Modern Emerging Biosensing Methodologies for the Early Diagnosis and Screening of Ovarian Cancer
Abstract
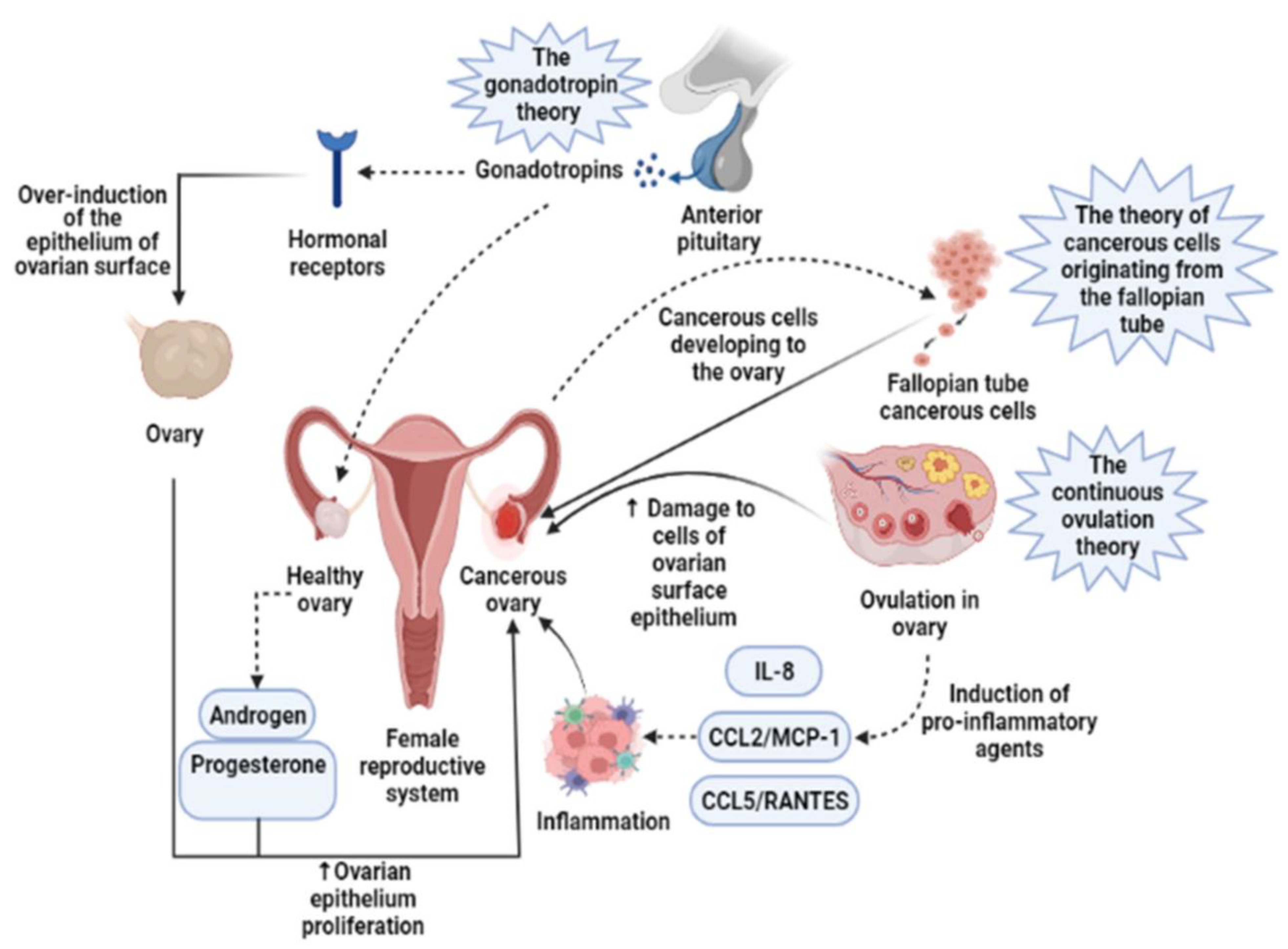
1. Introduction
1.1. CA-125
1.2. HE4
1.3. Human Prostasin (PSN)
1.4. Mesothelin
1.5. Osteopontin (OPN)
1.6. Kallikreins
1.7. Mucin 1 (MUC1)
1.8. Heat Shock Proteins (HSPs)
1.9. MicroRNAs (miRNAs)
1.10. Exosomes
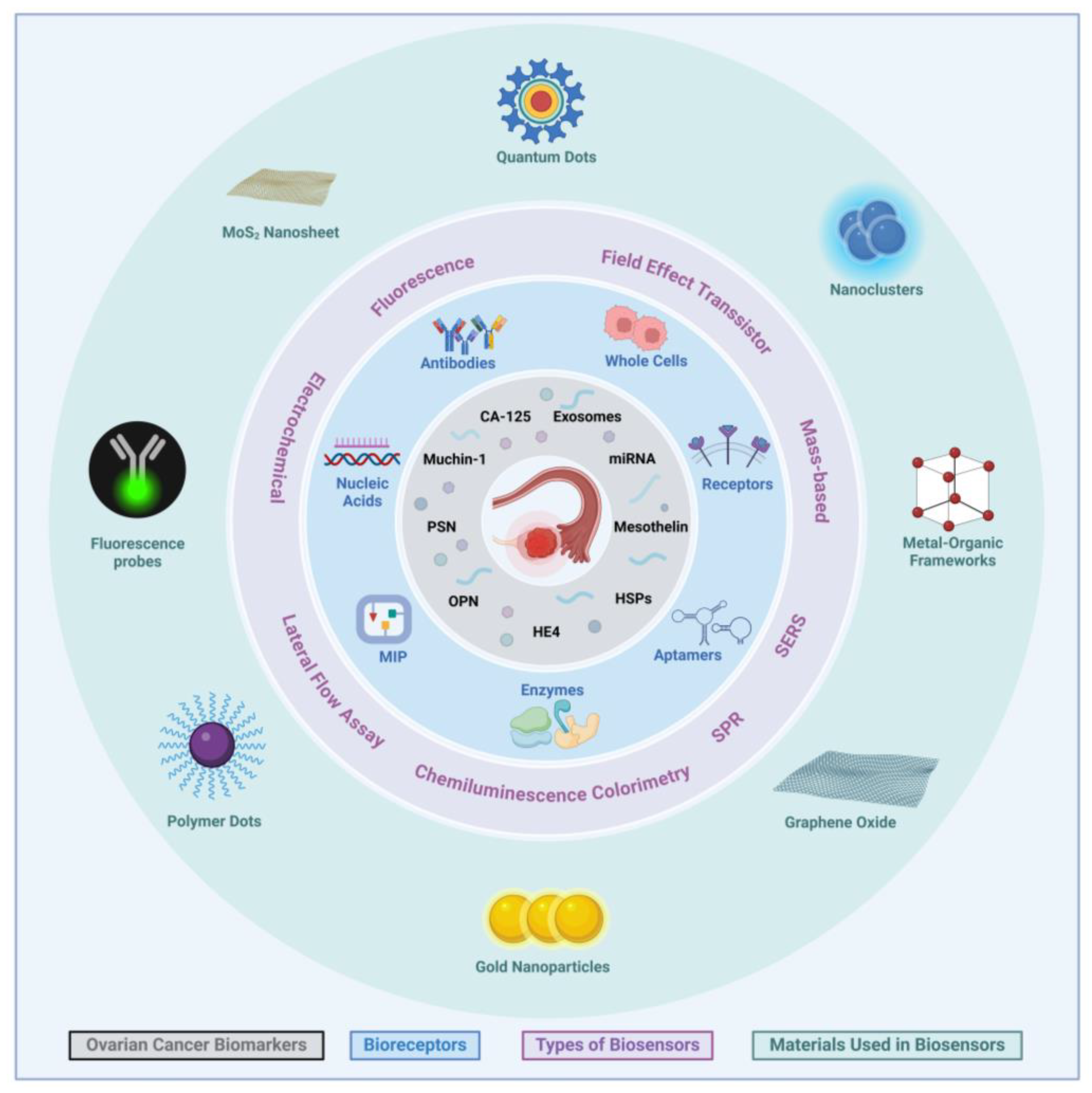
2. Biosensors
2.1. Optical Biosensors
2.1.1. Fluorescence Sensors
2.1.2. Optical Aptasensors
2.1.3. Colorimetric Biosensors
2.1.4. Surface Plasmon Resonance (SPR) Biosensors
2.1.5. Surface-Enhanced Raman Spectroscopy (SERS)-Based Biosensors
2.2. Electrochemical Biosensors
2.2.1. Label-Free and Sandwich-Type EC Biosensors
2.2.2. Electrochemical Aptasensors
3. Mass-Based Biosensors
4. Microfluid-Based Sensors
5. Paper-Based Lateral Flow Assay
6. Field Effect Transistor-Based Sensors
7. Molecular Imprinting Polymers as Bioreceptors
8. Discussion and Opportunities
9. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Giampaolino, P.; Foreste, V.; Della Corte, L.; Di Filippo, C.; Iorio, G.; Bifulco, G. Role of Biomarkers for Early Detection of Ovarian Cancer Recurrence. Gland Surg. 2020, 9, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, H.; Zhang, F.; Xu, Y.; Liang, W.; Huang, L. New Trends in Diagnosing and Treating Ovarian Cancer Using Nanotechnology. Front. Bioeng. Biotechnol. 2023, 11, 1160985. [Google Scholar] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian Cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Elias, K.M.; Guo, J.; Bast, R.C. Early Detection of Ovarian Cancer. Hematol. Oncol. Clin. 2018, 32, 903–914. [Google Scholar] [CrossRef]
- Sarojini, S.; Tamir, A.; Lim, H.; Li, S.; Zhang, S.; Goy, A.; Pecora, A.; Suh, K.S. Early Detection Biomarkers for Ovarian Cancer. J. Oncol. 2012, 2012, 709049. [Google Scholar] [CrossRef]
- Chinnappan, R.; Makhzoum, T.; Arai, M.; Hajja, A.; Abul Rub, F.; Alodhaibi, I.; Alfuwais, M.; Elahi, M.A.; Alshehri, E.A.; Ramachandran, L.; et al. Recent Advances in Biosensor Technology for Early-Stage Detection of Hepatocellular Carcinoma-Specific Biomarkers: An Overview. Diagnostics 2024, 14, 1519. [Google Scholar] [CrossRef]
- Ramachandran, L.; Abul Rub, F.; Hajja, A.; Alodhaibi, I.; Arai, M.; Alfuwais, M.; Makhzoum, T.; Yaqinuddin, A.; Al-Kattan, K.; Assiri, A.M.; et al. Biosensing of Alpha-Fetoprotein: A Key Direction toward the Early Detection and Management of Hepatocellular Carcinoma. Biosensors 2024, 14, 235. [Google Scholar] [CrossRef]
- Roointan, A.; Mir, T.A.; Wani, S.I.; Mati-ur-Rehman; Hussain, K.K.; Ahmed, B.; Abrahim, S.; Savardashtaki, A.; Gandomani, G.; Gandomani, M.; et al. Early Detection of Lung Cancer Biomarkers through Biosensor Technology: A Review. J. Pharm. Biomed. Anal. 2019, 164, 93–103. [Google Scholar] [CrossRef]
- Chinnappan, R.; Mir, T.A.; Alsalameh, S.; Makhzoum, T.; Alzhrani, A.; Alnajjar, K.; Adeeb, S.; Al Eman, N.; Ahmed, Z.; Shakir, I.; et al. Emerging Biosensing Methods to Monitor Lung Cancer Biomarkers in Biological Samples: A Comprehensive Review. Cancers 2023, 15, 3414. [Google Scholar] [CrossRef]
- Chinnappan, R.; Al Faraj, A.; Abdel Rahman, A.M.; Abu-Salah, K.M.; Mouffouk, F.; Zourob, M. Anti-VCAM-1 and Anti-IL4Rα Aptamer-Conjugated Super Paramagnetic Iron Oxide Nanoparticles for Enhanced Breast Cancer Diagnosis and Therapy. Molecules 2020, 25, 3437. [Google Scholar] [CrossRef]
- Eissa, S.; Chinnappan, R.; Zourob, M. Ultrasensitive Label-free Electrochemical Immunosensors for Multiple Cell Surface Biomarkers on Liver Cancer Stem Cells. Electroanalysis 2017, 29, 1994–2000. [Google Scholar] [CrossRef]
- Rezaei-Tazangi, F.; Roghani-Shahraki, H.; Khorsand Ghaffari, M.; Abolhasani Zadeh, F.; Boostan, A.; ArefNezhad, R.; Motedayyen, H. The Therapeutic Potential of Common Herbal and Nano-Based Herbal Formulations against Ovarian Cancer: New Insight into the Current Evidence. Pharmaceuticals 2021, 14, 1315. [Google Scholar] [CrossRef] [PubMed]
- Radu, M.R.; Prădatu, A.; Duică, F.; Micu, R.; Creţoiu, S.M.; Suciu, N.; Creţoiu, D.; Varlas, V.N.; Rădoi, V.E. Ovarian Cancer: Biomarkers and Targeted Therapy. Biomedicines 2021, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.Y.; Katsaros, D.; Scorilas, A.; Fracchioli, S.; Piccinno, R.; Rigault de la Longrais, I.A.; Howarth, D.J.; Diamandis, E.P. Prognostic Value of Human Kallikrein 10 Expression in Epithelial Ovarian Carcinoma. Clin. Cancer Res. 2001, 7, 2372–2379. [Google Scholar]
- Bast, R.C.; Feeney, M.; Lazarus, H.; Nadler, L.; Colvin, R.; Knapp, R. Reactivity of a Monoclonal Antibody with Human Ovarian Carcinoma. J. Clin. Investig. 1981, 68, 1331–1337. [Google Scholar] [CrossRef]
- Singer, G.; Oldt, R.; Cohen, Y.; Wang, B.G.; Sidransky, D.; Kurman, R.J.; Shih, I.-M. Mutations in BRAF and KRAS Characterize the Development of Low-Grade Ovarian Serous Carcinoma. J. Natl. Cancer Inst. 2003, 95, 484–486. [Google Scholar] [CrossRef]
- Peyssonnaux, C.; Eychène, A. The Raf/MEK/ERK Pathway: New Concepts of Activation. Biol. Cell 2001, 93, 53–62. [Google Scholar] [CrossRef]
- Ye, B.; Skates, S.; Mok, S.C.; Horick, N.K.; Rosenberg, H.F.; Vitonis, A.; Edwards, D.; Sluss, P.; Han, W.K.; Berkowitz, R.S.; et al. Proteomic-Based Discovery and Characterization of Glycosylated Eosinophil-Derived Neurotoxin and COOH-Terminal Osteopontin Fragments for Ovarian Cancer in Urine. Clin. Cancer Res. 2006, 12, 432–441. [Google Scholar] [CrossRef]
- Zhang, R.; Siu, M.K.Y.; Ngan, H.Y.S.; Chan, K.K.L. Molecular Biomarkers for the Early Detection of Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 12041. [Google Scholar] [CrossRef]
- Yu, J.X.; Chao, L.; Chao, J. Prostasin Is a Novel Human Serine Proteinase from Seminal Fluid. Purification, Tissue Distribution, and Localization in Prostate Gland. J. Biol. Chem. 1994, 269, 18843–18848. [Google Scholar]
- Costa, F.P.; Batista, E.L.; Zelmanowicz, A.; Svedman, C.; Devenz, G.; Alves, S.; da Silva, A.S.M.; Garicochea, B. Prostasin, a Potential Tumor Marker in Ovarian Cancer—A Pilot Study. Clinics 2009, 64, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Schummer, M.; Ng, W.V.; Bumgarner, R.E.; Nelson, P.S.; Schummer, B.; Bednarski, D.W.; Hassell, L.; Baldwin, R.L.; Karlan, B.Y.; Hood, L. Comparative Hybridization of an Array of 21,500 Ovarian cDNAs for the Discovery of Genes Overexpressed in Ovarian Carcinomas. Gene 1999, 238, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.K.; Rashid, M.; Bisht, M. Multiplexed Magnetic Nanoparticle-Antibody Conjugates (MNPs-ABS) Based Prognostic Detection of Ovarian Cancer Biomarkers, CA-125, β-2M and ApoA1 Using Fluorescence Spectroscopy with Comparison of Surface Plasmon Resonance (SPR) Analysis. Biosens. Bioelectron. 2015, 73, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-W.; Bae, S.M.; Lim, H.; Kim, Y.J.; Ahn, W.S. Development of Multiplexed Bead-Based Immunoassays for the Detection of Early Stage Ovarian Cancer Using a Combination of Serum Biomarkers. PLoS ONE 2012, 7, e44960. [Google Scholar] [CrossRef]
- Fassl, S.; Leisser, C.; Huettenbrenner, S.; Maier, S.; Rosenberger, G.; Strasser, S.; Grusch, M.; Fuhrmann, G.; Leuhuber, K.; Polgar, D.; et al. Transferrin Ensures Survival of Ovarian Carcinoma Cells When Apoptosis Is Induced by TNFalpha, FasL, TRAIL, or Myc. Oncogene 2003, 22, 8343–8355. [Google Scholar] [CrossRef]
- Ahmed, N.; Oliva, K.T.; Barker, G.; Hoffmann, P.; Reeve, S.; Smith, I.A.; Quinn, M.A.; Rice, G.E. Proteomic Tracking of Serum Protein Isoforms as Screening Biomarkers of Ovarian Cancer. Proteomics 2005, 5, 4625–4636. [Google Scholar] [CrossRef]
- Chang, M.C.; Chen, C.A.; Chen, P.J.; Chiang, Y.C.; Chen, Y.L.; Mao, T.L.; Lin, H.W.; Lin Chiang, W.H.; Cheng, W.F. Mesothelin Enhances Invasion of Ovarian Cancer by Inducing MMP-7 through MAPK/ERK and JNK Pathways. Biochem. J. 2012, 442, 293–302. [Google Scholar] [CrossRef]
- Hassan, R.; Kreitman, R.J.; Pastan, I.; Willingham, M.C. Localization of Mesothelin in Epithelial Ovarian Cancer. Appl. Immunohistochem. Mol. Morphol. AIMM 2005, 13, 243–247. [Google Scholar] [CrossRef]
- Hassan, R.; Ho, M. Mesothelin Targeted Cancer Immunotherapy. Eur. J. Cancer 2008, 44, 46–53. [Google Scholar] [CrossRef]
- Scholler, N.; Garvik, B.; Hayden-Ledbetter, M.; Kline, T.; Urban, N. Development of a CA125-Mesothelin Cell Adhesion Assay as a Screening Tool for Biologics Discovery. Cancer Lett. 2007, 247, 130–136. [Google Scholar] [CrossRef]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of Ovarian Cancer Antigen CA125/MUC16 to Mesothelin Mediates Cell Adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef] [PubMed]
- Badgwell, D.; Lu, Z.; Cole, L.; Fritsche, H.; Atkinson, E.N.; Somers, E.; Allard, J.; Moore, R.G.; Lu, K.H.; Bast, R.C. Urinary Mesothelin Provides Greater Sensitivity for Early Stage Ovarian Cancer than Serum Mesothelin, Urinary hCG Free Beta Subunit and Urinary hCG Beta Core Fragment. Gynecol. Oncol. 2007, 106, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Schorge, J.O.; Drake, R.D.; Lee, H.; Skates, S.J.; Rajanbabu, R.; Miller, D.S.; Kim, J.-H.; Cramer, D.W.; Berkowitz, R.S.; Mok, S.C. Osteopontin as an Adjunct to CA125 in Detecting Recurrent Ovarian Cancer. Clin. Cancer Res. 2004, 10, 3474–3478. [Google Scholar] [CrossRef] [PubMed]
- Drabovich, A.P.; Diamandis, E.P. Combinatorial Peptide Libraries Facilitate Development of Multiple Reaction Monitoring Assays for Low-Abundance Proteins. J. Proteome Res. 2010, 9, 1236–1245. [Google Scholar] [CrossRef]
- Pépin, D.; Shao, Z.-Q.; Huppé, G.; Wakefield, A.; Chu, C.-W.; Sharif, Z.; Vanderhyden, B.C. Kallikreins 5, 6 and 10 Differentially Alter Pathophysiology and Overall Survival in an Ovarian Cancer Xenograft Model. PLoS ONE 2011, 6, e26075. [Google Scholar] [CrossRef]
- Koh, S.C.L.; Huak, C.Y.; Lutan, D.; Marpuang, J.; Ketut, S.; Budiana, N.G.; Saleh, A.Z.; Aziz, M.F.; Winarto, H.; Pradjatmo, H.; et al. Combined Panel of Serum Human Tissue Kallikreins and CA-125 for the Detection of Epithelial Ovarian Cancer. J. Gynecol. Oncol. 2012, 23, 175–181. [Google Scholar] [CrossRef]
- Wang, T.; Gao, Y.; Wang, X.; Tian, J.; Li, Y.; Yu, B.; Huang, C.; Li, H.; Liang, H.; Irwin, D.M.; et al. Establishment of an Optimized CTC Detection Model Consisting of EpCAM, MUC1 and WT1 in Epithelial Ovarian Cancer and Its Correlation with Clinical Characteristics. Chin. J. Cancer Res. 2022, 34, 95–108. [Google Scholar] [CrossRef]
- Williams, K.A.; Terry, K.L.; Tworoger, S.S.; Vitonis, A.F.; Titus, L.J.; Cramer, D.W. Polymorphisms of MUC16 (CA125) and MUC1 (CA15.3) in Relation to Ovarian Cancer Risk and Survival. PLoS ONE 2014, 9, e88334. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, J.; Zhu, L.; Hu, Z.; Hou, R.; Liu, S.; Tan, M.; Liu, J.; Lin, B. Chemoresistance Is Associated with MUC1 and Lewis y Antigen Expression in Ovarian Epithelial Cancers. Int. J. Mol. Sci. 2013, 14, 11024–11033. [Google Scholar] [CrossRef]
- Fung, K.; Vivier, D.; Keinänen, O.; Sarbisheh, E.K.; Price, E.W.; Zeglis, B.M. 89Zr-Labeled AR20.5: A MUC1-Targeting ImmunoPET Probe. Molecules 2020, 25, 2315. [Google Scholar] [CrossRef]
- Pinheiro, S.P.; Hankinson, S.E.; Tworoger, S.S.; Rosner, B.A.; McKolanis, J.R.; Finn, O.J.; Cramer, D.W. Anti-MUC1 Antibodies and Ovarian Cancer Risk: Prospective Data from the Nurses’ Health Studies. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1595–1601. [Google Scholar] [CrossRef]
- Bodzek, P.; Partyka, R.; Damasiewicz-Bodzek, A. Antibodies against Hsp60 and Hsp65 in the Sera of Women with Ovarian Cancer. J. Ovarian Res. 2014, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Increased Expression of Heat Shock Protein 27 Correlates with Peritoneal Metastasis in Epithelial Ovarian Cancer—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/22534325/ (accessed on 6 December 2024).
- Albakova, Z.; Siam, M.K.S.; Sacitharan, P.K.; Ziganshin, R.H.; Ryazantsev, D.Y.; Sapozhnikov, A.M. Extracellular Heat Shock Proteins and Cancer: New Perspectives. Transl. Oncol. 2021, 14, 100995. [Google Scholar] [CrossRef] [PubMed]
- El Hayek, T.; Alnaser-Almusa, O.A.; Alsalameh, S.M.; Alhalabi, M.T.; Sabbah, A.N.; Alshehri, E.A.; Mir, T.A.; Mani, N.K.; Al-Kattan, K.; Chinnappan, R.; et al. Emerging Role of Exosomal microRNA in Liver Cancer in the Era of Precision Medicine; Potential and Challenges. Front. Mol. Biosci. 2024, 11, 1381789. [Google Scholar]
- Xu, X.; Qian, H.; Bo, X.; Liu, X.; Zhu, J. Liquid Biopsies for Ovarian Carcinoma: Potential Clinical Values of Circulating Acellular miRNA in Patients with Ovarian Cancer. Indian J. Pharm. Sci. 2020, 82, 121–126. [Google Scholar]
- Chinnappan, R.; Mohammed, R.; Yaqinuddin, A.; Abu-Salah, K.; Zourob, M. Highly Sensitive Multiplex Detection of microRNA by Competitive DNA Strand Displacement Fluorescence Assay. Talanta 2019, 200, 487–493. [Google Scholar] [CrossRef]
- Liu, M.X.; Siu, M.K.Y.; Liu, S.S.; Yam, J.W.P.; Ngan, H.Y.S.; Chan, D.W. Epigenetic Silencing of microRNA-199b-5p Is Associated with Acquired Chemoresistance via Activation of JAG1-Notch1 Signaling in Ovarian Cancer. Oncotarget 2014, 5, 944–958. [Google Scholar] [CrossRef]
- Liu, C.H.; Jing, X.N.; Liu, X.L.; Qin, S.Y.; Liu, M.W.; Hou, C.H. Tumor-Suppressor miRNA-27b-5p Regulates the Growth and Metastatic Behaviors of Ovarian Carcinoma Cells by Targeting CXCL1. J. Ovarian Res. 2020, 13, 92. [Google Scholar] [CrossRef]
- Zhou, P.; Xiong, T.; Yao, L.; Yuan, J. MicroRNA-665 Promotes the Proliferation of Ovarian Cancer Cells by Targeting SRCIN1. Exp. Ther. Med. 2020, 19, 1112–1120. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, S.; Liu, X. MicroRNA Profiling of Plasma Exosomes from Patients with Ovarian Cancer Using High-Throughput Sequencing. Oncol. Lett. 2019, 17, 5601–5607. [Google Scholar] [CrossRef]
- Yoshimura, A.; Sawada, K.; Nakamura, K.; Kinose, Y.; Nakatsuka, E.; Kobayashi, M.; Miyamoto, M.; Ishida, K.; Matsumoto, Y.; Kodama, M.; et al. Exosomal miR-99a-5p Is Elevated in Sera of Ovarian Cancer Patients and Promotes Cancer Cell Invasion by Increasing Fibronectin and Vitronectin Expression in Neighboring Peritoneal Mesothelial Cells. BMC Cancer 2018, 18, 1065. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, M.; Sachan, M.; Nara, S. Current Strategies for Early Epithelial Ovarian Cancer Detection Using miRNA as a Potential Tool. Front. Mol. Biosci. 2024, 11, 1361601. [Google Scholar] [CrossRef] [PubMed]
- Kartik Kumar, S.; Venkata Sasidhar, M. From Fluids to Forecasts: The Promise of Small Extracellular Vesicle miRNAs in Revolutionising Cancer Diagnostics. In Beyond the Blueprint—Decoding the Elegance of Gene Expression; IntechOpen: London, UK, 2024. [Google Scholar]
- Chinnappan, R.; Ramadan, Q.; Zourob, M. An Integrated Lab-on-a-Chip Platform for Pre-Concentration and Detection of Colorectal Cancer Exosomes Using Anti-CD63 Aptamer as a Recognition Element. Biosens. Bioelectron. 2023, 220, 114856. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhu, W.; Chen, Q.; Yuan, Y.; Wang, Y.; Wang, J.; Wu, X. Ovarian Cancer Cell-Secreted Exosomal miR-205 Promotes Metastasis by Inducing Angiogenesis. Theranostics 2019, 9, 8206–8220. [Google Scholar] [CrossRef]
- Chinnappan, R.; Ramadan, Q.; Zourob, M. Isolation and Detection of Exosomal Mir210 Using Carbon Nanomaterial-Coated Magnetic Beads. J. Funct. Biomater. 2023, 14, 441. [Google Scholar] [CrossRef]
- Yin, J.; Yan, X.; Yao, X.; Zhang, Y.; Shan, Y.; Mao, N.; Yang, Y.; Pan, L. Secretion of Annexin A3 from Ovarian Cancer Cells and Its Association with Platinum Resistance in Ovarian Cancer Patients. J. Cell. Mol. Med. 2012, 16, 337–348. [Google Scholar] [CrossRef]
- Li, N.; Lin, G.; Zhang, Y.; Zhang, Q.; Zhang, H. Exosome-Related Protein CRABP2 Is Upregulated in Ovarian Carcinoma and Enhances Cell Proliferation. Discov. Oncol. 2022, 13, 33. [Google Scholar] [CrossRef]
- Razmi, N.; Hasanzadeh, M. Current Advancement on Diagnosis of Ovarian Cancer Using Biosensing of CA 125 Biomarker: Analytical Approaches. TrAC Trends Anal. Chem. 2018, 108, 1–12. [Google Scholar] [CrossRef]
- Patel, P. Biosensors and Biomarkers: Promising Tools for Cancer Diagnosis. Int. J. Biosens. Bioelectron. 2017, 3, 313–316. [Google Scholar] [CrossRef][Green Version]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to Biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Kabashin, A.V.; Kravets, V.G.; Grigorenko, A.N. Label-Free Optical Biosensing: Going beyond the Limits. Chem. Soc. Rev. 2023, 52, 6554–6585. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, C.; Wang, C.; Wang, P.; Chang, X.; Han, L.; Zhang, Y. Multiple Biomarker Simultaneous Detection in Serum via a Nanomaterial-Functionalized Biosensor for Ovarian Tumor/Cancer Diagnosis. Micromachines 2022, 13, 2046. [Google Scholar] [CrossRef] [PubMed]
- Abou-Omar, M.N.; Attia, M.S.; Afify, H.G.; Amin, M.A.; Boukherroub, R.; Mohamed, E.H. Novel Optical Biosensor Based on a Nano-Gold Coated by Schiff Base Doped in Sol/Gel Matrix for Sensitive Screening of Oncomarker CA-125. ACS Omega 2021, 6, 20812–20821. [Google Scholar] [CrossRef]
- Zacharioudaki, D.-E.; Fitilis, I.; Kotti, M. Review of Fluorescence Spectroscopy in Environmental Quality Applications. Molecules 2022, 27, 4801. [Google Scholar] [CrossRef]
- De La Franier, B.; Thompson, M. Detection of the Ovarian Cancer Biomarker Lysophosphatidic Acid in Serum. Biosensors 2020, 10, 13. [Google Scholar] [CrossRef]
- Attia, M.; Ali, K.; El-Kemary, M.; Darwish, W. Phthalocyanine-Doped Polystyrene Fluorescent Nanocomposite as a Highly Selective Biosensor for Quantitative Determination of Cancer Antigen 125. Talanta 2019, 201, 185–193. [Google Scholar] [CrossRef]
- Bahari, D.; Babamiri, B.; Salimi, A. Ultrasensitive Molecularly Imprinted Fluorescence Sensor for Simultaneous Determination of CA125 and CA15–3 in Human Serum and OVCAR-3 and MCF-7 Cells Lines Using Cd and Ni Nanoclusters as New Emitters. Anal. Bioanal. Chem. 2021, 413, 4049–4061. [Google Scholar] [CrossRef]
- Verma, A.K.; Noumani, A.; Yadav, A.K.; Solanki, P.R. FRET Based Biosensor: Principle Applications Recent Advances and Challenges. Diagnostics 2023, 13, 1375. [Google Scholar] [CrossRef]
- Omer, W.E.; Abdelbar, M.F.; El-Kemary, N.M.; Fukata, N.; El-Kemary, M.A. Cancer Antigen 125 Assessment Using Carbon Quantum Dots for Optical Biosensing for the Early Diagnosis of Ovarian Cancer. RSC Adv. 2021, 11, 31047–31057. [Google Scholar] [CrossRef]
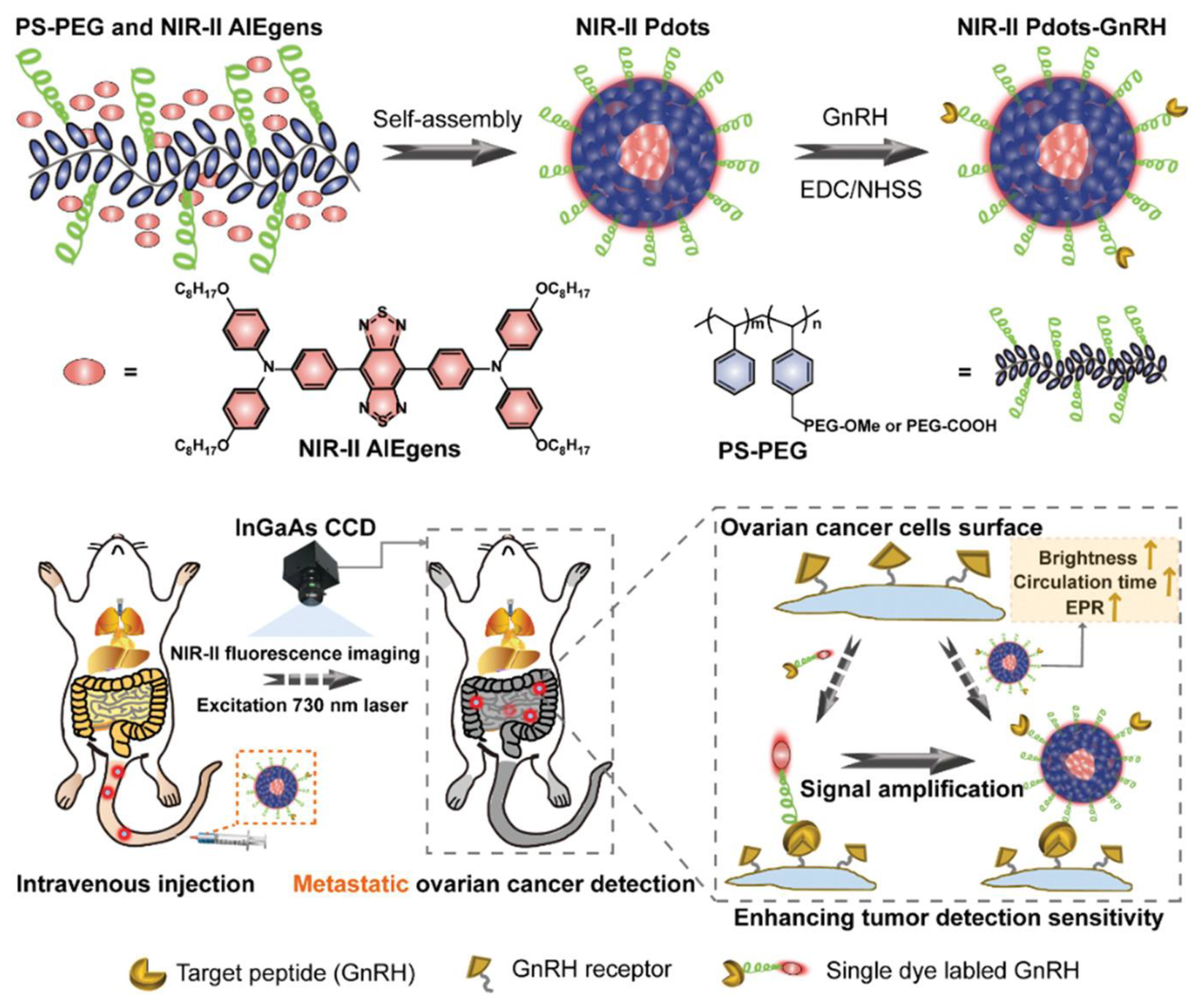
- Zhou, X.; Liu, Q.; Yuan, W.; Li, Z.; Xu, Y.; Feng, W.; Xu, C.; Li, F. Ultrabright NIR-II Emissive Polymer Dots for Metastatic Ovarian Cancer Detection. Adv. Sci. 2021, 8, 2000441. [Google Scholar] [CrossRef] [PubMed]
- Hada, A.-M.; Craciun, A.-M.; Focsan, M.; Borlan, R.; Soritau, O.; Todea, M.; Astilean, S. Folic Acid Functionalized Gold Nanoclusters for Enabling Targeted Fluorescence Imaging of Human Ovarian Cancer Cells. Talanta 2021, 225, 121960. [Google Scholar] [CrossRef] [PubMed]
- Alomran, N.; Chinnappan, R.; Alsolaiss, J.; Casewell, N.R.; Zourob, M. Exploring the Utility of ssDNA Aptamers Directed against Snake Venom Toxins as New Therapeutics for Snakebite Envenoming. Toxins 2022, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Alkhaldi, S.; Chinnappan, R.; Siddiqua, A.; Abduljabbar, M.; Abdel Rahman, A.M.; Dasouki, M.; Zourob, M. Selection, Characterization, and Electrochemical Biosensing Application of DNA Aptamers for Sepiapterin. Talanta 2020, 216, 120951. [Google Scholar] [CrossRef]
- Aljohani, M.M.; Chinnappan, R.; Eissa, S.; Alsager, O.A.; Weber, K.; Cialla-May, D.; Popp, J.; Zourob, M. In Vitro Selection of Specific DNA Aptamers Against the Anti-Coagulant Dabigatran Etexilate. Sci. Rep. 2018, 8, 13290. [Google Scholar] [CrossRef]
- Ng, A.; Chinnappan, R.; Eissa, S.; Liu, H.; Tlili, C.; Zourob, M. Selection, Characterization, and Biosensing Application of High Affinity Congener-Specific Microcystin-Targeting Aptamers. Environ. Sci. Technol. 2012, 46, 10697–10703. [Google Scholar] [CrossRef]
- Ibrahim, M.R.; Greish, Y.E. MOF-Based Biosensors for the Detection of Carcinoembryonic Antigen: A Concise Review. Molecules 2023, 28, 5970. [Google Scholar] [CrossRef]
- Han, R.; Sun, Y.; Dai, Y.; Gao, D.; Wang, X.; Luo, C. A Chemiluminescence Aptasensor for Sensitive Detection of Carcinoembryonic Antigen Based on Dual Aptamer-Conjugates Biorecognition. Sens. Actuators B Chem. 2021, 326, 128833. [Google Scholar] [CrossRef]
- Sohrabi, H.; Mahmoudi-Maleki, R.; Majidi, M.R.; Oroojalian, F.; Mokhtarzadeh, A.A.; de la Guardia, M. Recent Advances in Nanostructure-Enhanced Optical Assays Focused on Luminescence-Based Biosensors for Cancer Biomarkers. TrAC Trends Anal. Chem. 2024, 176, 117753. [Google Scholar] [CrossRef]
- Hasan, M.R.; Sharma, P.; Pilloton, R.; Khanuja, M.; Narang, J. Colorimetric Biosensor for the Naked-Eye Detection of Ovarian Cancer Biomarker PDGF Using Citrate Modified Gold Nanoparticles. Biosens. Bioelectron. X 2022, 11, 100142. [Google Scholar] [CrossRef]
- Xu, X.; Ji, J.; Chen, P.; Wu, J.; Jin, Y.; Zhang, L.; Du, S. Salt-Induced Gold Nanoparticles Aggregation Lights up Fluorescence of DNA-Silver Nanoclusters to Monitor Dual Cancer Markers Carcinoembryonic Antigen and Carbohydrate Antigen 125. Anal. Chim. Acta 2020, 1125, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Zhang, Z.; Liu, C.; Qi, Z. Gold-Silver Alloy Film Based Surface Plasmon Resonance Sensor for Biomarker Detection. Mater. Sci. Eng. 2020, 116, 111126. [Google Scholar] [CrossRef]
- Liu, C.; Zeng, X.; An, Z.; Yang, Y.; Eisenbaum, M.; Gu, X.; Jornet, J.M.; Dy, G.K.; Reid, M.E.; Gan, Q. Sensitive Detection of Exosomal Proteins via a Compact Surface Plasmon Resonance Biosensor for Cancer Diagnosis. ACS Sens. 2018, 3, 1471–1479. [Google Scholar] [PubMed]
- Szymanska, B.; Lukaszewski, Z.; Zelazowska-Rutkowska, B.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. An Spri Biosensor for Determination of the Ovarian Cancer Marker He4 in Human Plasma. Sensors 2021, 21, 3567. [Google Scholar] [CrossRef]
- Szymańska, B.; Lukaszewski, Z.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. A Biosensor for Determination of the Circulating Biomarker CA125/MUC16 by Surface Plasmon Resonance Imaging. Talanta 2020, 206, 120187. [Google Scholar]
- Oldak, L.; Sankiewicz, A.; Żelazowska-Rutkowska, B.; Cylwik, B.; Lukaszewski, Z.; Skoczylas, M.; Gorodkiewicz, E. Two SPRi Biosensors for the Determination of Cathepsin S in Blood Plasma. Talanta 2021, 225, 121900. [Google Scholar]
- Geka, G.; Kanioura, A.; Likodimos, V.; Gardelis, S.; Papanikolaou, N.; Kakabakos, S.; Petrou, P. SERS Immunosensors for Cancer Markers Detection. Materials 2023, 16, 3733. [Google Scholar] [CrossRef]
- Lan, T.; Zhao, Y.; Du, Y.; Ma, C.; Wang, R.; Zhang, Q.; Wang, S.; Wei, W.; Yuan, H.; Huang, Q. Fabrication of a Novel Au Star@ AgAu Yolk-Shell Nanostructure for Ovarian Cancer Early Diagnosis and Targeted Therapy. Int. J. Nanomed. 2023, 18, 3813–3824. [Google Scholar]
- Zhang, S.; Chen, Y.; Huang, Y.; Dai, H.; Lin, Y. Design and Application of Proximity Hybridization-Based Multiple Stimuli-Responsive Immunosensing Platform for Ovarian Cancer Biomarker Detection. Biosens. Bioelectron. 2020, 159, 112201. [Google Scholar]
- Ivanov, Y.D.; Kapustina, S.I.; Malsagova, K.A.; Goldaeva, K.V.; Pleshakova, T.O.; Galiullin, R.A.; Shumov, I.D.; Kozlov, A.F.; Glukhov, A.V.; Grabezhova, V.K. “Silicon-On-Insulator”-Based Biosensor for the Detection of MicroRNA Markers of Ovarian Cancer. Micromachines 2022, 14, 70. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Moammeri, A.; Shamsabadipour, A.; Moghaddam, Y.F.; Rahdar, A.; Pandey, S. Application of Various Optical and Electrochemical Nanobiosensors for Detecting Cancer Antigen 125 (CA-125): A Review. Biosensors 2023, 13, 99. [Google Scholar] [CrossRef]
- Ge, F.; Ding, W.; Han, C.; Zhang, L.; Liu, Q.; Zhao, J.; Luo, Z.; Jia, C.; Qu, P.; Zhang, L. Electrochemical Sensor for the Detection and Accurate Early Diagnosis of Ovarian Cancer. ACS Sens. 2024, 9, 2897–2906. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Lotay, N.; Thompson, M. Affinity-Based Electrochemical Biosensor with Antifouling Properties for Detection of Lysophosphatidic Acid, a Promising Early-Stage Ovarian Cancer Biomarker. Bioelectrochemistry 2023, 153, 108466. [Google Scholar] [CrossRef] [PubMed]
- Ekwujuru, E.U.; Olatunde, A.M.; Klink, M.J.; Ssemakalu, C.C.; Chili, M.M.; Peleyeju, M.G. Electrochemical and Photoelectrochemical Immunosensors for the Detection of Ovarian Cancer Biomarkers. Sensors 2023, 23, 4106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, R.; Wang, B.; He, C.; Bai, S.; Yan, H.; Yu, J.; Li, H.; Peng, B.; Gao, Z.; et al. Electrochemical Detection of Extracellular Vesicles for Early Diagnosis: A Focus on Disease Biomarker Analysis. Extracell. Vesicles Circ. Nucleic Acids 2024, 5, 165–179. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, T.; Hu, K.; Peng, Y.; Jia, X.; Yang, J.; Li, G. An Electrochemical Biosensor Designed with Entropy-Driven Autocatalytic DNA Circuits for Sensitive Detection of Ovarian Cancer-Derived Exosomes. Biosens. Bioelectron. 2024, 250, 116060. [Google Scholar] [CrossRef]
- Runprapan, N.; Wang, F.-M.; Ramar, A.; Yuan, C.-C. Role of Defects of Carbon Nanomaterials in the Detection of Ovarian Cancer Cells in Label-Free Electrochemical Immunosensors. Sensors 2023, 23, 1131. [Google Scholar] [CrossRef]
- Mu, W.; Wu, C.; Wu, F.; Gao, H.; Ren, X.; Feng, J.; Miao, M.; Zhang, H.; Chang, D.; Pan, H. Ultrasensitive and Label-Free Electrochemical Immunosensor for the Detection of the Ovarian Cancer Biomarker CA125 Based on CuCo-ONSs@AuNPs Nanocomposites. J. Pharm. Biomed. Anal. 2024, 243, 116080. [Google Scholar] [CrossRef]
- Yılmaz, M.; Bilgi, M. A Disposable Impedimetric Immunosensor for the Analysis of CA125 in Human Serum Samples. Biomed. Microdevices 2024, 26, 8. [Google Scholar] [CrossRef]
- Bilgi Kamaç, M.; Altun, M.; Yılmaz, M.; Yılmaz Aktan, A.; Aktan, S.; Sezgintürk, M.K. Point-of-Care Testing: A Disposable Label-Free Electrochemical CA125 and HE4 Immunosensors for Early Detection of Ovarian Cancer. Biomed. Microdevices 2023, 25, 18. [Google Scholar] [CrossRef]
- Cotchim, S.; Thavarungkul, P.; Kanatharana, P.; Thantipwan, T.; Jiraseree-amornkun, A.; Wannapob, R.; Limbut, W. A Portable Electrochemical Immunosensor for Ovarian Cancer Uses Hierarchical Microporous Carbon Material from Waste Coffee Grounds. Microchim. Acta 2023, 190, 232. [Google Scholar] [CrossRef]
- Foroozandeh, A.; SalarAmoli, H.; Abdouss, M.; Pourmadadi, M. Development of a Labeled-Free and Labeled Electrochemical Aptasensor for the Detection of Cancer Antigen 125 by Using Magnetic g-C3N4/MoS2 Nanocomposite. Sens. Actuators Rep. 2024, 7, 100195. [Google Scholar] [CrossRef]
- Bilgi Kamaç, M.; Altun, M.; Yilmaz, M.; Sezgintürk, M.K. A Label-Free Dual Immunosensor for the Simultaneous Electrochemical Determination of CA125 and HE4 Biomarkers for the Early Diagnosis of Ovarian Cancer. Anal. Bioanal. Chem. 2023, 415, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, Y.; Xia, Y.; Zhao, F.; Zeng, B. Simultaneous Detection of Ovarian Cancer-Concerned HE4 and CA125 Markers Based on Cu Single-Atom-Triggered CdS QDs and Eu MOF@Isoluminol ECL. Anal. Chem. 2023, 95, 4795–4802. [Google Scholar] [CrossRef]
- Krathumkhet, N.; Imae, T.; Wang, F.; Yuan, C.-C.; Manidae Lumban Gaol, J.; Paradee, N. Electrochemical Immunosensing by Carbon Ink/Carbon Dot/ZnO-Labeled-Ag@polypyrrole Composite Biomarker for CA-125 Ovarian Cancer Detection. Bioelectrochemistry 2023, 152, 108430. [Google Scholar] [CrossRef]
- Amirabadizadeh, M.; Siampour, H.; Abbasian, S.; Nikkhah, M.; Moshaii, A. Aptasensor for Ovarian Cancer Biomarker Detection Using Nanostructured Gold Electrodes. Microchim. Acta 2024, 191, 2. [Google Scholar] [CrossRef]
- Hu, C.; Qin, Z.; Fu, J.; Gao, Q.; Chen, C.; Tan, C.S.; Li, S. Aptamer-Based Carbohydrate Antigen 125 Sensor with Molybdenum Disulfide Functional Hybrid Materials. Anal. Biochem. 2023, 675, 115213. [Google Scholar] [CrossRef]
- Putri, A.D.; Murti, B.T.; Manga, Y.B.; Kanchi, S.; Huang, Y.; Peng, C.; Yang, P.; Hsieh, C. Whole-Cell Electrochemical Aptasensors for Cancer Diagnosis: Current Advances and Prospects. Adv. Sens. Res. 2024, 3, 2300151. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, W.; Yu, Y. Tetrahedral DNA Nanostructure Enhanced Toehold-Mediated Strand Displacement for Highly Sensitive Electrochemiluminescence Assay of CA125. Bioelectrochemistry 2024, 155, 108572. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.; Zhang, S.; Gao, L.; Lin, F.; Dai, H. Self-Reduced MXene-Metal Interaction Electrochemiluminescence Support with Synergistic Electrocatalytic and Photothermal Effects for the Bimodal Detection of Ovarian Cancer Biomarkers. J. Colloid Interface Sci. 2024, 661, 793–801. [Google Scholar] [CrossRef]
- Mei, X.; Zeng, Z.; Xu, W.; Yang, H.; Zheng, Y.; Gao, H.; Wu, C.; Zheng, Y.; Xu, Q.; Wang, G.; et al. Sandwich-Type Electrochemical Immunosensing of CA125 by Using Nanoribbon-like Ti3C2Tx MXenes and Toluidine Blue/UIO-66-NH2. Anal. Sci. 2024, 40, 1081–1087. [Google Scholar] [CrossRef]
- Maghiani, I.; Souza, L.V.; Bach-Toledo, L.; Faria, A.M.; Ortega, P.P.; Amoresi, R.A.C.; Simões, A.Z.; Mazon, T. Application of NiFe2O4 Nanoparticles towards the Detection of Ovarian Cancer Marker. Mater. Res. Bull. 2024, 177, 112835. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and Their Applications—A Review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef]
- Lim, H.J.; Saha, T.; Tey, B.T.; Tan, W.S.; Ooi, C.W. Quartz Crystal Microbalance-Based Biosensors as Rapid Diagnostic Devices for Infectious Diseases. Biosens. Bioelectron. 2020, 168, 112513. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, X.-H.; Shi, H.-S.; Mu, B.; Lv, Q. Rapid Detection of Ovarian Cancer from Immunized Serum Using a Quartz Crystal Microbalance Immunosensor. Asian Pac. J. Cancer Prev. APJCP 2012, 13, 3423–3426. [Google Scholar] [CrossRef]
- Joshi, H.C.; Kharkwal, H.; Kumar, A.; Gupta, P.K. Development of a Quartz Crystal Microbalance-Based Immunosensor for the Early Detection of Mesothelin in Cancer. Sens. Int. 2023, 4, 100248. [Google Scholar] [CrossRef]
- Onen, O.; Ahmad, A.A.; Guldiken, R.; Gallant, N.D. Surface Modification on Acoustic Wave Biosensors for Enhanced Specificity. Sensors 2012, 12, 12317–12328. [Google Scholar] [CrossRef]
- Huang, Y.; Das, P.K.; Bhethanabotla, V.R. Surface Acoustic Waves in Biosensing Applications. Sens. Actuators Rep. 2021, 3, 100041. [Google Scholar] [CrossRef]
- Alvarez, M.; Zinoviev, K.; Moreno, M.; Lechuga, L.M. Cantilever Biosensors. In Optical Biosensors; Elsevier: Amsterdam, The Netherlands, 2008; pp. 419–452. [Google Scholar] [CrossRef]
- Li, C.; Zhang, M.; Zhang, Z.; Tang, J.; Zhang, B. Microcantilever Aptasensor for Detecting Epithelial Tumor Marker Mucin 1 and Diagnosing Human Breast Carcinoma MCF-7 Cells. Sens. Actuators Chem. 2019, 297, 126759. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, L.; Liu, J.; Li, Z.; Li, J.; Zhou, W.; Wang, H.; Li, J.; Liu, D.; Yu, X. Multifunctional Microfluidic Chip for Cancer Diagnosis and Treatment. Nanotheranostics 2021, 5, 73. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A Microfluidic ExoSearch Chip for Multiplexed Exosome Detection towards Blood-Based Ovarian Cancer Diagnosis. Lab Chip 2016, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, M.M.; Chinnappan, R.; Alsager, O.A.; AlZabn, R.; Alhoshani, A.; Weber, K.; Cialla-May, D.; Popp, J.; Zourob, M. Mapping the Binding Region of Aptamer Targeting Small Molecule: Dabigatran Etexilate, an Anti-Coagulant. Talanta 2020, 218, 121132. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Arora, B.; Saxena, S.; Singh, S.; Palkar, P.; Goda, J.S.; Banerjee, R. Paper-Based Point of Care Diagnostics for Cancer Biomarkers. Sens. Diagn. 2024, 3, 504–535. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral Flow Assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Tripathi, P.; Sachan, M.; Nara, S. Novel ssDNA Ligand Against Ovarian Cancer Biomarker CA125 With Promising Diagnostic Potential. Front. Chem. 2020, 8, 400. [Google Scholar] [CrossRef]
- Kight, E.C.; Hussain, I.; Bowden, A.K.; Haselton, F.R. Recurrence Monitoring for Ovarian Cancer Using a Cell Phone-Integrated Paper Device to Measure the Ovarian Cancer Biomarker HE4/CRE Ratio in Urine. Sci. Rep. 2021, 11, 21945. [Google Scholar] [CrossRef]
- Liu, H.; Wu, M.-X.; Ding, S.-N. High-Density Gold Nanoparticles Implanted on Mg/Fe LDH Nanoflowers Assisted Lateral Flow Immuno-Dipstick Assay for Visual Detection of Human Epididymal Protein 4. Biosensors 2022, 12, 797. [Google Scholar] [CrossRef]
- Bayoumy, S.; Hyytiä, H.; Leivo, J.; Talha, S.M.; Huhtinen, K.; Poutanen, M.; Hynninen, J.; Perheentupa, A.; Lamminmäki, U.; Gidwani, K.; et al. Glycovariant-Based Lateral Flow Immunoassay to Detect Ovarian Cancer–Associated Serum CA125. Commun. Biol. 2020, 3, 460. [Google Scholar] [CrossRef]
- Ekman, M.; Salminen, T.; Raiko, K.; Soukka, T.; Gidwani, K.; Martiskainen, I. Spectrally Separated Dual-Label Upconversion Luminescence Lateral Flow Assay for Cancer-Specific STn-Glycosylation in CA125 and CA15-3. Anal. Bioanal. Chem. 2024, 416, 3251–3260. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Ding, S.-N. Duplex-Immunoassay of Ovarian Cancer Biomarker CA125 and HE4 Based Carbon Dot Decorated Dendritic Mesoporous Silica Nanoparticles. Analyst 2023, 148, 683–689. [Google Scholar] [CrossRef]
- Lee, C.-S.; Gwyther, R.E.A.; Freeley, M.; Jones, D.; Palma, M. Fabrication and Functionalisation of Nanocarbon-Based Field-Effect Transistor Biosensors. ChemBioChem 2022, 23, e202200282. [Google Scholar] [CrossRef]
- Eswaran, M.; Chokkiah, B.; Pandit, S.; Rahimi, S.; Dhanusuraman, R.; Aleem, M.; Mijakovic, I. A Road Map toward Field-Effect Transistor Biosensor Technology for Early Stage Cancer Detection. Small Methods 2022, 6, 2200809. [Google Scholar] [CrossRef]
- Sha, R.; Badhulika, S. Recent Advancements in Fabrication of Nanomaterial Based Biosensors for Diagnosis of Ovarian Cancer: A Comprehensive Review. Microchim. Acta 2020, 187, 181. [Google Scholar] [CrossRef]
- Panahi, A.; Ghafar-Zadeh, E. Emerging Field-Effect Transistor Biosensors for Life Science Applications. Bioengineering 2023, 10, 793. [Google Scholar] [CrossRef]
- Choi, J.; Seong, T.W.; Jeun, M.; Lee, K.H. Field-Effect Biosensors for On-Site Detection: Recent Advances and Promising Targets. Adv. Healthc. Mater. 2017, 6, 1700796. [Google Scholar] [CrossRef]
- Mansouri Majd, S.; Salimi, A. Ultrasensitive Flexible FET-Type Aptasensor for CA 125 Cancer Marker Detection Based on Carboxylated Multiwalled Carbon Nanotubes Immobilized onto Reduced Graphene Oxide Film. Anal. Chim. Acta 2018, 1000, 273–282. [Google Scholar] [CrossRef]
- Ji, H.; Wang, Z.; Wang, S.; Wang, C.; Zhang, K.; Zhang, Y.; Han, L. Highly Stable InSe-FET Biosensor for Ultra-Sensitive Detection of Breast Cancer Biomarker CA125. Biosensors 2023, 13, 193. [Google Scholar] [CrossRef]
- Shilla, P.; Verma, V.; Kumar, R.; Kumar, A. Effect of Band to Band Tunnelling (BTBT) on Multi-Gate Tunnel Field Effect Transistors (TFETs)-A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1033, 012018. [Google Scholar] [CrossRef]
- Choudhury, S.; Baishnab, K.L.; Guha, K.; Jakšić, Z.; Jakšić, O.; Iannacci, J. Modeling and Simulation of a TFET-Based Label-Free Biosensor with Enhanced Sensitivity. Chemosensors 2023, 11, 312. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular Imprinting: Perspectives and Applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Arabi, M.; Wang, X.; Wang, Y.; Chen, L. Molecular-Imprinting-Based Surface-Enhanced Raman Scattering Sensors. ACS Sens. 2020, 5, 601–619. [Google Scholar] [CrossRef]
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Ramanavicius, A.; Viter, R.; Ramanavicius, S. Molecularly Imprinted Polymers for the Determination of Cancer Biomarkers. Int. J. Mol. Sci. 2023, 24, 4105. [Google Scholar] [CrossRef]
- Kang, M.S.; Cho, E.; Choi, H.E.; Amri, C.; Lee, J.-H.; Kim, K.S. Molecularly Imprinted Polymers (MIPs): Emerging Biomaterials for Cancer Theragnostic Applications. Biomater. Res. 2023, 27, 45. [Google Scholar] [CrossRef]
- Liu, L.; Dong, C.; Li, X.; Li, S.; Ma, B.; Zhao, B.; Li, X.; Liang, Z.; Yang, K.; Zhang, L. Antibody-free Hydrogel with the Synergistic Effect of Cell Imprinting and Boronate Affinity: Toward the Selective Capture and Release of Undamaged Circulating Tumor Cells. Small 2020, 16, 1904199. [Google Scholar] [CrossRef]
- Diken Gür, S.; Bakhshpour, M.; Denizli, A. Nanoscale SPR Sensor for the Ultrasensitive Detection of the Ovarian Cancer Marker Carbohydrate Antigen 125. New J. Chem. 2022, 46, 7263–7270. [Google Scholar] [CrossRef]
- Rebelo, T.S.C.R.; Costa, R.; Brandão, A.T.S.C.; Silva, A.F.; Sales, M.G.F.; Pereira, C.M. Molecularly Imprinted Polymer SPE Sensor for Analysis of CA-125 on Serum. Anal. Chim. Acta 2019, 1082, 126–135. [Google Scholar] [CrossRef]
- Viswanathan, S.; Rani, C.; Ribeiro, S.; Delerue-Matos, C. Molecular Imprinted Nanoelectrodes for Ultra Sensitive Detection of Ovarian Cancer Marker. Biosens. Bioelectron. 2012, 33, 179–183. [Google Scholar] [CrossRef]
- Büyüktiryaki, S.; Say, R.; Denizli, A.; Ersöz, A. Phosphoserine Imprinted Nanosensor for Detection of Cancer Antigen 125. Talanta 2017, 167, 172–180. [Google Scholar] [CrossRef]
- Bast, R.C.; Badgwell, D.; Lu, Z.; Marquez, R.; Rosen, D.; Liu, J.; Baggerly, K.A.; Atkinson, E.N.; Skates, S.; Zhang, Z.; et al. New Tumor Markers: CA125 and Beyond. Int. J. Gynecol. Cancer 2005, 15 (Suppl. 3), 274–281. [Google Scholar] [CrossRef]
- Tai, J.; Fan, S.; Ding, S.; Ren, L. Gold Nanoparticles Based Optical Biosensors for Cancer Biomarker Proteins: A Review of the Current Practices. Front. Bioeng. Biotechnol. 2022, 10, 877193. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Q.; Xiao, Z.; Liu, M.; Zhu, Y.; Chen, Q.; Li, Y.; Ai, K. Nanomaterial-Based Biosensor Developing as a Route toward in Vitro Diagnosis of Early Ovarian Cancer. Mater. Today Bio 2022, 13, 100218. [Google Scholar] [CrossRef] [PubMed]
- Yonet-Tanyeri, N.; Ahlmark, B.Z.; Little, S.R. Advances in Multiplexed Paper-Based Analytical Devices for Cancer Diagnosis: A Review of Technological Developments. Adv. Mater. Technol. 2021, 6, 2001138. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-L.; Gong, T.-T.; Liu, F.-H.; Chen, H.-Y.; Xiao, Q.; Hou, Y.; Huang, Y.; Sun, H.-Z.; Shi, Y.; Gao, S.; et al. Artificial Intelligence Performance in Image-Based Ovarian Cancer Identification: A Systematic Review and Meta-Analysis. EClinicalMedicine 2022, 53, 101662. [Google Scholar] [CrossRef] [PubMed]
- Cunnea, P.; Gorgy, T.; Petkos, K.; Gowers, S.A.N.; Lu, H.; Morera, C.; Wu, W.; Lawton, P.; Nixon, K.; Leong, C.L.; et al. Clinical Value of Bioelectrical Properties of Cancerous Tissue in Advanced Epithelial Ovarian Cancer Patients. Sci. Rep. 2018, 8, 14695. [Google Scholar] [CrossRef]


| Biomarker | Materials Used | Method of Detection | Linear Range | LOD | Reference |
|---|---|---|---|---|---|
| CA-125 | Antibody-functionalized nanosized GO- | Fluorescence | 0.1–10 U/mL | 0.01 U/mL | [65] |
| CA-125 | Nanogold thin film doped into a sol−gel matrix | Fluorescence quenching | 2.0−127.0 U/mL | 1.45 U/mL | [66] |
| CA-125 | Ni-phthalocyanine complex doped in polystyrene matrix | Fluorescence quenching | 0.001–127 U/mL | 0.0001 | [69] |
| CA-125 | Ni and Cd nanoclusters | Fluorescence | 0.0005–40 U/mL | 50 µU/mL | [70] |
| CA-125 | Carbon Quantum dots entrapped in polymethyl methacrylate (PMMA) matrix | FRET | 0.01–129 U/mL | 0.66 U/mL | [72] |
| CA-125 | Red-emitting DNA-AgNCs with CA-125 aptamer (rDNA2-AgNCs-apta2)-Target induced AuNPs aggregation and fluorescence recovery from surface plasmon-enhanced energy transfer (SPEET) | Fluorescence | 0.01 and 2.0 U/mL | 0.015 U/mL | [83] |
| CA-125 | Gold–silver alloy film-based SPR (AuAg-SPR) sensor | SPR | 0.1–10 U/mL | 0.1 U/mL | [84] |
| CA-125 | Nanogold-functionalized copper–cobalt oxide nanosheets (CuCo-ONSs@AuNPs) as nanocomposites | Electrochemical | 1 × 10−7 U/mL to 1 × 10−3 U/mL | 3.9 × 10−8 U/mL | [100] |
| CA-125 | Screen-printed carbon electrodes modified with polytoluidine blue (PTB)/AuNps | Electrochemical | 5–100 pg/mL | 1.20 pg/mL | [101] |
| CA-125 | Hierarchical microporous carbon material fabricated from waste coffee grounds (WCG) modified screen-printed electrode decorated with AuNps | Electrochemical | 0.5–50.0 U/mL | 0.4 U/mL | [103] |
| CA-125 | Graphitic carbon nitrides/molybdenum disulfide/magnetic nanoparticles (g-C3N4/MoS2/Fe3O4) immobilized glassy carbon electrodes (GCEs) | Electrochemical | 2–10 U/mL | 0.215 U/mL | [104] |
| CA-125 | Eu metal–organic framework-loaded isoluminol-Au nanoparticles (Eu MOF@Isolu-Au NPs)/carboxyl-functionalized CdS quantum dots and N-doped porous carbon-anchored Cu single-atom catalyst | Electrochemiluminescence (ECL) | 0.005–500 ng/mL | 0.37 pg/mL | [106] |
| CA-125 | Carbon ink/carbon dot/zine oxide (C-ink/CD/ZnO)/silver@polypyrrole (Ag@PPy) | Electrochemical | 1 ag/mL–100 ng/mL | 0.1 fg/mL | [107] |
| CEA | Deal Aptamer labeled with metalloporphyrinic iron-based metal–organic framework, hemin@MIL-88B (Fe)—Apt1 and luminol-Ap2- Fe3O4@SiO2 adsorbed on magnetic carbon nanotubes (MCNTs) | Chemiluminescence | 0.01–100 ng/mL | 0.0015 ng/mL | [80] |
| CEA | Green-emitting DNA-AgNCs with CEA aptamer (gDNA1-AgNCsapta1)-Target induced AuNPs aggregation and fluorescence recovery from surface plasmon-enhanced energy transfer (SPEET) | Fluorescence | 0.01–0.9 ng/mL | 7.5 pg/mL | [83] |
| CEA | Antibody-functionalized nanosized GO- | Fluorescence | 10–100 pg/mL | ~1 pg/mL | [65] |
| platelet-derived growth factor (PDGF) | Aptamers conjugated AuNPs | Salt-induced AuNps aggregation colorimetry | 0.01–10 μg/mL | 0.01 μg/mL | [82] |
| lysophosphatidic acid (LPA) | Solid supported actin–gelsolin/dye complex | Action-dye displacement by LPA | 0–50 µM | 5 µM | [68] |
| lysophosphatidic acid (LPA) | Gelsolin–actin affinity-based system | Electrochemical | 0.01–10 µM | 0.7 µM | [95] |
| HE4 | Antibody-functionalized nanosized GO- | Fluorescence | 10–100 pg/mL | ~1 pg/mL | [65] |
| HE4 | Antibody-conjugated gold chip coated with cysteamine | Surface plasmon resonance imaging (SPRi) | 2–120 pM | 2 pM | [86] |
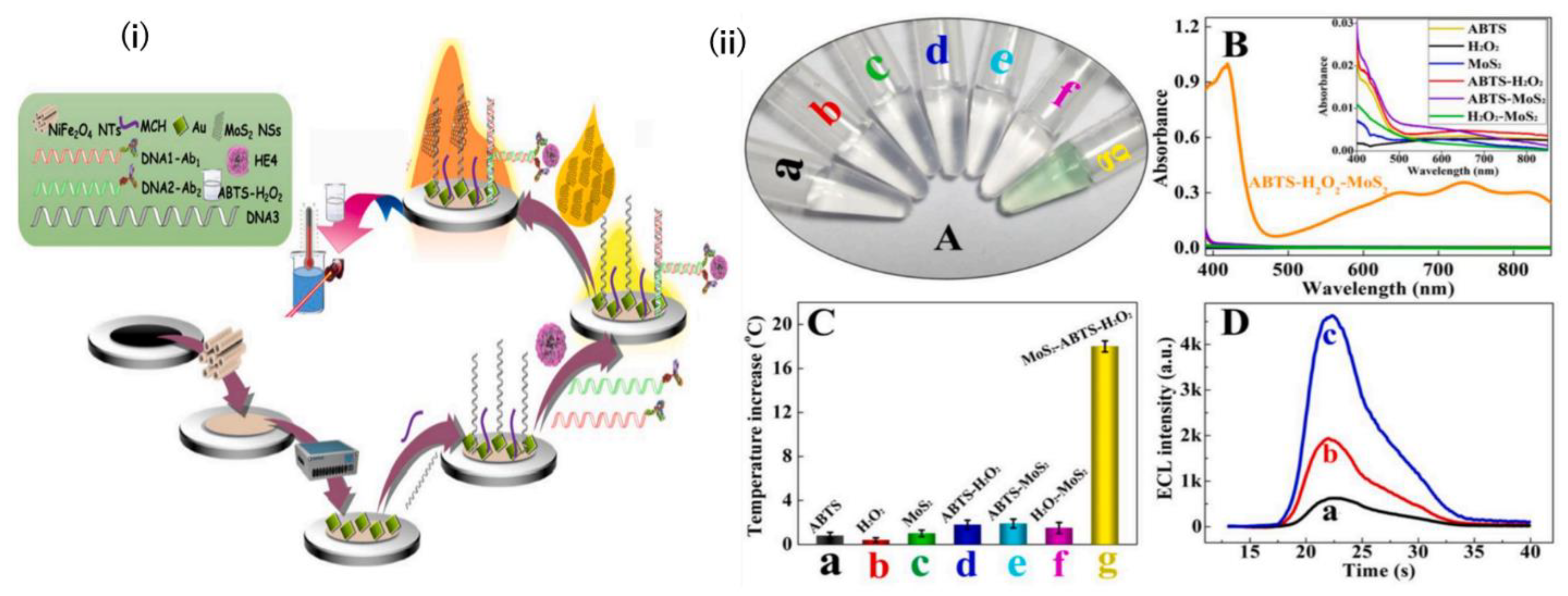
| HE4 | NiFe2O4 nanotubes (NTs)/Au nanoparticles (ed-Au NPs)/Thiolated captured DNA/Anti HER4 linked complementary DNA | Electrochemiluminescence (ECL) | 10−6 ng/mL–10 ng/mL | 3 × 10−7 ng/mL | [91] |
| HE4 | Eu metal–organic framework-loaded isoluminol-Au nanoparticles (Eu MOF@Isolu-Au NPs)/carboxyl-functionalized CdS quantum dots and N-doped porous carbon-anchored Cu single-atom catalyst | Electrochemiluminescence (ECL) | 0.005–500 ng/mL | 1.58 pg/mL | [106] |
| APF | Antibody-functionalized nanosized GO- | Fluorescence | 10–100 pg/mL | ~1 pg/mL | [65] |
| CYPA | SiO2-encapsulated Au star@AgAu yolk shell nanostructure (YSNS) | Surface-enhanced Raman scattering | 10−7 μg/mL–10−2 μg/mL | 7.76 × 10−10 μg/mL | [90] |
| MicroRNA (miRNA-21, miRNA-141, and miRNA-200a) | Silicon-on-insulator structures (SOI-NWs) | Electrochemical | 1.1 × 10−17 M–1.1 × 10−14 M | 1.1 × 10−16 M | [92] |
| Exosomes | Entropy-driven strand displacement reaction (EDR) process and DNAzymes-induced cleavages | Electrochemical | - | 30 particles/μL | [98] |
| Exosomes | Metal–organic frameworks assembled “double hook”-type aptamer | Electrochemical | 31 to 3.1 × 106 particles/μL | 12 particles/μL | [94] |
| mesothelin | Self-assembled monolayer cysteamine chip | Quartz Crystal Microbalance (QCM) | 100 pg/mL–50 ng/mL | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abul Rub, F.; Moursy, N.; Alhedeithy, N.; Mohamed, J.; Ifthikar, Z.; Elahi, M.A.; Mir, T.A.; Rehman, M.U.; Tariq, S.; Alabudahash, M.; et al. Modern Emerging Biosensing Methodologies for the Early Diagnosis and Screening of Ovarian Cancer. Biosensors 2025, 15, 203. https://doi.org/10.3390/bios15040203
Abul Rub F, Moursy N, Alhedeithy N, Mohamed J, Ifthikar Z, Elahi MA, Mir TA, Rehman MU, Tariq S, Alabudahash M, et al. Modern Emerging Biosensing Methodologies for the Early Diagnosis and Screening of Ovarian Cancer. Biosensors. 2025; 15(4):203. https://doi.org/10.3390/bios15040203
Chicago/Turabian StyleAbul Rub, Farah, Naseel Moursy, Nouf Alhedeithy, Juraij Mohamed, Zainab Ifthikar, Muhammad Affan Elahi, Tanveer Ahmed Mir, Mati Ur Rehman, Saima Tariq, Mubark Alabudahash, and et al. 2025. "Modern Emerging Biosensing Methodologies for the Early Diagnosis and Screening of Ovarian Cancer" Biosensors 15, no. 4: 203. https://doi.org/10.3390/bios15040203
APA StyleAbul Rub, F., Moursy, N., Alhedeithy, N., Mohamed, J., Ifthikar, Z., Elahi, M. A., Mir, T. A., Rehman, M. U., Tariq, S., Alabudahash, M., Chinnappan, R., & Yaqinuddin, A. (2025). Modern Emerging Biosensing Methodologies for the Early Diagnosis and Screening of Ovarian Cancer. Biosensors, 15(4), 203. https://doi.org/10.3390/bios15040203






