High-Resolution Tracking of Aging-Related Small Molecules: Bridging Pollutant Exposure, Brain Aging Mechanisms, and Detection Innovations
Abstract
1. Introduction
2. Possible Mechanisms by Which Environmental Pollutants Accelerate Brain Aging Through Bioactive Small Molecules
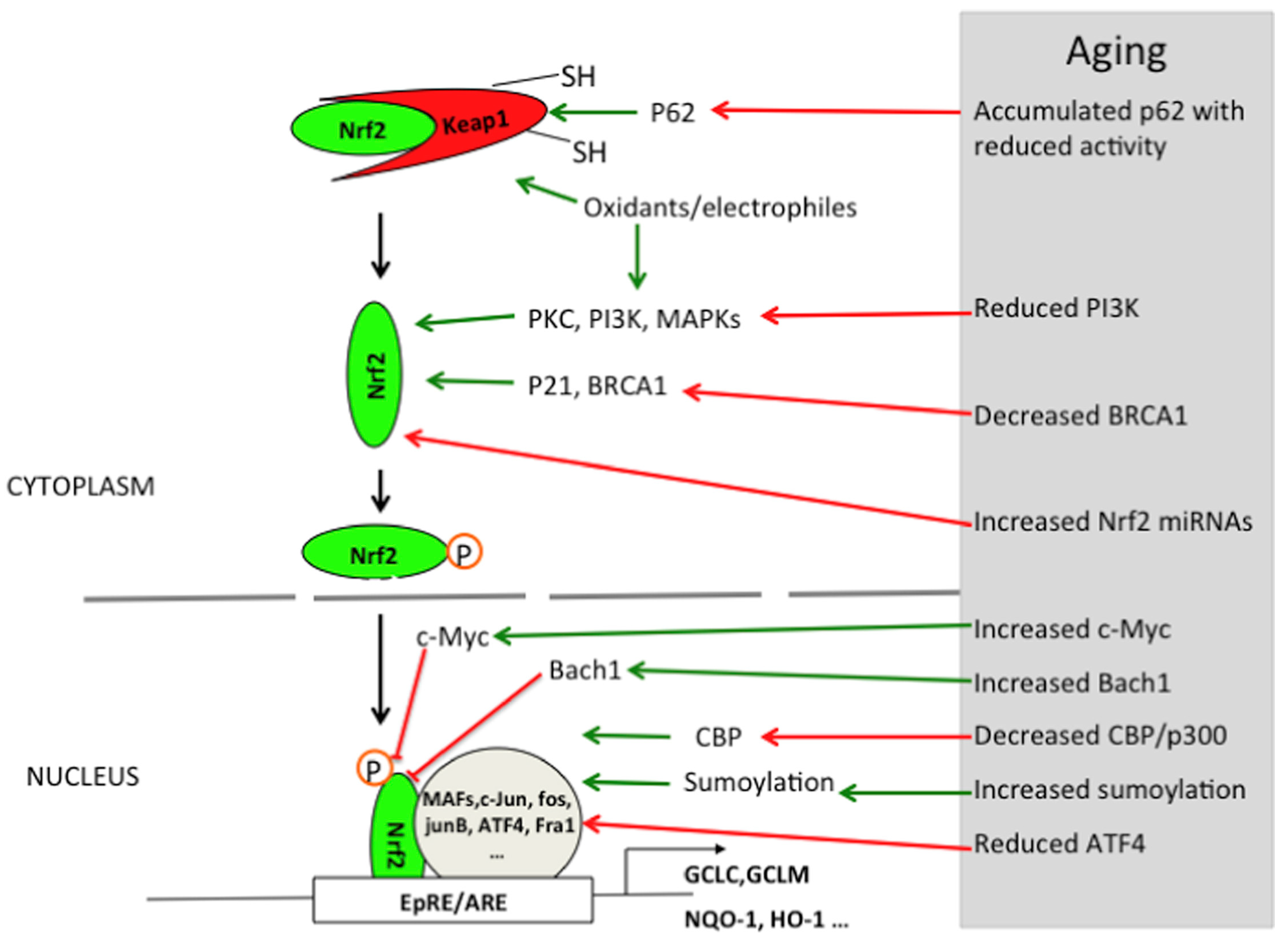
2.1. Oxidative Stress Signaling Pathway
2.1.1. The Enhancement of Oxidative Stress During Brain Aging
2.1.2. The Mechanisms by Which Environmental Pollutants Exacerbate Oxidative Stress
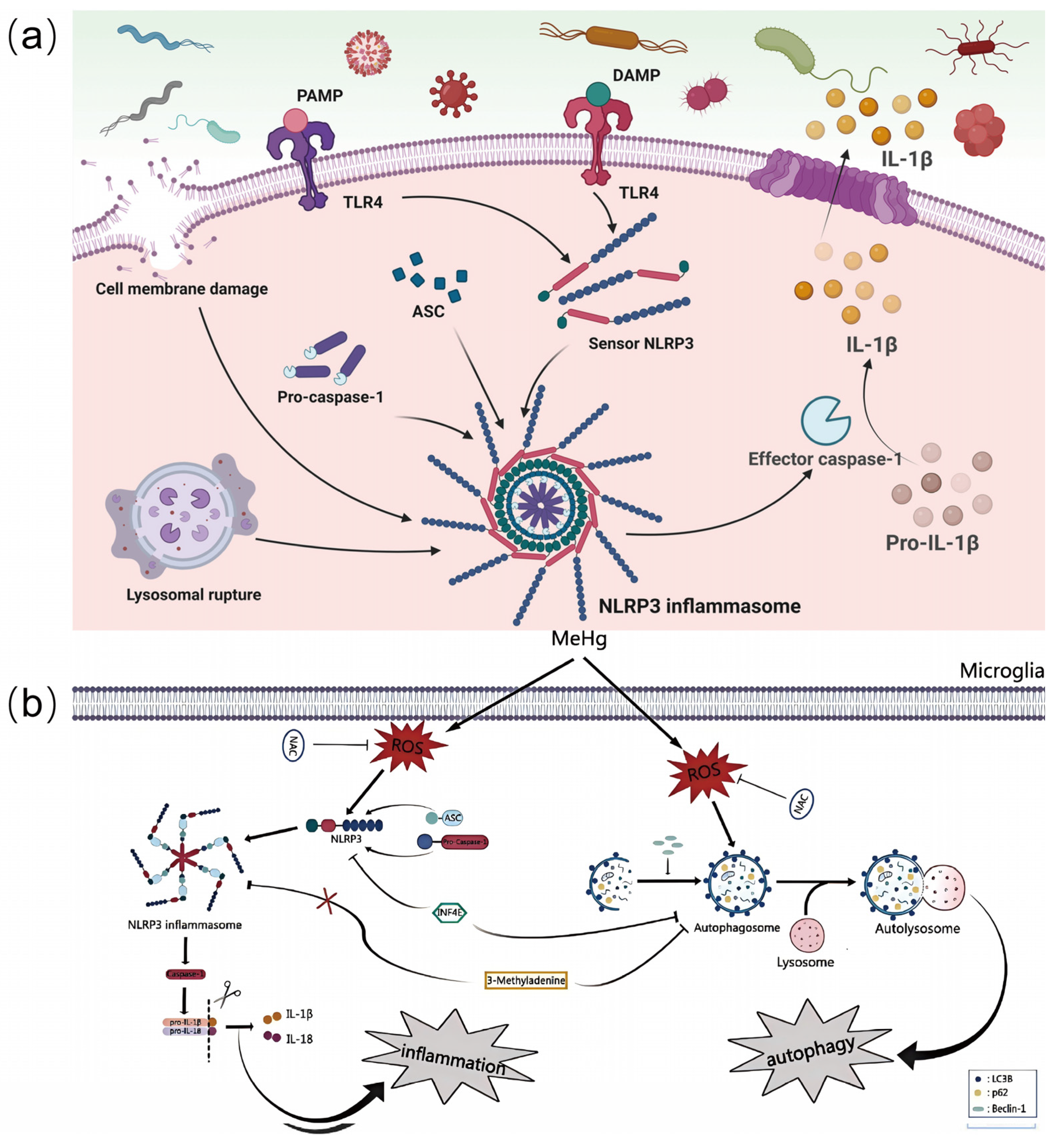
2.2. Neuroinflammation Signaling Pathways
2.2.1. The Enhancement of Neuroinflammation During Brain Aging
2.2.2. The Mechanisms by Which Environmental Pollutants Exacerbate Neuroinflammation
2.3. Mitochondrial Dysfunction Signaling Pathways
2.3.1. The Phenomenon of Mitochondrial Dysfunction During Brain Aging
2.3.2. The Mechanisms by Which Environmental Pollutants Exacerbate Mitochondrial Dysfunction

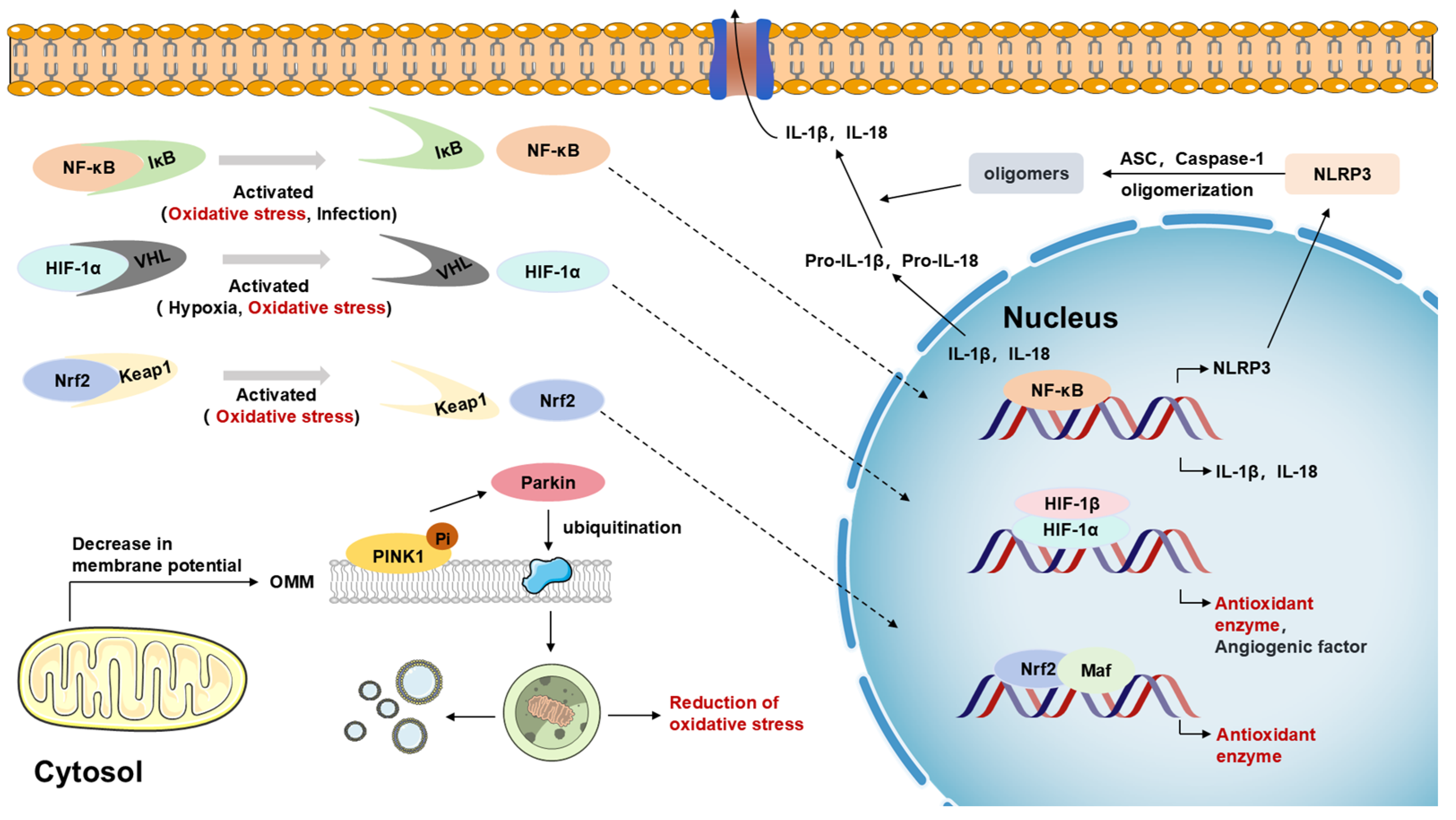
2.4. Other Related Mechanisms
2.4.1. Multiple Roles of Nrf2, NF-κB, and NLRP3 Inflammatory Vesicle Signaling Pathways
2.4.2. Critical Roles of Other Cell Signaling Pathways in Regulating Oxidative Stress, Neuroinflammation, and Mitochondrial Dysfunction
3. Multi-Level Network Construction of Pollutant Small Molecule-Brain Aging
3.1. A Multilayer Network Model of the Contaminant Small Molecule Signaling Pathway and Brain Aging
3.2. Intervention of Exogenous Bioactive Small Molecules
3.2.1. Antioxidant Quantum Dots
3.2.2. Other Interventions
| Form | Access | Pollutant | Performance | Specific Impacts | References |
|---|---|---|---|---|---|
| oxidative stress | Nrf2 | PM2.5 | increase | The expression of Nrf2 and its downstream antioxidant genes (e.g., NQO1, γ-GCS) is significantly increased | [28] |
| lambda-cyhalothrin | increase | Expression of Nrf2 and its downstream genes (e.g., HO-1, NQO1) is upregulated | [29] | ||
| BDE209 | increase | Activation of the Nrf2/GPX4 pathway | [10] | ||
| Cd | decrease | Inhibition of the Keap1–Nrf2 pathway and its downstream genes induces lipid peroxidation and ferroptosis | [130] | ||
| HIF-1α | MeHg | decrease | Reduces protein expression levels in astrocytes and inhibits the expression of its multiple downstream target genes | [32] | |
| Cr6⁺ | increase | Activates HIF-1α protein and promotes VEGF expression | [34] | ||
| PM2.5 | increase | Penetration into the lungs and BBB leads to aberrant activation of the HIF-1α signaling pathway, triggering SIRT1/HIF-1α-mediated ferroptosis | [35] | ||
| neuroinflammation | NF-κB | PM2.5 | increase | Increased expression of inflammatory factors and activation of NF-κB pathway | [44] |
| increase | |||||
| increase | |||||
| Pb | increase | Inhibits SIRT1 expression and promotes HMGB1 expression, which in turn activates NF-κB | [46] | ||
| increase | |||||
| β-HCH | increase | Regulation of NF-κB and induction of histone acetylation modifications | [45] | ||
| increase | |||||
| increase | |||||
| Fe | increase | Increased expression of α-synuclein in neuronal cells induced by the NF-κB regulatory pathway | [131] | ||
| increase | |||||
| NLRP3 | MeHg | increase | NLRP3 inflammatory vesicles are activated by oxidative stress | [11] | |
| PM2.5 | increase | NLRP3 inflammatory vesicles are activated by oxidative stress | [28] | ||
| polystyrene | increase | NLRP3 inflammatory vesicles are activated by oxidative stress | [78] | ||
| mitochondria | PINK1/Parkin | aluminum chloride | increase | Induces oxidative stress and activates mitochondrial autophagy | [58] |
| uBC | increase | Loss of mitochondrial membrane potential and decreased ATP levels | [12] | ||
| PM2.5 | increase | ROS overproduction and SOD2 expression | [132] | ||
| OXPHOS | MPTP | increase | Inhibits the electron transport chain and exacerbates mitochondrial dysfunction | [57] | |
| rotenone | increase | ||||
| paraquat | increase | ||||
| PM2.5 | increase | COX4I1 defects subsequently disrupt OXPHOS, leading to diminished ATP production and ROS accumulation | [133] | ||
| increase | High PM2.5 affects mitochondrial OXPHOS and proteins in the electron transport chain | [134] |
4. Detection of Aging-Related Bioactive Small Molecules Affected by Environmental Pollutants
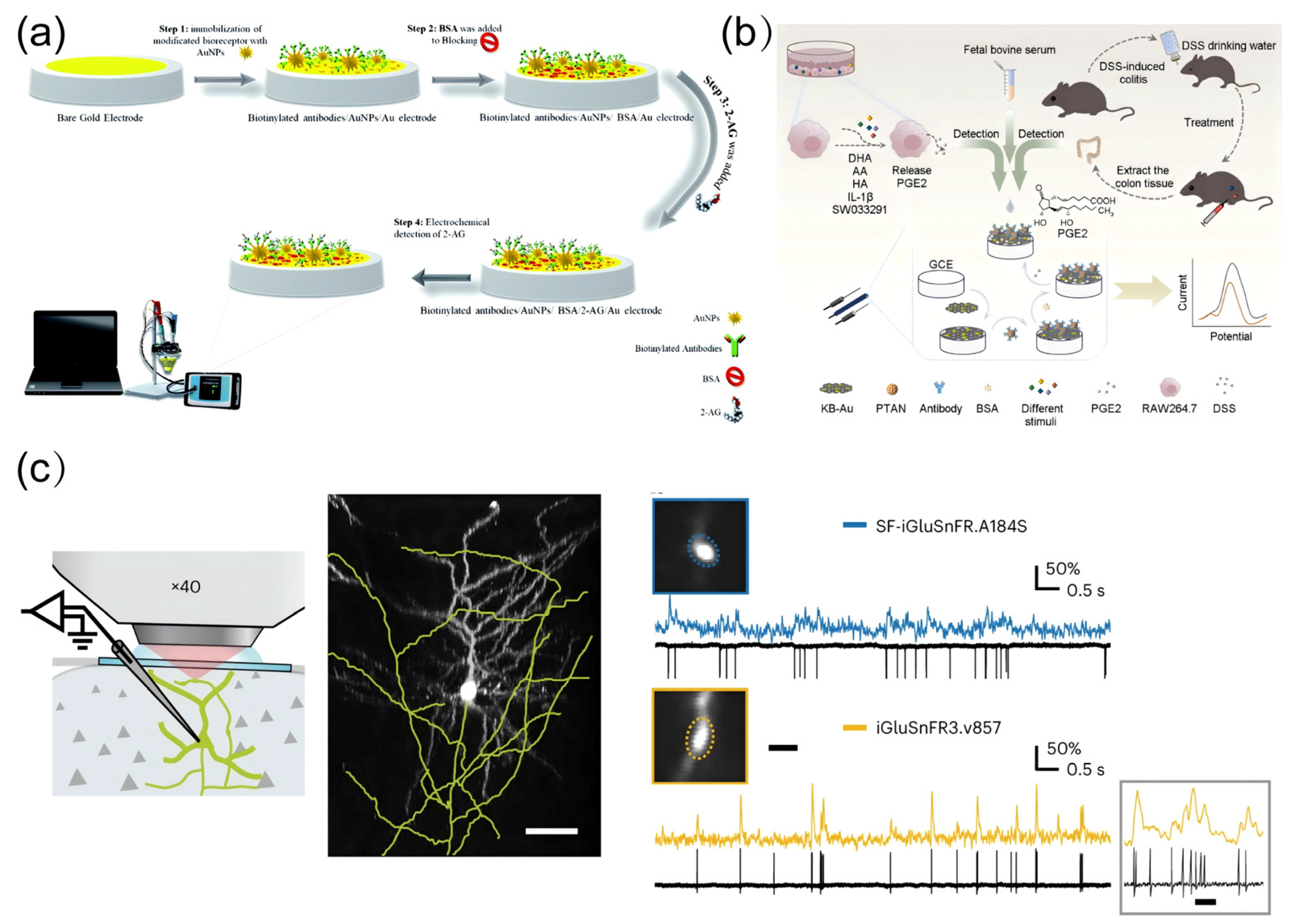
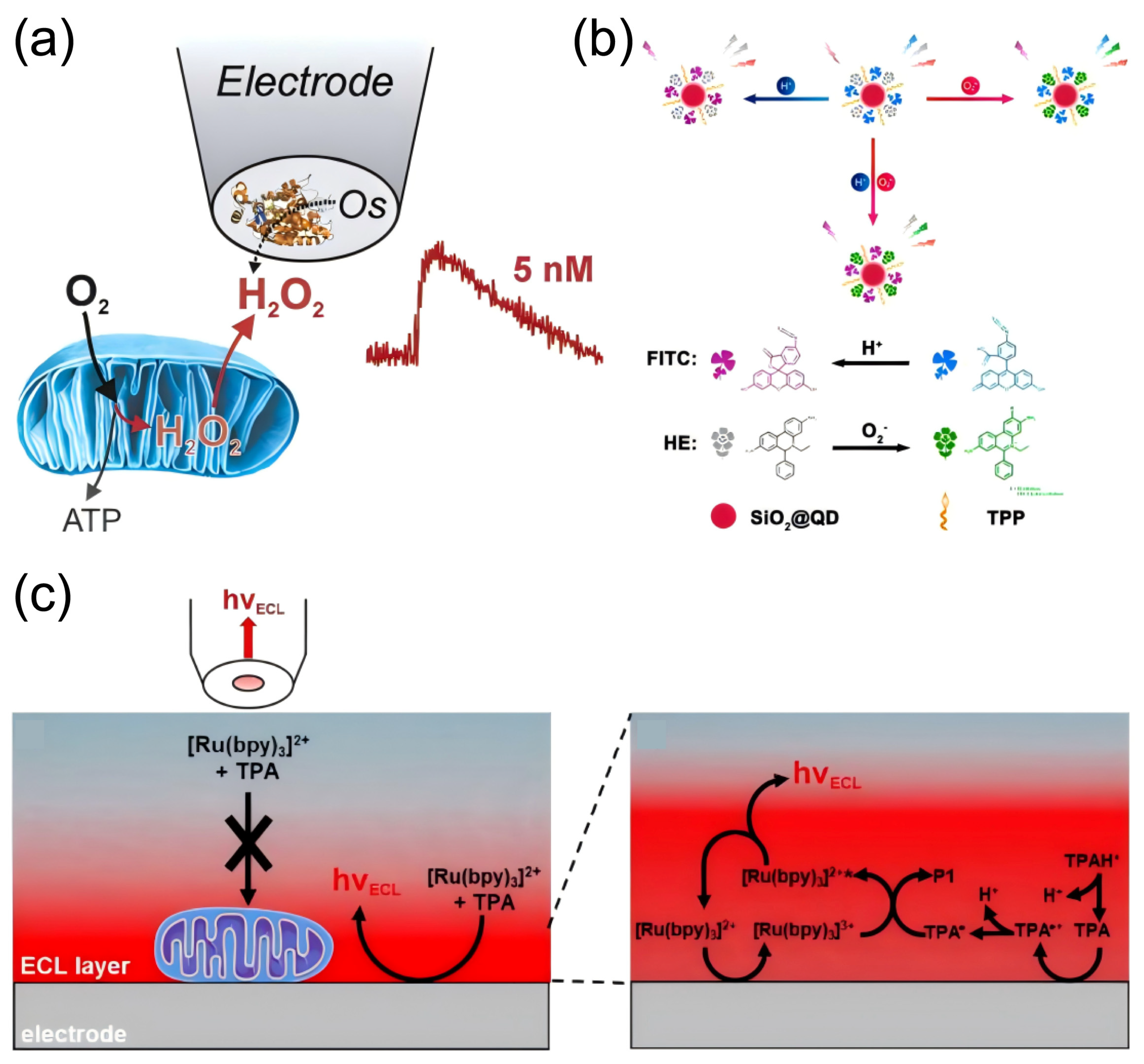
4.1. Detection of Oxidative Stress-Related Molecules
4.2. Detection of Neuroinflammation-Related Molecules
4.3. Detection of Mitochondrial Damage Biomarkers
| Small Molecule Type | Analyte | Type of Effect | Detection Methods | Innovation Point | Linear Range | Detection Limit | Reference |
|---|---|---|---|---|---|---|---|
| ROS | H2O2 | Pro-oxidant | Electrochemical Detection | Utilization of the natural mineral amphibole clay as an enzyme immobilization carrier | 0.2–150 μM | 0.05 ± 0.01 μM | [172] |
| O2−, •OH, and H2O2 | Pt-NWE electrodes | N/A | N/A | [173] | |||
| H2O2 | HRP embedded in crosslinked three-dimensional polymer matrices containing mobile Os redox mediators | 1–100 mM | N/A | [169] | |||
| RNS | ONOO−, NO•, NO2− | Pro-oxidant | Electrochemical detection | Nanoelectrode | N/A | N/A | [174] |
| AA | Antioxidant | ECL | Bipolar ratio response | 50–3 μM | 20 nM | [175] | |
| Electrochemical detection | SWCNT modified carbon fiber microelectrodes | N/A | N/A | [151] | |||
| Glu | Antioxidant | Fluorescent sensors | Superfolded GFP | N/A | N/A | [176] | |
| Electrochemical detection | Microbial sensors | 0–100 mM | 1 mM | [177] | |||
| Prostaglandin E2 | Pro-inflammatory | Electrochemical detection | PTAN-Ab complexes as signal amplification components | 10⁻5–106 fg/mL | 10⁻5 fg/mL | [163] | |
| 2-AG | Anti-inflammatory | Electrochemical detection | 2-AG specific antibody modified electrodes | 0.48–1 ngmL−1 | N/A | [165] | |
| O2− | Membrane Potential | Fluorescent probe | CdSe/ZnS quantum dots | N/A | N/A | [170] | |
| OXPHOS | Electron transport chain | Real-time cell analysis system | Sphere model | N/A | N/A | [178] | |
| E3 ubiquitin | Autophagic small molecule | Proteomics identification | BioE3 | N/A | N/A | [179] | |
| ATP | Membrane Potential | Electrochemical detection | An innovative impedance electrode structure | N/A | N/A | [180] | |
| LAC | Membrane Potential | Enzyme Chromatography, Voltammetry | N/A | N/A | [181] | ||
| PA | Membrane Potential | Bacterial luminescence methods | Biospecialty luminescent systems | N/A | N/A | [182] | |
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic Acid |
| ROS | Reactive Oxygen Species |
| ATP | Adenosine Triphosphate |
| Nrf2 | Nuclear Factor Erythroid 2–Related Factor 2 |
| ARE | Antioxidant Response Element |
| 4-HNE | 4-Hydroxynonenal |
| HIF-1α | Hypoxia-Inducible Factor 1 Alpha |
| Keap1 | Kelch-like ECH-associated protein 1 |
| PM2.5 | Particulate Matter less than 2.5 μm |
| Se-Met | Selenomethionine |
| GPX4 | Glutathione Peroxidase 4 |
| VHL | Von Hippel–Lindau |
| HO-1 | Heme Oxygenase 1 |
| NQO1 | NAD(P)H Quinone Dehydrogenase 1 |
| LDHA | Lactate Dehydrogenase A |
| VEGF | Vascular Endothelial Growth Factor |
| MeHg | Methylmercury |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| PI3K | Phosphoinositide 3-Kinase |
| Akt | Protein Kinase B |
| FoxO | Forkhead Box O |
| AMPK | AMP-Activated Protein Kinase |
| BDE209 | Decabromodiphenyl Ether |
| OS | Oxidative Stress |
| IκB | Inhibitor of Nuclear Factor Kappa B |
| TNF-α | Tumor Necrosis Factor Alpha |
| IL-6 | Interleukin 6 |
| NLRP3 | NOD-Like Receptor Family Pyrin Domain Containing 3 |
| IL-1β | Interleukin 1 Beta |
| IL-18 | Interleukin 18 |
| IKK | IκB Kinase |
| κB | Kappa Light Chain Enhancer of Activated B Cells |
| ASC | Apoptosis-Associated Speck-Like Protein Containing a CARD |
| BV2 | A murine microglial cell line |
| IL-8 | Interleukin 8 |
| β-HCH | Beta-Hexachlorocyclohexane |
| PINK1 | PTEN-Induced Kinase 1 |
| OXPHOS | Oxidative Phosphorylation |
| SOD2 | Superoxide Dismutase 2 |
| GPx | Glutathione Peroxidase |
| VDAC1 | Voltage-Dependent Anion Channel 1 |
| MFN2 | Mitofusin 2 |
| SQSTM1 | Sequestosome 1 |
| LC3-II | Microtubule-Associated Protein 1A/1B-Light Chain 3-II |
| ETC | Electron Transport Chain |
| MPTP | Mitochondrial Membrane Permeability Transition Pore |
| MPP⁺ | 1-Methyl-4-Phenylpyridinium |
| uBC | Ubiquitin C |
| MDA | Malondialdehyde |
| Mn-SOD | Manganese Superoxide Dismutase |
| NAC | N-Acetylcysteine |
| Mito-TEMPO | Mitochondria-Targeted Tempol |
| AMP | Adenosine Monophosphate |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1 Alpha |
| PS-MPs | Polystyrene Microplastics |
| MAMs | Mitochondria-Associated Membranes |
| CAT | Catalase |
| RNS | Reactive Nitrogen Species |
| GSH | Glutathione |
| AEA | Anandamide |
| PGE2 | Prostaglandin E2 |
| EP2 | Prostaglandin E Receptor 2 |
| NMR | Nuclear Magnetic Resonance |
| CB1 | Cannabinoid Receptor 1 |
| NMDA | N-Methyl-D-Aspartate |
| mTORC1 | Mechanistic Target of Rapamycin Complex 1 |
| TLR4 | Toll-Like Receptor 4 |
| JNK | c-Jun N-terminal Kinase |
| AQDs | Antioxidant quantum dots |
| BBB | Blood–brain barrier |
| SFN | Sulforaphane |
| CUR | Curcumin |
| CNPs | Carbon Nanoparticles |
| RTA-408 | A small molecule drug used for neuroprotection |
| SWCNT | Single-Walled Carbon Nanotube |
| HyPer7 | A genetically encoded hydrogen peroxide sensor |
| 2-AG | 2-Arachidonoylglycerol |
| PTAN | Poly(Thionine-Aniline) |
| iGluSnFR | A sensor for glutamate in real-time imaging |
| Pt-NWE | Platinum Nanowire Electrode |
| ECL | Electrochemiluminescence |
| GFP | Green Fluorescent Protein |
| BioE3 | A bioactive small molecule used in cellular research |
References
- Chen, S.M.; Cao, Z.; Nandi, A.; Counts, N.; Jiao, L.R.; Prettner, K.; Kuhn, M.; Seligman, B.; Tortorice, D.; Vigo, D.; et al. The global macroeconomic burden of Alzheimer’s disease and other dementias: Estimates and projections for 152 countries or territories. Lancet Glob. Health 2024, 12, e1534–e1543. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, G.Y.; Jing, W.Z.; Liu, J.; Liu, M. Global trends and regional differences in incidence and mortality of cardiovascular disease, 1990–2019: Findings from 2019 global burden of disease study. Eur. J. Prev. Cardiol. 2023, 30, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Kulick, E.R.; Wellenius, G.A.; Boehme, A.K.; Joyce, N.R.; Schupf, N.; Kaufman, J.D.; Mayeux, R.; Sacco, R.L.; Manly, J.J.; Elkind, M.S.V. Long-term exposure to air pollution and trajectories of cognitive decline among older adults. Neurology 2020, 94, E1782–E1792. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Fan, K.C.; Wu, C.D.; Pan, W.C.; Chen, J.C.; Chao, Y.P.; Lai, Y.J.; Chiu, Y.L.; Chuang, Y.F. Yearly change in air pollution and brain aging among older adults: A community-based study in Taiwan. Environ. Int. 2024, 190, 108876. [Google Scholar] [CrossRef]
- Hendriks, S.; Ranson, J.M.; Peetoom, K.; Lourida, I.; Tai, X.Y.; de Vugt, M.; Llewellyn, D.J.; Koehler, S. Risk Factors for Young-Onset Dementia in the UK Biobank. JAMA Neurol. 2024, 81, 134–142. [Google Scholar] [CrossRef]
- Iaccarino, L.; La Joie, R.; Lesman-Segev, O.H.; Lee, E.; Hanna, L.; Allen, I.E.; Hillner, B.E.; Siegel, B.A.; Whitmer, R.A.; Carrillo, M.C.; et al. Association Between Ambient Air Pollution and Amyloid Positron Emission Tomography Positivity in Older Adults With Cognitive Impairment. JAMA Neurol. 2021, 78, 197–207. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.B.; Chen, Z.H.; Dong, Y.T.; Jiang, Y.S.; Hua, J.M.; Liu, Y.F.; Osman, A.I.; Farghali, M.; Huang, L.P.; et al. Biomaterials technology and policies in the building sector: A review. Environ. Chem. Lett. 2024, 22, 715–750. [Google Scholar] [CrossRef]
- Wang, B.R.; Shi, J.Q.; Ge, N.N.; Ou, Z.; Tian, Y.Y.; Jiang, T.; Zhou, J.S.; Xu, J.; Zhang, Y.D. PM2.5 exposure aggravates oligomeric amyloid beta-induced neuronal injury and promotes NLRP3 inflammasome activation in an in vitro model of Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 132. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.Z.; Ma, J.C.; Zhao, Y.P. PM2.5 impairs neurobehavior by oxidative stress and myelin sheaths injury of brain in the rat. Environ. Pollut. 2018, 242, 994–1001. [Google Scholar] [CrossRef]
- Dong, B.W.; Jiang, Y.Y.; Shi, B.D.; Zhang, Z.Q.; Zhang, Z.W. Selenomethionine alleviates decabromodiphenyl ether-induced oxidative stress and ferroptosis via the NRF2/GPX4 pathway in the chicken brain. J. Hazard. Mater. 2024, 465, 133307. [Google Scholar] [CrossRef]
- Li, X.Y.; Ma, K.; Tian, T.T.; Pang, H.; Liu, T.X.; Li, M.; Li, J.L.; Luo, Z.X.; Hu, H.Y.; Hou, S.S.; et al. Methylmercury induces inflammatory response and autophagy in microglia through the activation of NLRP3 inflammasome. Environ. Int. 2024, 186, 108631. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Xue, W.L.; Kong, J.X.; Chen, Y.J.; Qiu, X.H.; An, X.Q.; Li, Y.; Wang, H.L.; An, J. Ultrafine black carbon caused mitochondrial oxidative stress, mitochondrial dysfunction and mitophagy in SH-SY5Y cells. Sci. Total. Environ. 2022, 813, 151899. [Google Scholar] [CrossRef] [PubMed]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef] [PubMed]
- Imarisio, A.; Yahyavi, I.; Gasparri, C.; Hassan, A.; Avenali, M.; Di Maio, A.; Buongarzone, G.; Galandra, C.; Picascia, M.; Filosa, A.; et al. Serum dysregulation of serine and glycine metabolism as predictive biomarker for cognitive decline in frail elderly subjects. Transl. Psychiatry 2024, 14, 281. [Google Scholar] [CrossRef]
- Chu, S.S.; Nguyen, H.A.; Lin, D.R.; Bhatti, M.; Jones-Tinsley, C.E.; Do, A.H.; Frostig, R.D.; Nenadic, Z.; Xu, X.M.; Lim, M.M.; et al. Development of highly sensitive, flexible dual L-glutamate and GABA microsensors for in vivo brain sensing. Biosens. Bioelectron. 2023, 222, 114941. [Google Scholar] [CrossRef]
- Marvin, J.S.; Borghuis, B.G.; Tian, L.; Cichon, J.; Harnett, M.T.; Akerboom, J.; Gordus, A.; Renninger, S.L.; Chen, T.W.; Bargmann, C.I.; et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 2013, 10, 162–170. [Google Scholar] [CrossRef]
- Floyd, R.A.; Hensley, K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 2002, 23, 795–807. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Cand, F.; Verdetti, J. Superoxide dismutase, glutathione peroxidase, catalase, and lipid peroxidation in the major organs of the aging rats. Free Radic. Biol. Med. 1989, 7, 59–63. [Google Scholar] [CrossRef]
- Jaganjac, M.; Milkovic, L.; Zarkovic, N.; Zarkovic, K. Oxidative stress and regeneration. Free Radic. Biol. Med. 2022, 181, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Pizzimenti, S.; Dianzani, M.U. Lipid peroxidation: Control of cell proliferation, cell differentiation and cell death. Mol. Asp. Med. 2008, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann-Stroissnigg, H.; Ling, Y.Y.; Zhao, J.; McGowan, S.J.; Zhu, Y.; Brooks, R.W.; Grassi, D.; Gregg, S.Q.; Stripay, J.L.; Dorronsoro, A.; et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 2017, 8, 422. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]
- Li, H.S.; Zhou, Y.N.; Li, L.; Li, S.F.; Long, D.; Chen, X.L.; Zhang, J.B.; Feng, L.; Li, Y.P. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol. 2019, 25, 101109. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Chen, G.H.; Song, C.C.; Pantopoulos, K.; Wei, X.L.; Zheng, H.; Luo, Z. Mitochondrial oxidative stress mediated Fe-induced ferroptosis via the NRF2-ARE pathway. Free Radic. Biol. Med. 2022, 180, 95–107. [Google Scholar] [CrossRef]
- Chu, C.; Zhang, H.; Cui, S.; Han, B.; Zhou, L.; Zhang, N.; Su, X.; Niu, Y.; Chen, W.; Chen, R.; et al. Ambient PM2.5 caused depressive-like responses through Nrf2/NLRP3 signaling pathway modulating inflammation. J. Hazard. Mater. 2019, 369, 180–190. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Guo, M.; Liu, Y.; Yu, H.; Xing, M. Environmentally relevant concentration of cypermethrin or/and sulfamethoxazole induce neurotoxicity of grass carp: Involvement of blood-brain barrier, oxidative stress and apoptosis. Sci. Total Environ. 2021, 762, 143054. [Google Scholar] [CrossRef]
- Chen, C.; Chen, B.; Lin, Y.; He, Q.; Yang, J.; Xiao, J.; Pan, Z.; Li, S.; Li, M.; Wang, F.; et al. Cardamonin attenuates iron overload-induced osteoblast oxidative stress through the HIF-1α/ROS pathway. Int. Immunopharmacol. 2024, 142, 112893. [Google Scholar] [CrossRef]
- Hui, Y.; Xia, K.; Zhong, J.; Zhang, Y.; Qiu, Q.; Chen, Z.; Wang, L.; Liu, X. SENP1 reduces oxidative stress and apoptosis in renal ischaemia-reperfusion injury by deSUMOylation of HIF-1α. J. Cell Mol. Med. 2024, 28, e70043. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Yang, B.; Zhou, Y.; Yin, C.; Liu, T.; Qian, H.; Xing, G.; Wang, S.; Li, F.; Zhang, Y.; et al. Acute Methylmercury Exposure and the Hypoxia-Inducible Factor-1α Signaling Pathway under Normoxic Conditions in the Rat Brain and Astrocytes in Vitro. Environ. Health Perspect. 2019, 127, 127006. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Paithankar, J.G.; Saini, S.; Dwivedi, S.; Sharma, A.; Chowdhuri, D.K. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 2021, 262, 128350. [Google Scholar] [CrossRef]
- Zheng, S.; Jiang, J.; Shu, Z.; Qiu, C.; Jiang, L.; Zhao, N.; Lin, X.; Qian, Y.; Liang, B.; Qiu, L. Fine particulate matter (PM(2.5)) induces testosterone disruption by triggering ferroptosis through SIRT1/HIF-1α signaling pathway in male mice. Free Radic. Biol. Med. 2024, 221, 40–51. [Google Scholar] [CrossRef]
- Mulero, M.C.; Huxford, T.; Ghosh, G. NF-κB, IκB, and IKK: Integral Components of Immune System Signaling. Adv. Exp. Med. Biol. 2019, 1172, 207–226. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Shao, B.Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Anilkumar, S.; Wright-Jin, E. NF-κB as an Inducible Regulator of Inflammation in the Central Nervous System. Cells 2024, 13, 485. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Liang, R.; Qi, X.; Cai, Q.; Niu, L.; Huang, X.; Zhang, D.; Ling, J.; Wu, Y.; Chen, Y.; Yang, P.; et al. The role of NLRP3 inflammasome in aging and age-related diseases. Immun. Ageing 2024, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.E.; Pike, G.B. MRI of healthy brain aging: A review. NMR Biomed. 2021, 34, e4564. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, W.; Xia, X.; Zhu, Y.; Ge, C.; Guo, X.; Zhang, N.; Chen, H.; Xu, S. Selenomethionine mitigate PM2.5-induced cellular senescence in the lung via attenuating inflammatory response mediated by cGAS/STING/NF-κB pathway. Ecotoxicol. Environ. Saf. 2022, 247, 114266. [Google Scholar] [CrossRef]
- Grieco, M.; Giorgi, A.; Giacovazzo, G.; Maggiore, A.; Ficchì, S.; d’Erme, M.; Mosca, L.; Mignogna, G.; Maras, B.; Coccurello, R. β-Hexachlorocyclohexane triggers neuroinflammatory activity, epigenetic histone post-translational modifications and cognitive dysfunction. Ecotoxicol. Environ. Saf. 2024, 279, 116487. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Li, J.Y.; Li, Z.C.; Wang, L.L.; Gan, C.L.; Chen, J.; Jiang, S.Y.; Aschner, M.; Ou, S.Y.; Jiang, Y.M. Sodium Para-aminosalicylic Acid Inhibits Lead-Induced Neuroinflammation in Brain Cortex of Rats by Modulating SIRT1/HMGB1/NF-κB Pathway. Neurochem. Res. 2023, 48, 238–249. [Google Scholar] [CrossRef]
- Eiyama, A.; Okamoto, K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr. Opin. Cell Biol. 2015, 33, 95–101. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, D.; Bankhead, A.; Neamati, N. Why All the Fuss about Oxidative Phosphorylation (OXPHOS)? J. Med. Chem. 2020, 63, 14276–14307. [Google Scholar] [CrossRef]
- Guo, J.; Chiang, W.C. Mitophagy in aging and longevity. IUBMB Life 2022, 74, 296–316. [Google Scholar] [CrossRef]
- Boveris, A.; Navarro, A. Brain mitochondrial dysfunction in aging. IUBMB Life 2008, 60, 308–314. [Google Scholar] [CrossRef]
- Chu, X.Y.; Wang, G.; Zhang, H.Y. ATP as an anti-aging agent: Beyond the energy reservoir. Drug Discov. Today 2021, 26, 2783–2785. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Wang, K.; Du, Y.; Li, P.; Guan, C.; Zhou, M.; Wu, L.; Liu, Z.; Huang, Z. Nanoplastics causes heart aging/myocardial cell senescence through the Ca(2+)/mtDNA/cGAS-STING signaling cascade. J. Nanobiotechnol. 2024, 22, 96. [Google Scholar] [CrossRef]
- Quinn, P.M.J.; Moreira, P.I.; Ambrósio, A.F.; Alves, C.H. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta. Neuropathol. Commun. 2020, 8, 189. [Google Scholar] [CrossRef]
- Vercellino, I.; Sazanov, L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2022, 23, 141–161. [Google Scholar] [CrossRef]
- Zolkipli-Cunningham, Z.; Falk, M.J. Clinical effects of chemical exposures on mitochondrial function. Toxicology 2017, 391, 90–99. [Google Scholar] [CrossRef]
- Cui, Y.; Song, M.; Xiao, B.; Huang, W.; Zhang, J.; Zhang, X.; Shao, B.; Han, Y.; Li, Y. PINK1/Parkin-Mediated Mitophagy Plays a Protective Role in the Bone Impairment Caused by Aluminum Exposure. J. Agric. Food Chem. 2021, 69, 6054–6063. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Sorrenti, V. Editorial of Special Issue “Protective and Detrimental Role of Heme Oxygenase-1”. Int. J. Mol. Sci. 2019, 20, 4744. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Wang, H.; Dong, J.; Wu, Y.; Zhang, H.; Fu, L.; Zhang, J. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate neuroinflammation and oxidative stress through the NRF2/NF-κB/NLRP3 pathway. CNS Neurosci. Ther. 2024, 30, e14454. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kitamura, M. Bidirectional regulation of NF-κB by reactive oxygen species: A role of unfolded protein response. Free Radic. Biol. Med. 2013, 65, 162–174. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017, 25, 11–24. [Google Scholar] [CrossRef]
- Bubici, C.; Papa, S.; Pham, C.G.; Zazzeroni, F.; Franzoso, G. The NF-kappaB-mediated control of ROS and JNK signaling. Histol. Histopathol. 2006, 21, 69–80. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wang, H.; Zhang, X.; Chen, Y.; Chen, G. Microglial pyroptosis in hippocampus mediates sevolfurane-induced cognitive impairment in aged mice via ROS-NLRP3 inflammasome pathway. Int. Immunopharmacol. 2023, 116, 109725. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Q.; Han, B.; Chen, Y.; Qiao, X.; Wang, L. CD36 promotes NLRP3 inflammasome activation via the mtROS pathway in renal tubular epithelial cells of diabetic kidneys. Cell Death Dis. 2021, 12, 523. [Google Scholar] [CrossRef]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 2022, 55, 1370–1385.e1378. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Halling, J.F.; Pilegaard, H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- Yang, K.; Wu, J.; Li, S.; Wang, S.; Zhang, J.; Wang, Y.P.; Yan, Y.S.; Hu, H.Y.; Xiong, M.F.; Bai, C.B.; et al. NTRK1 knockdown induces mouse cognitive impairment and hippocampal neuronal damage through mitophagy suppression via inactivating the AMPK/ULK1/FUNDC1 pathway. Cell Death Discov. 2023, 9, 404. [Google Scholar] [CrossRef]
- Wu, S.; Zou, M.H. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4987. [Google Scholar] [CrossRef]
- Petsouki, E.; Cabrera, S.N.S.; Heiss, E.H. AMPK and NRF2: Interactive players in the same team for cellular homeostasis? Free Radic. Biol. Med. 2022, 190, 75–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, K.; Wang, D.; Wang, Y.; Lu, H.; Zhao, H.; Xing, M. Polystyrene microplastics-induced cardiotoxicity in chickens via the ROS-driven NF-κB-NLRP3-GSDMD and AMPK-PGC-1α axes. Sci. Total Environ. 2022, 840, 156727. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, C.; Chen, F.; Zhang, A.; Wang, W. The mystery of methylmercury-perturbed calcium homeostasis: AMPK-DRP1-dependent mitochondrial fission initiates ER-mitochondria contact formation. Sci. Total Environ. 2024, 923, 171398. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Huang, Y.F.; Wang, G.; Ding, L.; Bai, Z.R.; Leng, Y.; Tian, J.W.; Zhang, J.Z.; Li, Y.Q.; Ahmad; Qin, Y.H.; et al. Lactate-upregulated NADPH-dependent NOX4 expression via HCAR1/PI3K pathway contributes to ROS-induced osteoarthritis chondrocyte damage. Redox Biol. 2023, 67, 102867. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jia, S.; Yang, Y.; Piao, L.; Wang, Z.; Jin, Z.; Bai, L. Exercise induced meteorin-like protects chondrocytes against inflammation and pyroptosis in osteoarthritis by inhibiting PI3K/Akt/NF-κB and NLRP3/caspase-1/GSDMD signaling. Biomed. Pharmacother. 2023, 158, 114118. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.; Bagchi, A.K.; Ludke, A.L.; Sharma, A.K.; Singal, P.K. Akt regulates IL-10 mediated suppression of TNFα-induced cardiomyocyte apoptosis by upregulating Stat3 phosphorylation. PLoS ONE 2011, 6, e25009. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, B.; Li, Y.; Zhang, Y.; Shao, J.; Wu, P.; Xu, C.; Chen, G.; Shi, H. Activation of RARα Receptor Attenuates Neuroinflammation After SAH via Promoting M1-to-M2 Phenotypic Polarization of Microglia and Regulating Mafb/Msr1/PI3K-Akt/NF-κB Pathway. Front. Immunol. 2022, 13, 839796. [Google Scholar] [CrossRef]
- Ji, L.L.; Yeo, D. Mitochondrial dysregulation and muscle disuse atrophy. F1000Research 2019, 8, 1621. [Google Scholar] [CrossRef]
- Yuan, J.; Deng, Y.; Zhang, Y.; Gan, X.; Gao, S.; Hu, H.; Hu, S.; Hu, J.; Liu, H.; Li, L.; et al. Bmp4 inhibits goose granulosa cell apoptosis via PI3K/AKT/Caspase-9 signaling pathway. Anim. Reprod. Sci. 2019, 200, 86–95. [Google Scholar] [CrossRef]
- He, Y.; Zheng, Z.; Liu, C.; Li, W.; Zhao, L.; Nie, G.; Li, H. Inhibiting DNA methylation alleviates cisplatin-induced hearing loss by decreasing oxidative stress-induced mitochondria-dependent apoptosis via the LRP1-PI3K/AKT pathway. Acta. Pharm. Sin. B 2022, 12, 1305–1321. [Google Scholar] [CrossRef]
- Xue, Y.; Cheng, X.; Ma, Z.Q.; Wang, H.P.; Zhou, C.; Li, J.; Zhang, D.L.; Hu, L.L.; Cui, Y.F.; Huang, J.; et al. Polystyrene nanoplastics induce apoptosis, autophagy, and steroidogenesis disruption in granulosa cells to reduce oocyte quality and fertility by inhibiting the PI3K/AKT pathway in female mice. J. Nanobiotechnol. 2024, 22, 460. [Google Scholar] [CrossRef]
- Yin, H.; Zuo, Z.; Yang, Z.; Guo, H.; Fang, J.; Cui, H.; Ouyang, P.; Chen, X.; Chen, J.; Geng, Y.; et al. Nickel induces autophagy via PI3K/AKT/mTOR and AMPK pathways in mouse kidney. Ecotoxicol. Environ. Saf. 2021, 223, 112583. [Google Scholar] [CrossRef]
- Hoye, A.T.; Davoren, J.E.; Wipf, P.; Fink, M.P.; Kagan, V.E. Targeting mitochondria. Acc. Chem. Res. 2008, 41, 87–97. [Google Scholar] [CrossRef]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, H.; Wang, C.; Jiao, F.; Xu, H.; Wang, X.; Luan, W.; Ma, F.; Ni, L.; Tang, X.; et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. 2019, 33, 12515–12527. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xu, W.; Li, H.; Dong, B.; Geng, H.; Jin, J.; Han, D.; Liu, H.; Zhu, X.; Yang, Y.; et al. Vitamin C Attenuates Oxidative Stress, Inflammation, and Apoptosis Induced by Acute Hypoxia through the Nrf2/Keap1 Signaling Pathway in Gibel Carp (Carassius gibelio). Antioxidants 2022, 11, 935. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Ji, W.; Zhang, Y.; Wu, F.; Tang, Q.; Wei, H.; Mao, L.; Zhang, M. Zwitterionic Polydopamine Engineered Interface for In Vivo Sensing with High Biocompatibility. Angew. Chem. Int. Ed. 2020, 59, 23445–23449. (In English) [Google Scholar] [CrossRef]
- Gan, L.; Camarena, V.; Mustafi, S.; Wang, G. Vitamin C Inhibits Triple-Negative Breast Cancer Metastasis by Affecting the Expression of YAP1 and Synaptopodin 2. Nutrients 2019, 11, 2997. [Google Scholar] [CrossRef]
- Jin, J.; Ji, W.; Li, L.; Zhao, G.; Wu, W.; Wei, H.; Ma, F.; Jiang, Y.; Mao, L. Electrochemically Probing Dynamics of Ascorbate during Cytotoxic Edema in Living Rat Brain. J. Am. Chem. Soc. 2020, 142, 19012–19016. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, X.; Zhu, Y.; Yue, Q.; Ji, W. Electrochemical sensing of transient ascorbate fluctuation under hypoxic stress in live rat brain. Talanta 2025, 282, 126996. [Google Scholar] [CrossRef]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Tsuge, K.; Inazumi, T.; Shimamoto, A.; Sugimoto, Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int. Immunol. 2019, 31, 597–606. [Google Scholar] [CrossRef]
- Bauminger, H.; Zaidan, H.; Akirav, I.; Gaisler-Salomon, I. Anandamide Hydrolysis Inhibition Reverses the Long-Term Behavioral and Gene Expression Alterations Induced by MK-801 in Male Rats: Differential CB1 and CB2 Receptor-Mediated Effects. Schizophr. Bull. 2022, 48, 795–803. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Hampson, A.J.; Bornheim, L.M.; Scanziani, M.; Yost, C.S.; Gray, A.T.; Hansen, B.M.; Leonoudakis, D.J.; Bickler, P.E. Dual effects of anandamide on NMDA receptor-mediated responses and neurotransmission. J. Neurochem. 1998, 70, 671–676. [Google Scholar] [CrossRef]
- Ha, J.; Guan, K.L.; Kim, J. AMPK and autophagy in glucose/glycogen metabolism. Mol. Asp. Med. 2015, 46, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Jiang, Z.; Luo, P.; Liu, L.; Huang, Y.; Wang, H.; Wang, Y.; Long, L.; Tan, X.; et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat. Commun. 2019, 10, 2538. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, Y.; Yuan, X.; Li, F.; Guo, L.; Wu, B. REV-ERBα integrates colon clock with experimental colitis through regulation of NF-κB/NLRP3 axis. Nat. Commun. 2018, 9, 4246. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Zhang, W.; He, J.; Xu, B.; Lei, B.; Wang, Z.; Cates, C.; Rousselle, T.; Li, J. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metabolism 2018, 83, 256–270. [Google Scholar] [CrossRef]
- Ivankovic, D.; Chau, K.Y.; Schapira, A.H.; Gegg, M.E. Mitochondrial and lysosomal biogenesis are activated following PINK1/parkin-mediated mitophagy. J. Neurochem. 2016, 136, 388–402. [Google Scholar] [CrossRef]
- Zhu, D.; Zhong, J.; Gong, X.; Wu, X. Augmenter of liver regeneration reduces mitochondria-derived ROS and NLRP3 inflammasome activation through PINK1/Parkin-mediated mitophagy in ischemia-reperfusion-induced renal tubular injury. Apoptosis 2023, 28, 335–347. [Google Scholar] [CrossRef]
- Huang, H.; Shen, Z.F.; Chen, B.Y.; Wang, X.Y.; Xia, Q.N.; Ge, Z.G.; Wang, Y.G.; Li, X. Selenium-doped two-photon fluorescent carbon nanodots for in-situ free radical scavenging in mitochondria. J. Colloid Interface Sci. 2020, 567, 402–409. [Google Scholar] [CrossRef]
- Yang, X.Y.; Tang, X.L.; Jia, G.Y.; Wang, Y.; Yang, L.; Li, Y.Y.; Wu, M.J.; Zhang, Z.; Yu, Y.M.; Xiao, Y.; et al. Multifunctional Carbon Quantum Dots: Iron Clearance and Antioxidation for Neuroprotection in Intracerebral Hemorrhage Mice. ACS Appl. Mater. Interfaces J. 2023, 15, 56820–56833. [Google Scholar] [CrossRef]
- Wu, Z.X.; Xia, W.B.; Ou, L.L.; Zheng, L.; Hou, B.Y.; Pan, T.H.; Sun, W.J.; Koole, L.H.; Shao, Y.Q.; Qi, L. Utilization of Nitrogen-Doped Graphene Quantum Dots to Neutralize ROS and Modulate Intracellular Antioxidant Pathways to Improve Dry Eye Disease Therapy. Int. J. Nanomed. 2024, 19, 2691–2708. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Pan, Y.; Tang, S.J.; Guo, T.W.; Yang, L.; Chen, G. Carbon quantum dots amplify beneficial effects of EGCG against neural injuries by NLRP3 inflammasome after intracerebral hemorrhage. Int. J. Pharm. 2025, 671, 125281. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lie, Q.S.; Liu, Y.A.; Jia, Z.; Gong, Y.C.; Yuan, X.Y.; Liu, J. Multifunctional Selenium Quantum Dots for the Treatment of Alzheimer’s Disease by Reducing Aβ-Neurotoxicity and Oxidative Stress and Alleviate Neuroinflammation. Acs. Appl. Mater. Interfaces J. 2021, 13, 30261–30273. [Google Scholar] [CrossRef]
- Krunic, M.; Ristic, B.; Bosnjak, M.; Paunovic, V.; Tovilovic-Kovacevic, G.; Zogovic, N.; Mircic, A.; Markovic, Z.; Todorovic-Markovic, B.; Jovanovic, S.; et al. Graphene quantum dot antioxidant and proautophagic actions protect SH-SY5Y neuroblastoma cells from oxidative stress-mediated apoptotic death. Free Radic. Biol. Med. 2021, 177, 167–180. [Google Scholar] [CrossRef]
- Wu, T.S.; Liang, X.; Liu, X.; Li, Y.M.; Wang, Y.T.; Kong, L.; Tang, M. Induction of ferroptosis in response to graphene quantum dots through mitochondrial oxidative stress in microglia. Part. Fibre Toxicol. 2020, 17, 167–180. [Google Scholar] [CrossRef]
- Shen, J.J.; Sun, Y.; Liu, X.Z.; Chai, Y.M.; Wang, C.Y.; Xu, J. Nerve Regeneration Potential of Antioxidant-Modified Black Phosphorus Quantum Dots in Peripheral Nerve Injury. ACS Nano 2024, 18, 23518–23536. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Fluorescent quantum dots-based hydrogels: Synthesis, fabrication and multimodal biosensing. Talanta Open 2023, 8, 100243. [Google Scholar] [CrossRef]
- Kurungottu, P.; Thomas, M.B.; Lalitha, M.M.; Ganesh, P.; Gnanadhas, D.P.; Chakravortty, D.; Raichur, A.M.; Kurapati, R. Biodegradable Nanocomposite of ZnS (Mn) Quantum Dots Immobilized Graphene Oxide for bioimaging applications. Nanotheranostics 2024, 8, 150–162. [Google Scholar] [CrossRef]
- Han, M.K.; Zhao, J.B.; Fabian, J.M.; Evans, S.; Mustafa, S.; Ruan, Y.L.; Wiederman, S.; Ebendorff-Heidepriem, H. Cytoplasmic delivery of quantum dots via microelectrophoresis technique. Electrophoresis 2021, 42, 1247–1254. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Marvi, P.K.; Hassan, S.; Sherazee, M.; Mahana, M.; Tang, X.W.S.; Srinivasan, S.; Rajabzadeh, A.R. Silicene-Based Quantum Dots Nanocomposite Coated Functional UV Protected Textiles With Antibacterial and Antioxidant Properties: A Versatile Solution for Healthcare and Everyday Protection. Adv. Healthc. Mater. 2025, 14, e2404911. [Google Scholar] [CrossRef]
- Qi, W.X.; Liu, Y.H.; Dong, N.J.; Li, M.T.; Zhou, J.X.; Xie, Y.J.; Chang, Q.; Luo, B.X.; Celia, C.; Wang, J.; et al. Multifunctional Carbon Quantum Dots for Monitoring and Therapy of Bacterial Infected Wounds. Adv. Healthc. Mater. 2025, 4810, e2403670. [Google Scholar] [CrossRef] [PubMed]
- Bhaloo, A.; Nguyen, S.; Lee, B.H.; Valimukhametova, A.; Gonzalez-Rodriguez, R.; Sottile, O.; Dorsky, A.; Naumov, A.V. Doped Graphene Quantum Dots as Biocompatible Radical Scavenging Agents. Antioxidantien 2023, 12, 1536. [Google Scholar] [CrossRef] [PubMed]
- Amouzadeh Tabrizi, M. A Facile Method for the Fabrication of the Microneedle Electrode and Its Application in the Enzymatic Determination of Glutamate. Biosens 2023, 13, 828. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, C.; Zhou, Y.; Wang, Q.; Jiang, Y.; Bai, Y.; Qian, H.; Xing, G.; Wang, S.; Li, F.; et al. Curcumin protects against methylmercury-induced cytotoxicity in primary rat astrocytes by activating the Nrf2/ARE pathway independently of PKCδ. Toxicology 2019, 425, 152248. [Google Scholar] [CrossRef]
- Marquardt, J.U.; Gomez-Quiroz, L.; Arreguin Camacho, L.O.; Pinna, F.; Lee, Y.H.; Kitade, M.; Domínguez, M.P.; Castven, D.; Breuhahn, K.; Conner, E.A.; et al. Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. J. Hepatol. 2015, 63, 661–669. [Google Scholar] [CrossRef]
- Chiao, Y.A.; Kolwicz, S.C.; Basisty, N.; Gagnidze, A.; Zhang, J.; Gu, H.; Djukovic, D.; Beyer, R.P.; Raftery, D.; MacCoss, M.; et al. Rapamycin transiently induces mitochondrial remodeling to reprogram energy metabolism in old hearts. Aging 2016, 8, 314–327. [Google Scholar] [CrossRef]
- Sun, X.; Xie, Z.; Hu, B.; Zhang, B.; Ma, Y.; Pan, X.; Huang, H.; Wang, J.; Zhao, X.; Jie, Z.; et al. The Nrf2 activator RTA-408 attenuates osteoclastogenesis by inhibiting STING dependent NF-κb signaling. Redox Biol. 2020, 28, 101309. [Google Scholar] [CrossRef]
- Hao, J.; Zhou, J.; Hu, S.; Zhang, P.; Wu, H.; Yang, J.; Zhao, B.; Liu, H.; Lin, H.; Chi, J.; et al. RTA 408 ameliorates diabetic cardiomyopathy by activating Nrf2 to regulate mitochondrial fission and fusion and inhibiting NF-κB-mediated inflammation. Am. J. Physiol. Cell Physiol. 2024, 326, C331–C347. [Google Scholar] [CrossRef]
- Abeti, R.; Baccaro, A.; Esteras, N.; Giunti, P. Novel Nrf2-Inducer Prevents Mitochondrial Defects and Oxidative Stress in Friedreich’s Ataxia Models. Front Cell Neurosci. 2018, 12, 188. [Google Scholar] [CrossRef]
- Lan, Y.; Hu, L.; Feng, X.; Wang, M.; Yuan, H.; Xu, H. Synergistic effect of PS-MPs and Cd on male reproductive toxicity: Ferroptosis via Keap1-Nrf2 pathway. J. Hazard. Mater. 2024, 461, 132584. [Google Scholar] [CrossRef]
- Angelova, D.M.; Brown, D.R. Microglia and the aging brain: Are senescent microglia the key to neurodegeneration? J. Neurochem. 2019, 151, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Ning, R.; Li, Y.; Du, Z.; Li, T.; Sun, Q.; Lin, L.; Xu, Q.; Duan, J.; Sun, Z. The mitochondria-targeted antioxidant MitoQ attenuated PM(2.5)-induced vascular fibrosis via regulating mitophagy. Redox Biol. 2021, 46, 102113. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Gao, F.; Xiao, Y.; Liang, J.; Mao, Z.; Gan, C.; Song, H.; Du, M.; Wang, M.; Tian, M.; et al. PM(2.5)-derived exosomal long noncoding RNA PAET participates in childhood asthma by enhancing DNA damage via m(6)A-dependent OXPHOS regulation. Environ. Int. 2024, 183, 108386. [Google Scholar] [CrossRef] [PubMed]
- You, Y.A.; Park, S.; Kwon, E.; Kim, Y.A.; Hur, Y.M.; Lee, G.I.; Kim, S.M.; Song, J.M.; Kim, M.S.; Kim, Y.J.; et al. Maternal PM2.5 exposure is associated with preterm birth and gestational diabetes mellitus, and mitochondrial OXPHOS dysfunction in cord blood. Environ. Sci. Pollut. Res. Int. 2024, 31, 10565–10578. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, C.; Liu, C.; Fan, Z.; Yang, S.; Song, H.; Hao, R. Advanced electrochemical detection methodologies for assessing neuroactive substance variability induced by environmental pollutants exposure. Environ. Technol. Innov. 2025, 37, 103965. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, S.; Jin, S.Y.; Gao, T.M. Extracellular ATP Is a Homeostatic Messenger That Mediates Cell-Cell Communication in Physiological Processes and Psychiatric Diseases. Biol. Psychiatry 2025, 97, 41–53. [Google Scholar] [CrossRef]
- Willard, S.S.; Koochekpour, S. Glutamate, glutamate receptors, and downstream signaling pathways. Int. J. Biol. Sci. 2013, 9, 948–959. [Google Scholar] [CrossRef]
- Menegollo, M.; Tessari, I.; Bubacco, L.; Szabadkai, G. Determination of ATP, ADP, and AMP Levels by Reversed-Phase High-Performance Liquid Chromatography in Cultured Cells. Methods Mol. Biol. 2019, 1925, 223–232. [Google Scholar] [CrossRef]
- Law, A.S.; Hafen, P.S.; Brault, J.J. Liquid chromatography method for simultaneous quantification of ATP and its degradation products compatible with both UV-Vis and mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1206, 123351. [Google Scholar] [CrossRef]
- Drake, D.M.; Shapiro, A.M.; Wells, P.G. Measurement of the Oxidative DNA Lesion 8-Oxoguanine (8-oxoG) by ELISA or by High-Performance Liquid Chromatography (HPLC) with Electrochemical Detection. Methods Mol. Biol. 2019, 1965, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, P.; Ghosh, S.; Patra, S.; Mandal, M.; Das, S. Comparing Ag-O coordinated AgMOF-5 and Ag-N coordinated Ag nanosphere catalytic polymers for real time monitoring of H(2)O(2) level in cancer cells. Biosens. Bioelectron. 2025, 271, 117056. [Google Scholar] [CrossRef]
- Zhai, R.; Fang, B.; Lai, Y.; Peng, B.; Bai, H.; Liu, X.; Li, L.; Huang, W. Small-molecule fluorogenic probes for mitochondrial nanoscale imaging. Chem. Soc. Rev. 2023, 52, 942–972. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Z.; Zou, H.-q.; Hu, S.-l.; Wang, D.-z.; Liu, J.-s.; Wang, L.-d. Electrochemical determination of glutamic pyruvic transaminase using a microfluidic chip. Microfluid. Nanofluidics 2017, 21, 27. [Google Scholar] [CrossRef]
- Greve, H.J.; Dunbar, A.L.; Lombo, C.G.; Ahmed, C.; Thang, M.; Messenger, E.J.; Mumaw, C.L.; Johnson, J.A.; Kodavanti, U.P.; Oblak, A.L.; et al. The bidirectional lung brain-axis of amyloid-β pathology: Ozone dysregulates the peri-plaque microenvironment. Brain 2023, 146, 991–1005. [Google Scholar] [CrossRef]
- Singh, S.A.; Suresh, S.; Vellapandian, C. Ozone-induced neurotoxicity: In vitro and in vivo evidence. Ageing Res. Rev. 2023, 91, 102045. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Chen, J.; Rodopoulou, S.; Strak, M.; de Hoogh, K.; Andersen, Z.J.; Bellander, T.; Brandt, J.; Fecht, D.; Forastiere, F.; et al. Long-term air pollution exposure and malignant intracranial tumours of the central nervous system: A pooled analysis of six European cohorts. Br. J. Cancer 2023, 129, 656–664. [Google Scholar] [CrossRef]
- Mo, S.; Wang, Y.; Peng, M.; Wang, Q.; Zheng, H.; Zhan, Y.; Ma, Z.; Yang, Z.; Liu, L.; Hu, K.; et al. Sex disparity in cognitive aging related to later-life exposure to ambient air pollution. Sci. Total Environ. 2023, 886, 163980. [Google Scholar] [CrossRef]
- Zhao, S.; Zang, G.; Zhang, Y.; Liu, H.; Wang, N.; Cai, S.; Durkan, C.; Xie, G.; Wang, G. Recent advances of electrochemical sensors for detecting and monitoring ROS/RNS. Biosens. Bioelectron. 2021, 179, 113052. [Google Scholar] [CrossRef]
- Pak, V.V.; Ezeriņa, D.; Lyublinskaya, O.G.; Pedre, B.; Tyurin-Kuzmin, P.A.; Mishina, N.M.; Thauvin, M.; Young, D.; Wahni, K.; Martínez Gache, S.A.; et al. Ultrasensitive Genetically Encoded Indicator for Hydrogen Peroxide Identifies Roles for the Oxidant in Cell Migration and Mitochondrial Function. Cell Metab. 2020, 31, 642–653. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, Y.; Wei, H.; Yu, P.; Jiang, Y.; Mao, L. Electrochemical Monitoring of Propagative Fluctuation of Ascorbate in the Live Rat Brain during Spreading Depolarization. Angew. Chem. Int. Ed. 2019, 58, 6616–6619. (In English) [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Ji, W.; Jiang, Y.; Yu, P.; Mao, L. Deep Learning for Voltammetric Sensing in a Living Animal Brain. Angew. Chem. Int. Ed. 2021, 60, 23777–23783. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, L.; Ye, L.; Jiang, Y.; Li, Q.; Zhang, H.; Zhang, R.; Li, H.; Yu, D.; Zhang, R.; et al. PP2A-mTOR-p70S6K/4E-BP1 axis regulates M1 polarization of pulmonary macrophages and promotes ambient particulate matter induced mouse lung injury. J. Hazard. Mater. 2022, 424, 127624. [Google Scholar] [CrossRef]
- Ning, J.; Pei, Z.; Wang, M.; Hu, H.; Chen, M.; Liu, Q.; Wu, M.; Yang, P.; Geng, Z.; Zheng, J.; et al. Site-specific Atg13 methylation-mediated autophagy regulates epithelial inflammation in PM2.5-induced pulmonary fibrosis. J. Hazard. Mater. 2023, 457, 131791. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Jin, S.; Lu, W.; Zhang, J.; Zhang, L.; Tao, F.; Zhang, Y.; Hu, X.; Liu, Q. The mechanisms, hallmarks, and therapies for brain aging and age-related dementia. Sci. Bull. 2024, 69, 3756–3776. [Google Scholar] [CrossRef]
- Friberg, S.; Lindblad, C.; Zeiler, F.A.; Zetterberg, H.; Granberg, T.; Svenningsson, P.; Piehl, F.; Thelin, E.P. Fluid biomarkers of chronic traumatic brain injury. Nat. Rev. Neurol. 2024, 20, 671–684. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Cai, T.; Shang, K.; Wang, X.; Qi, X.; Liu, R.; Wang, X. Integration of Glutamate Dehydrogenase and Nanoporous Gold for Electrochemical Detection of Glutamate. Biosens 2023, 13, 1023. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, W.; Zhang, S.; Gao, N.; Xu, T.; Wang, X.; Zhang, M. Vitamin D Inhibits the Early Aggregation of α-Synuclein and Modulates Exocytosis Revealed by Electrochemical Measurements. Angew. Chem. Int. Ed. 2022, 61, e202111853. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kohansal, F.; Mobed, A.; Ansari, R.; Hasanzadeh, M.; Ahmadalipour, A.; Shadjou, N. An innovative electrochemical immuno-platform towards ultra-sensitive monitoring of 2-arachidonoyl glycerol in samples from rats with sleep deprivation: Bioanalysis of endogenous cannabinoids using biosensor technology. RSC Adv. 2022, 12, 14154–14166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, C.; Li, X.; Ren, D.; Zheng, M.; Zhang, S.; Yuan, F.; Du, X.; Zhang, Z. Inflammation assessment and therapeutic monitoring based on highly sensitive and multi-level electrochemical detection of PGE2. Biosens. Bioelectron. 2024, 262, 116539. [Google Scholar] [CrossRef]
- Aggarwal, A.; Liu, R.; Chen, Y.; Ralowicz, A.J.; Bergerson, S.J.; Tomaska, F.; Mohar, B.; Hanson, T.L.; Hasseman, J.P.; Reep, D.; et al. Glutamate indicators with improved activation kinetics and localization for imaging synaptic transmission. Nat. Methods 2023, 20, 925–934. [Google Scholar] [CrossRef]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 2020, 21, e49799. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Ramos-Campo, D.J.; Belinchón-deMiguel, P.; Martinez-Guardado, I.; Dalamitros, A.A.; Yáñez-Sepúlveda, R.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines 2023, 11, 2488. [Google Scholar] [CrossRef]
- Kotrys, A.V.; Durham, T.J.; Guo, X.A.; Vantaku, V.R.; Parangi, S.; Mootha, V.K. Single-cell analysis reveals context-dependent, cell-level selection of mtDNA. Nature 2024, 629, 458–466. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, X.; Hochrein, S.M.; Eckstein, M.; Gubert, G.F.; Knöpper, K.; Mansilla, A.M.; Öner, A. Doucet-Ladevèze, R.; Schmitz, W.; et al. Mitochondrial dysfunction promotes the transition of precursor to terminally exhausted T cells through HIF-1α-mediated glycolytic reprogramming. Nat. Commun. 2023, 14, 6858. [Google Scholar] [CrossRef]
- Suraniti, E.; Ben-Amor, S.; Landry, P.; Rigoulet, M.; Fontaine, E.; Bottari, S.; Devin, A.; Sojic, N.; Mano, N.; Arbault, S. Electrochemical monitoring of the early events of hydrogen peroxide production by mitochondria. Angew. Chem. Int. Ed. 2014, 53, 6655–6658. (In English) [Google Scholar] [CrossRef]
- Huang, H.; Dong, F.; Tian, Y. Mitochondria-Targeted Ratiometric Fluorescent Nanosensor for Simultaneous Biosensing and Imaging of O2•– and pH in Live Cells. Anal. Chem. 2016, 88, 12294–12302. [Google Scholar] [CrossRef]
- Ma, Y.; Colin, C.; Descamps, J.; Arbault, S.; Sojic, N. Shadow Electrochemiluminescence Microscopy of Single Mitochondria. Angew. Chem. Int. Ed. 2021, 60, 18742–18749. (In English) [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, H.; Wu, P.; Gong, Z.; Xu, G.; Cai, C. Electrochemical detection of extracellular hydrogen peroxide released from RAW 264.7 murine macrophage cells based on horseradish peroxidase-hydroxyapatite nanohybrids. Analyst 2011, 136, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, X.W.; Liao, Q.L.; Wu, W.T.; Liu, Y.L.; Huang, W.H. Electrochemical Monitoring of Paclitaxel-Induced ROS Release from Mitochondria inside Single Cells. Small 2019, 15, e1901787. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, K.; Yu, Y.; Rotenberg, S.A.; Amatore, C.; Mirkin, M.V. Direct Electrochemical Measurements of Reactive Oxygen and Nitrogen Species in Nontransformed and Metastatic Human Breast Cells. J. Am. Chem. Soc. 2017, 139, 13055–13062. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Peng, Z.; Li, Y. A ratiometric electrochemiluminescence sensing platform for robust ascorbic acid analysis based on a molecularly imprinted polymer modified bipolar electrode. Biosens. Bioelectron. 2020, 167, 112490. [Google Scholar] [CrossRef]
- Marvin, J.S.; Scholl, B.; Wilson, D.E.; Podgorski, K.; Kazemipour, A.; Müller, J.A.; Schoch, S.; Quiroz, F.J.U.; Rebola, N.; Bao, H.; et al. Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR. Nat. Methods 2018, 15, 936–939. [Google Scholar] [CrossRef]
- Sirca, D.; Vardeu, A.; Pinna, M.; Diana, M.; Enrico, P. A robust, state-of-the-art amperometric microbiosensor for glutamate detection. Biosens. Bioelectron. 2014, 61, 526–531. [Google Scholar] [CrossRef]
- Beerkens, A.P.M.; Boreel, D.F.; Nathan, J.A.; Neuzil, J.; Cheng, G.; Kalyanaraman, B.; Hardy, M.; Adema, G.J.; Heskamp, S.; Span, P.N.; et al. Characterizing OXPHOS inhibitor-mediated alleviation of hypoxia using high-throughput live cell-imaging. Cancer Metab. 2024, 12, 13. [Google Scholar] [CrossRef]
- Barroso-Gomila, O.; Merino-Cacho, L.; Muratore, V.; Perez, C.; Taibi, V.; Maspero, E.; Azkargorta, M.; Iloro, I.; Trulsson, F.; Vertegaal, A.C.O.; et al. BioE3 identifies specific substrates of ubiquitin E3 ligases. Nat. Commun. 2023, 14, 7656. [Google Scholar] [CrossRef]
- Goldenberg, S.J.; Veriabo, N.J.; Soslau, G. A micromethod to measure platelet aggregation and atp release by impedance. Thromb. Res. 2001, 103, 57–61. [Google Scholar] [CrossRef]
- Pundir, C.S.; Narwal, V.; Batra, B. Determination of lactic acid with special emphasis on biosensing methods: A review. Biosens. Bioelectron. 2016, 86, 777–790. [Google Scholar] [CrossRef]
- Xuan, G.; Lu, X.; Wang, J.; Lin, H.; Liu, H. Determination of pyruvic acid concentration using a bioluminescence system from Photobacterium leiognathi. Photochem. Photobiol. Sci. 2015, 14, 1163–1168. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Yang, S.; Song, H.; Sun, Z.; Wang, K.; Zhu, Y.; Yang, C.; Hao, R.; Cao, Y. High-Resolution Tracking of Aging-Related Small Molecules: Bridging Pollutant Exposure, Brain Aging Mechanisms, and Detection Innovations. Biosensors 2025, 15, 242. https://doi.org/10.3390/bios15040242
Yu K, Yang S, Song H, Sun Z, Wang K, Zhu Y, Yang C, Hao R, Cao Y. High-Resolution Tracking of Aging-Related Small Molecules: Bridging Pollutant Exposure, Brain Aging Mechanisms, and Detection Innovations. Biosensors. 2025; 15(4):242. https://doi.org/10.3390/bios15040242
Chicago/Turabian StyleYu, Keying, Sirui Yang, Hongxu Song, Zhou Sun, Kaichao Wang, Yuqi Zhu, Chengkai Yang, Rongzhang Hao, and Yuanyuan Cao. 2025. "High-Resolution Tracking of Aging-Related Small Molecules: Bridging Pollutant Exposure, Brain Aging Mechanisms, and Detection Innovations" Biosensors 15, no. 4: 242. https://doi.org/10.3390/bios15040242
APA StyleYu, K., Yang, S., Song, H., Sun, Z., Wang, K., Zhu, Y., Yang, C., Hao, R., & Cao, Y. (2025). High-Resolution Tracking of Aging-Related Small Molecules: Bridging Pollutant Exposure, Brain Aging Mechanisms, and Detection Innovations. Biosensors, 15(4), 242. https://doi.org/10.3390/bios15040242





