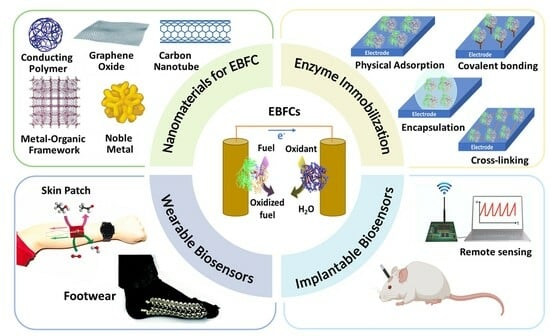
Recent Advances in Enzymatic Biofuel Cells to Power Up Wearable and Implantable Biosensors
Abstract
1. Introduction
2. Enzymatic Biofuel Cells
2.1. EBFC Fundamentals and Directions
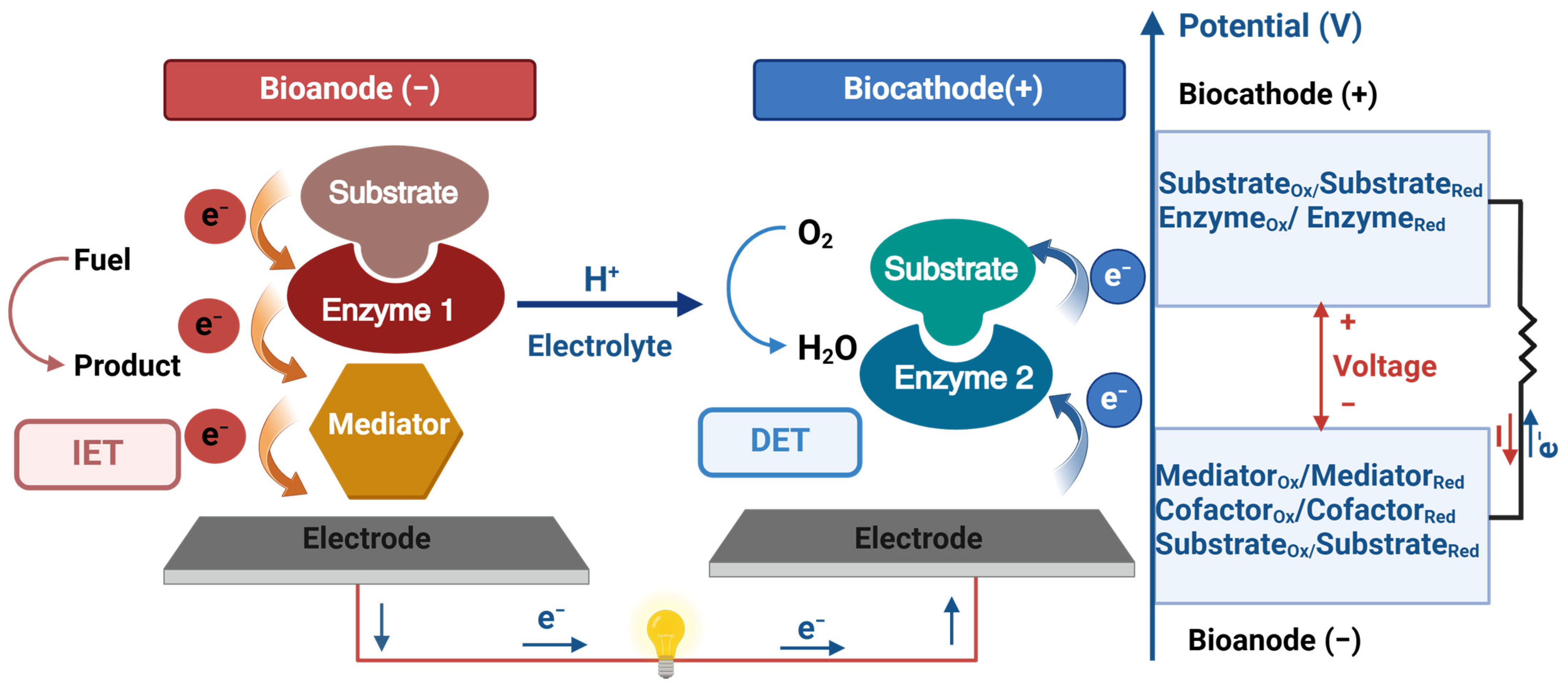
2.2. Electron Transfer Mechanisms
2.3. Enzyme Immobilization Strategies
2.3.1. Physical Adsorption
2.3.2. Covalent Binding
2.3.3. Encapsulation
2.3.4. Cross-Linking
3. Nanomaterials for EBFCs
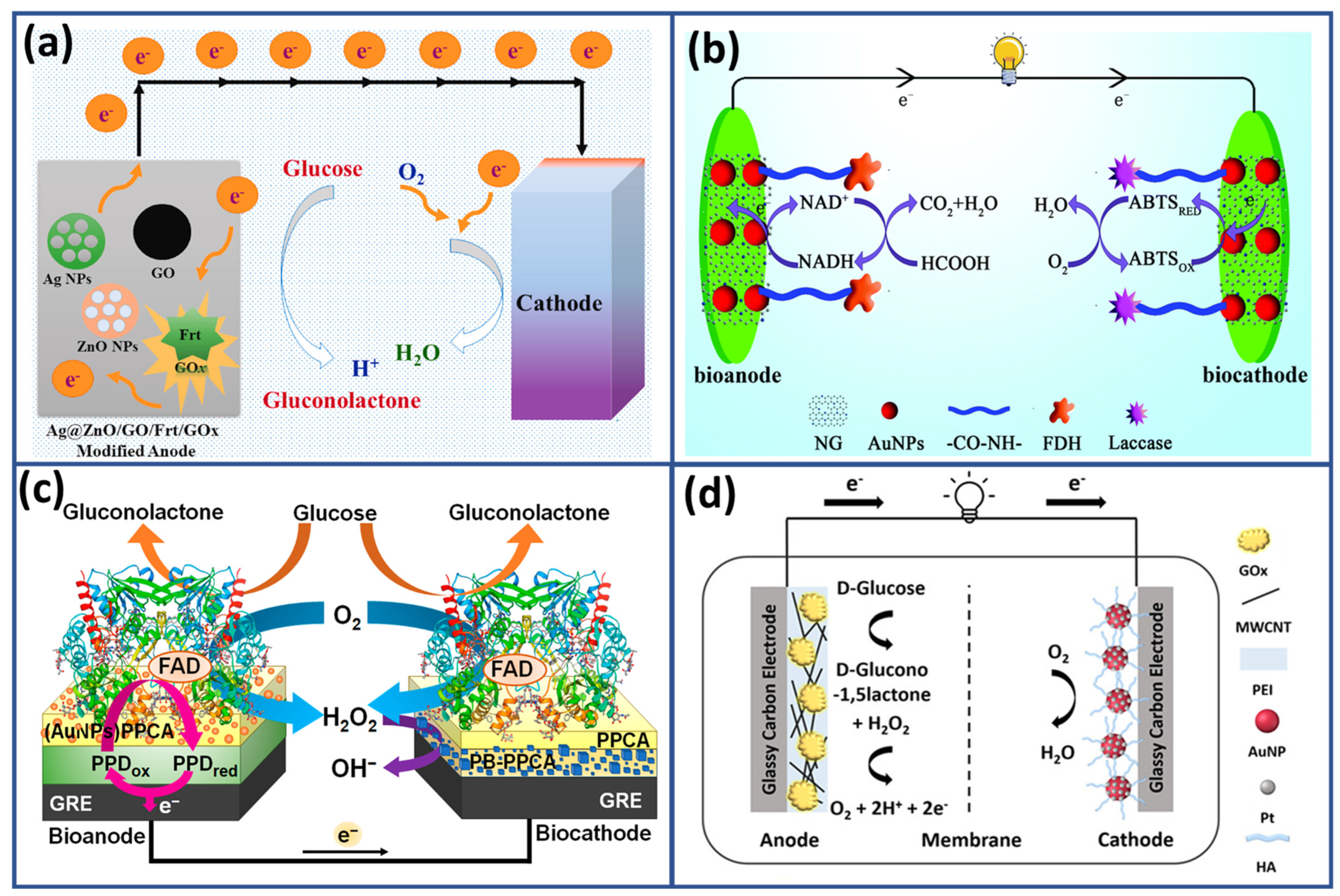
3.1. Carbon-Based Nanomaterials
3.2. Noble Metals
3.3. Conducting Polymers Based Bioelectrodes
3.4. Metal–Organic Frameworks Based Bioelectrodes
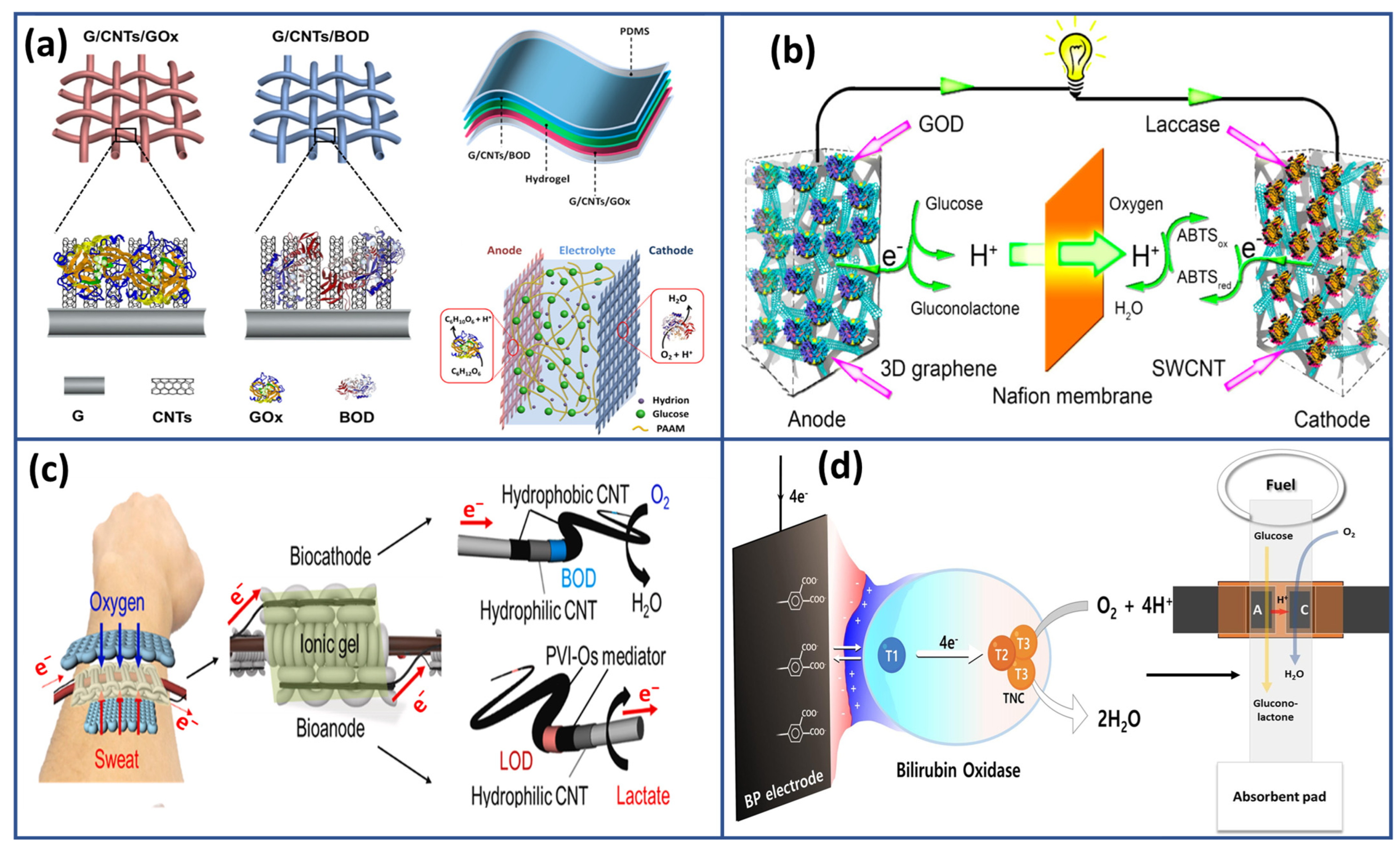
4. EBFCs Based Wearable SPB for Sweat Analysis
4.1. Self-Powered Neurotransmitter Biosensor
4.2. Self-Powered Glucose Biosensor

4.3. Self-Powered Lactate Biosensor

4.4. Self-Powered Ethanol Biosensor
5. EBFCs-Based Implantable SPBs
6. Challenges and Trends
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| Abbreviation | Definition |
| EBFCs | Enzymatic biofuel cells |
| BFCs | Biological fuel cells |
| SPBs | Self-powered biosensors |
| DET | Direct Electron transfer |
| IET | Indirect electron transfer |
| SAMs | self-assembled monolayers |
| EDC | 1-ethyl-3(3-dimethylaminopropyl) carbodiimide |
| CNT | Carbon nanotube |
| GOx | Glucose oxidase |
| BOx | Bilirubin oxidase |
| GDH | Glucose dehydrogenase |
| MWCNTs | Multiwalled carbon nanotubes |
| SWNTs | Single-walled carbon nanotubes |
| MOF | Metal–organic framework |
| CNDs | Carbon nanodots |
| BP | Bucky paper |
| GO | graphene oxide |
| rGO | Reduced graphene oxide |
| ABTS | 3-Aminopropyltriethoxysilane |
| BOD | Bilirubin oxidase |
| 4-APA | 4-amino phthalic acid |
| CS | Chitosan |
| CNCs | carbon nanochips |
| GCE | Glassy carbon electrode |
| Pt | Platinum |
| FRT | ferritin |
| AgNPs | Silver nanoparticles |
| ZnO | Zinc oxide |
| NADH | nicotinamide adenine dinucleotide with hydrogen |
| NG | Nitrogen-doped graphene |
| AuNPs | Gold nanoparticles |
| FDH | Formate dehydrogenase |
| PPCA | Poly(pyrrole-2-carboxylic acid |
| CPs | Conducting polymers |
| NGQDs | N-doped graphene quantum dots |
| PANI | Polyaniline |
| PB | Prussian blue |
| EPD | Bottom-up electrophoretic deposition |
| FTIR | Fourier-transform infrared spectroscopy |
| HRP | Horseradish peroxidase |
| SEM | Scanning electron microscopy |
| PQQ | pyrroloquinoline quinone |
| NQ | 4-naphthoquinone |
| LOx | Lactate oxidase |
| PSS | Polystyrene sulfonate |
| AOx | Alcohol oxidase |
| ADH | Alcohol dehydrogenase |
| PVP | Polyvinylpyrrolidone |
| GBFC | Glucose Biofuel Cell |
| PPO | Polyphenol oxidase |
| ABS | Animal Brain Stimulator |
References
- Liu, Z.; Yang, J.; Wang, H.; Zhang, J.; Bai, H.; Peng, B.; Ai, W.; Du, H.; Li, L.; Chen, P. Recent Progress in Mitochondrial Biofuel Cells. J. Electroanal. Chem. 2023, 950, 117881. [Google Scholar] [CrossRef]
- Babadi, A.A.; Bagheri, S.; Hamid, S.B.A. Progress on Implantable Biofuel Cell: Nano-Carbon Functionalization for Enzyme Immobilization Enhancement. Biosens. Bioelectron. 2016, 79, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Yahiro, A.T.; Lee, S.M.; Kimble, D.O. Bioelectrochemistry: I. Enzyme Utilizing Bio-Fuel Cell Studies. Biochim. Biophys. Acta (BBA) Spec. Sect. Biophys. Subj. 1964, 88, 375–383. [Google Scholar] [CrossRef]
- Gamella, M.; Koushanpour, A.; Katz, E. Biofuel Cells—Activation of Micro- and Macro-Electronic Devices. Bioelectrochemistry 2018, 119, 33–42. [Google Scholar] [CrossRef]
- Rewatkar, P.; Hitaishi, V.P.; Lojou, E.; Goel, S. Enzymatic Fuel Cells in a Microfluidic Environment: Status and Opportunities. A Mini Review. Electrochem. Commun. 2019, 107, 106533. [Google Scholar] [CrossRef]
- ul Haque, S.; Duteanu, N.; Ciocan, S.; Nasar, A.; Inamuddin. A Review: Evolution of Enzymatic Biofuel Cells. J. Environ. Manag. 2021, 298, 113483. [Google Scholar] [CrossRef]
- Farzin, M.A.; Naghib, S.M.; Rabiee, N. Advancements in Bio-Inspired Self-Powered Wireless Sensors: Materials, Mechanisms, and Biomedical Applications. ACS Biomater. Sci. Eng. 2024, 10, 1262–1301. [Google Scholar] [CrossRef]
- Li, Q.; Gao, M.; Sun, X.; Wang, X.; Chu, D.; Cheng, W.; Xi, Y.; Lu, Y. All-in-One Self-Powered Wearable Biosensors Systems. Mater. Sci. Eng. R Rep. 2025, 163, 100934. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Sempionatto, J.R.; Barfidokht, A.; Mishra, R.K.; Campbell, A.S.; Hubble, L.J.; Wang, J. Wearable Bioelectronics: Enzyme-Based Body-Worn Electronic Devices. Acc. Chem. Res. 2018, 51, 2820–2828. [Google Scholar] [CrossRef]
- Cosnier, S.; Gross, A.J.; Le Goff, A.; Holzinger, M. Recent Advances on Enzymatic Glucose/Oxygen and Hydrogen/Oxygen Biofuel Cells: Achievements and Limitations. J. Power Sources 2016, 325, 252–263. [Google Scholar] [CrossRef]
- Mazurenko, I.; de Poulpiquet, A.; Lojou, E. Recent Developments in High Surface Area Bioelectrodes for Enzymatic Fuel Cells. Curr. Opin. Electrochem. 2017, 5, 74–84. [Google Scholar] [CrossRef]
- Wang, Z.L. Self-Powered Nanosensors and Nanosystems. Adv. Mater. 2012, 24, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Arechederra, R.L.; Minteer, S.D. Self-Powered Sensors. Anal. Bioanal. Chem. 2011, 400, 1605–1611. [Google Scholar] [CrossRef]
- Katz, E.; Bückmann, A.F.; Willner, I. Self-Powered Enzyme-Based Biosensors. J. Am. Chem. Soc. 2001, 123, 10752–10753. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Mukasa, D.; Zhang, H.; Gao, W. Self-Powered Wearable Biosensors. Acc. Mater. Res. 2021, 2, 184–197. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, R.; Fan, Z.; Lin, Y. Self-Powered and Wearable Biosensors for Healthcare. Mater. Today Energy 2022, 23, 100900. [Google Scholar] [CrossRef]
- Zhao, C.; Gai, P.; Song, R.; Chen, Y.; Zhang, J.; Zhu, J.-J. Nanostructured Material-Based Biofuel Cells: Recent Advances and Future Prospects. Chem. Soc. Rev. 2017, 46, 1545–1564. [Google Scholar] [CrossRef]
- Luqman, M.; Alharthi, M.A.; Shakeel, N.; Imran Ahamed, M.; Inamuddin. A Quick, Easy and Green Hydrothermal Method for the Development of a Bioanode Based on a Ternary Nanocomposite for Enzymatic Biofuel Cell Applications. Mater. Sci. Eng. B 2024, 300, 117107. [Google Scholar] [CrossRef]
- Holzinger, M. Carbon-Based Nanostructured Bio-Assemblies for Bioelectrochemical Applications. Biomed. Mater. Devices 2024, 2, 208–224. [Google Scholar] [CrossRef]
- Ding, J.; Luo, W.; Wu, T.; Cai, S.; Pan, Z.; Li, H.; Tu, B.; Fang, Q.; Yan, X.; Yang, R. Atomic-Thick Porous Pd Nanosheets with Antioxidant Enzyme-like Activities and Photothermal Properties for Potential Alzheimer’s Disease Treatment. Nano Today 2024, 54, 102121. [Google Scholar] [CrossRef]
- Bilge, S.; Dogan-Topal, B.; Gürbüz, M.M.; Ozkan, S.A.; Sınağ, A. Recent Trends in Core/Shell Nanoparticles: Their Enzyme-Based Electrochemical Biosensor Applications. Microchim. Acta 2024, 191, 240. [Google Scholar] [CrossRef] [PubMed]
- Tawalbeh, M.; Javed, R.M.N.; Al-Othman, A.; Almomani, F. The Novel Advancements of Nanomaterials in Biofuel Cells with a Focus on Electrodes’ Applications. Fuel 2022, 322, 124237. [Google Scholar] [CrossRef]
- Xiao, X. The Direct Use of Enzymatic Biofuel Cells as Functional Bioelectronics. eScience 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, Y.; Song, H.; Wang, X.; Zhang, Y.-H.P.J.; Zhu, Z. A Wearable and Flexible Lactic-Acid/O2 Biofuel Cell with an Enhanced Air-Breathing Biocathode. Biosens. Bioelectron. 2024, 246, 115845. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Feng, Q.; Zhang, Y.; Lv, P.; Wei, Q. Flexible Bioelectrode via In-Situ Growth of MOF/Enzyme on Electrospun Nanofibers for Stretchable Enzymatic Biofuel Cell. Chem. Eng. J. 2022, 440, 135719. [Google Scholar] [CrossRef]
- Li, Z.; Wu, R.; Chen, K.; Gu, W.; Zhang, Y.-H.P.J.; Zhu, Z. Enzymatic Biofuel Cell-Powered Iontophoretic Facial Mask for Enhanced Transdermal Drug Delivery. Biosens. Bioelectron. 2023, 223, 115019. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yun, J.-H.; Park, Y.B.; Hyeon, J.S.; Jang, Y.; Choi, Y.-B.; Kim, H.-H.; Kang, T.M.; Ovalle, R.; Baughman, R.H.; et al. Two-Ply Carbon Nanotube Fiber-Typed Enzymatic Biofuel Cell Implanted in Mice. IEEE Trans. NanoBioscience 2020, 19, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Nishaa, V.; Spoorthi, B.V.; Soumya, B.T.; Meda, U.S.; Desai, V.S. Powering Implantable Medical Devices with Biological Fuel Cells. ECS Trans. 2022, 107, 19197. [Google Scholar] [CrossRef]
- Yue, O.; Wang, X.; Xie, L.; Bai, Z.; Zou, X.; Liu, X. Biomimetic Exogenous “Tissue Batteries” as Artificial Power Sources for Implantable Bioelectronic Devices Manufacturing. Adv. Sci. 2024, 11, 2307369. [Google Scholar] [CrossRef]
- Singh, R.; Kaur, N.; Singh, M. Bio-Compatible Bio-Fuel Cells for Medical Devices. Mater. Today Proc. 2021, 44, 242–249. [Google Scholar] [CrossRef]
- Yimamumaimaiti, T.; Lu, X.; Zhang, J.-R.; Wang, L.; Zhu, J.-J. Efficient Blood-Toleration Enzymatic Biofuel Cell via In Situ Protection of an Enzyme Catalyst. ACS Appl. Mater. Interfaces 2020, 12, 41429–41436. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.T.C.; Frasco, M.F.; Barbosa, S.G.; Peixoto, L.; Alves, M.M.; Sales, M.G.F. Enzymatic Self-Powered Biosensing Devices. In Bioelectrochemical Interface Engineering; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 505–519. ISBN 978-1-119-61110-3. [Google Scholar]
- Oh, H.; Park, J.; Park, G.; Baek, J.; Youn, J.; Yang, S.; Na, J.; Kim, D.; Lim, J.; Lee, H.; et al. Design Strategies for High Performance of Proton Exchange Fuel Cells with Ti-Sputtered Carbon Nanotube Sheet Functional Layer. Adv. Funct. Mater. 2024, 35, 2412311. [Google Scholar] [CrossRef]
- Monkrathok, J.; Janphuang, P.; Suphachiaraphan, S.; Kampaengsri, S.; Kamkaew, A.; Chansaenpak, K.; Lisnund, S.; Blay, V.; Pinyou, P. Enhancing Glucose Biosensing with Graphene Oxide and Ferrocene-Modified Linear Poly(Ethylenimine). Biosensors 2024, 14, 161. [Google Scholar] [CrossRef]
- Gu, C.; Gai, P.; Li, F. Construction of Biofuel Cells-Based Self-Powered Biosensors via Design of Nanocatalytic System. Nano Energy 2022, 93, 106806. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Chen, X.; Ding, M.; Eskandari, A.; Fairen-Jimenez, D.; Giménez-Marqués, M.; Gref, R.; Lin, W.; Luo, T.; Forgan, R.S. Metal–Organic Frameworks for Biological Applications. Nat. Rev. Methods Primers 2024, 4, 42. [Google Scholar] [CrossRef]
- Dey, T.; Chauhan, I.; Dutta, S. Flexible and Stretchable Electrodes in Biofuel Cells for Sustainable Power. ACS Appl. Electron. Mater. 2024, 6, 4016–4029. [Google Scholar] [CrossRef]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in Biosensors for Continuous Glucose Monitoring Towards Wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef]
- Sahoo, J.; Sharma, R.; Pachauri, V.; Gandhi, S. Biomimetic/Bioderived Nanoengineered Interfaces for Biosensor Applications: A Review. ACS Appl. Nano Mater. 2024, 7, 19854–19875. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. A Biofuel Cell with Electrochemically Switchable and Tunable Power Output. J. Am. Chem. Soc. 2003, 125, 6803–6813. [Google Scholar] [CrossRef]
- Meyer, J.; Meyer, L.; Kara, S. Enzyme Immobilization in Hydrogels: A Perfect Liaison for Efficient and Sustainable Biocatalysis. Eng. Life Sci. 2021, 22, 165–177. [Google Scholar] [CrossRef]
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, Techniques, and Applications of Biocatalyst Immobilization for Industrial Application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Wan, L.-S.; Liu, Z.-M.; Huang, X.-J.; Xu, Z.-K. Enzyme Immobilization on Electrospun Polymer Nanofibers: An Overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Cavalcante, A.L.G.; de Sousa, I.G.; Neto, F.S.; dos Santos, J.C.S. Current Status and Future Perspectives of Supports and Protocols for Enzyme Immobilization. Catalysts 2021, 11, 1222. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.; Hu, S.; Gou, L.; Chen, R. Physical Origin of Adsorption Heat and Its Significance in the Isotherm Equation. Int. J. Heat Mass Transf. 2024, 220, 124914. [Google Scholar] [CrossRef]
- Duan, S.; Shen, S.; Li, G.; Ling, X.; Shen, P. Statistical Physics Modelling of Adsorption Isotherms of Water Vapour on Shale: Stereographic, Energetic and Thermodynamic Investigations. Asia-Pacific J. Chem. Eng. 2024, 19, e3062. [Google Scholar] [CrossRef]
- Patti, S.; Magrini Alunno, I.; Pedroni, S.; Riva, S.; Ferrandi, E.E.; Monti, D. Advances and Challenges in the Development of Immobilized Enzymes for Batch and Flow Biocatalyzed Processes. ChemSusChem 2024, e202402007. [Google Scholar] [CrossRef]
- Serleti, A.; Xiao, X.; Shortall, K.; Magner, E. Use of Self-Assembled Monolayers for the Sequential and Independent Immobilisation of Enzymes. ChemElectroChem 2021, 8, 3911–3916. [Google Scholar] [CrossRef]
- Liu, S.; Bilal, M.; Rizwan, K.; Gul, I.; Rasheed, T.; Iqbal, H.M.N. Smart Chemistry of Enzyme Immobilization Using Various Support Matrices—A Review. Int. J. Biol. Macromol. 2021, 190, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Fredj, Z.; Sawan, M. Advanced Nanomaterials-Based Electrochemical Biosensors for Catecholamines Detection: Challenges and Trends. Biosensors 2023, 13, 211. [Google Scholar] [CrossRef]
- Bellino, M.G.; Soler-Illia, G.J.A.A. Nano-Designed Enzyme–Functionalized Hierarchical Metal–Oxide Mesoporous Thin Films: En Route to Versatile Biofuel Cells. Small 2014, 10, 2834–2839. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhao, Z.; Jiang, B.; Chen, J. Enhancing Enzyme Immobilization: Fabrication of Biosilica-Based Organic-Inorganic Composite Carriers for Efficient Covalent Binding of D-Allulose 3-Epimerase. Int. J. Biol. Macromol. 2024, 265, 130980. [Google Scholar] [CrossRef]
- Mirsalami, S.M.; Mirsalami, M.; Ghodousian, A. Techniques for Immobilizing Enzymes to Create Durable and Effective Biocatalysts. Results Chem. 2024, 7, 101486. [Google Scholar] [CrossRef]
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M. A Comprehensive Review of the Covalent Immobilization of Biomolecules onto Electrospun Nanofibers. Nanomaterials 2020, 10, 2142. [Google Scholar] [CrossRef] [PubMed]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme Entrapment, Biocatalyst Immobilization without Covalent Attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Z.; Cao, J.; Zhao, X.; Ye, L.; Wang, G. Self-Adhesive Wearable Poly (Vinyl Alcohol)-Based Hybrid Biofuel Cell Powered by Human Bio-Fluids. Biosens. Bioelectron. 2024, 247, 115930. [Google Scholar] [CrossRef]
- Sakalauskiene, L.; Popov, A.; Kausaite-Minkstimiene, A.; Ramanavicius, A.; Ramanaviciene, A. The Impact of Glucose Oxidase Immobilization on Dendritic Gold Nanostructures on the Performance of Glucose Biosensors. Biosensors 2022, 12, 320. [Google Scholar] [CrossRef]
- Blout, A.; Pulpytel, J.; Mori, S.; Arefi-Khonsari, F.; Méthivier, C.; Pailleret, A.; Jolivalt, C. Carbon Nanowalls Functionalization for Efficient O2 Reduction Catalyzed by Laccase Using Design of Experiment. Appl. Surf. Sci. 2021, 547, 149112. [Google Scholar] [CrossRef]
- Ren, S.; Wang, F.; Gao, H.; Han, X.; Zhang, T.; Yuan, Y.; Zhou, Z. Recent Progress and Future Prospects of Laccase Immobilization on MOF Supports for Industrial Applications. Appl. Biochem. Biotechnol. 2024, 196, 1669–1684. [Google Scholar] [CrossRef]
- Sha, Z.; Ling, T.; Yang, W.; Xie, H.; Wang, C.; Sun, S. Microfluidic Synthesis and Accurate Immobilization of Low-Density QD-Encoded Magnetic Microbeads for Multiplex Immunoassay. J. Mater. Chem. B 2024, 12, 11230–11236. [Google Scholar] [CrossRef]
- Imam, H.T.; Hill, K.; Reid, A.; Mix, S.; Marr, P.C.; Marr, A.C. Supramolecular Ionic Liquid Gels for Enzyme Entrapment. ACS Sustain. Chem. Eng. 2023, 11, 6829–6837. [Google Scholar] [CrossRef]
- Shchipunov, Y. Biomimetic Sol–Gel Chemistry to Tailor Structure, Properties, and Functionality of Bionanocomposites by Biopolymers and Cells. Materials 2024, 17, 224. [Google Scholar] [CrossRef]
- Zebda, A.; Gondran, C.; Le Goff, A.; Holzinger, M.; Cinquin, P.; Cosnier, S. Mediatorless High-Power Glucose Biofuel Cells Based on Compressed Carbon Nanotube-Enzyme Electrodes. Nat. Commun. 2011, 2, 370. [Google Scholar] [CrossRef]
- Abellanas-Perez, P.; Carballares, D.; Fernandez-Lafuente, R.; Rocha-Martin, J. Glutaraldehyde Modification of Lipases Immobilized on Octyl Agarose Beads: Roles of the Support Enzyme Loading and Chemical Amination of the Enzyme on the Final Enzyme Features. Int. J. Biol. Macromol. 2023, 248, 125853. [Google Scholar] [CrossRef]
- Radwan, I.T.; Sayed-Ahmed, M.Z.; Ghazawy, N.A.; Alqahtani, S.S.; Ahmad, S.; Alam, N.; Alkhaibari, A.M.; Ali, M.S.; Selim, A.; AbdelFattah, E.A. Effect of Nanostructure Lipid Carrier of Methylene Blue and Monoterpenes as Enzymes Inhibitor for Culex pipiens. Sci. Rep. 2023, 13, 12522. [Google Scholar] [CrossRef] [PubMed]
- Davidson-Rozenfeld, G.; Chen, X.; Qin, Y.; Ouyang, Y.; Sohn, Y.S.; Li, Z.; Nechushtai, R.; Willner, I. Stiffness-Switchable, Biocatalytic pH-Responsive DNA-Functionalized Polyacrylamide Cryogels and Their Mechanical Applications. Adv. Funct. Mater. 2024, 34, 2306586. [Google Scholar] [CrossRef]
- Marchianò, V.; Tricase, A.; Ditaranto, N.; Macchia, E.; d’Ingeo, S.; Franco, C.D.; Scamarcio, G.; Torsi, L.; Bollella, P. High Voltage Flexible Glucose/O2 Fully Printed Hydrogel-Based Enzymatic Fuel Cell. J. Phys. D Appl. Phys. 2024, 57, 135503. [Google Scholar] [CrossRef]
- Xia, H.; Li, N.; Zhong, X.; Jiang, Y. Metal-Organic Frameworks: A Potential Platform for Enzyme Immobilization and Related Applications. Front. Bioeng. Biotechnol. 2020, 8, 695. [Google Scholar] [CrossRef]
- Nadar, S.S.; Vaidya, L.; Rathod, V.K. Enzyme Embedded Metal Organic Framework (Enzyme–MOF): De Novo Approaches for Immobilization. Int. J. Biol. Macromol. 2020, 149, 861–876. [Google Scholar] [CrossRef]
- Ye, N.; Kou, X.; Shen, J.; Huang, S.; Chen, G.; Ouyang, G. Metal-Organic Frameworks: A New Platform for Enzyme Immobilization. ChemBioChem 2020, 21, 2585–2590. [Google Scholar] [CrossRef]
- Chen, N.; Chang, B.; Shi, N.; Yan, W.; Lu, F.; Liu, F. Cross-Linked Enzyme Aggregates Immobilization: Preparation, Characterization, and Applications. Crit. Rev. Biotechnol. 2023, 43, 369–383. [Google Scholar] [CrossRef]
- Lee, Y.L.; Jaafar, N.R.; Ling, J.G.; Huyop, F.; Abu Bakar, F.D.; Rahman, R.A.; Illias, R.M. Cross-Linked Enzyme Aggregates of Polyethylene Terephthalate Hydrolyse (PETase) from Ideonella sakaiensis for the Improvement of Plastic Degradation. Int. J. Biol. Macromol. 2024, 263, 130284. [Google Scholar] [CrossRef] [PubMed]
- Fredj, Z.; Singh, B.; Bahri, M.; Qin, P.; Sawan, M. Enzymatic Electrochemical Biosensors for Neurotransmitters Detection: Recent Achievements and Trends. Chemosensors 2023, 11, 388. [Google Scholar] [CrossRef]
- Hong, J.; Jung, D.; Park, S.; Oh, Y.; Oh, K.K.; Lee, S.H. Immobilization of Laccase via Cross-Linked Enzyme Aggregates Prepared Using Genipin as a Natural Cross-Linker. Int. J. Biol. Macromol. 2021, 169, 541–550. [Google Scholar] [CrossRef]
- Qian, J.; Huang, A.; Zhu, H.; Ding, J.; Zhang, W.; Chen, Y. Immobilization of Lipase on Silica Nanoparticles by Adsorption Followed by Glutaraldehyde Cross-Linking. Bioprocess Biosyst. Eng. 2023, 46, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, S.; Pandey, L.M.; Chandra, P. Nanoengineered Material Based Biosensing Electrodes for Enzymatic Biofuel Cells Applications. Mater. Sci. Energy Technol. 2018, 1, 38–48. [Google Scholar] [CrossRef]
- ul Haque, S.; Nasar, A.; Duteanu, N.; Pandey, S.; Inamuddin. Carbon Based-Nanomaterials Used in Biofuel Cells—A Review. Fuel 2023, 331, 125634. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chia, W.Y.; Tang, D.Y.Y.; Show, P.L.; Chew, K.W.; Chen, W.-H. Nanomaterials Utilization in Biomass for Biofuel and Bioenergy Production. Energies 2020, 13, 892. [Google Scholar] [CrossRef]
- Jin, J.; Guo, J.; Guo, J.; Li, D. Carbon-Based Biosensor in Point of Care Setting. Adv. Sens. Res. 2024, 3, 2400037. [Google Scholar] [CrossRef]
- de Poulpiquet, A.; Ciaccafava, A.; Lojou, E. New Trends in Enzyme Immobilization at Nanostructured Interfaces for Efficient Electrocatalysis in Biofuel Cells. Electrochim. Acta 2014, 126, 104–114. [Google Scholar] [CrossRef]
- Zhang, J.; Lovell, J.F.; Shi, J.; Zhang, Y. Nanomaterials for Co-Immobilization of Multiple Enzymes. BMEMat 2024, 3, e12080. [Google Scholar] [CrossRef]
- Wen, D.; Eychmüller, A. Enzymatic Biofuel Cells on Porous Nanostructures. Small 2016, 12, 4649–4661. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Cheng, Y.; Jiang, S.P. Effect of Carbon Nanotubes on Direct Electron Transfer and Electrocatalytic Activity of Immobilized Glucose Oxidase. ACS Omega 2018, 3, 667–676. [Google Scholar] [CrossRef]
- Bilal, M.; Singh, A.K.; Iqbal, H.M.N.; Zdarta, J.; Chrobok, A.; Jesionowski, T. Enzyme-Linked Carbon Nanotubes as Biocatalytic Tools to Degrade and Mitigate Environmental Pollutants. Environ. Res. 2024, 241, 117579. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, U.; Gentil, S.; Courvoisier-Dezord, E.; Rousselot-Pailley, P.; Thomas, F.; Tron, T.; Goff, A.L. Laccase-Catalyzed Functionalization of Phenol-Modified Carbon Nanotubes: From Grafting of Metallopolyphenols to Enzyme Self-Immobilization. J. Mater. Chem. A 2023, 11, 10850–10856. [Google Scholar] [CrossRef]
- Barik, S.; Dash, A.K.; Saharay, M. Immobilization of Cellulase Enzymes on Single-Walled Carbon Nanotubes for Recycling of Enzymes and Better Yield of Bioethanol Using Computer Simulations. J. Chem. Inf. Model. 2023, 63, 5192–5203. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, X.-X.; Liu, H.-M.; Li, X.; Luo, X.-J.; Liao, X.; Zhao, Y. Covalent Immobilization of Tyrosinase on Magnetic Multi-Walled Carbon Nanotubes for Inhibitor Screening. J. Sep. Sci. 2023, 46, 2300195. [Google Scholar] [CrossRef]
- Gai, P.; Zhang, S.; Yu, W.; Li, H.; Li, F. Light-Driven Self-Powered Biosensor for Ultrasensitive Organophosphate Pesticide Detection via Integration of the Conjugated Polymer-Sensitized CdS and Enzyme Inhibition Strategy. J. Mater. Chem. B 2018, 6, 6842–6847. [Google Scholar] [CrossRef]
- Ruff, A.; Pinyou, P.; Nolten, M.; Conzuelo, F.; Schuhmann, W. A Self-Powered Ethanol Biosensor. ChemElectroChem 2017, 4, 890–897. [Google Scholar] [CrossRef]
- Miyake, T.; Yoshino, S.; Yamada, T.; Hata, K.; Nishizawa, M. Self-Regulating Enzyme−Nanotube Ensemble Films and Their Application as Flexible Electrodes for Biofuel Cells. J. Am. Chem. Soc. 2011, 133, 5129–5134. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Liu, M.; Lu, H.; Zhao, G. A Novel Self-Powered Aptasensor for Environmental Pollutants Detection Based on Simple and Efficient Enzymatic Biofuel Cell. Sens. Actuators B Chem. 2020, 305, 127468. [Google Scholar] [CrossRef]
- Yan, Y.; Zheng, W.; Su, L.; Mao, L. Carbon-Nanotube-Based Glucose/O2 Biofuel Cells. Adv. Mater. 2006, 18, 2639–2643. [Google Scholar] [CrossRef]
- Du, B.; Zhang, Z.; Lu, G.; Feng, Y.; Liu, M. Simple and Portable Self-Powered Sensor Using Ultrasonically Dispersed Graphene as an Electron Transfer Promoter for Ultra-Trace Atrazine Monitoring. ACS EST Water 2023, 3, 2140–2150. [Google Scholar] [CrossRef]
- Kabir, M.H.; Marquez, E.; Djokoto, G.; Parker, M.; Weinstein, T.; Ghann, W.; Uddin, J.; Ali, M.M.; Alam, M.M.; Thompson, M.; et al. Energy Harvesting by Mesoporous Reduced Graphene Oxide Enhanced the Mediator-Free Glucose-Powered Enzymatic Biofuel Cell for Biomedical Applications. ACS Appl. Mater. Interfaces 2022, 14, 24229–24244. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yao, Y.; Lv, T.; Yang, Y.; Liu, Y.; Chen, T. Flexible and Stretchable Enzymatic Biofuel Cell with High Performance Enabled by Textile Electrodes and Polymer Hydrogel Electrolyte. Nano Lett. 2022, 22, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.P.; Chen, Y.; Chen, P. Three-Dimensional Graphene-Carbon Nanotube Hybrid for High-Performance Enzymatic Biofuel Cells. ACS Appl. Mater. Interfaces 2014, 6, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Liu, X.; Kaji, T.; Nishina, Y.; Miyake, T. Fiber-Crafted Biofuel Cell Bracelet for Wearable Electronics. Biosens. Bioelectron. 2021, 179, 113107. [Google Scholar] [CrossRef]
- Kim, S.; Ji, J.; Kwon, Y. Paper-Type Membraneless Enzymatic Biofuel Cells Using a New Biocathode Consisting of Flexible Buckypaper Electrode and Bilirubin Oxidase Based Catalyst Modified by Electrografting. Appl. Energy 2023, 339, 120978. [Google Scholar] [CrossRef]
- Singh, A.; Kafle, S.R.; Sharma, M.; Kim, B.S. Comprehensive Review on Multifaceted Carbon Dot Nanocatalysts: Sources and Energy Applications. Catalysts 2023, 13, 1446. [Google Scholar] [CrossRef]
- Zhao, M.; Gao, Y.; Sun, J.; Gao, F. Mediatorless Glucose Biosensor and Direct Electron Transfer Type Glucose/Air Biofuel Cell Enabled with Carbon Nanodots. Anal. Chem. 2015, 87, 2615–2622. [Google Scholar] [CrossRef]
- Kang, Z.; Jiao, K.; Yu, C.; Dong, J.; Peng, R.; Hu, Z.; Jiao, S. Direct Electrochemistry and Bioelectrocatalysis of Glucose Oxidase in CS/CNC Film and Its Application in Glucose Biosensing and Biofuel Cells. RSC Adv. 2017, 7, 4572–4579. [Google Scholar] [CrossRef]
- Kang, Z.; Jiao, K.; Cheng, J.; Peng, R.; Jiao, S.; Hu, Z. A Novel Three-Dimensional Carbonized PANI1600@CNTs Network for Enhanced Enzymatic Biofuel Cell. Biosens. Bioelectron. 2018, 101, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ren, G.; Wang, W.A.; Hu, Z. Rational Design of N-Doped CNTs@C3N4 Network for Dual-Capture of Biocatalysts in Enzymatic Glucose/O2 Biofuel Cells. Nanoscale 2021, 13, 7774–7782. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Han, Y.; Yu, Y.; Xu, M.; Zhang, X.; Huang, L.; Dong, S. RGO/Au NPs/N-Doped CNTs Supported on Nickel Foam as an Anode for Enzymatic Biofuel Cells. Biosens. Bioelectron. 2017, 97, 34–40. [Google Scholar] [CrossRef]
- Perveen, R.; Nasar, A.; Inamuddin; Kanchi, S.; Kashmery, H.A. Development of a Ternerry Condunting Composite (PPy/Au/CNT@Fe3O4) Immobilized FRT/GOD Bioanode for Glucose/Oxygen Biofuel Cell Applications. Int. J. Hydrogen Energy 2021, 46, 3259–3269. [Google Scholar] [CrossRef]
- Chung, M.; Nguyen, T.L.; Tran, T.Q.N.; Yoon, H.H.; Kim, I.T.; Kim, M.I. Ultrarapid Sonochemical Synthesis of Enzyme-Incorporated Copper Nanoflowers and Their Application to Mediatorless Glucose Biofuel Cell. Appl. Surf. Sci. 2018, 429, 203–209. [Google Scholar] [CrossRef]
- Bollella, P.; Fusco, G.; Stevar, D.; Gorton, L.; Ludwig, R.; Ma, S.; Boer, H.; Koivula, A.; Tortolini, C.; Favero, G.; et al. A Glucose/Oxygen Enzymatic Fuel Cell Based on Gold Nanoparticles Modified Graphene Screen-Printed Electrode. Proof-of-Concept in Human Saliva. Sens. Actuators B Chem. 2018, 256, 921–930. [Google Scholar] [CrossRef]
- Umasankar, Y.; Adhikari, B.-R.; Chen, A. Effective Immobilization of Alcohol Dehydrogenase on Carbon Nanoscaffolds for Ethanol Biofuel Cell. Bioelectrochemistry 2017, 118, 83–90. [Google Scholar] [CrossRef]
- Koyappayil, A.; Yagati, A.K.; Lee, M.-H. Recent Trends in Metal Nanoparticles Decorated 2D Materials for Electrochemical Biomarker Detection. Biosensors 2023, 13, 91. [Google Scholar] [CrossRef]
- Quinson, J.; Kunz, S.; Arenz, M. Surfactant-Free Colloidal Syntheses of Precious Metal Nanoparticles for Improved Catalysts. ACS Catal. 2023, 13, 4903–4937. [Google Scholar] [CrossRef]
- Wen, L.; Sun, K.; Liu, X.; Yang, W.; Li, L.; Jiang, H.-L. Electronic State and Microenvironment Modulation of Metal Nanoparticles Stabilized by MOFs for Boosting Electrocatalytic Nitrogen Reduction. Adv. Mater. 2023, 35, 2210669. [Google Scholar] [CrossRef]
- Cao, L.; Chen, J.; Pang, J.; Qu, H.; Liu, J.; Gao, J. Research Progress in Enzyme Biofuel Cells Modified Using Nanomaterials and Their Implementation as Self-Powered Sensors. Molecules 2024, 29, 257. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ji, J.; Lee, J.J.; Kim, C.; Kwon, Y. Density Functional Theory-Based Predictions and Experimental Evaluations of Ferrocene Derivatives Considered as Mediator for Anodic Catalysts of Glucose and Oxygen Enzymatic Biofuel Cells. Int. J. Energy Res. 2023, 2023, e7697293. [Google Scholar] [CrossRef]
- Ahamed, M.I.; Khan, I.M.; Inamuddin; Rezakazemi, M. Silver Nanoparticles Anchored on Zinc Oxide Synthesized via Green Route as Scaffold for Enzymatic Biofuel Cell Application. Int. J. Hydrogen Energy 2024, 52, 681–693. [Google Scholar] [CrossRef]
- Gai, P.; Ji, Y.; Chen, Y.; Zhu, C.; Zhang, J.; Zhu, J.-J. A Nitrogen-Doped Graphene/Gold Nanoparticle/Formate Dehydrogenase Bioanode for High Power Output Membrane-Less Formic Acid/O2 Biofuel Cells. Analyst 2015, 140, 1822–1826. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Kaminskas, A.; Gayda, G.; Ramanaviciene, A. Towards a Self-Powered Amperometric Glucose Biosensor Based on a Single-Enzyme Biofuel Cell. Biosensors 2024, 14, 138. [Google Scholar] [CrossRef]
- Han, H.H.; Jung, S.-M.; Kim, S.-K.; Lee, G.-H.; Kim, S.-J.; Kim, Y.-T.; Hahn, S.K. Bimetallic Electrocatalyst of Hyaluronate-Au@Pt for Durable Oxygen Reduction in Biofuel Cells. ACS Appl. Energy Mater. 2022, 5, 12475–12484. [Google Scholar] [CrossRef]
- Saputra, H.A.; Jannath, K.A.; Kim, K.B.; Park, D.-S.; Shim, Y.-B. Conducting Polymer Composite-Based Biosensing Materials for the Diagnosis of Lung Cancer: A Review. Int. J. Biol. Macromol. 2023, 252, 126149. [Google Scholar] [CrossRef]
- Kuznetsova, L.S.; Arlyapov, V.A.; Plekhanova, Y.V.; Tarasov, S.E.; Kharkova, A.S.; Saverina, E.A.; Reshetilov, A.N. Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells. Polymers 2023, 15, 3783. [Google Scholar] [CrossRef]
- Sun, G.; Wei, X.; Zhang, D.; Huang, L.; Liu, H.; Fang, H. Immobilization of Enzyme Electrochemical Biosensors and Their Application to Food Bioprocess Monitoring. Biosensors 2023, 13, 886. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Chang, C.-C.; Chang, L.-Y.; Wang, P.-C.; Kanokpaka, P.; Yeh, M.-H. Self-Powered Triboelectric Sensor with N-Doped Graphene Quantum Dots Decorated Polyaniline Layer for Non-Invasive Glucose Monitoring in Human Sweat. Nano Energy 2023, 112, 108505. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Deng, X.; Li, J.; Huang, S.; Jin, X.; Zhu, X. Self-Encapsulated Enzyme through in-Situ Growth of Polypyrrole for High-Performance Enzymatic Biofuel Cell. Chem. Eng. J. 2022, 429, 132148. [Google Scholar] [CrossRef]
- Wang, T.; Ran, Y.; He, Y.; Shi, L.; Zeng, B.; Zhao, F. Self-Powered Photoelectrochemical/Visual Sensing Platform Based on PEDOT/BiOBr0.8I0.2 Organic-Inorganic Hybrid Material and MWCNTs/SnS2 Heterojunction for the Ultrasensitive Detection of Programmed Death Ligand-1. Biosens. Bioelectron. 2023, 237, 115558. [Google Scholar] [CrossRef]
- ul Haque, S.; Duteanu, N.; Nasar, A.; Inamuddin. Polythiophene-Titanium Oxide (PTH-TiO2) Nanocomposite: As an Electron Transfer Enhancer for Biofuel Cell Anode Construction. J. Power Sources 2022, 520, 230867. [Google Scholar] [CrossRef]
- Lin, X.; Song, D.; Shao, T.; Xue, T.; Hu, W.; Jiang, W.; Zou, X.; Liu, N. A Multifunctional Biosensor via MXene Assisted by Conductive Metal–Organic Framework for Healthcare Monitoring. Adv. Funct. Mater. 2024, 34, 2311637. [Google Scholar] [CrossRef]
- Hou, H.; Wang, L.; Gao, Y.; Ping, J.; Zhao, F. Recent Advances in Metal-Organic Framework-Based Nanozymes and Their Enabled Optical Biosensors for Food Safety Analysis. TrAC Trends Anal. Chem. 2024, 173, 117602. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A Review on Metal-Organic Frameworks: Synthesis and Applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.-L.; Xiong, J.; Zong, M.-H.; Lou, W.-Y. Metal-Organic Frameworks as Novel Matrices for Efficient Enzyme Immobilization: An Update Review. Coord. Chem. Rev. 2020, 406, 213149. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, L.; Liu, D.; Du, W.; Wang, Y. Progress & Prospect of Metal-Organic Frameworks (MOFs) for Enzyme Immobilization (Enzyme/MOFs). Renew. Sustain. Energy Rev. 2018, 91, 793–801. [Google Scholar] [CrossRef]
- Akpinar, I.; Wang, X.; Fahy, K.; Sha, F.; Yang, S.; Kwon, T.; Das, P.J.; Islamoglu, T.; Farha, O.K.; Stoddart, J.F. Biomimetic Mineralization of Large Enzymes Utilizing a Stable Zirconium-Based Metal-Organic Frameworks. J. Am. Chem. Soc. 2024, 146, 5108–5117. [Google Scholar] [CrossRef]
- Chen, G.; Kou, X.; Huang, S.; Tong, L.; Shen, Y.; Zhu, W.; Zhu, F.; Ouyang, G. Modulating the Biofunctionality of Metal–Organic-Framework-Encapsulated Enzymes through Controllable Embedding Patterns. Angew. Chem. Int. Ed. 2020, 59, 2867–2874. [Google Scholar] [CrossRef]
- Wang, X.; Lan, P.C.; Ma, S. Metal–Organic Frameworks for Enzyme Immobilization: Beyond Host Matrix Materials. ACS Cent. Sci. 2020, 6, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-Y.; Zhang, J.; Hsu, Y.-C.; Lin, H.; Han, Z.; Pang, J.; Yang, Z.; Liang, R.-R.; Shi, W.; Zhou, H.-C. Bioinspired Framework Catalysts: From Enzyme Immobilization to Biomimetic Catalysis. Chem. Rev. 2023, 123, 5347–5420. [Google Scholar] [CrossRef]
- Aggarwal, V.; Solanki, S.; Malhotra, B.D. Applications of Metal–Organic Framework-Based Bioelectrodes. Chem. Sci. 2022, 13, 8727–8743. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Sene, S.; Mousty, C.; Serre, C.; Chaussé, A.; Legrand, L.; Steunou, N. Design of Laccase–Metal Organic Framework-Based Bioelectrodes for Biocatalytic Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 20012–20022. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, Q.; Lu, K.; Huang, J.; Zhang, Y.; Hou, Y.; Qiao, H.; Li, D.; Wei, Q. Encapsulating Enzyme into Metal-Organic Framework during in-Situ Growth on Cellulose Acetate Nanofibers as Self-Powered Glucose Biosensor. Biosens. Bioelectron. 2021, 171, 112690. [Google Scholar] [CrossRef]
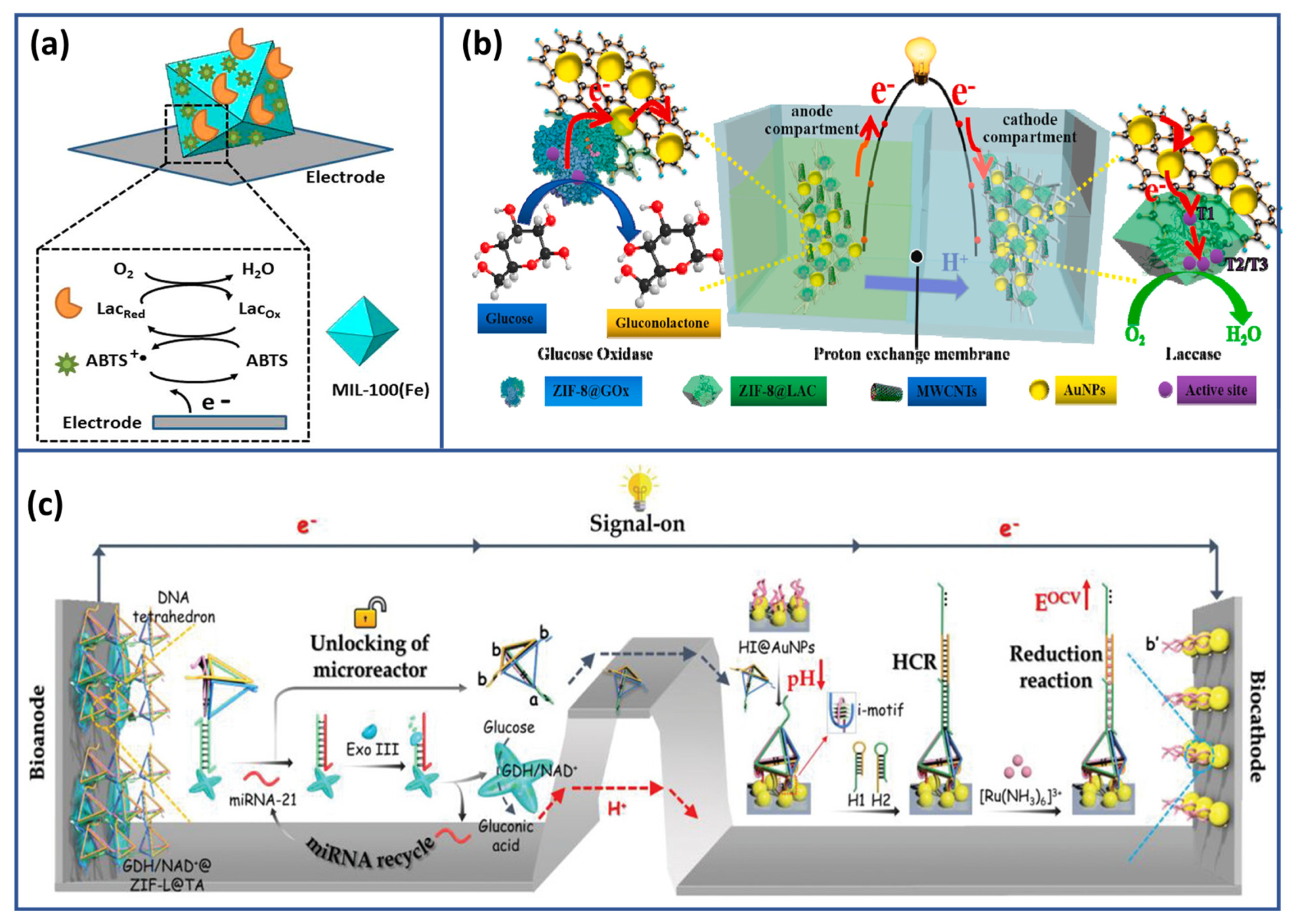
- Yan, Y.; Guo, L.; Geng, H.; Bi, S. Hierarchical Porous Metal-Organic Framework as Biocatalytic Microreactor for Enzymatic Biofuel Cell-Based Self-Powered Biosensing of MicroRNA Integrated with Cascade Signal Amplification. Small 2023, 19, 2301654. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Zhang, Y.; Lv, P.; Feng, Q.; Wei, Q. Encapsulation of Enzyme by Metal-Organic Framework for Single-Enzymatic Biofuel Cell-Based Self-Powered Biosensor. Nano Energy 2020, 68, 104308. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, L.; Zhang, Z.; Guo, S.; Shang, H.; Li, Y.; Liu, J. Wearable Biofuel Cells Based on the Classification of Enzyme for High Power Outputs and Lifetimes. Biosens. Bioelectron. 2019, 124–125, 40–52. [Google Scholar] [CrossRef]
- Fu, L.; Liu, J.; Hu, Z.; Zhou, M. Recent Advances in the Construction of Biofuel Cells Based Self-Powered Electrochemical Biosensors: A Review. Electroanalysis 2018, 30, 2535–2550. [Google Scholar] [CrossRef]
- Tan, P.; Zou, Y.; Fan, Y.; Li, Z. Self-Powered Wearable Electronics. Wearable Technol. 2020, 1, e5. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device Integration of Electrochemical Biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, X. An Overview of Recent Analysis and Detection of Acetylcholine. Anal. Biochem. 2021, 632, 114381. [Google Scholar] [CrossRef] [PubMed]
- Bodur, O.C.; Hasanoğlu Özkan, E.; Çolak, Ö.; Arslan, H.; Sarı, N.; Dişli, A.; Arslan, F. Preparation of Acetylcholine Biosensor for the Diagnosis of Alzheimer’s Disease. J. Mol. Struct. 2021, 1223, 129168. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, L.; Wang, S.; Jiang, X.; Ma, F.; Zhao, J.; Meng, W.; Gao, L. A New Acetylcholine Optical Fiber Biosensor Based on Gold Film-GNRs Resonance Coupling Enhancement. IEEE Sens. J. 2024, 24, 4557–4564. [Google Scholar] [CrossRef]
- Moreira, F.T.C.; Sale, M.G.F.; Di Lorenzo, M. Towards Timely Alzheimer Diagnosis: A Self-Powered Amperometric Biosensor for the Neurotransmitter Acetylcholine. Biosens. Bioelectron. 2017, 87, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Surme, S.; Ergun, C.; Gul, S.; Akyel, Y.K.; Gul, Z.M.; Ozcan, O.; Ipek, O.S.; Akarlar, B.A.; Ozlu, N.; Taskin, A.C.; et al. TW68, Cryptochromes Stabilizer, Regulates Fasting Blood Glucose Levels in Diabetic Ob/Ob and High Fat-Diet-Induced Obese Mice. Biochem. Pharmacol. 2023, 218, 115896. [Google Scholar] [CrossRef]
- Fischer, C.; Fraiwan, A.; Choi, S. A 3D Paper-Based Enzymatic Fuel Cell for Self-Powered, Low-Cost Glucose Monitoring. Biosens. Bioelectron. 2016, 79, 193–197. [Google Scholar] [CrossRef]
- Song, Y.; Wang, C. High-Power Biofuel Cells Based on Three-Dimensional Reduced Graphene Oxide/Carbon Nanotube Micro-Arrays. Microsyst. Nanoeng. 2019, 5, 46. [Google Scholar] [CrossRef]
- Chansaenpak, K.; Kamkaew, A.; Lisnund, S.; Prachai, P.; Ratwirunkit, P.; Jingpho, T.; Blay, V.; Pinyou, P. Development of a Sensitive Self-Powered Glucose Biosensor Based on an Enzymatic Biofuel Cell. Biosensors 2021, 11, 16. [Google Scholar] [CrossRef]
- Cho, E.; Mohammadifar, M.; Choi, S. A Single-Use, Self-Powered, Paper-Based Sensor Patch for Detection of Exercise-Induced Hypoglycemia. Micromachines 2017, 8, 265. [Google Scholar] [CrossRef]
- Ding, S.; Saha, T.; Yin, L.; Liu, R.; Khan, M.I.; Chang, A.-Y.; Lee, H.; Zhao, H.; Liu, Y.; Nazemi, A.S.; et al. A Fingertip-Wearable Microgrid System for Autonomous Energy Management and Metabolic Monitoring. Nat. Electron. 2024, 7, 788–799. [Google Scholar] [CrossRef]
- Veenuttranon, K.; Kaewpradub, K.; Jeerapan, I. Screen-Printable Functional Nanomaterials for Flexible and Wearable Single-Enzyme-Based Energy-Harvesting and Self-Powered Biosensing Devices. Nano-Micro Lett. 2023, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, G.; Kulkarni, T. A Self-Powered Glucose Biosensing System. Biosens. Bioelectron. 2016, 78, 45–50. [Google Scholar] [CrossRef]
- Slaughter, G.; Kulkarni, T. Highly Selective and Sensitive Self-Powered Glucose Sensor Based on Capacitor Circuit. Sci. Rep. 2017, 7, 1471. [Google Scholar] [CrossRef] [PubMed]
- Nasu, Y.; Aggarwal, A.; Le, G.N.T.; Vo, C.T.; Kambe, Y.; Wang, X.; Beinlich, F.R.M.; Lee, A.B.; Ram, T.R.; Wang, F.; et al. Lactate Biosensors for Spectrally and Spatially Multiplexed Fluorescence Imaging. Nat. Commun. 2023, 14, 6598. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Xu, X.; Hu, C.; Zhang, W.; Zhang, Y.; Ma, C.; Xu, P. Pyruvate Producing Biocatalyst with Constitutive NAD-Independent Lactate Dehydrogenases. Process Biochem. 2010, 45, 1912–1915. [Google Scholar] [CrossRef]
- Daboss, E.V.; Shcherbacheva, E.V.; Tikhonov, D.V.; Karyakin, A.A. On-Body Hypoxia Monitor Based on Lactate Biosensors with a Tunable Concentration Range. J. Electroanal. Chem. 2023, 935, 117330. [Google Scholar] [CrossRef]
- Currano, L.J.; Sage, F.C.; Hagedon, M.; Hamilton, L.; Patrone, J.; Gerasopoulos, K. Wearable Sensor System for Detection of Lactate in Sweat. Sci. Rep. 2018, 8, 15890. [Google Scholar] [CrossRef]
- Jeerapan, I.; Sempionatto, J.R.; Pavinatto, A.; You, J.-M.; Wang, J. Stretchable Biofuel Cells as Wearable Textile-Based Self-Powered Sensors. J. Mater. Chem. A 2016, 4, 18342–18353. [Google Scholar] [CrossRef]
- Hickey, D.P.; Reid, R.C.; Milton, R.D.; Minteer, S.D. A Self-Powered Amperometric Lactate Biosensor Based on Lactate Oxidase Immobilized in Dimethylferrocene-Modified LPEI. Biosens. Bioelectron. 2016, 77, 26–31. [Google Scholar] [CrossRef]
- Baingane, A.; Slaughter, G. Self-Powered Electrochemical Lactate Biosensing. Energies 2017, 10, 1582. [Google Scholar] [CrossRef]
- Santiago-Malagón, S.; Río-Colín, D.; Azizkhani, H.; Aller-Pellitero, M.; Guirado, G.; del Campo, F.J. A Self-Powered Skin-Patch Electrochromic Biosensor. Biosens. Bioelectron. 2021, 175, 112879. [Google Scholar] [CrossRef]
- Chen, X.; Yin, L.; Lv, J.; Gross, A.J.; Le, M.; Gutierrez, N.G.; Li, Y.; Jeerapan, I.; Giroud, F.; Berezovska, A.; et al. Stretchable and Flexible Buckypaper-Based Lactate Biofuel Cell for Wearable Electronics. Adv. Funct. Mater. 2019, 29, 1905785. [Google Scholar] [CrossRef]
- Varlinskaya, E.I.; Spear, L.P. Social Consequences of Ethanol: Impact of Age, Stress, and Prior History of Ethanol Exposure. Physiol. Behav. 2015, 148, 145–150. [Google Scholar] [CrossRef]
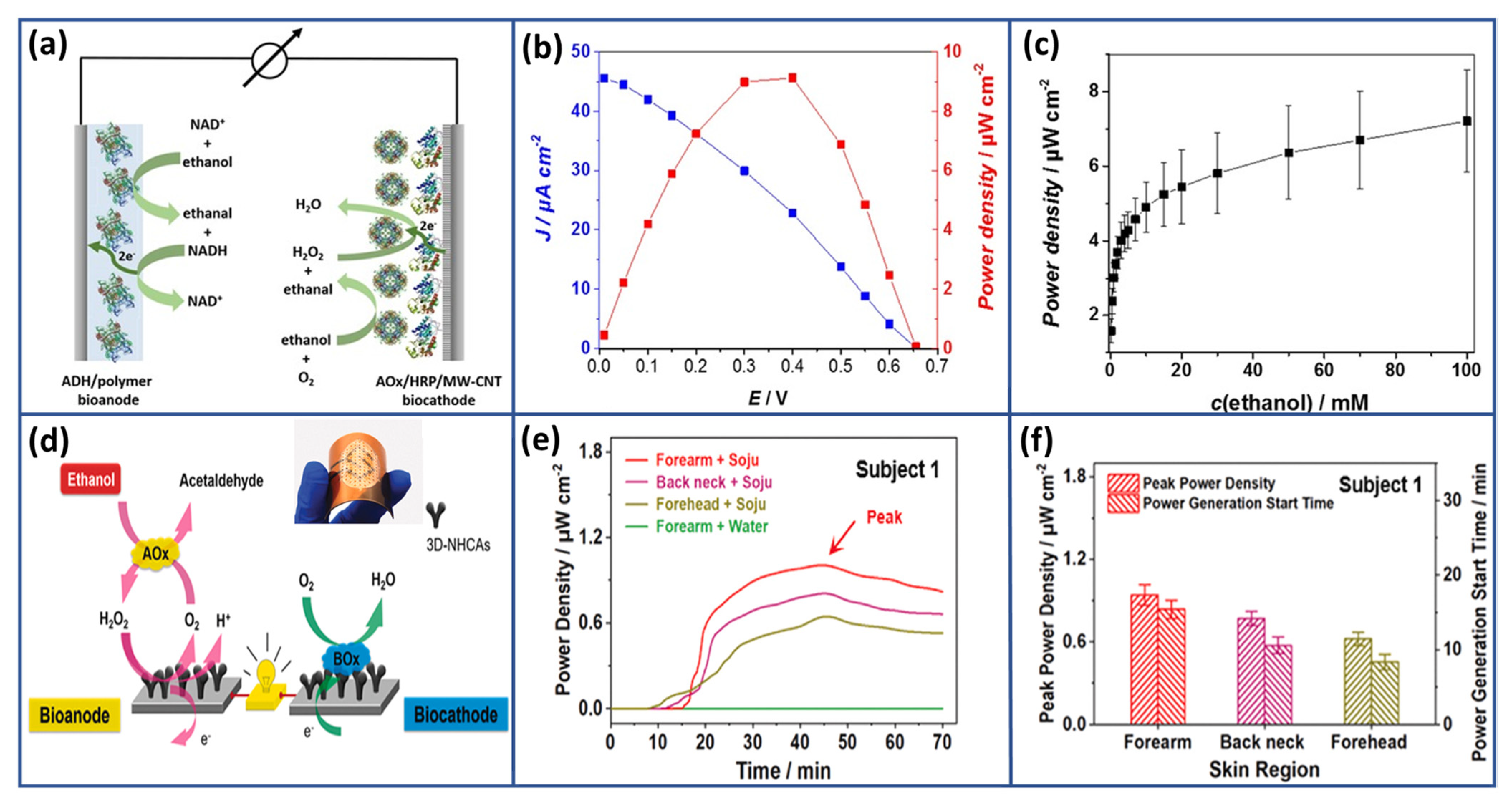
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A. Enzymatic Biofuel Cell Based on Anode and Cathode Powered by Ethanol. Biosens. Bioelectron. 2008, 24, 761–766. [Google Scholar] [CrossRef]
- Lau, C.; Moehlenbrock, M.J.; Arechederra, R.L.; Falase, A.; Garcia, K.; Rincon, R.; Minteer, S.D.; Banta, S.; Gupta, G.; Babanova, S.; et al. Paper Based Biofuel Cells: Incorporating Enzymatic Cascades for Ethanol and Methanol Oxidation. Int. J. Hydrogen Energy 2015, 40, 14661–14666. [Google Scholar] [CrossRef]
- Sun, M.; Gu, Y.; Pei, X.; Wang, J.; Liu, J.; Ma, C.; Bai, J.; Zhou, M. A Flexible and Wearable Epidermal Ethanol Biofuel Cell for On-Body and Real-Time Bioenergy Harvesting from Human Sweat. Nano Energy 2021, 86, 106061. [Google Scholar] [CrossRef]
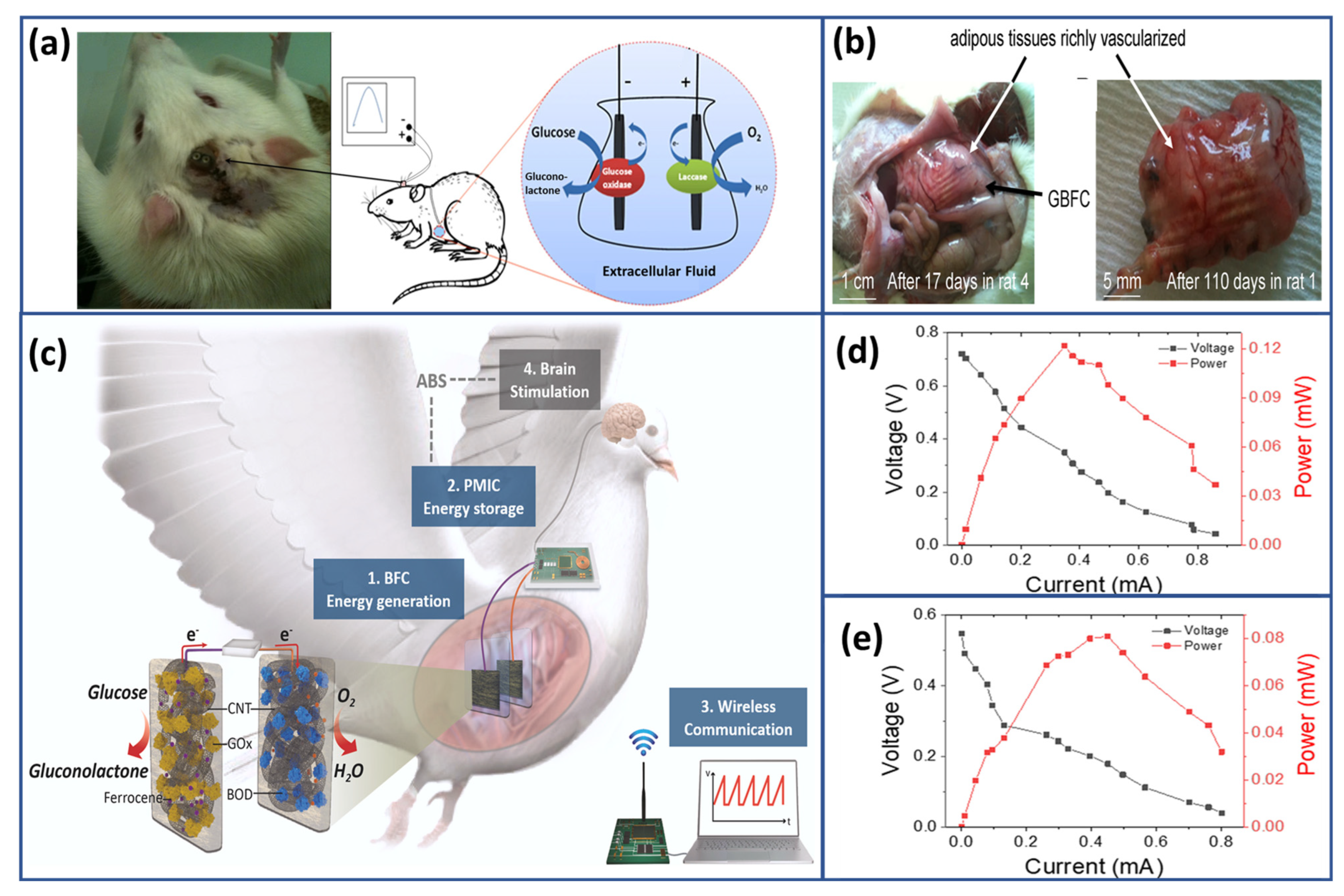
- Cinquin, P.; Gondran, C.; Giroud, F.; Mazabrard, S.; Pellissier, A.; Boucher, F.; Alcaraz, J.-P.; Gorgy, K.; Lenouvel, F.; Mathé, S.; et al. A Glucose BioFuel Cell Implanted in Rats. PLoS ONE 2010, 5, e10476. [Google Scholar] [CrossRef]
- Zebda, A.; Cosnier, S.; Alcaraz, J.-P.; Holzinger, M.; Le Goff, A.; Gondran, C.; Boucher, F.; Giroud, F.; Gorgy, K.; Lamraoui, H.; et al. Single Glucose Biofuel Cells Implanted in Rats Power Electronic Devices. Sci. Rep. 2013, 3, 1516. [Google Scholar] [CrossRef]
- Lee, D.; Jeong, S.H.; Yun, S.; Kim, S.; Sung, J.; Seo, J.; Son, S.; Kim, J.T.; Susanti, L.; Jeong, Y.; et al. Totally Implantable Enzymatic Biofuel Cell and Brain Stimulator Operating in Bird through Wireless Communication. Biosens. Bioelectron. 2021, 171, 112746. [Google Scholar] [CrossRef]
- Menassol, G.; Dubois, L.; Nadolska, M.; Vadgama, P.; Martin, D.K.; Zebda, A. A Biocompatible Iron Doped Graphene Based Cathode for an Implantable Glucose Biofuel Cell. Electrochim. Acta 2023, 439, 141627. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; You, J.-M.; Kim, N.-H.; Gu, Y.; Kumar, R.; Mohan, A.M.V.; Kurniawan, J.; Imani, S.; Nakagawa, T.; Parish, B.; et al. Soft, Stretchable, High Power Density Electronic Skin-Based Biofuel Cells for Scavenging Energy from Human Sweat. Energy Environ. Sci. 2017, 10, 1581–1589. [Google Scholar] [CrossRef]
- Serag, E.; El-Maghraby, A.; El Nemr, A. Recent Developments in the Application of Carbon-Based Nanomaterials in Implantable and Wearable Enzyme-Biofuel Cells. Carbon Lett. 2022, 32, 395–412. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Su, B.S.Q.; Song, R.; Zhang, J.-R.; Zhu, J.-J. Enzymatic Biofuel Cell: Opportunities and Intrinsic Challenges in Futuristic Applications. Adv. Energy Sustain. Res. 2021, 2, 2100031. [Google Scholar] [CrossRef]
- Nasar, A.; Perveen, R. Applications of Enzymatic Biofuel Cells in Bioelectronic Devices—A Review. Int. J. Hydrogen Energy 2019, 44, 15287–15312. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, S.; Fan, P.; Li, X.; Ying, Y.; Ping, J.; Pan, Y. Implantable Electrochemical Microsensors for In Vivo Monitoring of Animal Physiological Information. Nano-Micro Lett. 2023, 16, 49. [Google Scholar] [CrossRef]
- Ghodhbane, M.; Beneventi, D.; Zebda, A.; Dubois, L.; Alcaraz, J.-P.; Boucher, F.; Boutonnat, J.; Menassol, G.; Chaussy, D.; Belgacem, N. 3D Printed Cathodes for Implantable Abiotic Biofuel Cells. J. Power Sources 2023, 580, 233356. [Google Scholar] [CrossRef]
- De la Paz, E.; Maganti, N.H.; Trifonov, A.; Jeerapan, I.; Mahato, K.; Yin, L.; Sonsa-ard, T.; Ma, N.; Jung, W.; Burns, R.; et al. A Self-Powered Ingestible Wireless Biosensing System for Real-Time in Situ Monitoring of Gastrointestinal Tract Metabolites. Nat. Commun. 2022, 13, 7405. [Google Scholar] [CrossRef]
- Xiao, X.; Xia, H.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the Challenges of Enzymatic (Bio)Fuel Cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef]
- Huang, W.; Zulkifli, M.Y.B.; Chai, M.; Lin, R.; Wang, J.; Chen, Y.; Chen, V.; Hou, J. Recent Advances in Enzymatic Biofuel Cells Enabled by Innovative Materials and Techniques. Exploration 2023, 3, 20220145. [Google Scholar] [CrossRef]
- Ruff, A.; Conzuelo, F.; Schuhmann, W. Bioelectrocatalysis as the Basis for the Design of Enzyme-Based Biofuel Cells and Semi-Artificial Biophotoelectrodes. Nat. Catal. 2020, 3, 214–224. [Google Scholar] [CrossRef]
- Dutta, S.; Patil, R.; Dey, T. Electron Transfer-Driven Single and Multi-Enzyme Biofuel Cells for Self-Powering and Energy Bioscience. Nano Energy 2022, 96, 107074. [Google Scholar] [CrossRef]
- Kalyana Sundaram, S.d.; Hossain, M.M.; Rezki, M.; Ariga, K.; Tsujimura, S. Enzyme Cascade Electrode Reactions with Nanomaterials and Their Applicability towards Biosensor and Biofuel Cells. Biosensors 2023, 13, 1018. [Google Scholar] [CrossRef] [PubMed]
- Kausaite-Minkstimiene, A.; Kaminskas, A.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. Development of a New Biocathode for a Single Enzyme Biofuel Cell Fuelled by Glucose. Sci. Rep. 2021, 11, 18568. [Google Scholar] [CrossRef]
- Xu, C.; Song, Y.; Han, M.; Zhang, H. Portable and Wearable Self-Powered Systems Based on Emerging Energy Harvesting Technology. Microsyst. Nanoeng. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Wang, Y.H.; Huang, K.; Huang, K.J.; Jiang, H.; Wang, X.M. Enzyme-Based Biofuel Cells for Biosensors and in Vivo Power Supply. Nano Energy 2021, 84, 105853. [Google Scholar] [CrossRef]
- Scholte, N.T.B.; van Ravensberg, A.E.; Shakoor, A.; Boersma, E.; Ronner, E.; de Boer, R.A.; Brugts, J.J.; Bruining, N.; van der Boon, R.M.A. A Scoping Review on Advancements in Noninvasive Wearable Technology for Heart Failure Management. npj Digit. Med. 2024, 7, 279. [Google Scholar] [CrossRef]


| Immobilization Strategies | Advantages | Drawbacks |
|---|---|---|
| Physical Adsorption | Simple and quick method; Minimal alteration to enzyme structure; Reversible process | Weak enzyme attachment; Potential desorption over time; Limited stability and reusability |
| Covalent Binding | Strong and stable enzyme attachment; Enhanced reusability; Greater resistance to environmental conditions | Chemical modification of enzyme may affect activity; Labor-intensive and time-consuming process; Limited enzyme loading capacity |
| Encapsulation | Protects enzyme from harsh environments; Allows for controlled release; Good stability and reusability | Diffusion limitations for substrates and products; Potential mass transfer issues; Complex fabrication processes and materials |
| Cross-Linking | Provides stability to enzymes; Allows for moderate reusability; Retention of enzyme activity | Limited control over cross-linking density; Possibility of enzyme inactivation during cross-linking; Can alter enzyme conformation and activity |
| Bioanode | Biocathode | Biofuel | Current Density | Power Density | Ref |
|---|---|---|---|---|---|
| CS/GOx/CNCs/GCE | Pt sheet | Glucose | 434 μA cm−2 | 55 μW cm−2 | [101] |
| Nafion/GOx/PANI1600@GO/GCE | CNFs/GCE | Glucose | 2.48 mA cm−2 | 1.12 mW cm−2 | [102] |
| GOx/CNTs@C3N4/GCE | LOx/N-CNTs@C3N4/GCE | Glucose | 1.21 mA cm−2 | 0.57 mW cm−2 | [103] |
| rGO/AuNPs/N-doped CNTs | Pt electrode | Glucose | NR | 235 µW cm−2 | [104] |
| GCE/PPy/Au/CNT@Fe3O4/FRT/GOD | Au Electrode | Glucose | 6.01 mA cm−2 | 1.32 mW cm−2 | [105] |
| Graphene/SPE/GDH | Graphene/SPE/LOx | Ethanol | 88 μA cm−2 | 5.16 μW cm−2 | [107] |
| GCE/MWCNT/GOx nanoflowers | GCE/MWCNT/LOx nanoflowers | Glucose | 1.62 mA cm−2 | 200 μW cm−2 | [106] |
| SWCNTs wrapped with rGO/Alcohol dehydrogenase | NR | Ethanol | 165 μA cm−2 | NR | [108] |
| Energy Source | Pros | Cons | Challenges to Address | Wearable Applications | Implantable Applications |
|---|---|---|---|---|---|
| Neuro-transmitters (e.g., Acetylcholine, Dopamine) |
|
|
|
|
|
| Glucose |
|
|
|
|
|
| Lactate |
|
|
|
|
|
| Ethanol |
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fredj, Z.; Rong, G.; Sawan, M. Recent Advances in Enzymatic Biofuel Cells to Power Up Wearable and Implantable Biosensors. Biosensors 2025, 15, 218. https://doi.org/10.3390/bios15040218
Fredj Z, Rong G, Sawan M. Recent Advances in Enzymatic Biofuel Cells to Power Up Wearable and Implantable Biosensors. Biosensors. 2025; 15(4):218. https://doi.org/10.3390/bios15040218
Chicago/Turabian StyleFredj, Zina, Guoguang Rong, and Mohamad Sawan. 2025. "Recent Advances in Enzymatic Biofuel Cells to Power Up Wearable and Implantable Biosensors" Biosensors 15, no. 4: 218. https://doi.org/10.3390/bios15040218
APA StyleFredj, Z., Rong, G., & Sawan, M. (2025). Recent Advances in Enzymatic Biofuel Cells to Power Up Wearable and Implantable Biosensors. Biosensors, 15(4), 218. https://doi.org/10.3390/bios15040218