The Usefulness of the Glucose Management Indicator in Evaluating the Quality of Glycemic Control in Patients with Type 1 Diabetes Using Continuous Glucose Monitoring Sensors: A Cross-Sectional, Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Clinical, Laboratory and Anthropometric Measurements
2.3. Continuous Glucose Monitoring
2.4. Statistical Analysis
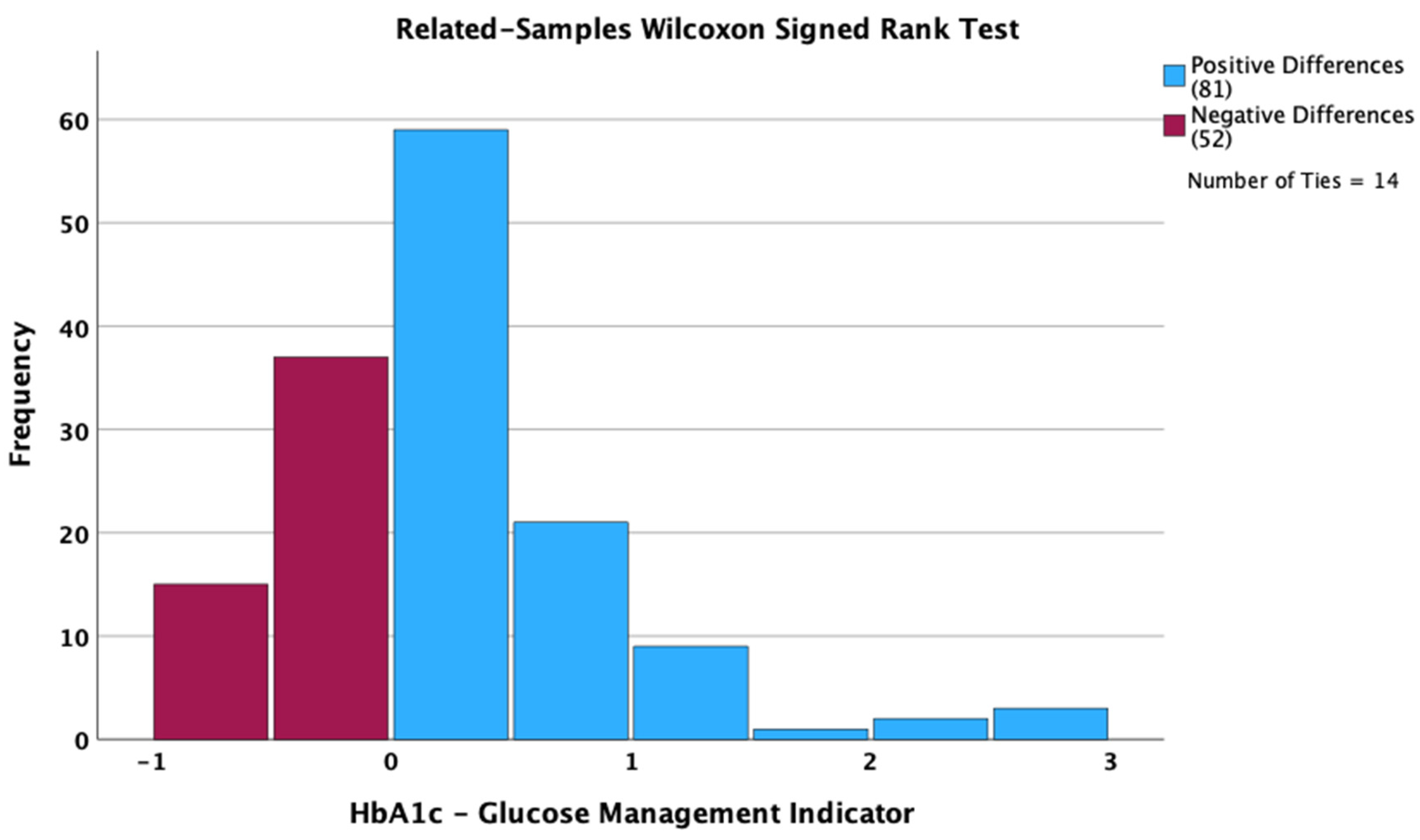
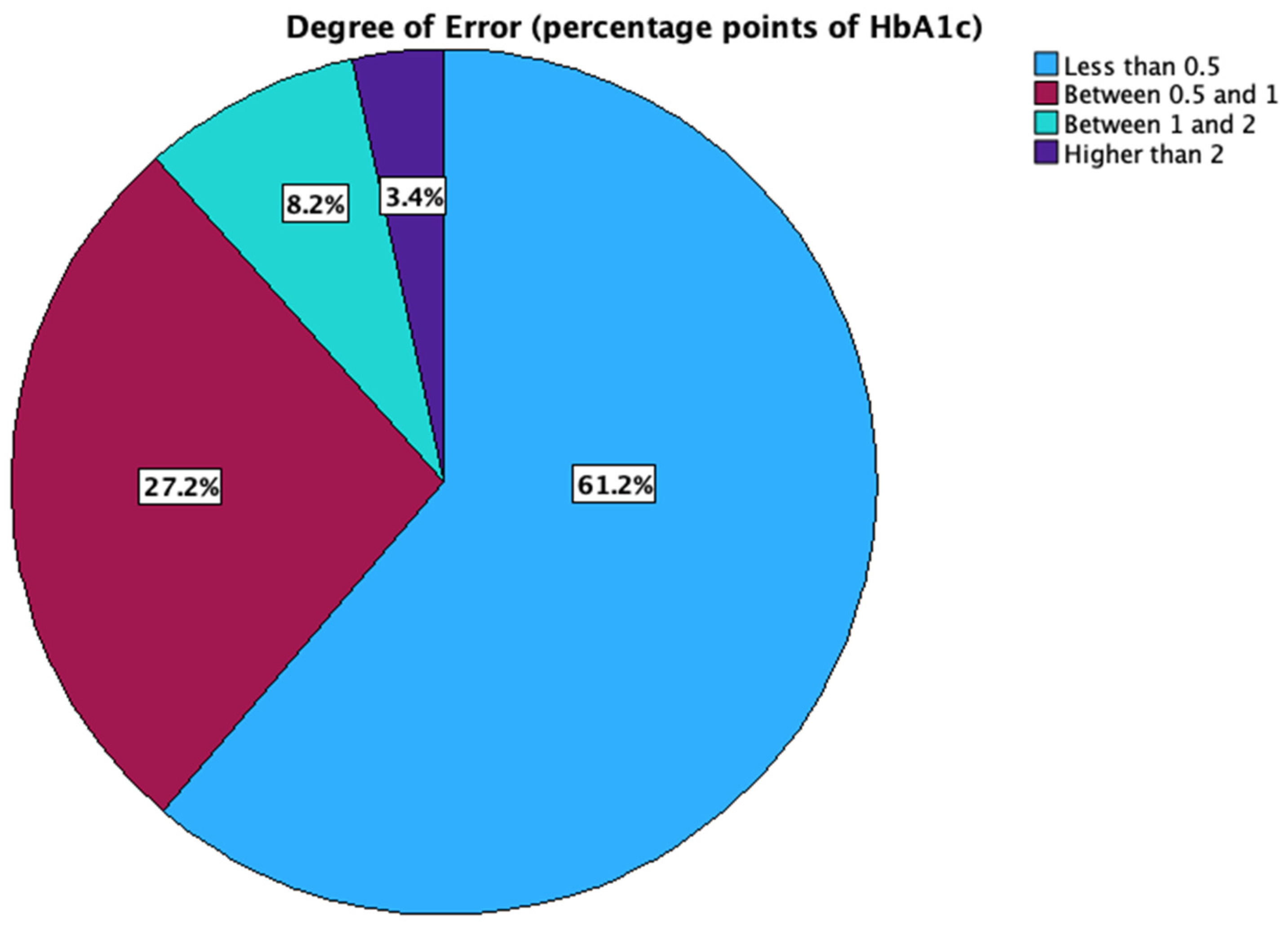
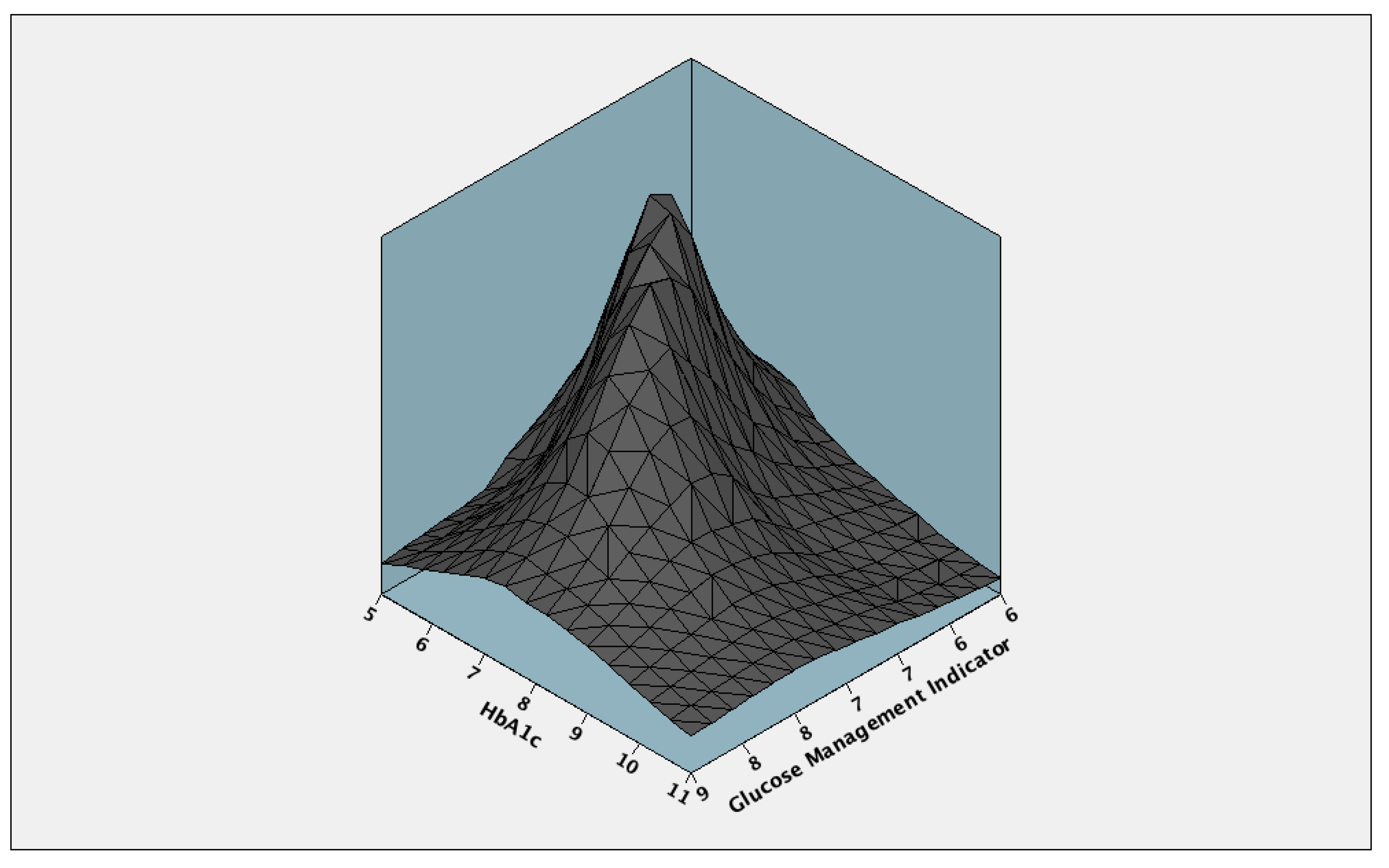
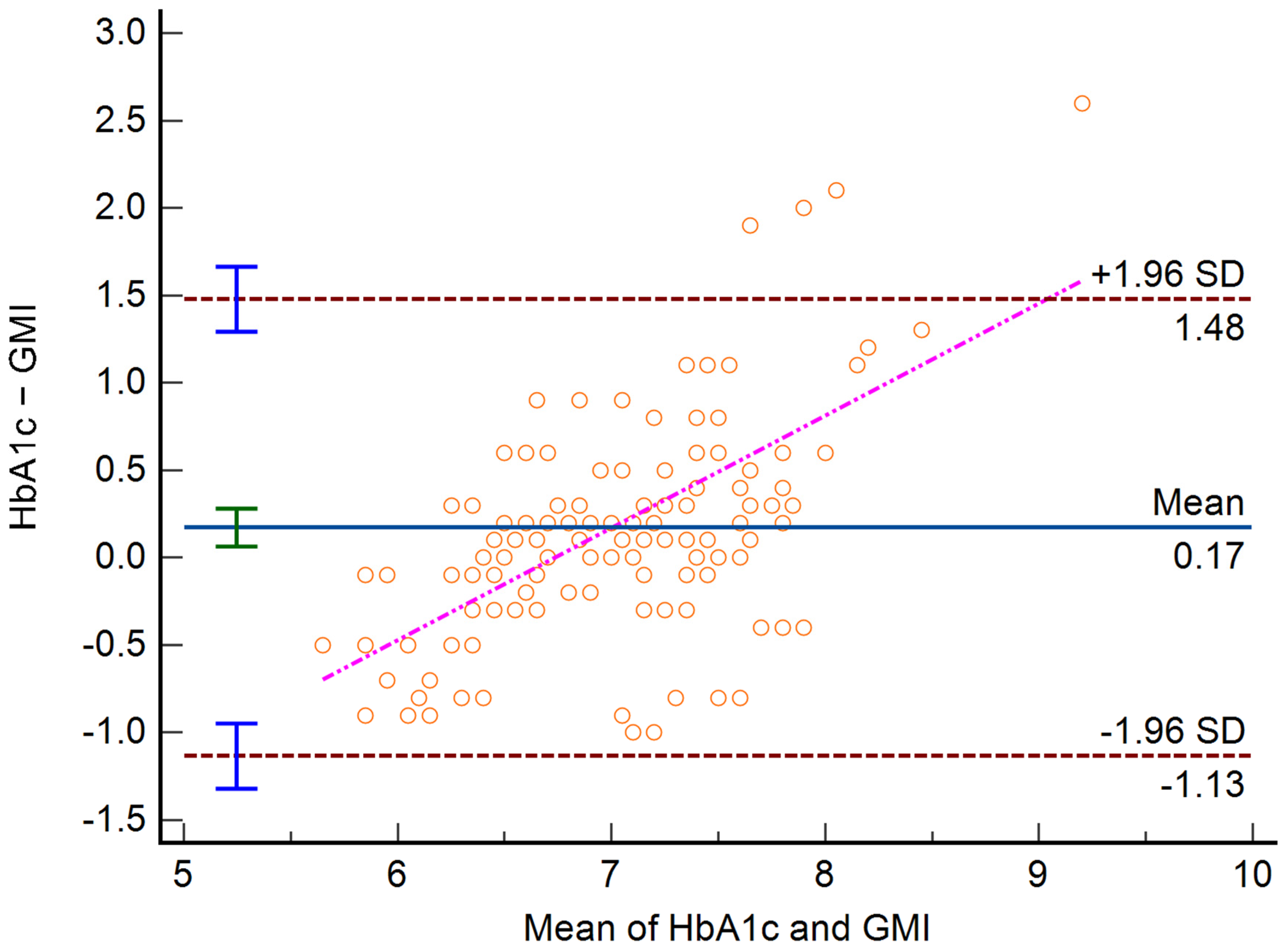
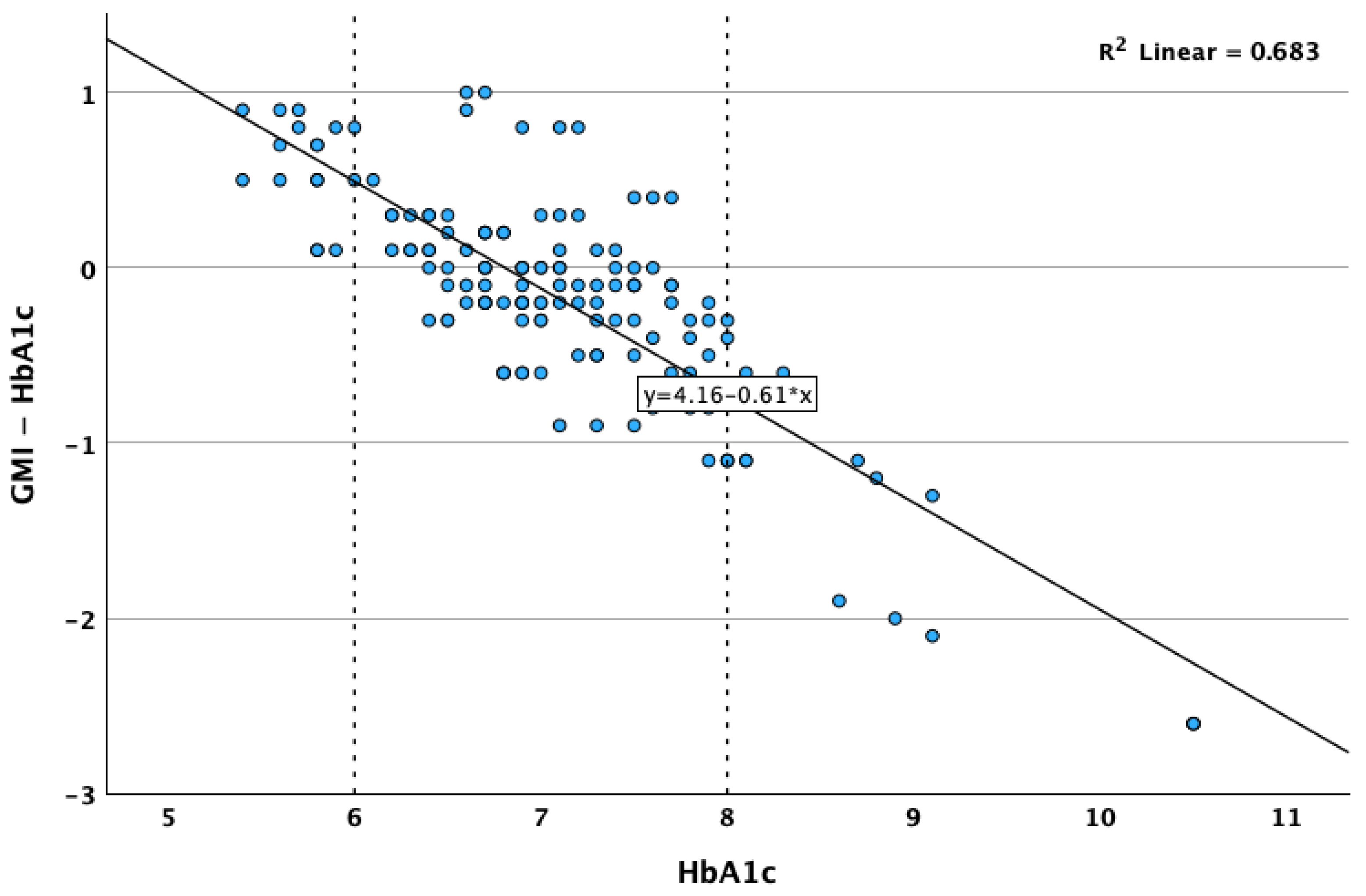
3. Results
Predictors for Differences Between GMI and HbA1c
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Facchinetti, A. Continuous Glucose Monitoring Sensors for Diabetes Management: A Review of Technologies and Applications. Diabetes Metab. J. 2019, 43, 383. [Google Scholar] [CrossRef] [PubMed]
- Funtanilla, V.D.; Candidate, P.; Caliendo, T.; Hilas, O. Continuous Glucose Monitoring: A Review of Available Systems. Pharm. Ther. 2019, 44, 550–553. [Google Scholar]
- Villena Gonzales, W.; Mobashsher, A.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.M. Using Continuous Glucose Monitoring in Clinical Practice. Clin. Diabetes 2020, 38, 429–438. [Google Scholar] [CrossRef]
- Rossetti, P.; Bondia, J.; Vehí, J.; Fanelli, C.G. Estimating Plasma Glucose from Interstitial Glucose: The Issue of Calibration Algorithms in Commercial Continuous Glucose Monitoring Devices. Sensors 2010, 10, 10936–10952. [Google Scholar] [CrossRef]
- Basu, A.; Slama, M.Q.; Nicholson, W.T.; Langman, L.; Peyser, T.; Carter, R.; Basu, R. Continuous Glucose Monitor Interference With Commonly Prescribed Medications: A Pilot Study. J. Diabetes Sci. Technol. 2017, 11, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Janapala, R.N.; Jayaraj, J.S.; Fathima, N.; Kashif, T.; Usman, N.; Dasari, A.; Jahan, N.; Sachmechi, I. Continuous Glucose Monitoring Versus Self-Monitoring of Blood Glucose in Type 2 Diabetes Mellitus: A Systematic Review with Meta-Analysis. Cureus 2019, 11, e5634. [Google Scholar] [CrossRef]
- Gomez, A.M.; Umpierrez, G.E. Continuous Glucose Monitoring in Insulin-Treated Patients in Non-ICU Settings. J. Diabetes Sci. Technol. 2014, 8, 930–936. [Google Scholar] [CrossRef]
- Wilmot, E.G.; Choudhary, P.; Leelarathna, L.; Baxter, M. Glycaemic Variability: The Under-recognized Therapeutic Target in Type 1 Diabetes Care. Diabetes Obes. Metab. 2019, 21, 2599–2608. [Google Scholar] [CrossRef]
- Friedman, J.G.; Cardona Matos, Z.; Szmuilowicz, E.D.; Aleppo, G. Use of Continuous Glucose Monitors to Manage Type 1 Diabetes Mellitus: Progress, Challenges, and Recommendations. Pharmgenom. Pers. Med. 2023, 16, 263–276. [Google Scholar] [CrossRef]
- Bolli, G. Physiological Insulin Replacement in Type 1 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2001, 109, S317–S332. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Han, K.; Park, S.; Yu, J.H.; Seo, J.A.; Kim, N.H.; Yoo, H.J.; Kim, S.G.; Choi, K.M.; Baik, S.H.; et al. Glucose Variability and the Risks of Stroke, Myocardial Infarction, and All-Cause Mortality in Individuals with Diabetes: Retrospective Cohort Study. Cardiovasc. Diabetol. 2020, 19, 144. [Google Scholar] [CrossRef]
- Slieker, R.C.; van der Heijden, A.A.W.H.; Nijpels, G.; Elders, P.J.M.; ’t Hart, L.M.; Beulens, J.W.J. Visit-to-Visit Variability of Glycemia and Vascular Complications: The Hoorn Diabetes Care System Cohort. Cardiovasc. Diabetol. 2019, 18, 170. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Pan, Y.; Zhou, K.; Liu, H.; Zhong, S. Correlation Between Glycemic Variability and Diabetic Complications: A Narrative Review. Int. J. Gen. Med. 2023, 16, 3083–3094. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S83–S96. [Google Scholar] [CrossRef]
- Langendam, M.; Luijf, Y.M.; Hooft, L.; DeVries, J.H.; Mudde, A.H.; Scholten, R.J. Continuous Glucose Monitoring Systems for Type 1 Diabetes Mellitus. Cochrane Database Syst. Rev. 2012, 2012, CD008101. [Google Scholar] [CrossRef]
- Bergenstal, R.M.; Beck, R.W.; Close, K.L.; Grunberger, G.; Sacks, D.B.; Kowalski, A.; Brown, A.S.; Heinemann, L.; Aleppo, G.; Ryan, D.B.; et al. Glucose Management Indicator (GMI): A New Term for Estimating A1C From Continuous Glucose Monitoring. Diabetes Care 2018, 41, 2275–2280. [Google Scholar] [CrossRef]
- Grimsmann, J.M.; von Sengbusch, S.; Freff, M.; Ermer, U.; Placzek, K.; Danne, T.; Hammer, E.; Holl, R.W. Glucose Management Indicator Based on Sensor Data and Laboratory HbA1c in People With Type 1 Diabetes From the DPV Database: Differences by Sensor Type. Diabetes Care 2020, 43, e111–e112. [Google Scholar] [CrossRef]
- Riddlesworth, T.D.; Beck, R.W.; Gal, R.L.; Connor, C.G.; Bergenstal, R.M.; Lee, S.; Willi, S.M. Optimal Sampling Duration for Continuous Glucose Monitoring to Determine Long-Term Glycemic Control. Diabetes Technol. Ther. 2018, 20, 314–316. [Google Scholar] [CrossRef]
- Radin, M.S. Pitfalls in Hemoglobin A1c Measurement: When Results May Be Misleading. J. Gen. Intern. Med. 2014, 29, 388–394. [Google Scholar] [CrossRef]
- Bae, J.C.; Suh, S.; Jin, S.; Kim, S.W.; Hur, K.Y.; Kim, J.H.; Min, Y.; Lee, M.; Lee, M.K.; Jeon, W.S.; et al. Hemoglobin A1c Values Are Affected by Hemoglobin Level and Gender in Non-anemic Koreans. J. Diabetes Investig. 2014, 5, 60–65. [Google Scholar] [CrossRef]
- Gomez-Peralta, F.; Choudhary, P.; Cosson, E.; Irace, C.; Rami-Merhar, B.; Seibold, A. Understanding the Clinical Implications of Differences between Glucose Management Indicator and Glycated Haemoglobin. Diabetes Obes. Metab. 2022, 24, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.J.; Low, C.; Jones, T.W.; Fournier, P.A. Contribution of an Intrinsic Lag of Continuous Glucose Monitoring Systems to Differences in Measured and Actual Glucose Concentrations Changing at Variable Rates in Vitro. J. Diabetes Sci. Technol. 2010, 4, 1393–1399. [Google Scholar] [CrossRef]
- Leelarathna, L.; Beck, R.W.; Bergenstal, R.M.; Thabit, H.; Hovorka, R. Glucose Management Indicator (GMI): Insights and Validation Using Guardian 3 and Navigator 2 Sensor Data. Diabetes Care 2019, 42, e60–e61. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Baidal, D. The Management of Type 1 Diabetes; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; Endotext: South Dartmouth, MA, USA, 2021. [Google Scholar]
- Gong, B.; Yang, W.; Xing, Y.; Lai, Y.; Shan, Z. Global, Regional, and National Burden of Type 1 Diabetes in Adolescents and Young Adults. Pediatr. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Clenciu, D.; Cojan, T.S.T.; Dijmarescu, A.L.; Ene, C.G.; Davitoiu, D.V.; Baleanu, V.D.; Ciora, C.A.; Socea, B.; Voiculescu, D.-I.; Nedelcuta, R.-M.; et al. Diabetic Retinopathy in Relation with EGDR Value in Patients with Type 1 Diabetes Mellitus. Rev. De Chim. 2019, 70, 1434–1438. [Google Scholar] [CrossRef]
- Amzolini, A.M.; Forţofoiu, M.C.; Barău Abu-Alhija, A.; Vladu, I.M.; Clenciu, D.; Mitrea, A.; Forţofoiu, M.; Matei, D.; Enăchescu, V.; Predescu, O.I.; et al. Triglyceride and Glucose Index: A Useful Tool for Non-Alcoholic Liver Disease Assessed by Liver Biopsy in Patients with Metabolic Syndrome? Rom. J. Morphol. Embryol. 2022, 62, 475–480. [Google Scholar] [CrossRef]
- Patel, N.; Sethi, Y.; Kaka, N.; Kaiwan, O.; Gupta, I.; Shaheen, R.S.; Sapoor, S.; Chopra, H.; Popoviciu, M.S.; Emran, T.B.; et al. Acute Hepatitis of Unknown Origin in Pediatric Age Group: Recent Outbreaks and Approach to Management. J. Clin. Med. 2022, 12, 9. [Google Scholar] [CrossRef]
- Caramori, M.L.; Mauer, M. Diabetes and Nephropathy. Curr. Opin. Nephrol. Hypertens 2003, 12, 273–282. [Google Scholar] [CrossRef]
- Prázný, M.; Škrha, J.; Šoupal, J.; Škrha, J. Short-Term and Long-Term Glycemic Variability and Its Relationship to Microvascular Complications of Diabetes. Vnitr. Lek. 2016, 62, S85–S93. [Google Scholar]
- O’Connell, J.M.; Manson, S.M. Understanding the Economic Costs of Diabetes and Prediabetes and What We May Learn About Reducing the Health and Economic Burden of These Conditions. Diabetes Care 2019, 42, 1609–1611. [Google Scholar] [CrossRef] [PubMed]
- Smith-Palmer, J.; Brändle, M.; Trevisan, R.; Orsini Federici, M.; Liabat, S.; Valentine, W. Assessment of the Association between Glycemic Variability and Diabetes-Related Complications in Type 1 and Type 2 Diabetes. Diabetes Res. Clin. Pract. 2014, 105, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Šoupal, J.; Škrha, J.; Fajmon, M.; Horová, E.; Mráz, M.; Škrha, J.; Prázný, M. Glycemic Variability Is Higher in Type 1 Diabetes Patients with Microvascular Complications Irrespective of Glycemic Control. Diabetes Technol. Ther. 2014, 16, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Popoviciu, M.S.; Kaka, N.; Sethi, Y.; Patel, N.; Chopra, H.; Cavalu, S. Type 1 Diabetes Mellitus and Autoimmune Diseases: A Critical Review of the Association and the Application of Personalized Medicine. J. Pers. Med. 2023, 13, 422. [Google Scholar] [CrossRef]
- Mader, J.K.; Gölz, S.; Bilz, S.; Bramlage, P.; Danne, T. Controlling Glycemic Variability in People Living with Type 1 Diabetes Receiving Insulin Glargine 300 U/ML (Gla-300). BMJ Open Diabetes Res. Care 2022, 10, e002898. [Google Scholar] [CrossRef]
- Suh, S.; Kim, J.H. Glycemic Variability: How Do We Measure It and Why Is It Important? Diabetes Metab. J. 2015, 39, 273. [Google Scholar] [CrossRef] [PubMed]
- Lautsch, D.; Boggs, R.; Wang, T.; Gonzalez, C.; Milligan, G.; Rajpathak, S.; Malkani, S.; McLeod, E.; Carroll, J.; Higgins, V. Individualized HbA1c Goals, and Patient Awareness and Attainment of Goals in Type 2 Diabetes Mellitus: A Real-World Multinational Survey. Adv. Ther. 2022, 39, 1016–1032. [Google Scholar] [CrossRef]
- Wang, M.; Hng, T.-M. HbA1c: More than Just a Number. Aust. J. Gen. Pract. 2021, 50, 628–632. [Google Scholar] [CrossRef]
- Chehregosha, H.; Khamseh, M.E.; Malek, M.; Hosseinpanah, F.; Ismail-Beigi, F. A View Beyond HbA1c: Role of Continuous Glucose Monitoring. Diabetes Ther. 2019, 10, 853–863. [Google Scholar] [CrossRef]
- Hussain, N. Implications of Using HBA1C as a Diagnostic Marker for Diabetes. Diabetol. Int. 2016, 7, 18–24. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark Insights 2016, 11, BMI.S38440. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.G.; Coyne, K.; Aleppo, G.; Szmuilowicz, E.D. Beyond A1C: Exploring Continuous Glucose Monitoring Metrics in Managing Diabetes. Endocr. Connect 2023, 12, e230085. [Google Scholar] [CrossRef]
- Selvin, E. The Glucose Management Indicator: Time to Change Course? Diabetes Care 2024, 47, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Dunn, T.C.; Xu, Y.; Bergenstal, R.M.; Ogawa, W.; Ajjan, R.A. Personalized Glycated Hemoglobin in Diabetes Management: Closing the Gap with Glucose Management Indicator. Diabetes Technol. Ther. 2023, 25, S-65–S-74. [Google Scholar] [CrossRef]
- Danne, T.; Nimri, R.; Battelino, T.; Bergenstal, R.M.; Close, K.L.; DeVries, J.H.; Garg, S.; Heinemann, L.; Hirsch, I.; Amiel, S.A.; et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017, 40, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Lacy, M.E.; Wellenius, G.A.; Sumner, A.E.; Correa, A.; Carnethon, M.R.; Liem, R.I.; Wilson, J.G.; Sacks, D.B.; Jacobs, D.R.; Carson, A.P.; et al. Association of Sickle Cell Trait With Hemoglobin A 1c in African Americans. JAMA 2017, 317, 507. [Google Scholar] [CrossRef]
- Guo, W.; Zhou, Q.; Jia, Y.; Xu, J. Increased Levels of Glycated Hemoglobin A1c and Iron Deficiency Anemia: A Review. Med. Sci. Monit. 2019, 25, 8371–8378. [Google Scholar] [CrossRef]
- Aggarwal, N.; Rai, A.K.; Kupfer, Y.; Tessler, S. Immeasurable Glycosylated Haemoglobin: A Marker for Severe Haemolysis. BMJ Case Rep. 2013, 2013, bcr2013200307. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Advanced Glycation End Products in the Pathogenesis of Chronic Kidney Disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef]
- Sehrawat, T.; Jindal, A.; Kohli, P.; Thour, A.; Kaur, J.; Sachdev, A.; Gupta, Y. Utility and Limitations of Glycated Hemoglobin (HbA1c) in Patients with Liver Cirrhosis as Compared with Oral Glucose Tolerance Test for Diagnosis of Diabetes. Diabetes Ther. 2018, 9, 243–251. [Google Scholar] [CrossRef]
- Perlman, J.E.; Gooley, T.A.; McNulty, B.; Meyers, J.; Hirsch, I.B. HbA1c and Glucose Management Indicator Discordance: A Real-World Analysis. Diabetes Technol. Ther. 2021, 23, 253–258. [Google Scholar] [CrossRef]
- Akintola, A.A.; Noordam, R.; Jansen, S.W.; de Craen, A.J.; Ballieux, B.E.; Cobbaert, C.M.; Mooijaart, S.P.; Pijl, H.; Westendorp, R.G.; van Heemst, D. Accuracy of Continuous Glucose Monitoring Measurements in Normo-Glycemic Individuals. PLoS ONE 2015, 10, e0139973. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L.; Schoemaker, M.; Schmelzeisen-Redecker, G.; Hinzmann, R.; Kassab, A.; Freckmann, G.; Reiterer, F.; Del Re, L. Benefits and Limitations of MARD as a Performance Parameter for Continuous Glucose Monitoring in the Interstitial Space. J. Diabetes Sci. Technol. 2020, 14, 135–150. [Google Scholar] [CrossRef]
- Liu, H.; Yang, D.; Deng, H.; Xu, W.; Lv, J.; Zhou, Y.; Luo, S.; Zheng, X.; Liang, H.; Yao, B.; et al. Impacts of Glycemic Variability on the Relationship between Glucose Management Indicator from IPro TM 2 and Laboratory Hemoglobin A1c in Adult Patients with Type 1 Diabetes Mellitus. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820931664. [Google Scholar] [CrossRef] [PubMed]
- Leelarathna, L.; Thabit, H.; Hovorka, R.; Evans, M. Estimated HbA 1c and Glucose Management Indicator (GMI): Are They the Same? Diabet. Med. 2021, 38. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yeung, S.L.A.; Schooling, C.M. Sex differences in the association of fasting glucose with HbA1c, and their consequences for mortality: A Mendelian randomization study. eBioMedicine 2022, 84, 104259. [Google Scholar] [CrossRef]
- Obeagu, E.I.; Igwe, M.C.; Obeagu, G.U. Oxidative stress’s impact on red blood cells: Unveiling implications for health and disease. Medicine 2024, 103, e37360. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]



| GMI-HbA1c | Absolute difference (GMI-HbA1c) | HbA1c | Standard Deviation of Glycemia | Time In Range | ||
|---|---|---|---|---|---|---|
| Percentage Error (GMI vs. HbA1c) | Correlation Coefficient | −0.205 | ||||
| p-value | 0.013 | |||||
| Absolute difference (GMI-HbA1c) | Correlation Coefficient | −0.295 | -- | |||
| p-value | <0.001 | . | ||||
| HbA1c | Correlation Coefficient | −0.707 | 0.254 | -- | ||
| p-value | <0.001 | 0.002 | . | |||
| Standard Deviation of Glycemia | Correlation Coefficient | −0.296 | 0.189 | 0.656 | -- | |
| p-value | <0.001 | 0.022 | <0.001 | . | ||
| Time In Range | Correlation Coefficient | 0.248 | −0.190 | −0.637 | −0.794 | -- |
| p-value | 0.002 | 0.021 | 0.000 | 0.000 | . | |
| Coefficient of variation | Correlation Coefficient | −0.299 | 0.161 | 0.349 | 0.801 | −0.513 |
| p-value | <0.001 | 0.051 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar, S.; Potre, O.; Ionita, I.; Reurean-Pintilei, D.-V.; Timar, R.; Herascu, A.; Avram, V.F.; Timar, B. The Usefulness of the Glucose Management Indicator in Evaluating the Quality of Glycemic Control in Patients with Type 1 Diabetes Using Continuous Glucose Monitoring Sensors: A Cross-Sectional, Multicenter Study. Biosensors 2025, 15, 190. https://doi.org/10.3390/bios15030190
Lazar S, Potre O, Ionita I, Reurean-Pintilei D-V, Timar R, Herascu A, Avram VF, Timar B. The Usefulness of the Glucose Management Indicator in Evaluating the Quality of Glycemic Control in Patients with Type 1 Diabetes Using Continuous Glucose Monitoring Sensors: A Cross-Sectional, Multicenter Study. Biosensors. 2025; 15(3):190. https://doi.org/10.3390/bios15030190
Chicago/Turabian StyleLazar, Sandra, Ovidiu Potre, Ioana Ionita, Delia-Viola Reurean-Pintilei, Romulus Timar, Andreea Herascu, Vlad Florian Avram, and Bogdan Timar. 2025. "The Usefulness of the Glucose Management Indicator in Evaluating the Quality of Glycemic Control in Patients with Type 1 Diabetes Using Continuous Glucose Monitoring Sensors: A Cross-Sectional, Multicenter Study" Biosensors 15, no. 3: 190. https://doi.org/10.3390/bios15030190
APA StyleLazar, S., Potre, O., Ionita, I., Reurean-Pintilei, D.-V., Timar, R., Herascu, A., Avram, V. F., & Timar, B. (2025). The Usefulness of the Glucose Management Indicator in Evaluating the Quality of Glycemic Control in Patients with Type 1 Diabetes Using Continuous Glucose Monitoring Sensors: A Cross-Sectional, Multicenter Study. Biosensors, 15(3), 190. https://doi.org/10.3390/bios15030190






