Nanobody-Based Immunoassays for the Detection of Food Hazards—A Review
Abstract
1. Introduction
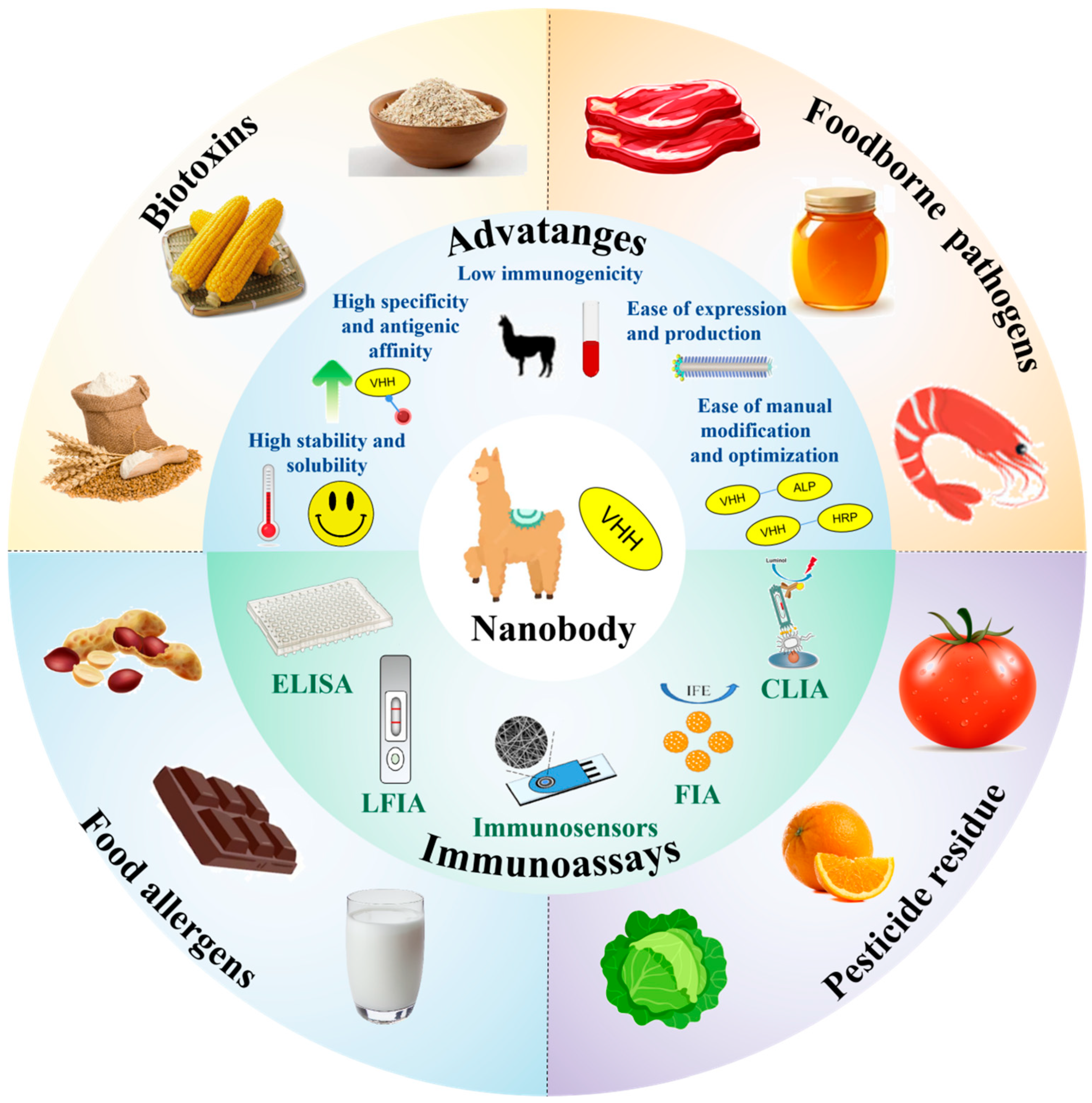
2. Overview of Nb
2.1. Brief History of Nb
2.2. Unique Properties of Nbs
2.2.1. High Stability and Solubility
2.2.2. Low Immunogenicity
2.2.3. High Specificity and Antigenic Affinity
2.2.4. Ease of Expression and Production
2.2.5. Ease of Manual Modification and Optimization
3. Immunoassay and Immunosensor
4. Nb-Based Immunoassay Applications in Detecting Food Hazards
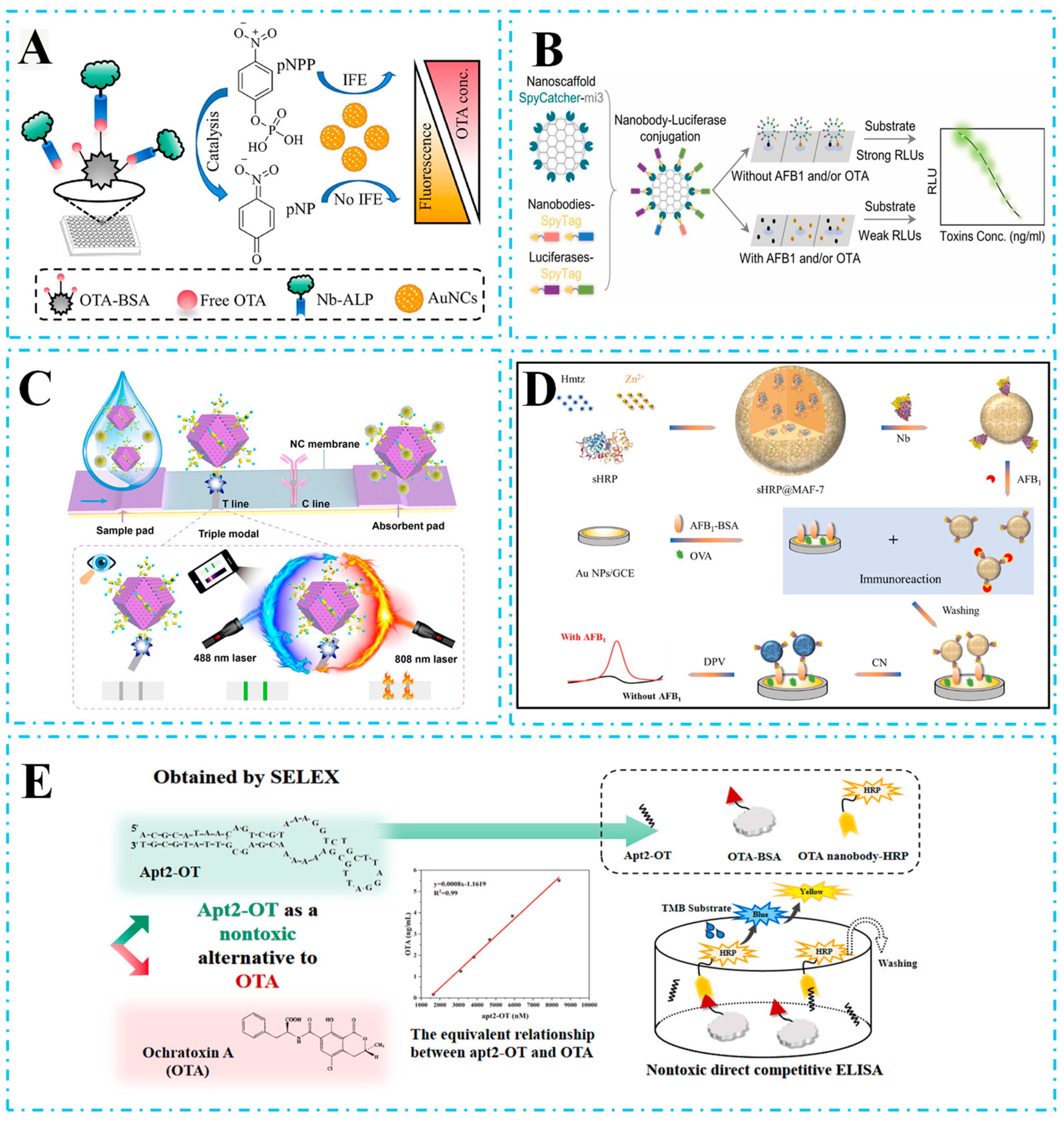
4.1. Detection of Biotoxins
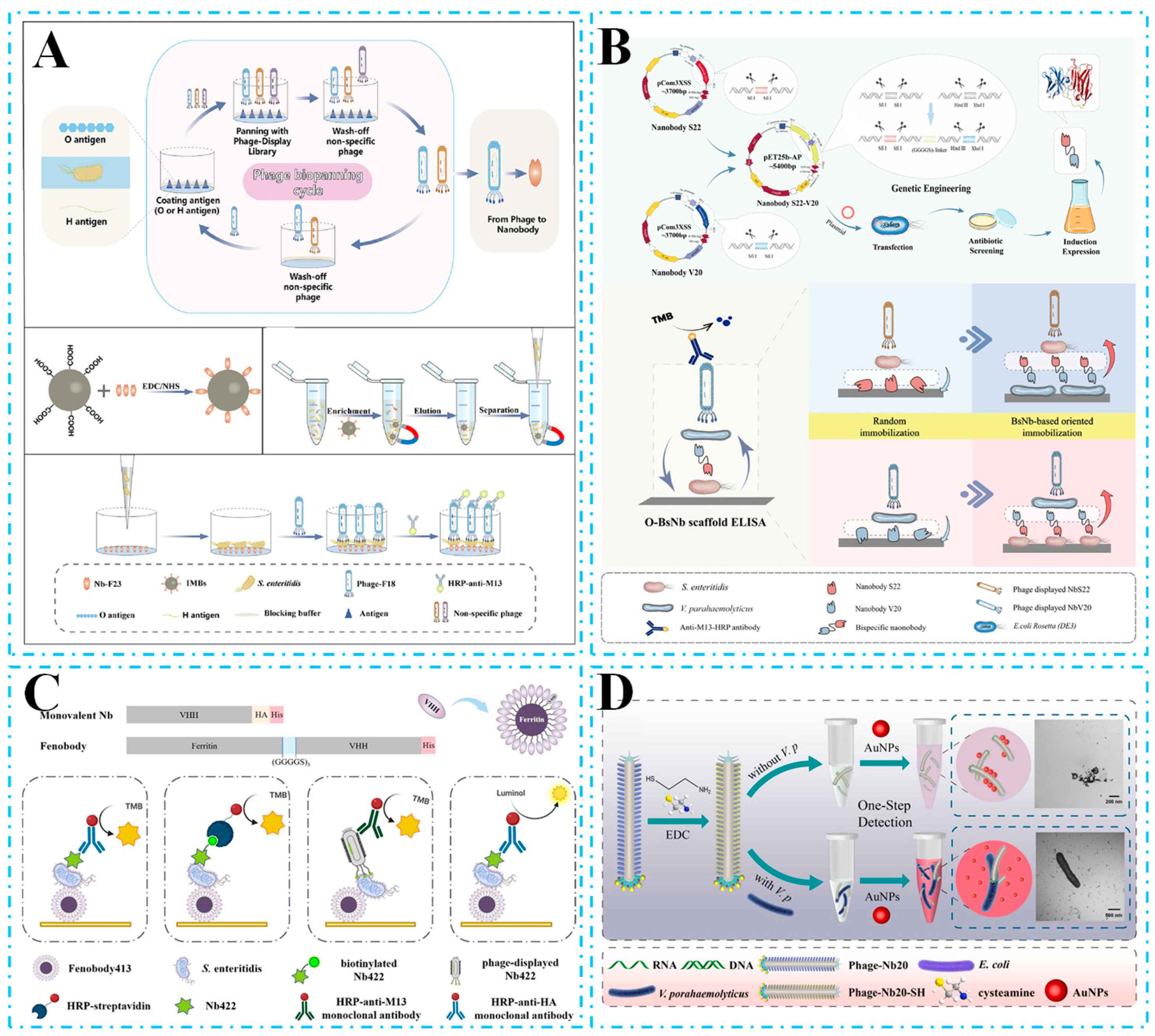
4.2. Detection of Foodborne Pathogens
4.3. Detection of Pesticide Residues
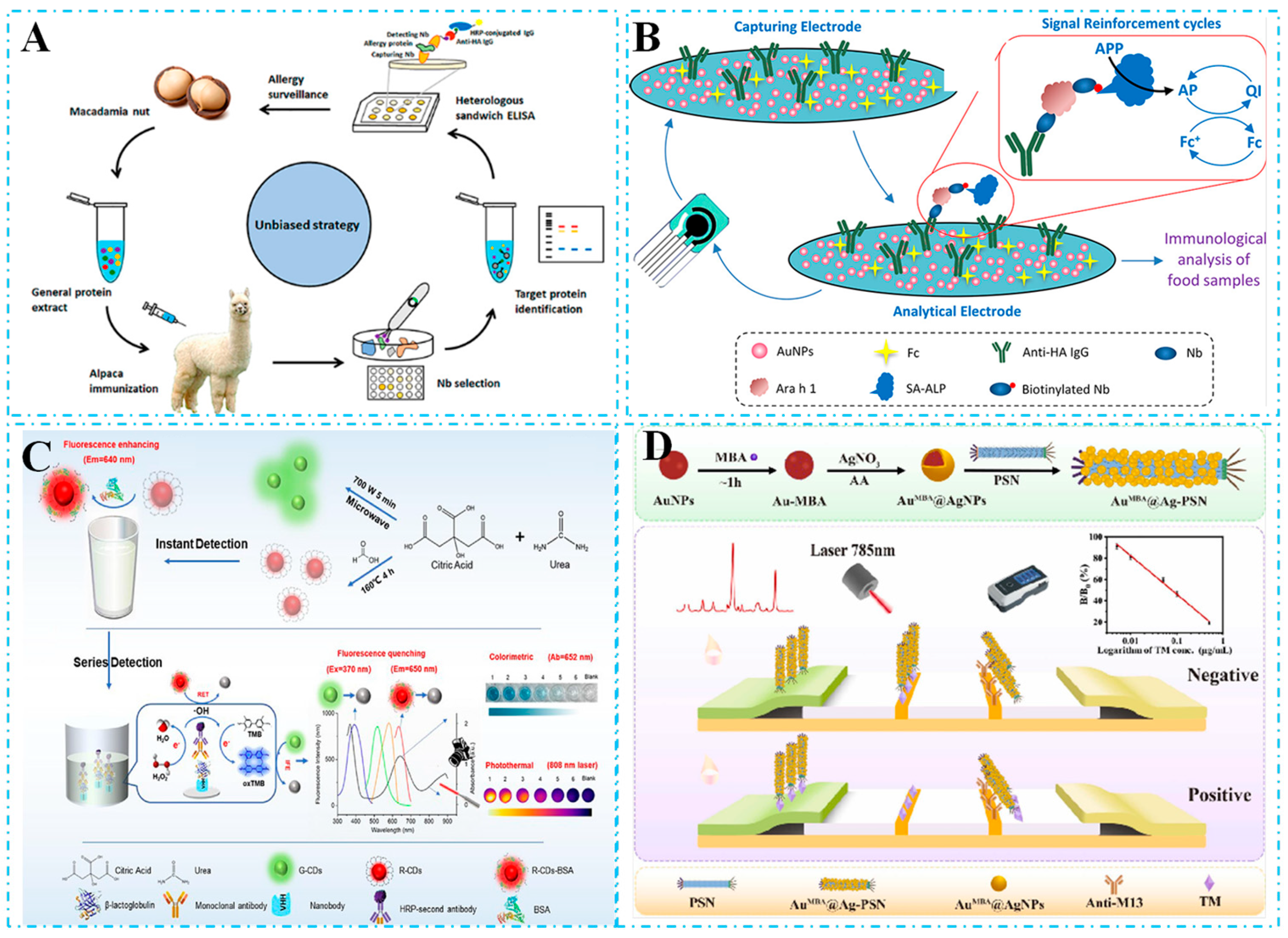
4.4. Detection of Food Allergens
5. Challenges of Nb-Based Immunoassay from Laboratory to Field
- (I)
- Lack of technological maturity: unlike traditional antibodies, nanobodies usually rely on prokaryotic expression systems (e.g., E. coli), which are prone to the formation of inclusion bodies, leading to loss of activity, whereas eukaryotic systems (yeast and mammalian cells), although soluble expression is much better, are expensive (the cost of a single expression on a laboratory scale can be up to 3–5 times that of traditional antibodies).
- (II)
- Lack of standardization: nanobody-based testing methods have not yet formed unified standards and specifications, making it difficult to compare and verify results between different laboratories or companies.
- (III)
- Low market acceptance: users of on-site testing lack understanding of nanobody technology, and the commercial market is still dominated by traditional monoclonal/polyclonal antibodies.
6. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Li, G.; Wu, D.; Li, X.; Yu, Y.; Luo, P.; Chen, J.; Dai, C.; Wu, Y. Recent advances in emerging nanomaterials based food sample pretreatment methods for food safety screening. TrAC Trends Anal. Chem. 2019, 121, 115669. [Google Scholar] [CrossRef]
- Lv, M.; Liu, Y.; Geng, J.; Kou, X.; Xin, Z.; Yang, D. Engineering nanomaterials-based biosensors for food safety detection. Biosens. Bioelectron. 2018, 106, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Mead, P.S.; Slutsker, L.; Dietz, V.; McCaig, L.F.; Bresee, J.S.; Shapiro, C.; Griffin, P.M.; Tauxe, R.V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999, 5, 607–625. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.H.; Ren, D.B.; Sun, L.P.; Zhuang, Y.L.; Yi, L.Z.; Wang, S. Recent advances in nanomaterial-assisted electrochemical sensors for food safety analysis. Food Front. 2022, 3, 453–479. [Google Scholar] [CrossRef]
- Li, Y.; Man, S.; Ye, S.; Liu, G.; Ma, L. CRISPR-Cas-based detection for food safety problems: Current status, challenges, and opportunities. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3770–3798. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef]
- Li, T.; Shang, D.W.; Gao, S.W.; Wang, B.; Kong, H.; Yang, G.Z.; Shu, W.D.; Xu, P.L.; Wei, G. Two-Dimensional Material-Based Electrochemical Sensors/Biosensors for Food Safety and Biomolecular Detection. Biosensors 2022, 12, 314. [Google Scholar] [CrossRef]
- Rocha, D.F.D.; Oliveira, M.D.; Furlong, E.B.; Junges, A.; Paroul, N.; Valduga, E.; Backes, G.T.; Zeni, J.; Cansian, R.L. Evaluation of the TLC quantification method and occurrence of deoxynivalenol in wheat flour of southern Brazil. Food Addit. Contam. Part A-Chem. Anal. Control Expo. Risk Assess. 2017, 34, 2220–2229. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Mañes, J.; Berrada, H. Development of a GC–MS/MS strategy to determine 15 mycotoxins and metabolites in human urine. Talanta 2014, 128, 125–131. [Google Scholar] [CrossRef]
- Keskin, E.; Eyupoglu, O.E. Determination of mycotoxins by HPLC, LC-MS/MS and health risk assessment of the mycotoxins in bee products of Turkey. Food Chem. 2023, 400, 134086. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Lin, W.; Chang, H.; Li, B. QuEChERS-HPLC-MS for the Determination of Coronatine Residues in Paddy Field Environment and Five Common Fruits and Vegetables. J. Food Compos. Anal. 2024, 135, 106705. [Google Scholar] [CrossRef]
- Tang, X.Q.; Li, X.; Li, P.W.; Zhang, Q.; Li, R.; Zhang, W.; Ding, X.X.; Lei, J.W.; Zhang, Z.W. Development and Application of an Immunoaffinity Column Enzyme Immunoassay for Mycotoxin Zearalenone in Complicated Samples. PLoS ONE 2014, 9, e85606. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wu, A.; Xu, L.; Xu, C.; Liu, L.; Kuang, H.; Xu, X. Recent progress on lateral flow immunoassays in foodborne pathogen detection. Food Biosci. 2023, 52, 102475. [Google Scholar] [CrossRef]
- Sena-Torralba, A.; Pallás-Tamarit, Y.; Morais, S.; Maquieira, Á. Recent advances and challenges in food-borne allergen detection. TrAC Trends Anal. Chem. 2020, 132, 116050. [Google Scholar] [CrossRef]
- Yao, J.J.; Wang, Z.X.; Guo, L.L.; Xu, X.X.; Liu, L.Q.; Xu, L.G.; Song, S.S.; Xu, C.L.; Kuang, H. Advances in immunoassays for organophosphorus and pyrethroid pesticides. Trac-Trends Anal. Chem. 2020, 131, 116022. [Google Scholar] [CrossRef]
- Goldman, E.R.; Liu, J.L.; Zabetakis, D.; Anderson, G.P. Enhancing Stability of Camelid and Shark Single Domain Antibodies: An Overview. Front. Immunol. 2017, 8, 865. [Google Scholar] [CrossRef]
- Peltomaa, R.; Barderas, R.; Benito-Peña, E.; Moreno-Bondi, M.C. Recombinant antibodies and their use for food immunoanalysis. Anal. Bioanal. Chem. 2022, 414, 193–217. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Avila, D.; Hughes, M.; Hughes, A.; McKinney, E.C.; Flajnik, M.F. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature 1995, 374, 168–173. [Google Scholar] [CrossRef]
- Salvador, J.P.; Vilaplana, L.; Marco, M.P. Nanobody: Outstanding features for diagnostic and therapeutic applications. Anal. Bioanal. Chem. 2019, 411, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, L.; Wang, A.; Jin, Y.; Zhou, D. Nanobodies: The potential application in bacterial treatment and diagnosis. Biochem. Pharmacol. 2023, 214, 115640. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.I.A.; Amorim, C.G.; Abu Qatouseh, L.F.; Montenegro, M. Nanobody-based immunodiagnostics: A systematic review of nanobody integration in diagnostics and deep insight into electrochemical immunoassays. Microchem. J. 2024, 196, 109628. [Google Scholar] [CrossRef]
- Gao, S.; Yang, W.; Zheng, X.; Wang, T.; Zhang, D.; Zou, X. Advances of nanobody-based immunosensors for detecting food contaminants. Trends Food Sci. Technol. 2025, 156, 104871. [Google Scholar] [CrossRef]
- Maddur, M.S.; Lacroix-Desmazes, S.; Dimitrov, J.D.; Kazatchkine, M.D.; Bayry, J.; Kaveri, S.V. Natural Antibodies: From First-Line Defense Against Pathogens to Perpetual Immune Homeostasis. Clin. Rev. Allergy Immunol. 2020, 58, 213–228. [Google Scholar] [CrossRef]
- Uhlen, M.; Bandrowski, A.; Carr, S.; Edwards, A.; Ellenberg, J.; Lundberg, E.; Rimm, D.L.; Rodriguez, H.; Hiltke, T.; Snyder, M.; et al. A proposal for validation of antibodies. Nat. Methods 2016, 13, 823–827. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497, reprinted in J. Immunol. 2005, 174, 2453–2455. [Google Scholar] [CrossRef]
- Liu, J.K. The history of monoclonal antibody development—Progress, remaining challenges and future innovations. Ann. Med. Surg. 2014, 3, 113–116. [Google Scholar] [CrossRef]
- Khodabakhsh, F.; Behdani, M.; Rami, A.; Kazemi-Lomedasht, F. Single-Domain Antibodies or Nanobodies: A Class of Next-Generation Antibodies. Int. Rev. Immunol. 2018, 37, 316–322. [Google Scholar] [CrossRef]
- Duggan, S. Caplacizumab: First Global Approval. Drugs 2018, 78, 1639–1642. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Arbabi Ghahroudi, M.; Desmyter, A.; Wyns, L.; Hamers, R.; Muyldermans, S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997, 414, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.M.; Renisio, J.G.; Prompers, J.J.; van Platerink, C.J.; Cambillau, C.; Darbon, H.; Frenken, L.G. Thermal unfolding of a llama antibody fragment: A two-state reversible process. Biochemistry 2001, 40, 74–83. [Google Scholar] [CrossRef]
- Cortez-Retamozo, V.; Backmann, N.; Senter, P.D.; Wernery, U.; De Baetselier, P.; Muyldermans, S.; Revets, H. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res. 2004, 64, 2853–2857. [Google Scholar] [CrossRef]
- Rossotti, M.A.; Bélanger, K.; Henry, K.A.; Tanha, J. Immunogenicity and humanization of single-domain antibodies. FEBS J. 2022, 289, 4304–4327. [Google Scholar] [CrossRef]
- Wenzel, E.V.; Bosnak, M.; Tierney, R.; Schubert, M.; Brown, J.; Dübel, S.; Efstratiou, A.; Sesardic, D.; Stickings, P.; Hust, M. Human antibodies neutralizing diphtheria toxin in vitro and in vivo. Sci. Rep. 2020, 10, 571. [Google Scholar] [CrossRef]
- Eyer, L.; Hruška, K.J.V.M. Single-domain antibody fragments derived from heavy-chain antibodies: A review. Vet. Med. 2018, 57, 439–513. [Google Scholar] [CrossRef]
- Liu, M.L.; Liang, X.M.; Jin, M.Y.; Huang, H.W.; Luo, L.; Wang, H.; Shen, X.; Xu, Z.L. Food-Borne Biotoxin Neutralization in Vivo by Nanobodies: Current Status and Prospects. J. Agric. Food Chem. 2024, 72, 10753–10771. [Google Scholar] [CrossRef]
- Schütze, K.; Petry, K.; Hambach, J.; Schuster, N.; Fumey, W.; Schriewer, L.; Röckendorf, J.; Menzel, S.; Albrecht, B.; Haag, F.; et al. CD38-Specific Biparatopic Heavy Chain Antibodies Display Potent Complement-Dependent Cytotoxicity Against Multiple Myeloma Cells. Front. Immunol. 2018, 9, 2553. [Google Scholar] [CrossRef]
- Chames, P.; Baty, D. Bispecific antibodies for cancer therapy: The light at the end of the tunnel? mAbs 2009, 1, 539–547. [Google Scholar] [CrossRef]
- Els Conrath, K.; Lauwereys, M.; Wyns, L.; Muyldermans, S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J. Biol. Chem. 2001, 276, 7346–7350. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh-Ghassabeh, G.; Devoogdt, N.; De Pauw, P.; Vincke, C.; Muyldermans, S. Nanobodies and their potential applications. Nanomedicine 2013, 8, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niessner, R.; Tang, D.; Knopp, D. Nanoparticle-based immunosensors and immunoassays for aflatoxins. Anal. Chim. Acta 2016, 912, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Raghav, R.; O’Kennedy, R.; Srivastava, S. Advances in ovarian cancer diagnosis: A journey from immunoassays to immunosensors. Enzym. Microb. Technol. 2016, 89, 15–30. [Google Scholar] [CrossRef]
- Manaf, B.A.A.; Hong, S.P.; Rizwan, M.; Arshad, F.; Gwenin, C.; Ahmed, M.U. Recent advancement in sensitive detection of carcinoembryonic antigen using nanomaterials based immunosensors. Surf. Interfaces 2023, 36, 102596. [Google Scholar] [CrossRef]
- Turner, A.P.F. Immunosensors: The next generation. Nat. Biotechnol. 1997, 15, 421. [Google Scholar] [CrossRef]
- Sawant, S.N. 13—Development of Biosensors from Biopolymer Composites. In Biopolymer Composites in Electronics; Sadasivuni, K.K., Ponnamma, D., Kim, J., Cabibihan, J.J., AlMaadeed, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 353–383. [Google Scholar]
- Vashist, S.K.; Luong, J.H.T. Chapter 2—Antibody Immobilization and Surface Functionalization Chemistries for Immunodiagnostics. In Handbook of Immunoassay Technologies; Vashist, S.K., Luong, J.H.T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 19–46. [Google Scholar]
- Luppa, P.B. Immunosensor Technology-Principles and Applications. Immunosensor-Technologie-Grundlagen und Anwendungen. J. Lab. Med. 2001, 25, 388–398. [Google Scholar] [CrossRef]
- Ju, H.; Lai, G.; Yan, F. 1—Introduction. In Immunosensing for Detection of Protein Biomarkers; Ju, H., Lai, G., Yan, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–30. [Google Scholar]
- Wang, Z.; Guo, Y.; Xianyu, Y. Applications of self-assembly strategies in immunoassays: A review. Coord. Chem. Rev. 2023, 478, 214974. [Google Scholar] [CrossRef]
- Parak, M.; Asgari, A.; Nourian, Y.H.; Ghanei, M. A review of poisoning with various types of biotoxins and its common clinical symptoms. Toxicon 2024, 240, 107629. [Google Scholar] [CrossRef]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Mohan, K.; Karthick Rajan, D.; Pillay, A.A.; Palanisami, T.; Sathishkumar, P.; Conterno, L. Distribution, toxicity, interactive effects, and detection of ochratoxin and deoxynivalenol in food: A review. Food Chem. 2022, 378, 131978. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, Z.S.; Tan, C.Y.; Guo, Y.P.; Li, L.; Ren, H.L.; Li, Y.S.; Hu, P.; Gong, S.; Zhou, Y.; et al. Contamination of commercially available seafood by key diarrhetic shellfish poisons along the coast of China. Environ. Sci. Pollut. Res. 2015, 22, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Mijović, B.; Codd, G.A.; Meriluoto, J. Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Arch. Toxicol. 2017, 91, 621–650. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.; Mendoza, M.C.; Rodicio, M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef]
- Xu, C.; Yang, Y.; Liu, L.; Li, J.; Liu, X.; Zhang, X.; Liu, Y.; Zhang, C.; Liu, X. Microcystin-LR nanobody screening from an alpaca phage display nanobody library and its expression and application. Ecotoxicol. Environ. Saf. 2018, 151, 220–227. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Y.-Y.; Wan, D.-B.; Li, J.-D.; Liang, Y.-F.; Li, Z.-F.; Shen, Y.-D.; Xu, Z.-L.; Yang, J.-Y.; Wang, H.; et al. Nanobodies for accurate recognition of iso-tenuazonic acid and development of sensitive immunoassay for contaminant detection in foods. Food Control 2022, 136, 108835. [Google Scholar] [CrossRef]
- Wang, W.; Gu, G.; Yin, R.; Fu, J.; Jing, M.; Shen, Z.; Lai, D.; Wang, B.; Zhou, L. A Nanobody-Based Immunoassay for Detection of Ustilaginoidins in Rice Samples. Toxins 2022, 14, 659. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, J.; Li, Y.; He, T.; Yang, Y.; Liu, M. Development of a biotinylated nanobody for sensitive detection of aflatoxin B1 in cereal via ELISA. Talanta 2022, 239, 123125. [Google Scholar] [CrossRef]
- Zuo, H.; Wang, X.; Liu, W.; Chen, Z.; Liu, R.; Yang, H.; Xia, C.; Xie, J.; Sun, T.; Ning, B. Nanobody-based magnetic chemiluminescence immunoassay for one-pot detection of ochratoxin A. Talanta 2023, 258, 124388. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Zhang, P.; Wan, Y.; Chang, G.; Xu, X.; Ruan, F.; Zhou, T.; Zhao, Q.; Zhang, M.; et al. Facile construction of sandwich ELISA based on double-nanobody for specific detection of α-hemolysin in food samples. Talanta 2024, 274, 126021. [Google Scholar] [CrossRef]
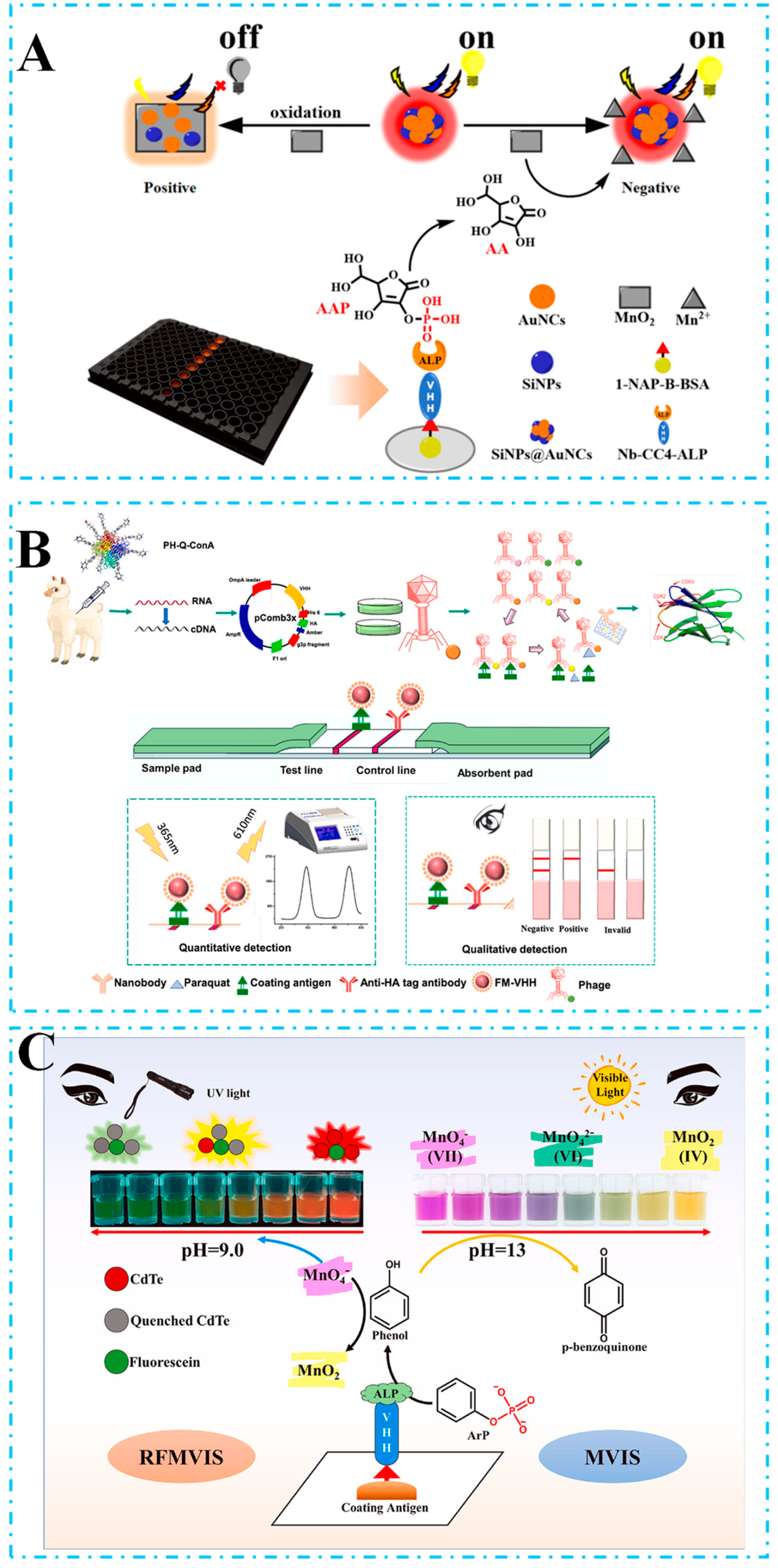
- Wang, X.; Wang, Y.; Wang, Y.; Chen, Q.; Liu, X. Nanobody-alkaline phosphatase fusion-mediated phosphate-triggered fluorescence immunoassay for ochratoxin a detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117617. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yang, X.; Shi, S.; Chen, X.; Sun, Z.; Xu, Z.; Liu, X. Inner filter effect-based fluorescence immunoassay using nanobody-alkaline phosphatase fusion and gold nanoclusters for detecting ochratoxin A in pepper. Food Control 2023, 153, 109961. [Google Scholar] [CrossRef]
- Ni, Y.; Rosier, B.J.H.M.; van Aalen, E.A.; Hanckmann, E.T.L.; Biewenga, L.; Pistikou, A.-M.M.; Timmermans, B.; Vu, C.; Roos, S.; Arts, R.; et al. A plug-and-play platform of ratiometric bioluminescent sensors for homogeneous immunoassays. Nat. Commun. 2021, 12, 4586. [Google Scholar] [CrossRef]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered Luciferase Reporter from a Deep Sea Shrimp Utilizing a Novel Imidazopyrazinone Substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef]
- Bao, K.; Liu, X.; Liao, Y.; Liu, Z.; Cao, H.; Wu, L.; Chen, Q. Nanobody-Nanoluciferase Fusion Protein-Enabled Immunoassay for Ochratoxin A in Coffee with Enhanced Specificity and Sensitivity. Toxins 2022, 14, 713. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.-F.; Yang, Y.-Y.; Wan, D.-B.; Vasylieva, N.; Zhang, Y.-Q.; Cai, J.; Wang, H.; Shen, Y.-D.; Xu, Z.-L.; et al. Chemiluminescent Enzyme Immunoassay and Bioluminescent Enzyme Immunoassay for Tenuazonic Acid Mycotoxin by Exploitation of Nanobody and Nanobody–Nanoluciferase Fusion. Anal. Chem. 2020, 92, 11935–11942. [Google Scholar] [CrossRef]
- Wu, S.; Xu, J.; Chen, W.; Wang, F.; Tan, X.; Zou, X.; Zhou, W.; Huang, W.; Zheng, Y.; Wang, S.; et al. Protein nanoscaffold enables programmable nanobody-luciferase immunoassembly for sensitive and simultaneous detection of aflatoxin B1 and ochratoxin A. J. Hazard. Mater. 2024, 462, 132701. [Google Scholar] [CrossRef]
- Pang, J.; Ren, W.; He, B.; Tu, Z.J.C. Development of a Rapid Gold Nanoflowers Immunochromatographic Test Strip Based on the Nanobody for Detection of Aflatoxin B1. ChemistrySelect 2023, 8, e202300913. [Google Scholar] [CrossRef]
- Yang, E.; Liu, Q.; Huang, G.; Liu, J.; Wei, W. Engineering nanobodies for next-generation molecular imaging. Drug Discov. Today 2022, 27, 1622–1638. [Google Scholar] [CrossRef]
- Wang, X.; Sun, T.; Shen, W.; Liu, M.; Liu, W.; Zuo, H.; Zhang, Y.; Geng, L.; Wang, W.; Shao, C.; et al. A lateral flow immunochromatographic assay based on nanobody-oriented coupling strategy for aflatoxin B1 detection. Sens. Actuators B Chem. 2023, 394, 134419. [Google Scholar] [CrossRef]
- He, D.; Du, Z.; Wang, Y.; Xu, E.; Jin, Z.; Wu, Z. Quantitative detection of Campylobacter jejuni with a core-satellite assemblies-based dual-modular aptasensor. Food Control 2022, 135, 108828. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Li, Y.; Cui, Y.; Jia, C.; Wang, J.; Pan, J.; Yu, G.; Zhang, X.; Wang, X.; et al. The “umbrella of tolerance”: Nanobodies-armed photothermal lateral flow immunoassay for the detection of staphylococcal enterotoxin B. Chem. Eng. J. 2023, 470, 144273. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Zhang, Q.; Li, P.; Tang, X. Self-Assembly Multivalent Fluorescence-Nanobody Coupled Multifunctional Nanomaterial with Colorimetric Fluorescence and Photothermal to Enhance Immunochromatographic Assay. ACS Nano 2023, 17, 19359–19371. [Google Scholar] [CrossRef]
- Wu, L.; Jiao, L.; Xue, D.; Li, Y.; Han, Y.; Ouyang, W.; Chen, Q. Nanozyme and bifunctional nanobody-based colorimetric-SERS dual-mode Immunosensor for microcystin-LR detection. Food Chem. 2025, 464, 141574. [Google Scholar] [CrossRef]
- Zuo, J.; Yan, T.; Tang, X.; Zhang, Q.; Li, P. Dual-Modal Immunosensor Made with the Multifunction Nanobody for Fluorescent/Colorimetric Sensitive Detection of Aflatoxin B1 in Maize. ACS Appl. Mater. Interfaces 2023, 15, 2771–2780. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, X.; Wang, W.; Liu, C.; Yang, W.; Wang, D. Nanobody@Biomimetic mineralized MOF as a sensing immunoprobe in detection of aflatoxin B1. Biosens. Bioelectron. 2023, 220, 114906. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, X.; Su, B.; Chen, Q.; Cao, H.; Yun, Y.; Xu, Y.; Hammock, B.D. Ultrasensitive and rapid detection of ochratoxin A in agro-products by a nanobody-mediated FRET-based immunosensor. J. Hazard. Mater. 2020, 387, 121678. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Q.; Nidiaye, S.; Yan, H.; Zhang, W.; Tang, X.; Li, P. Development of a specific anti-idiotypic nanobody for monitoring aflatoxin M1 in milk and dairy products. Microchem. J. 2021, 167, 106326. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.-F.; Wan, D.-B.; Vasylieva, N.; Shen, Y.-D.; Xu, Z.-L.; Yang, J.-Y.; Gettemans, J.; Wang, H.; Hammock, B.D.; et al. Enhanced Non-Toxic Immunodetection of Alternaria Mycotoxin Tenuazonic Acid Based on Ferritin-Displayed Anti-Idiotypic Nanobody-Nanoluciferase Multimers. J. Agric. Food Chem. 2021, 69, 4911–4917. [Google Scholar] [CrossRef]
- Cai, C.; Liu, Y.; Tang, X.; Zhang, W.; Zhang, Q.; Li, P. Development of a toxin-free competitive immunoassay for aflatoxin M1 based on a nanobody as surrogate calibrator. LWT 2023, 182, 114829. [Google Scholar] [CrossRef]
- Li, P.; Deng, S.; Zech Xu, Z. Toxicant substitutes in immunological assays for mycotoxins detection: A mini review. Food Chem. 2021, 344, 128589. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-T.; Fu, H.-J.; Huang, J.-J.; Luo, L.; Lei, H.-T.; Shen, Y.-D.; Chen, Z.-J.; Wang, H.; Xu, Z.-L. Mimotope-Based Immunoassays for the Rapid Analysis of Mycotoxin: A Review. J. Agric. Food Chem. 2021, 69, 11743–11752. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, Z.; He, Z.; Xie, X.; Liu, X. Combination of nanobody and peptidomimetic to develop novel immunoassay platforms for detecting ochratoxin A in cereals. Food Chem. 2023, 429, 137018. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liu, X.; Li, Y.; Hou, J.; Liu, H.; Wu, Q.; Liu, J. Aptamers for nanobodies: A nontoxic alternative to toxic ochratoxin A in immunoassays. Biosens. Bioelectron. 2024, 248, 115995. [Google Scholar] [CrossRef]
- Mao, F.; He, Z.; Sun, Z.; Zhang, S.; Cao, H.; Liu, X. Plasmonic enzyme immunoassay via nanobody-driven controllable aggregation of gold nanoparticles for detection of ochratoxin A in pepper. Food Chem. 2024, 453, 139623. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Li, M.; Tian, Y.; Zhou, T.; Yu, Y.; Zheng, W.; Wang, X. A bifunctional protein RANbody based on nanobody facilitates dual-mode immunoassay of Staphylococcal enterotoxin B in food samples. Sens. Actuators B Chem. 2024, 418, 136295. [Google Scholar] [CrossRef]
- Xie, X.; He, Z.; Qu, C.; Sun, Z.; Cao, H.; Liu, X. Nanobody/NanoBiT system-mediated bioluminescence immunosensor for one-step homogeneous detection of trace ochratoxin A in food. J. Hazard. Mater. 2022, 437, 129435. [Google Scholar] [CrossRef]
- Tang, X.; Catanante, G.; Huang, X.; Marty, J.-L.; Wang, H.; Zhang, Q.; Li, P. Screen-printed electrochemical immunosensor based on a novel nanobody for analyzing aflatoxin M1 in milk. Food Chem. 2022, 383, 132598. [Google Scholar] [CrossRef]
- Xie, G.; Lu, Y.; Li, W.; He, Z.; Sun, Z.; Xie, X.; Liu, X. Simultaneous heptamerization of nanobody and alkaline phosphatase by self-assembly and its application for ultrasensitive immunodetection of small molecular contaminants in agro-products. Food Control 2022, 141, 109156. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Zuo, H.; Shen, W.; Zhang, Y.; Liu, R.; Geng, L.; Wang, W.; Shao, C.; Sun, T. Development of a magnetic separation immunoassay with high sensitivity and time-saving for detecting aflatoxin B1 in agricultural crops using nanobody. Eur. Food Res. Technol. 2023, 249, 1125–1136. [Google Scholar] [CrossRef]
- Sun, T.; Zhao, Z.; Liu, W.; Xu, Z.; He, H.; Ning, B.; Jiang, Y.; Gao, Z. Development of sandwich chemiluminescent immunoassay based on an anti-staphylococcal enterotoxin B Nanobody–Alkaline phosphatase fusion protein for detection of staphylococcal enterotoxin B. Anal. Chim. Acta 2020, 1108, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kalyoussef, S.; Feja, K.N. Foodborne Illnesses. Adv. Pediatr. 2014, 61, 287–312. [Google Scholar] [CrossRef] [PubMed]
- Braden, C.R.; Tauxe, R.V. Emerging trends in foodborne diseases. Infect. Dis. Clin. N. Am. 2013, 27, 517–533. [Google Scholar] [CrossRef]
- Maguire van Seventer, J.; Hamer, D.H. Foodborne Diseases. In International Encyclopedia of Public Health, 2nd ed.; Quah, S.R., Ed.; Academic Press: Oxford, UK, 2017; pp. 160–173. [Google Scholar]
- Zhao, X.; Lin, C.W.; Wang, J.; Oh, D.H. Advances in rapid detection methods for foodborne pathogens. J. Microbiol. Biotechnol. 2014, 24, 297–312. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, Y. Etiological Agents Implicated in Foodborne Illness World Wide. Food Sci. Anim. Resour. 2021, 41, 1–7. [Google Scholar] [CrossRef]
- Park, H.-Y.; Kim, C.-R.; Huh, I.-S.; Jung, M.-Y.; Seo, E.-Y.; Park, J.-H.; Lee, D.; Yang, J.-M. Staphylococcus aureus Colonization in Acute and Chronic Skin Lesions of Patients with Atopic Dermatitis. Ann. Dermatol. 2013, 25, 410–416. [Google Scholar] [CrossRef]
- Teklemariam, A.D.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Alghamdi, M.A.; Filimban, A.A.R.; Al Mutiri, A.S.; Al-Alyani, A.M.; Alseghayer, M.S.; Almaneea, A.M.; et al. Human Salmonellosis: A Continuous Global Threat in the Farm-to-Fork Food Safety Continuum. Foods 2023, 12, 1756. [Google Scholar] [CrossRef]
- Letchumanan, V.; Chan, K.G.; Lee, L.H. Vibrio parahaemolyticus: A review on the pathogenesis, prevalence, and advance molecular identification techniques. Front. Microbiol. 2014, 5, 705. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Recent Advances in Microfluidics-Based Electrochemical Sensors for Foodborne Pathogen Detection. Biosensors 2023, 13, 246. [Google Scholar] [CrossRef]
- Atkins, K.L.; Burman, J.D.; Chamberlain, E.S.; Cooper, J.E.; Poutrel, B.; Bagby, S.; Jenkins, A.T.A.; Feil, E.J.; van den Elsen, J.M.H.S. aureus IgG-binding proteins SpA and Sbi: Host specificity and mechanisms of immune complex formation. Mol. Immunol. 2008, 45, 1600–1611. [Google Scholar] [CrossRef]
- Baker, M. Reproducibility crisis: Blame it on the antibodies. Nature 2015, 521, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Chen, Q.; Li, Y.; Xiong, Y.; Xu, Y.; Hu, N.; Tao, Y. Identification and characterization of species-specific nanobodies for the detection of Listeria monocytogenes in milk. Anal. Biochem. 2016, 493, 1–7. [Google Scholar] [CrossRef] [PubMed]
- He, Y.X.; Ren, Y.R.; Guo, B.; Yang, Y.F.; Ji, Y.W.; Zhang, D.H.; Wang, J.L.; Wang, Y.R.; Wang, H. Development of a specific nanobody and its application in rapid and selective determination of Salmonella enteritidis in milk. Food Chem. 2020, 310, 125942. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Y.; Gu, J.; Yang, F.; Wu, S.; Zhang, C.; Ji, X.; Lv, H.; Muyldermans, S.; Wang, S. Selection of specific nanobodies to develop an immuno-assay detecting Staphylococcus aureus in milk. Food Chem. 2021, 353, 129481. [Google Scholar] [CrossRef]
- Bai, M.; Wang, Y.; Zhang, C.; Wang, Y.; Wei, J.; Liao, X.; Wang, J.; Anfossi, L.; Wang, Y. Nanobody-based immunomagnetic separation platform for rapid isolation and detection of Salmonella enteritidis in food samples. Food Chem. 2023, 424, 136416. [Google Scholar] [CrossRef]
- Anderson, G.P.; Liu, J.L.; Shriver-Lake, L.C.; Zabetakis, D.; Sugiharto, V.A.; Chen, H.W.; Lee, C.R.; Defang, G.N.; Wu, S.L.; Venkateswaran, N.; et al. Oriented Immobilization of Single-Domain Antibodies Using SpyTag/SpyCatcher Yields Improved Limits of Detection. Anal. Chem. 2019, 91, 9424–9429. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, J.; Wang, Y.; Wang, P.; Ji, Y.; Liu, B.; Wang, J.; González-Sapienza, G.; Wang, Y. Development of a streptavidin-bridged enhanced sandwich ELISA based on self-paired nanobodies for monitoring multiplex Salmonella serogroups. Anal. Chim. Acta 2022, 1203, 339705. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, P.; Liao, X.; Dai, Y.; Yu, Q.; Yu, G.; Zhang, Y.; Wei, J.; Jing, Y.; et al. Enhancing Oriented Immobilization Efficiency: A One-for-Two Organism-Bispecific Nanobody Scaffold for Highly Sensitive Detection of Foodborne Pathogens. Anal. Chem. 2023, 95, 17135–17142. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.L.; Bai, M.F.; Wang, Y.; Liao, X.R.; Zhang, Y.; Wang, P.; Wei, J.; Zhang, H.Y.; Wang, J.L.; et al. An ultrasensitive sandwich chemiluminescent enzyme immunoassay based on phage-mediated double-nanobody for detection of Salmonella Typhimurium in food. Sens. Actuators B-Chem. 2022, 352, 131058. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, X.R.; Yu, G.G.; Wei, J.; Wang, P.; Wang, Y.Q.; Jing, Y.N.; Wang, J.M.; Chen, P.Y.; Wang, J.L.; et al. Phage-Displayed Nanobody as a Sensitive Nanoprobe to Enhance Chemiluminescent Immunoassay for Cronobacter sakazakii Detection in Dairy Products. Anal. Chem. 2023, 95, 13698–13707. [Google Scholar] [CrossRef]
- Wade, J.; Rimbault, C.; Ali, H.; Ledsgaard, L.; Rivera-de-Torre, E.; Abou Hachem, M.; Boddum, K.; Mirza, N.; Bohn, M.-F.; Sakya, S.A.; et al. Generation of Multivalent Nanobody-Based Proteins with Improved Neutralization of Long α-Neurotoxins from Elapid Snakes. Bioconjug. Chem. 2022, 33, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhang, Y.; Liang, Y.; Zhang, L.; Wang, P.; Wei, J.; Yin, X.; Wang, J.; Wang, H.; Wang, Y. Enhanced sandwich immunoassay based on bivalent nanobody as an efficient immobilization approach for foodborne pathogens detection. Anal. Chim. Acta 2024, 1289, 342209. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Jiang, B.; Guan, Z.; He, J.; Yang, D.; Xie, N.; Nie, G.; Xie, C.; Yan, X. Fenobody: A Ferritin-Displayed Nanobody with High Apparent Affinity and Half-Life Extension. Anal. Chem. 2018, 90, 5671–5677. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wang, J.; Guo, B.; Bai, M.; Zhang, Y.; Yu, G.; Wang, P.; Wei, J.; Wang, J.; Yan, X.; et al. Enhancing Nanobody Immunoassays through Ferritin Fusion: Construction of a Salmonella-Specific Fenobody for Improved Avidity and Sensitivity. J. Agric. Food Chem. 2024, 72, 14967–14974. [Google Scholar] [CrossRef]
- Wang, P.; Yu, G.; Wei, J.; Liao, X.; Zhang, Y.; Ren, Y.; Zhang, C.; Wang, Y.; Zhang, D.; Wang, J.; et al. A single thiolated-phage displayed nanobody-based biosensor for label-free detection of foodborne pathogen. J. Hazard. Mater. 2023, 443, 130157. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Liu, Z.; Bai, M.; Wang, J.; Wang, Y. Nanobody-based immunochromatographic biosensor for colorimetric and photothermal dual-mode detection of foodborne pathogens. Sens. Actuators B Chem. 2022, 369, 132371. [Google Scholar] [CrossRef]
- He, Q.; Pan, J.; Xu, Z.; Hammock, B.D.; Li, D. Development of a nanobody-based immunoassay for the detection of Escherichia coli O157:H7 in food samples. Food Chem. 2025, 473, 142987. [Google Scholar] [CrossRef]
- Yan, H.; Fu, J.; Tang, X.; Wang, D.; Zhang, Q.; Li, P. Sensitivity enhancement of paper-based sandwich immunosensor via nanobody immobilization instead of IgG antibody, taking aflatoxingenetic fungi as an analyte example. Sens. Actuators B Chem. 2022, 373, 132760. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Balali-Mood, K.; Moodi, M.; Balali-Mood, B. Health aspects of organophosphorous pesticides in asian countries. Iran. J. Public Health 2012, 41, 1–14. [Google Scholar]
- Nougadère, A.; Reninger, J.-C.; Volatier, J.-L.; Leblanc, J.-C. Chronic dietary risk characterization for pesticide residues: A ranking and scoring method integrating agricultural uses and food contamination data. Food Chem. Toxicol. 2011, 49, 1484–1510. [Google Scholar] [CrossRef] [PubMed]
- Lerro, C.C.; Hofmann, J.N.; Andreotti, G.; Koutros, S.; Parks, C.G.; Blair, A.; Albert, P.S.; Lubin, J.H.; Sandler, D.P.; Beane Freeman, L.E. Dicamba use and cancer incidence in the agricultural health study: An updated analysis. Int. J. Epidemiol. 2020, 49, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Asthana, A.; Upadhyay, K. Kinetic-spectrophotometric determination of methyl parathion in water and vegetable samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 101, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Sun, X.; Li, L.; Li, D.; Wang, M.; Shi, H. Toxicity effects of procymidone, iprodione and their metabolite of 3,5-dichloroaniline to zebrafish. Chemosphere 2021, 272, 129577. [Google Scholar] [CrossRef]
- Xu, L.Y.; Abd El-Aty, A.M.; Eun, J.B.; Shim, J.H.; Zhao, J.; Lei, X.M.; Gao, S.; She, Y.X.; Jin, F.; Wang, J.; et al. Recent Advances in Rapid Detection Techniques for Pesticide Residue: A Review. J. Agric. Food Chem. 2022, 70, 13093–13117. [Google Scholar] [CrossRef]
- Xu, B.; Wang, K.; Vasylieva, N.; Zhou, H.; Xue, X.; Wang, B.; Li, Q.X.; Hammock, B.D.; Xu, T. Development of a nanobody-based ELISA for the detection of the insecticides cyantraniliprole and chlorantraniliprole in soil and the vegetable bok choy. Anal. Bioanal. Chem. 2021, 413, 2503–2511. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, H.; Fu, Y.; Wang, Z.; Gao, Q.; Yang, D.; Kang, J.; Chen, L.; An, Z.; Hammock, B.D.; et al. Establishment of an indirect competitive immunoassay for the detection of dicamba based on a highly specific nanobody. Sci. Total Environ. 2024, 917, 170567. [Google Scholar] [CrossRef]
- Liu, M.-L.; Chen, Z.-J.; Huang, X.-Q.; Wang, H.; Zhao, J.-L.; Shen, Y.-D.; Luo, L.; Wen, X.-W.; Hammock, B.; Xu, Z.-L. A bispecific nanobody with high sensitivity/efficiency for simultaneous determination of carbaryl and its metabolite 1-naphthol in the soil and rice samples. Environ. Pollut. 2023, 335, 122265. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Zhang, Y.-F.; Chen, J.-L.; Lin, Z.-S.; Wu, M.-F.; Shen, Y.-D.; Luo, L.; Wang, H.; Wen, X.-W.; Hammock, B.; et al. Production and Characterization of Biotinylated Anti-fenitrothion Nanobodies and Development of Sensitive Fluoroimmunoassay. J. Agric. Food Chem. 2022, 70, 4102–4111. [Google Scholar] [CrossRef]
- Lv, T.; Wang, B.; Xu, N.; Shang, B.; Liu, N.; Su, C.; Yang, C.; Li, H.; Xu, Z.; Sun, C. Gold nanoclusters-manganese dioxide composite-based fluorescence immunoassay for sensitive monitoring of fenitrothion degradation in Chinese cabbage. Food Chem. 2023, 412, 135551. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Wu, H.-L.; Shen, Y.-D.; Wang, H.; Zhang, Y.-F.; Hammock, B.; Li, Z.-F.; Luo, L.; Lei, H.-T.; Xu, Z.-L. Phosphate-triggered ratiometric fluoroimmunoassay based on nanobody-alkaline phosphatase fusion for sensitive detection of 1-naphthol for the exposure assessment of pesticide carbaryl. J. Hazard. Mater. 2022, 424, 127411. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Pang, J.; Wang, Y.; Bi, C.; Xu, Z.; Shen, Y.; Yang, J.; Wang, H.; Sun, Y. Nanobodies-based colloidal gold immunochromatographic assay for specific detection of parathion. Anal. Chim. Acta 2024, 1310, 342717. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; He, X.T.; Xu, Z.L.; Deng, H.; Shen, Y.D.; Luo, L.; Shen, X.; Chen, Z.J.; Hammock, B.; Wang, H. Development of a Biotinylated Nanobody-Based Gold Nanoparticle Immunochromatographic Assay for the Detection of Procymidone in Crops. J. Agric. Food Chem. 2023, 71, 13137–13146. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Li, L.-H.; Wang, Y.; Wang, H.; Xu, Z.-L.; Tian, Y.-X.; Sun, Y.-M.; Yang, J.-Y.; Shen, Y.-D. Ultrasensitive and rapid colorimetric detection of paraquat via a high specific VHH nanobody. Biosens. Bioelectron. 2022, 205, 114089. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.-J.; Zhang, J.-X.; Wang, H.; Wang, Y.; Zeng, X.; Xu, Z.-L.; Yang, J.-Y.; Xiao, Z.-L.; Hammock, B.D.; Wen, P. A highly sensitive electrochemical immunosensor based on electrospun nanocomposite for the detection of parathion. Food Chem. 2023, 404, 134371. [Google Scholar] [CrossRef]
- Liu, M.-L.; Zeng, X.; Deng, H.; Wang, Y.; Zhang, Y.-F.; Shen, Y.-D.; Luo, L.; Wang, H.; Chen, Z.-J.; Xu, Z.-L. Phosphate-triggered ratiometric multicolor immunosensor based on nanobody-alkaline phosphatase fusion protein for sensitive detection of fenitrothion. Sens. Actuators B Chem. 2022, 373, 132734. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Huang, A.-J.; Luo, L.; Xu, Z.-L.; Wang, H. Simple dual-readout immunosensor based on phosphate-triggered and potassium permanganate for visual detection of fenitrothion. Biosens. Bioelectron. 2024, 246, 115872. [Google Scholar] [CrossRef]
- Liang, Y.-F.; Li, J.-D.; Fang, R.-Y.; Xu, Z.-L.; Luo, L.; Chen, Z.-J.; Yang, J.-Y.; Shen, Y.-D.; Ueda, H.; Hammock, B.; et al. Design of an Antigen-Triggered Nanobody-Based Fluorescence Probe for PET Immunoassay to Detect Quinalphos in Food Samples. Anal. Chem. 2023, 95, 12321–12328. [Google Scholar] [CrossRef]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Soller, L.; La Vieille, S.; Cameron, S.B.; Mak, R.; Cook, V.E.; Gerdts, J.; Chan, E.S. Allergic reactions to emerging food allergens in Canadian children. Allergy Asthma Clin. Immunol. 2021, 17, 71. [Google Scholar] [CrossRef]
- Koplin, J.J.; Mills, E.N.; Allen, K.J. Epidemiology of food allergy and food-induced anaphylaxis: Is there really a Western world epidemic? Curr. Opin. Allergy Clin. Immunol. 2015, 15, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.H.; Li, A.; Lv, H.; Ji, X.M.; Hu, Y.Z.; Wang, S. Nanobody-based food allergen surveillance: Current status and prospects. Food Qual. Saf. 2024, 8, fyae018. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Nie, L.; Lin, J.; Wu, S.; Li, S.; Wu, J.; Ji, X.; Lv, H.; Muyldermans, S.; et al. Exploration of Specific Nanobodies as Immunological Reagents to Detect Milk Allergen of β-Lactoglobulin without Interference of Hydrolytic Peptides. J. Agric. Food Chem. 2022, 70, 15271–15282. [Google Scholar] [CrossRef]
- Li, S.; Nie, L.; Wang, Y.; Wang, Y.; Fan, D.; Wang, J.; Hu, Y.; Dong, L.; Zhang, Y.; Wang, S. Detection of β-lactoglobulin under different thermal-processing conditions by immunoassay based on nanobody and monoclonal antibody. Food Chem. 2023, 424, 136337. [Google Scholar] [CrossRef]
- Rodríguez-Camejo, C.; Delfin-Riela, T.; Rossotti, M.A.; Puyol, A.; Echaides, C.; Hernández, A.; González-Sapienza, G. A highly sensitive nanobody-based ELISA for bovine β-lactoglobulin to classified donated human milk destined to susceptible newborns. Food Control 2023, 153, 109910. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, S.; Wang, Y.; Lin, J.; Sun, Y.; Zhang, C.; Gu, J.; Yang, F.; Lv, H.; Ji, X.; et al. Unbiased Immunization Strategy Yielding Specific Nanobodies against Macadamia Allergen of Vicilin-like Protein for Immunoassay Development. J. Agric. Food Chem. 2021, 69, 5178–5188. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, C.; Lin, J.; Wang, Y.; Wu, S.; Sun, Y.; Zhang, B.; Lv, H.; Ji, X.; Lu, Y.; et al. Selection of specific nanobodies against peanut allergen through unbiased immunization strategy and the developed immuno-assay. Food Sci. Hum. Wellness 2023, 12, 745–754. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, J.; Peng, L.; Wang, Y.; Wu, S.; Ji, X.; Lv, H.; Wu, J.; Zhang, Y.; Wang, S. Nanobody-Based Electrochemical Immunoassay for Sensitive Detection of Peanut Allergen Ara h 1. J. Agric. Food Chem. 2023, 71, 7535–7545. [Google Scholar] [CrossRef]
- Li, S.; Nie, L.; Yang, L.; Fan, D.; Wang, J.; Hu, Y.; Zhang, Y.; Wang, S. “Fluorescence-wavelength” label-free POCT tandem with “fluorescence-photothermal” nanobody-immunosensor for detecting BSA and β-lactoglobulin. Food Chem. 2024, 430, 137019. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, X.; He, Z.; Wu, L.; Xie, X.; Sun, Z.; Zhang, S.; Cao, H.; Hammock, B.D.; Liu, X. Colorimetric and surface-enhanced Raman scattering dual-mode lateral flow immunosensor using phage-displayed shark nanobody for the detection of crustacean allergen tropomyosin. J. Hazard. Mater. 2024, 468, 133821. [Google Scholar] [CrossRef]
- Yao, C.; Hu, Y.; Liu, Q.; Liu, J.M.; Ji, X.; Lv, H.; Wang, S. Nanobody mediated dual-mode immunoassay for detection of peanut allergen Ara h 3. Food Chem. 2024, 436, 137679. [Google Scholar] [CrossRef]
| Principle | Target | Detection Technique | Detection Label | LOD | IC50 | Linear Range | Sample | Reference |
|---|---|---|---|---|---|---|---|---|
| ELISA | Alternaria mycotoxins tenuazonic acid | IC-ELISA | Nb(B3G3) | 0.09 ng/mL | 1.3 ng/mL | - | Rice, flour, and bread | [59] |
| Ustilaginoidins | IC-ELISA | Nb-B15 | - | 11.86 µg/mL and 11.22 µg/mL | 3.41~19.98 µg/mL and 1.17~32.13 µg/mL | Rice | [60] | |
| Ochratoxin A | Dc-PEIA | Nb 28 | Instrumental LOD: 0.275 ng/mL Visual LOD: 1.56 ng/mL | 10.84 ng/mL | 5.18~29.32 ng/mL | Black pepper and white pepper | [88] | |
| Aflatoxin B1 | BA-ELISA | Nb 26 | 0.04 ng/mL | 0.21 ng/mL | - | Wheat and corn | [61] | |
| Ochratoxin A | MBS-ELISA | Nb-2G | 0.07 ng/mL | 1.17 ng/mL | 248.8 pg/mL~5.28 ng/mL | Mung bean, buckwheat, and sorghum rice | [62] | |
| α-hemolysin | Sandwich ELISA | HLA 39 HLA 17 | 10 ng/mL | - | 10~1000 ng/mL | Milk and pork | [63] | |
| Fluorescence immunoassay | Ochratoxin A | Nb-AP-induced PT-FIA | Nb-AP | 0.12 ng/mL | 0.46 ng/mL | 0.2~1.26 ng/mL | Barley | [64] |
| Ochratoxin A | IFE-FLIA | Nb-ALP | 0.018 μg/kg | 0.22 ng/mL | 0.11~0.53 ng/mL | Pepper | [65] | |
| Staphylococcal enterotoxin B | Dual-mode immunoassay | SEB57 SEB27-vHRRP | Colorimetric mode: 0.12 ng/mL Fluorescence mode: 0.24 ng/mL | - | 0.31~2500 ng/mL | Milk, pork | [89] | |
| Bioluminescent immunoassay | Ochratoxin A | BLEIA | Nb 28-Nluc | 3.7 ng/mL | - | - | Coffee | [68] |
| Tenuazonic acid | CLEIA/BLEIA | Nb39−Nluc | 0.3 ng/mL 1.1 ng/mL | 8.6 ng/mL 9.3 ng/mL | - | Rice, flour, and apple juice | [69] | |
| Aflatoxin B1 and ochratoxin A | SA-BLEIA | Nb 28 and Nb 26 | AFB1: 0.053 ng/mL OTA: 0.051 ng/mL | AFB1: 0.452 ng/mL OTA: 0.147 ng/mL | - | Cereal powders and spiked cereal | [70] | |
| LFIA | Aflatoxin B1 | AuNPs-ICTS | G8-DIG | 0.1 ng/mL | 5.46 ng/mL | 1.02~27.86 ng/mL | Corn | [71] |
| Aflatoxin B1 | Nb-LFIA | Nb@QD | 0.095 ng/mL | 0.85 ng/mL | - | Oat | [73] | |
| Staphylococcal enterotoxin B | NLFIA | anti-SEB Nb7 | Colorimetric mode: 1.68 ng/mL Photothermal mode: 0.58 ng/mL | - | 1~128 ng/mL | Milk, milk powder, and pork | [75] | |
| Aflatoxin B1 | TLFIA | Nb 26-EGFP-H6 | Colorimetric signals: 0.0012 ng/mL Fluorescent signals: 0.0094 ng/mL Photothermal signals: 0.252 ng/mL | - | 0.05~100 ng/mL, 0.25~60 ng/mL, and 1~500 ng/mL | Maize | [76] | |
| Immunosensor | Microcystin-LR | Multimodal biosensors | A2.3-SBP | 0.26 ng/mL | - | 1.0~500 ng/mL | Lake water samples | [77] |
| Aflatoxin B1 | Fluorescent–colorimetric immunosensor | Nb26-EGFP | 0.0024 ng/mL | - | - | Corn | [78] | |
| Aflatoxin B1 | Immunoensor | Nb G8 | 20.0 fg/mL | - | 50.0 fg/mL~20.0 ng/mL | Flour and rice | [79] | |
| Ochratoxin A | Nb-FRET immunosensor | Nb 28 | 5 pg/mL | - | - | Rice, oats, barley, and wheat | [80] | |
| Ochratoxin A | Bioluminescence immunosensor | Nb 28 | 0.01 ng/mL | 0.31 ng/mL | 0.04~2.23 ng/mL | Barley, oats, and rice | [90] | |
| Nontoxic immunoassay | Aflatoxin M1 | C-ELISA | Nb C4 | 0.05 ng/mL | 0.25 ng/mL | 0.10 ng/mL~0.60 ng/mL | Milk, yogurt, and milk powder | [81] |
| Tenuazonic acid | BLEIA | AId-Nb NLuc | 0.7 ng/mL | 6.5 ng/mL | - | Rice, flour, and bread | [82] | |
| Aflatoxin M1 | Toxin-free ELISA | Nb C4 | 0.035 ng/mL | - | 0.045~0.329 ng/mL | Milk and yogurt | [83] | |
| Aflatoxin M1 | Electrochemical immunosensor | Nb 4–1-1 | 0.09 ng/mL | - | 0.25~5.0 ng/mL | Milk | [91] | |
| Ochratoxin A | APN-ELISA | Nb-C4bpα | 0.027 ng/mL | 0.169 ng/mL | 0.058~0.471 ng/mL | Barley, oats, and rice | [86] | |
| Ochratoxin A | IC-ELISA | apt 2-OT | 0.23 ng/mL | - | 0.25~10.50 ng/mL | Flour, corn, and meal | [87] | |
| Enhanced colorimetric enzyme immunoassay | Ochratoxin A | Colorimetric enzyme immunoassay | Nb-ALP-C4bpα | 0.018 ng/mL | 0.081 ng/mL | 0.036~0.175 ng/mL | Barley, oats, and rice | [92] |
| CLEIA | Aflatoxin B1 | MB-CLEIA | Nb-ALP | 0.743 pg/mL | 0.33 ng/mL | 7.23 pg/mL~12.38 ng/mL | Oats, corn, and oil sample | [93] |
| Staphylococcal enterotoxin B | Sandwich CLIA | Nb37-ALP | 1.44 ng/mL | 8.59± 0.37 ng/mL | 3.12~50 ng/mL | Pure milk, water, and serum | [94] |
| Principle | Target | Detection Technique | Detection Label | LOD | Linear Range | Sample | Reference |
|---|---|---|---|---|---|---|---|
| ELISA | Salmonella enteritidis | Sandwich ELISA | Nb13 | 1.4 × 105 CFU/mL | - | Whole milk, skimmed milk, and walnut milk | [107] |
| Staphylococcus aureus | Sandwich ELISA | Nb147 and biotinylated Nb147 | 1.4 × 105 CFU/mL | 104~1010 CFU/mL | Milk | [108] | |
| Salmonella enteritidis | IMS-ELISA | Nb-F23 | 3.2 × 103 CFU/mL | 1.4 × 104~5.9 × 105 CFU/mL | Chicken meat, cabbage, tomato, and apple juice | [109] | |
| E. coli O157:H7 | Sandwich ELISA | VHH | 8.7 × 103 CFU/mL | - | Orange juice, milk, and beef | [121] | |
| Salmonella Enteritidis, Salmonella Typhimurium, Salmonella London, Salmonella Paratyphi B, and Salmonella Hadar | SAB-ELISA | bi-Nb01 | 6.31 × 103 CFU/mL 9.15 × 103 CFU/mL 4.23 × 103 CFU/mL 7.31 × 103 CFU/mL 7.25 × 103 CFU/mL | - | Milk, honey, pork, and lettuce | [111] | |
| Salmonella spp. and V. parahaemolyticus. | O-ELISA | O-BsNb | Salmonella spp.: 3.33 × 103 CFU/mL V. parahaemolyticus.: 6.35 × 104 CFU/mL | - | Shrimp and chicken | [112] | |
| CLISA | Salmonella Typhimurium | P-CLISA | Nb1 and Nb9 | 3.63 × 103 CFU/mL | 5.1 × 103~1.2 × 106 CFU/mL | Juice, honey, milk, and pork samples | [113] |
| Cronobacter sakazakii | P-CLISA | Cs-Nb 1 and Cs-Nb 2 | 1.04 × 104 CFU/mL | - | Milk powder and whole milk | [114] | |
| Salmonella | BNb-ELISA | Nb413 and Nb422 | 2.364 × 103 CFU/mL | - | Ham sausage, beef, and shrimp | [116] | |
| S. Enteritidis | FbNb-ELISA FbBio-ELISA FbP-ELISA FbNb-CLISA | Nb422 and biotinyiated Nb422 | 3.56 × 104 CFU/mL 5.83 × 105 CFU/mL 4.42 × 105 CFU/mL 2.94 × 103 CFU/mL | - | Juice, ham sausage, and honey | [118] | |
| Immunosensor | V. parahaemolyticus | Nb-based biosensor | Phage–Nb-SH | 104 CFU/mL | - | Shrimp | [119] |
| Salmonella Typhimurium | KNb-DITS | K0.27MnO2·0. 54 H2O@Au@Nb9 | Colorimetric mode: 104 CFU/mL Photothermal mode: 103 CFU/mL | - | Juice, honey, and chocolate | [120] | |
| Aflatoxingenetic fungi | Time-resolved fluorescence immunoassay | PO8-VHH | 0.035 μg/mL | 0.085~323.56 μg/mL and 0.23~327.55 μg/mL | Blank peanut | [122] |
| Principle | Target | Detection Technique | Detection Label | LOD | IC50 | Linear Range | Sample | Reference |
|---|---|---|---|---|---|---|---|---|
| ELISA | Insecticides cyantraniliprole and chlorantraniliprole | C-ELISA | NbC1 and NbC2 | 0.2 ng mL | 1.2 and 1.5 ng/mL | 0.4~6.1 ng/mL | Bok choy | [130] |
| Dicamba | ic-ELISA | Nb-242 | - | 0.93 μg/mL | 0.11~8.01 μg/mL | Tap water and soil | [131] | |
| Carbaryl and 1-naphthol | Bic-ELISA | G4S-C-N-VHH | 0.8 ng/mL and 0.4 ng/mL | 18.8/6.3 ng/mL | 2.1~270.9 ng/mL 1.1~112.0 ng/mL | Soil and rice | [132] | |
| Fluorescence immunoassay | Fenitrothion | FIA | VHHjd8-BT | 0.03 ng/mL | 1.4 ng/mL | 0.078~100 ng/mL | Chinese cabbage, lettuce, and tangerine | [133] |
| Fenitrothion | FIA | Nb-ALP | 5.78 pg/mL | - | 0.00001~100 ng/mL | Tap water, river water, apple, chinese cabbage, lettuce, rice, and tomato | [134] | |
| Quinalphos | PET | Nb-R29W | 0.007 μg/mL | 0.063 μg/mL | 0.015~0.255 μg/mL | Chinese cabbage and cucumber | [142] | |
| LFIA | Parathion | GICA | VHH9 | 0.15 ng/mL | 2.39 ng/mL | 0.47~10.58 ng/mL | Chinese cabbage, orange, and cucumber | [136] |
| Procymidone | BtNb-ICA | GNP@NbFM5-Bt | 0.88 ng/mL | 6.04 ng/mL | 1.95~18.67 ng/mL | Chives, cucumbers, and tomatoes | [137] | |
| Paraquat | TRFICA | FM-VHH | 0.0090 ng/mL | 0.0588 ng/mL | 0.0201~0.165 ng/mL | Chinese cabbage, pear, blood, urine, rice, and corn | [138] | |
| Immunosensor | Parathion | Electrochemical immunosensor | VHH9-HRP | 2.26 pg/mL | - | 0.01~100 ng/mL | Cucumber, orange, and cabbage | [139] |
| Fenitrothion | Multicolor immunosensor | VHHjd8ALP | MRVIA: 3.0 ng/mL MRFIA: 1.3 ng/mL | MRVIA: 6.7 ng/mL MRFIA: 6.2 ng/mL | MRVIA: 4.7~11.6 ng/mL MRFIA: 2.6~19.5 ng/mL | Apple, cabbage, and cucumber | [140] | |
| Fenitrothion | Multicolor immunosensor | VHHjd8ALP | MVIS: 11.2 ng/mL FMVIS: 7.4 ng/mL | MVIS: 70.7 ng/mL FMVIS: 12.1 ng/mL | MVIS: 17.3~197.5 ng/mL FMVIS: 7.7~16.1 ng/mL | Apple, Chinese cabbage, and cucumber | [141] |
| Principle | Target | Detection Technique | Detection Label | LOD | Linear Range | Sample | Reference |
|---|---|---|---|---|---|---|---|
| ELISA | β-lactoglobulin | cELISA/sELISA | Nb 82 | 4.55 ng/mL 13.82 ng/mL | 39~10,000 ng/mL 29.7~1250 ng/mL | Milk, oatmeal, and candy | [148] |
| β-lactoglobulin | sandwich ELISA | Nb 82 | 0.24 ng/mL | 0.01~10 μg/mL | Milk and beverage | [149] | |
| β-lactoglobulin | sandwich ELISA | HA-Nb | 40 pg/mL | 3000 pg/mL | Human milk | [150] | |
| Macadamia protein | sandwich ELISA | Nb 139 H and Nb 68 HA | 27.1 ng/mL | 0.442~2800 μg/mL | Skimmed milk | [151] | |
| Ara h 3 | sandwich ELISA | Nb P43 | 53.13 ng/mL | 0.2~10.6 μg/mL | Skim milk | [152] | |
| Biosensor | Ara h 1 | Nb-μTEI | Nb152 | 0.86 ng/mL | 4.5~55 ng/mL | Milk and chocolate | [153] |
| BSA and β-lactoglobulin | “fluorescence–photothermal” immunosensor | Nb82 | fluorescence mode: 0.034 ng/mL wavelength mode: 0.075 ng/mL | fluorescence mode: 0.1 ng/mL~0.1 μg/mL wavelength mode: 0.1 ng/mL~0.1 μg/mL | Milk and beverage | [154] | |
| Ara h 3 | colorimetry with ratiometric fluorescence immunoassay | Nb P43 | 6.61 ng/mL and 9.79 ng/mL | 10~1200 ng/mL | Peanut allergy Ara h 3 and fried peanuts | [156] | |
| Tropomyosin | CM/SERS-LFI | AuMBA@AgNPs | 0.0026 μg/mL (SERS mode) and 0.0057 μg/mL (colorimetric mode) visual LOD 0.01 μg/mL | 0.005~0.5 μg/mL | Bread, cookies, and cheese | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Xu, Z.; He, Q.; Pan, J.; Zhang, Y.; El-Sheikh, E.-S.A.; Hammock, B.D.; Li, D. Nanobody-Based Immunoassays for the Detection of Food Hazards—A Review. Biosensors 2025, 15, 183. https://doi.org/10.3390/bios15030183
Li W, Xu Z, He Q, Pan J, Zhang Y, El-Sheikh E-SA, Hammock BD, Li D. Nanobody-Based Immunoassays for the Detection of Food Hazards—A Review. Biosensors. 2025; 15(3):183. https://doi.org/10.3390/bios15030183
Chicago/Turabian StyleLi, Wenkai, Zhihao Xu, Qiyi He, Junkang Pan, Yijia Zhang, El-Sayed A. El-Sheikh, Bruce D. Hammock, and Dongyang Li. 2025. "Nanobody-Based Immunoassays for the Detection of Food Hazards—A Review" Biosensors 15, no. 3: 183. https://doi.org/10.3390/bios15030183
APA StyleLi, W., Xu, Z., He, Q., Pan, J., Zhang, Y., El-Sheikh, E.-S. A., Hammock, B. D., & Li, D. (2025). Nanobody-Based Immunoassays for the Detection of Food Hazards—A Review. Biosensors, 15(3), 183. https://doi.org/10.3390/bios15030183






