Application of an Electrochemical Sensor Based on Nitrogen-Doped Biochar Loaded with Ruthenium Oxide for Heavy Metal Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization
2.2. The Preparation of Raw Materials
2.3. Preparation of Modified Electrodes
2.4. Determination of Cu2+ and Pb2+ Solutions at Different Concentrations
2.5. Ion Selectivity Experiments
2.6. Analysis of Samples
3. Results
3.1. Structural Characterization of RuO2-NC-Modified Electrodes
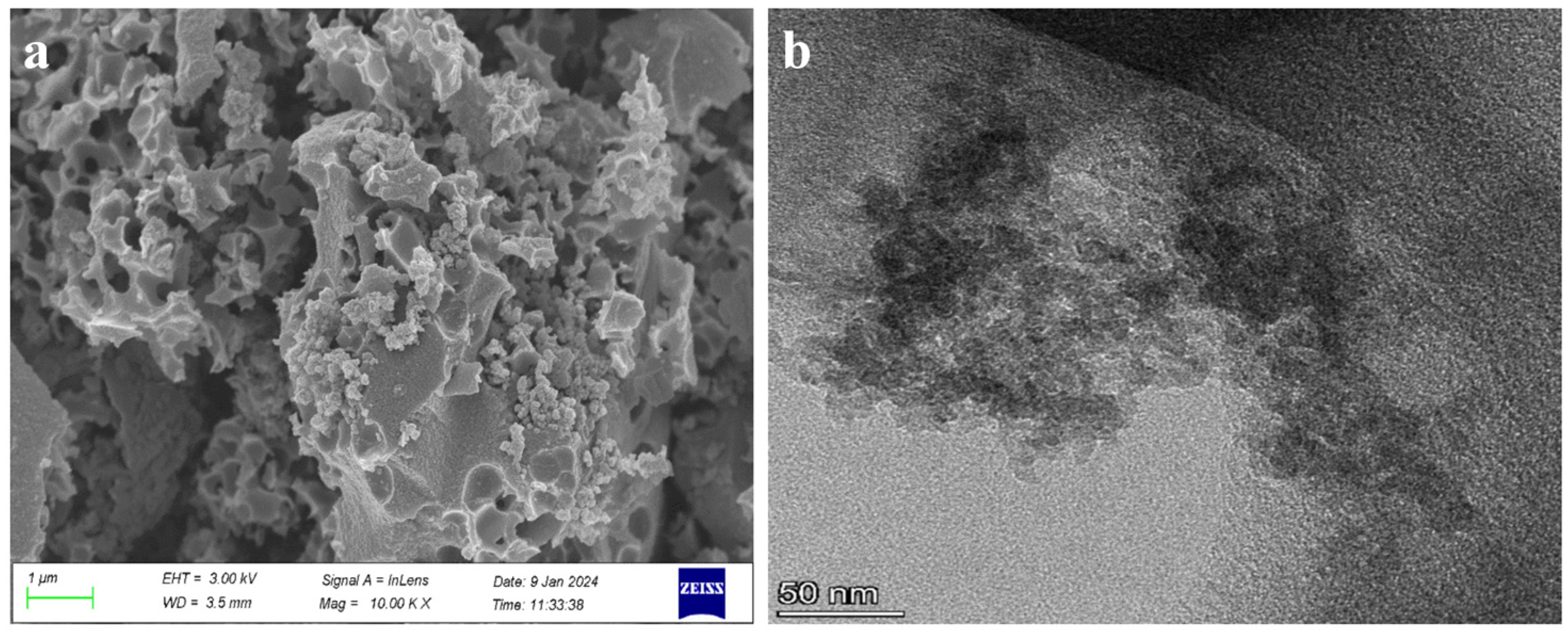
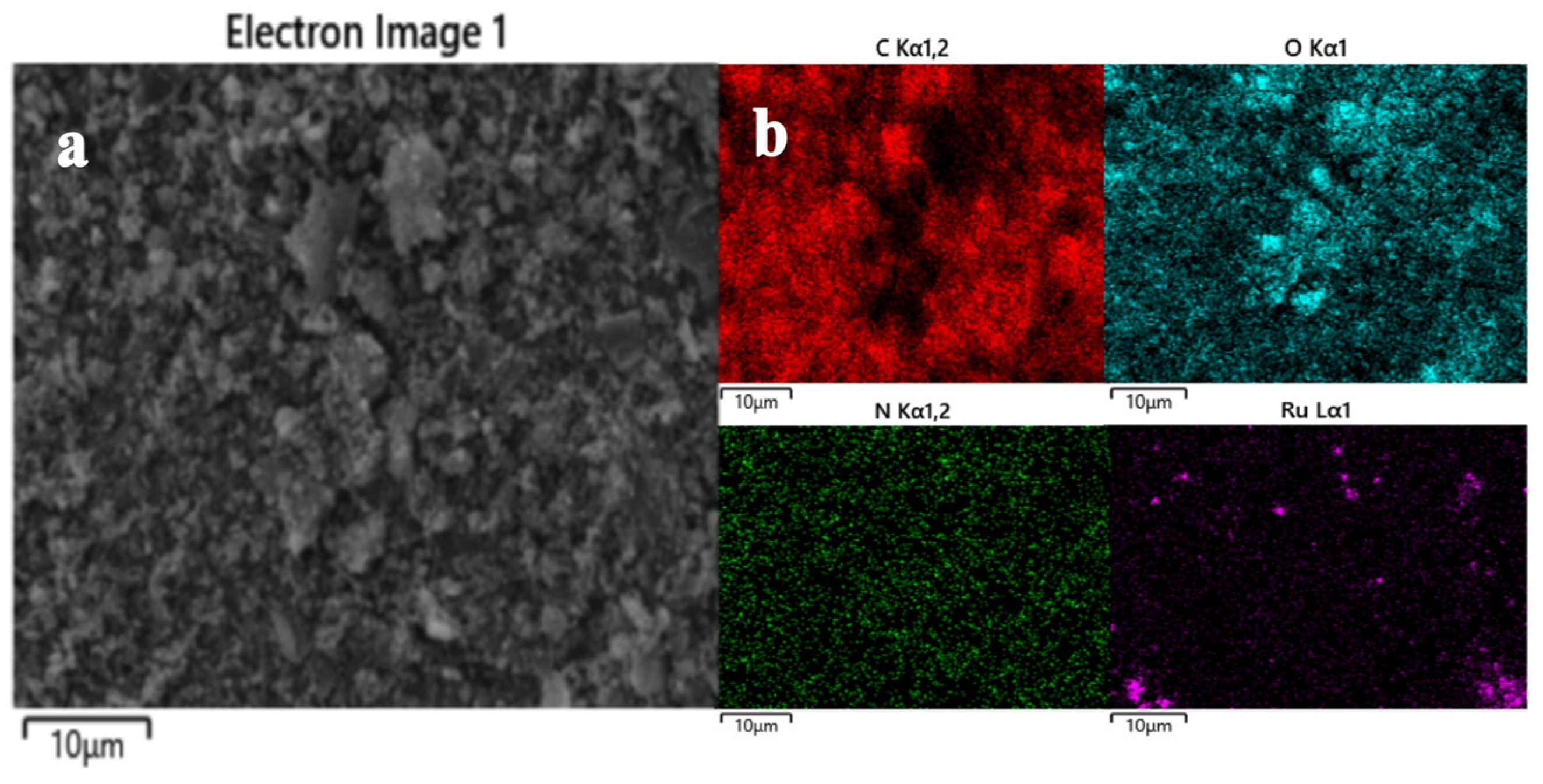
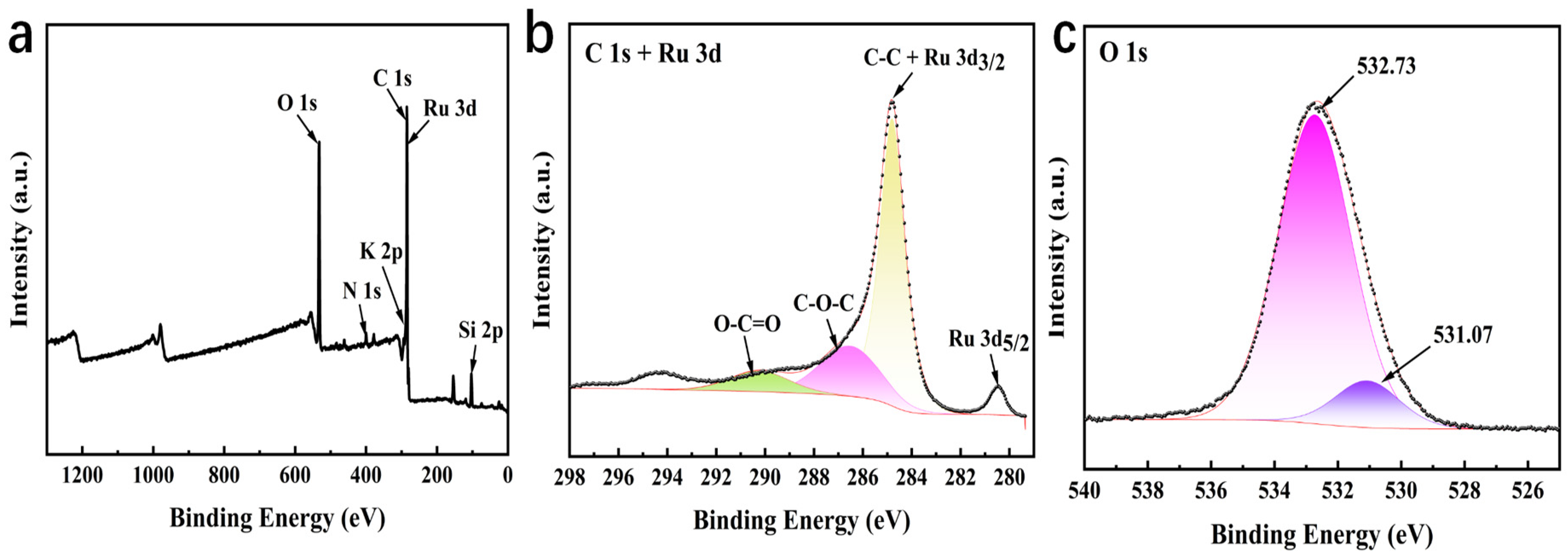
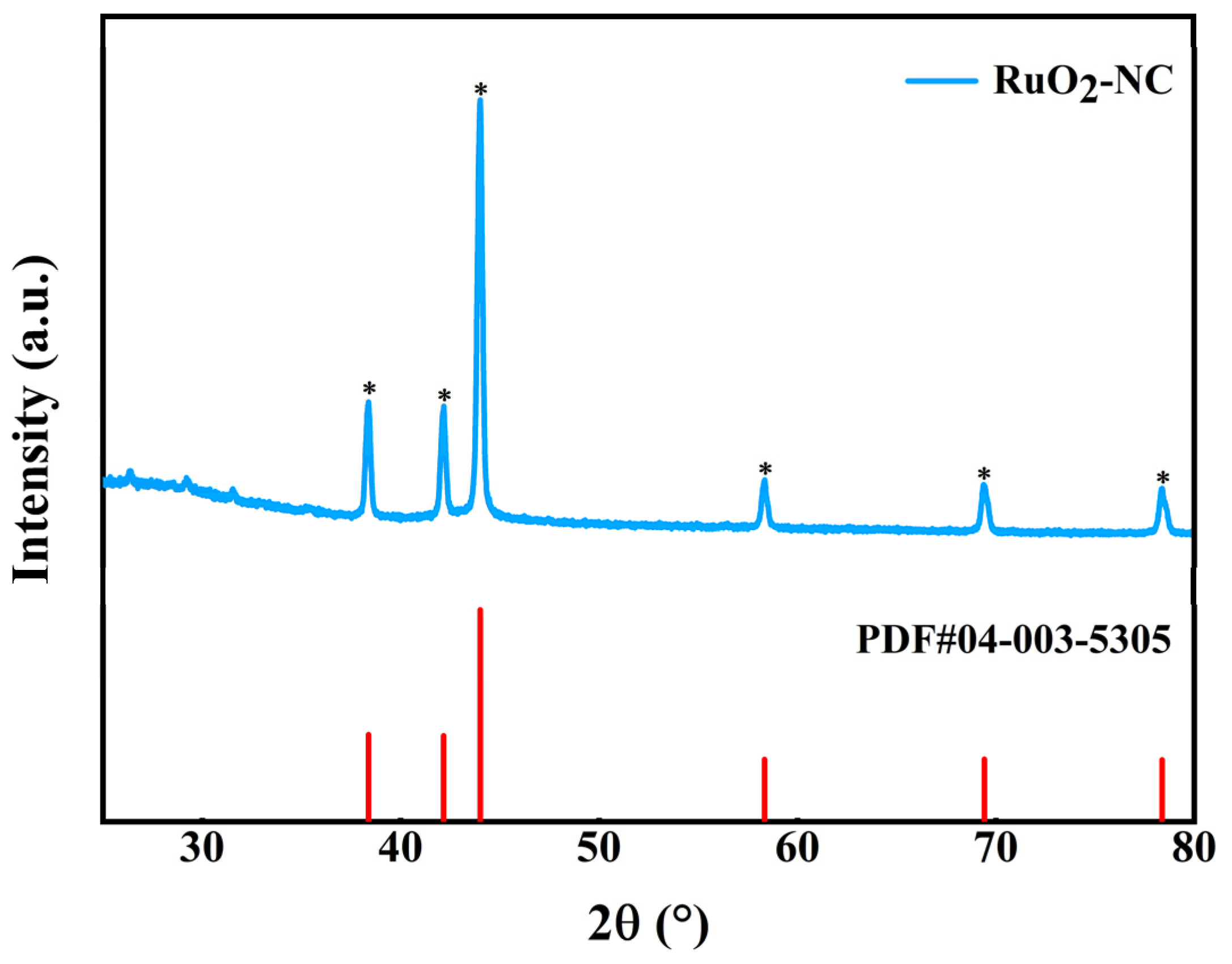
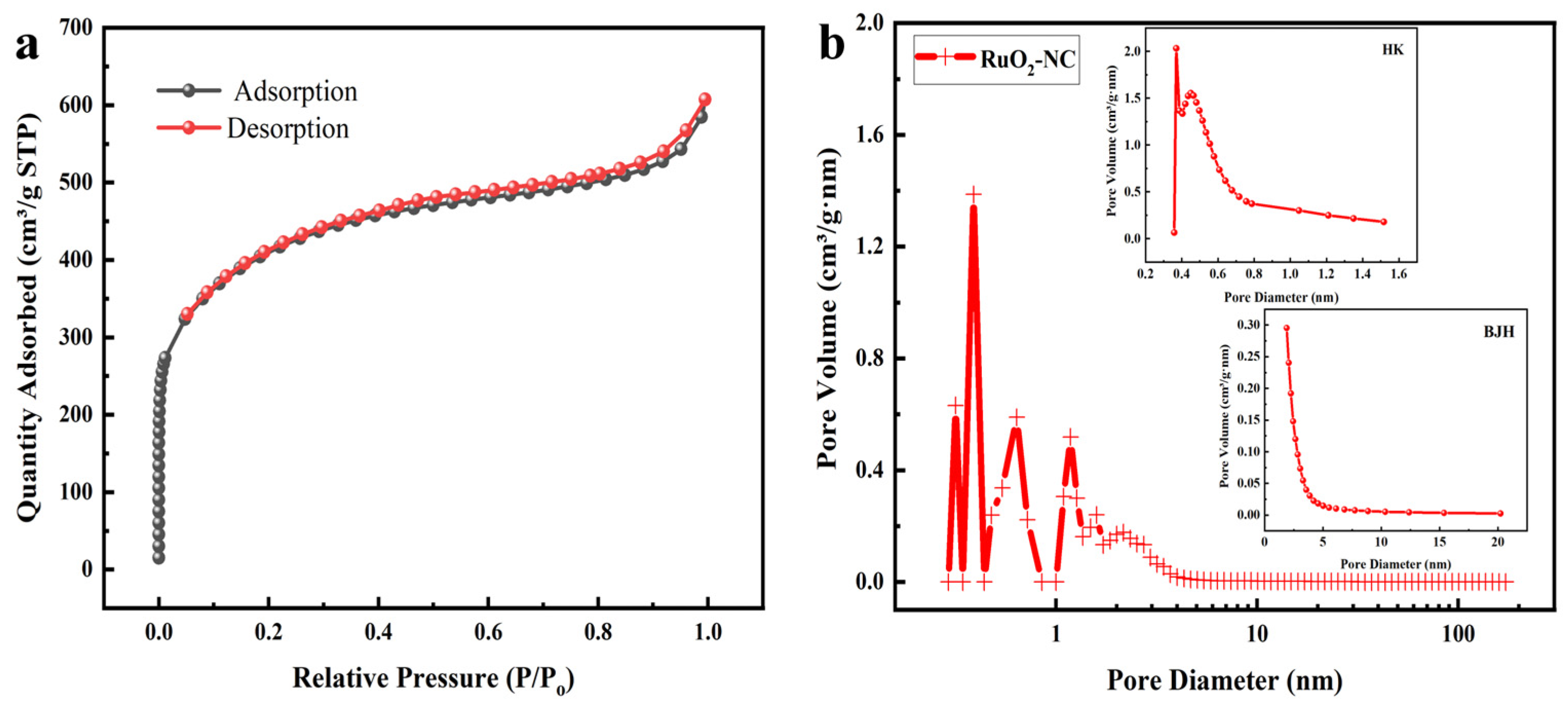
3.1.1. Physical Representation
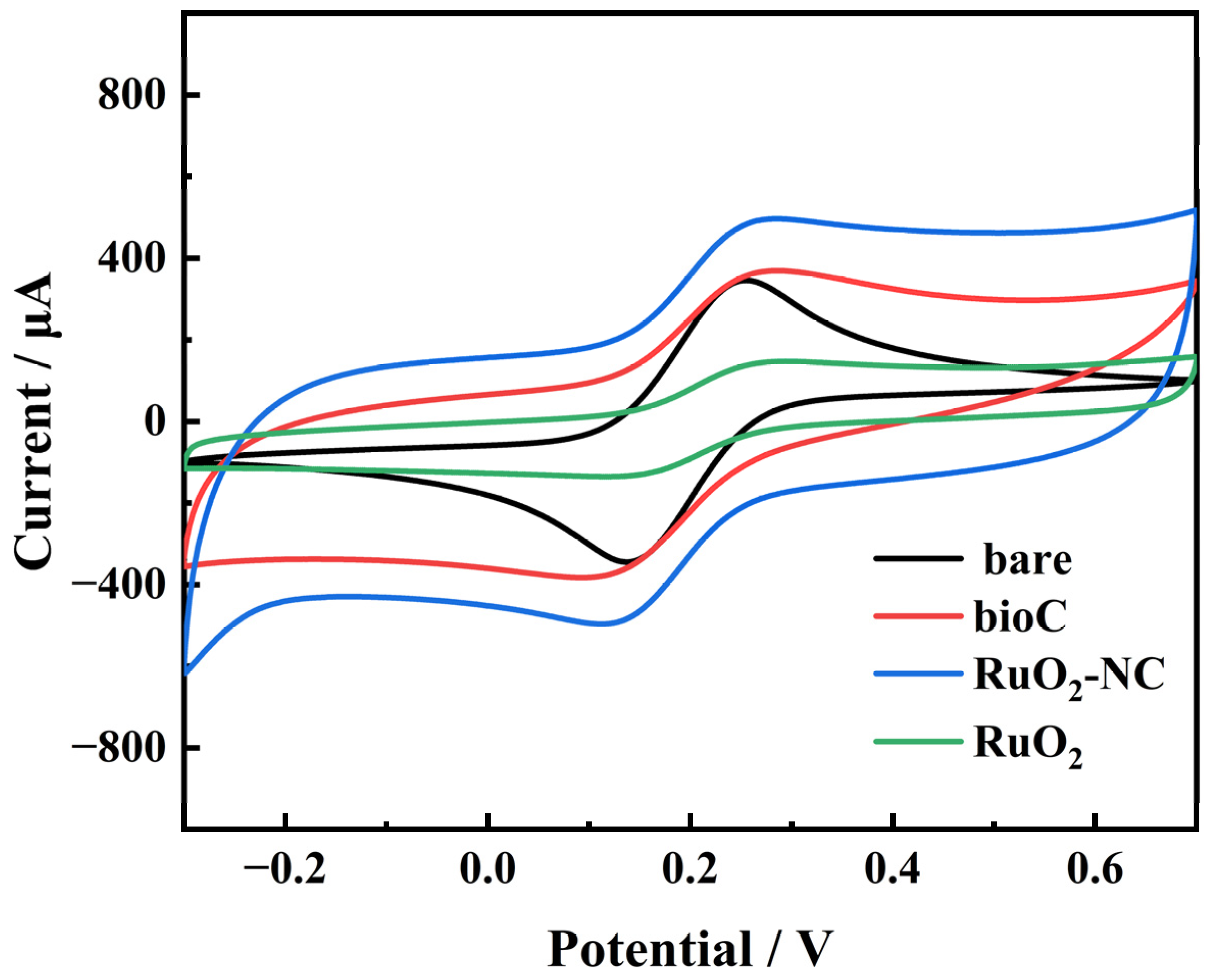
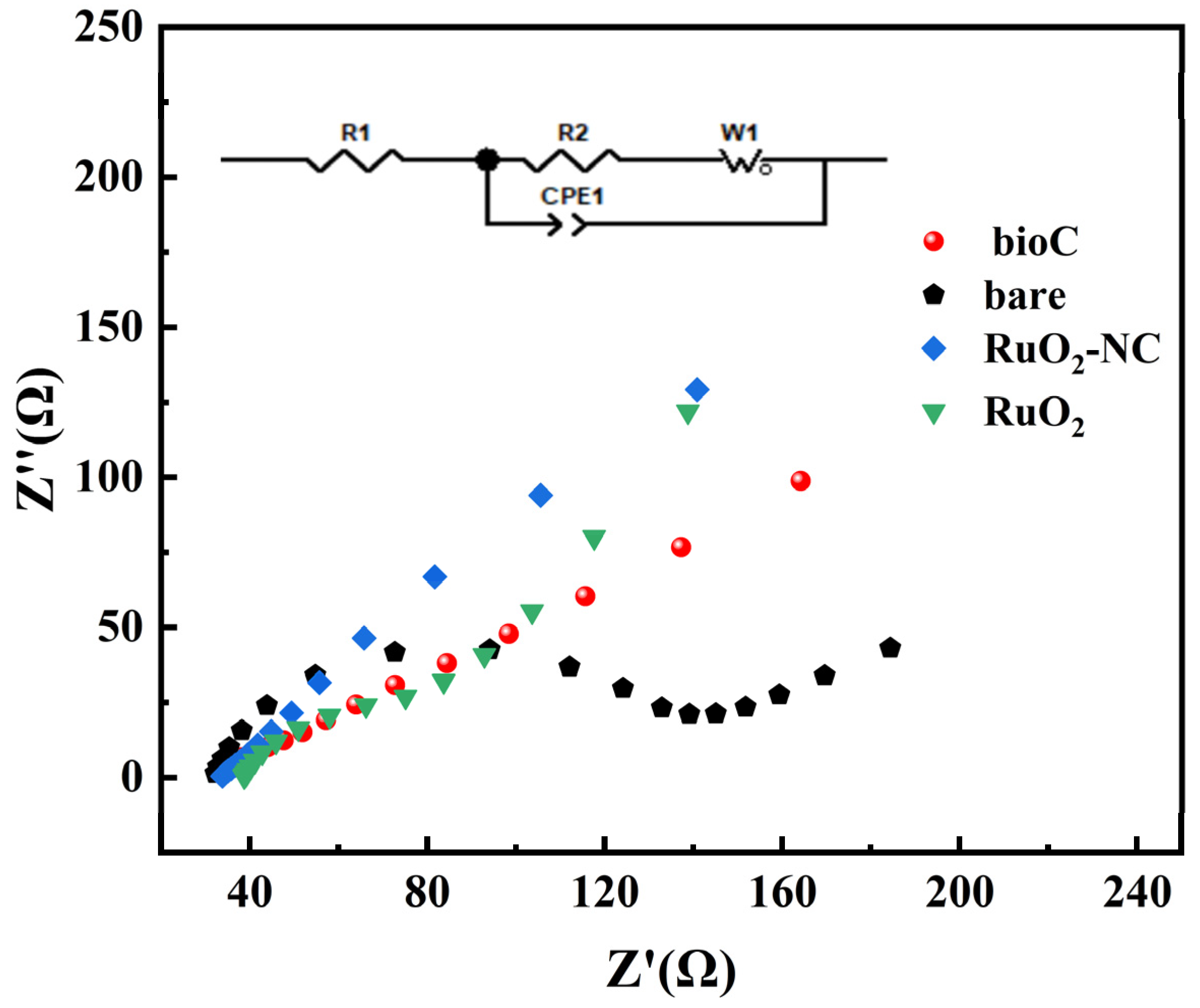
3.1.2. Electrochemical Characteristics
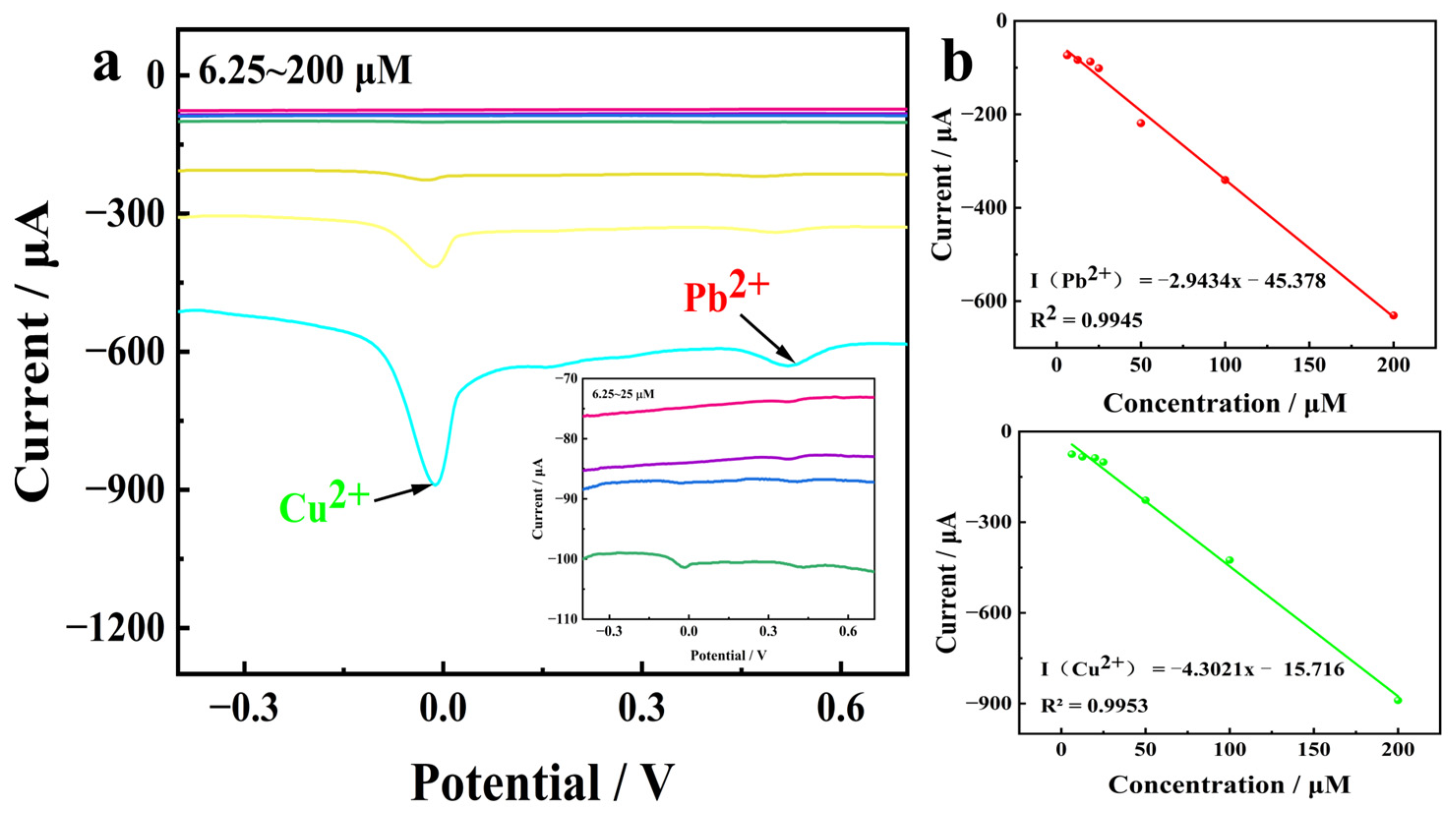
3.2. Linear Relationship Between Concentrations of Copper and Lead Ions
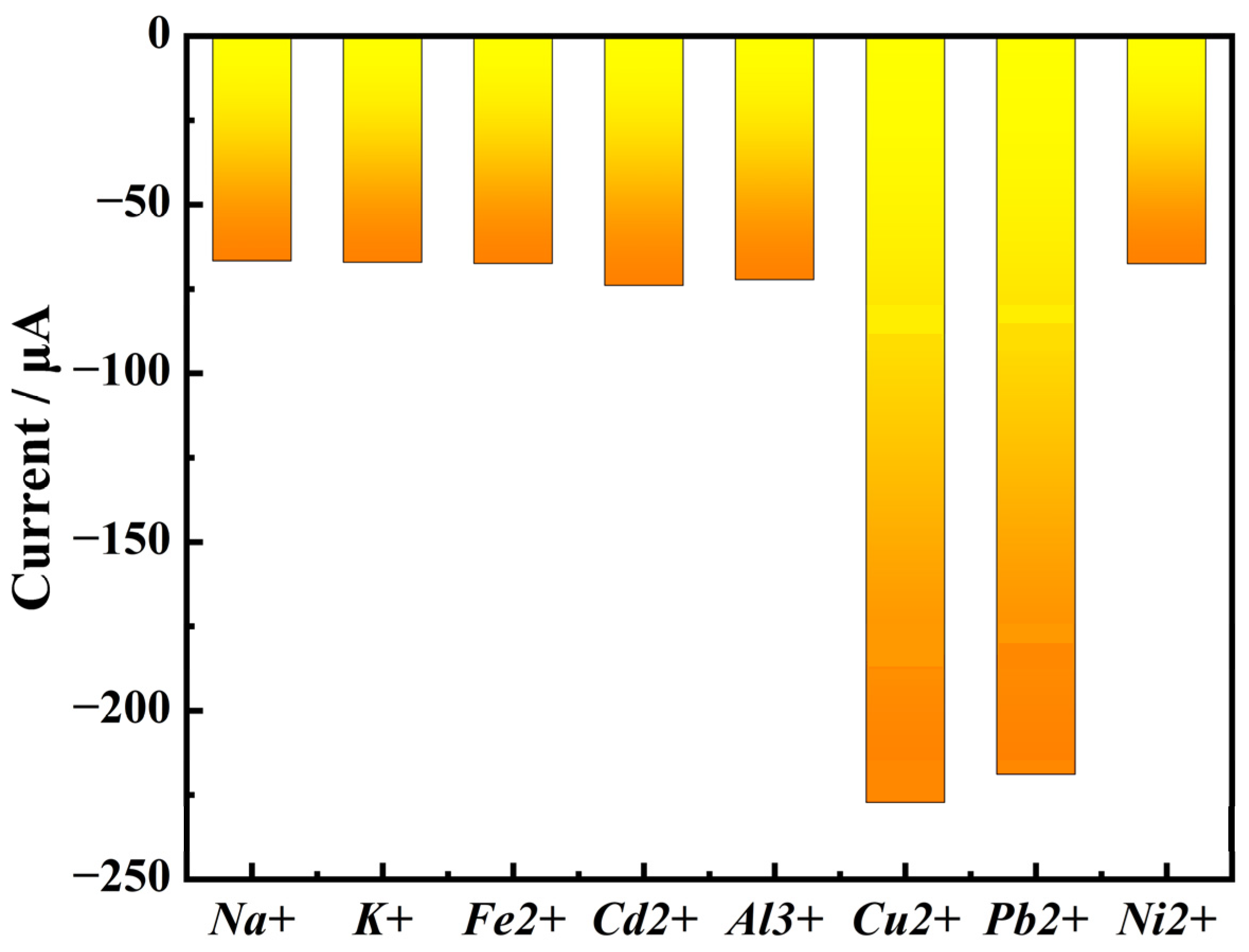
3.3. Ion Selectivity Experiment Results
3.4. Detection of Copper and Lead Ions in Samples by Electrochemical Sensor
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ebrahimi, M.; Hosseini-Monfared, H.; Javanbakht, M.; Mahdi, F. Biomass-derived nanostructured carbon materials for high-performance supercapacitor electrodes. Biomass Convers. Biorefin. 2024, 14, 17363–17380. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, Y.; Zhang, L.; Xie, Y.; Ye, X. Facile synthesis of three-dimensional porous carbon for high-performance supercapacitors. J. Alloys Compd. 2019, 787, 1–8. [Google Scholar] [CrossRef]
- El Nemr, A.; Khaled, A.; Abdelwahab, O.; El-Sikaily, A. Treatment of wastewater containing toxic chromium using new activated carbon developed from date palm seed. J. Hazard. Mater. 2008, 152, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, R.; Vairamuthu, R.; Ganapathy, A. Use of chemically activated cotton nut shell carbon for the removal of fluoride contaminated drinking water: Kinetics evaluation. Chin. J. Chem. Eng. 2015, 23, 710–721. [Google Scholar] [CrossRef]
- Kurniawan, A.; Sisnandy, V.O.A.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Performance of durian shell waste as high capacity biosorbent for Cr(VI) removal from synthetic wastewater. Ecol. Eng. 2011, 37, 940–947. [Google Scholar] [CrossRef]
- Akram, M.; Khan, B.; Imran, M.; Ahmad, I.; Ajaz, H.; Tahir, M.; Rabbani, F.; Kaleem, I.; Nadeem Akhtar, M.; Ahmad, N.; et al. Biosorption of lead by cotton shells powder: Characterization and equilibrium modeling study. Int. J. Phytoremediat. 2019, 21, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zhang, Y.; Meng, D.; Liu, X.; Zhang, Z.; Gao, P.; Lin, A.; Hou, L. Removal of sulfadiazine from aqueous solution by in-situ activated biochar derived from cotton shell. Environ. Res. 2020, 191, 110104. [Google Scholar] [CrossRef]
- Rumjit, N.P.; Samsudin, N.A.; Low, F.W.; Thomas, P.; Lai, C.W.; Velayudhaperumal Chellam, P.; Bin Johan, M.R.; Lim, Y.-C.; Amin, N.; Tiong, S.K. Kinetic and isotherm studies on adsorptive removal of sulfates by cotton shell derived biochar: Recovery of sulfates from marcasite soil. Sustain. Chem. Pharm. 2021, 20, 100361. [Google Scholar] [CrossRef]
- Zhou, L.; Ding, H.; Yan, F.; Guo, W.; Su, B. Electrochemical detection of Alzheimer’s disease related substances in biofluids by silica nanochannel membrane modified glassy carbon electrodes. Analyst 2018, 143, 4756–4763. [Google Scholar] [CrossRef]
- Shen, Y.; Xue, Y.; Xia, X.; Zeng, S.; Zhang, J.; Li, K. Metallic-like boron-modified bio-carbon electrodes for simultaneous electroanalysis for Cd2+, Pb2+ and Cu2+: Theoretical insight into the role of CxBOy(H). Carbon 2023, 214, 118350. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, S.; Qu, B.; Li, D.; Zhou, R. Migration and oxidation of vanadium atom and dimer supported on anatase TiO2(1 0 1) surface. Appl. Surf. Sci. 2021, 565, 150517. [Google Scholar] [CrossRef]
- Wu, T.; Li, L.; Song, G.; Ran, M.; Lu, X.; Liu, X. An ultrasensitive electrochemical sensor based on cotton carbon fiber composites for the determination of superoxide anion release from cells. Microchim. Acta 2019, 186, 198. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Zhou, L.; Tian, Z.; He, Y.; Shu, R. Hydrogenolysis of cornstalk lignin in supercritical ethanol over N-doped micro-mesoporous biochar supported Ru catalyst. Fuel Process. Technol. 2022, 231, 107218. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, D.; Li, H.; Xu, Z.; Sun, H.; Li, J.; Wang, L.; Shen, L. Energy release from RuO2//RuO2 supercapacitors under dynamic discharge conditions. Electrochim. Acta 2021, 367, 137455. [Google Scholar] [CrossRef]
- Wojtyła, S.; Baran, T. Photocatalytic H2 production over RuO2@ZnS and RuO2@CuS nanostructures. Int. J. Hydrogen Energy 2019, 44, 14624–14634. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Nayak, A.; Wang, Y.; Shan, B.; Quan, X.; Meyer, T.J. Steering CO2 electroreduction toward ethanol production by a surface-bound Ru polypyridyl carbene catalyst on N-doped porous carbon. Proc. Natl. Acad. Sci. USA 2019, 116, 26353–26358. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Inoue, S.; Watanabe, K. XRD and electrochemical measurements of RuO2 powder treated by using a mechanical grinding method. J. Alloys Compd. 2003, 358, 177–181. [Google Scholar] [CrossRef]
- Jebakumar Immanuel Edison, T.N.; Atchudan, R.; Lee, Y.R. Facile synthesis of carbon encapsulated RuO2 nanorods for supercapacitor and electrocatalytic hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 2323–2329. [Google Scholar] [CrossRef]
- Atanasoska, L.; Atanasoski, R.; Trasatti, S. XPS and AES study of mixed layers of RuO2 and IrO2. Vacuum 1990, 40, 91–94. [Google Scholar] [CrossRef]
- He, R.; Xu, G.; Wu, Y.; Shi, K.; Tang, H.; Ma, P.; Zeng, J.; Bai, Y.; Chen, S. Nano-sized RuO2 electrocatalyst improves the electrochemical performance for hydrogen oxidation reaction. Int. J. Hydrogen Energy 2019, 44, 5940–5947. [Google Scholar] [CrossRef]
- Hoffmann, V.; Rodriguez Correa, C.; Sachs, S.; del Pilar Sandoval-Rojas, A.; Qiao, M.; Brown, A.; Zimmermann, M.; Rodriguez Estupiñan, J.; Cortes, M.; Moreno-Piraján, J.; et al. Activated Carbon from Corncobs Doped with RuO2 as Biobased Electrode Material. Electron. Mater. 2021, 2, 324–343. [Google Scholar] [CrossRef]
- Silva, R.C.; Gouveia, A.F.; Sczancoski, J.C.; Santos, R.S.; Sá, J.L.S.; Longo, E.; Cavalcante, L.S. Electronic Structure, Morphological Aspects, Optical and Electrochemical Properties of RuO2 Nanocrystals. Electron. Mater. Lett. 2019, 15, 645–653. [Google Scholar] [CrossRef]
- Kim, K.C.; Yoon, T.-U.; Bae, Y.-S. Applicability of using CO2 adsorption isotherms to determine BET surface areas of microporous materials. Microporous Mesoporous Mater. 2016, 224, 294–301. [Google Scholar] [CrossRef]
- Batonneau-Gener, I.; Sachse, A. Determination of the Exact Microporous Volume and BET Surface Area in Hierarchical ZSM-5. J. Phys. Chem. C 2019, 123, 4235–4242. [Google Scholar] [CrossRef]
- Gu, P.; Tan, F.; Zhao, W.; Dong, J.; Zhou, S.; Hao, X.; Gu, S.; Liu, G.; Wang, D. Facile post-quaternization of conjugated microporous polymer for markedly efficient photodegradation and artificial photosynthesis of H2O2. Sep. Purif. Technol. 2025, 360, 130928. [Google Scholar] [CrossRef]
- Eraković, S.; Pavlović, M.M.; Stopić, S.; Stevanović, J.; Mitrić, M.; Friedrich, B.; Panić, V. Interactive promotion of supercapacitance of rare earth/CoO3-based spray pyrolytic perovskite microspheres hosting the hydrothermal ruthenium oxide. Electrochim. Acta 2019, 321, 134721. [Google Scholar] [CrossRef]
- Kuratani, K.; Kiyobayashi, T.; Kuriyama, N. Influence of the mesoporous structure on capacitance of the RuO2 electrode. J. Power Sources 2009, 189, 1284–1291. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Wang, C.; Chen, Y.; Ma, B.; Zhang, J. Surface morphology and electrochemical properties of RuO2-doped Ti/IrO2-ZrO2 anodes for oxygen evolution reaction. J. Alloys Compd. 2019, 778, 593–602. [Google Scholar] [CrossRef]
- Palma-Goyes, R.E.; Vazquez-Arenas, J.; Ostos, C.; Ferraro, F.; Torres-Palma, R.A.; Gonzalez, I. Microstructural and electrochemical analysis of Sb2O5 doped-Ti/RuO2-ZrO2 to yield active chlorine species for ciprofloxacin degradation. Electrochim. Acta 2016, 213, 740–751. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, İ.A.; Karaman, O.; Karaman, C. The production of rGO/ RuO2 aerogel supercapacitor and analysis of its electrochemical performances. Ceram. Int. 2021, 47, 34514–34520. [Google Scholar] [CrossRef]
- Yang, S.; Wang, R.; Zheng, J.; Han, W.; Lu, J.; Zhao, P.; Mao, X.; Fan, H. Remote Sensing-Based Monitoring of Cotton Growth and Its Response to Meteorological Factors. Sustainability 2024, 16, 3992. [Google Scholar] [CrossRef]
- Vitale, G.S.; Scavo, A.; Zingale, S.; Tuttolomondo, T.; Santonoceto, C.; Pandino, G.; Lombardo, S.; Anastasi, U.; Guarnaccia, P. Agronomic Strategies for Sustainable Cotton Production: A Systematic Literature Review. Agriculture 2024, 14, 1597. [Google Scholar] [CrossRef]
- Monteiro, E.; Ferreira, S. Biomass Waste for Energy Production. Energies 2022, 15, 5943. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, Y.; Chen, N.; Chen, B. A Review of the Efficient and Thermal Utilization of Biomass Waste. Sustainability 2024, 16, 9506. [Google Scholar] [CrossRef]
- Francioso, O. Current and future perspectives for biomass waste management and utilization. Sci. Rep. 2024, 14, 9635. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Zhang, X. The Future of Graphene: Preparation from Biomass Waste and Sports Applications. Molecules 2024, 29, 1825. [Google Scholar] [CrossRef]
| Sample | Metal Ion | Background Quantity/μM | Added Quantity/μM | Measured Quantity/μM | Recovery % | Average Recovery % | RSD |
|---|---|---|---|---|---|---|---|
| Viola tianshanica Maxim | Cu2+ | 39.06 | 40 | 79.13 | 100.18 | 100.49 | 1.63% |
| 40 | 79.09 | 100.08 | |||||
| 40 | 79.05 | 99.98 | |||||
| 55 | 93.42 | 98.84 | |||||
| 55 | 93.48 | 98.95 | |||||
| 55 | 93.36 | 98.73 | |||||
| 80 | 121.01 | 102.44 | |||||
| 80 | 121.07 | 102.51 | |||||
| 80 | 121.23 | 102.71 | |||||
| Pb2+ | 44.67 | 55 | 100.57 | 101.64 | 101.27 | 0.55% | |
| 55 | 100.64 | 101.76 | |||||
| 55 | 100.65 | 101.78 | |||||
| 75 | 119.92 | 101.67 | |||||
| 75 | 119.88 | 101.61 | |||||
| 75 | 120.68 | 101.35 | |||||
| 120 | 165.42 | 100.63 | |||||
| 120 | 165.22 | 100.46 | |||||
| 120 | 165.29 | 100.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhao, Y.; Wang, Z.; Tao, J.; Yang, M.; Li, C.; Zhang, X.; Sun, S.; Zhao, N. Application of an Electrochemical Sensor Based on Nitrogen-Doped Biochar Loaded with Ruthenium Oxide for Heavy Metal Detection. Biosensors 2025, 15, 160. https://doi.org/10.3390/bios15030160
Li L, Zhao Y, Wang Z, Tao J, Yang M, Li C, Zhang X, Sun S, Zhao N. Application of an Electrochemical Sensor Based on Nitrogen-Doped Biochar Loaded with Ruthenium Oxide for Heavy Metal Detection. Biosensors. 2025; 15(3):160. https://doi.org/10.3390/bios15030160
Chicago/Turabian StyleLi, Le, Yonghong Zhao, Zhengjiu Wang, Jiale Tao, Manying Yang, Chen Li, Xiaoqian Zhang, Shiguo Sun, and Na Zhao. 2025. "Application of an Electrochemical Sensor Based on Nitrogen-Doped Biochar Loaded with Ruthenium Oxide for Heavy Metal Detection" Biosensors 15, no. 3: 160. https://doi.org/10.3390/bios15030160
APA StyleLi, L., Zhao, Y., Wang, Z., Tao, J., Yang, M., Li, C., Zhang, X., Sun, S., & Zhao, N. (2025). Application of an Electrochemical Sensor Based on Nitrogen-Doped Biochar Loaded with Ruthenium Oxide for Heavy Metal Detection. Biosensors, 15(3), 160. https://doi.org/10.3390/bios15030160





