ratner, N.; Mandler, D. Electrochemical detection of low concentrations of mercury in water using gold nanoparticles. Anal. Chem. 2015, 87, 5148–5155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, H.; Wu, Z.; Xue, Y.; Zhang, X.; He, Y.; Li, X.; Yuan, Z. A novel graphene-DNA biosensor for selective detection of mercury ions. Biosens. Bioelectron. 2013, 48, 180–187. [Google Scholar] [CrossRef]
- Fang, S.; Dong, X.; Zhang, Y.; Kang, M.; Liu, S.; Yan, F.; He, L.; Feng, X.; Wang, P.; Zhang, Z. One-step synthesis of porous cuprous oxide microspheres on reduced graphene oxide for selective detection of mercury ions. New J. Chem. 2014, 38, 5935–5942. [Google Scholar] [CrossRef]
- Estrela, P.; Paul, D.; Song, Q.; Stadler, K.; Wang, L.; Huq, E.; Davis, J.; Ko Ferrigno, P.; Migliorato, P. Label-free sub-picomolar protein detection with field-effect transistors. Anal. Chem. 2010, 82, 3531–3536. [Google Scholar] [CrossRef]
- Formisano, N.; Bhalla, N.; Heeran, M.; Reyes Martinez, J.; Sarkar, A.; Laabei, M.; Jolly, P.; Bowen, R.; Taylor, T.; Flitsch, S.; et al. Inexpensive and fast pathogenic bacteria screening using field-effect transistors. Biosens. Bioelectron. 2016, 85, 103–109. [Google Scholar] [CrossRef]
- Aliakbarinodehi, N.; Jolly, P.; Bhalla, N.; Miodek, A.; De Micheli, G.; Estrela, P.; Carrara, S. Aptamer-based field-effect biosensor for tenofovir detection. Sci. Rep. 2017, 7, 44409. [Google Scholar] [CrossRef]
- Hung, C.; Cheng, J.; Yang, F.; Lo, P. Investigation of extended-gate field-effect transistor pH sensors based on different-temperature-annealed bi-layer MWCNTs-In2O3 films. Nanoscale Res. Lett. 2014, 9, 502. [Google Scholar] [CrossRef]
- Marchenko, V.; Soldatkin, O.; Kasap, O.; Kurc, A.; Soldatkin, P.; Dzyadevych, V. Creatinine deiminase adsorption onto silicalite-modified pH-FET for creation of new creatinine-sensitive biosensor. Nanoscale Res. Lett. 2016, 11, 173. [Google Scholar] [CrossRef]
- Park, J.; Kwon, S.; Lee, H.; Song, S.; Park, H.; Jang, J. Ultrasensitive flexible graphene based field-effect transistor (FET)-type bioelectronic nose. Nano Lett. 2012, 12, 5082–5090. [Google Scholar] [CrossRef] [PubMed]
- Knopfmacher, O.; Hammock, L.; Appleton, L.; Schwartz, G.; Mei, J.; Lei, T.; Pei, J.; Bao, Z. Highly stable organic polymer field-effect transistor sensor for selective detection in the marine environment. Nat. Commun. 2014, 5, 2954. [Google Scholar] [CrossRef] [PubMed]
- Dan, Y.; Lu, Y.; Kybert, J.; Luo, Z.; Johnson, T. Intrinsic response of graphene vapor sensors. Nano Lett. 2009, 9, 1472–1475. [Google Scholar] [CrossRef]
- He, Q.; Sudibya, G.; Yin, Z.; Wu, S.; Li, H.; Boey, F.; Huang, W.; Chen, P.; Zhang, H. Centimeter-long and large-scale micropatterns of reduced graphene oxide films: Fabrication and sensing applications. ACS Nano 2010, 4, 3201–3208. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, X.; Shi, Y.; Li, M.; Li, J.; Chen, P. Nanoelectronic biosensors based on CVD grown graphene. Nanoscale 2010, 2, 1485–1488. [Google Scholar] [CrossRef]
- Myung, S.; Solanki, A.; Kim, C.; Park, J.; Kim, S.; Lee, B. Graphene-encapsulated nanoparticle-based biosensor for the selective detection of cancer biomarkers. Adv. Mater. 2011, 23, 2221–2225. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Jeon, S.; Kim, M.; Kim, S.; Kim, K.; Bien, F.; Hong, Y.; Park, U. Highly transparent and stretchable field-effect transistor sensors using graphene-nanowire hybrid nanostructures. Adv. Mater. 2015, 27, 3292–3297. [Google Scholar] [CrossRef]
- Han, D.; Chand, R.; Kim, S. Microscale loop-mediated isothermal amplification of viral DNA with real-time monitoring on solution-gated graphene FET microchip. Biosens. Bioelectron. 2017, 93, 220–225. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Zhu, Y.; Zhou, X.; Xiang, Y.; He, M.; Zeng, S. Fully integrated graphene electronic biosensor for label-free detection of lead (II) ion based on G-quadruplex structure-switching. Biosens. Bioelectron. 2017, 89 Pt 2, 758–763. [Google Scholar] [CrossRef]
- Kotlowski, C.; Larisika, M.; Guerin, M.; Kleber, C.; Krober, T.; Mastrogiacomo, R.; Nowak, C.; Pelosi, P.; Schutz, S.; Schwaighofer, A.; et al. Fine discrimination of volatile compounds by graphene-immobilized odorant-binding proteins. Sens. Actuat. B Chem. 2018, 256, 564–572. [Google Scholar] [CrossRef]
- Mansouri Majd, S.; Salimi, A. Ultrasensitive flexible FET-type aptasensor for CA 125 cancer marker detection based on carboxylated multiwalled carbon nanotubes immobilized onto reduced graphene oxide film. Anal. Chim. Acta 2018, 1000, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, S.; Zhang, C.; Yue, W.; Zou, Y.; Wang, G.; Liu, H.; Zhang, X.; Li, M.; Zhu, Z.; et al. Ultrasensitive label-free detection of dna hybridization by sapphire-based graphene field-effect transistor biosensor. Appl. Surf. Sci. 2018, 427, 1114–1119. [Google Scholar] [CrossRef]
- Tu, J.; Gan, Y.; Liang, T.; Hu, Q.; Wang, Q.; Ren, T.; Sun, Q.; Wan, H.; Wang, P. Graphene FET array biosensor based on ssDNA aptamer for ultrasensitive Hg2+ detection in environmental pollutants. Front. Chem. 2018, 6, 333. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Liu, G. Electrochemical biosensor using DNA embedded phosphorothioate modified RNA for mercury ion determination. ACS Sens. 2018, 3, 624–631. [Google Scholar] [CrossRef]
- Dickinson, G.; Dillon, T. Raman spectra of solutions of some ionized substances. Proc. Natl. Acad. Sci. USA 1929, 15, 334–337. [Google Scholar] [CrossRef][Green Version]
- Salant, O.; Sandow, A. Raman scattering from HCL liquid. Science 1929, 69, 357. [Google Scholar] [CrossRef]
- Chen, Q.; Fu, Y.; Zhang, W.; Ye, S.; Zhang, H.; Xie, F.; Gong, L.; Wei, Z.; Jin, H.; Chen, J. Highly sensitive detection of glucose: A quantitative approach employing nanorods assembled plasmonic substrate. Talanta 2017, 165, 516–521. [Google Scholar] [CrossRef]
- Uchiyama, T.; Miura, T.; Takeuchi, H.; Dairaku, T.; Komuro, T.; Kawamura, T.; Kondo, Y.; Benda, L.; Sychrovsky, V.; Bour, P.; et al. Raman spectroscopic detection of the T-Hg II-T base pair and the ionic characteristics of mercury. Nucleic Acids Res. 2012, 40, 5766–5774. [Google Scholar] [CrossRef]
- Kneipp, J.; Kneipp, H.; Kneipp, K. SERS—A single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 2008, 37, 1052–1060. [Google Scholar] [CrossRef]
- Feng, S.; Zheng, Z.; Xu, Y.; Lin, J.; Chen, G.; Weng, C.; Lin, D.; Qiu, S.; Cheng, M.; Huang, Z.; et al. A noninvasive cancer detection strategy based on gold nanoparticle surface-enhanced raman spectroscopy of urinary modified nucleosides isolated by affinity chromatography. Biosens. Bioelectron. 2017, 91, 616–622. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, H.; Zhang, L.; Wu, T.; Sun, L.; Jiang, D.; Du, Y. In situ preparation of Ag nanoparticles by laser photoreduction as sers substrate for determination of Hg2+. J. Raman Spectrosc. 2017, 48, 399–404. [Google Scholar] [CrossRef]
- Sun, Z.; Du, J.; Jing, C. Recent progress in detection of mercury using surface enhanced Raman spectroscopy—A review. J. Environ. Sci. 2016, 39, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Esmaielzadeh Kandjani, A.; Sabri, M.; Mohammad-Taheri, M.; Bansal, V.; Bhargava, K. Detect, remove and reuse: A new paradigm in sensing and removal of Hg (II) from wastewater via SERS-active ZnO/Ag nanoarrays. Environ. Sci. Technol. 2015, 49, 1578–1584. [Google Scholar] [CrossRef]
- Lu, Y.; Zhong, J.; Yao, G.; Huang, Q. A label-free sers approach to quantitative and selective detection of mercury (II) based on dna aptamer-modified SiO2@Au core/shell nanoparticles. Sens. Actuat. A Phys. 2018, 258, 365–372. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Lai, Y.; Wu, C.; Sun, S.; He, Y.; Ma, H. Ultrasensitive and selective detection of copper (II) and mercury (II) ions by dye-coded silver nanoparticle-based SERS probes. Biosens. Bioelectron. 2013, 39, 82–87. [Google Scholar] [CrossRef]
- Ding, X.; Kong, L.; Wang, J.; Fang, F.; Li, D.; Liu, J. Highly sensitive SERS detection of Hg2+ ions in aqueous media using gold nanoparticles/graphene heterojunctions. ACS Appl. Mater. Interfaces 2013, 5, 7072–7078. [Google Scholar] [CrossRef]
- Ma, W.; Sun, M.; Xu, L.; Wang, L.; Kuang, H.; Xu, C. A SERS active gold nanostar dimer for mercury ion detection. Chem. Commun. 2013, 49, 4989–4991. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Zong, S.; Chen, H.; Zhu, D.; Wu, L.; Hu, G.; Cui, Y. SERS detection and removal of mercury(II)/silver(I) using oligonucleotide-functionalized core/shell magnetic silica sphere@Au nanoparticles. ACS Appl. Mater. Inter. 2014, 6, 7371–7379. [Google Scholar] [CrossRef]
- Xu, L.; Yin, H.; Ma, W.; Kuang, H.; Wang, L.; Xu, C. Ultrasensitive SERS detection of mercury based on the assembled gold nanochains. Biosens. Bioelectron. 2015, 67, 472–476. [Google Scholar] [CrossRef]
- Sun, B.; Jiang, X.; Wang, H.; Song, B.; Zhu, Y.; Wang, H.; Su, Y.; He, Y. Surface-enhancement Raman scattering sensing strategy for discriminating trace mercuric ion (II) from real water samples in sensitive, specific, recyclable, and reproducible manners. Anal. Chem. 2015, 87, 1250–1256. [Google Scholar] [CrossRef]
- Yi, Z.; Li, Y.; Liu, J.; Jin, Y.; Chu, X.; Yu, Q. Design of label-free, homogeneous biosensing platform based on plasmonic coupling and surface-enhanced Raman scattering using unmodified gold nanoparticles. Biosens. Bioelectron. 2013, 43, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Lim, Y.; Kim, J.; Piao, L.; Chung, T.D. Mercury(ii) detection by SERS based on a single gold microshell. Chem. Commun. 2010, 46, 5587–5589. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, T.; Wu, Z.; Yu, R. Novel ratiometric surface-enhanced raman spectroscopy aptasensor for sensitive and reproducible sensing of Hg2+. Biosens. Bioelectron. 2018, 99, 646–652. [Google Scholar] [CrossRef]
- Qing, G.; Ling, W.; Chen, X. Aptameric sers sensor for Hg2+ analysis using silver nanoparticles. Chin. Chem. Lett. 2009, 20, 1475–1477. [Google Scholar]
- Stiles, L.; Dieringer, A.; Shah, C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef]
- Liu, X.; Liu, M.; Lu, Y.; Wu, C.; Xu, Y.; Lin, D.; Lu, D.; Zhou, T.; Feng, S. Facile Ag-film based surface enhanced Raman spectroscopy using DNA molecular switch for ultra-sensitive mercury ions detection. Nanomaterials 2018, 8, 596. [Google Scholar] [CrossRef]
- Shaban, M. In-situ SERS detection of Hg2+/Cd2+ and congo red adsorption using spiral CNTs/Brass nails. Nanomaterials 2022, 12, 3778. [Google Scholar] [CrossRef]
- Tian, C.; Zhao, L.; Zhu, J.; Zhang, S. Ultrasensitive detection of trace Hg2+ by SERS aptasensor based on dual recycling amplification in water environment. J. Hazard. Mater. 2021, 416, 126251. [Google Scholar] [CrossRef]
- Zhang, R.; Lv, S.; Gong, Y.; Li, Y.; Ding, C. Sensitive determination of Hg(II) based on a hybridization chain recycling amplification reaction and surface-enhanced Raman scattering on gold nanoparticles. Microchim. Acta 2018, 185, 363. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Sun, Y.; Chen, B.; Hu, F.; Guo, C.; Yang, T. Recent Advances in Colorimetric Sensors Based on Gold Nanoparticles for Pathogen Detection. Biosensors 2022, 13, 29. [Google Scholar] [CrossRef]
- Tessaro, L.; Aquino, A.; Panzenhagen, P.; Joshi, N.; Conte-Junior, C.A. A systematic review of the advancement on colorimetric nanobiosensors for SARS-CoV-2 detection. J. Pharm. Biomed. Anal. 2023, 222, 115087. [Google Scholar] [CrossRef]
- Fakayode, O.; Walgama, C.; Fernand Narcisse, E.; Grant, C. Electrochemical and colorimetric nanosensors for detection of heavy metal ions: A review. Sensors 2023, 23, 9080. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Z.; Qi, J.; You, J.; Ma, J.; Chen, L. Colorimetric detection of heavy metal ions with various chromogenic materials: Strategies and applications. J. Hazard. Mater. 2023, 441, 129889. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, F.; Harmonis, A.; Pratiwi, R.; Hasanah, N. Gold nanoparticle-based colorimetric sensors: Properties and application in detection of heavy metals and biological molecules. Sensors 2023, 23, 8172. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, J.; Li, H. Chromogenic mechanisms of colorimetric sensors based on gold nanoparticles. Biosensors 2023, 13, 801. [Google Scholar] [CrossRef]
- Cho, H.; Jung, H.; Heo, H.; Lee, Y.; Jeong, Y.; Lee, H. Gold nanoparticles as exquisite colorimetric transducers for water pollutant detection. ACS Appl. Mater. Inter. 2023, 15, 19785–19806. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Accelerated color change of gold nanoparticles assembled by DNAzymes for simple and fast colorimetric Pb2+ detection. J. Am. Chem. Soc. 2004, 126, 12298–12305. [Google Scholar] [CrossRef]
- Knecht, R.; Sethi, M. Bio-inspired colorimetric detection of Hg2+ and Pb2+ heavy metal ions using Au nanoparticles. Anal. Bioanal. Chem. 2009, 394, 33–46. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Y.; Wu, M.; Dong, M.; Li, J. Label-free colorimetric sensor for mercury(II) and DNA on the basis of mercury(II) switched-on the oxidase-mimicking activity of silver nanoclusters. Anal. Chim. Acta 2015, 871, 1–8. [Google Scholar] [CrossRef]
- Tan, L.; Chen, Z.; Zhang, C.; Wei, X.; Lou, T.; Zhao, Y. Colorimetric detection of Hg2+ based on the growth of aptamer-coated AuNPs: The effect of prolonging aptamer strands. Small 2017, 13, 1603370. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cai, Y.; Zhu, Y.; Zheng, L.; Ding, J.; Quan, Y.; Wang, L.; Qi, B. Highly sensitive colorimetric sensor for Hg(2+) detection based on cationic polymer/DNA interaction. Biosens. Bioelectron. 2015, 69, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Saputri, A.; Zubaidah, U.; Kenanga, P.; Jatmika, C.; Pratiwi, R.; Dhumale, A. Development of a colorimetric paper sensor for Hg2+ detection in water using cyanuric acid-conjugated gold nanoparticles. Molecules 2023, 28, 6527. [Google Scholar] [CrossRef]
- Shao, X.; Yang, D.; Wang, M.; Yue, Q. A colorimetric detection of Hg2+ based on gold nanoparticles synthesized oxidized N-methylpyrrolidone as a reducing agent. Sci. Rep. 2023, 13, 22208. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tang, G.; Tan, H. Colorimetric determination of mercury(II) via the inhibition by ssDNA of the oxidase-like activity of a mixed valence state cerium-based metal-organic framework. Microchim. Acta 2018, 185, 475. [Google Scholar] [CrossRef]
- Wang, X.; Hou, T.; Dong, S.; Liu, X.; Li, F. Fluorescence biosensing strategy based on mercury ion-mediated DNA conformational switch and nicking enzyme-assisted cycling amplification for highly sensitive detection of carbamate pesticide. Biosens. Bioelectron. 2016, 77, 644–649. [Google Scholar] [CrossRef]
- Wang, S.; Lin, B.; Chen, L.; Li, N.; Xu, J.; Wang, J.; Yang, Y.; Qi, Y.; She, Y.; Shen, X.; et al. Branch-migration based fluorescent probe for highly sensitive detection of mercury. Anal. Chem. 2018, 90, 11764–11769. [Google Scholar] [CrossRef]
- Tian, Y.; Xin, C.; Liu, S.; Liu, Y.; Liu, S. Affinity binding-induced Hg2+ release and quantum dot doping for general, label-free, and homogenous fluorescence protein assay. ACS Sens. 2018, 3, 1401–1408. [Google Scholar] [CrossRef]
- Zhan, S.; Xu, H.; Zhang, D.; Xia, B.; Zhan, X.; Wang, L.; Lv, J.; Zhou, P. Fluorescent detection of Hg2+ and Pb2+ using GeneFinder™ and an integrated functional nucleic acid. Biosens. Bioelectron. 2015, 72, 95–99. [Google Scholar] [CrossRef]
- Chu-Mong, K.; Thammakhet, C.; Thavarungkul, P.; Kanatharana, P.; Buranachai, C. A FRET based aptasensor coupled with non-enzymatic signal amplification for mercury (II) ion detection. Talanta 2016, 155, 305–313. [Google Scholar] [CrossRef]
- Ren, X.; Xu, Q.H. Highly sensitive and selective detection of mercury ions by using oligonucleotides, DNA intercalators, and conjugated polymers. Langmuir ACS J. Surf. Colloids 2009, 25, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ding, J.; Liu, J. 2-Aminopurine-modified DNA homopolymers for robust and sensitive detection of mercury and silver. Biosens. Bioelectron. 2017, 87, 171–177. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, L.; Zhou, X.; Qin, J.; Yang, C. Designing label-free DNA sequences to achieve controllable turn-off/on fluorescence response for Hg2+ detection. Chem. Commun. 2011, 47, 8010–8012. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, X.; Jin, X.; Wu, Z.; Chen, X.; Qiu, J. Function of graphene oxide as the "nanoquencher" for Hg2+ detection using an exonuclease I-assisted biosensor. Int. J. Mol. Sci. 2022, 23, 6326. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, N.; Shi, Z.; Fang, C.; Wang, Z. Dual fluorescence resonance energy transfer assay between tunable upconversion nanoparticles and controlled gold nanoparticles for the simultaneous detection of Pb2+ and Hg2+. Talanta 2014, 128, 327–336. [Google Scholar] [CrossRef]
- Li, K.; Hu, Y.; Niu, G.; Huang, W.; Zeng, M. A fluorescent DNA based probe for Hg(II) based on thymine-Hg(II)-thymine interaction and enrichment via magnetized graphene oxide. Mikrochim. Acta 2018, 185, 207. [Google Scholar] [CrossRef]
- Huang, J.; Wang, F.; Liu, J. Cleavable molecular beacon for Hg(2+) detection based on phosphorothioate RNA modifications. Anal. Chem. 2015, 87, 6890–6895. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Hu, B.; Gao, C.; Li, Z.; Sun, Z.; You, J. A FRET-based ratiometric fluorescent probe for Hg2+ detection in aqueous solution and bioimaging in multiple samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 121965. [Google Scholar] [CrossRef]
- Huang, J.; van Ballegooie, C.; Liu, J. Hg(2+) detection using a phosphorothioate RNA probe adsorbed on graphene oxide and a comparison with thymine-rich DNA. Analyst 2016, 141, 3788–3793. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Costa-García, A. Recent advances in the electrochemical detection of mercury. Curr. Opin. Electrochem. 2017, 3, 91–96. [Google Scholar] [CrossRef]
- Zhu, Z.; Su, Y.; Li, J.; Li, D.; Zhang, J.; Song, S.; Zhao, Y.; Li, G.; Fan, C. Highly sensitive electrochemical sensor for mercury(II) ions by using a mercury-specific oligonucleotide probe and gold nanoparticle-based amplification. Anal. Chem. 2009, 81, 7660–7666. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Wang, Z.; White, D.; Star, A. Machine learning-assisted calibration of Hg2+ sensors based on carbon nanotube field-effect transistors. Biosens. Bioelectron. 2021, 180, 113085. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, C.; Chen, D.; Wang, J.; Ji, Y.; Liang, N.; Gao, H.; Cheng, S.; Liu, H. Ultrasensitive and stable all graphene field-effect transistor-based Hg2+ sensor constructed by using different covalently bonded RGO films assembled by different conjugate linking molecules. SmartMat 2021, 2, 213–225. [Google Scholar] [CrossRef]
- Yan, Z.; Yuen, M.; Hu, L.; Sun, P.; Lee, C. Advances for the colorimetric detection of Hg2+ in aqueous solution. RSC Adv. 2014, 4, 48373–48388. [Google Scholar] [CrossRef]
- Kumar, N.; Bhalla, V.; Kumar, M. Resonance energy transfer-based fluorescent probes for Hg2+, Cu2+ and Fe2+/Fe3+ ions. Analyst 2014, 139, 543–558. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).