Abstract
Cardiovascular diseases have long been a major challenge to human health, and the treatment differences caused by individual variability remain unresolved. In recent years, personalized cardiovascular drug therapy has attracted widespread attention. This paper reviews the strategies for achieving personalized cardiovascular drug therapy through traditional dynamic monitoring and multidimensional data integration and analysis. It focuses on key technologies for dynamic monitoring, dynamic monitoring based on individual differences, and multidimensional data integration and analysis. By systematically reviewing the relevant literature, the main challenges in current research and the proposed potential directions for future studies were summarized.
1. Introduction
Cardiovascular diseases are a group of common chronic conditions that affect the function of the heart and blood vessels, including coronary heart disease [1], hypertension [2], and heart failure [3]. These diseases often lead to blood circulation disorders, which, in severe cases, may result in strokes [4] or even life-threatening situations [5]. Due to their high mortality and disability rates, cardiovascular diseases pose a significant burden on individual health and societal healthcare resources [6]. Comprehensive management of cardiovascular diseases, from prevention to follow-up, is crucial, as it not only helps reduce incidence rates but also improves patients’ long-term prognosis [7]. In recent years, cardiovascular diseases have shown trends of steadily increasing incidence, a noticeable shift toward younger populations, a higher proportion of comorbidities with other chronic diseases, and insufficient treatment adherence [8,9,10]. These factors further complicate this public health issue. Against this backdrop, the prevention and treatment of cardiovascular diseases are of paramount importance [11,12].
Pharmacological treatment of cardiovascular diseases has achieved remarkable success in reducing mortality and improving patient prognosis. However, its complexity and challenges remain prominent. This complexity spans various stages, including disease diagnosis, treatment, and efficacy monitoring, further underscoring the necessity and importance of personalized treatment [13]. In terms of diagnosis, the etiology and clinical manifestations of cardiovascular diseases are highly complex, and some patients may present with atypical symptoms, which poses significant challenges to early diagnosis [14,15]. In terms of treatment, pharmacological therapy is one of the most common interventions. However, due to the narrow therapeutic window of certain drugs, even slight deviations in dosage can lead to severe consequences, imposing stringent requirements on treatment precision. Consequently, frequent efficacy monitoring and treatment adjustments based on monitoring results become essential [16,17].
Nevertheless, drug efficacy is often influenced by multiple factors, such as the patient’s genetic background, metabolic capacity, and underlying comorbidities [18,19,20]. These factors result in significant variability in the sensitivity and efficacy of the same medication among different patients. While some patients may experience significant therapeutic benefits, others may encounter insufficient efficacy or severe adverse reactions [21]. Furthermore, for cardiovascular disease patients with comorbidities (e.g., diabetes), combination therapy is a common treatment strategy. However, drug–drug interactions may reduce efficacy or exacerbate adverse effects, adding further complexity to treatment [22]. At the same time, traditional efficacy monitoring methods also have certain limitations. For example, some patients may have a fear of or discomfort with blood sampling, especially those sensitive to needles. Frequent invasive blood sampling for monitoring may reduce patient adherence [23,24]. Therefore, for medications requiring real-time dynamic dose adjustment, the intermittent monitoring methods widely used in clinical practice (e.g., periodic blood tests) may be inadequate for capturing real-time changes in drug concentrations and efficacy. For example, adjusting the dosage of anticoagulant medications typically relies on monitoring the international normalized ratio (INR). Generally, in the treatment of venous thromboembolism, INR is monitored daily during the initial phase of treatment, and the warfarin dosage is adjusted accordingly. Once INR stabilizes, the monitoring interval can be extended to a maximum of 12 weeks after three months [25]. Medications like warfarin require precise dosage adjustments but are easily influenced by factors such as diet, other medications, and genetic factors. Although experts recommend that patients’ time in therapeutic range (TTR) should reach at least 65%, this is rarely achieved in practice [26]. This delay in monitoring restricts the ability to dynamically adjust treatment plans, potentially resulting in therapeutic adjustments lagging behind the patient’s actual needs [26,27,28,29]. A study has shown that patients who perform weekly self-testing (PST) have significantly higher TTR compared to those who test irregularly (every 2–4 weeks) (74% vs. 68.9%, p < 0.0001), with a lower incidence of extreme INR values [30]. This indicates that timely and frequent monitoring is more conducive to INR adjustment.
In summary, the complexity of pharmacological treatment, the challenges in diagnosis, and the limitations of traditional monitoring methods in cardiovascular disease management all highlight the core value of personalized treatment [31,32]. By dynamically monitoring patients’ physiological biomarkers and related data, and integrating multidimensional data analysis to enable dose adjustments, personalized treatment offers a crucial pathway forward (Figure 1). It allows for the development of optimized treatment plans tailored to the specific conditions of each patient, thereby improving efficacy, reducing adverse reactions, and enhancing long-term prognosis. This represents not only a key direction in the development of cardiovascular disease treatment but also a critical breakthrough path in addressing current complexities and challenges [32,33]. This review will systematically elaborate on the current status and prospects of dynamic monitoring in cardiovascular pharmacological treatment from the following four aspects: key technologies for dynamic monitoring, dynamic monitoring based on individual differences, multidimensional data integration and analysis, as well as challenges and future developments. This review aims to explore the critical pathways and strategies for achieving personalized pharmacological treatment, providing new insights and inspiration for the precise treatment of cardiovascular diseases.
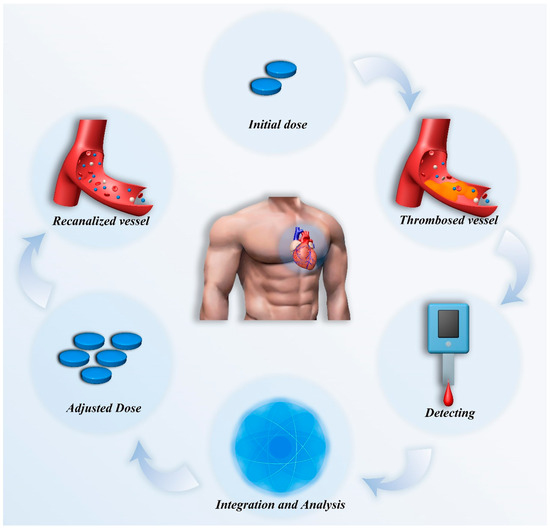
Figure 1.
Schematic diagram of dynamic monitoring combined with multidimensional data analysis for adjusting medication in thrombotic patients as an example.
2. Key Technologies for Dynamic Monitoring
Monitoring technologies play a crucial role in cardiovascular drug monitoring and efficacy evaluation. Especially in clinical applications, these technologies have been widely adopted and have had a profound impact [34,35]. At present, the key technologies for dynamic monitoring of cardiovascular drug efficacy include sensor technology, microfluidic chip technology, and the application of nanomaterials [36]. These technologies enable high-sensitivity and multi-parameter detection [37,38]. As technology continues to advance, these methods have not only improved monitoring efficiency but also significantly expanded their potential applications in personalized medicine. Continuous monitoring, real-time monitoring, flexibility, portability, accuracy, and sensitivity have gradually become key characteristics that enable monitoring technologies to play a crucial role in personalized cardiovascular treatment. Through these characteristics, monitoring technology can more comprehensively collect individualized patient information, thereby providing a solid foundation for accurate diagnosis and the scientific development of personalized treatment plans [39].
Based on the sensor principle classification [40], this article will further explore its dynamic monitoring application in personalized drug therapy. Some studies of core sensing technologies such as electrochemical sensors [41], optical sensors [42], magnetic sensors [43], and pressure sensors [44], with their high sensitivity [45], rapid response [45], and non-invasive characteristics [46], not only enable real-time monitoring of drug concentrations [47], metabolites [48], and key indicators in the blood [28] but also assess the regulatory effects of drugs on cardiovascular function [49]. With the advancement of material science and microscale analytical techniques, these methods provide more precise and efficient solutions for drug efficacy evaluation and adverse reaction monitoring [50,51]. The application of these technologies (Figure 2) significantly enhances the precision and safety of cardiovascular drug treatments, offering patients more efficient and convenient monitoring methods [35,52].
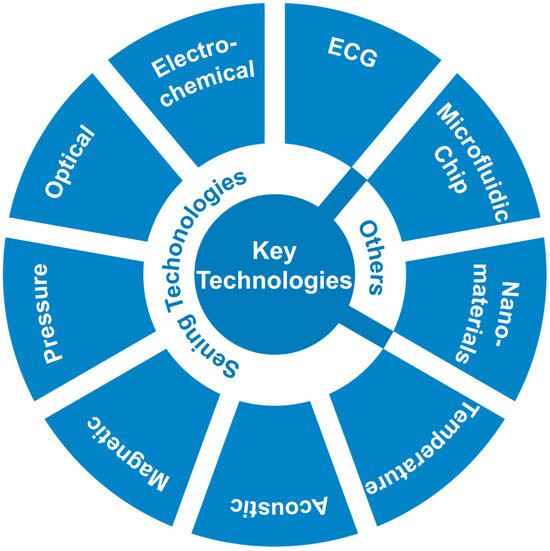
Figure 2.
Key technologies that are used for dynamic monitoring.
2.1. Sensing Technologies
2.1.1. ECG Sensor
Electrocardiogram (ECG) sensors are typically based on the principle of electrode detection, measuring a patient’s cardiac activity data (such as rhythm and waveforms) by recording the electrical signals generated by the heart with each beat [53]. They are often implemented as electrocardiographs, capable of collecting and analyzing ECG data in real-time. Clinically, ECG sensors are widely used to diagnose arrhythmias and other cardiac conditions, as well as to monitor the patient’s heart health [52]. Additionally, in the evaluation of cardiovascular drugs, ECG sensors can detect the effects of medications on cardiac electrical activity, helping to identify potential arrhythmias or cardiotoxic reactions [54].
Compared to indirect measurement sensors (such as devices that monitor peripheral physiological signals through photoplethysmography (PPG) sensors to indirectly measure cardiac activity), ECG sensors can directly detect the electrical activity of the heart, offering higher signal accuracy [55]. Continuous ECG monitoring of patients can more comprehensively capture individual variations in cardiac electrical activity. Currently, the commonly used clinical method, Ambulatory ECG Monitoring, enables continuous recording and analysis of patient data for 24 h or longer through portable devices (such as Holter monitors or wearable ECG sensors), providing data support for personalized treatment. This includes diagnosing arrhythmias, evaluating drug efficacy, or predicting cardiovascular events [56]. However, during the data collection process, signal quality may be affected by factors such as motion artifacts [57], changes in skin resistance [58], and environmental electromagnetic interference [59], which can disrupt the collection and analysis of personalized physiological data. For instance, signal artifacts caused by poorly adhered electrodes may be mistakenly identified by computer algorithms as atrial fibrillation (AF), potentially leading to misdiagnosis [60]. Therefore, optimizing sensor design and improving signal processing algorithms are effective approaches to enhancing the reliability and accuracy of the data.
ECG sensors, as an essential medical device, are widely used in hospitals and emergency settings with the traditional 12-lead ECG still serving as the gold standard for diagnosing cardiovascular diseases such as arrhythmia and myocardial ischemia. According to a relevant clinical guideline [61], the traditional 12-lead ECG continues to play a vital role in diagnosing atrial fibrillation (AF). At the same time, the guideline mentions the potential use of continuous monitoring devices, such as ambulatory ECG, for evaluating and diagnosing AF in selected patients, reflecting the personalized nature of AF diagnostic assessments. Furthermore, implantable ECG devices, such as implantable loop recorders, are mentioned as advanced technologies that may assist in detecting subclinical AF. For atrial tachycardia after pulmonary vein isolation, recent studies employing continuous implantable recorders to monitor surgical procedures have identified the incidence of early postoperative arrhythmia recurrence and confirmed a correlation between early and late recurrences [61]. This demonstrates the potential of continuous implantable recorders to help physicians gain a more comprehensive understanding of postoperative arrhythmia recurrence patterns, thereby providing a basis for more precise treatment and management. In addition, a clinical guideline [62] emphasizes the importance of selecting appropriate monitoring tools based on the specific conditions of different patients. For instance, for patients who have experienced systemic thromboembolic events but have no known history of atrial fibrillation, implantable cardiac monitors are considered a reasonable choice to improve detection sensitivity (Recommendation Class: 2a, Evidence Level: B-R). For patients requiring AF monitoring, the use of high-quality consumer ECG monitoring devices is recommended to track recurrence (Recommendation Class: 2a, Evidence Level: B-R) [62].
2.1.2. Electrochemical Sensors
Electrochemical sensors achieve precise measurement of analytes by detecting changes in electrochemical signals, such as current, caused by target molecules [63]. In recent years, this technology has been widely applied in cardiovascular drug monitoring, particularly excelling in real-time monitoring of drug metabolism and key blood parameters [64]. Electrochemical sensors can detect the concentration of cardiovascular drugs (e.g., anticoagulants), providing critical guidance for optimizing drug dosages [65]. Additionally, they can monitor metabolites produced during drug metabolism, aiding in the assessment of patient responses to medications [66]. By immobilizing specific enzymes (e.g., glucose oxidase) on the sensor surface [67], electrochemical sensors can accurately detect metabolites produced during the metabolism of anticoagulants like warfarin [68]. In evaluating the effects of drugs on cardiac function, electrochemical sensors can monitor dynamic changes in electrolytes such as potassium and sodium ions in the blood in real-time [69,70]. Moreover, by detecting reactive oxygen species levels in the blood, these sensors can assess the regulatory effects of cardiovascular drugs on oxidative stress [71]. Notably, integrating electrochemical sensors into portable devices enables point-of-care testing (POCT), providing efficient and reliable technical support for clinicians to quickly adjust drug dosages [72].
Through an efficient electrochemical transduction mechanism, high-sensitivity and fast-response electrochemical sensors with clinical application potential can be developed. Romele et al. developed an ion sensing amplifier based on complementary organic electrochemical transistors (OECT), capable of real-time, multi-scale, high-sensitivity detection of ion concentration changes, with a sensitivity larger than 2300 mV V−1 dec−1 and a response time of 11 s [73]. Song et al. developed a wireless, non-invasive smart contact lens that integrates an electrochemical cholesterol biosensor with NFC wireless communication technology to enable real-time monitoring by detecting cholesterol levels in tear fluid. Animal experiments validated the correlation between cholesterol levels in tear fluid and those in blood, providing a novel non-invasive solution for the diagnosis and monitoring of hyperlipidemia and related cardiovascular diseases. Compared to traditional cholesterol detection methods, this smart contact lens offers a more convenient and continuous data support system for personalized monitoring through non-invasive, real-time cholesterol level tracking [74]. Electrochemical biosensing platforms play a critical role in precision medicine by utilizing biological receptors such as antibodies, aptamers, and enzymes to detect molecular biomarkers at different omics levels beyond metabolic markers. This enhances the resolution of individualized diagnosis and treatment for patients. With their design diversity and flexibility, these platforms can simultaneously detect drugs and biomarkers related to efficacy or toxicity (e.g., infection, inflammation, kidney function), thereby optimizing treatment plans and enabling personalized precision therapy [75]. Currently, electrochemical sensors in clinical cardiovascular disease measurements may be affected by the complexity of biological samples (e.g., interfering substances in the blood [76]) and sensor stability (e.g., sensitivity degradation due to prolonged use [76,77]), thereby affecting treatment. For instance, a decline in sensor stability (such as reduced sensitivity over time [76]) may lead to inaccurate follow-up data for patients, which, in turn, could affect physicians’ assessment of disease progression and adjustments to personalized treatment plans. Therefore, it is necessary to enhance measurement accuracy and reliability by optimizing sensor surfaces (e.g., anti-interference [78]) and improving sensor materials (e.g., using nanomaterials to enhance stability and sensitivity [77]).
Cardiovascular disease patients often have comorbid diabetes, making blood glucose monitoring critically important. Electrochemical sensors also play a significant role in this area, such as using electrochemical sensors to detect HbA1c [79]. According to a relevant international clinical guideline, diabetes screening is recommended for all cardiovascular disease patients, as undiagnosed diabetes is common in this population. The guideline points out the importance of optimizing blood glucose control after cardiovascular events and mentions the need for further research to determine whether optimizing blood glucose control in cardiovascular disease patients with diabetes, through technologies like continuous glucose monitoring, can improve clinical outcomes. Implementing various blood glucose management strategies is particularly important for diabetic patients at high cardiovascular risk. These strategies include setting personalized HbA1c targets, minimizing hypoglycemic episodes, and limiting blood glucose fluctuations [80]. This underscores the significant potential of developing electrochemical sensors for continuous HbA1c monitoring in advancing personalized blood glucose control in the future.
2.1.3. Optical Sensors
Optical sensors analyze target molecules by detecting changes in light absorption, reflection, scattering, or fluorescence signals [81]. In recent years, these sensors have been widely applied in evaluating the efficacy of cardiovascular drugs, demonstrating the significant value of their potential application [82]. They can assess the regulatory effects of lipid-lowering drugs on cholesterol and lipid levels in the blood [83], while fluorescence labeling techniques enable dynamic tracking of drug distribution and metabolism within the body [84]. For example, fluorescence labeling can be used to monitor the improvement of vascular endothelial function caused by antihypertensive drugs [85]. Additionally, integrated into wearable devices, optical sensors can also provide real-time monitoring of heart rate and blood oxygen levels, indirectly assessing the cardiovascular protective effects of drugs [86].
Compared to electrochemical sensors [87], optical sensors do not require the use of chemical reagents or electrolyte solutions, avoiding the complexity of chemical reactions while enabling non-invasive operation [88]. Detection of cTnI can be used to identify late-stage AMI patients. Yu Li et al. developed an ultrasensitive detection method based on aptamer-based rolling circle amplification (RCA) and graphene oxide-based fluorescence resonance energy transfer (GO-FRET). This method can detect cardiac troponin I (cTnI) with a detection limit of 14.40 pg/mL, offering high sensitivity and specificity, making it suitable for clinical sample analysis [89]. In addition, it is reported that a graphene quantum dots fluorescence resonance energy transfer (FRET)-based sensor was developed for the rapid detection of the myocardial infarction (heart attack) biomarker cTnI. This sensor achieves specific detection through the covalent conjugation of antibodies with GQDs, with a detection limit as low as 0.192 pg/mL and a detection time of only 10 min, featuring high sensitivity and rapid response [90]. The high sensitivity and rapid response of this technology are of great significance for achieving early and accurate diagnosis of myocardial infarction, as well as providing timely and targeted treatment plans for patients. Overall, factors affecting the measurement accuracy of optical sensors mainly include light interference from the environment and potential interfering substances in the sample (such as non-target molecules or impurities) [91]. Effective solutions to these interference factors (such as light shielding techniques [92] and enhanced specificity recognition [93]) can significantly improve the accuracy and reliability of measurement data, providing individuals with more precise information. Among these, optical fiber sensors, by integrating recognition elements, can efficiently detect disease-related biomarkers, enabling real-time diagnosis. The flexibility and compactness of optical fibers allow them to support wearable or implantable devices, enabling continuous monitoring of human health information and showing promising prospects in precision medicine. However, fiber-optic biosensors face challenges such as low throughput, bulky device size, and potential risks of harm to the human body. In the future, driven by advancements in medical learning algorithms, novel materials, and optical engineering, next-generation fiber-optic sensors are expected to deliver superior diagnostic and therapeutic performance, better meeting the demands of precision medicine [94].
Myocardial infarction (heart attack) is commonly associated with coronary artery diseases, including acute coronary syndrome (ACS) [95]. Optical sensors can be used to detect related biomarkers (such as cTnI). A relevant clinical guideline [96] mentions the importance of high-sensitivity cardiac troponin (hs-cTn) detection in assessing patients with ACS. Additionally, the guideline mentions there is a trial [97] utilizing intravascular ultrasound (IVUS) and optical coherence tomography (OCT) to guide percutaneous coronary intervention (PCI), which, compared to angiography-guided approaches, can reduce target vessel failure. The study and practice demonstrate that optical sensors not only play a critical role in detecting biomarkers (such as cTnI) in ACS patients but also show extensive application and development prospects in intravascular imaging and interventional treatment guidance. This multidimensional application further underscores the potential of optical technology in the diagnosis and treatment of cardiovascular diseases [96].
2.1.4. Pressure Sensors
Pressure sensors are typically based on principles such as piezoelectricity [98], capacitance [99], or strain gauges [100] and are used to monitor pressure changes within blood vessels or heart chambers, thereby evaluating the therapeutic effects of cardiovascular drugs [101]. Commonly used clinical pressure sensors include catheter-based pressure sensors [102] and cuff-based blood pressure sensors [103]. In addition, implantable pressure sensors [104] have also demonstrated certain application potential. For instance, implantable pressure sensors enable long-term monitoring of intracardiac pressure, thereby having the potential to evaluate the efficacy of drugs for heart failure [104].
Compared to optical and magnetic sensors, pressure sensors are characterized by their direct contact with physical pressure, making them suitable for contact-based measurements and capable of accurately reflecting changes in gas or liquid pressure [105]. Li et al. developed a high-performance flexible pressure sensor based on the soft strain effect, utilizing a flexible conductive film stuck on a hollow electrode as a soft strain gauge to generate and amplify tensile strain. This sensor can be used to detect pulse wave signals from deep and peripheral arteries. It features ultra-high sensitivity (~636.8 kPa−1), a wide detection range (0.1–56 kPa), and excellent stability (no significant performance degradation after 10,000 cycles at 10 kPa). It addresses the issue of sensitivity reduction under high pressure in traditional pressure sensors, providing a new approach for the early diagnosis of cardiovascular diseases and health monitoring [106]. The hemodynamic signals of peripheral arteries tend to weaken as blood flows toward the extremities, primarily due to the progressive narrowing of blood vessels and the rise in flow resistance. Moreover, for patients with peripheral vascular diseases, their blood flow characteristics are often more complex, which places higher demands on the sensitivity of sensors [107]. Sensors with excellent performance (ultra-high sensitivity and wide detection range [106]) are expected to address the limitations of traditional devices in detecting weak signals and the inconvenience of long-term monitoring. These sensors offer new possibilities for real-time, high-precision monitoring of pulse waves and blood pressure, enabling more sensitive capture of peripheral arterial signals. This contributes to the early diagnosis of cardiovascular diseases such as atherosclerosis, helps avoid missed diagnoses caused by insufficient detection, and provides reliable support for personalized health management. In addition, the existence of white-coat hypertension and masked hypertension further highlights the importance of sensors capable of precise long-term blood pressure monitoring. These sensors can provide technological support for better addressing individual differences in hypertension diagnosis [108]. Currently, pressure sensor measurements are influenced by factors such as the balance between sensitivity and detection range [109], mechanical performance and stability [110], as well as material and structural design [111]. In the future, through the optimization of materials [112], structural design [113,114], and signal processing technologies [115], it is expected that the measurement accuracy and stability of pressure sensors can be significantly improved, meeting the needs of multi-scenario applications.
A relevant clinical guideline [116] mentions that wearing an ambulatory blood pressure monitor (ABPM) is commonly used to supplement blood pressure readings obtained in a clinical setting. The device is typically configured to measure blood pressure every 15 to 30 min during the day and every 15 min to 1 h at night [117]. ABPM provides estimates of average blood pressure over the monitoring period, as well as daytime and nighttime averages. It can also identify the extent of nocturnal ‘dipping’, identify the early-morning BP surge pattern, estimate BP variability, and help identify symptomatic hypotension [118]. Moreover, many newer oscillometric devices can automatically inflate multiple times (at intervals of from 1 to 2 min), reducing disruption to the patient. While traditional methods remain dominant, there is growing evidence supporting the use of automated office blood pressure measurement techniques [119]. Additionally, the guideline recommends the use of ABPM or HBPM (home blood pressure monitoring) in specific cases. For example, for adults with white coat hypertension, regular monitoring using ABPM or HBPM is recommended to detect progression to sustained hypertension (Class of Recommendation [COR]: IIa, Level of Evidence [LOE]: C-LD) [116]. This reflects a shift in blood pressure monitoring from traditional office-based measurements to dynamic and automated methods, with greater emphasis on improving continuity and accuracy in measurements. Furthermore, by utilizing dynamic blood pressure monitoring and automated devices, it becomes possible to more precisely capture individual blood pressure variation patterns, thereby guiding personalized treatment plans.
2.1.5. Magnetic Sensors
Magnetic sensors, by detecting magnetic nanoparticles or changes in magnetic fields, have become a cutting-edge technology for the precise identification of target substances, molecules, or biomarkers [120]. Clinical magnetocardiography (MCG) [121] and magnetoencephalography (MEG) [122]) can be used to detect weak biomagnetic fields. These techniques evaluate the activity of the heart and brain by detecting the subtle magnetic fields generated by their electrical activity, with magnetic sensors being an indispensable component of these technologies [123].
Previously, superconducting quantum interference devices (SQUIDs) have been a key technology supporting MCG. Magnetoresistive (MR) sensor technology has now achieved sensitivities suitable for MCG. Magnetoresistive materials exhibit resistance changes in response to external magnetic fields, and, when incorporated into sensor designs, they can provide extremely high sensitivity, reaching 5146%/mT [124]. In addition, it is reported that a high-sensitive 1D photonic crystal magnetic field sensor leverages Fano/Tamm resonance in the far-infrared region, offering new possibilities for magnetic field monitoring in the biomedical field. This sensor demonstrated a maximum sensitivity of 57 nm/Tesla under magnetic field strengths ranging from 20 to 140 Tesla [125]. Such high sensitivity reflects the sensor’s strong responsiveness to changes in magnetic field strength, showcasing its great potential in detecting subtle magnetic field variations. High-sensitivity magnetic sensors hold promising prospects in personalized medical devices, such as detecting weak biomagnetic fields, enabling more precise diagnostic and therapeutic solutions. However, due to their high sensitivity to magnetic field changes, magnetic sensors are prone to electromagnetic interference (EMI). To mitigate the impact of external EMI, shielding designs can be employed [126].
Magnetic sensors also serve as critical components in magnetic resonance imaging (MRI) systems, assisting MRI equipment in achieving high-precision imaging [127]. MRI is frequently mentioned in clinical guidelines [128] as an important tool for diagnosing and managing cardiomyopathies. The guideline further mentions that newly developed rapid cardiac magnetic resonance (CMR) techniques allow even very young children to undergo scans without the need for general anesthesia [128,129]. This indicates that, with advancements in rapid CMR technology, MRI will further enable non-invasive and safe diagnostics for special populations, such as children, expanding its applications in personalized medicine. At the same time, the related component—magnetic sensors—has the potential to evolve accordingly.
2.1.6. Acoustic Sensors
Acoustic sensors can evaluate the efficacy of cardiovascular drugs indirectly by detecting changes in the propagation characteristics of sound waves (e.g., velocity, frequency, or echoes) within blood vessels or the heart, reflecting hemodynamic parameters or tissue conditions [130]. In recent years, acoustic sensors have been increasingly applied in the diagnosis and treatment of cardiovascular diseases, owing to their non-invasive nature, high sensitivity, and real-time monitoring capabilities, showcasing tremendous potential for further development [131].
Compared to traditional magnetic field sensors, acoustic sensors are less susceptible to interference in strong magnetic fields or complex electromagnetic environments, such as near MRI equipment, thereby maintaining high sensitivity [132]. A highly sensitive acoustic sensor based on electrospun piezoelectric nanofibers that utilizes the principle of converting sound waves into electrical signals has been reported. This sensor can detect low-frequency sound waves and noise with a sensitivity of up to 266 mV Pa−1, which is five times higher than that of traditional piezoelectric PVDF films [133]. A developed wearable ultrasound device was reported that employs a piezoelectric transducer array and a deep learning model to enable real-time, continuous cardiac function assessment. It can measure key cardiac performance indicators, such as left ventricular ejection fraction and cardiac output, making it suitable for dynamic cardiovascular monitoring and stress testing. The device demonstrates high consistency with commercial equipment in measuring critical cardiac indicators (e.g., ejection fraction and cardiac output) with minimal discrepancies. Its dynamic range reaches 63.2 dB, exceeding the 60 dB threshold required for medical diagnostics, indicating excellent resolution of contrast between different tissues [134]. The high dynamic range capability allows for clear differentiation of subtle contrasts between cardiac tissue and low-reflectivity structures such as blood [135], ensuring precise detection of pathological regions and functional abnormalities and providing richer imaging details in personalized cardiovascular disease diagnosis and treatment [136]. This portable, high-precision sensor, capable of continuous and real-time cardiac function monitoring, not only exhibits strong applicability in daily health monitoring but also provides high-quality and reliable individual data for personalized cardiovascular disease management. Overall, factors currently affecting acoustic sensor measurements include the sensor’s placement [137], environmental noise levels [138], patient activity status [139], and calibration accuracy [140]. Optimizing sensor design and performance to address these factors has become a key challenge in enhancing their effectiveness in personalized monitoring. Among them, wearable acoustic sensors, due to their portability, are advantageous for long-term monitoring. Future challenges include improving material biocompatibility and performance, further miniaturizing sensor designs, and establishing human sensor networks [141].
Echocardiography is a core tool for assessing cardiac structure and function, aiding in the confirmation of heart failure diagnoses [142]. In a clinical guideline for the diagnosis and treatment of acute and chronic heart failure, transthoracic echocardiography is recommended as a key diagnostic test for evaluating cardiac function in all patients with suspected chronic heart failure (Class I recommendation, Level of Evidence C) [143]. Additionally, other relevant guidelines point out that for patients presenting cardiogenic shock, hemodynamic instability, or suspected mechanical complications, urgent ultrasound examinations should be performed, potentially including bedside ultrasound assessments conducted by properly trained clinicians [96,144]. This reflects the critical role of bedside ultrasound in rapid diagnosis and evaluation. With its real-time capability and portability, bedside ultrasound is expected to play an even greater role in personalized medicine in the future.
2.1.7. Temperature Sensors
Temperature sensors detect temperature changes or thermal conductivity properties to indirectly reflect physiological parameters related to cardiovascular drug effects, such as blood flow, tissue metabolism, or inflammatory responses, thereby enabling efficacy monitoring [145]. In recent years, these sensors have gained increasing attention in the research and application of cardiovascular disease treatment [146].
The working principle of temperature sensors differs from that of sensors that rely on receiving reflected signals [147,148]. Temperature sensors typically measure temperature by directly contacting the object or environment being measured to sense changes in thermal energy. However, some non-contact temperature sensors achieve measurement by detecting infrared radiation [149]. With technological advancements, the application of flexible temperature sensors has become increasingly widespread [147]. Deng et al. developed a flexible wearable thermal sensor based on the principle of thermal anemometric flow, which can be used for continuous monitoring of blood flow changes in the vascular access of hemodialysis patients. This sensor detects changes in blood flow through a non-invasive method, featuring rapid response capabilities (with an overall response time ranging from a few seconds to several tens of seconds), and has demonstrated its practicality in both human and animal experiments [150]. In comparison, one of the commonly used clinical techniques, the thermodilution method used in right heart catheterization (RHC), is one of the important techniques for measuring cardiac output (CO) and is based on the principle of temperature sensing. It typically involves invasive procedures [151]. Compared to traditional techniques, the non-invasive sensor with rapid response and continuous monitoring holds significant application value in the personalized diagnosis and treatment of cardiovascular diseases. Its non-invasive design helps improve patient compliance, and its monitoring precision and timeliness provide support for early intervention, thereby enhancing the patient treatment experience and optimizing outcomes [152,153]. Key factors currently affecting temperature sensor monitoring include the thickness and thermal conductivity of the vessel wall, the thickness of the skin layer, and variations in blood flow rate [154,155]. In addition, for flexible temperature sensors, geometric design and attachment position significantly influence monitoring effectiveness [156,157,158].
In a clinical guideline [143] for the diagnosis and treatment of acute and chronic heart failure, RHC is recommended for patients with heart failure thought to result from constrictive pericarditis, restrictive cardiomyopathy, congenital heart disease, or high output states (class IIa). Additionally, it can be considered for selected patients with HFpEF to aid in confirming their diagnosis (class IIb) [143]. This reflects the importance of RHC in the diagnosis of heart failure, as well as the critical role of related sensor technologies (such as temperature sensors), which directly impact the accuracy and reliability of diagnostics.
2.2. Microfluidic Chip Technology
Microfluidic chip technology manipulates fluid flow on a microscale chip to precisely simulate biochemical reactions and metabolic processes within the body [159]. In recent years, it has been widely applied in cardiovascular drug screening [160] and metabolism research [161], playing an especially important role in personalized medicine.
Microfluidic chips significantly enhance the efficiency of drug screening and monitoring while reducing reliance on animal experiments, providing a powerful tool for new drug development [162]. For example, by simulating liver metabolism on the chip [163,164,165], researchers can study the effects of antihypertensive drug metabolites on blood vessels. Through the design of microchannels on the chip, the technology can detect the impact of drugs on the adhesion [166] and migration capabilities [167] of vascular endothelial cells. Additionally, with multi-channel microfluidic systems, it is possible to investigate interactions between drugs and various proteins, enabling a comprehensive evaluation of drug safety [168,169]. This technology not only advances drug development but also lays a solid foundation for precision medicine and personalized treatment [170,171]. Microfluidic technology (LOC devices) combined with induced pluripotent stem cells (iPSCs) can generate personalized cardiovascular models. This integration enables researchers to simulate real cardiovascular environments on a chip, facilitating the study of disease mechanisms and drug screening. Through this technology, drug screening and efficacy prediction can be tailored to the specific biological characteristics of individual patients, thereby optimizing treatment plans. It provides a powerful tool and platform for achieving precision medicine and personalized cardiovascular drug therapy [172].
2.3. Nanomaterials Technology
Nanomaterials not only demonstrate great potential in the field of novel cardiovascular therapeutic drugs but also serve as a powerful tool for enhancing sensor performance [173]. The unique physicochemical properties of nanomaterials, such as nanoparticles, nanotubes, or quantum dots, significantly enhance the performance of sensors [174]. In recent years, nanomaterials have been widely applied in the development of cardiovascular drug sensors [175,176], greatly improving their sensitivity and selectivity [176].
The integration of nanomaterials allows sensors to detect target molecules at ultra-low concentrations, advancing the early diagnosis of cardiovascular diseases and the development of personalized treatments [177,178,179]. For instance, these materials enable the detection of cardiovascular drugs, such as statins, at extremely low concentrations, thereby optimizing drug dosage [180]. Incorporating gold nanoparticles into electrochemical sensors enhances the sensitivity for detecting anticoagulant drugs [181]. Sensors modified with carbon nanotubes can monitor real-time changes in blood cholesterol levels with enhanced sensitivity [182].
3. Dynamic Monitoring Based on Individual Differences
Cardiovascular drugs are mainly categorized into anticoagulants [183], antihypertensives [184], antiarrhythmics [185], and lipid-lowering agents [186], and other classes, which achieve therapeutic effects by regulating blood coagulation [187], blood pressure control [32], cardiac electrical activity [188], and lipid metabolism [189]. These drugs are suitable for patients with thrombotic diseases [190], hypertension [191], arrhythmias [192], and hypercholesterolemia [193], helping to reduce the risk of cardiovascular events. When using these medications, due to individual differences (such as genetics, metabolic capacity, and disease conditions), it is essential to consider the effective dosage and potential adverse reactions. Therefore, during treatment, measuring biomarkers or physiological parameters related to efficacy and adverse reactions in the patient’s body is crucial (Figure 3) [194,195,196].
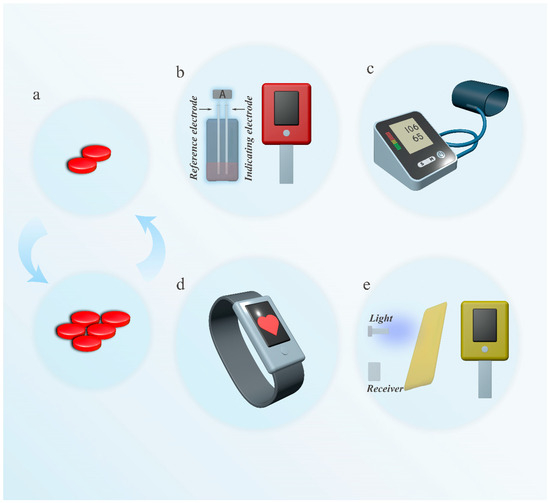
Figure 3.
Examples of common dynamic monitoring to adjust medication for cardiovascular disease. (a). Schematic diagram of optimizing dosage. (b). Measurement of INR using a coagulation analyzer (left image: illustrating possible electrochemical principles) (c). A blood pressure monitor used to measure blood pressure. (d). An electronic watch that monitors heart rate. (e). Cholesterol meter for measuring cholesterol.
3.1. Dynamic Monitoring of Anticoagulant Drugs
3.1.1. Mechanism of Action and Metabolic Pathways of Warfarin
Warfarin is a classic oral anticoagulant whose mechanism of action involves inhibiting vitamin K epoxide reductase-VKORC1, thereby disrupting the synthesis of coagulation factors II, VII, IX, and X to achieve an anticoagulant effect [197,198]. It is widely used for the prevention and treatment of various thrombotic disorders, including deep vein thrombosis [199], pulmonary embolism (PE) [200], and thromboembolism prevention [201] in patients with atrial fibrillation, and thrombosis risk management following artificial heart valve replacement surgery [202].
However, warfarin’s metabolic pathway is complex and primarily depends on the cytochrome P450 enzyme (CYP2C9) in the liver [203]. The efficiency of CYP2C9 metabolism is significantly influenced by genetic polymorphisms, leading to considerable individual variability in metabolic capacity based on genotype [204,205]. This variability results in substantial differences in warfarin concentrations in the bloodstream, making its therapeutic window extremely narrow [16,206]. Dose adjustments must be made with great caution: excessive dosing can lead to severe bleeding risks, while underdosing may fail to effectively prevent thrombus formation [207,208]. Thus, monitoring warfarin’s pharmacodynamics is critical to ensuring both safety and efficacy [209].
3.1.2. Dynamic Monitoring of INR Values
The International Normalized Ratio (INR) is a crucial indicator for evaluating the anticoagulant effect of warfarin [210], and its monitoring is essential for optimizing treatment outcomes and reducing the risk of bleeding or thrombosis [211]. Electrochemical sensors (Figure 3b) achieve real-time monitoring of INR values by detecting the activity of coagulation factors in the blood. These sensors rely on electrical signals generated from reactions on the electrode surface, enabling rapid and accurate reflection of coagulation status. Portable electrochemical sensor devices have been widely used in clinical and home monitoring, assisting patients and doctors in timely adjustments of warfarin dosages to reduce the risks of bleeding or thrombosis [212]. Williams et al. developed a fully printed portable prothrombin time (PT) sensor based on the principle of electrochemical impedance detection. This low-cost, rapid, and precise point-of-care testing (POCT) system is designed for monitoring blood coagulation status. By optimizing the frequency and using flexible substrates, the sensor can stably detect the blood coagulation process and shows potential for integration into wearable devices [213].
Optical sensors, on the other hand, monitor INR values, detecting changes in the optical properties of blood samples, such as absorbance, scattering [214,215,216]. In practical applications, optical detection techniques have been integrated into portable or bedside testing devices (e.g., optical coagulation analyzers) for routine patient monitoring [217].
Chan et al. developed a low-cost PT/INR detection system based on micro-mechanical motion tracking using a smartphone’s vibration motor and camera. This system employs the vibration motor to drive small copper particles in blood or plasma and uses the camera to capture changes in particle motion. By combining video analysis algorithms, the system detects changes in sample viscosity during coagulation, enabling measurements of coagulation time and INR values. This innovative approach provides an economical and practical testing solution for resource-limited settings. This is achieved by enabling the system to operate on older smartphones, such as second-hand iPhone 5s devices released in 2013, which are priced at just $35. By making coagulation testing more accessible, this method has the potential to improve the time within the therapeutic range for anticoagulation users, especially in rural areas [218].
3.2. Dynamic Monitoring of Antihypertensive Drugs
3.2.1. Mechanism of Action of β-Blockers
β-blockers are a class of drugs that exert their effects by blocking β-adrenergic receptors [219]. Their primary mechanism involves reducing heart rate and myocardial contractility, thereby decreasing myocardial oxygen consumption and effectively controlling blood pressure [220]. These drugs are widely used in the treatment of hypertension [221], angina pectoris [222], and heart failure [223], significantly improving patient outcomes [224].
However, the therapeutic effects of β-blockers vary among individuals due to differences in patient characteristics, and dose adjustments must be made with particular caution. Excessive doses may lead to hypotension [225] or bradycardia [226], while insufficient doses may fail to achieve the desired therapeutic effect. Therefore, monitoring physiological indicators such as heart rate and blood pressure is crucial for optimizing treatment regimens, enhancing efficacy, and reducing the risk of adverse effects [227].
3.2.2. Dynamic Monitoring Heart Rate and Blood Pressure Data
Indicators (including uncommon indicators) for evaluating the effects of β-blockers include heart rate [228], blood pressure [229], heart rate variability [230], cardiac output [231,232], lactate levels [233], and ECG [234] parameters. Monitoring techniques can assess the drug’s effectiveness to a certain extent by focusing on core indicators. By tracking changes in patients’ heart rate and blood pressure (Figure 3c), scientific evidence can be provided for adjusting β-blocker dosages [235]. For instance, portable blood pressure monitoring devices [236] and wearable heart rate sensors [86] can record patients’ physiological data and medication dosages can be optimized through algorithmic analysis [237]. Mai et al. developed an intelligent drug infusion system by combining active disturbance rejection control and deep reinforcement learning in a closed-loop control method, achieving precise regulation and automated management of postoperative patients’ mean arterial pressure [238].
3.3. Dynamic Monitoring of Antiarrhythmic Drugs
3.3.1. Mechanism of Action of Antiarrhythmic Drugs
Arrhythmias are often closely related to abnormalities in cardiac electrical activity, such as disruptions in the generation or conduction of action potentials in myocardial cells, leading to excessively fast, slow, or irregular heart rhythms [239,240]. The treatment of arrhythmias primarily relies on regulating the function of ion channels in myocardial cells. For example, sodium channel blockers can slow the rate of action potential upstroke [241,242], while calcium channel blockers can reduce the excitability and conductivity of myocardial cells [243,244], thereby restoring normal cardiac rhythm. However, the complexity and variability of arrhythmic conditions [245,246] may lead to imprecise control of the disease. Therefore, monitoring cardiac electrical activity is necessary to guide the use of medications.
3.3.2. Dynamic Monitoring of Heart Rhythm
The treatment of arrhythmias and the optimization of drug dosages often rely on the monitoring of multiple cardiac electrophysiological parameters [247]. Generally, monitoring technologies assess the state of a patient’s cardiac electrical activity by recording key parameters such as heart rate (Figure 3d) [248], rhythm [249], ECG waveforms [250], and heart rate variability [251]. These indicators reflect the rhythmicity, conductivity, and stability of electrical activity in the heart, aiding physicians in analyzing the type, severity, and dynamic trends of arrhythmias [252]. This provides a scientific basis for adjusting the dosage of antiarrhythmic drugs [253].
The emergence of devices such as Holter monitors and wearable ECG sensors has made real-time, continuous heart rhythm data collection possible [254]. These devices not only capture the frequency and severity of arrhythmias but also provide crucial references for personalized medication management [255,256]. For instance, in patients with atrial fibrillation, heart rhythm data monitored by sensors can guide the dosage of antiarrhythmic drugs (e.g., amiodarone), helping to avoid risks such as bradycardia or loss of rhythm control [257,258,259].
3.4. Dynamic Monitoring the Efficacy of Lipid-Lowering Drugs
3.4.1. Mechanism of Action and Adverse Reactions of Statins
Statins are used to lower low-density lipoprotein cholesterol (LDL-C) levels in the blood, with the aim of preventing and treating atherosclerotic cardiovascular disease (ASCVD) [260,261,262]. Their primary mechanism involves the inhibition of HMG-CoA reductase, which reduces cholesterol synthesis, thereby achieving lipid-lowering effects [263]. Although statins play a crucial role in both primary and secondary prevention of cardiovascular diseases [264,265], their use may lead to adverse reactions such as rhabdomyolysis [266,267,268]. These adverse effects are closely associated with elevated creatine kinase (CK) levels [269,270]. Therefore, during treatment, it is essential to regularly monitor patients’ cholesterol and CK levels to ensure both the efficacy and safety of the medication [270,271].
3.4.2. Dynamic Monitoring of Cholesterol Level
The techniques used to monitor cholesterol levels include various types [272,273], among which optical sensors (Figure 3e) have demonstrated significant application value in cardiovascular disease management due to their high sensitivity and specificity [274]. Optical sensors can rapidly evaluate cholesterol levels by detecting the absorbance or fluorescence signals of cholesterol molecules in the blood [275,276,277]. This sensor technology can be integrated into portable detection devices, providing cholesterol data for patients and doctors, thereby aiding in the optimization of statin dosage adjustments [277].
Ni et al. synthesized a novel fluorescent compound, FDD (fluorescent digitonin derivative), which achieves non-invasive detection of skin cholesterol by specifically binding to cholesterol and emitting fluorescence signals. This method can be used for atherosclerosis risk assessment. By exciting cholesterol-bound FDD with 405 nm light and detecting fluorescence intensity changes, cholesterol levels can be quantitatively analyzed [278].
3.4.3. Dynamic Monitoring of Creatine Kinase Levels
To promptly detect the potential risk of rhabdomyolysis associated with statin use in the treatment of atherosclerotic cardiovascular disease (ASCVD), monitoring technologies can be employed to track CK (creatine kinase) levels [279]. Among these, electrochemical sensors [280,281] and optical sensors [282,283] are ideal monitoring tools due to their high sensitivity and rapid response capabilities.
Electrochemical sensors have the advantage of enabling precise measurements of CK [279,284], while optical sensors offer non-invasive assessment [285]. These sensor technologies enable monitoring of CK levels, offering critical support for the early warning of rhabdomyolysis [284]. By dynamically tracking changes in CK levels, physicians can adjust medication dosages or switch treatment plans in time, thereby maximizing patient safety during drug administration.
4. Multi-Dimensional Data Integration and Analysis
The integration of multidimensional data holds significant importance in modern medicine, particularly in the diagnosis and treatment of cardiovascular diseases, where its value is increasingly evident [286]. By comprehensively integrating various layers of data—such as biomarkers, omics data, and external factors like environment and lifestyle—it is possible to reflect a patient’s health status holistically, from the molecular to the individual level [287]. In modern healthcare, with the growing demand for personalized medicine, data integration technologies are gradually becoming one of the key drivers for optimizing drug therapies and achieving precision medicine. Effectively integrating multidimensional biological data to support the optimization of drug therapies has become a critical focus of research [288,289].
In this context, pharmacokinetic and pharmacodynamic models (PK/PD models) serve as essential tools for data integration and have been widely used in drug dosage design and efficacy evaluation [290]. Meanwhile, drug–target networks provide a novel perspective for systematically analyzing the interactions between drugs and their targets. These network models not only optimize drug design and combination therapy strategies but also offer critical theoretical support for precision treatment [291]. Moreover, with the rapid advancement of artificial intelligence, machine learning has gradually become a pivotal tool in precision medicine. By leveraging multidimensional data, machine learning can uncover hidden correlations between datasets and dynamically predict drug responses and adverse effects, thereby providing scientific support for the development of personalized treatment plans (Figure 4a) [292]. The following sections will explore how PK/PD models, drug–target networks, and machine learning utilize multidimensional data integration to achieve more efficient precision medicine.
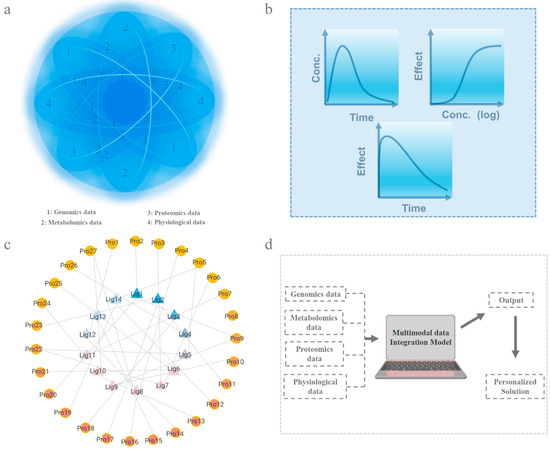
Figure 4.
Integration and analysis of multidimensional data. (a). A schematic diagram of the integration and analysis of multidimensional data. (b). Schematic diagram of PK/PD model (Con.: Concentration). (c). Schematic diagram of drug–target network (Lig: ligand molecules, Pro: protein receptor). (d). Diagram of machine learning (multidimensional data integration and analysis through specific models).
4.1. Pharmacokinetic/Pharmacodynamic Models
PK/PD models (Figure 4b) can be a core tool for optimizing drug therapy. PK models describe the processes of absorption, distribution, metabolism, and excretion of drugs in the body, while PD models reveal the mechanisms of drug action on their targets [293]. However, the accuracy of these models heavily relies on high-quality data, and individual variability (e.g., genetic profiles and metabolic characteristics) [294] may limit their applicability to all patients. Furthermore, in complex pathological conditions, the application of these models remains constrained, as they may not fully account for drug interactions in cases of multiple comorbidities [295,296].
However, analyzing PK and PD models only may overlook the limitations in achieving a comprehensive understanding of individual responses to drug actions. By further integrating multidimensional measurement data, these models have the potential to quantitatively describe the behavior and mechanisms of drugs within the body [297,298]. Such models can also be used to predict drug efficacy or adverse reactions [297]. The models thus established can not only help to understand the processes of drug action but also guide personalized medicine and optimize treatment regimens. Individual differences in drug metabolism rates due to genetic factors pose significant challenges to precision medicine [299]. With the development of genomics and metabolomics, integrating patients’ genetic information with PK/PD models offers a novel solution for personalized healthcare [300]. A patient’s genome can reveal gene variations related to drug metabolism and targets [301], while physiological data and biomarkers further reflect individual differences in metabolism rates, drug clearance, and target sensitivity [302]. Moreover, the application of sensor technology enables continuous monitoring of patients’ physiological parameters [303]. These high-frequency, accurate data not only capture the dynamic changes in a patient’s condition but also record immediate physiological responses, providing critical evidence for personalized treatment [303]. For example, in cardiovascular disease management, PK/PD models that combine genomic information with real-time monitoring data have the potential to significantly enhance drug efficacy and reduce the incidence of adverse reactions. The application of this technology not only provides a safer and more effective solution for the precise treatment of cardiovascular diseases but also lays the foundation for remote healthcare and intelligent management of chronic diseases, further advancing the development of personalized medicine [300].
4.2. Drug–Target Networks
Drug–target networks (Figure 4c) are a tool that constructs network models of drug–target relationships to systematically analyze drug mechanisms of action and target interactions [304]. They are based on the interactions between drugs and targets, using network topology to uncover multi-target mechanisms in complex diseases, thereby optimizing drug design and combination strategies [291,305]. The complex topology of drug–target networks reflects the polypharmacology of many drugs [306]. For example, statins (such as atorvastatin) act on the key target HMG-CoA reductase, effectively lowering cholesterol levels. Furthermore, it has been revealed that the potential anti-inflammatory and vascular function-improving effects of statins offer more precise guidance for clinical medication [307]. In cardiovascular drug development, drug–target networks may indicate potential disease targets, enabling more drug candidates [308]. Additionally, it can analyze the potential of multi-drug combinations to optimize combination therapy and enhance therapeutic outcomes. In clinical applications, drug–target networks can assist in evaluating the target coverage of drugs and potential drug interactions, providing a scientific basis for personalized treatment [307].
As an essential tool in systems biology, drug–target networks play a crucial role in cardiovascular drug therapy. By integrating multi-level biological data, drug–target networks provide a comprehensive analysis of the complex interactions between drugs and biological systems, offering a scientific basis for personalized treatment [309,310]. Through network analysis, drug–target networks can be utilized to design combination therapy strategies targeting multiple sites, thereby achieving more effective therapeutic outcomes [311]. For instance, in complex diseases such as hypertension combined with diabetes, network analysis can uncover the complex relationships of different drugs to act on targets (e.g., insulin receptors and angiotensin receptors) while comprehensively evaluating possible drug–drug interactions [312,313]. This approach offers a novel perspective for precision medicine, enabling the development of more targeted combination therapies that not only enhance treatment efficacy but also minimize side effects, ultimately improving overall patient outcomes [314]. Theoretically, by combining real-time monitoring of biomarkers and drug–target networks, we can dynamically adjust treatment plans, significantly enhancing the precision and safety of drug therapy [297]. Moreover, drug–target networks have the potential to assess individual differences in drug metabolism and response by incorporating patient-specific metabolic enzyme activity or genetic polymorphisms [315]. In clinical application, this approach has the potential to quantify a patient’s efficiency in metabolizing cardiovascular therapy drugs while incorporating the expression levels of targets to comprehensively evaluate the actual effects of the drug in the body. This integration significantly enhances the precision of personalized drug dosage design. As a core tool for cardiovascular disease drug development and treatment optimization, drug–target networks provide innovative technical support and research perspectives for precision medicine.
4.3. Machine Learning
Machine learning (Figure 4d) is a technology that uses algorithms and statistical models to identify patterns and make predictions from data. Its advantages lie in its ability to quickly process massive amounts of data and extract valuable information while also possessing self-optimization and continuous learning capabilities, thereby improving prediction accuracy over time [316,317]. In the clinical field, machine learning has developed rapidly and is widely applied in disease diagnosis, treatment optimization, and personalized medicine, significantly enhancing medical efficiency and precision [318,319]. Currently, machine learning is being increasingly applied in cardiovascular treatment, including heart disease risk prediction [320], electrocardiogram anomaly detection [15], and personalized drug dosage adjustment [321], providing significant support for the precise treatment of cardiovascular diseases. For example, a relevant study has conducted continuous dynamic heart rhythm monitoring for 30 days on patients with stroke risk factors but no known atrial fibrillation, analyzing their ECG data using artificial intelligence (AI) algorithms. The research indicates that the artificial intelligence (AI) algorithm-guided targeted screening approach is more efficient than traditional methods and can improve the effectiveness of atrial fibrillation screening [322]. In addition, adverse reactions to cardiovascular drugs can lead to severe consequences, such as fatal bleeding caused by anticoagulants [323] or acute hypotension and arrhythmia induced by certain antihypertensive drugs [324], posing life-threatening risks to patients. Traditional methods often rely on clinical observation and patient self-reporting to identify adverse reactions [325]. However, these methods have difficulty capturing early physiological changes or individual differences, which may lead to missed opportunities for timely intervention. Machine learning has demonstrated exceptional potential in predicting drug responses and adverse reactions to help patients avoid drug-related risks and improve the safety and effectiveness of treatments [326,327].
In recent years, the application of deep learning, a subset of machine learning, in cardiology has become increasingly widespread, with its importance reflected in its powerful predictive capabilities. For instance, deep neural networks (DNNs), as a sophisticated form of artificial neural networks, have emerged as a powerful tool in the field of cardiology, capable of automatically learning features and making predictions from raw data. These models, characterized by their multi-layer input and output structures, excel at handling unlabeled and unstructured data. By extracting key features from various high-dimensional data sources, DNNs have played a crucial role in advancing data-driven discoveries [328]. At present, artificial intelligence has made considerable progress in the interpretation of medical imaging, but its integration into broader healthcare fields remains significantly limited. However, clinical practice often requires the integration of multimodal data for analysis, making the development of AI models capable of multidimensional data integration and analysis quite urgent [328]. As a commonly used learning technique in deep learning, the Transformer model has demonstrated excellent performance and significant potential in integrating multimodal data [329]. A study reported a Transformer model based on the self-attention mechanism, which utilized patient features from the Cleveland Heart Disease dataset (e.g., age, blood pressure, cholesterol, etc.) to predict cardiovascular diseases. Unlike traditional recurrent neural network (RNN) methods, this model leveraged the advantages of position-level attention mechanisms and self-attention layers to learn the representation of the entire sequence, enabling the identification and evaluation of the relative importance of various sequence components, thereby enhancing predictive effectiveness. Its predictive accuracy reached as high as 96.51% and demonstrated strong interpretability, aiding doctors in understanding key features [330]. Integrating patients’ genomics, metabolomics, proteomics data, and real-time physiological data can be a crucial method to improve treatment outcomes in cardiovascular personalized medicine. For example, although atrial fibrillation (AF), long QT syndrome (LQTS), and Brugada syndrome (BrS) have different underlying causes, and current guidelines do not yet recommend genetic testing for familial atrial fibrillation; it is important to note that the role of genetic testing in the personalized management of these arrhythmias is becoming increasingly significant. This is because pathogenic or likely pathogenic variants in multiple disease-causing genes can contribute to the development of the final arrhythmia phenotype [331]. Therefore, by integrating multi-dimensional data, like patient genomics or/and metabolomics information [332], with real-time physiological data, machine learning models such as the Transformer, which excel in multidimensional data integration and analysis, have the potential to achieve highly accurate predictions. Through deep learning and feature extraction techniques, they can uncover potential correlations among different data types [333]. For instance, deep learning models can effectively determine a patient’s sensitivity to specific drugs, assisting doctors in formulating more precise treatment plans [334,335]. However, the integration and standardization of multimodal data remain a major challenge. Due to the significant differences in the sources and formats of data across different dimensions, the process of unifying and analyzing these data is extremely complex. In addition, improving the interpretability of deep learning models is also a critical issue. In the diagnosis of cardiovascular diseases or the evaluation of drug efficacy, understanding the basis of model decisions is essential, as it directly impacts the credibility and practical value of these models in clinical applications [336].
5. Challenges and Future Prospects
5.1. Complexity of Cardiovascular Pharmacotherapy
The treatment of cardiovascular diseases is highly complex, requiring not only long-term medication but also managing adverse reactions such as drug side effects and resistance [337,338]. Moreover, due to the interplay of multiple pathological mechanisms, cardiovascular diseases are often accompanied by other related conditions such as diabetes, further complicating treatment [339,340]. Consequently, combination therapy has become a critical treatment strategy. However, the metabolic pathways of different drugs may interact with each other, potentially affecting efficacy and safety. Individual differences in genetic background, age, sex, and lifestyle lead to variations in drug absorption, metabolism, and excretion, resulting in significant differences in metabolite levels or potential risks among patients [315,341]. Future advancements in implementing personalized therapy aim to optimize drug selection and dosage adjustments based on the specific conditions of each patient. This approach can reduce the risk of drug interactions, enhance therapeutic efficacy and safety, minimize side effects, and improve patient compliance and quality of life [342,343].
5.2. Advances in Monitoring Technologies for Cardiovascular Drug Efficacy
Given the complexity of cardiovascular disease treatment, timely assessment and adjustment of drug regimens is an effective solution, with monitoring technologies serving as a critical tool to achieve this goal [342]. However, many current sensors still suffer from limitations in detection sensitivity and specificity, such as low accuracy in detecting specific biomarkers or limited ability to distinguish target molecules. These shortcomings not only hinder precise adjustments to drug regimens but may also lead to misjudgments and erroneous results [344]. To overcome these deficiencies, future research may focus on the development of novel materials and the optimization of sensor design to enhance sensor performance [345]. Additionally, due to drug–drug interactions, there is an increasing demand for the simultaneous detection of multiple biomarkers. The capability for multi-parameter synchronous detection is likely to become a key trend in the development of sensor technology [346,347]. Facing the complex data generated by individualized responses, integrating advanced data processing methods (e.g., machine learning) to comprehensively analyze and predict monitoring results holds promise for achieving precise combination therapy and personalized treatment [292].
Furthermore, optimizing the efficacy of cardiovascular disease treatment may also involve the application of implantable devices. Since implantable devices are in direct contact with the body, they offer higher accuracy in certain aspects [348,349]. However, during long-term use, these devices are often affected by the surrounding physiological environment, such as encapsulation, material aging, performance degradation, and energy leakage. These post-implantation challenges highlight the urgent need for advancements in new material development, optimization of surface functionalization technologies, and improvements in self-powering technologies [350,351,352,353]. By addressing these critical issues, the safety and widespread adoption of implantable devices in long-term use is expected to improve significantly, providing more reliable solutions for cardiovascular disease treatment [354].
5.3. Challenges and Solutions in Clinical Translation
Although sensor technology, as a rapidly developing and highly focused area in monitoring methods, has demonstrated excellent performance in laboratory settings, its clinical application validation still faces numerous challenges [355]. Firstly, there are significant differences in experimental design and data processing methods for current sensor monitoring, which affect the comparability and consistency of data in cardiovascular drug monitoring technologies. Therefore, establishing an analytical model to evaluate such data would help improve the efficiency of technology comparison and enhance the scientific rigor of performance evaluation [356,357]. Secondly, some sensors are tested in overly specific subjects or application scenarios, limiting their applicability to diverse patient groups and varied application contexts. Thus, developing technologies compatible with diverse populations and scenarios will become a trend. By integrating advanced data processing methods, these technologies can further enable personalized and precise treatments [292,358,359]. Finally, many studies remain at the preclinical stage and have yet to progress into extensive clinical trials. The simplified physiological environments simulated in vitro often fail to fully reflect the complex physiological dynamics in vivo, leading to significant differences in sensor performance between real biological environments and laboratory conditions [360]. Therefore, further evaluation of their stability and reliability in complex bodily fluid environments is necessary. To promote the clinical translation of sensor technologies, combining standardized yet diversified experimental designs with validation in complex bodily fluid environments may become a key solution [292,357,361].
5.4. Development Direction of Cardiovascular Drug Therapy
To achieve personalized treatment, dynamically monitoring the impact of cardiovascular drugs on individuals’ multi-level physiological indicators may be a key solution, with data processing being an indispensable part of this process [362,363]. The challenges in these areas mainly focus on addressing the limitations of current sensor performance and data analysis capabilities [364,365]. These shortcomings can be tackled through approaches such as developing new materials (e.g., nanomaterials), optimizing sensor design, improving surface functionalization technologies, and enhancing energy supply techniques [366,367,368]. By integrating and deeply analyzing multidimensional data, the value of individual data can be maximized, enabling dynamic adjustments to medication regimens to achieve precise personalized treatment goals [369]. Furthermore, it may even become possible to establish precise dosages, and specific medication plans before administration. Looking forward, the development of cardiovascular drug efficacy evaluation will continue to advance toward personalized treatment, laying a foundation for safe, efficient, and individualized therapeutic solutions by accurately monitoring patients’ drug responses and their dynamic changes [292,297,343,370].
Author Contributions
Conceptualization, R.L.; Visualization, R.L., Z.H. and Y.L.; Writing—original draft preparation, R.L. and Z.H.; Writing—review and editing, R.L. and Y.Z; Funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The authors appreciate the support of the Science and Technology Development Fund, Macau SAR (0021/2022/A1, 006/2022/ALC, 0007/2021/AKP), and Research Grant MYRG2022-00088-IAPME from the Research & Development Administration Office at the University of Macau.
Acknowledgments
All individuals included in this section have consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Poulter, N. Coronary heart disease is a multifactorial disease. Am. J. Hypertens. 1999, 12, 92S–95S. [Google Scholar] [CrossRef]
- Hamilton, G.A. Measuring adherence in a hypertension clinical trial. Eur. J. Cardiovasc. Nurs. 2003, 2, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Kamel, H.; Healey, J.S. Cardioembolic stroke. Circ. Res. 2017, 120, 514–526. [Google Scholar] [CrossRef]
- Reich, P.; DeSilva, R.A.; Lown, B.; Murawski, B.J. Acute psychological disturbances preceding life-threatening ventricular arrhythmias. Jama 1981, 246, 233–235. [Google Scholar] [CrossRef]
- Woodruff, R.C.; Tong, X.; Khan, S.S.; Shah, N.S.; Jackson, S.L.; Loustalot, F.; Vaughan, A.S. Trends in cardiovascular disease mortality rates and excess deaths, 2010–2022. Am. J. Prev. Med. 2024, 66, 582–589. [Google Scholar] [CrossRef]
- Lopez, E.; Ballard, B.; Jan, A. Cardiovascular Disease. StatPearls-NCBI Bookshelf. National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535419/ (accessed on 28 August 2023).
- Gooding, H.C.; Gidding, S.S.; Moran, A.E.; Redmond, N.; Allen, N.B.; Bacha, F.; Burns, T.L.; Catov, J.M.; Grandner, M.A.; Harris, K.M. Challenges and opportunities for the prevention and treatment of cardiovascular disease among young adults: Report from a National Heart, Lung, and Blood Institute Working Group. J. Am. Heart Assoc. 2020, 9, e016115. [Google Scholar] [CrossRef]
- Wagner, M.; Gelbrich, G.; Kircher, J.; Kotseva, K.; Wood, D.; Morbach, C.; Leyh, R.; Ertl, G.; Karmann, W.; Stoerk, S. Secondary prevention in younger vs. older coronary heart disease patients—Insights from the German subset of the EUROASPIRE IV survey. Int. J. Behav. Med. 2018, 25, 283–293. [Google Scholar] [CrossRef]
- Antza, C.; Gallo, A.; Boutari, C.; Ershova, A.; Gurses, K.M.; Lewek, J.; Mirmaksudov, M.; Silbernagel, G.; Sandstedt, J.; Lebedeva, A. Prevention of cardiovascular disease in young adults: Focus on gender differences. A collaborative review from the EAS Young Fellows. Atherosclerosis 2023, 384, 117272. [Google Scholar] [CrossRef]
- Lee, H.; Yano, Y.; Cho, S.M.J.; Heo, J.E.; Kim, D.-W.; Park, S.; Lloyd-Jones, D.M.; Kim, H.C. Adherence to antihypertensive medication and incident cardiovascular events in young adults with hypertension. Hypertension 2021, 77, 1341–1349. [Google Scholar] [CrossRef]
- Rippe, J.M.; Angelopoulos, T.J. Lifestyle strategies for risk factor reduction, prevention and treatment of cardiovascular disease. In Lifestyle Medicine, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 19–36. [Google Scholar]
- Yin, T.; Duan, J.; Xu, D.; Huang, M.; Yin, D. Precision individualized medication strategies and challenges for cardiovascular diseases. Precis. Medicat. 2024, 1, 7–15. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Emerging risk biomarkers in cardiovascular diseases and disorders. J. Lipids 2015, 2015, 971453. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Noseworthy, P.A.; Attia, Z.I.; Friedman, P.A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 2021, 18, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Nolin, T.D. On warfarin use in kidney disease: A therapeutic window of opportunity? Am. J. Kidney Dis. 2010, 56, 805–808. [Google Scholar] [CrossRef]
- Fahmi, A.M.; Elewa, H.; El Jilany, I. Warfarin dosing strategies evolution and its progress in the era of precision medicine, a narrative review. Int. J. Clin. Pharm. 2022, 44, 599–607. [Google Scholar] [CrossRef]
- Bellanca, C.M.; Augello, E.; Cantone, A.F.; Di Mauro, R.; Attaguile, G.A.; Di Giovanni, V.; Condorelli, G.A.; Di Benedetto, G.; Cantarella, G.; Bernardini, R. Insight into Risk Factors, Pharmacogenetics/Genomics, and Management of Adverse Drug Reactions in Elderly: A Narrative Review. Pharmaceuticals 2023, 16, 1542. [Google Scholar] [CrossRef]
- Langmia, I.M.; Just, K.S.; Yamoune, S.; Brockmöller, J.; Masimirembwa, C.; Stingl, J.C. CYP2B6 functional variability in drug metabolism and exposure across populations—Implication for drug safety, dosing, and individualized therapy. Front. Genet. 2021, 12, 692234. [Google Scholar] [CrossRef]
- Gervasini, G.; Benítez, J.; Carrillo, J.A. Pharmacogenetic testing and therapeutic drug monitoring are complementary tools for optimal individualization of drug therapy. Eur. J. Clin. Pharmacol. 2010, 66, 755–774. [Google Scholar] [CrossRef]
- Alomar, M.J. Factors affecting the development of adverse drug reactions. Saudi Pharm. J. 2014, 22, 83–94. [Google Scholar] [CrossRef]
- Munger, M.A. Polypharmacy and combination therapy in the management of hypertension in elderly patients with co-morbid diabetes mellitus. Drugs Aging 2010, 27, 871–883. [Google Scholar] [CrossRef]
- Zijp, T.R.; Izzah, Z.; Åberg, C.; Gan, C.T.; Bakker, S.J.; Touw, D.J.; Van Boven, J.F. Clinical value of emerging bioanalytical methods for drug measurements: A scoping review of their applicability for medication adherence and therapeutic drug monitoring. Drugs 2021, 81, 1983–2002. [Google Scholar] [CrossRef] [PubMed]
- Delahaye, L.; Veenhof, H.; Koch, B.C.; Alffenaar, J.-W.C.; Linden, R.; Stove, C. Alternative sampling devices to collect dried blood microsamples: State-of-the-art. Ther. Drug Monit. 2021, 43, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Witt, D.M.; Clark, N.P.; Kaatz, S.; Schnurr, T.; Ansell, J.E. Guidance for the practical management of warfarin therapy in the treatment of venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 187–205. [Google Scholar] [CrossRef]
- Schein, J.R.; White, C.M.; Nelson, W.W.; Kluger, J.; Mearns, E.S.; Coleman, C.I. Vitamin K antagonist use: Evidence of the difficulty of achieving and maintaining target INR range and subsequent consequences. Thromb. J. 2016, 14, 1–10. [Google Scholar] [CrossRef]
- Bruttomesso, D.; Laviola, L.; Avogaro, A.; Bonora, E.; Del Prato, S.; Frontoni, S.; Orsi, E.; Rabbone, I.; Sesti, G.; Purrello, F. The use of real time continuous glucose monitoring or flash glucose monitoring in the management of diabetes: A consensus view of Italian diabetes experts using the Delphi method. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 421–431. [Google Scholar] [CrossRef]
- Frost, M.C.; Meyerhoff, M.E. Real-time monitoring of critical care analytes in the bloodstream with chemical sensors: Progress and challenges. Annu. Rev. Anal. Chem. 2015, 8, 171–192. [Google Scholar] [CrossRef]
- Godoy, L.C.; Tomlinson, G.; Abumuamar, A.M.; Farkouh, M.E.; Rudolph, M.; Billia, F.; Cohn, I.; Marcus, G.; Kim, R.H.; Rao, V. Association between time to therapeutic INR and length of stay following mechanical heart valve surgery. J. Card. Surg. 2022, 37, 62–69. [Google Scholar] [CrossRef]
- DeSantis, G.; Hogan-Schlientz, J.; Liska, G.; Kipp, S.; Sallee, R.; Wurster, M.; Kupfer, K.; Ansell, J. STABLE results: Warfarin home monitoring achieves excellent INR control. Am. J. Manag. Care 2014, 20, 202–209. [Google Scholar]
- Leopold, J.A.; Loscalzo, J. Emerging role of precision medicine in cardiovascular disease. Circ. Res. 2018, 122, 1302–1315. [Google Scholar] [CrossRef]
- Savoia, C.; Volpe, M.; Grassi, G.; Borghi, C.; Agabiti Rosei, E.; Touyz, R.M. Personalized medicine—A modern approach for the diagnosis and management of hypertension. Clin. Sci. 2017, 131, 2671–2685. [Google Scholar] [CrossRef]
- Rumsfeld, J.S.; Joynt, K.E.; Maddox, T.M. Big data analytics to improve cardiovascular care: Promise and challenges. Nat. Rev. Cardiol. 2016, 13, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Rogers, A.J.; Johnson, K.W.; Wang, Z.; Turakhia, M.P.; Halperin, J.L.; Narayan, S.M. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat. Rev. Cardiol. 2021, 18, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Walsh III, J.A.; Topol, E.J.; Steinhubl, S.R. Novel wireless devices for cardiac monitoring. Circulation 2014, 130, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Misra, R. Nanomaterials in microfluidics for disease diagnosis and therapy development. Mater. Technol. 2019, 34, 92–116. [Google Scholar] [CrossRef]
- Hemdan, M.; Ali, M.A.; Doghish, A.S.; Mageed, S.S.A.; Elazab, I.M.; Khalil, M.M.; Mabrouk, M.; Das, D.B.; Amin, A.S. Innovations in biosensor technologies for healthcare diagnostics and therapeutic drug monitoring: Applications, recent progress, and future research challenges. Sensors 2024, 24, 5143. [Google Scholar] [CrossRef]
- Bendre, A.; Bhat, M.P.; Lee, K.-H.; Altalhi, T.; Alruqi, M.A.; Kurkuri, M. Recent developments in microfluidic technology for synthesis and toxicity-efficiency studies of biomedical nanomaterials. Mater. Today Adv. 2022, 13, 100205. [Google Scholar] [CrossRef]
- Xue, Z.; Gai, Y.; Wu, Y.; Liu, Z.; Li, Z. Wearable mechanical and electrochemical sensors for real-time health monitoring. Commun. Mater. 2024, 5, 211. [Google Scholar] [CrossRef]
- Lin, R.; Lei, M.; Ding, S.; Cheng, Q.; Ma, Z.; Wang, L.; Tang, Z.; Zhou, B.; Zhou, Y. Applications of flexible electronics related to cardiocerebral vascular system. Mater. Today Bio 2023, 23, 100787. [Google Scholar] [CrossRef]
- Teymourian, H.; Parrilla, M.; Sempionatto, J.R.; Montiel, N.F.; Barfidokht, A.; Van Echelpoel, R.; De Wael, K.; Wang, J. Wearable electrochemical sensors for the monitoring and screening of drugs. ACS Sens. 2020, 5, 2679–2700. [Google Scholar] [CrossRef]
- Garzón, V.; Pinacho, D.G.; Bustos, R.-H.; Garzón, G.; Bustamante, S. Optical biosensors for therapeutic drug monitoring. Biosensors 2019, 9, 132. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Xiao, S.; Liu, Y.; Bai, M.; Gong, L.; Zhao, J.; Chen, D. Revolutionizing precision medicine: Exploring wearable sensors for therapeutic drug monitoring and personalized therapy. Biosensors 2023, 13, 726. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qi, J.; Fan, S.; Qiao, Z.; Yeo, J.C.; Lim, C.T. Flexible wearable sensors for cardiovascular health monitoring. Adv. Healthc. Mater. 2021, 10, 2100116. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qi, H.; Ma, Y.; Deng, Y.; Liu, S.; Jie, Y.; Jing, J.; He, J.; Zhang, X.; Wheatley, L. A flexible and physically transient electrochemical sensor for real-time wireless nitric oxide monitoring. Nat. Commun. 2020, 11, 3207. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Alquié, G.; Deshours, F.; Kokabi, H.; Shubair, R.M. Non-invasive real-time monitoring of glucose level using novel microwave biosensor based on triple-pole CSRR. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 1407–1420. [Google Scholar] [CrossRef]
- Ogata, G.; Ishii, Y.; Asai, K.; Sano, Y.; Nin, F.; Yoshida, T.; Higuchi, T.; Sawamura, S.; Ota, T.; Hori, K. A microsensing system for the in vivo real-time detection of local drug kinetics. Nat. Biomed. Eng. 2017, 1, 654–666. [Google Scholar] [CrossRef]
- Ma’mun, A.; Abd El-Rahman, M.K.; Abd El-Kawy, M. Real-time potentiometric sensor; an innovative tool for monitoring hydrolysis of chemo/bio-degradable drugs in pharmaceutical sciences. J. Pharm. Biomed. Anal. 2018, 154, 166–173. [Google Scholar] [CrossRef]
- Fuad, N.M.; Kaslin, J.; Wlodkowic, D. Lab-on-a-Chip imaging micro-echocardiography (iμEC) for rapid assessment of cardiovascular activity in zebrafish larvae. Sens. Actuators B Chem. 2018, 256, 1131–1141. [Google Scholar] [CrossRef]
- Yuan, S.; Yuan, H.; Hay, D.C.; Hu, H.; Wang, C. Revolutionizing Drug Discovery: The Impact of Distinct Designs and Biosensor Integration in Microfluidics-Based Organ-on-a-Chip Technology. Biosensors 2024, 14, 425. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, H.; Yi, C.; Zheng, Y.; Tan, Z.; Jia, X.; Liu, Z. Recent progress of non-invasive in vitro diagnosis using electrochemical analysis strategy and wearable microfluidic devices applied to exocrine secretion sampling. TrAC Trends Anal. Chem. 2024, 172, 117561. [Google Scholar] [CrossRef]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; Martin, S.S.; Muse, E.D.; Turakhia, M.P.; Tarakji, K.G. Smart wearable devices in cardiovascular care: Where we are and how to move forward. Nat. Rev. Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef]
- Sharma, P.; Imtiaz, S.A.; Rodriguez-Villegas, E. Acoustic sensing as a novel wearable approach for cardiac monitoring at the wrist. Sci. Rep. 2019, 9, 20079. [Google Scholar] [CrossRef] [PubMed]
- Coker, S.J.; Batey, A.J.; Lightbown, I.D.; Díaz, M.E.; Eisner, D.A. Effects of mefloquine on cardiac contractility and electrical activity in vivo, in isolated cardiac preparations, and in single ventricular myocytes. Br. J. Pharmacol. 2000, 129, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Weiler, D.T.; Villajuan, S.O.; Edkins, L.; Cleary, S.; Saleem, J.J. Wearable heart rate monitor technology accuracy in research: A comparative study between PPG and ECG technology. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting, Wollongong, Australia, 26–29 November 2017; pp. 1292–1296. [Google Scholar]
- Fabian, D.; Ahmed, I. Ambulatory ECG monitoring. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pérez-Riera, A.R.; Barbosa-Barros, R.; Daminello-Raimundo, R.; de Abreu, L.C. Main artifacts in electrocardiography. Ann. Noninvasive Electrocardiol. 2018, 23, e12494. [Google Scholar] [CrossRef]
- Casal, L.; Mura, G.L. Skin-electrode impedance measurement during ECG acquisition: Method’s validation. J. Phys. Conf. Ser. 2016, 705, 012006. [Google Scholar] [CrossRef]
- Laudon, M.K.; Webster, J.G.; Frayne, R.; Grist, T.M. Minimizing interference from magnetic resonance imagers during electrocardiography. IEEE Trans. Biomed. Eng. 1998, 45, 160–164. [Google Scholar] [CrossRef]
- Bogun, F.; Anh, D.; Kalahasty, G.; Wissner, E.; Serhal, C.B.; Bazzi, R.; Weaver, W.D.; Schuger, C. Misdiagnosis of atrial fibrillation and its clinical consequences. Am. J. Med. 2004, 117, 636–642. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.; De Potter, T.J.; Dwight, J.; Guasti, L.; Hanke, T. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, ehae176. [Google Scholar]
- Members, W.C.; Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2024, 83, 109–279. [Google Scholar]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of bio-electrochemical sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
- Qin, S.-N.; Jie, Z.-Q.; Chen, L.-Y.; Zheng, J.-X.; Xie, Y.; Feng, L.; Chen, Z.-M.; Salminen, K.; Sun, J.-J. Real-time monitoring of daunorubicin pharmacokinetics with nanoporous electrochemical aptamer-based sensors in vivo. Sens. Actuators B Chem. 2024, 411, 135710. [Google Scholar] [CrossRef]
- Mruthunjaya, A.K.; Torriero, A.A. Electrochemical Monitoring in Anticoagulation Therapy. Molecules 2024, 29, 1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Harwood, G.J.; Pouton, C.W. Amperometric enzyme biosensors for the analysis of drugs and metabolites. Adv. Drug Deliv. Rev. 1996, 18, 163–191. [Google Scholar] [CrossRef]
- Ghoneim, M.; Tawfik, A. Assay of anti-coagulant drug warfarin sodium in pharmaceutical formulation and human biological fluids by square-wave adsorptive cathodic stripping voltammetry. Anal. Chim. Acta 2004, 511, 63–69. [Google Scholar] [CrossRef]
- Wisedchaisri, G.; Gamal El-Din, T.M. Druggability of voltage-gated sodium channels—Exploring old and new drug receptor sites. Front. Pharmacol. 2022, 13, 858348. [Google Scholar] [CrossRef]
- Seshadri, D.R.; Li, R.T.; Voos, J.E.; Rowbottom, J.R.; Alfes, C.M.; Zorman, C.A.; Drummond, C.K. Wearable sensors for monitoring the physiological and biochemical profile of the athlete. NPJ Digit. Med. 2019, 2, 72. [Google Scholar] [CrossRef]
- Deshpande, A.S.; Muraoka, W.; Andreescu, S. Electrochemical sensors for oxidative stress monitoring. Curr. Opin. Electrochem. 2021, 29, 100809. [Google Scholar] [CrossRef]
- Bansal, H.; Kohli, R.K.; Saluja, K.; Chaurasia, A. Recent advancements in biomedical research in the era of AI and ML. Artif. Intell. Comput. Dyn. Biomed. Res. 2022, 8, 1–20. [Google Scholar]
- Romele, P.; Gkoupidenis, P.; Koutsouras, D.A.; Lieberth, K.; Kovács-Vajna, Z.M.; Blom, P.W.; Torricelli, F. Multiscale real time and high sensitivity ion detection with complementary organic electrochemical transistors amplifier. Nat. Commun. 2020, 11, 3743. [Google Scholar] [CrossRef]
- Song, H.; Shin, H.; Seo, H.; Park, W.; Joo, B.J.; Kim, J.; Kim, J.; Kim, H.K.; Kim, J.; Park, J.U. Wireless non-invasive monitoring of cholesterol using a smart contact lens. Adv. Sci. 2022, 9, 2203597. [Google Scholar] [CrossRef]
- Campuzano, S.; Barderas, R.; Moreno-Casbas, M.T.; Almeida, Á.; Pingarrón, J.M. Pursuing precision in medicine and nutrition: The rise of electrochemical biosensing at the molecular level. Anal. Bioanal. Chem. 2024, 416, 2151–2172. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhong, A. Electrochemical detection strategies for cardiovascular disease biomarkers: Applications in troponin I and troponin T assays. Int. J. Electrochem. Sci. 2024, 19, 100675. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Liang, Y.; Yang, J.; Lin, Y.; Li, Y. Microneedle-Based Electrochemical Array Patch for Ultra-Antifouling and Ultra-Anti-Interference Monitoring of Subcutaneous Oxygen. Anal. Chem. 2024, 97, 373–381. [Google Scholar] [CrossRef]
- Mandali, P.K.; Prabakaran, A.; Annadurai, K.; Krishnan, U.M. Trends in quantification of HbA1c using electrochemical and point-of-care analyzers. Sensors 2023, 23, 1901. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes: Developed by the task force on the management of cardiovascular disease in patients with diabetes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Amjad, A.; Xian, X. Optical Sensors for Transdermal Biomarker Detection: A Review. Biosens. Bioelectron. 2024, 267, 116844. [Google Scholar] [CrossRef]
- Pereira, R.H.A.; Prado, A.R.; Caro, L.F.C.D.; Zanardo, T.É.C.; Alencar, A.P.; Nogueira, B.V. A non-linear mathematical model using optical sensor to predict heart decellularization efficacy. Sci. Rep. 2019, 9, 12211. [Google Scholar] [CrossRef]
- Jena, P.V.; Roxbury, D.; Galassi, T.V.; Akkari, L.; Horoszko, C.P.; Iaea, D.B.; Budhathoki-Uprety, J.; Pipalia, N.; Haka, A.S.; Harvey, J.D. A carbon nanotube optical reporter maps endolysosomal lipid flux. ACS Nano 2017, 11, 10689–10703. [Google Scholar] [CrossRef]
- Sun, T.; Zhao, H.; Hu, L.; Shao, X.; Lu, Z.; Wang, Y.; Ling, P.; Li, Y.; Zeng, K.; Chen, Q. Enhanced optical imaging and fluorescent labeling for visualizing drug molecules within living organisms. Acta Pharm. Sin. B 2024, 14, 2428–2446. [Google Scholar] [CrossRef]
- Nakayama, A.; Otani, A.; Inokuma, T.; Tsuji, D.; Mukaiyama, H.; Nakayama, A.; Itoh, K.; Otaka, A.; Tanino, K.; Namba, K. Development of a 1, 3a, 6a-triazapentalene derivative as a compact and thiol-specific fluorescent labeling reagent. Commun. Chem. 2020, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating sources of inaccuracy in wearable optical heart rate sensors. NPJ Digit. Med. 2020, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical sensors. Anal. Chem. 2010, 82, 4723–4741. [Google Scholar] [CrossRef] [PubMed]
- Sarkodie, K.; Fergusson-Rees, A.; Diaz, P. A review of the application of non-intrusive infrared sensing for gas–liquid flow characterization. J. Comput. Multiph. Flows 2018, 10, 43–56. [Google Scholar] [CrossRef]
- Li, Y.; Dai, W.; Lv, X.; Deng, Y. Aptamer-based rolling circle amplification coupled with graphene oxide-based fluorescence resonance energy transfer for sensitive detection of cardiac troponin I. Anal. Methods 2018, 10, 1767–1773. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Kumar, V.; Kumar, A.; Kaur, I. Graphene quantum dots FRET based sensor for early detection of heart attack in human. Biosens. Bioelectron. 2016, 79, 495–499. [Google Scholar] [CrossRef]
- Ulber, R.; Frerichs, J.-G.; Beutel, S. Optical sensor systems for bioprocess monitoring. Anal. Bioanal. Chem. 2003, 376, 342–348. [Google Scholar] [CrossRef]
- Liu, H.; Yang, C.; Ma, J.; Xu, M. Multimodal soft sensor integrated with hydrogel-optoelectronic for wrist motion monitoring. Measurement 2025, 239, 115486. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Denizli, A. Recent advances in optical biosensing approaches for biomarkers detection. Biosens. Bioelectron. X 2022, 12, 100269. [Google Scholar] [CrossRef]
- Luo, Z.; Li, M.; Kong, X.; Li, Y.; Li, W.; Tian, Z.; Cao, Q.; Zaman, M.H.; Li, Y.; Xiao, W. Advance on fiber optic-based biosensors for precision medicine: From diagnosis to therapy. Interdiscip. Med. 2023, 1, e20230022. [Google Scholar] [CrossRef]
- Ambrose, J.A.; Singh, M. Pathophysiology of coronary artery disease leading to acute coronary syndromes. F1000prime Rep. 2015, 7, 08. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M. 2025 ACC/AHA/ACEP/NAEMSP/SCAI guideline for the management of patients with acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Choi, K.H.; Song, Y.B.; Lee, J.-Y.; Lee, S.-J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J. Intravascular imaging–guided or angiography-guided complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Su, Y.; Joe, P.; Yona, R.; Liu, Y.; Kim, Y.-S.; Huang, Y.; Damadoran, A.R.; Xia, J.; Martin, L.W. Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nat. Commun. 2014, 5, 4496. [Google Scholar] [CrossRef]
- Boutry, C.M.; Kaizawa, Y.; Schroeder, B.C.; Chortos, A.; Legrand, A.; Wang, Z.; Chang, J.; Fox, P.; Bao, Z. A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat. Electron. 2018, 1, 314–321. [Google Scholar] [CrossRef]
- Hasenkamp, W.; Forchelet, D.; Pataky, K.; Villard, J.; Lintel, H.V.; Bertsch, A.; Wang, Q.; Renaud, P. Polyimide/SU-8 catheter-tip MEMS gauge pressure sensor. Biomed. Microdevices 2012, 14, 819–828. [Google Scholar] [CrossRef]
- Huang, S.; Gao, Y.; Hu, Y.; Shen, F.; Jin, Z.; Cho, Y. Recent development of piezoelectric biosensors for physiological signal detection and machine learning assisted cardiovascular disease diagnosis. RSC Adv. 2023, 13, 29174–29194. [Google Scholar] [CrossRef]
- Davidson, C.J.; Bonow, R.O. Cardiac catheterization. Libby P 1997, 10, 18. [Google Scholar]
- Pilz, N.; Picone, D.; Patzak, A.; Opatz, O.; Lindner, T.; Fesseler, L.; Heinz, V.; Bothe, T. Cuff-based blood pressure measurement: Challenges and solutions. Blood Press. 2024, 33, 2402368. [Google Scholar] [CrossRef]
- Abraham, W.T.; Perl, L. Implantable hemodynamic monitoring for heart failure patients. J. Am. Coll. Cardiol. 2017, 70, 389–398. [Google Scholar] [CrossRef]
- Meng, K.; Xiao, X.; Wei, W.; Chen, G.; Nashalian, A.; Shen, S.; Xiao, X.; Chen, J. Wearable pressure sensors for pulse wave monitoring. Adv. Mater. 2022, 34, 2109357. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, Y.; Yang, Y.; Zheng, L.; Luo, L.; Gao, J.; Jiang, H.; Song, J.; Xu, M.; Wang, X. The soft-strain effect enabled high-performance flexible pressure sensor and its application in monitoring pulse waves. Research 2022, 2022, 0002. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.K.; Flanigan, D.P.T.; White, S.M.; Rice, T.B. A real-time blood flow measurement device for patients with peripheral artery disease. J. Vasc. Interv. Radiol. 2021, 32, 453–458. [Google Scholar] [CrossRef]
- Mancia, G.; Bombelli, M.; Seravalle, G.; Grassi, G. Diagnosis and management of patients with white-coat and masked hypertension. Nat. Rev. Cardiol. 2011, 8, 686–693. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Q.; Wang, Y.; Zhang, W.; Liu, J.; Xu, D.; Xia, X.; Hu, S.; Bian, B.; Zhao, Y. 3D printing wide detection range and high sensitivity flexible pressure sensor and its applications. React. Funct. Polym. 2024, 196, 105840. [Google Scholar] [CrossRef]
- Ma, Q.; Sun, L.; Wang, B. A strain-insensitive quantitative pressure sensor on your dynamic tissue. Matter 2022, 5, 782–784. [Google Scholar] [CrossRef]
- Li, F.; Wang, H.; Nan, S.; Yang, Y.; Wang, Z.; Zhu, R.; Zhang, T.; Zhang, J. Flexible pressure sensors tuned by interface structure design–Numerical and experimental study. Appl. Surf. Sci. 2023, 638, 158021. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, X.; Chen, S.C.; Zhao, N. Emerging technologies of flexible pressure sensors: Materials, modeling, devices, and manufacturing. Adv. Funct. Mater. 2019, 29, 1808509. [Google Scholar] [CrossRef]
- Basov, M.; Prigodskiy, D.M. Investigation of high-sensitivity piezoresistive pressure sensors at ultra-low differential pressures. IEEE Sens. J. 2020, 20, 7646–7652. [Google Scholar] [CrossRef]
- Basov, M.; Prigodskiy, D. Development of high-sensitivity piezoresistive pressure sensors for−0.5…+ 0.5 kPa. J. Micromech. Microeng. 2020, 30, 105006. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Wang, Z.; Yan, H. Novel method for processing the dynamic calibration signal of pressure sensor. Sensors 2015, 15, 17748–17766. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (vol 71, pg 2199, 2018). J. Am. Coll. Cardiol. 2018, 71, 2273. [Google Scholar]
- Pickering, T.G.; Shimbo, D.; Haas, D. Ambulatory blood-pressure monitoring. N. Engl. J. Med. 2006, 354, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Tunis, S.; Kendall, P.; Londner, M.; Whyte, J. Decision Memo for Ambulatory Blood Pressure Monitoring (CAG-00067N). 2001. Available online: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=Y&NCAId=294 (accessed on 5 March 2025).
- Leung, A.A.; Daskalopoulou, S.S.; Dasgupta, K.; McBrien, K.; Butalia, S.; Zarnke, K.B.; Nerenberg, K.; Harris, K.C.; Nakhla, M.; Cloutier, L. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can. J. Cardiol. 2017, 33, 557–576. [Google Scholar] [CrossRef]
- Wang, M.; Jin, L.; Hang-Mei Leung, P.; Wang-Ngai Chow, F.; Zhao, X.; Chen, H.; Pan, W.; Liu, H.; Li, S. Advancements in magnetic nanoparticle-based biosensors for point-of-care testing. Front. Bioeng. Biotechnol. 2024, 12, 1393789. [Google Scholar] [CrossRef]
- Roth, B.J. The magnetocardiogram. Biophys. Rev. 2024, 5, 021305. [Google Scholar] [CrossRef]
- Proudfoot, M.; Woolrich, M.W.; Nobre, A.C.; Turner, M.R. Magnetoencephalography. Pract. Neurol. 2014, 14, 336–343. [Google Scholar] [CrossRef]
- Khan, W.U.; Alissa, M.; Abouzied, A.S.; Alsugoor, M.H.; Sullivan, M. Navigating Sensor-Skin Coupling Challenges in Magnetic-Based Blood Pressure Monitoring: Innovations and Clinical Implications for Hypertension and Aortovascular Disease Management. Curr. Probl. Cardiol. 2024, 50, 102964. [Google Scholar] [CrossRef]
- Lau, S.; Petković, B.; Haueisen, J. Optimal magnetic sensor vests for cardiac source imaging. Sensors 2016, 16, 754. [Google Scholar] [CrossRef]
- Elsayed, H.A.; Medhat, M.; Hajjiah, A.; Alfassam, H.E.; Abukhadra, M.R.; Mehaney, A. A promising high-sensitive 1D photonic crystal magnetic field sensor based on the coupling of Fano\Tamm resonance in far IR region. Sci. Rep. 2025, 15, 1977. [Google Scholar] [CrossRef]
- Zhu, R.; Li, Z.; Deng, G.; Yu, Y.; Shui, J.; Yu, R.; Pan, C.; Liu, X. Anisotropic magnetic liquid metal film for wearable wireless electromagnetic sensing and smart electromagnetic interference shielding. Nano Energy 2022, 92, 106700. [Google Scholar] [CrossRef]
- Sobol, W.T. Recent advances in MRI technology: Implications for image quality and patient safety. Saudi J. Ophthalmol. 2012, 26, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Steeden, J.A.; Quail, M.; Gotschy, A.; Mortensen, K.H.; Hauptmann, A.; Arridge, S.; Jones, R.; Muthurangu, V. Rapid whole-heart CMR with single volume super-resolution. J. Cardiovasc. Magn. Reson. 2020, 22, 56. [Google Scholar] [CrossRef]
- Grogan, S.P.; Mount, C.A. Ultrasound physics and instrumentation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Zhang, T.; Liu, N.; Xu, J.; Liu, Z.; Zhou, Y.; Yang, Y.; Li, S.; Huang, Y.; Jiang, S. Flexible electronics for cardiovascular healthcare monitoring. Innovation 2023, 4, 100485. [Google Scholar] [CrossRef]
- Madore, B.; Hess, A.T.; van Niekerk, A.M.; Hoinkiss, D.C.; Hucker, P.; Zaitsev, M.; Afacan, O.; Günther, M. External hardware and sensors, for improved MRI. J. Magn. Reson. Imaging 2023, 57, 690–705. [Google Scholar] [CrossRef]
- Lang, C.; Fang, J.; Shao, H.; Ding, X.; Lin, T. High-sensitivity acoustic sensors from nanofibre webs. Nat. Commun. 2016, 7, 11108. [Google Scholar] [CrossRef]
- Hu, H.; Huang, H.; Li, M.; Gao, X.; Yin, L.; Qi, R.; Wu, R.S.; Chen, X.; Ma, Y.; Shi, K. A wearable cardiac ultrasound imager. Nature 2023, 613, 667–675. [Google Scholar] [CrossRef]
- Shung, K.K.; Sigelmann, R.A.; Reid, J.M. Scattering of ultrasound by blood. IEEE Trans. Biomed. Eng. 1976, 23, 460–467. [Google Scholar] [CrossRef]
- Degirmenci, A.; Perrin, D.P.; Howe, R.D. High dynamic range ultrasound imaging. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 721–729. [Google Scholar] [CrossRef]
- Burk, R.S.; Parker, A.; Sievers, L.; Rooney, M.B.; Pepperl, A.; Schubert, C.M.; Grap, M.J. Effects of position and operator on high-frequency ultrasound scan quality. Intensive Crit. Care Nurs. 2015, 31, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Wessels, P.W.; Basten, T.G. Design aspects of acoustic sensor networks for environmental noise monitoring. Appl. Acoust. 2016, 110, 227–234. [Google Scholar] [CrossRef]
- Hou, X.; Liu, C. Rope Jumping Strength Monitoring on Smart Devices via Passive Acoustic Sensing. Sensors 2022, 22, 9739. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-W.; Prager, R.W.; Gee, A.H.; Treece, G.M. Freehand 3D ultrasound calibration: A review. In Advanced Imaging in Biology and Medicine: Technology, Software Environments, Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 47–84. [Google Scholar]
- Liu, T.; Mao, Y.; Dou, H.; Zhang, W.; Yang, J.; Wu, P.; Li, D.; Mu, X. Emerging Wearable Acoustic Sensing Technologies. Adv. Sci. 2025, 12, 2408653. [Google Scholar] [CrossRef]
- Edler, I.; Lindström, K. The history of echocardiography. Ultrasound Med. Biol. 2004, 30, 1565–1644. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Cardiovasc. Comput. Tomogr. 2022, 16, 54–122. [Google Scholar] [CrossRef]
- Sessler, D.I. Temperature monitoring and perioperative thermoregulation. Anesthesiology 2008, 109, 318. [Google Scholar] [CrossRef]
- Tansey, E.A.; Johnson, C.D. Recent advances in thermoregulation. Adv. Physiol. Educ. 2015, 39, 139–148. [Google Scholar] [CrossRef]
- Moser, Y.; Gijs, M.A. Miniaturized flexible temperature sensor. J. Microelectromech. Syst. 2007, 16, 1349–1354. [Google Scholar] [CrossRef]
- Rai, V.K. Temperature sensors and optical sensors. Appl. Phys. B 2007, 88, 297–303. [Google Scholar] [CrossRef]
- Zhao, Y.; Bergmann, J.H. Non-contact infrared thermometers and thermal scanners for human body temperature monitoring: A systematic review. Sensors 2023, 23, 7439. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Arafa, H.M.; Yang, T.; Albadawi, H.; Fowl, R.J.; Zhang, Z.; Kandula, V.; Ramesh, A.; Correia, C.; Huang, Y. A soft thermal sensor for the continuous assessment of flow in vascular access. Nat. Commun. 2025, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, G.M.; Ciarka, A.; Biniecka, M.; Ceranowicz, P. Right heart catheterization—Background, physiological basics, and clinical implications. J. Clin. Med. 2019, 8, 1331. [Google Scholar] [CrossRef]
- Scholte, N.T.; van Ravensberg, A.E.; Shakoor, A.; Boersma, E.; Ronner, E.; de Boer, R.A.; Brugts, J.J.; Bruining, N.; van der Boon, R.M. A scoping review on advancements in noninvasive wearable technology for heart failure management. NPJ Digit. Med. 2024, 7, 279. [Google Scholar] [CrossRef]
- Lin, J.; Fu, R.; Zhong, X.; Yu, P.; Tan, G.; Li, W.; Zhang, H.; Li, Y.; Zhou, L.; Ning, C. Wearable sensors and devices for real-time cardiovascular disease monitoring. Cell Rep. Phys. Sci. 2021, 2, 100541. [Google Scholar] [CrossRef]
- Wei, D.; Saidel, G.M.; Jones, S.C. Optimal design of a thermistor probe for surface measurement of cerebral blood flow. IEEE Trans. Biomed. Eng. 1990, 37, 1159–1172. [Google Scholar] [CrossRef]
- Bill, A. Studies of the heated thermocouple principle for determinations of blood flow in tissues. Acta Physiol. Scand. 1962, 55, 111–126. [Google Scholar] [CrossRef]
- Mimoun, B.; Van Der Horst, A.; Dekker, R.; Van Der Voort, D.; Rutten, M.; Van de Vosse, F. Thermal flow sensors on flexible substrates for minimally invasive medical instruments. In Proceedings of the SENSORS, 2012 IEEE, Taipei, Taiwan, 28–31 October 2012; pp. 1–4. [Google Scholar]
- Su, Y.; Ma, C.; Chen, J.; Wu, H.; Luo, W.; Peng, Y.; Luo, Z.; Li, L.; Tan, Y.; Omisore, O.M. Printable, highly sensitive flexible temperature sensors for human body temperature monitoring: A review. Nanoscale Res. Lett. 2020, 15, 1–34. [Google Scholar] [CrossRef]
- Lu, D.; Li, S.; Yang, Q.; Arafa, H.M.; Xu, Y.; Yan, Y.; Ostojich, D.; Bai, W.; Guo, H.; Wu, C. Implantable, wireless, self-fixing thermal sensors for continuous measurements of microvascular blood flow in flaps and organ grafts. Biosens. Bioelectron. 2022, 206, 114145. [Google Scholar] [CrossRef]
- Leung, C.M.; De Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Ribas, J.; Sadeghi, H.; Manbachi, A.; Leijten, J.; Brinegar, K.; Zhang, Y.S.; Ferreira, L.; Khademhosseini, A. Cardiovascular organ-on-a-chip platforms for drug discovery and development. Appl. Vitr. Toxicol. 2016, 2, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Odijk, M.; Baumann, A.; Lohmann, W.; Van Den Brink, F.; Olthuis, W.; Karst, U.; Van Den Berg, A. A microfluidic chip for electrochemical conversions in drug metabolism studies. Lab A Chip 2009, 9, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-chip: A new paradigm for drug development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef]
- Shen, J.X.; Youhanna, S.; Zandi Shafagh, R.; Kele, J.; Lauschke, V.M. Organotypic and microphysiological models of liver, gut, and kidney for studies of drug metabolism, pharmacokinetics, and toxicity. Chem. Res. Toxicol. 2019, 33, 38–60. [Google Scholar] [CrossRef]
- Donoghue, L.; Nguyen, K.T.; Graham, C.; Sethu, P. Tissue chips and microphysiological systems for disease modeling and drug testing. Micromachines 2021, 12, 139. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Kwon, K.W.; Jang, K.-J.; Park, M.C.; Lee, J.S.; Suh, K.Y. Adhesion assays of endothelial cells on nanopatterned surfaces within a microfluidic channel. Anal. Chem. 2010, 82, 3016–3022. [Google Scholar] [CrossRef]
- Van der Meer, A.D.; Vermeul, K.; Poot, A.A.; Feijen, J.; Vermes, I. A microfluidic wound-healing assay for quantifying endothelial cell migration. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, H719–H725. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, X.; Chen, Z.; Luo, Y.; Lu, Y.; Liu, T.; Wu, Z.; Jin, Y.; Zhao, W.; Lin, B. A cell lines derived microfluidic liver model for investigation of hepatotoxicity induced by drug-drug interaction. Biomicrofluidics 2019, 13, 024101. [Google Scholar] [CrossRef]
- Sikanen, T.M.; Kiiski, I.; Ollikainen, E. Microfluidic Analysis Techniques for Safety Assessment of Pharmaceutical Nano-and Microsystems. In Characterization of Pharmaceutical Nano and Microsystems; Wiley: Hoboken, NJ, USA, 2021; pp. 97–135. [Google Scholar]
- Ayuso, J.M.; Virumbrales-Muñoz, M.; Lang, J.M.; Beebe, D.J. A role for microfluidic systems in precision medicine. Nat. Commun. 2022, 13, 3086. [Google Scholar] [CrossRef] [PubMed]
- Low, L.A.; Tagle, D.A. ‘You-on-a-chip’for precision medicine. Expert Rev. Precis. Med. Drug Dev. 2018, 3, 137–146. [Google Scholar] [CrossRef]
- Bax, M.; Thorpe, J.; Romanov, V. The future of personalized cardiovascular medicine demands 3D and 4D printing, stem cells, and artificial intelligence. Front. Sens. 2023, 4, 1294721. [Google Scholar] [CrossRef]
- Pala, R.; Pattnaik, S.; Busi, S.; Nauli, S.M. Nanomaterials as novel cardiovascular theranostics. Pharmaceutics 2021, 13, 348. [Google Scholar] [CrossRef]
- Philip, A.; Kumar, A.R. The performance enhancement of surface plasmon resonance optical sensors using nanomaterials: A review. Coord. Chem. Rev. 2022, 458, 214424. [Google Scholar] [CrossRef]
- Sharma, P.A.; Maheshwari, R.; Tekade, M.; Kumar Tekade, R. Nanomaterial based approaches for the diagnosis and therapy of cardiovascular diseases. Curr. Pharm. Des. 2015, 21, 4465–4478. [Google Scholar] [CrossRef]
- Garg, M.; Gupta, A.; Sharma, A.L.; Singh, S. Advancements in 2D materials based biosensors for oxidative stress biomarkers. ACS Appl. Bio Mater. 2021, 4, 5944–5960. [Google Scholar] [CrossRef]
- Gunache, R.O.; Bounegru, A.V.; Apetrei, C. Determination of atorvastatin with voltammetric sensors based on nanomaterials. Inventions 2021, 6, 57. [Google Scholar] [CrossRef]
- Martins de Oliveira, A.; Matias Silva, R.; Dias da Silva, A.; Silva, T.A. Electroanalysis of Statin Drugs: A Review on the Electrochemical Sensor Architectures Ranging from Classical to Modern Systems. Crit. Rev. Anal. Chem. 2024, 1–20. [Google Scholar] [CrossRef]
- Nenna, A.; Nappi, F.; Larobina, D.; Verghi, E.; Chello, M.; Ambrosio, L. Polymers and nanoparticles for statin delivery: Current use and future perspectives in cardiovascular disease. Polymers 2021, 13, 711. [Google Scholar] [CrossRef]
- Roșca, R.O.; Bounegru, A.V.; Apetrei, C. Quantification of Statins in Pharmaceutical Products Using Screen-Printed Sensors Based of Multi-Walled Carbon Nanotubes and Gold Nanoparticles. Inventions 2023, 8, 111. [Google Scholar] [CrossRef]
- Yawari, I.; Kaykhaii, M. Determination of (S)-warfarin using an activated screen printed gold electrode modified with gold nanoparticles and an enantioselective molecularly imprinted polymer. Anal. Methods 2017, 9, 6583–6589. [Google Scholar] [CrossRef]
- Taogesi; Wu, H.Y.; Murata, M.; Ren, H.F.; Endo, H. Carbon Nanotube-Enhanced Enzyme Sensor for Real-Time Monitoring of Cholesterol Levels in Free-Swimming Fish. Sens. Mater. 2015, 27, 805–815. [Google Scholar]
- Rettie, A.E.; Tai, G.Y. The pharmacogenomics of Warfarin—Closing in on personalized medicine. Mol. Interv. 2006, 6, 223–227. [Google Scholar] [CrossRef]
- Collier, D.J.; Taylor, M.; Godec, T.; Shiel, J.; James, R.; Chowdury, Y.; Ebano, P.; Monk, V.; Patel, M.; Pheby, J.; et al. Personalized Antihypertensive Treatment Optimization With Smartphone-Enabled Remote Precision Dosing of Amlodipine During the COVID-19 Pandemic (PERSONAL-CovidBP Trial). J. Am. Heart Assoc. 2024, 13, 030749. [Google Scholar] [CrossRef]
- Zhu, W.D.; Mazzanti, A.; Voelker, T.L.; Hou, P.P.; Moreno, J.D.; Angsutararux, P.; Naegle, K.M.; Priori, S.G.; Silva, J.R. Predicting Patient Response to the Antiarrhythmic Mexiletine Based on Genetic Variation Personalized Medicine for Long QT Syndrome. Circ. Res. 2019, 124, 539–552. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Iezzi, A.; Paglia, G.; Banfi, C. Pharmacometabolomics for the Study of Lipid-Lowering Therapies: Opportunities and Challenges. Int. J. Mol. Sci. 2023, 24, 3291. [Google Scholar] [CrossRef]
- Brown, R.E.; Dorion, R.P.; Trowbridge, C.; Stammers, A.H.; Fitt, W.; Davis, J. Algorithmic and Consultative Integration of Transfusion Medicine and Coagulation: A Personalized Medicine Approach with Reduced Blood Component Utilization. Ann. Clin. Lab. Sci. 2011, 41, 211–216. [Google Scholar]
- Entcheva, E.; Kay, M.W. Cardiac optogenetics: A decade of enlightenment. Nat. Rev. Cardiol. 2021, 18, 349–367. [Google Scholar] [CrossRef]
- Abeltino, A.; Hatem, D.; Serantoni, C.; Riente, A.; De Giulio, M.M.; De Spirito, M.; De Maio, F.; Maulucci, G. Unraveling the Gut Microbiota: Implications for Precision Nutrition and Personalized Medicine. Nutrients 2024, 16, 3806. [Google Scholar] [CrossRef]
- Valke, L.; Rijpma, S.; Meijer, D.; Schols, S.E.M.; van Heerde, W.L. Thrombin generation assays to personalize treatment in bleeding and thrombotic diseases. Front. Cardiovasc. Med. 2022, 9, 1033416. [Google Scholar] [CrossRef] [PubMed]
- Kotchen, T.A. Historical Trends and Milestones in Hypertension Research A Model of the Process of Translational Research. Hypertension 2011, 58, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.L. Treating arrhythmias with adjunctive magnesium: Identifying future research directions. Eur. Heart J.-Cardiovasc. Pharmacother. 2017, 3, 108–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Havel, R.J.; Hunninghake, D.B.; Illingworth, D.R.; Lees, R.S.; Stein, E.A.; Tobert, J.A.; Bacon, S.R.; Bolognese, J.A.; Frost, P.H.; Lamkin, G.E.; et al. Lovastatin (mevinolin) in the treatment of heterozygous familial hypercholesterolemia—A multicenter study. Ann. Intern. Med. 1987, 107, 609–615. [Google Scholar] [CrossRef]
- Dunn, K.E.; Barrett, F.S.; Brands, B.; Marsh, D.C.; Bigelow, G.E. Individual differences in human opioid abuse potential as observed in a human laboratory study. Drug Alcohol Depend. 2019, 205, 107688. [Google Scholar] [CrossRef]
- Joseph, G.; Marott, J.L.; Torp-Pedersen, C.; Biering-Sørensen, T.; Nielsen, G.; Christensen, A.E.; Johansen, M.B.; Schnohr, P.; Sogaard, P.; Mogelvang, R. Dose-Response Association Between Level of Physical Activity and Mortality in Normal, Elevated, and High Blood Pressure. Hypertension 2019, 74, 1307–1315. [Google Scholar] [CrossRef]
- Dagenais, G.R.; Pais, P.; Gao, P.; Roshandel, G.; Malekzadeh, R.; Joseph, P.; Yusuf, S. Fixed dose combination therapies in primary cardiovascular disease prevention in different groups: An individual participant meta-analysis. Heart 2023, 109, 1372–1379. [Google Scholar] [CrossRef]
- Wallin, R.; Wajih, N.; Hutson, S.M. VKORC1: A warfarin-sensitive enzyme in vitamin K metabolism and biosynthesis of vitamin K-dependent blood coagulation factors. Vitam Horm 2008, 78, 227–246. [Google Scholar] [CrossRef]
- Rost, S.; Fregin, A.; Ivaskevicius, V.; Conzelmann, E.; Hörtnagel, K.; Pelz, H.J.; Lappegard, K.; Seifried, E.; Scharrer, I.; Tuddenham, E.G.; et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature 2004, 427, 537–541. [Google Scholar] [CrossRef]
- Fiessinger, J.N.; Huisman, M.V.; Davidson, B.L.; Bounameaux, H.; Francis, C.W.; Eriksson, H.; Lundström, T.; Berkowitz, S.D.; Nyström, P.; Thorsén, M.; et al. Ximelagatran vs. low-molecular-weight heparin and warfarin for the treatment of deep vein thrombosis: A randomized trial. Jama 2005, 293, 681–689. [Google Scholar] [CrossRef]
- Kostadima, E.; Zakynthinos, E. Pulmonary embolism: Pathophysiology, diagnosis, treatment. Hell. J. Cardiol 2007, 48, 94–107. [Google Scholar]
- Ridker, P.M.; Goldhaber, S.Z.; Danielson, E.; Rosenberg, Y.; Eby, C.S.; Deitcher, S.R.; Cushman, M.; Moll, S.; Kessler, C.M.; Elliott, C.G.; et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N. Engl. J. Med. 2003, 348, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Grzymala-Lubanski, B.; Labaf, A.; Englund, E.; Svensson, P.J.; Själander, A. Mechanical heart valve prosthesis and warfarin—Treatment quality and prognosis. Thromb. Res. 2014, 133, 795–798. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Korzekwa, K.R.; Jones, J.P.; Rettie, A.E.; Trager, W.F. Structural forms of phenprocoumon and warfarin that are metabolized at the active site of CYP2C9. Arch. Biochem. Biophys. 1999, 372, 16–28. [Google Scholar] [CrossRef]
- Schwarz, U.I. Clinical relevance of genetic polymorphisms in the human CYP2C9 gene. Eur. J. Clin. Invest. 2003, 33, 23–30. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Huang, S.Q.; Su, H.H.; Zhou, S.F. Genetic polymorphism of the human cytochrome P450 2C9 gene and its clinical significance. Curr. Drug Metab. 2009, 10, 781–834. [Google Scholar] [CrossRef]
- Watanabe, E. Small therapeutic window of warfarin in Japanese population. J. Cardiol. 2015, 65, 171–172. [Google Scholar] [CrossRef]
- Merli, G.J.; Tzanis, G. Warfarin: What are the clinical implications of an out-of-range-therapeutic international normalized ratio? J. Thromb. Thrombolysis 2009, 27, 293–299. [Google Scholar] [CrossRef]
- Lee, M.T.; Klein, T.E. Pharmacogenetics of warfarin: Challenges and opportunities. J. Hum. Genet. 2013, 58, 334–338. [Google Scholar] [CrossRef]
- Gao, X.; Passman, R. Stroke Prevention in Atrial Fibrillation. Curr. Cardiol. Rep. 2022, 24, 1765–1774. [Google Scholar] [CrossRef]
- Mohamed, S.; Mei Fong, C.; Jie Ming, Y.; Naila Kori, A.; Abdul Wahab, S.; Mohd Ali, Z. Evaluation of an Initiation Regimen of Warfarin for International Normalized Ratio Target 2.0 to 3.0. J. Pharm. Technol. 2021, 37, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Brasen, C.L.; Madsen, J.S.; Parkner, T.; Brandslund, I. Home Management of Warfarin Treatment Through a Real-Time Supervised Telemedicine Solution: A Randomized Controlled Trial. Telemed. J. e-Health 2019, 25, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.F.; Castro-López, V.; Killard, A.J. Coagulation monitoring devices: Past, present, and future at the point of care. TrAC Trends Anal. Chem. 2013, 50, 85–95. [Google Scholar] [CrossRef]
- Williams, N.X.; Carroll, B.; Noyce, S.G.; Hobbie, H.A.; Joh, D.Y.; Rogers, J.G.; Franklin, A.D. Fully printed prothrombin time sensor for point-of-care testing. Biosens. Bioelectron. 2021, 172, 112770. [Google Scholar] [CrossRef]
- Tripathi, M.M.; Egawa, S.; Wirth, A.G.; Tshikudi, D.M.; Van Cott, E.M.; Nadkarni, S.K. Clinical evaluation of whole blood prothrombin time (PT) and international normalized ratio (INR) using a Laser Speckle Rheology sensor. Sci. Rep. 2017, 7, 9169. [Google Scholar] [CrossRef]
- Mohammadi Aria, M.; Erten, A.; Yalcin, O. Technology Advancements in Blood Coagulation Measurements for Point-of-Care Diagnostic Testing. Front. Bioeng. Biotechnol. 2019, 7, 395. [Google Scholar] [CrossRef]
- Gupta, P.; Mohan, A.; Mishra, A.; Nair, A.; Chowdhury, N.; Balekai, D.; Rai, K.; Prabhakar, A.; Saiyed, T. Multiplexed fluorescence and scatter detection with single cell resolution using on-chip fiber optics for droplet microfluidic applications. Microsyst. Nanoeng. 2024, 10, 35. [Google Scholar] [CrossRef]
- Dusse, L.M.; Oliveira, N.C.; Rios, D.R.; Marcolino, M.S. Point-of-care test (POCT) INR: Hope or illusion? Rev. Bras. Cir. Cardiovasc. 2012, 27, 296–301. [Google Scholar] [CrossRef]
- Chan, J.; Michaelsen, K.; Estergreen, J.K.; Sabath, D.E.; Gollakota, S. Micro-mechanical blood clot testing using smartphones. Nat. Commun. 2022, 13, 831. [Google Scholar] [CrossRef]
- Ripley, T.L.; Saseen, J.J. β-blockers: A review of their pharmacological and physiological diversity in hypertension. Ann. Pharmacother. 2014, 48, 723–733. [Google Scholar] [CrossRef]
- Oliver, E.; Mayor, F., Jr.; D’Ocon, P. Beta-blockers: Historical Perspective and Mechanisms of Action. Rev. Esp. Cardiol. 2019, 72, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; McAlister, F.A. Re-examining the efficacy of beta-blockers for the treatment of hypertension: A meta-analysis. Cmaj 2006, 174, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- de Muinck, E.D.; Lie, K.I. Safety and efficacy of beta-blockers in the treatment of stable angina pectoris. J. Cardiovasc. Pharmacol. 1990, 16, S123–S128. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Rossignol, P. Beta-blockers in heart failure patients with severe chronic kidney disease-time for a randomized controlled trial? Nephrol. Dial. Transpl. 2020, 35, 728–731. [Google Scholar] [CrossRef]
- Chatterjee, S.; Biondi-Zoccai, G.; Abbate, A.; D’Ascenzo, F.; Castagno, D.; Van Tassell, B.; Mukherjee, D.; Lichstein, E. Benefits of β blockers in patients with heart failure and reduced ejection fraction: Network meta-analysis. Bmj 2013, 346, f55. [Google Scholar] [CrossRef]
- Ko, D.T.; Hebert, P.R.; Coffey, C.S.; Curtis, J.P.; Foody, J.M.; Sedrakyan, A.; Krumholz, H.M. Adverse effects of beta-blocker therapy for patients with heart failure: A quantitative overview of randomized trials. Arch. Intern. Med. 2004, 164, 1389–1394. [Google Scholar] [CrossRef]
- Bateman, B.T.; Patorno, E.; Desai, R.J.; Seely, E.W.; Mogun, H.; Maeda, A.; Fischer, M.A.; Hernandez-Diaz, S.; Huybrechts, K.F. Late Pregnancy β Blocker Exposure and Risks of Neonatal Hypoglycemia and Bradycardia. Pediatrics 2016, 138, e20160731. [Google Scholar] [CrossRef]
- Niu, W.; Qi, Y. A meta-analysis of randomized controlled trials assessing the impact of beta-blockers on arterial stiffness, peripheral blood pressure and heart rate. Int. J. Cardiol. 2016, 218, 109–117. [Google Scholar] [CrossRef]
- Thackray, S.D.; Ghosh, J.M.; Wright, G.A.; Witte, K.K.; Nikitin, N.P.; Kaye, G.C.; Clark, A.L.; Tweddel, A.; Cleland, J.G. The effect of altering heart rate on ventricular function in patients with heart failure treated with beta-blockers. Am. Heart J. 2006, 152, 713.e9–713.e13. [Google Scholar] [CrossRef]
- Pucci, G.; Ranalli, M.G.; Battista, F.; Schillaci, G. Effects of β-Blockers With and Without Vasodilating Properties on Central Blood Pressure: Systematic Review and Meta-Analysis of Randomized Trials in Hypertension. Hypertension 2016, 67, 316–324. [Google Scholar] [CrossRef]
- Aronson, D.; Burger, A.J. Effect of beta-blockade on heart rate variability in decompensated heart failure. Int. J. Cardiol. 2001, 79, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.E.; Cockcroft, J.R. The vasodilatory beta-blockers. Curr. Hypertens. Rep. 2007, 9, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Delpón, E. Optimization of beta-blockers’ pharmacology. J. Cardiovasc. Pharmacol. 1990, 16, S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Contenti, J.; Occelli, C.; Corraze, H.; Lemoël, F.; Levraut, J. Long-Term β-Blocker Therapy Decreases Blood Lactate Concentration in Severely Septic Patients. Crit. Care Med. 2015, 43, 2616–2622. [Google Scholar] [CrossRef]
- Love, J.N.; Enlow, B.; Howell, J.M.; Klein-Schwartz, W.; Litovitz, T.L. Electrocardiographic changes associated with beta-blocker toxicity. Ann. Emerg. Med. 2002, 40, 603–610. [Google Scholar] [CrossRef]
- Oba, Y.; Kabutoya, T.; Kohro, T.; Imai, Y.; Kario, K.; Sato, H.; Nochioka, K.; Nakayama, M.; Fujita, H.; Mizuno, Y.; et al. Relationships Among Heart Rate, β-Blocker Dosage, and Prognosis in Patients With Coronary Artery Disease in a Real-World Database Using a Multimodal Data Acquisition System. Circ. J. 2023, 87, 336–344. [Google Scholar] [CrossRef]
- Graettinger, W.F.; Lipson, J.L.; Cheung, D.G.; Weber, M.A. Validation of portable noninvasive blood pressure monitoring devices: Comparisons with intra-arterial and sphygmomanometer measurements. Am. Heart J. 1988, 116, 1155–1160. [Google Scholar] [CrossRef]
- Galiè, N.; Seeger, W.; Naeije, R.; Simonneau, G.; Rubin, L.J. Comparative analysis of clinical trials and evidence-based treatment algorithm in pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004, 43, 81s–88s. [Google Scholar] [CrossRef]
- Mai, V.; Alattas, K.A.; Bouteraa, Y.; Ghaderpour, E.; Mohammadzadeh, A. Personalized blood pressure control by machine learning for remote patient monitoring. IEEE Access 2024, 12, 83994–84004. [Google Scholar] [CrossRef]
- Goodacre, S.; Irons, R. Atrial arrhythmias. Bmj 2002, 324, 594–597. [Google Scholar] [CrossRef]
- Keating, M.T.; Sanguinetti, M.C. Molecular and cellular mechanisms of cardiac arrhythmias. Cell 2001, 104, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Kodama, I.; Toyama, J.; Takanaka, C.; Yamada, K. Block of activated and inactivated sodium channels by class-I antiarrhythmic drugs studied by using the maximum upstroke velocity (Vmax) of action potential in guinea-pig cardiac muscles. J. Mol. Cell. Cardiol. 1987, 19, 367–377. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, C.; Pavlovic, D.; Rajpoot, K.; Winter, J. Examination of the effects of conduction slowing on the upstroke of optically recorded action potentials. Front. Physiol. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Zhou, Y.; Liu, Z.; Liu, R. Pharmacological mechanism of natural drugs and their active ingredients in the treatment of arrhythmia via calcium channel regulation. Biomed. Pharmacother. 2023, 160, 114413. [Google Scholar] [CrossRef]
- Fleckenstein, A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu. Rev. Pharmacol. Toxicol. 1977, 17, 149–166. [Google Scholar] [CrossRef]
- Tejera, E.; Nieto-Villar, J.; Rebelo, I. Unexpected heart rate variability complexity in the aging process of arrhythmic subjects. Commun. Nonlinear Sci. Numer. Simul. 2010, 15, 1858–1863. [Google Scholar] [CrossRef]
- Ni, H.; Morotti, S.; Grandi, E. A heart for diversity: Simulating variability in cardiac arrhythmia research. Front. Physiol. 2018, 9, 958. [Google Scholar] [CrossRef]
- Campbell, T.; Williams, K. Therapeutic drug monitoring: Antiarrhythmic drugs. Br. J. Clin. Pharmacol. 2001, 52, 21–33. [Google Scholar] [CrossRef]
- Zaza, A.; Ronchi, C.; Malfatto, G. Arrhythmias and heart rate: Mechanisms and significance of a relationship. Arrhythmia Electrophysiol. Rev. 2018, 7, 232. [Google Scholar] [CrossRef]
- Fu, D.-G. Cardiac arrhythmias: Diagnosis, symptoms, and treatments. Cell Biochem. Biophys. 2015, 73, 291–296. [Google Scholar] [CrossRef]
- Sahoo, S.; Dash, M.; Behera, S.; Sabut, S. Machine learning approach to detect cardiac arrhythmias in ECG signals: A survey. Irbm 2020, 41, 185–194. [Google Scholar] [CrossRef]
- Reed, M.J.; Robertson, C.; Addison, P. Heart rate variability measurements and the prediction of ventricular arrhythmias. Qjm 2005, 98, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sperelakis, N. Electrical properties of embryonic heart cells. In Electrical Phenomena in the Heart; Academic Press: New York, NY, USA, 1972; pp. 1–61. [Google Scholar]
- Bigger, J.T., Jr.; Sahar, D.I. Clinical types of proarrhythmic response to antiarrhythmic drugs. Am. J. Cardiol. 1987, 59, E2–E9. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Li, J.; Cui, C.; Wang, X.; Gao, H.; Liu, C.; Chen, M. A wearable real-time telemonitoring electrocardiogram device compared with traditional Holter monitoring. J. Biomed. Res. 2020, 35, 238. [Google Scholar] [CrossRef]
- Chaudhary, M.H.; Dev, S.; Kumari, A.; Kanwal, K.; Jadav, D.N.; Rasool, S.; Saleem, M.T.; Bhagat, R.; Prachi, F.; Puri, P. Holistic approaches to arrhythmia management: Combining medication, ablation, and device interventions. Cureus 2023, 15, 45958. [Google Scholar] [CrossRef]
- Shusterman, V.; London, B. Personalized ECG monitoring and adaptive machine learning. J. Electrocardiol. 2024, 82, 131–135. [Google Scholar] [CrossRef]
- Siddoway, L.A. Amiodarone: Guidelines for use and monitoring. Am. Fam. Physician 2003, 68, 2189–2197. [Google Scholar]
- Roy, D.; Talajic, M.; Dorian, P.; Connolly, S.; Eisenberg, M.J.; Green, M.; Kus, T.; Lambert, J.; Dubuc, M.; Gagné, P. Amiodarone to prevent recurrence of atrial fibrillation. N. Engl. J. Med. 2000, 342, 913–920. [Google Scholar] [CrossRef]
- Mujović, N.; Dobrev, D.; Marinković, M.; Russo, V.; Potpara, T.S. The role of amiodarone in contemporary management of complex cardiac arrhythmias. Pharmacol. Res. 2020, 151, 104521. [Google Scholar] [CrossRef]
- Banach, M.; Penson, P.E. Statins and LDL-C in secondary prevention—So much progress, so far to go. JAMA Netw. Open 2020, 3, e2025675. [Google Scholar] [CrossRef]
- Ferhatbegović, L.; Mršić, D.; Kušljugić, S.; Pojskić, B. LDL-C: The only causal risk factor for ASCVD. Why is it still overlooked and underestimated? Curr. Atheroscler. Rep. 2022, 24, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Underberg, J.; Toth, P.P.; Rodriguez, F. LDL-C target attainment in secondary prevention of ASCVD in the United States: Barriers, consequences of nonachievement, and strategies to reach goals. Postgrad. Med. 2022, 134, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Reiner, Ž. Statins in the primary prevention of cardiovascular disease. Nat. Rev. Cardiol. 2013, 10, 453–464. [Google Scholar] [CrossRef]
- Vrecer, M.; Turk, S.; Drinovec, J.; Mrhar, A. Use of statins in primary and secondary prevention of coronary heart disease and ischemic stroke: Meta-analysis of randomized trials. In Database of Abstracts of Reviews of Effects (DARE): Quality-Assessed Reviews; Centre for Reviews and Dissemination: York, UK, 2003. [Google Scholar]
- Tournadre, A. Statins, myalgia, and rhabdomyolysis. Jt. Bone Spine 2020, 87, 37–42. [Google Scholar] [CrossRef]
- Sakamoto, K.; Kimura, J. Mechanism of statin-induced rhabdomyolysis. J. Pharmacol. Sci. 2013, 123, 289–294. [Google Scholar] [CrossRef]
- Antons, K.A.; Williams, C.D.; Baker, S.K.; Phillips, P.S. Clinical perspectives of statin-induced rhabdomyolysis. Am. J. Med. 2006, 119, 400–409. [Google Scholar] [CrossRef]
- Safari, S.; Yousefifard, M.; Hashemi, B.; Baratloo, A.; Forouzanfar, M.M.; Rahmati, F.; Motamedi, M.; Najafi, I. The value of serum creatine kinase in predicting the risk of rhabdomyolysis-induced acute kidney injury: A systematic review and meta-analysis. Clin. Exp. Nephrol. 2016, 20, 153–161. [Google Scholar] [CrossRef]
- Luckoor, P.; Salehi, M.; Kunadu, A. Exceptionally high creatine kinase (CK) levels in multicausal and complicated rhabdomyolysis: A case report. Am. J. Case Rep. 2017, 18, 746. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef]
- Narwal, V.; Deswal, R.; Batra, B.; Kalra, V.; Hooda, R.; Sharma, M.; Rana, J. Cholesterol biosensors: A review. Steroids 2019, 143, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.M.; Park, J.-D.; Kang, H.-C.; Lee, J.-J. Recent development in nanomaterial-based electrochemical sensors for cholesterol detection. Chemosensors 2021, 9, 98. [Google Scholar] [CrossRef]
- Budiyanto, M.; Purnomo, A.; Sabtiawan, W.; Yasin, M. Development of optical fiber sensor performance as a tool to determine cholesterol concentration. J. Phys. Conf. Ser. 2019, 1417, 012005. [Google Scholar] [CrossRef]
- Li, Q.; Ding, L.; Zhang, Y.; Wu, T. A cholesterol optical fiber sensor based on CQDs-COD/CA composite. IEEE Sens. J. 2022, 22, 6247–6255. [Google Scholar] [CrossRef]
- Joshi, V.; Hussain, S.; Dua, S.; Arora, N.; Mir, S.H.; Rydzek, G.; Senthilkumar, T. Oligomer sensor nanoarchitectonics for “turn-on” fluorescence detection of cholesterol at the nanomolar level. Molecules 2022, 27, 2856. [Google Scholar] [CrossRef]
- Guo, L.; Chen, S.; Yu, Y.-L.; Wang, J.-H. A smartphone optical device for point-of-care testing of glucose and cholesterol using Ag NPs/UiO-66-NH2-based ratiometric fluorescent probe. Anal. Chem. 2021, 93, 16240–16247. [Google Scholar] [CrossRef]
- Ni, J.; Liu, Y.; Hong, H.; Kong, X.; Han, Y.; Zhang, L.; Zhang, Y.; Zhang, Y.; Hua, C.; Wang, Q. A novel fluorescent digitonin derivative for non-invasive skin cholesterol detection: Potential application in atherosclerosis screening. RSC Adv. 2022, 12, 18397–18406. [Google Scholar] [CrossRef]
- Demirbakan, B.; Sezgintürk, M.K. An electrochemical immunosensor based on graphite paper electrodes for the sensitive detection of creatine kinase in actual samples. J. Electroanal. Chem. 2022, 921, 116656. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Zhang, Y.; Li, Z.; Xiao, H.; Cui, Y. Electrochemical protein-based biosensors for creatine kinase: A review. IEEE Sens. J. 2022, 22, 10280–10291. [Google Scholar] [CrossRef]
- Li, H.; Nie, W. Electrochemical sensors for creatine kinase detection in blood serum based on glucose oxidase modified multiwalled carbon nanotubes. Int. J. Electrochem. Sci. 2024, 19, 100447. [Google Scholar] [CrossRef]
- Ferreira, A.L.; de Lima, L.F.; Moraes, A.S.; Rubira, R.J.; Constantino, C.J.; Leite, F.L.; Delgado-Silva, A.O.; Ferreira, M. Development of a novel biosensor for creatine kinase (CK-MB) using surface plasmon resonance (SPR). Appl. Surf. Sci. 2021, 554, 149565. [Google Scholar] [CrossRef]
- John, R.V.; Devasiya, T.; VR, N.; Adigal, S.; Lukose, J.; Kartha, V.; Chidangil, S. Cardiovascular biomarkers in body fluids: Progress and prospects in optical sensors. Biophys. Rev. 2022, 14, 1023–1050. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.T.; Dutra, R.A.; Noronha, J.P.; Sales, M.G.F. Novel sensory surface for creatine kinase electrochemical detection. Biosens. Bioelectron. 2014, 56, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, M.; Wu, D.; Wu, H.-F. Synthesis of Fluorescent Titanium Nanoclusters at ambient temperature for highly sensitive and selective detection of Creatine Kinase MM in myocardial infarction. Colloids Surf. B Biointerfaces 2022, 217, 112594. [Google Scholar] [CrossRef]
- Arneson, D.; Shu, L.; Tsai, B.; Barrere-Cain, R.; Sun, C.; Yang, X. Multidimensional integrative genomics approaches to dissecting cardiovascular disease. Front. Cardiovasc. Med. 2017, 4, 8. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Pan, D.; Wang, X.; Xu, Y.; Yan, J.; Wang, L.; Yang, X.; Yang, M.; Liu, G.P. Applications of multi-omics analysis in human diseases. MedComm 2023, 4, e315. [Google Scholar] [CrossRef]
- Ullah, A.; Kumar, M.; Sayyar, M.; Sapna, F.; John, C.; Memon, S.; Qureshi, K.; Agbo, E.C.; Ariri, H.I.; Chukwu, E.J. Revolutionizing cardiac care: A comprehensive narrative review of cardiac rehabilitation and the evolution of cardiovascular medicine. Cureus 2023, 15, e46469. [Google Scholar] [CrossRef]
- Namasivayam, V.; Senguttuvan, N.; Saravanan, V.; Palaniappan, S.; Kathiravan, M.K. Artificial intelligence and its application in cardiovascular disease management. In Machine Learning and Systems Biology in Genomics and Health; Springer: Berlin/Heidelberg, Germany, 2022; pp. 189–236. [Google Scholar]
- Garralda, E.; Dienstmann, R.; Tabernero, J. Pharmacokinetic/pharmacodynamic modeling for drug development in oncology. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 210–215. [Google Scholar] [CrossRef]
- Yıldırım, M.A.; Goh, K.-I.; Cusick, M.E.; Barabási, A.-L.; Vidal, M. Drug—Target network. Nat. Biotechnol. 2007, 25, 1119–1126. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision medicine, AI, and the future of personalized health care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Andes, D. Pharmacokinetics and pharmacodynamics of antifungals. Infect. Dis. Clin. 2006, 20, 679–697. [Google Scholar] [CrossRef] [PubMed]
- Tyson, R.J.; Park, C.C.; Powell, J.R.; Patterson, J.H.; Weiner, D.; Watkins, P.B.; Gonzalez, D. Precision dosing priority criteria: Drug, disease, and patient population variables. Front. Pharmacol. 2020, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Ma, C.; Wang, Z.; Miao, Y. A review of local anesthetic-induced heart toxicity using human induced pluripotent stem cell-derived cardiomyocytes. Mol. Cell. Probes 2024, 76, 101965. [Google Scholar] [CrossRef] [PubMed]
- Raz, I.; Riddle, M.C.; Rosenstock, J.; Buse, J.B.; Inzucchi, S.E.; Home, P.D.; Del Prato, S.; Ferrannini, E.; Chan, J.C.; Leiter, L.A. Personalized management of hyperglycemia in type 2 diabetes: Reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2013, 36, 1779–1788. [Google Scholar] [CrossRef]
- Marques, L.; Costa, B.; Pereira, M.; Silva, A.; Santos, J.; Saldanha, L.; Silva, I.; Magalhães, P.; Schmidt, S.; Vale, N. Advancing precision medicine: A review of innovative In Silico approaches for drug development, clinical pharmacology and personalized healthcare. Pharmaceutics 2024, 16, 332. [Google Scholar] [CrossRef]
- Niu, J.; Straubinger, R.M.; Mager, D.E. Pharmacodynamic drug–drug interactions. Clin. Pharmacol. Ther. 2019, 105, 1395–1406. [Google Scholar] [CrossRef]
- Vesell, E.S. Genetic and environmental factors affecting drug disposition in man. Clin. Pharmacol. Ther. 1977, 22, 659–679. [Google Scholar] [CrossRef]
- Knights, J.; Chanda, P.; Sato, Y.; Kaniwa, N.; Saito, Y.; Ueno, H.; Zhang, A.; Ramanathan, M. Vertical integration of pharmacogenetics in population PK/PD modeling: A novel information theoretic method. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Zhou, Y.; Arribas, G.H.; Turku, A.; Jürgenson, T.; Mkrtchian, S.; Krebs, K.; Wang, Y.; Svobodova, B.; Milani, L.; Schulte, G. Rare genetic variability in human drug target genes modulates drug response and can guide precision medicine. Sci. Adv. 2021, 7, eabi6856. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Jackson, S.H. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br. J. Clin. Pharmacol. 2004, 57, 6–14. [Google Scholar] [CrossRef]
- Reddy, N.; Verma, N.; Dungan, K. Monitoring technologies-continuous glucose monitoring, mobile technology, biomarkers of glycemic control. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2023. [Google Scholar]
- Tanoli, Z.; Alam, Z.; Vähä-Koskela, M.; Ravikumar, B.; Malyutina, A.; Jaiswal, A.; Tang, J.; Wennerberg, K.; Aittokallio, T. Drug Target Commons 2.0: A community platform for systematic analysis of drug–target interaction profiles. Database 2018, 2018, bay083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huai, Y.; Miao, Z.; Qian, A.; Wang, Y. Systems pharmacology for investigation of the mechanisms of action of traditional Chinese medicine in drug discovery. Front. Pharmacol. 2019, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Vogt, I.; Mestres, J. Drug-target networks. Mol. Inform. 2010, 29, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Zhao, S.; Iyengar, R. Systems pharmacology: Network analysis to identify multiscale mechanisms of drug action. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 505–521. [Google Scholar] [CrossRef]
- Breitenbach, T.; Lorenz, K.; Dandekar, T. How to steer and control ERK and the ERK signaling cascade exemplified by looking at cardiac insufficiency. Int. J. Mol. Sci. 2019, 20, 2179. [Google Scholar] [CrossRef]
- Katoh, M. Therapeutics targeting FGF signaling network in human diseases. Trends Pharmacol. Sci. 2016, 37, 1081–1096. [Google Scholar] [CrossRef]
- Shi, S.-H.; Cai, Y.-P.; Cai, X.-J.; Zheng, X.-Y.; Cao, D.-S.; Ye, F.-Q.; Xiang, Z. A network pharmacology approach to understanding the mechanisms of action of traditional medicine: Bushenhuoxue formula for treatment of chronic kidney disease. PLoS ONE 2014, 9, e89123. [Google Scholar] [CrossRef]
- Bakris, G.; Ali, W.; Parati, G. ACC/AHA versus ESC/ESH on hypertension guidelines: JACC guideline comparison. J. Am. Coll. Cardiol. 2019, 73, 3018–3026. [Google Scholar] [CrossRef]
- Bakris, G.L. A practical approach to achieving recommended blood pressure goals in diabetic patients. Arch. Intern. Med. 2001, 161, 2661–2667. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, Y.; Huang, W.; Zhou, H.; Zhang, W. Drug-microbiota interactions: An emerging priority for precision medicine. Signal Transduct. Target. Ther. 2023, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, X.; Cao, X.; Huang, C.; Liu, E.; Qian, S.; Liu, X.; Wu, Y.; Dong, F.; Qiu, C.-W. Artificial intelligence: A powerful paradigm for scientific research. Innovation 2021, 2, 100179. [Google Scholar] [CrossRef]
- Bohr, A.; Memarzadeh, K. The rise of artificial intelligence in healthcare applications. In Artificial Intelligence in Healthcare; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–60. [Google Scholar]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Khalifa, M.; Albadawy, M. Artificial Intelligence for Clinical Prediction: Exploring Key Domains and Essential Functions. Comput. Methods Programs Biomed. Update 2024, 5, 100148. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Khanna, N.N.; Laird, J.R.; Nicolaides, A.; Faa, G.; Johri, A.M.; Mantella, L.E.; Fernandes, J.F.E.; Teji, J.S. Artificial intelligence for cardiovascular disease risk assessment in personalised framework: A scoping review. EClinicalMedicine 2024, 73, 102660. [Google Scholar] [CrossRef]
- Xue, L.; He, S.; Singla, R.K.; Qin, Q.; Ding, Y.; Liu, L.; Ding, X.; Bediaga-Bañeres, H.; Arrasate, S.; Durado-Sanchez, A. Machine learning guided prediction of warfarin blood levels for personalized medicine based on clinical longitudinal data from cardiac surgery patients: A prospective observational study. Int. J. Surg. 2024, 110, 6528–6540. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Attia, Z.I.; Behnken, E.M.; Giblon, R.E.; Bews, K.A.; Liu, S.; Gosse, T.A.; Linn, Z.D.; Deng, Y.; Yin, J. Artificial intelligence-guided screening for atrial fibrillation using electrocardiogram during sinus rhythm: A prospective non-randomised interventional trial. Lancet 2022, 400, 1206–1212. [Google Scholar] [CrossRef]
- Lip, G.Y.; Frison, L.; Halperin, J.L.; Lane, D.A. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: The HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J. Am. Coll. Cardiol. 2011, 57, 173–180. [Google Scholar]
- Verdecchia, P.; Angeli, F.; Reboldi, G. Hypertension and atrial fibrillation: Doubts and certainties from basic and clinical studies. Circ. Res. 2018, 122, 352–368. [Google Scholar] [CrossRef]
- Page, R.L.; O’Bryant, C.L.; Cheng, D.; Dow, T.J.; Ky, B.; Stein, C.M.; Spencer, A.P.; Trupp, R.J.; Lindenfeld, J. Drugs that may cause or exacerbate heart failure: A scientific statement from the American Heart Association. Circulation 2016, 134, e32–e69. [Google Scholar] [CrossRef] [PubMed]
- Denfeld, Q.E.; Turrise, S.; MacLaughlin, E.J.; Chang, P.-S.; Clair, W.K.; Lewis, E.F.; Forman, D.E.; Goodlin, S.J.; on behalf of the American Heart Association Cardiovascular Disease in Older Populations Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health; et al. Preventing and managing falls in adults with cardiovascular disease: A scientific statement from the American Heart Association. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e000108. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, Y.; Chen, Y.; Sun, J.; Zhao, Z.; Chen, X.-w.; Matheny, M.E.; Xu, H. Large-scale prediction of adverse drug reactions using chemical, biological, and phenotypic properties of drugs. J. Am. Med. Inform. Assoc. 2012, 19, e28–e35. [Google Scholar] [CrossRef] [PubMed]
- Marvasti, T.B.; Gao, Y.; Murray, K.R.; Hershman, S.; McIntosh, C.; Moayedi, Y. Unlocking tomorrow’s health care: Expanding the clinical scope of wearables by applying artificial intelligence. Can. J. Cardiol. 2024, 40, 1934–1945. [Google Scholar] [CrossRef]
- Antikainen, E.; Linnosmaa, J.; Umer, A.; Oksala, N.; Eskola, M.; van Gils, M.; Hernesniemi, J.; Gabbouj, M. Transformers for cardiac patient mortality risk prediction from heterogeneous electronic health records. Sci. Rep. 2023, 13, 3517. [Google Scholar] [CrossRef]
- Rahman, A.U.; Alsenani, Y.; Zafar, A.; Ullah, K.; Rabie, K.; Shongwe, T. Enhancing heart disease prediction using a self-attention-based transformer model. Sci. Rep. 2024, 14, 514. [Google Scholar] [CrossRef]
- Giudicessi, J.R.; Ackerman, M.J.; Fatkin, D.; Kovacic, J.C. Precision medicine approaches to cardiac arrhythmias: JACC focus seminar 4/5. J. Am. Coll. Cardiol. 2021, 77, 2573–2591. [Google Scholar] [CrossRef]
- Acosta, J.N.; Falcone, G.J.; Rajpurkar, P.; Topol, E.J. Multimodal biomedical AI. Nat. Med. 2022, 28, 1773–1784. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lasalde-Ramírez, J.A.; Mahato, K.; Wang, J.; Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 2022, 6, 899–915. [Google Scholar] [CrossRef]
- Quazi, S. Artificial intelligence and machine learning in precision and genomic medicine. Med. Oncol. 2022, 39, 120. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, J.; Han, D.; Zhu, H. From machine learning to deep learning: Progress in machine intelligence for rational drug discovery. Drug Discov. Today 2017, 22, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Moshawrab, M.; Adda, M.; Bouzouane, A.; Ibrahim, H.; Raad, A. Reviewing multimodal machine learning and its use in cardiovascular diseases detection. Electronics 2023, 12, 1558. [Google Scholar] [CrossRef]
- Benetos, A.; Petrovic, M.; Strandberg, T. Hypertension management in older and frail older patients. Circ. Res. 2019, 124, 1045–1060. [Google Scholar] [CrossRef]
- NIo, A. The Dangers of Polypharmacy and the Case for Deprescribing in Older Adults. 2022. Available online: https://www.nia.nih.gov/news/dangers-polypharmacy-and-case-deprescribing-older-adults (accessed on 10 January 2025).
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Care, D. Cardiovascular disease and risk management: Standards of medical care in diabetesd 2021. Diabetes Care 2021, 44, S125–S150. [Google Scholar]
- Wiggins, B.S.; Saseen, J.J.; Page, R.L.; Reed, B.N.; Sneed, K.; Kostis, J.B.; Lanfear, D.; Virani, S.; Morris, P.B. Recommendations for management of clinically significant drug-drug interactions with statins and select agents used in patients with cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2016, 134, e468–e495. [Google Scholar] [CrossRef]
- Sugandh, F.; Chandio, M.; Raveena, F.; Kumar, L.; Karishma, F.; Khuwaja, S.; Memon, U.A.; Bai, K.; Kashif, M.; Varrassi, G. Advances in the management of diabetes mellitus: A focus on personalized medicine. Cureus 2023, 15, e43697. [Google Scholar] [CrossRef]
- Puccetti, M.; Pariano, M.; Schoubben, A.; Giovagnoli, S.; Ricci, M. Biologics, theranostics, and personalized medicine in drug delivery systems. Pharmacol. Res. 2024, 201, 107086. [Google Scholar] [CrossRef]
- Ahmad, A.; Imran, M.; Ahsan, H. Biomarkers as biomedical bioindicators: Approaches and techniques for the detection, analysis, and validation of novel Biomarkers of diseases. Pharmaceutics 2023, 15, 1630. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, N.; Huang, Z.a.; Lai, J.; Liu, J.n.; Deng, G.; Wang, X.; Zhao, W. High-Sensitivity Force Sensors Based on Novel Materials. Adv. Devices Instrum. 2023, 4, 0019. [Google Scholar] [CrossRef]
- Klebes, A.; Ates, H.C.; Verboket, R.D.; Urban, G.A.; von Stetten, F.; Dincer, C.; Früh, S.M. Emerging multianalyte biosensors for the simultaneous detection of protein and nucleic acid biomarkers. Biosens. Bioelectron. 2024, 244, 115800. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsyst. Nanoeng. 2023, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sapna, F.; Raveena, F.; Chandio, M.; Bai, K.; Sayyar, M.; Varrassi, G.; Khatri, M.; Kumar, S.; Mohamad, T. Advancements in heart failure management: A comprehensive narrative review of emerging therapies. Cureus 2023, 15, e46486. [Google Scholar] [CrossRef] [PubMed]
- Ignatiuk, B.; Baccillieri, M.; Frigo, G.; Marinaccio, L.; Cassinadri, E.; Montemurro, D.; Verlato, R.; Marchese, D.; Pasquetto, G. Costs and quality of care optimization in the remote monitoring of cardiac implantable electronic devices (CIEDs). A picture from Veneto, Northern Italy. Eur. Heart J. 2020, 41, ehaa946.3537. [Google Scholar] [CrossRef]
- Zhao, J.; Ghannam, R.; Htet, K.O.; Liu, Y.; Law, M.k.; Roy, V.A.; Michel, B.; Imran, M.A.; Heidari, H. Self-Powered implantable medical devices: Photovoltaic energy harvesting review. Adv. Healthc. Mater. 2020, 9, 2000779. [Google Scholar] [CrossRef]
- Shuvo, M.M.H.; Titirsha, T.; Amin, N.; Islam, S.K. Energy harvesting in implantable and wearable medical devices for enduring precision healthcare. Energies 2022, 15, 7495. [Google Scholar] [CrossRef]
- Zhang, S.; Bick, M.; Xiao, X.; Chen, G.; Nashalian, A.; Chen, J. Leveraging triboelectric nanogenerators for bioengineering. Matter 2021, 4, 845–887. [Google Scholar] [CrossRef]
- Le, H.T.; Haque, R.I.; Ouyang, Z.; Lee, S.W.; Fried, S.I.; Zhao, D.; Qiu, M.; Han, A. MEMS inductor fabrication and emerging applications in power electronics and neurotechnologies. Microsyst. Nanoeng. 2021, 7, 59. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Y.; Qu, X.; Liu, Y.; Cheng, S.; Zhang, Z.; Shan, Y.; Luo, R.; Weng, S.; Li, H. A self-powered intracardiac pacemaker in swine model. Nat. Commun. 2024, 15, 507. [Google Scholar] [CrossRef]
- Yogev, D.; Goldberg, T.; Arami, A.; Tejman-Yarden, S.; Winkler, T.E.; Maoz, B.M. Current state of the art and future directions for implantable sensors in medical technology: Clinical needs and engineering challenges. APL Bioeng. 2023, 7, 031506. [Google Scholar] [CrossRef]
- Alugubelli, N.; Abuissa, H.; Roka, A. Wearable devices for remote monitoring of heart rate and heart rate variability—What we know and what is coming. Sensors 2022, 22, 8903. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.; Van den Eynde, J.; Chinni, B.; Cedars, A.; Sen, D.G.; Kutty, S.; Manlhiot, C. Data Quality Degradation on Prediction Models Generated From Continuous Activity and Heart Rate Monitoring: Exploratory Analysis Using Simulation. JMIR Cardio 2023, 7, e40524. [Google Scholar] [CrossRef] [PubMed]
- Colomer-Lahiguera, S.; Gentizon, J.; Christofis, M.; Darnac, C.; Serena, A.; Eicher, M. Achieving Comprehensive, Patient-Centered Cancer Services: Optimizing the Role of Advanced Practice Nurses at the Core of Precision Health. Semin. Oncol. Nurs. 2024, 40, 151629. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Shakyawar, S.K.; Sharma, M.; Kaushik, S. Big data in healthcare: Management, analysis and future prospects. J. Big Data 2019, 6, 1–25. [Google Scholar] [CrossRef]
- Soto, R.J.; Schoenfisch, M.H. Preclinical performance evaluation of percutaneous glucose biosensors: Experimental considerations and recommendations. J. Diabetes Sci. Technol. 2015, 9, 978–984. [Google Scholar] [CrossRef]
- Flynn, C.D.; Chang, D.; Mahmud, A.; Yousefi, H.; Das, J.; Riordan, K.T.; Sargent, E.H.; Kelley, S.O. Biomolecular sensors for advanced physiological monitoring. Nat. Rev. Bioeng. 2023, 1, 560–575. [Google Scholar] [CrossRef]
- Tyler, J.; Choi, S.W.; Tewari, M. Real-time, personalized medicine through wearable sensors and dynamic predictive modeling: A new paradigm for clinical medicine. Curr. Opin. Syst. Biol. 2020, 20, 17–25. [Google Scholar] [CrossRef]
- Chen, C.; Ding, S.; Wang, J. Digital health for aging populations. Nat. Med. 2023, 29, 1623–1630. [Google Scholar] [CrossRef]
- Ahmed, Z.; Mohamed, K.; Zeeshan, S.; Dong, X. Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database 2020, 2020, baaa010. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface functionalization–The way for advanced applications of smart materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Fan, S.; Zhu, B.; El-Hout, S.I.; Zhang, J.; Chen, C. Recent progress on photothermal nanomaterials: Design, mechanism, and applications. Green Energy Environ. 2024; in press. [Google Scholar]
- Vitorino, R. Navigating the omics landscape in precision medicine: A bidirectional approach to patient care. Oral Oncol. Rep. 2024, 12, 100660. [Google Scholar] [CrossRef]
- Duan, X.-P.; Qin, B.-D.; Jiao, X.-D.; Liu, K.; Wang, Z.; Zang, Y.-S. New clinical trial design in precision medicine: Discovery, development and direction. Signal Transduct. Target. Ther. 2024, 9, 57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).