Nanoparticulate Copper Cluster-Mediated Biosensing of Cardiac Biomolecular Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Methods
2.3. Synthesis of Copper Nanoparticles (CuNPs)
2.4. Synthesis of Troponin I Monoclonal Antibody-Conjugated Copper Nanoparticles
2.5. Synthesis of Troponin T Monoclonal Antibody-Conjugated Copper Nanoparticles
2.6. Detection of Troponin I and T by the Troponin I and Tmonoclonal Antibody-Conjugated Copper Nanoparticles
3. Results
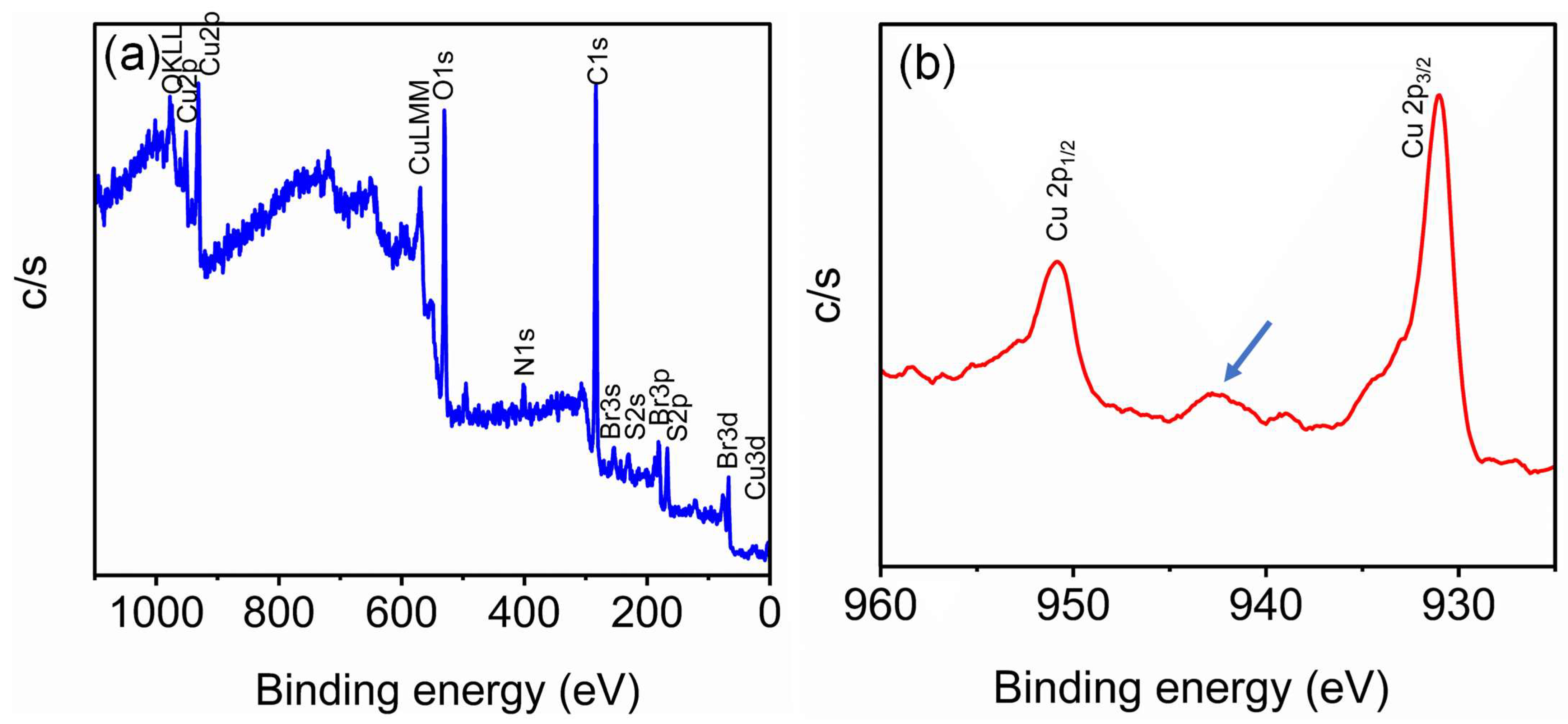
3.1. Synthesis and Characterization of Copper Nanoparticles
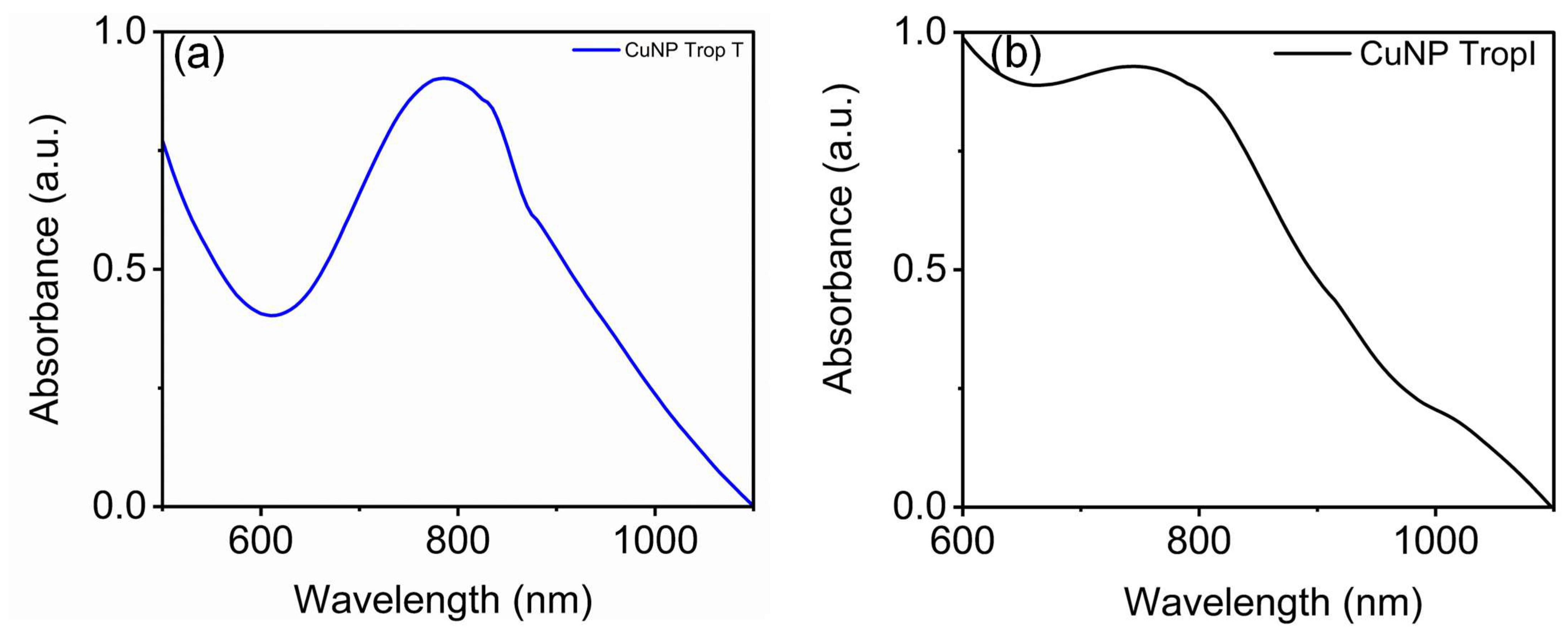
3.2. Development of CuNP Sensor for Troponin I and Troponin T
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adhikary, D.; Barman, S.; Ranjan, R.; Stone, H. A Systematic Review of Major Cardiovascular Risk Factors: A Growing Global Health Concern. Cureus 2022, 14, e30119. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Jeukendrup, A.E. Heart Rate Monitoring: Applications and Limitations. Sports Med. 2003, 33, 517–538. [Google Scholar] [CrossRef] [PubMed]
- Skeik, N.; Patel, D.C. A Review of Troponins in Ischemic Heart Disease and Other Conditions. Int. J. Angiol. 2007, 16, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, Y.; Apple, F.S.; Mahler, S.A.; Body, R.; Collinson, P.O.; Jaffe, A.S.; on behalf of the International Federation of Clinical Chemistry and Laboratory Medicine Committee on the Clinical Application of Cardiac Biomarkers. High-Sensitivity Cardiac Troponin and the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guidelines for the Evaluation and Diagnosis of Acute Chest Pain. Circulation 2022, 146, 569–581. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, W.; Wang, K.; Han, Z.; Yang, C. Methods for Detecting of Cardiac Troponin I Biomarkers for Myocardial Infarction Using Biosensors: A Narrative Review of Recent Research. J. Thorac. Dis. 2023, 15, 5112–5121. [Google Scholar] [CrossRef]
- Lim, G.B. A Wearable Sensor to Measure Troponin I Levels. Nat. Rev. Cardiol. 2023, 20, 286. [Google Scholar] [CrossRef]
- Nair, L.V.; Philips, D.S.; Jayasree, R.S.; Ajayaghosh, A. A Near-Infrared Fluorescent Nanosensor (AuC@Urease) for the Selective Detection of Blood Urea. Small 2013, 9, 2673–2677. [Google Scholar] [CrossRef]
- Durgadas, C.V.; Lakshmi, V.N.; Sharma, C.P.; Sreenivasan, K. Sensing of Lead Ions Using Glutathione Mediated End to End Assembled Gold Nanorod Chains. Sens. Actuators B Chem. 2011, 156, 791–797. [Google Scholar] [CrossRef]
- Rahman, P.P.M.S.; Joseph, M.; Hanas, T.; Nair, L.V. End to End Aligned Surface-Modified Gold Nanorod for the Detection of Calcium. Mater. Lett. 2022, 325, 132906. [Google Scholar] [CrossRef]
- Pathiriparambath, M.S.R.; Joseph, M.; Manog, M.; Thomas, V.; Tharayil, H.; Nair, L.V. Glutamic Acid Modified Gold Nanorod Sensor for the Detection of Calcium Ions in Neuronal Cells. ChemBioChem 2024, 25, e202400009. [Google Scholar] [CrossRef]
- Nair, L.V.; Nair, R.V.; Jayasree, R.S. Cadmium Selenium Quantum Dot Based Nanosensor with Femto Molar Level Sensitivity for the Detection of the Pesticide Endosulfan. J. Polym. Sci. Eng. 2023, 6, 3208. [Google Scholar] [CrossRef]
- Nair, R.V.; Radhakrishna Pillai Suma, P.; Jayasree, R.S. A Dual Signal On-off Fluorescent Nanosensor for the Simultaneous Detection of Copper and Creatinine. Mater. Sci. Eng. C 2020, 109, 110569. [Google Scholar] [CrossRef]
- Santhakumar, H.; Nair, R.V.; Philips, D.S.; Shenoy, S.J.; Thekkuveettil, A.; Ajayaghosh, A.; Jayasree, R.S. Real Time Imaging and Dynamics of Hippocampal Zn2+ under Epileptic Condition Using a Ratiometric Fluorescent Probe. Sci. Rep. 2018, 8, 9069. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.V.; Thomas, T.; Kuttoth, H.; Karthikeyan, A.; Nair, B.G.; Sandhyarani, N. Cu2+-Mediated Aggregation of Gold Nanoparticles as an Optical Probe for the Detection of Endotoxin. Langmuir 2022, 38, 10826–10835. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Sohouli, E.; Dehkordi, Z.S. Electrochemical Sensor Based on Mesoporous G-C3N4/N-CNO/Gold Nanoparticles for Measuring Oxycodone. Sci. Rep. 2024, 14, 17221. [Google Scholar] [CrossRef]
- Qin, R.; Zhang, Y.; Xu, H.; Nie, P. Gold Nanoparticle-Modified Electrodes for Electrochemical Sensing of Ammonia Nitrogen in Water. ACS Appl. Nano Mater. 2024, 7, 577–593. [Google Scholar] [CrossRef]
- Shams, N.; Lim, H.N.; Hajian, R.; Yusof, N.A.; Abdullah, J.; Sulaiman, Y.; Ibrahim, I.; Huang, N.M. Electrochemical Sensor Based on Gold Nanoparticles/Ethylenediamine-Reduced Graphene Oxide for Trace Determination of Fenitrothion in Water. RSC Adv. 2016, 6, 89430–89439. [Google Scholar] [CrossRef]
- Yu, A.; Liang, Z.; Cho, J.; Caruso, F. Nanostructured Electrochemical Sensor Based on Dense Gold Nanoparticle Films. Nano Lett. 2003, 3, 1203–1207. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Zhang, L.; Zhang, Y.; Zhang, H.; Zhang, C.; Xuan, X.; Wang, M.; Zhang, J.; Yuan, Y. A Novel Graphene-Based Nanomaterial Modified Electrochemical Sensor for the Detection of Cardiac Troponin I. Front. Chem. 2021, 9, 680593. [Google Scholar] [CrossRef]
- Chen, J.N.; Hasabnis, G.K.; Akin, E.; Gao, G.; Usha, S.P.; Süssmuth, R.; Altintas, Z. Developing Innovative Point-of-Care Electrochemical Sensors Empowered by Cardiac Troponin I-Responsive Nanocomposite Materials. Sens. Actuators B Chem. 2024, 417, 136052. [Google Scholar] [CrossRef]
- Hasabnis, G.K.; Altintas, Z. Cardiac Troponin I-Responsive Nanocomposite Materials for Voltammetric Monitoring of Acute Myocardial Infarction. ACS Omega 2024, 9, 30737–30750. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, D.; Kaur, I.; Kumar, A. Ultrasensitive Cardiac Troponin I Antibody Based Nanohybrid Sensor for Rapid Detection of Human Heart Attack. Int. J. Biol. Macromol. 2017, 95, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Surya, S.G.; Majhi, S.M.; Agarwal, D.K.; Lahcen, A.A.; Yuvaraja, S.; Chappanda, K.N.; Salama, K.N. A Label-Free Aptasensor FET Based on Au Nanoparticle Decorated Co3O4 Nanorods and a SWCNT Layer for Detection of Cardiac Troponin T Protein. J. Mater. Chem. B 2020, 8, 18–26. [Google Scholar] [CrossRef]
- Periyakaruppan, A.; Gandhiraman, R.P.; Meyyappan, M.; Koehne, J.E. Label-Free Detection of Cardiac Troponin-I Using Carbon Nanofiber Based Nanoelectrode Arrays. Anal. Chem. 2013, 85, 3858–3863. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khoshfetrat, S.M.; Mirzaeizadeh, Z.; Kabiri, S.; Rezaie, J.; Omidfar, K. Electrochemical Immunosensor for Determination of Cardiac Troponin I Using Two-Dimensional Metal-Organic Framework/Fe3O4–COOH Nanosheet Composites Loaded with Thionine and pCTAB/DES Modified Electrode. Talanta 2022, 237, 122911. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Darius, E.; Lien, M.-C.; Yeh, I.-H.; Shi, H.-F.; Huang, Y.-H.; Chen, C.-H.; Chen, H.-W.; Su, C.-Y.; Hsu, R.-Y.; et al. Two-Dimensional Cs2 AgBiBr6-Based Biosensor for Selective and Sensitive Detection of Cardiac Biomarker Troponin I. ACS Appl. Nano Mater. 2023, 6, 23022–23028. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Joseph, M.; Rahman Pathiripparambath, M.S.; Thomas, V.; Tharayil, H.; Jayasree, R.S.; Nair, L.V. Porphyrin and Doxorubicin Mediated Nanoarchitectonics of Copper Clusters: A Bimodal Theranostics for Cancer Diagnosis and Treatment in Vitro. J. Mater. Chem. B 2024, 12, 720–729. [Google Scholar] [CrossRef]
- Joseph, M.; Pathiripparambath, M.S.R.; Tharayil, H.; Jayasree, R.S.; Nair, L.V. Copper Nanocluster Enables Simultaneous Photodynamic and Chemo Therapy for Effective Cancer Diagnosis and Treatment in Vitro. ChemMedChem 2022, 17, e202200201. [Google Scholar] [CrossRef]
- Stebunov, Y.V.; Yakubovsky, D.I.; Fedyanin, D.Y.; Arsenin, A.V.; Volkov, V.S. Superior Sensitivity of Copper-Based Plasmonic Biosensors. Langmuir 2018, 34, 4681–4687. [Google Scholar] [CrossRef]
- Qing, Z.; Bai, A.; Xing, S.; Zou, Z.; He, X.; Wang, K.; Yang, R. Progress in Biosensor Based on DNA-Templated Copper Nanoparticles. Biosens. Bioelectron. 2019, 137, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, L.; Xu, R.; Ma, Y.; Ma, L. Facile Fabrication of Biosensors Based on Cu Nanoparticles Modified As-Grown CVD Graphene for Non-Enzymatic Glucose Sensing. J. Electroanal. Chem. 2019, 853, 113527. [Google Scholar] [CrossRef]
- Li, K.; Xu, X.; Liu, W.; Yang, S.; Huang, L.; Tang, S.; Zhang, Z.; Wang, Y.; Chen, F.; Qian, K. A Copper-Based Biosensor for Dual-Mode Glucose Detection. Front. Chem. 2022, 10, 861353. [Google Scholar] [CrossRef]
- Cui, J.; Han, H.; Piao, J.; Shi, H.; Zhou, D.; Gong, X.; Chang, J. Construction of a Novel Biosensor Based on the Self-Assembly of Dual-Enzyme Cascade Amplification-Induced Copper Nanoparticles for Ultrasensitive Detection of MicroRNA153. ACS Appl. Mater. Interfaces 2020, 12, 34130–34136. [Google Scholar] [CrossRef]
- Soganci, T.; Ayranci, R.; Harputlu, E.; Ocakoglu, K.; Acet, M.; Farle, M.; Unlu, C.G.; Ak, M. An Effective Non-Enzymatic Biosensor Platform Based on Copper Nanoparticles Decorated by Sputtering on CVD Graphene. Sens. Actuators B Chem. 2018, 273, 1501–1507. [Google Scholar] [CrossRef]
- Campu, A.; Muresan, I.; Craciun, A.-M.; Cainap, S.; Astilean, S.; Focsan, M. Cardiac Troponin Biosensor Designs: Current Developments and Remaining Challenges. Int. J. Mol. Sci. 2022, 23, 7728. [Google Scholar] [CrossRef]
- Abdorahim, M.; Rabiee, M.; Alhosseini, S.N.; Tahriri, M.; Yazdanpanah, S.; Alavi, S.H.; Tayebi, L. Nanomaterials-Based Electrochemical Immunosensors for Cardiac Troponin Recognition: An Illustrated Review. TrAC Trends Anal. Chem. 2016, 82, 337–347. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumar, P.; Hussain, M.; Das, A.K.; Nayak, G.C. Synthesis and Characterization of CuO Nanoparticles Using Strong Base Electrolyte through Electrochemical Discharge Process. Bull. Mater. Sci. 2016, 39, 469–478. [Google Scholar] [CrossRef]
- Raul, P.K.; Senapati, S.; Sahoo, A.K.; Umlong, I.M.; Devi, R.R.; Thakur, A.J.; Veer, V. CuO Nanorods: A Potential and Efficient Adsorbent in Water Purification. RSC Adv. 2014, 4, 40580–40587. [Google Scholar] [CrossRef]
- Nzilu, D.M.; Madivoli, E.S.; Makhanu, D.S.; Wanakai, S.I.; Kiprono, G.K.; Kareru, P.G. Green Synthesis of Copper Oxide Nanoparticles and Its Efficiency in Degradation of Rifampicin Antibiotic. Sci. Rep. 2023, 13, 14030. [Google Scholar] [CrossRef]
- Jana, J.; Ganguly, M.; Pal, T. Enlightening Surface Plasmon Resonance Effect of Metal Nanoparticles for Practical Spectroscopic Application. RSC Adv. 2016, 6, 86174–86211. [Google Scholar] [CrossRef]
- Jain, P.K.; El-Sayed, M.A. Surface Plasmon Resonance Sensitivity of Metal Nanostructures: Physical Basis and Universal Scaling in Metal Nanoshells. J. Phys. Chem. C 2007, 111, 17451–17454. [Google Scholar] [CrossRef]
- Yu, H.; Peng, Y.; Yang, Y.; Li, Z.-Y. Plasmon-Enhanced Light–Matter Interactions and Applications. Npj Comput. Mater. 2019, 5, 45. [Google Scholar] [CrossRef]
- Roh, S.; Chung, T.; Lee, B. Overview of the Characteristics of Micro- and Nano-Structured Surface Plasmon Resonance Sensors. Sensors 2011, 11, 1565–1588. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Jhuang, L.-S.; Kumar, G.; Chen, F.-C. Localized Surface Plasmon Resonance of Copper Nanoparticles Improves the Performance of Quasi-Two-Dimensional Perovskite Light-Emitting Diodes. Dye. Pigment. 2021, 188, 109204. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, B.; Wang, S.; Liu, H.; Wang, J.; Si, M. Highly Stable Localized Surface Plasmon Resonance of Cu Nanoparticles Obtained via Oxygen Plasma Irradiation. Nanoscale 2024, 16, 9748–9753. [Google Scholar] [CrossRef]
- Baral, T.; Datta, C.; Das, S. Cu Nanoparticle-Based Solution and Paper Strips for Colorimetric and Visual Detection of Heavy Metal Ions. ACS Omega 2022, 7, 37279–37285. [Google Scholar] [CrossRef]
- Song, Y.; Cho, D.; Venkateswarlu, S.; Yoon, M. Systematic Study on Preparation of Copper Nanoparticle Embedded Porous Carbon by Carbonization of Metal–Organic Framework for Enzymatic Glucose Sensor. RSC Adv. 2017, 7, 10592–10600. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, M.; Li, C. Copper-Based Nanomaterials for Cancer Imaging and Therapy. Bioconjugate Chem. 2016, 27, 1188–1199. [Google Scholar] [CrossRef]
- Wei, Q.; Pan, Y.; Zhang, Z.; Yan, S.; Li, Z. Copper-Based Nanomaterials for Biomedical Applications. Chem. Eng. J. 2024, 483, 149040. [Google Scholar] [CrossRef]
- Frangioni, J. In Vivo Near-Infrared Fluorescence Imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, L.M.; DeLouise, L.A. Whole Blood Optical Biosensor. Biosens. Bioelectron. 2007, 23, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Zhu, M.; Kuang, Z.; Li, X.; Xu, F.; Miao, S.; Zhang, Z.; Lou, X.; Li, H.; et al. Electrochemical Biosensors for Whole Blood Analysis: Recent Progress, Challenges, and Future Perspectives. Chem. Rev. 2023, 123, 7953–8039. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, L.V.; Wheeler, J.; Ha, Y.; Jones, K.M.; Jones, J.; Thomas, V. Nanoparticulate Copper Cluster-Mediated Biosensing of Cardiac Biomolecular Markers. Biosensors 2025, 15, 237. https://doi.org/10.3390/bios15040237
Nair LV, Wheeler J, Ha Y, Jones KM, Jones J, Thomas V. Nanoparticulate Copper Cluster-Mediated Biosensing of Cardiac Biomolecular Markers. Biosensors. 2025; 15(4):237. https://doi.org/10.3390/bios15040237
Chicago/Turabian StyleNair, Lakshmi V., Jarred Wheeler, Yaelyn Ha, Kimberly M. Jones, Jesse Jones, and Vinoy Thomas. 2025. "Nanoparticulate Copper Cluster-Mediated Biosensing of Cardiac Biomolecular Markers" Biosensors 15, no. 4: 237. https://doi.org/10.3390/bios15040237
APA StyleNair, L. V., Wheeler, J., Ha, Y., Jones, K. M., Jones, J., & Thomas, V. (2025). Nanoparticulate Copper Cluster-Mediated Biosensing of Cardiac Biomolecular Markers. Biosensors, 15(4), 237. https://doi.org/10.3390/bios15040237





