Nanomaterial Engineered Biosensors and Stimulus–Responsive Platform for Emergency Monitoring and Intelligent Diagnosis
Abstract
1. Introduction
2. Classification and Characteristics of Nanomaterials
2.1. Metal Nanomaterials
2.2. Semiconductor Nanomaterials
2.3. Carbon–Based Nanomaterials
2.4. Two–Dimensional Nanomaterials
2.5. Stimulus–Responsive Functional Nanomaterials
3. Nanomaterial Biosensors Based on Different Detection Mechanisms
3.1. Optical Sensing
3.1.1. Local Surface Plasmon Resonance (LSPR)
3.1.2. Surface–Enhanced Raman Scattering (SERS)
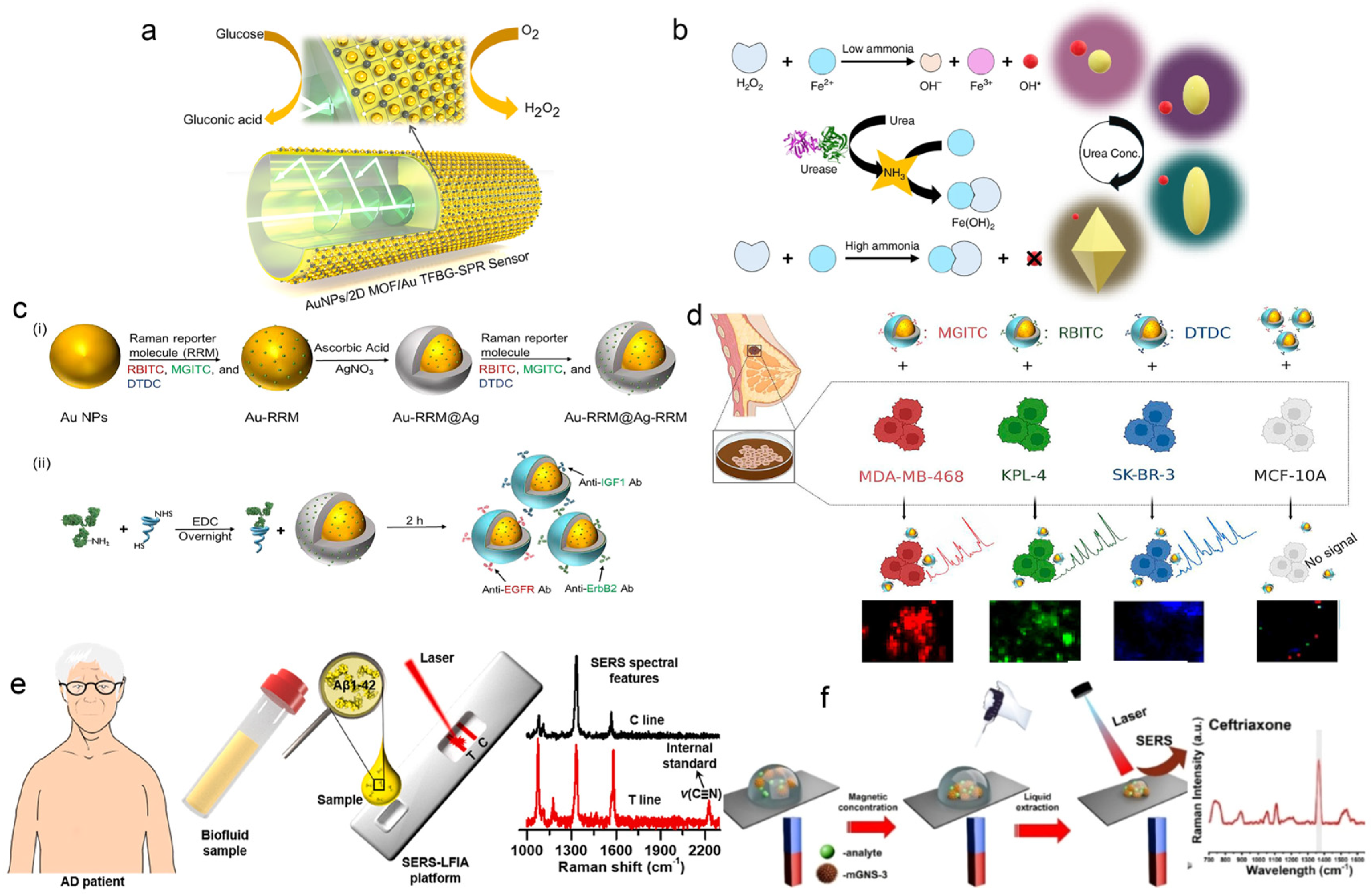
3.1.3. Fluorescence and Electrochemiluminescence (ECL)
3.2. Electrochemical Sensing
3.2.1. Label–Free Biomolecular Detection of Graphene Field–Effect Transistors (Gfet)
3.2.2. Electrochemical Synergistic Effect of Gold Nanoparticle Composites
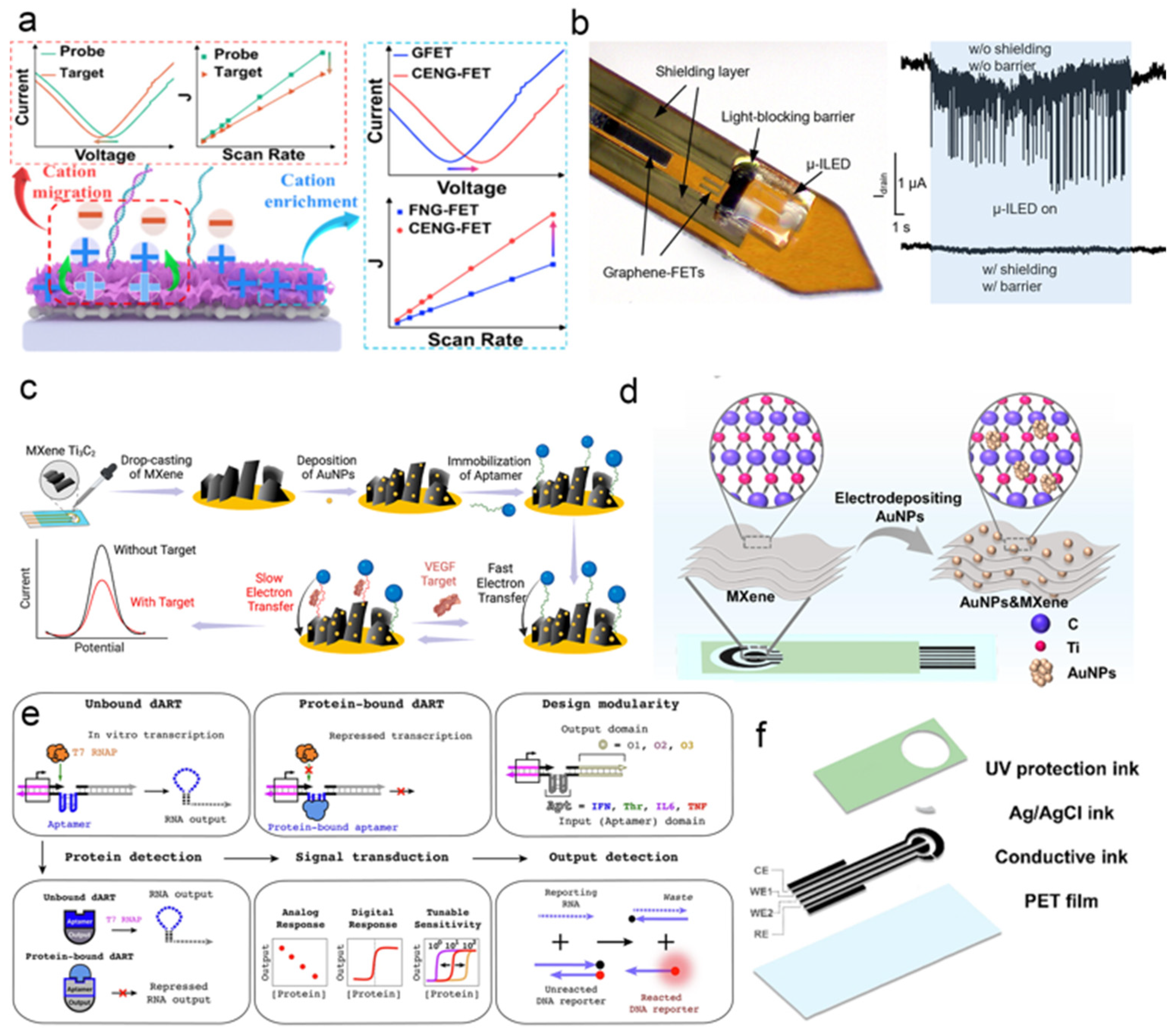
3.2.3. Aptamer Modified Electrode Sensing
3.3. Emerging Detection Sensing
3.3.1. Crispr–Cas12a System Integrated with Nanomaterials
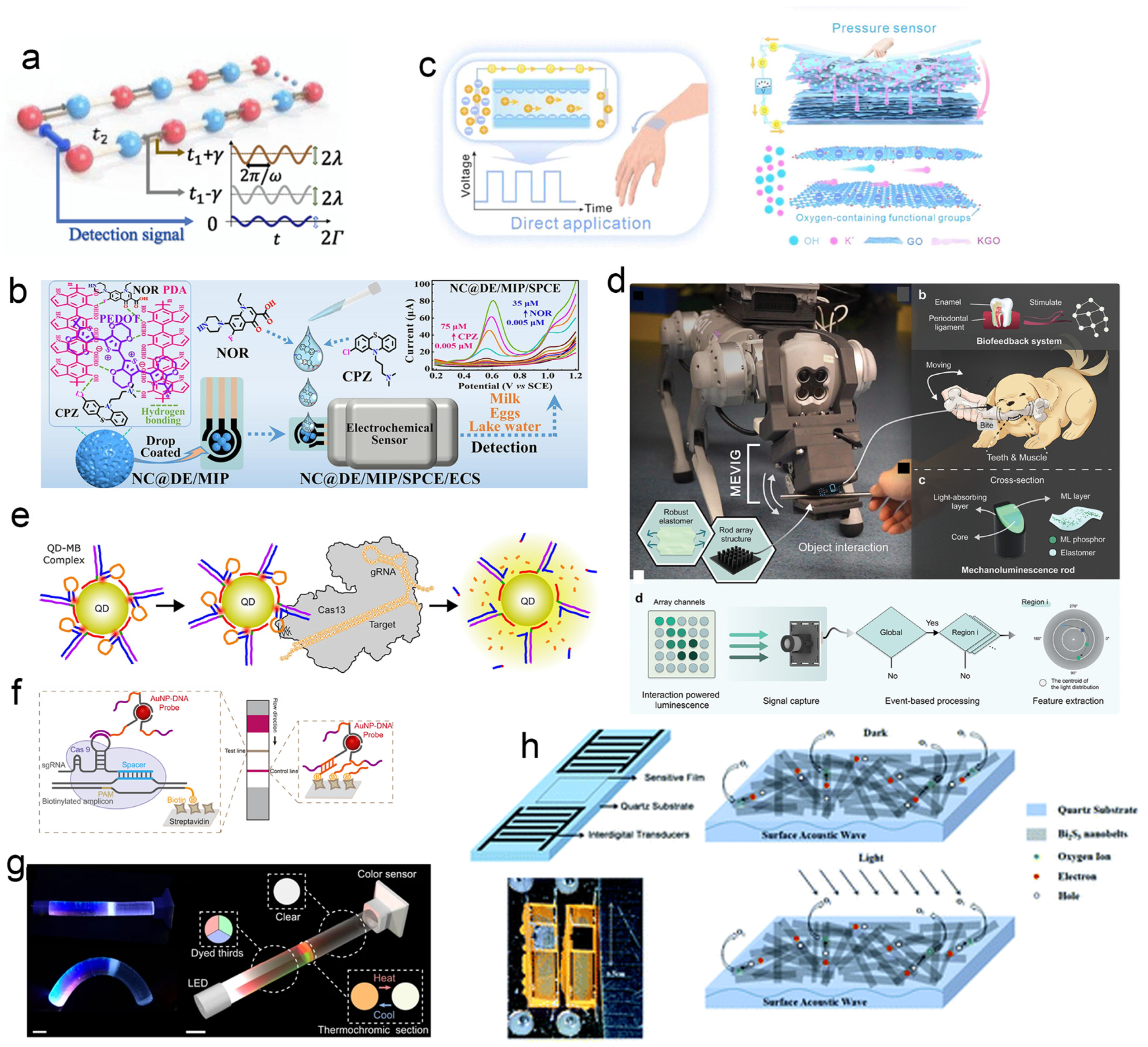
3.3.2. Frequency–Sensitive and Multimodal Sensing
3.3.3. Surface Acoustic Wave (Saw) Sensing
4. Application Fields of Biosensing Nanomaterials
4.1. Medical Diagnosis and Precision Medicine
4.1.1. Early Cancer Screening
4.1.2. Rapid Pathogen Identification
4.1.3. Wearable Devices
4.2. Environmental and Food Safety Monitoring
4.3. Emerging Cross–Disciplinary Fields
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rawat, S.; Phogat, P.; Shreya; Chand, B. Advances in nanomaterial–based biosensors: Innovations, challenges, and emerging applications. Mater. Today Commun. 2025, 48, 113334. [Google Scholar] [CrossRef]
- Xu, J.; Ma, J.; Peng, Y.; Cao, S.; Zhang, S.; Pang, H. Applications of metal nanoparticles/metal–organic frameworks composites in sensing field. Chin. Chem. Lett. 2023, 34, 107527. [Google Scholar] [CrossRef]
- Pan, M.; Yang, J.; Liu, K.; Yin, Z.; Ma, T.; Liu, S.; Xu, L.; Wang, S. Noble Metal Nanostructured Materials for Chemical and Biosensing Systems. Nanomaterials 2020, 10, 209. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Sensors and biosensors based on metal oxide nanomaterials. TrAC Trends Anal. Chem. 2019, 121, 115690. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef]
- Li, S.–S.; Wang, A.–J.; Yuan, P.–X.; Mei, L.–P.; Zhang, L.; Feng, J.–J. Heterometallic nanomaterials: Activity modulation, sensing, imaging and therapy. Chem. Sci. 2022, 13, 5505–5530. [Google Scholar] [CrossRef]
- Yan, T.; Wang, Z.; Pan, Z.–J. Flexible strain sensors fabricated using carbon–based nanomaterials: A review. Curr. Opin. Solid State Mater. Sci. 2018, 22, 213–228. [Google Scholar] [CrossRef]
- Ravi, S.N.; Rajendran, S.; Madhumathi, G.S.; Packirisamy, A.S.B.; Vallinayagam, S.; Khan, A.A.; Malik, A. Carbon nanomaterials: Pioneering innovations in bioimaging and biosensing technologies. J. Mol. Struct. 2024, 1316, 138987. [Google Scholar] [CrossRef]
- Gupta, B.D.; Pathak, A.; Semwal, V. Carbon–Based Nanomaterials for Plasmonic Sensors: A Review. Sensors 2019, 19, 3536. [Google Scholar] [CrossRef] [PubMed]
- Britto, J.S.J.; Guan, X.; Tran, T.K.A.; Lei, Z.; Bahadur, R.; Patel, V.; Zhang, X.; Wong, S.L.; Vinu, A. Emerging Multifunctional Carbon–Nanomaterial–Based Biosensors for Cancer Diagnosis. Small Sci. 2024, 4, 2300221. [Google Scholar] [CrossRef]
- Joshi, A.; Slaughter, G. Electrochemical carbon–based sensors for non–enzymatic uric acid sensing. Microchem. J. 2025, 208, 112331. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z. Transition metal compounds: From properties, applications to wettability regulation. Adv. Colloid Interface Sci. 2023, 321, 103027. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, S.; Peng, S.; Jia, H.; Pang, J.; Ibarlucea, B.; Hou, C.; Cao, Y.; Zhou, W.; Liu, H.; et al. Toward Smart Sensing by MXene. Small 2023, 19, 2206126. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, L.; Zhai, D.; Sun, L.; Zhai, S.; Zhou, W.; Wang, X.; Deng, W.–Q.; Wu, H. MXene–Derived Metal–Organic Framework@MXene Heterostructures toward Electrochemical NO Sensing. Small 2022, 18, 2204942. [Google Scholar] [CrossRef]
- Navitski, I.; Ramanaviciute, A.; Ramanavicius, S.; Pogorielov, M.; Ramanavicius, A. MXene–Based Chemo–Sensors and Other Sensing Devices. Nanomaterials 2024, 14, 447. [Google Scholar] [CrossRef]
- Qu, L.; Xu, Y.; Cui, W.; Wu, L.; Feng, Y.; Gu, Y.; Pan, H. Trends in conductive MOFs for sensing: A review. Anal. Chim. Acta 2025, 1336, 343307. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Kumari, S.; Yadav, P.; Kanoo, P.; Chakraborty, A. Metal–organic frameworks (MOFs) and MOF composites based biosensors. Coord. Chem. Rev. 2024, 519, 216102. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Chen, Y.; Li, H.–Y.; Liu, H. Construction of semiconductor nanocomposites for room–temperature gas sensors. Nanoscale 2024, 16, 12883–12908. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, G.; Bapna, K.; Shivagan, D.D. Semiconductor–metal–oxide–based nano–composites for humidity sensing applications. Mater. Res. Bull. 2023, 158, 112053. [Google Scholar] [CrossRef]
- Betty, C.A.; Choudhury, S.; Shah, A. Nanostructured metal oxide semiconductors and composites for reliable trace gas sensing at room temperature. Surf. Interfaces 2023, 36, 102560. [Google Scholar] [CrossRef]
- Saber, O. Fabrication of a novel reversible optical nanocomposite for high temperature sensing. Opt. Mater. 2025, 168, 117411. [Google Scholar] [CrossRef]
- Bouali, W.; Genc, A.A.; Erk, N.; Uzcan, F.; Soylak, M. Highly sensitive electrochemical detection of the tyrosine kinase inhibitor nintedanib using a novel BaTiO3@MnO2 nanocomposite–based sensor. Electrochim. Acta 2025, 539, 147097. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Gas adsorption properties of graphene–based materials. Adv. Colloid Interface Sci. 2017, 243, 46–59. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Zhang, L.; Li, X. Plasmonic biosensing based on non–noble–metal materials. Chin. Chem. Lett. 2018, 29, 54–60. [Google Scholar] [CrossRef]
- Ma, X.; Sun, H.; Wang, Y.; Wu, X.; Zhang, J. Electronic and optical properties of strained noble metals: Implications for applications based on LSPR. Nano Energy 2018, 53, 932–939. [Google Scholar] [CrossRef]
- Castro, R.C.; Ribeiro, D.S.M.; Santos, J.L.M. Visual detection using quantum dots sensing platforms. Coord. Chem. Rev. 2021, 429, 213637. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Z.; Zhang, Y.; Li, H.–Y.; Gao, N.; Tian, Z.; Zhou, L.; Zhang, B.; Tang, J.; Zhang, J.; et al. MoS2 Nanosheets Sensitized with Quantum Dots for Room–Temperature Gas Sensors. Nano–Micro Lett. 2020, 12, 59. [Google Scholar] [CrossRef]
- La Spada, L.; Vegni, L. Electromagnetic Nanoparticles for Sensing and Medical Diagnostic Applications. Materials 2018, 11, 603. [Google Scholar] [CrossRef]
- Conteduca, D. Photonic Biosensors: Detection, Analysis and Medical Diagnostics. Biosensors 2022, 12, 238. [Google Scholar] [CrossRef]
- Gao, D.–H.; Yu, Q.–C.; Kebeded, M.A.; Zhuang, Y.–Y.; Huang, S.; Jiao, M.–Z.; He, X.–J. Advances in modification of metal and noble metal nanomaterials for metal oxide gas sensors: A review. Rare Met. 2025, 44, 1443–1496. [Google Scholar] [CrossRef]
- Pascariu, P.; Gherasim, C.; Airinei, A. Metal Oxide Nanostructures (MONs) as Photocatalysts for Ciprofloxacin Degradation. Int. J. Mol. Sci. 2023, 24, 9564. [Google Scholar] [CrossRef]
- Berwal, P.; Sihag, S.; Rani, S.; Kumar, A.; Jatrana, A.; Singh, P.; Dahiya, R.; Kumar, A.; Dhillon, A.; Sanger, A.; et al. Hybrid Metal Oxide Nanocomposites for Gas–Sensing Applications: A Review. Ind. Eng. Chem. Res. 2023, 62, 14835–14852. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Z.; Yao, Q.; Xie, J. Luminescent metal nanoclusters: Biosensing strategies and bioimaging applications. Aggregate 2021, 2, 114–132. [Google Scholar] [CrossRef]
- Zhong, S.-J.; Chen, K.-Y.; Wang, S.-L.; Manshaii, F.; Jing, N.; Wang, K.-D.; Liu, S.-C.; Zhou, Y.-L. Metal–based nanowires in electrical biosensing. Rare Met. 2024, 43, 6233–6254. [Google Scholar] [CrossRef]
- Feng, J.; Gao, C.; Yin, Y. Stabilization of noble metal nanostructures for catalysis and sensing. Nanoscale 2018, 10, 20492–20504. [Google Scholar] [CrossRef]
- Tan, X.; Sheng, R.; Liu, Z.; Li, W.; Yuan, R.; Tao, Y.; Yang, N.; Ge, L. Assembly of Metal–Phenolic Networks onto Microbubbles for One–Step Generation of Functional Microcapsules. Small 2024, 20, 2305325. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liu, H.; Richardson, J.J.; Xu, W.; Chen, J.; Zhou, J.; Caruso, F. Metal–phenolic network composites: From fundamentals to applications. Chem. Soc. Rev. 2024, 53, 10800–10826. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Wang, F.; Luo, X.; Zhang, W.; Lyu, Y.; Yang, H.; Cai, R.; Tan, W. On–demand controlled bidirectional DNAzyme path for ultra–sensitive heavy metal ion detection. Chem. Sci. 2024, 15, 18170–18178. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhu, B.; Bai, C.; Gu, B.; Lv, C.; Zhang, J. Thickness–modulated optical nonlinearity of colloidal CdSe–CdS core–shell nanoplatelets: Large two–photon absorption and self–focusing effects. Nanoscale 2023, 15, 17996–18003. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, Y.; Jiang, X.; Su, Y.; Liu, H.; Gao, J. The quantum confinement effects on the electronic properties of monolayer GeS nanoribbon with tube–edged reconstruction. Nanotechnology 2022, 33, 345202. [Google Scholar] [CrossRef]
- Qin, N.; Han, H.; Guan, G.; Han, M.–Y. Structurally altered size, composition, shape and interface–dependent optical properties of quantized nanomaterials. Nano Res. 2024, 17, 10543–10569. [Google Scholar] [CrossRef]
- Yang, J.–N.; Wang, J.–J.; Yin, Y.–C.; Yao, H.–B. Mitigating halide ion migration by resurfacing lead halide perovskite nanocrystals for stable light–emitting diodes. Chem. Soc. Rev. 2023, 52, 5516–5540. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, S.; Zhang, X. Surface–controlled carbon dots with high brightness and tunable emission towards fluorescent anti–counterfeiting and pH sensors. Microchem. J. 2024, 207, 112262. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; He, Q.; Hui, Y.; Xu, C.; Wang, B.; Shan, F.; Zhang, J.; Shao, J. Bidirectional, Multilayer MXene/Polyimide Aerogels for Ultra–Broadband Microwave Absorption. Adv. Mater. 2024, 36, 2401733. [Google Scholar] [CrossRef] [PubMed]
- Rex, T.; Baumert, S.; Hepp, A.; Fernández, G.; Strassert, C.A. Adaptive photoluminescence through a bioinspired antioxidative mechanism. Chem. Sci. 2024, 15, 18881–18887. [Google Scholar] [CrossRef]
- Moulin, E.; Faour, L.; Carmona–Vargas, C.C.; Giuseppone, N. From Molecular Machines to Stimuli–Responsive Materials. Adv. Mater. 2020, 32, 1906036. [Google Scholar] [CrossRef]
- Fang, H.; Xu, S.; Wang, Y.; Yang, H.; Su, D. Endogenous stimuli–responsive drug delivery nanoplatforms for kidney disease therapy. Colloids Surf. B Biointerfaces 2023, 232, 113598. [Google Scholar] [CrossRef]
- Xiao, R.; Zhou, G.; Wen, Y.; Ye, J.; Li, X.; Wang, X. Recent advances on stimuli–responsive biopolymer–based nanocomposites for drug delivery. Compos. Part B Eng. 2023, 266, 111018. [Google Scholar] [CrossRef]
- Shi, J.; Wang, H.; Liu, R.; Yan, J.; Jiang, M.; Xu, Y.; Zhang, Z.; Si, H.; Wang, S.; Jiang, S. Synergistic integration of 2D MOF films and gold nanoparticles for multifunctional surface plasmon resonance sensing. Talanta 2026, 297, 128629. [Google Scholar] [CrossRef]
- Elbalaawy, A.Y.; Kim, M.–J.; Shaban, S.M.; Hafez, E.; Elmasry, M.R.; Kim, D.–H. pH–Inhibited Fenton etching of gold nanobipyramids: A multicolor approach for enhanced urea detection. Microsyst. Nanoeng. 2025, 11, 114. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, W.; Zhou, Y.; Cui, H.; Xing, Y.; Yu, F.; Wang, R. Highly sensitive surface–enhanced Raman scattering (SERS) imaging for phenotypic diagnosis and therapeutic evaluation of breast cancer. Chem. Eng. J. 2023, 459, 141502. [Google Scholar] [CrossRef]
- Liu, X.; Su, X.; Chen, M.; Xie, Y.; Li, M. Self–calibrating surface–enhanced Raman scattering–lateral flow immunoassay for determination of amyloid–β biomarker of Alzheimer’s disease. Biosens. Bioelectron. 2024, 245, 115840. [Google Scholar] [CrossRef]
- Ding, S.–Y.; You, E.–M.; Tian, Z.–Q.; Moskovits, M. Electromagnetic theories of surface–enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Sarfraz, N.; Khan, I. Plasmonic Gold Nanoparticles (AuNPs): Properties, Synthesis and their Advanced Energy, Environmental and Biomedical Applications. Chem. Asian J. 2021, 16, 720–742. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Thorsen, T.L.; Sanchez, S.; Zhao, Y.; Vo–Dinh, T. Multibranched Magnetic Core–Shell Gold Nanostars for In Situ Solution–Based SERS Detection. ACS Appl. Nano Mater. 2025, 8, 12393–12403. [Google Scholar] [CrossRef]
- Fang, X.; Xie, Z.; Sharshir, S.; Lei, H.; Zheng, J.; Zheng, B.; Lin, X.; Liu, X.; Yuan, Z. Lead Halide Perovskite Nanocrystals in Metal–Organic Frameworks: Synthesis, Properties, and Applications. Adv. Sci. 2025, 12, e05407. [Google Scholar] [CrossRef]
- Liu, H.; Mou, B.; Li, J.; Tian, N.; Feng, Y.; Cui, X.; Kapitonov, Y.; Liang, H.; You, C.; Li, Y.; et al. Recent progress on high–precision construction of nanoarchitectured SERS substrates for ultrasensitive bio–medical sensors. Adv. Powder Mater. 2025, 4, 100300. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, J.–G.; Ma, F.; Zhang, C.–Y. Advances in single–molecule fluorescent nanosensors. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1716. [Google Scholar] [CrossRef]
- Rassaei, L.; Xu, G.; Ding, Z.; Mathwig, K. Electrochemiluminescence: Fundamentals to Applications. ChemElectroChem 2017, 4, 1571. [Google Scholar] [CrossRef]
- Yao, J.; Li, L.; Li, P.; Yang, M. Quantum dots: From fluorescence to chemiluminescence, bioluminescence, electrochemiluminescence, and electrochemistry. Nanoscale 2017, 9, 13364–13383. [Google Scholar] [CrossRef]
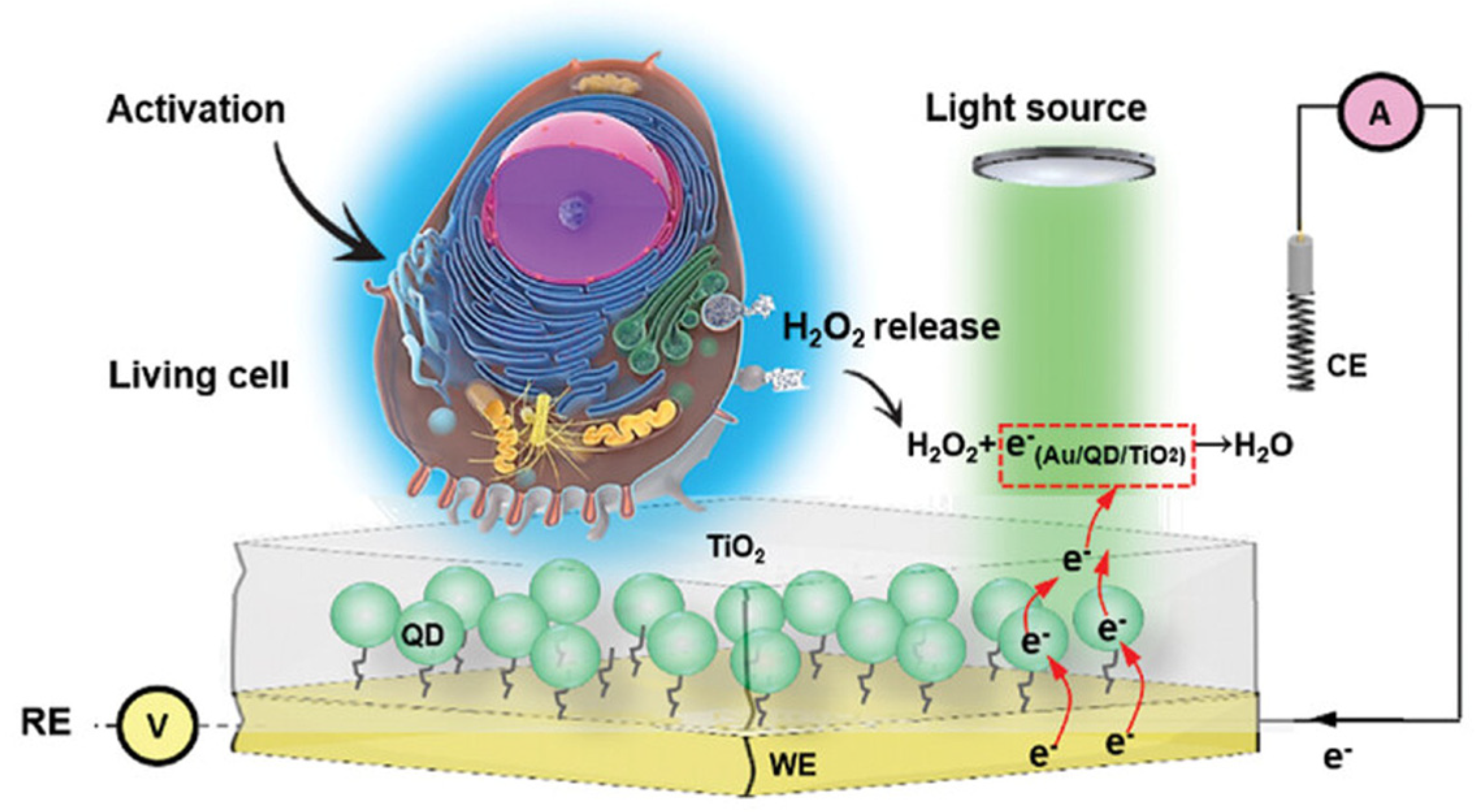
- Zhao, S.; Yue, Z.; Zhu, D.; Harberts, J.; Blick, R.H.; Zierold, R.; Lisdat, F.; Parak, W.J. Quantum Dot/TiO2 Nanocomposite–Based Photoelectrochemical Sensor for Enhanced H2O2 Detection Applied for Cell Monitoring and Visualization. Small 2024, 20, 2401703. [Google Scholar] [CrossRef]
- Li, C.; Hang, T.; Jin, Y. Atomically Fe–anchored MOF–on–MOF nanozyme with differential signal amplification for ultrasensitive cathodic electrochemiluminescence immunoassay. Exploration 2023, 3, 20220151. [Google Scholar] [CrossRef]
- Hang, T.; Zhang, C.; Pei, F.; Yang, M.; Wang, F.; Xia, M.; Hao, Q.; Lei, W. Magnetism–Functionalized Lanthanide MOF–on–MOF with Plasmonic Differential Signal Amplification for Ultrasensitive Fluorescence Immunoassays. ACS Sens. 2024, 9, 6779–6788. [Google Scholar] [CrossRef]
- Shen, Z.; Li, W.; Tang, W.; Jiang, X.; Qi, K.; Liu, H.; Xu, W.; Xu, W.; Zang, S.; Zhen, K.; et al. Fluorophor Embedded MOFs Steering Gas Ultra–Recognition. Adv. Funct. Mater. 2024, 34, 2401631. [Google Scholar] [CrossRef]
- Song, Q.; Li, Q.; Yan, J.; Song, Y. Echem methods and electrode types of the current in vivo electrochemical sensing. RSC Adv. 2022, 12, 17715–17739. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, R.; Zeng, H.; Wang, X.; Luo, S.; Li, W.; Luo, X.; Yang, T. Nucleic acid–based ratiometric electrochemiluminescent, electrochemical and photoelectrochemical biosensors: A review. Microchim. Acta 2019, 186, 405. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar–Zadeh, E. Biosensing based on field–effect transistors (FET): Recent progress and challenges. TrAC Trends Anal. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.; Zhang, X.; Sun, T.; Huo, P.; Cui, X.; Man, B.; Yang, C.; Wei, D. Cation Enrichment Effect Modulated Nafion/Graphene Field–Effect Transistor for Ultrasensitive RNA Detection. Nano Lett. 2024, 24, 16245–16252. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Zhang, X.; Xue, X.; Homer, L.; Jos, G.; Weng, Z.; Li, H.; Kim, I.; Zhang, Y. An Implantable, Ultralow Distortion Bioelectronic Interface Integrating Light–Emitting Diodes and Graphene Field–Effect Transistors. ACS Nano 2025, 19, 29228–29241. [Google Scholar] [CrossRef]
- Elancheziyan, M.; Singh, M.; Won, K. Gold Nanoparticle–Embedded Thiol–Functionalized Ti3C2Tx MXene for Sensitive Electrochemical Sensing of Ciprofloxacin. Nanomaterials 2024, 14, 1655. [Google Scholar] [CrossRef]
- Rajput, P.; Sinha, R.K.; Devi, P. Double transition metal carbide Mo2TiC2Tx MXene architectured with in–situ synthesized gold nanoparticles for SERS enhancement. Inorg. Chem. Commun. 2025, 174, 114047. [Google Scholar] [CrossRef]
- Duan, H.; Tang, S.–Y.; Goda, K.; Li, M. Enhancing the sensitivity and stability of electrochemical aptamer–based sensors by AuNPs@MXene nanocomposite for continuous monitoring of biomarkers. Biosens. Bioelectron. 2024, 246, 115918. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.; Liu, Y.; Liu, Z.; Li, Z.; Li, Z.; Chen, X.; Liu, J.; Chen, Z.; Mo, G.; et al. A Gold Nanoparticles and MXene Nanocomposite Based Electrochemical Sensor for Point–of–Care Monitoring of Serum Biomarkers. ACS Nano 2025, 19, 16980–16994. [Google Scholar] [CrossRef]
- Lee, H.; Xie, T.; Kang, B.; Yu, X.; Schaffter, S.W.; Schulman, R. Plug–and–play protein biosensors using aptamer–regulated in vitro transcription. Nat. Commun. 2024, 15, 7973. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, W.; Cao, W.; Zhang, X. Non–Hermitian Floquet Topological Sensors for Ultrasensitive Detection of Dynamic Signals. Phys. Rev. Lett. 2025, 135, 106601. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Abdurexit, A.; Jamal, R.; Abdiryim, T.; Li, J.; Liu, J.; Wang, Z.; Song, K.; Li, Z.; et al. Polydopamine–PEDOT–based portable molecularly imprinted sensor for simultaneous ultrasensitive determination of chlorpromazine and norfloxacin. Chem. Eng. J. 2025, 521, 166633. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Q.; Wang, M.; Lu, H.; Chen, Z.; Liu, J.; Chen, C.; Wang, S.; Ma, Y.; Yue, Y. Osmotic Energy Directly Driving Flexible All–Solid–State 2D Nanofluidic Pressure Sensors. Adv. Mater. 2025, 37, e06990. [Google Scholar] [CrossRef]
- Sou, K.–W.; Chan, W.–S.; Lei, K.–C.; Wang, Z.; Li, S.; Peng, D.; Ding, W. A Bio–Inspired Event–Driven Mechanoluminescent Visuotactile Sensor for Intelligent Interactions. Adv. Funct. Mater. 2025, 35, 2420872. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual–RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Fan, C.; Chen, H.–Y. Biosensing: CRISPR–powered diagnostics. Nat. Biomed. Eng. 2017, 1, 0091. [Google Scholar] [CrossRef]
- Barrangou, R.; Doudna, J.A. Applications of CRISPR technologies in research and beyond. Nat. Biotechnol. 2016, 34, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Gao, Y.; Zhou, N.; Wang, B. CRISPR–powered RNA sensing in vivo. Trends Biotechnol. 2024, 42, 1601–1614. [Google Scholar] [CrossRef]
- Liu, G. Advancing CRISPR/Cas Biosensing with Integrated Devices. ACS Sens. 2025, 10, 575–576. [Google Scholar] [CrossRef]
- Fu, R.; Xianyu, Y. Gold Nanomaterials–Implemented CRISPR–Cas Systems for Biosensing. Small 2023, 19, 2300057. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiong, E.; Tian, T.; Cheng, M.; Lin, W.; Wang, H.; Zhang, G.; Sun, J.; Zhou, X. Clustered Regularly Interspaced Short Palindromic Repeats/Cas9–Mediated Lateral Flow Nucleic Acid Assay. ACS Nano 2020, 14, 2497–2508. [Google Scholar] [CrossRef] [PubMed]
- Lysne, D.P.; Stewart, M.H.; Susumu, K.; Leski, T.A.; Stenger, D.A.; Medintz, I.L.; Díaz, S.A.; Green, C.M. Quantum dot molecular beacons achieve sub-10 pM CRISPR–Cas detection in field–ready assays. Sci. Rep. 2025, 15, 27950. [Google Scholar] [CrossRef]
- Baines, R.; Zuliani, F.; Chennoufi, N.; Joshi, S.; Kramer–Bottiglio, R.; Paik, J. Multi–modal deformation and temperature sensing for context–sensitive machines. Nat. Commun. 2023, 14, 7499. [Google Scholar] [CrossRef]
- Ashley, B.K.; Sui, J.; Javanmard, M.; Hassan, U. Multi–modal sensing with integrated machine learning to differentiate specific leukocytes targeted by electrically sensitive hybrid particles. Biosens. Bioelectron. 2023, 241, 115661. [Google Scholar] [CrossRef]
- Mujahid, A.; Dickert, F.L. Surface Acoustic Wave (SAW) for Chemical Sensing Applications of Recognition Layers. Sensors 2017, 17, 2716. [Google Scholar] [CrossRef]
- Perez–Cortes, L.; Hernandez–Rodriguez, C.; Mazingue, T.; Lomello–Tafin, M. Functionality of Surface Acoustic Wave (SAW) transducer for palladium–platinum–based hydrogen sensor. Sens. Actuators A Phys. 2016, 251, 35–41. [Google Scholar] [CrossRef]
- Li, C.; Kan, H.; Luo, J.; Fu, C.; Zhou, J.; Liu, X.; Wang, W.; Wei, Q.; Fu, Y. A high performance surface acoustic wave visible light sensor using novel materials: Bi2S3 nanobelts. RSC Adv. 2020, 10, 8936–8940. [Google Scholar] [CrossRef]
- Maduraiveeran, G. Nanomaterials–based portable electrochemical sensing and biosensing systems for clinical and biomedical applications. J. Anal. Sci. Technol. 2022, 13, 35. [Google Scholar] [CrossRef]
- Uniyal, S.; Choudhary, K.; Sachdev, S.; Kumar, S. Nano–bio fusion: Advancing biomedical applications and biosensing with functional nanomaterials. Opt. Laser Technol. 2024, 168, 109938. [Google Scholar] [CrossRef]
- Gao, F.; Li, F.; Wang, J.; Yu, H.; Li, X.; Chen, H.; Wang, J.; Qin, D.; Li, Y.; Liu, S.; et al. SERS–Based Optical Nanobiosensors for the Detection of Alzheimer’s Disease. Biosensors 2023, 13, 880. [Google Scholar] [CrossRef]
- Kazi, R.N.; Hasani, I.W.; Khafaga, D.S.R.; Kabba, S.; Farhan, M.; Aatif, M.; Muteeb, G.; Fahim, Y.A. Nanomedicine: The Effective Role of Nanomaterials in Healthcare from Diagnosis to Therapy. Pharmaceutics 2025, 17, 987. [Google Scholar] [CrossRef]
- Bigham, A.; Zarepour, A.; Khosravi, A.; Iravani, S.; Zarrabi, A. Sustainable nanomaterials for precision medicine in cancer therapy. Mater. Today Sustain. 2024, 27, 100865. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
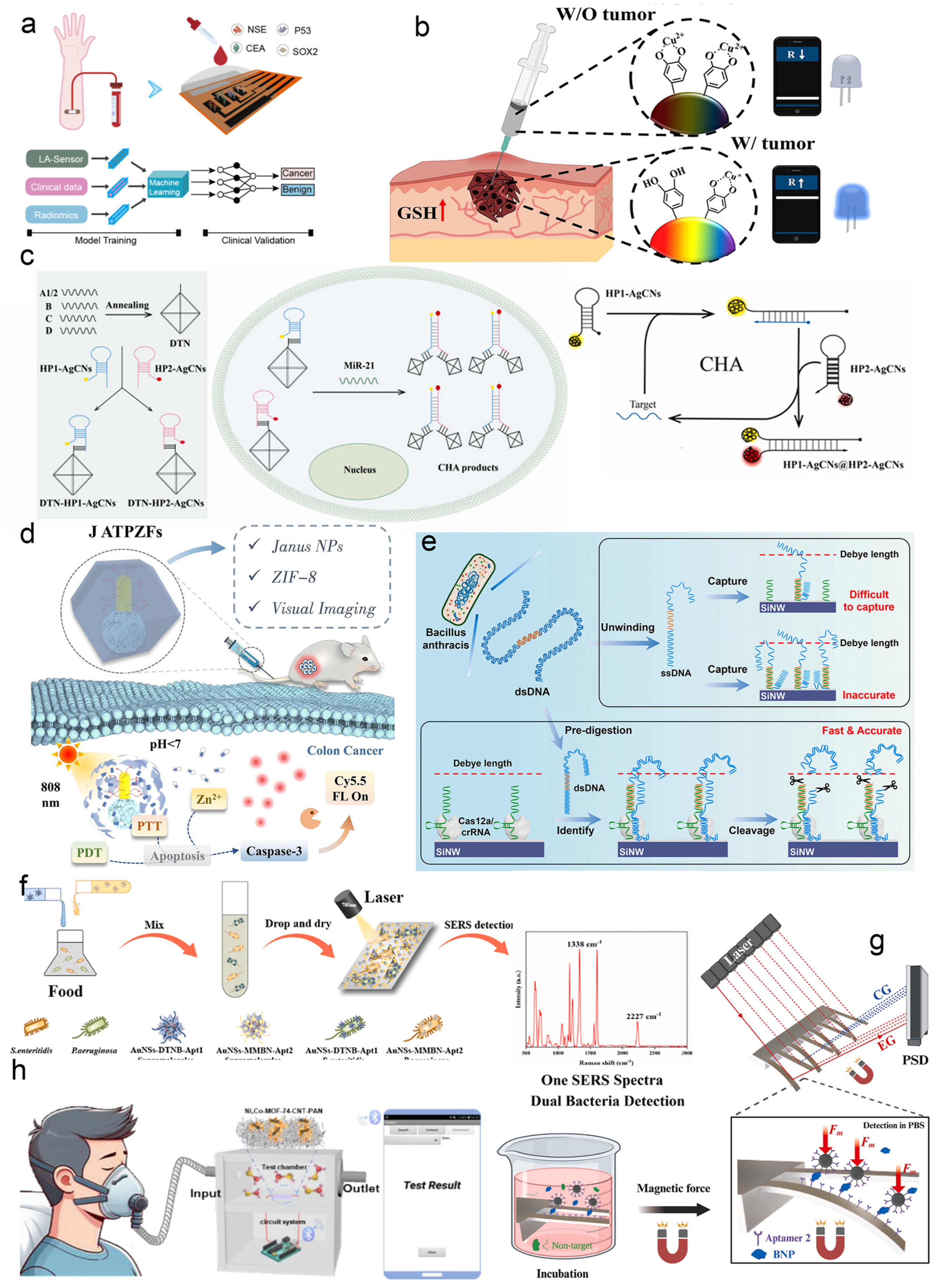
- Cai, Y.; Ke, L.; Du, A.; Dong, J.; Gai, Z.; Gao, L.; Yang, X.; Han, H.; Du, M.; Qiang, G.; et al. Machine Learning–Assisted Multimodal Early Screening of Lung Cancer Based on a Multiplexed Laser–Induced Graphene Immunosensor. ACS Nano 2025, 19, 25697–25709. [Google Scholar] [CrossRef]
- Kim, A.H.; Jeon, S.; Roy, K.; Kim, T.M.; Jin, E.–J.; Park, S.Y. Fabrication of low–viscosity injectable mineralized hydrogel sensor with redox–activated hybrid nanoparticle structure for cancer detection. Chem. Eng. J. 2024, 490, 151859. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, L.; Jiang, M.; Bao, Y.; Fan, M.; Shen, H.; Zhang, X.; Liu, Z.; Liu, M.; Ran, X. Enzyme–free nano–sensor for high–sensitivity miRNA detection and tumor imaging based on multicolor silver nanoclusters pair and DNA–mediated CHA reactions. Sens. Actuators B Chem. 2025, 427, 137222. [Google Scholar] [CrossRef]
- Yang, J.; Ma, M.; Zhao, C.; Chen, Y.; Sun, J.; Sun, Y.; Ma, P.; Jiao, S.; Song, D. Janus NPs@MOFs–based nanosensors for real–time visual monitoring and enhanced therapy of colon cancer. Chem. Eng. J. 2025, 508, 161091. [Google Scholar] [CrossRef]
- Li, Y.; Dou, Y.; Lu, Z.; Wang, Y.; Zhou, H.; Li, T. CRISPR/Cas12a–functionalized silicon nanowires field–effect transistor sensor for ultra–sensitive detection of pathogen nucleic acids. Biosens. Bioelectron. 2025, 289, 117936. [Google Scholar] [CrossRef]
- Cheng, N.; Deng, Y.; Cao, X.; Tao, Y. Hotspot–enhanced SERS Biosensor: Triplex noble nanoparticle–aptamer supramolecules on micropatterned silicon for simultaneous detection of foodborne pathogens. Biosens. Bioelectron. 2025, 289, 117901. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, L.; Qiao, Z.; Yang, Y.; Wu, W.; Wang, C.; Chen, J.; Wu, S.; Zhang, Q. Ultrasensitive magnetic nanomechanical biosensors for simultaneous detection of multiple cardiovascular disease biomarkers in a single blood drop. Biosens. Bioelectron. 2025, 280, 117448. [Google Scholar] [CrossRef]
- Zhao, J.; Li, P.; Zhang, Q.; Ye, Z.; Wang, Y.; Tian, J.; Xu, Z.; Peng, N.; Ren, H.; Zhang, X. MOF nanoflowers–based flexible portable NO sensors for human airway inflammation detection. Chem. Eng. J. 2024, 499, 156184. [Google Scholar] [CrossRef]
- Yoon, J.; Kwon, N.; Lee, Y.; Kim, S.; Lee, T.; Choi, J.–W. Nanotechnology–Based Wearable Electrochemical Biosensor for Disease Diagnosis. ACS Sens. 2025, 10, 1675–1689. [Google Scholar] [CrossRef] [PubMed]
- Jayathilaka, W.A.D.M.; Qi, K.; Qin, Y.; Chinnappan, A.; Serrano–García, W.; Baskar, C.; Wang, H.; He, J.; Cui, S.; Thomas, S.W.; et al. Significance of Nanomaterials in Wearables: A Review on Wearable Actuators and Sensors. Adv. Mater. 2019, 31, 1805921. [Google Scholar] [CrossRef] [PubMed]
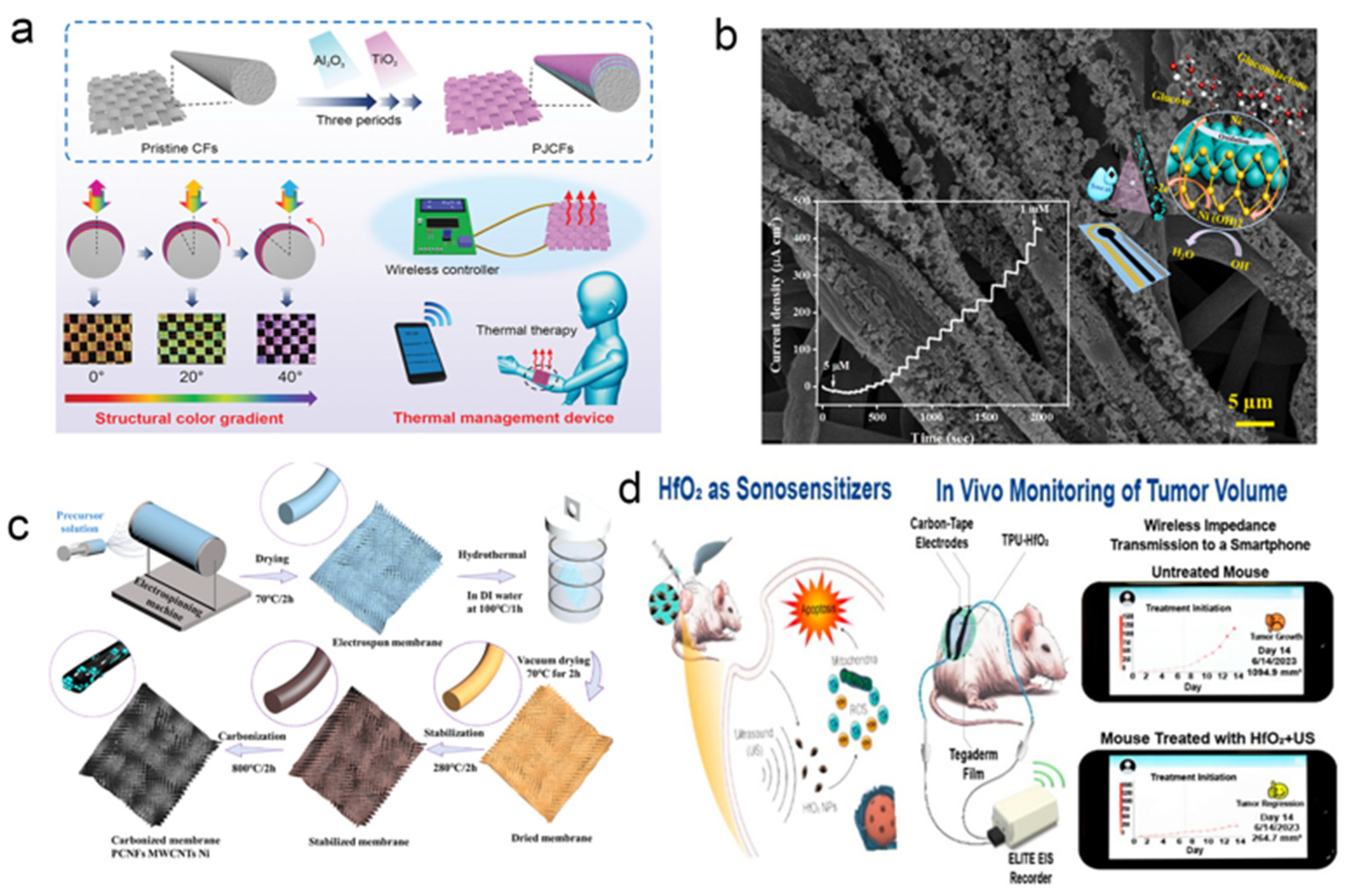
- Zhao, K.; Cheng, J.; Sun, N.; Wang, Y.; Huang, H.; Zhang, S.; Niu, W. Photonic Janus Carbon Fibers with Structural Color Gradient for Multicolored, Wirelessly Wearable Thermal Management Devices. Adv. Mater. Technol. 2022, 7, 2101057. [Google Scholar] [CrossRef]
- Chandran, M.; Veerapandian, M.; Dhanasekaran, B.; Govindaraju, S.; Yun, K. Advanced nanomaterials for health monitoring and diagnostics in next–generation wearable sensors. Mater. Sci. Eng. R Rep. 2025, 165, 101015. [Google Scholar] [CrossRef]
- Ali, S.; Abdalla, I.; Chen, G.; Xiang, H.; Zhu, M. Non–invasive wearable nanoporous device for real–time monitoring of glucose in sweat. Compos. Part B Eng. 2025, 303, 112613. [Google Scholar] [CrossRef]
- Siboro, P.Y.; Sharma, A.K.; Lai, P.–J.; Jayakumar, J.; Mi, F.–L.; Chen, H.–L.; Chang, Y.; Sung, H.–W. Harnessing HfO2 Nanoparticles for Wearable Tumor Monitoring and Sonodynamic Therapy in Advancing Cancer Care. ACS Nano 2024, 18, 2485–2499. [Google Scholar] [CrossRef]
- Bouazzara, H.; Chaibi, R. Diatoms and nanotechnology, current trends in nanomaterials applications and environmental monitoring. Environ. Monit. Assess. 2025, 197, 793. [Google Scholar] [CrossRef] [PubMed]
- Kidanemariam, A.; Cho, S. Recent Advances in Metal–Organic Framework–Based Nanozymes for Intelligent Microbial Biosensing: A Comprehensive Review of Biomedical and Environmental Applications. Biosensors 2025, 15, 437. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Wang, C.; Wang, Q.; Zhao, W.; He, Z.; Zheng, Y.; Jing, Y.; Sun, X.; Zhang, S. State of the art overview wearable biohazard gas sensors based on nanosheets for environment monitoring applications. Trends Environ. Anal. Chem. 2023, 40, e00215. [Google Scholar] [CrossRef]
- Chang, A.; Uy, C.; Xiao, X.; Xiao, X.; Chen, J. Self–powered environmental monitoring via a triboelectric nanogenerator. Nano Energy 2022, 98, 107282. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, K.; Yu, D.–G.; Zheng, X.; Huang, C. Advances in Biosensing and Environmental Monitoring Based on Electrospun Nanofibers. Adv. Fiber Mater. 2022, 4, 404–435. [Google Scholar] [CrossRef]
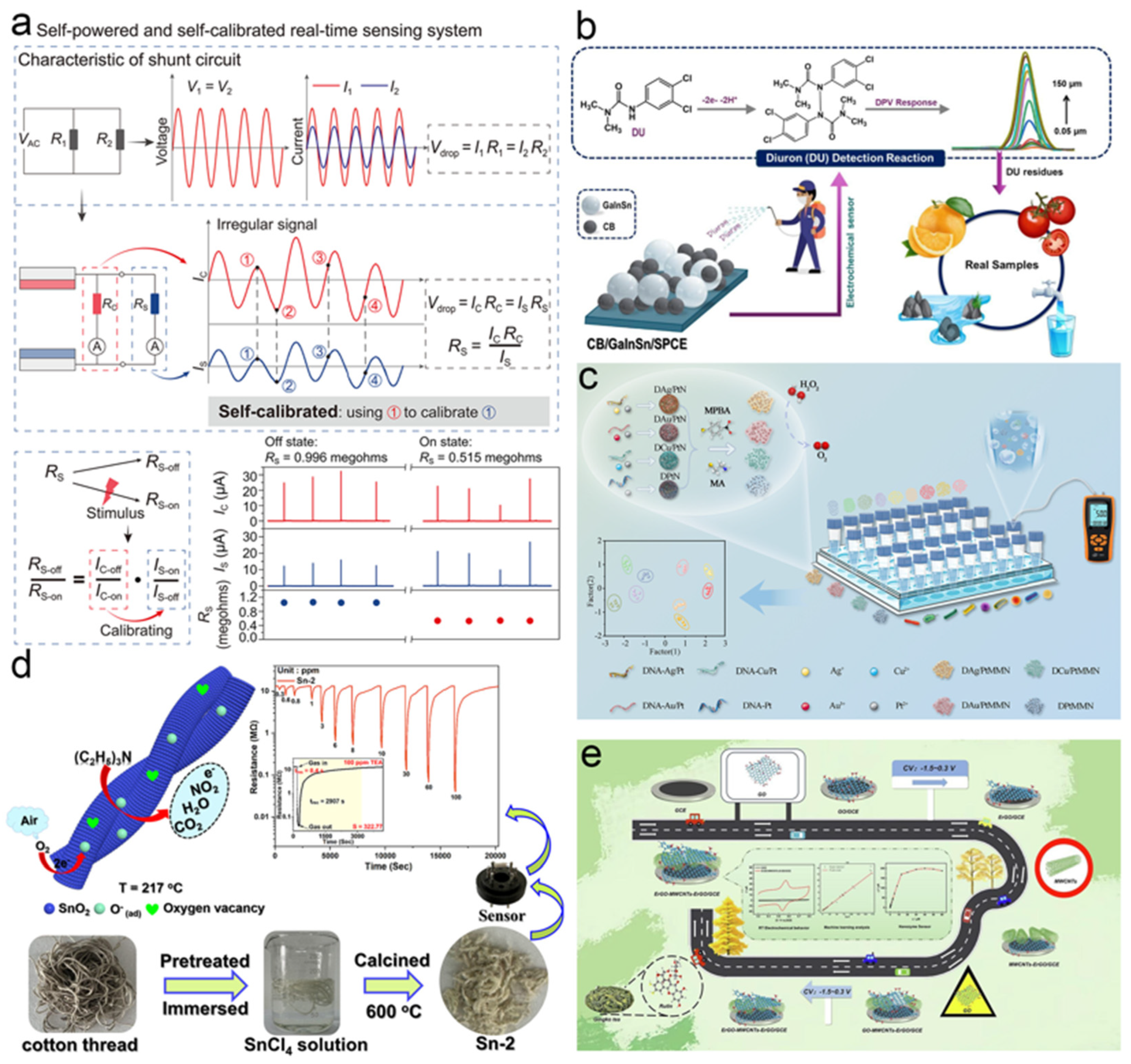
- Ma, J.; Sun, H.; Chu, Z.; Zhao, J.; Yang, Z.; Liu, S.; Wen, J.; Qin, Y. Self–powered and self–calibrated sensing system for real–time environmental monitoring. Sci. Adv. 2025, 11, eadw3745. [Google Scholar] [CrossRef] [PubMed]
- Mutharani, B.; Ranganathan, P.; Chang, Y.–H.; Chiu, F.–C. Development of carbon black–percolated GaInSn liquid metal networks for high–sensitive electrochemical detection of diuron in environmental samples. Food Chem. 2025, 494, 146139. [Google Scholar] [CrossRef]
- Jiao, J.; Kang, Q.; Ma, C.; Lin, T.; Xiao, Z.; Du, T.; Wang, N.; Du, X.; Wang, S. Pressure Sensor Array Based on Four DNA–Nanoenzymes with Catalase–like Activity for Portable Multiple Detection of Foodborne Pathogens. J. Agric. Food Chem. 2025, 73, 1694–1702. [Google Scholar] [CrossRef]
- Wang, J.–H.; Zhang, X.–N.; Lin, K.; Li, W.–X.; Song, B.–Y.; Ye, F.; Fu, Y.; Xu, Y.–M. Waste cotton thread–derived SnO2 microfibres for enhanced sensing to trace triethylamine and application in meat freshness detection. Chem. Eng. J. 2025, 521, 167085. [Google Scholar] [CrossRef]
- Cheng, C.; Li, M.; Shi, C.; Hu, S.; Chen, M.; Cheng, Y.; Cao, Q.; Wang, X.; Kong, X.; He, Z.; et al. Hierarchical all–carbon nanozyme architectures for enhanced intelligent rutin detection. Chem. Eng. J. 2025, 514, 163289. [Google Scholar] [CrossRef]
- Shi, S.; Ming, Y.; Wu, H.; Zhi, C.; Yang, L.; Meng, S.; Si, Y.; Wang, D.; Fei, B.; Hu, J. A Bionic Skin for Health Management: Excellent Breathability, In Situ Sensing, and Big Data Analysis. Adv. Mater. 2024, 36, 2306435. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, Y.; Gu, M.; Yang, Z.; Zhan, L.; Du, Z. Sensing and Stimulation Applications of Carbon Nanomaterials in Implantable Brain–Computer Interface. Int. J. Mol. Sci. 2023, 24, 5182. [Google Scholar] [CrossRef]
- Guo, M.; Huang, K.; Xu, W. Third Generation Whole–Cell Sensing Systems: Synthetic Biology Inside, Nanomaterial Outside. Trends Biotechnol. 2021, 39, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Zhou, H.; Xu, S.; Lee, C. Advances in Machine–Learning Enhanced Nanosensors: From Cloud Artificial Intelligence Toward Future Edge Computing at Chip Level. Small Struct. 2024, 5, 2300325. [Google Scholar] [CrossRef]
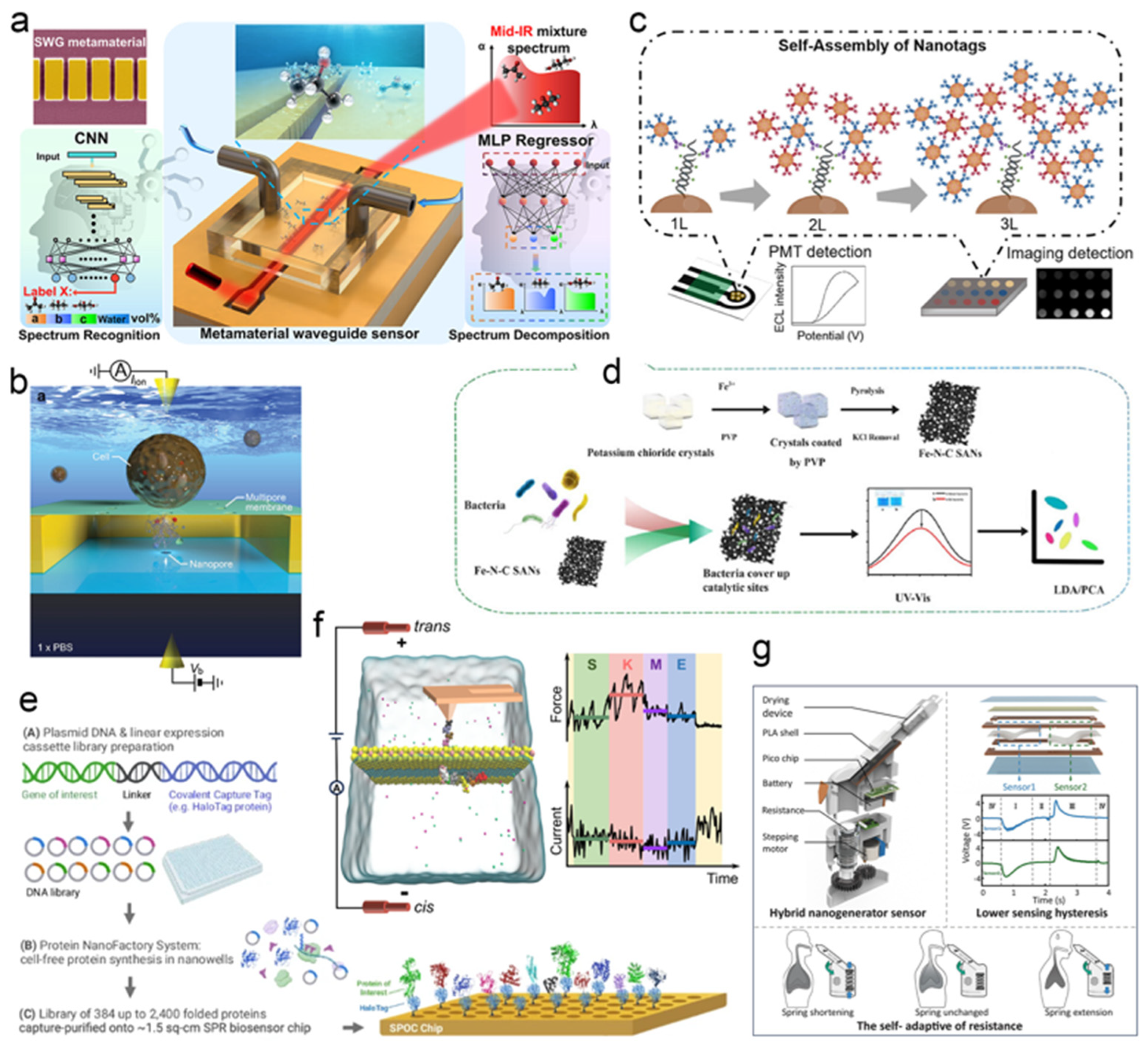
- Zhou, J.; Zhang, Z.; Dong, B.; Ren, Z.; Liu, W.; Lee, C. Midinfrared Spectroscopic Analysis of Aqueous Mixtures Using Artificial–Intelligence–Enhanced Metamaterial Waveguide Sensing Platform. ACS Nano 2023, 17, 711–724. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Liu, Y.; Khan, M.A.; Zhao, H.; Cao, H.; Ye, D. Identification of multiple foodborne pathogens using single–atom nanozyme colorimetric sensor arrays and machine learning. Chem. Eng. J. 2025, 511, 162115. [Google Scholar] [CrossRef]
- Xue, Z.; Tan, P.; Xue, J.; Xi, Y.; Liu, M.; Zou, Y.; Zheng, Q.; Li, Z.; Wu, Y. Adaptive respiratory muscle trainer based on hybrid nanogenerator sensor and artificial intelligence. InfoMat 2025, 7, e70004. [Google Scholar] [CrossRef]
- Tsutsui, M.; Yokota, K.; Arima, A.; Washio, T.; Baba, Y.; Kawai, T. Detecting Single Molecule Deoxyribonucleic Acid in a Cell Using a Three–Dimensionally Integrated Nanopore. Small Methods 2021, 5, 2100542. [Google Scholar] [CrossRef]
- Nevedomskaya, E.; Haendler, B. From Omics to Multi–Omics Approaches for In–Depth Analysis of the Molecular Mechanisms of Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 6281. [Google Scholar] [CrossRef]
- Cao, X.; Yuan, C.; Yu, Q.; Wu, J.; Ju, H. Highly Sensitive and Diversified Electrochemiluminescence DNA Methylation Biosensing Platform Based on Self–Assembly of Nanotags. Anal. Chem. 2025, 97, 9920–9926. [Google Scholar] [CrossRef]
- Zhang, Z.; Si, W. MoS2/MoSe2 Planar Heterostructure Nanoslits for Protein Sequencing. ACS Appl. Nano Mater. 2025, 8, 8274–8282. [Google Scholar] [CrossRef]
- Agu, C.V.; Cook, R.L.; Martelly, W.; Gushgari, L.R.; Mohan, M.; Takulapalli, B. Multiplexed proteomic biosensor platform for label–free real–time simultaneous kinetic screening of thousands of protein interactions. Commun. Biol. 2025, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, T.; Wang, H.; Wang, Z.; Hou, L.; Jiang, J.; Xu, T. Applications of nanomaterial technology in biosensing. J. Sci. Adv. Mater. Devices 2024, 9, 100694. [Google Scholar] [CrossRef]
- Welch, E.C.; Powell, J.M.; Clevinger, T.B.; Fairman, A.E.; Shukla, A. Advances in Biosensors and Diagnostic Technologies Using Nanostructures and Nanomaterials. Adv. Funct. Mater. 2021, 31, 2104126. [Google Scholar] [CrossRef]
- Cho, Y.; Choi, Y.; Jang, Y.; Seong, H. Nanomaterial–Enhanced Biosensing: Mechanisms and Emerging Applications. Adv. Healthc. Mater. 2025, 2500189. [Google Scholar] [CrossRef] [PubMed]
- Budd, J.; Miller, B.S.; Manning, E.M.; Lampos, V.; Zhuang, M.; Edelstein, M.; Rees, G.; Emery, V.C.; Stevens, M.M.; Keegan, N.; et al. Digital technologies in the public–health response to COVID-19. Nat. Med. 2020, 26, 1183–1192. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Panigrahi, D.; Rewatkar, P.; Haick, H. Role of Machine Learning Assisted Biosensors in Point–of–Care–Testing For Clinical Decisions. ACS Sens. 2024, 9, 4495–4519. [Google Scholar] [CrossRef]
| Material Type | Representative Materials | Advantage | Sensing Mechanism |
|---|---|---|---|
| Metallic nanomaterial | AgNWs, AuNPs | Conductivity, SPR effect | Resistance variation, LSPR |
| Carbon–based nanomaterials | Graphene, CNTs | High specific surface area, electrical performance, mechanical flexibility | Adsorption of target substance causes changes in resistance or FET channel characteristics |
| Two–dimensional transition metal carbon/nitride | MXene | High metallic conductivity, hydrophilicity, and abundant surface functional groups | Surface adsorption causes changes in conductivity; Synergistic effect is generated during composite |
| Semiconductor metal oxide | ZnO, TiO2 | Sensitive to gases and mature preparation processes | The target gas undergoes an oxidation–reduction reaction with the material surface, causing a change in resistance |
| Conductive polymer | PANI, PPy, PEDOT | Great flexibility, easy to modify | Conductivity changes during doping/dedoping process |
| New composite materials | Multiple combinations of materials | Synergy: Comprehensive advantages of each component | Heterojunction interface charge transfer, conductive pathway modulation, sensitization effect, inhibition of nano layer restacking |
| Type | Common Materials | Properties and Uses |
|---|---|---|
| Noble metal nanoparticles | Au, Ag, Pt, Pd | SPR/LSPR, used for sensing and imaging; catalysis; antibacterial (Ag) |
| Metal oxide nanoparticles | ZnO, TiO2, Fe3O4 | Semiconductority, photocatalysis, ultraviolet absorption, superparamagnetism |
| Alloy nanoparticles | Au–Ag, Pt–Ni | Tunable plasma resonance characteristics; synergistic catalytic effect, better than single metal |
| Metal nanoclusters | Gold clusters, silver clusters | Molecular like discrete energy levels, fluorescence emission, used for fluorescence labeling and sensing |
| Special morphology structure | Nanorods, nanoshells, nanostars, nanocubes, nanowires | Its plasma resonance peak strongly depends on shape |
| Type | Common Materials | Properties and Uses |
|---|---|---|
| Quantum dots (QDs) | CdSe, CdTe, PbS, InP | Size dependent fluorescence, used in biological imaging, display technology, photodetectors |
| Nanowires | Si, ZnO, GaN, InAs | Great electronic transmission, constructing (FET) biosensors, nanoscale electronic devices |
| Nanosheets | Transition metal chalcogenides (TMDs) | Unique band structure, interaction between light and matter, flexible electronic, optoelectronic devices |
| Perovskite nanocrystals | CsPbBr3, FAPbI3 | High fluorescence quantum yield, color purity, and tunable bandgap, QLED and solar cells |
| Type | Structural Characteristics | Properties |
|---|---|---|
| Graphene | Single–layer honeycomb lattice composed, sp2 hybridized carbon atoms arranged | High carrier mobility, high specific surface area, outstanding mechanical strength, excellent thermal conductivity, good flexibility |
| Carbon nanotubes (CNTs) | Single–walled carbon nanotubes (SWCNTs), multi walled carbon nanotubes (MWCNTs) | One dimensional quantum transport effect, high aspect ratio, excellent mechanical strength (>steel), high electrical and thermal conductivity |
| Fullerene | A football shaped hollow cage structure composed of 60 carbon atoms (C60) | Three dimensional electron delocalization, great electron acceptor properties, soluble in organic solvents |
| Carbon dots (CDs) & graphene quantum dots (GQDs) | CDs: composed of amorphous or nanocrystalline carbon nuclei and surface functional groups; GQDs: small–sized graphene sheets | Size dependent fluorescence luminescence, good photostability, high biocompatibility, easy surface functionalization |
| Nanodiamonds (NDs) | The diamond structure formed by sp3 hybridized bonding of carbon atoms | High hardness, excellent biocompatibility, chemical inertness, negative electron affinity, nitrogen vacancy color center |
| Type | Structural Characteristics | Properties |
|---|---|---|
| Graphene | Zero bandgap semimetal | High electrical and thermal conductivity, high mechanical strength |
| Transition metal chalcogenides | Sandwich | From indirect bandgap to direct bandgap (in monolayer), with adjustable bandgap (1–2 eV), strong light matter interaction |
| MXene | Two–dimensional metal carbon/nitride | High metallic conductivity, hydrophilicity, electrochemical performance |
| Xenes (Monoxene) | Various types of single element two–dimensional atomic crystals | Many have Dirac cones or topological properties, unstable in air |
| Stimulus Type | Representative Materials | Response Mechanism | Typical Applications |
|---|---|---|---|
| Endogenous stimulation | |||
| pH | Polyelectrolytes, hydrazone bonds, ketal bonds | Materials undergo protonation, hydrolysis, or structural collapse under acidic conditions | Tumor targeted drug release |
| Enzyme | Peptide/protein based materials, specific enzyme substrate linking chains | The material is specifically cleaved by enzymes | Highly specific diagnosis and treatment |
| Redox | Polymers and selenides containing disulfide bonds (–S–S–) | Disulfide bonds and other substances break at high concentrations of GSH | Intracellular release |
| Temperature | PNIPAM and temperature sensitive polymers | The material has the lowest critical solution temperature (LCST) | Thermal triggered drug release |
| External stimulation | |||
| Light | Gold nanorods/shells, upconversion nanoparticles, azobenzene | Specific wavelength laser irradiation produces photothermal effect or photochemical reaction | Photothermal/photodynamic therapy, remote precise controlled release |
| Magnetic field | Fe3O4 nanoparticles | External alternating magnetic field application causes magnetic nanoparticles to produce magnetocaloric effect | Magnetic hyperthermia, magnetic targeting |
| Ultrasonic wave | Microbubbles, liposomes | Ultrasonic irradiation produces mechanical or thermal effects that damage the structure of the carrier | Non–invasive trigger drug release, drug release, and gene delivery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, B.; Chen, Y.; Jiang, H.; Liu, X.; Wang, X. Nanomaterial Engineered Biosensors and Stimulus–Responsive Platform for Emergency Monitoring and Intelligent Diagnosis. Biosensors 2025, 15, 789. https://doi.org/10.3390/bios15120789
Fang B, Chen Y, Jiang H, Liu X, Wang X. Nanomaterial Engineered Biosensors and Stimulus–Responsive Platform for Emergency Monitoring and Intelligent Diagnosis. Biosensors. 2025; 15(12):789. https://doi.org/10.3390/bios15120789
Chicago/Turabian StyleFang, Bo, Yuanyuan Chen, Hui Jiang, Xiaohui Liu, and Xuemei Wang. 2025. "Nanomaterial Engineered Biosensors and Stimulus–Responsive Platform for Emergency Monitoring and Intelligent Diagnosis" Biosensors 15, no. 12: 789. https://doi.org/10.3390/bios15120789
APA StyleFang, B., Chen, Y., Jiang, H., Liu, X., & Wang, X. (2025). Nanomaterial Engineered Biosensors and Stimulus–Responsive Platform for Emergency Monitoring and Intelligent Diagnosis. Biosensors, 15(12), 789. https://doi.org/10.3390/bios15120789







