Progress in Electrode Modifiers for Nitrite Electrochemical Sensing Applications
Abstract
1. Introduction
2. Progress in Nitrite Detection
2.1. Zinc Oxide (ZnO)-Based Materials
2.2. Co3O4-Based Materials
2.3. CeO2-Based Materials
2.4. Iron Oxide-Based Materials
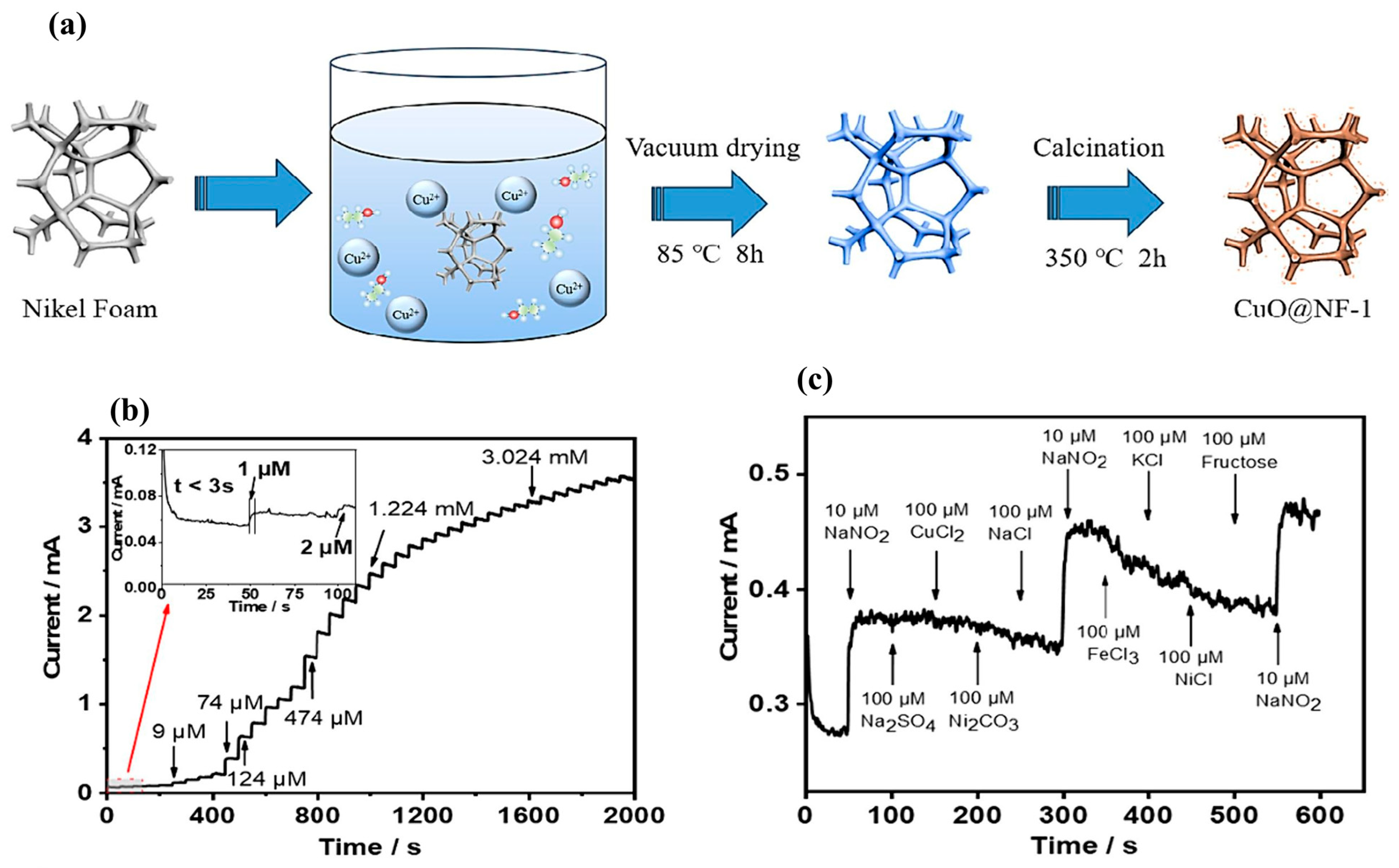
2.5. CuO-Based Materials
2.6. TiO2-Based Materials
2.7. MoS2-Based Materials
2.8. Polymer-Based Materials
2.9. MOF/ZIF-Based Materials
2.10. gCN-Based Materials
2.11. CNTs-Based Materials
2.12. GO and rGO-Based Materials
2.13. MXene-Based Materials
3. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guerrieri, N.; Mazzini, S.; Borgonovo, G. Food Plants and Environmental Contamination: An Update. Toxics 2024, 12, 365. [Google Scholar] [CrossRef]
- Sandri, E.; Broccolo, A.; Piredda, M. Analysis of the Importance of Food Sustainability and the Consumption of Organic and Local Products in the Spanish Population. Sustainability 2025, 17, 991. [Google Scholar] [CrossRef]
- Chai, D.; Meng, T.; Zhang, D. Influence of Food Safety Concerns and Satisfaction with Government Regulation on Organic Food Consumption of Chinese Urban Residents. Foods 2022, 11, 2965. [Google Scholar] [CrossRef]
- Gajdár, J.; Gaspar, S.R.; Almeida, M.G. Trends in nitrite detection: Recent advances in electrochemical sensor technologies. TrAC Trends Anal. Chem. 2025, 183, 118105. [Google Scholar] [CrossRef]
- Pavitra, V.; Praveen, B.M.; Nagaraju, G. Exploring the Spondias mombin (Hog plum) mediated ZnWO4-CuWO4 nanocomposite for photocatalysis and electrochemical nitrite sensing. Mater. Chem. Phys. 2023, 293, 126882. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, H.; Barman, V.; Mohil, R. Mixed metal oxide nanocomposites for enhanced electrochemical detection of nitrite in food products. J. Food Compos. Anal. 2025, 139, 107137. [Google Scholar] [CrossRef]
- Jeevika, A.; Mariyappan, V.; Kim, Y.; Chen, Y.-S.; Iimura, K.-I. Interfacial nanostructure engineering: Hydrothermal-assisted synthesis of GdMn2O5/GdMnO3 for enhanced nitrite detection in water samples. J. Environ. Chem. Eng. 2025, 13, 115623. [Google Scholar] [CrossRef]
- Liu, H.; Sun, H.; Zhao, P.; Shang, K.; Fang, M.; Tan, X.; Yu, L.; Ma, B. Enhanced electrochemical detection of nitrite ions by CTAB-modified hexagonal Fe-doped SnS2 nanosheets in water. Mater. Res. Bull. 2025, 186, 113344. [Google Scholar] [CrossRef]
- Doğan, H.O.; Albayrak, Ö.F. Ag nanoparticles-decorated CuBi2O4–rGO electrodes as an amperometric sensor for electrochemical determination of nitrite. Synth. Met. 2023, 298, 117445. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Duan, C.; Chen, G.; Liu, Y.; Lu, M. A high-performance electrochemical sensor based on dendritic Au/Zn modified carbon cloth for the determination of nitrite in aquaculture. Sci. Total Environ. 2024, 950, 175346. [Google Scholar] [CrossRef]
- Li, E.; Fu, J.; Zhou, S.; Wei, J.; Zhou, S.; Yang, Y.; Jia, Z.; Su, X. Construction of nitrite electrochemical sensor by in-situ deposition of FeOOH nanomaterials on carbon cloth modified with green DBD technology. Microchem. J. 2024, 205, 111264. [Google Scholar] [CrossRef]
- Chih, P.-S.; Wang, W.-R.; Chen, C.-Y.; Yan, P.-R.; Mahmudiono, T.; Lee, C.-C.; Chen, H.-L. Influence of cooking and storage conditions on the formation of N-nitrosamines in processed meats and pickled fish. LWT 2025, 224, 117822. [Google Scholar] [CrossRef]
- Hu, H.; Hu, F.; Wang, X.; Shi, X. Paper-based sensor with electro-modified chitosan/silver nanoparticles for rapid and sensitive nitrite detection. J. Environ. Chem. Eng. 2024, 12, 112858. [Google Scholar] [CrossRef]
- Jiang, J.; Bao, S.; Lv, J.; Yu, X. Preparation of Pd–Co bimetallic nanoparticles supported on graphene for rapid electrochemical detection of nitrite. Int. J. Electrochem. Sci. 2022, 17, 220118. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Bressi, V.; Espro, C.; Iannazzo, D.; Piperopoulos, E.; Neri, G. Electrochemical determination of nitrites and sulfites by using waste-derived nanobiochar. J. Electroanal. Chem. 2023, 928, 117071. [Google Scholar] [CrossRef]
- Ibrahim, N.; Hefnawy, M.A.; Fadlallah, S.A.; Medany, S.S. Recent advances in electrochemical approaches for detection of nitrite in food samples. Food Chem. 2025, 462, 140962. [Google Scholar] [CrossRef] [PubMed]
- Paramparambath, S.; Geetha, M.; Alahzm, A.M.; Al-Ejji, M.; Sadasivuni, K.K. Innovative smart colorimetric sensor for nitrite detection in poultry packaging. Discov. Appl. Sci. 2024, 6, 263. [Google Scholar] [CrossRef]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 851, 51–70. [Google Scholar] [CrossRef]
- Piknova, B.; Park, J.W.; Cassel, K.S.; Gilliard, C.N.; Schechter, A.N. Measuring nitrite and nitrate, metabolites in the nitric oxide pathway, in biological materials using the chemiluminescence method. J. Vis. Exp. 2016, 118, 54879. [Google Scholar]
- Wang, X.; Adams, E.; Van Schepdael, A. A fast and sensitive method for the determination of nitrite in human plasma by capillary electrophoresis with fluorescence detection. Talanta 2012, 97, 142–144. [Google Scholar] [CrossRef]
- Liu, G.; Guo, H.; Zhao, W.; Yan, H.; Zhang, E.; Gao, L. Advancements in Preprocessing and Analysis of Nitrite and Nitrate since 2010 in Biological Samples: A Review. Molecules 2023, 28, 7122. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, Y.; Zhou, B.; Xu, H. Nitrite: From Application to Detection and Development. Appl. Sci. 2024, 14, 9027. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, G.; Geng, Y.; Chen, F.; Zhu, H.; Zhao, C.; Suo, H.; Ding, L. A highly selective copper–tin alloy nanosheet/carbon-paper sensitive electrode for detecting nitrite at low electrochemical reduction potential based on a binary cooperative strategy. Chem. Eng. J. 2025, 512, 162199. [Google Scholar] [CrossRef]
- Sheikh, T.; Nagendran, V.; Vasant, K.S.; Mallya, U.; Mutalik, S.; Khan, F.; Nayak, S.; Sudhakara, S.M. Acacia auriculiformis-mediated synthesis of silver nanoparticles for the sensitive and rapid electrochemical sensing of nitrite in water sample. Microchem. J. 2025, 211, 113162. [Google Scholar] [CrossRef]
- Shafi, I.; Liang, E.; Li, B. Self-assembled BiVO4 nanorods: A fascinating electrode material for highly efficient pseudocapacitors and electrochemical nitrite sensors. J. Phys. Chem. Solids 2022, 162, 110517. [Google Scholar] [CrossRef]
- Alsaiari, M.; Saleem, A.; Alsaiari, R.; Muhammad, N.; Latif, U.; Tariq, M.; Almohana, A.; Rahim, A. SiO2/Al2O3/C grafted 3-n-propylpyridinium silsesquioxane chloride-based non-enzymatic electrochemical sensor for determination of carcinogenic nitrite in food products. Food Chem. 2022, 369, 130970. [Google Scholar] [CrossRef]
- Shi, H.; Fu, L.; Chen, F.; Zhao, S.; Lai, G. Preparation of highly sensitive electrochemical sensor for detection of nitrite in drinking water samples. Environ. Res. 2022, 209, 112747. [Google Scholar] [CrossRef] [PubMed]
- Dorovskikh, S.I.; Pelevina, A.A.; Klyamer, D.D.; Volchek, V.V.; Sukhikh, A.S.; Korotaev, E.V.; Morozova, N.B.; Basova, T.V. Sensors based on iron phthalocyanine films decorated with platinum nanoparticles and carbon rods for electrochemical detection of nitrites. Appl. Surf. Sci. 2023, 640, 158300. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Yang, W.; Sun, X. Surfactant-free synthesis of 2D Cu nanoflakes as electrochemical sensors and their applications for detection of formaldehyde, nitrite and glucose. J. Food Compos. Anal. 2024, 131, 106245. [Google Scholar] [CrossRef]
- Arivazhagan, M.; Saetang, S.; Permwong, W.; Jakmunee, J. Electrochemical sensors for sensitive and selective detection of nitrite based on flexible gold microneedle-like nanodendrites modified electrode. Electrochim. Acta 2024, 507, 145148. [Google Scholar] [CrossRef]
- Wang, W.; Cai, Q.; Dai, C.; Li, J.; Xu, H.; Zhang, W.; Chen, Y.; Hu, J. Construction of an electrochemical sensor based on a conductive Ni3(HHTP)2 nanowires array functionalized with Ag nanoparticles@WS2 QDs for nitrite detection. Food Chem. 2025, 473, 143126. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, X.; Yang, C.; Yang, W.; Liu, G.; Li, Y.; Zhang, G.; Zhao, X. Surfactant-free synthesis of CuBr NPs decorated by Pt for glucose and nitrite sensors. J. Ind. Eng. Chem. 2023, 124, 323–330. [Google Scholar] [CrossRef]
- Arularasu, M.V.; Rajendran, T.V.; Vignesh, R.; Nelson, V.K.; Yusuf, S.M. A screen-printed carbon electrode modified with a green synthesized gold nanoparticle for selective detection of nitrite in drinking water sample. Sens. Bio-Sens. Res. 2025, 48, 100781. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, Y.; Zhou, G.; Gao, J.; Jiang, H.; Zhao, C.; Suo, H. Electron structure modulation of cerium(III)-doped copper nanoparticles on carbon cloth for boosted electrocatalytic sensing of nitrite. J. Alloys Compd. 2025, 1014, 178801. [Google Scholar] [CrossRef]
- Hasan, M.R.; Islam, T.; Hasan, M.M.; Chowdhury, A.-N.; Ahammad, A.J.S.; Reaz, A.H.; Roy, C.K.; Shah, S.S.; Al-Imran; Aziz, M.A. Evaluating the electrochemical detection of nitrite using a platinum nanoparticle coated jute carbon modified glassy carbon electrode and voltammetric analysis. J. Phys. Chem. Solids 2022, 165, 110659. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Hefnawy, M.A.; Alamro, F.S.; Ahmed, M.A.; Al-Faze, R.; Mostafa, A.M.; Ahmed, H.A.; Medany, S.S. Nickel–manganese spinel oxide nanoparticles on graphite felt for ultrasensitive nitrite detection in milk samples. Microchem. J. 2025, 213, 113844. [Google Scholar] [CrossRef]
- Sari, N.P.; Mulyawati, M.; Syahputra, M.Y.; Madurani, K.A.; Kurniawan, F. A novel ultrasensitive nitrite ion detection using tungsten trioxide-modified gold electrode. Electrochim. Acta 2024, 497, 144590. [Google Scholar] [CrossRef]
- Maleki, A.; Amini, N.; Rezaee, R.; Safari, M.; Marzban, N.; Seifi, M. Fabrication of Cu@Ag core–shell/nafion/polyalizarin: Applications to simultaneous electrocatalytic oxidation and reduction of nitrite in water samples. Heliyon 2025, 11, e40979. [Google Scholar] [CrossRef]
- Lin, Z.; Cheng, S.; Li, H.; Li, L. A novel, rapidly preparable and easily maintainable biocathode electrochemical biosensor for the continuous and stable detection of nitrite in water. Sci. Total Environ. 2022, 806, 150945. [Google Scholar] [CrossRef] [PubMed]
- Ndebele, N.; Nyokong, T. The use of carbon-based nanomaterials conjugated to cobalt phthalocyanine complex in the electrochemical detection of nitrite. Diam. Relat. Mater. 2023, 132, 109672. [Google Scholar] [CrossRef]
- Manjari, G.; Saran, S.; Radhakrishanan, S.; Rameshkumar, P.; Pandikumar, A.; Devipriya, S.P. Facile Green Synthesis of Ag–Cu Decorated ZnO Nanocomposite for Effective Removal of Toxic Organic Compounds and Efficient Detection of Nitrite Ions. J. Environ. Manag. 2020, 262, 110282. [Google Scholar] [CrossRef]
- Rashed, M.A.; Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsaiari, M.; Al-Assiri, M.S. rGO/ZnO/Nafion Nanocomposite as Highly Sensitive and Selective Amperometric Sensor for Detecting Nitrite Ions (NO2−). J. Taiwan Inst. Chem. Eng. 2020, 112, 345–356. [Google Scholar] [CrossRef]
- Cheng, Z.; Song, H.; Zhang, X.; Cheng, X.; Xu, Y.; Zhao, H.; Gao, S.; Huo, L. Morphology Control of ZnO by Adjusting the Solvent and Non-Enzymatic Nitrite Ions Electrochemical Sensor Constructed with Stir Bar-Shaped ZnO/Nafion Nanocomposite. Sens. Actuators B Chem. 2021, 346, 130525. [Google Scholar] [CrossRef]
- Cheng, Z.; Song, H.; Zhang, X.; Cheng, X.; Xu, Y.; Zhao, H.; Gao, S.; Huo, L. Enhanced Non-Enzymatic Nitrite Electrochemical Sensing Property Based on Stir Bar-Shaped ZnO Nanorods Decorated with Nitrogen-Doped Reduced Graphene Oxide. Sens. Actuators B Chem. 2022, 355, 131313. [Google Scholar] [CrossRef]
- Cheng, Z.; Song, H.; Zhang, X.; Cheng, X.; Xu, Y.; Zhao, H.; Gao, S.; Huo, L. Non-Enzymatic Nitrite Amperometric Sensor Fabricated with Near-Spherical ZnO Nanomaterial. Colloids Surf. B Biointerfaces 2022, 211, 112313. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.S.H.; Khan, M.M.R.; Shohag, M.R.H.; Rahman, S.; Paul, S.K.; Rahman, M.M.; Asiri, A.M.; Rahman, M.M. Easy Synthesis of PPy/TiO2/ZnO Composites with Superior Photocatalytic Performance, Efficient Supercapacitors and Nitrite Sensor. Heliyon 2023, 9, e19564. [Google Scholar] [CrossRef]
- Sudhakara, S.M.; Devendrachari, M.C.; Khan, F.; Thippeshappa, S.; Kotresh, H.M.N. Highly Sensitive and Selective Detection of Nitrite by Polyaniline Linked Tetra Amino Cobalt(II) Phthalocyanine Surface Functionalized ZnO Hybrid Electrocatalyst. Surf. Interfaces 2023, 36, 102565. [Google Scholar] [CrossRef]
- Cheng, Z.; Song, H.; Li, Z.; Chen, L.; Hu, H.; Lv, Y.; Liu, Z.; Wu, Y.; Lu, Y.; Han, D.; et al. Hierarchical Composites Based on Near-Spherical ZnO Attached on Nitrogen-Doped Reduced Graphene Oxide for Enhanced Nitrite Electrochemical Sensing Property. Microchem. J. 2024, 197, 109764. [Google Scholar] [CrossRef]
- Duan, C.; Chen, G.; Wang, Z.; Li, H.; Zhang, Z.; Liu, Y.; Lu, M. An Ultra-Sensitive Electrochemical Sensing Platform Based on Nanoflower-Like Au/ZnO Array on Carbon Cloth for Rapid Detection of Nitrite Residues in Food Samples. Food Chem. 2024, 437, 137892. [Google Scholar] [CrossRef]
- Song, H.; Cheng, Z.; Hu, H.; Li, Z.; Nan, H.; Ma, N.; Wang, G.; He, T.; Wang, L.; Lu, Y.; et al. Controllable Synthesis of Cross-Linked 3D ZnO Nanosheet toward a Nitrite Electrochemical Sensor. Electrochim. Acta 2024, 498, 14464. [Google Scholar] [CrossRef]
- Song, H.; Hu, H.; Li, Z.; Zhang, F.; An, J.; Su, M.; Tian, Q.; Li, X.; Huo, L.; Cheng, Z. DFT Investigation on the Effect of ZnO Microstructure on Nitrite Catalytic Performance and Its Application in Food. Microchem. J. 2025, 209, 112819. [Google Scholar] [CrossRef]
- Ndala, A.; Itota, B.; Chamier, J.; Ray, S.; Sunday, C.; Chowdhury, M. Novel (CH6N3+, NH3+)-Functionalized and Nitrogen Doped Co3O4 Thin Film Electrochemical Sensor for Nanomolar Detection of Nitrite in Neutral pH. Electrochim. Acta 2021, 388, 138556. [Google Scholar] [CrossRef]
- Zhe, T.; Li, M.; Li, F.; Li, R.; Bai, F.; Bu, T.; Jia, P.; Wang, L. Integrating Electrochemical Sensor Based on MoO3/Co3O4 Heterostructure for Highly Sensitive Sensing of Nitrite in Sausages and Water. Food Chem. 2022, 367, 130666. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, T.; Wang, J.; Feng, J.; Zhao, S.; Chen, H.; Gong, L.; Wang, Z.; Zhang, Y.; Yin, S.; et al. Hydrothermal Synthesis of Iron Foam-Supported Co3O4-Based Sensor Electrodes for Electrochemical Detection of Nitrite. Mater. Today Commun. 2025, 43, 111656. [Google Scholar] [CrossRef]
- Manibalan, G.; Murugadoss, G.; Hazra, S.; Marimuthu, R.; Manikandan, C.; Ramalingam, R.J.; Kumar, M.R. A Facile Synthesis of Sn-Doped CeO2 Nanoparticles: High Performance Electrochemical Nitrite Sensing Application. Inorg. Chem. Commun. 2022, 135, 109096. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, J. Two-Step Hydrothermal and Ultrasound-Assisted Synthesis of CB/NiCo2S4@CeO2 Composites for High-Sensitivity Electrochemical Detection of Nitrite. Microchem. J. 2022, 181, 107717. [Google Scholar] [CrossRef]
- Çelebi, N.; Temur, E.; Doğan, H.Ö.; Yüksel, A.K. The Electrochemical Fabrication of Cu@CeO2-rGO Electrode for High-Performance Electrochemical Nitrite Sensor. Diam. Relat. Mater. 2024, 143, 110907. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Gao, J.; Zhao, C.; Suo, H. Three-Dimensional CeO2 Nanosheets/CuO Nanoflowers p–n Heterostructure Supported on Carbon Cloth as Electrochemical Sensor for Sensitive Nitrite Detection. Anal. Chim. Acta 2025, 1336, 343526. [Google Scholar] [CrossRef]
- Riahifar, V.; Haghnazari, N.; Keshavarzi, F.; Nasri, F. Design of a High-Sensitive Electrochemical Sensor Based on Immobilized Cysteine on Fe3O4@Au Core–Shell Nanoparticles and Reduced Graphene Oxide Nanocomposite for Nitrite Monitoring. Microchem. J. 2021, 166, 106217. [Google Scholar] [CrossRef]
- Song, X.C.; Zheng, Y.F.; Wang, L. A Sensor Based on NiO/Fe2O3 Modified GCE Electrode for the Detection of Nitrite. J. Electroanal. Chem. 2023, 944, 117672. [Google Scholar] [CrossRef]
- Luo, L.; Xu, Q.-Q.; Chen, K.-K.; Liu, Z.-G.; Guo, Z. Carbon Spheres Decorated with Fe2O3/Fe3C/Fe Composite Nanoparticles for Highly Sensitive and Selective Electrochemical Determination of Nitrite (NO2−). Sens. Actuators B Chem. 2023, 382, 133488. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Guo, W. Electrochemical Sensor for Sensitive Nitrite and Sulfite Detection in Milk Based on Acid-Treated Fe3O4@SiO2 Nanoparticles. Food Chem. 2024, 430, 137004. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Chen, Y.; Xing, K.; Liu, Q. Electrochemical Sensor Based on Fe3O4@Au/MOF-P2W17V Composite Modified Glassy Carbon Electrode for Food Nitrite Detection. J. Food Compos. Anal. 2024, 136, 106792. [Google Scholar] [CrossRef]
- Saeed, M.; Marwani, H.M.; Shalauddin, M.; Alfaifi, S.Y.; Akhter, S.; Alzahrani, K.A.; Basirun, W.J.; Rahman, M.M. Sensitive Detection of Unsafe Nitrite Chemical Based on GO@Fe2O3/Y2O3 Nanocomposite by Electrochemical Approach for Environmental Assessment. J. Ind. Eng. Chem. 2025, 144, 552–564. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Wang, T.; Li, L.; Gong, J.; Zhang, L.; Chen, W. Copper Oxide Nanoleaves Covered with Loose Nickel Oxide Nanoparticles for Sensitive and Selective Non-Enzymatic Nitrite Sensors. Mater. Res. Bull. 2022, 149, 111712. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Gao, J.; Zhang, L.; Zhao, C.; Suo, H. In situ fabrication of copper oxide nanoparticles decorated carbon cloth for efficient electrocatalytic detection of nitrite. Microchem. J. 2023, 194, 109302. [Google Scholar] [CrossRef]
- Deng, J.; Ren, X.; Yang, H.; Qiu, T.; Wang, Z.; Zhang, Y.; Miao, C.; Fontaine, O.; Zhu, Y.; Chen, S. Vacuum-solvent thermal synthesis of nickel foam-supported CuO-based sensor electrode for good electrochemical detection of nitrite. Microchem. J. 2024, 200, 110337. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Yang, S.; Shan, J. A novel electrochemical sensor based on TiO2–Ti3C2TX/CTAB/chitosan composite for the detection of nitrite. Electrochim. Acta 2020, 359, 136938. [Google Scholar] [CrossRef]
- Elfiky, M.; Salahuddin, N. Advanced sensing platform for nanomolar detection of food preservative nitrite in sugar byproducts based on 3D mesoporous nanorods of montmorillonite/TiO2–ZnO hybrids. Microchem. J. 2021, 170, 106582. [Google Scholar] [CrossRef]
- Raghu, G.K.; Ravishankar, T.N.; Ramakrishnappa, T.; Kumar, S.; Patri, S.B.; Supritha, K.M.; Pandurangappa, M. Designing of Au doped TiO2 nanoparticles as an electrocatalyst for nitrite sensor. Results Chem. 2023, 5, 100964. [Google Scholar] [CrossRef]
- Pak, J.S.; Jang, P.H.; Pak, K.M.; Yang, W.-C. Electrochemical detection of nitrite on PANI–TiO2/Pt nanocomposite–modified carbon paste electrodes using TOPSIS and Taguchi methods. ACS Omega 2024, 9, 30583–30593. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Li, Y.W.; Shan, Q.; Wu, W. Ni nanosheets evenly distributed on MoS2 for selective electrochemical detection of nitrite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126865. [Google Scholar] [CrossRef]
- Zou, H.L.; Qin, L.Y.; Luo, H.Q.; Li, B.L.; Li, N.B. High-valence Mo(VI) derived from in-situ oxidized MoS2 nanosheets enables enhanced electrochemical responses for nitrite measurements. Sens. Actuators B Chem. 2021, 337, 129812. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, H.; Wang, B.; Shi, F.; Yan, L.; Zeng, L.; Li, L.; He, S.; Sun, W. An electrochemical biosensor based on hemoglobin and FeS@MoS2–C nanocomposite for nitrite, hydrogen peroxide and bromate detection. Int. J. Electrochem. Sci. 2022, 17, 221023. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, Q.; Li, J.; Hong, C.; Zhao, Z.; Xu, H.; Hu, J. Synthesis and enhanced electrochemical properties of AuNPs@MoS2/rGO hybrid structures for highly sensitive nitrite detection. Microchem. J. 2022, 172, 106904. [Google Scholar] [CrossRef]
- Anindya, W.; Wahyuni, W.T.; Rafi, M.; Putra, B.R. Electrochemical sensor based on graphene oxide/PEDOT:PSS composite modified glassy carbon electrode for environmental nitrite detection. Int. J. Electrochem. Sci. 2023, 18, 100034. [Google Scholar] [CrossRef]
- Wang, L.; Fan, Z.; Yue, F.; Zhang, S.; Qin, S.; Luo, C.; Pang, L.; Zhao, J.; Du, J.; Jin, B.; et al. Flower-like 3D MoS2 microsphere/2D C3N4 nanosheet composite for highly sensitive electrochemical sensing of nitrite. Food Chem. 2024, 430, 137027. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Zuo, J.; Lan, J.; Jiang, Z.; Xiao, C.; Wang, X.; Zuo, Y. A novel electrochemical sensor with NiSx@MoS2 composite for efficient NO2− sensing. Food Chem. 2025, 462, 140947. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Alam, M.M.; Ahmed, J.; Asiri, A.M.; Algethami, J.S.; Alkorbi, A.S.; Madkhali, O.; Aljabri, M.D.; Rahman, M.M.; Harraz, F.A. Electrochemical detection of nitrite (NO2) with PEDOT:PSS modified gold/PPy–C/carbon nitride nanocomposites by electrochemical approach. J. Ind. Eng. Chem. 2023, 121, 519–528. [Google Scholar] [CrossRef]
- Wahyuni, W.T.; Rahman, H.A.; Afifah, S.; Anindya, W.; Hidayat, R.A.; Khalil, M.; Fan, B.; Putra, B.R. Comparison of the analytical performance of two different electrochemical sensors based on a composite of gold nanorods with carbon nanomaterials and PEDOT:PSS for the sensitive detection of nitrite in processed meat products. RSC Adv. 2024, 14, 24856–24873. [Google Scholar] [CrossRef] [PubMed]
- Abbas, W.; Zafar, F.; Taleb, M.F.A.; Ameen, M.; Sami, A.; Mazhar, M.E.; Akhtar, N.; Fazal, M.W.; Ibrahim, M.M.; El-Bahy, Z.M. Machine learning trained poly(3,4-ethylenedioxythiophene) functionalized carbon matrix suspended Cu nanoparticles for precise monitoring of nitrite from pickled vegetables. Food Chem. 2024, 460, 140395. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Chen, Y.; Wang, D.; Liu, Q.; Zhong, J.; Zhang, Z.; Lü, D. The novel nanozyme-based electrochemical-driven electrochromic visual biosensor based on PEDOT:PSS/RGO conductive film for rapid detection of nitrite in food samples. Food Chem. 2025, 481, 143971. [Google Scholar] [CrossRef] [PubMed]
- Kaladevi, G.; Meenakshi, S.; Wilson, P.; Pandian, K.; Gopinath, S.C.B. Interfacial polymerization to synthesize AuNPs@PPy/rGO nanocomposites for the simultaneous voltammetric determination of hydrazine and nitrite in water samples. Microchem. J. 2025, 214, 113955. [Google Scholar] [CrossRef]
- Zhang, W.; Ge, C.-Y.; Jin, L.; Yoon, S.; Kim, W.; Xu, G.-R.; Jang, H. Nickel nanoparticles incorporated Co, N co-doped carbon polyhedron derived from core–shell ZIF-8@ZIF-67 for electrochemical sensing of nitrite. J. Electroanal. Chem. 2021, 887, 115163. [Google Scholar] [CrossRef]
- Lu, S.; Jia, H.; Hummel, M.; Wu, Y.; Wang, K.; Qi, X.; Gu, Z. Two-dimensional conductive phthalocyanine-based metal–organic frameworks for electrochemical nitrite sensing. RSC Adv. 2021, 11, 4472–4477. [Google Scholar] [CrossRef]
- Yang, N.; Zhou, X.; Qi, X.; Li, J.; Fang, W.; Xue, H.; Yang, Z. A nitrite sensor based on bimetallic zeolitic imidazole framework derived Co/porous carbon nanorods. Microchem. J. 2022, 182, 107910. [Google Scholar] [CrossRef]
- Feng, L.; Zou, M.; Lv, X.; Min, X.; Lin, X.; Ni, Y. Facile synthesis of ZIF-67C@RGO/NiNPs nanocomposite for electrochemical non-enzymatic sensing platform of nitrite. Microchem. J. 2022, 179, 107508. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, X.; Yin, Y.; Xue, H.; Fang, W. Metal-organic framework derived rod-like Co@carbon for electrochemical detection of nitrite. J. Alloys Compd. 2022, 911, 164915. [Google Scholar] [CrossRef]
- Saeb, E.; Asadpour-Zeynali, K. A novel ZIF-8@ZIF-67/Au core–shell metal–organic framework nanocomposite as a highly sensitive electrochemical sensor for nitrite determination. Electrochim. Acta 2022, 417, 140278. [Google Scholar] [CrossRef]
- Ambaye, A.D.; Muchindu, M.; Jijana, A.; Mishra, S.; Nxumalo, E. Screen-printed electrode system based on carbon black/copper–organic framework hybrid nanocomposites for the electrochemical detection of nitrite. Mater. Today Commun. 2023, 35, 105567. [Google Scholar] [CrossRef]
- Zhe, T.; Shen, S.; Li, F.; Li, R.; Li, M.; Ma, K.; Xu, K.; Jia, P.; Wang, L. Bimetallic–MOF-derived crystalline–amorphous interfacial sites for highly efficient nitrite sensing. Food Chem. 2023, 402, 134228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, Y.; Sun, M.; Li, S. Non-enzymatic electrochemical sensor based on ionic liquid [BMIM][PF6] functionalized zirconium–copper bimetallic MOF composite for the detection of nitrite in food samples. Food Chem. 2024, 456, 140023. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, T.; Gopalram, K.; Nagashree, K.L.; Venkatesan, S. Electrochemical sensing of nitrite by Cu and Zn based metal–organic frameworks—A green synthesis approach. J. Mol. Struct. 2025, 1343, 142801. [Google Scholar] [CrossRef]
- Arul, C.; Veerapandi, G.; Sekar, C. Selective and simultaneous electrochemical detection of nitrite and nitrate ions using Ag–MOF: Food and water analyses. Food Chem. 2025, 484, 144457. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Kong, L.-X.; Guo, X.; Lin, X.; Li, Y.; Jiang, J.; Pang, H. Construction of copper oxide/carbon nanocomposites derived from Cu–MOF and their application in electrochemical nitrite sensors. Mater. Today Chem. 2025, 45, 102707. [Google Scholar] [CrossRef]
- Amali, R.K.A.; Lim, H.N.; Ibrahim, I.; Zainal, Z.; Ahmad, S.A.A. Energy-efficient green synthesis of metal-organic frameworks for effective electrochemical nitrite sensing. Appl. Mater. Today 2023, 33, 101871. [Google Scholar] [CrossRef]
- Yang, Z.; Gong, Z.; Fan, M.; Zhang, W.; Xue, H.; Fang, W. Trimetallic zeolitic imidazolate framework-derived hollow structure as a sensing material for nitrite electrochemical detection. Colloids Surf. A 2023, 678, 132513. [Google Scholar] [CrossRef]
- Shi, X.; Pan, Y.; Liu, C. Polysaccharides derived from Ganoderma lucidum applied to the extraction chitosan and development of electrochemical sensor based on extracted chitosan and graphitic carbon nitride modified electrode for determination nitrite in food samples. Int. J. Electrochem. Sci. 2024, 19, 100446. [Google Scholar] [CrossRef]
- Adiraju, A.; Jalasutram, A.; Al-Hamry, A.; Talbi, M.; Wang, J.; Tegenkamp, C.; Kanoun, O. Laser-induced fibers and copper phthalocyanine modified laser-induced graphene electrodes for sensitive and selective electrochemical detection of nitrite. RSC Adv. 2024, 14, 28648–28658. [Google Scholar] [CrossRef]
- Pandiyarajan, C.; Rameshkumar, P.; SaravanaVadivu, A.; Hatshan, M.R.; Puthiaraj, P.; Shanthi, C.; Murugesan, S. Gold nanoparticles distributed graphitic carbon nitride embedded with silicate sol-gel for improved electrochemical sensing of nitrite ions. Inorg. Chem. Commun. 2025, 179, 114816. [Google Scholar] [CrossRef]
- Nasraoui, S.; Al-Hamry, A.; Teixeira, P.R.; Ameur, S.; Paterno, L.G.; Ben Ali, M.; Kanoun, O. Electrochemical sensor for nitrite detection in water samples using flexible laser-induced graphene electrodes functionalized by CNT decorated by Au nanoparticles. J. Electroanal. Chem. 2021, 880, 114893. [Google Scholar] [CrossRef]
- Karimi-Takallo, A.; Dianat, S.; Hatefi-Mehrjardi, A. Fabrication and electrochemical study of K(1,1′-(1,4 Butanediyl)dipyridinium)2[PW11O39Co(H2O)]/MWCNTs-COOH nanohybrid immobilized on glassy carbon for electrocatalytic detection of nitrite. J. Electroanal. Chem. 2021, 886, 115139. [Google Scholar] [CrossRef]
- Paramasivam, S.S.; Mariappan, S.A.; Sethy, N.K.; Manickam, P. Enzyme mimetic electrochemical sensor for salivary nitrite detection using copper chlorophyllin and carbon nanotubes-functionalized screen printed electrodes. Mater. Adv. 2023, 4, 6223–6232. [Google Scholar] [CrossRef]
- Han, E.; Li, L.; Gao, T.; Pan, Y.; Cai, J. Nitrite determination in food using electrochemical sensor based on self-assembled MWCNTs/AuNPs/poly-melamine nanocomposite. Food Chem. 2024, 437, 137773. [Google Scholar] [CrossRef] [PubMed]
- AIT Ramdane, K.; AIT Ramdane-Terbouche, C.; Terbouche, A.; Lakhdari, H.; Mezaoui, D.; Hauchard, D. Innovative electrochemical sensor based on platinum group metal complexes for determination of nitrite: Application for simultaneous detection of nitrite and tartrazine. J. Environ. Chem. Eng. 2024, 12, 114653. [Google Scholar] [CrossRef]
- Shahzad, U.; Marwani, H.M.; Rabbee, M.F.; Alfaifi, S.Y.; Alzahrani, K.A.; Khan, M.M.R.; Rahman, M. Efficient sensitive detection of nitrite with Binary Y/Fe-modified multiwalled carbon nanotube nanocomposite by electrochemical approaches. Mater. Chem. Phys. 2024, 328, 130000. [Google Scholar] [CrossRef]
- Kusonpan, P.; Kunpatee, K.; Chailapakul, O.; Kalcher, K.; Ortner, A.; Chaiyo, S.; Samphao, A. A simple manually rotated paper-based analytical device with electrochemical sensors for the determination of nitrite and nitrate. Talanta 2025, 292, 127919. [Google Scholar] [CrossRef]
- Wahyuni, W.T.; Rahman, H.A.; Hidayat, R.A.; Aris, A.; Khalil, M.; Takai-Yamashita, C.; Putra, B.R. Electrochemical sensors based on gold nanoparticles and nanocomposite of MWCNT-OH/graphene for detecting hydrazine and nitrite simultaneously. Next Mater. 2025, 8, 100677. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Zhou, J.; Wang, L. Construction of a highly sensitive non-enzymatic nitrite sensor using electrochemically reduced holey graphene. Anal. Chim. Acta 2018, 1043, 28–34. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, J. Non-enzymatic electrochemical sensor for nitrite based on a graphene oxide–polyaniline–Au nanoparticles nanocomposite. Microchem. J. 2021, 164, 106034. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, J.; Hu, C. Laser-scribed graphene sensors on nail polish with tunable composition for electrochemical detection of nitrite and glucose. Sens. Actuators B Chem. 2022, 357, 131394. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, J.; Ling, Y.; Yu, S.; Li, S.; Wu, X.; Zhang, Z. A facile and efficient nitrite electrochemical sensor based on N, O co-doped porous graphene film. Microchem. J. 2022, 178, 107361. [Google Scholar] [CrossRef]
- Paisanpisuttisin, A.; Poonwattanapong, P.; Rakthabut, P.; Ariyasantichai, P.; Prasittichai, C.; Siriwatcharapiboon, W. Sensitive electrochemical sensor based on nickel/PDDA/reduced graphene oxide modified screen-printed carbon electrode for nitrite detection. RSC Adv. 2022, 12, 29491–29502. [Google Scholar] [CrossRef]
- Qin, Y.; He, K.; Chen, B.; Ji, Z.; Wang, J. Ultrathin 2D/2D nanohybrid for efficient electrochemical detection of nitrite. Appl. Surf. Sci. 2023, 636, 157776. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, Y.; Chen, H.; Zhang, Y.; Chen, X.; Lu, M. Reduced graphene oxide-wrapped La0.8Sr0.2MnO3 microspheres sensing electrode for highly sensitive nitrite detection. Talanta 2023, 260, 124644. [Google Scholar] [CrossRef]
- Liu, Z.; Shan, X.; Xue, Q.; Liu, Y.; He, L.; Xie, H. Efficient detection of nitrite in water based on an Au/NiO/Rh trimetallic composite modified laser-induced graphene electrode prepared by one-step electrodeposition. Chem. Eng. J. 2023, 473, 145486. [Google Scholar] [CrossRef]
- Wang, J.; Shan, X.; Xue, Q.; Liu, Y.; Liu, Z.; He, L.; Wang, X.; Zhu, C. Detection of nitrite in water using Glycine-modified nanocarbon and Au nanoparticles co-modified flexible laser-induced graphene electrode. Inorg. Chem. Commun. 2023, 152, 110652. [Google Scholar] [CrossRef]
- Wang, R.; Cai, Z.; Zhu, H.; Wang, L.; Tan, Y.; Zhu, Z.; He, H.; He, Y.; Chang, G. In2O3 electrochemical transistors based on PtAu4/RGO nanocomposites functionalized gate for highly sensitive nitrite detection. J. Electroanal. Chem. 2024, 971, 118572. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Selvaraj, S.C.; Noh, J.-H.; Ko, T.H.; Kim, B.-S. A portable highly uniform and reproducible microflower CuS/rGO hybrid sensor: An effective electrochemical and DFT evaluation method for nitrite in water. J. Environ. Chem. Eng. 2023, 11, 110057. [Google Scholar] [CrossRef]
- Guo, X.; Fan, Y. Determination of nitrite in food specimens using electrochemical sensor based on polyneutral red modified reduced graphene oxide paste electrode. Int. J. Electrochem. Sci. 2023, 18, 100290. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Sun, Y.; Yang, Z.; Liu, J. Fabrication non-enzymatic electrochemical sensor based on methyl red and graphene oxide nanocomposite modified carbon paste electrode for determination of nitrite in food samples. Int. J. Electrochem. Sci. 2023, 18, 100097. [Google Scholar] [CrossRef]
- Yu, R.; Chen, M.; Li, X.; Liu, J.; Cheng, Z.; Zhong, H.; Qian, H. An ultrasensitive electrochemical sensor based on boron-doped porous graphene for efficient detection of nitrite in food. J. Food Compos. Anal. 2024, 132, 106314. [Google Scholar] [CrossRef]
- Daldal, P.A.; Doğan, H.Ö.; Topcu, K.C. Sensitive and selective nitrite sensor based on CuCo2O4-rGO nanocomposite synthesized by one-pot electrochemical method in pastırma. Diam. Relat. Mater. 2025, 157, 112550. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Qiao, J.; Dong, S.; Liang, Q.; Shao, S. Ultrasensitive determination of nitrite based on electrochemical platform of AuNPs deposited on PDDA-modified MXene nanosheets. Talanta 2021, 221, 121605. [Google Scholar] [CrossRef] [PubMed]
- Ezhil Vilian, A.T.; Umapathi, R.; Hwang, S.-K.; Huh, Y.S.; Han, Y.-K. Pd–Cu nanospheres supported on Mo2C for the electrochemical sensing of nitrites. J. Hazard. Mater. 2021, 408, 124914. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Waterhouse, G.I.N.; Qiao, X.; Sun, Y.; Xu, Z. Sensitive analytical detection of nitrite using an electrochemical sensor with STAB-functionalized Nb2C@MWCNTs for signal amplification. Food Chem. 2022, 372, 131356. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Han, G.; Cai, J.; Wang, X. Au@Carbon quantum Dots-MXene nanocomposite as an electrochemical sensor for sensitive detection of nitrite. J. Colloid Interface Sci. 2022, 607, 1313–1322. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.-J.; Ahmed, F.; Kumar, S. Deep eutectic solvents-assisted synthesis of NiFe-LDH/Mo2C nanocomposite for electrochemical determination of nitrite. J. Mol. Liq. 2023, 369, 120785. [Google Scholar] [CrossRef]
- Habibi, B.; Makani, S.; Mohammad-Rezaei, R. Co/Zn ZIFs with MXene and MWCNTs nanocomposites for electro-detection of nitrite ions: Effect of composite substrate. Res. Chem. 2025, 15, 102215. [Google Scholar] [CrossRef]






| Electrode Material | LOD (µM) | Linear Range (µM) | Sensitivity | Sensing Method | References |
|---|---|---|---|---|---|
| Ag-Cu@ZnO | 17 | 0 to 1500 | - | LSV | [41] |
| rGO/ZnO/GCE | 1.18 | 200 to 4000 | 0.3156 μA μM−1 cm−2 | LSV | [42] |
| rGO/ZnO/GCE | 1.36 | 20 to 520 | 0.2754 μA μM−1 cm−2 | Amperometry | [42] |
| ZnO/Nafion/GCE | 0.62 | 0.8 to 4860 | 0.392 μA μM−1 cm−2 | LSV | [43] |
| ZnO/Nafion/GCE | 0.21 | 0.3 to 6140 | 0.524 μA μM−1 cm−2 | Amperometry | [43] |
| Sb-ZnO/N-rGO/GCE | 0.43 | 0.4 to 900 and 1000 to 5000 | 397.6 μA mM−1 cm−2 | LSV | [44] |
| Sb-ZnO/N-rGO/GCE | 0.13 | 0.2 to 1200 and 1500 to 7800 | 294.4 μA mM−1 cm−2 | Chronoamperometry | [44] |
| Near-spherical ZnO/GCE | 0.89 | 1.9 to 800 and 1080 to 5900 | 0.646 μA μM−1 cm−2 | LSV | [45] |
| Near-spherical ZnO/GCE | 0.39 | 0.6 to 220 and 460 to 5500 | 0.785 μA μM−1 cm−2 | Chronoamperometry | [45] |
| PPy/TiO2/ZnO/GCE | 0.14 | 1 to 20 | 70.3234 μA μM−1 cm−2 | LSV | [46] |
| PA-TaCoPc@ZnO | 0.021 | 1 to 10 | 6.3575 μA μM−1 cm−2 | Chronoamperometry | [47] |
| ns-ZnO/N-rGO/GCE | 0.29 | 0.1 to 4600 | 405.8 μA mM−1 cm−2 | LSV | [48] |
| ns-ZnO/N-rGO/GCE | 0.08 | 0.037 to 5900 | 301.9 μA mM−1 cm−2 | Chronoamperometry | [48] |
| Ms−Au/ZnO@Pt−CC | 0.09 | 0.2 to 4986 | 5677 μA mM−1 cm−2 | Amperometry | [49] |
| cl-ZnO-nafion/GCE | 0.32 | 0.00095 to 0.515 and 0.667 to 5.41 | 1336.1 µA mM−1 cm−2 | LSV | [50] |
| cl-ZnO-nafion/GCE | 0.26 | 0.0008 to 0.462 and 0.608 to 7.84 | 824.6 µA mM−1 cm−2 | Chronoamperometry | [50] |
| Electrode Material | LOD (µM) | Linear Range (µM) | Sensitivity | Sensing Method | References |
|---|---|---|---|---|---|
| ZnO-nf-nafion/GCE | 0.28 | 0.0008 to 4.81 | 345.5 µA mM−1 cm−2 | LSV | [51] |
| ZnO-nf-nafion/GCE | 0.11 | 0.0002 to 0.453 and 0.57 to 8.5 | 763.4 µA mM−1 cm−2 | Chronoamperometry | [51] |
| L-Arginine/Co3O4/FTO | 0.00195 | 10 to 16,000 | 158 μA mM−1 | Amperometry | [52] |
| MoO3/Co3O4/CC | 0.075 | - | 1704.1 μA mM−1 cm−2 | Amperometry | [53] |
| Sn-CeO2 | 0.016 | 10 to 6000 | 245.4 and 89.53 µA mM−1 | Chronoamperometry | [55] |
| CB/NiCo2S4@CeO2/GCE | 0.003 | 0.2 to 7400 | 470 µA mM−1 cm−2 | Amperometry | [56] |
| Cu@CeO2−rGO | 0.0101 | 10 to 2000 | 1963.2 µA mM−1 cm−2 | Amperometry | [57] |
| CeO2 NSs/CuO NFs/CC | 0.0347 | 0.1 to 4000 | 11,610 µA mM−1 cm−2 | Amperometry | [58] |
| Fe3O4@Au@Cys/rGO/GCE | 0.008 | 0.03 to 2215 | - | DPV | [59] |
| NiO/Fe2O3/GCE | 0.05 | 5 to 500 | - | DPV/Amperometry | [60] |
| CSs@Fe2O3/Fe3C/Fe | 0.06 | 1 to 2540 | 451.85 µA mM−1 cm−2 | Amperometry | [61] |
| Fe3O4@SiO2(acid-treated)/GCE | 3.33 | 10 to 1000 | - | DPV | [62] |
| Fe3O4@Au/MOF-(P2W17V)6/GCE | 0.532 | 0.01 to 100 | 11.682 µA µM−1 cm−2 | CV | [63] |
| GO@Fe2O3/Y2O3 NCs/Nafion/GCE | 2250 | 0.74 M to 1.09 M | 73.83966 µA mM−1 cm−2 | LSV | [64] |
| Electrode Material | LOD (µM) | Linear Range (µM) | Sensitivity | Sensing Method | References |
|---|---|---|---|---|---|
| CuO/NiO/FTO | 0.013 | 1 to 1800 | 7.2 mA mM−1 cm−2 | Amperometry | [65] |
| CuO NPs/CC | 0.043 | 0.5 to 3000 | 1656 μA mM−1 cm−2 | Amperometry | [66] |
| CuO@NF-1 | 28.7 | 1 to 4250 | 2.402 mA mM−1 cm−2 | Chronoamperometry | [67] |
| Ti3C2TX/CTAB/CS/GCE | 0.85 | 3 to 250 and 250 to 1250 | - | DPV | [68] |
| 1.0% [MTZ2] GPS | 0.00012 | 0.0004 to 0.01 | - | SWV | [69] |
| Au-doped TiO2 NPs | 0.095 | 3.3 to 120 | - | SWV | [70] |
| Ni/MoS2/GCE | 2.74 | 20 to 1000 | 0.01509 μA μM−1 | DPV | [72] |
| Oxidized MoS2 Nanosheets | 0.028 | 1 to 386 | - | Chronoamperometry | [73] |
| Au4.5NPs@MoS2 /rGO/GCE | 0.804 | 0.2 to 2600 and 2600 to 16,000 | 0.805 and 0.468 μA μM−1 cm−2 | Amperometry | [75] |
| GO/PEDOT:PSS/GCE | 0.5 | 1 to 200 | - | DPV | [76] |
| 3D MoS2/2D C3N4/GCE | 0.065 | 0.1 to 1100 | - | DPV | [77] |
| Electrode Material | LOD (µM) | Linear Range (µM) | Sensitivity | Sensing Method | References |
|---|---|---|---|---|---|
| Au-PPy-C/g-C3N4 NCs/GCE | 1.11 | 1.5 to 22.5 | 91.19 μA μM−1 cm−2 | DPV | [79] |
| AuNRs/ErGO/PEDOT:PSS/GCE | 0.2 | 0.8 to 100 | 0.0451 μA μM−1 | DPV | [80] |
| AuNRs/ErGO/PEDOT:PSS/GCE | 0.08 | 0.2 to 100 | 0.0634 μA μM−1 | DPV | [80] |
| PEDOT-C@Cu-NPs | 3.91 | 5 to 580 | 0.6372 μA μM−1 cm−2 | Amperometry | [81] |
| AuNPs@PPy/rGO/GCE | 0.0165 | 0.005 to 82 | - | DPV | [83] |
| Ni/Co,N-CP/GCE | 0.094 | 1 to 500 | - | Chronoamperometry | [84] |
| CoN-PCRs-0.6 | 0.14 | 0.2 to 4000 and 4000 to 10,000 | - | Amperometry | [86] |
| ZIF-67C@RGO/NiNPs/GCE | 0.086 | 0.2 to 123 and 123 to 473 | - | Amperometry | [87] |
| CoN-CRs/MGCE | 0.17 | 0.5 to 4000 and 4000 to 8000 | 1.03 and 0.82 μA μM−1 cm−2 | Amperometry | [88] |
| CB/Cu-MOF/SPCE | 0.084 | 1 to 200 | - | LSV | [90] |
| C-A Zn/Co-Fe PNSs@CC | 0.44 | - | - | Amperometry | [91] |
| ZrCu-MOF-818/ILs | 0.148 | 6 to 5030 | - | DPV | [92] |
| Cu-MOF | 16.39 | 0.05 to 1000 | 2.91 μA μM−1 cm−2 | Chronoamperometry | [93] |
| ZIF-8 | 24.48 | 0.05 to 1000 | 1.56 μA μM−1 cm−2 | Chronoamperometry | [93] |
| Ag-MOF/GCE | 0.045 | 4 to 4040 | - | SWV | [94] |
| Ag-MOF/GCE | 23 | 5 to 5900 | - | CV | [94] |
| Ni-PDCA/SPCE | 0.052 | 0.1 to 1000 | 240 µA mM−1 cm−2 | DPV/Amperometry | [96] |
| H-CoMnN-PCs | 0.097 | 0.1 to 1500 and 1500 to 12,000 | 1.2644 and 0.9073 μA μM−1 cm−2 | Amperometry | [97] |
| Electrode Material | LOD (µM) | Linear Range (µM) | Sensitivity | Sensing Method | References |
|---|---|---|---|---|---|
| Chitosan/g-C3N4/GCE | 0.021 | 40 to 2000 | - | DPV | [98] |
| Chitosan/g-C3N4/GCE | 0.010 | 20 to 4230 | - | Amperometry | [98] |
| GCE/(EDAS/g-C3N4-Au)NCM | 0.6 | 10 to 375 | 0.0696 µA µM−1 cm−2 | Amperometry | [100] |
| LIG/f-MWCNT-AuNPs | 0.9 | 10 to 140 | - | SWV | [101] |
| 1,1′-(1,4-Butanediyl)dipyridinium (bdpy)PW11Co/MWCNTs-COOH/GCE | 0.63 | 10 to 1600 | 17.9 µA mM−1 | Amperometry | [102] |
| GCE/MWCNTs/AuNPs/PM. MWCNTs | 0.041 | 0.4 to 1475 | - | DPV/Amperometry | [104] |
| MEC/CG-MWCNT-Pd−2E-Peaemp | 0.39 | 2.48 to 909.12 | - | DPV | [105] |
| Y/Fe-MWCNT/GCE | 0.027 M | 1 to 1.66 M | 6.205 µA mM−1 cm−2 | LSV | [106] |
| CuPc-N-MWCNTs-SPCE | 10 | 50 to 1000 | - | DPV | [107] |
| AuNPs/MWCNT-OH/graphene/GCE | 3.64 | 40 to 1000 | - | DPV | [108] |
| ERHG/GCE | 0.054 | 0.2 to 10,000 | 0.311 µA µM−1 cm−2 | Amperometry | [109] |
| GO-PANI-AuNPs/GCE | 0.17 | 0.0005 to 0.24 and 0.24 to 2.58 | Amperometry | [110] | |
| Cu2+/NP-LIG | 0.9 | 2 to 1000 | - | DPV | [111] |
| hematene/GO | 2 | 2 to 1000 | - | CV/i-t | [114] |
| LSM/rGO | 0.016 | 2 to 100 and 100 to 5000 | 0.041 and 0.039 µA µM−1 cm−2 | Amperometry | [115] |
| Au/NiO/Rh/LIG | 0.3 | 1 to 1000 | 2292.99 µA mM−1 cm−2 | LSV | [116] |
| Au/NiO/LG | 0.98 | 2 to 850 | 2104.64 µA mM−1 cm−2 | LSV | [116] |
| Au/Rh/LIG | 1.06 | 3 to 800 | 1850.32 µA mM−1 cm−2 | LSV | [116] |
| rGO/CuS | 0.0022 | 5 to 8000 | 2002 µA mM−1 cm−2 | Amperometry | [119] |
| B-PG | 1.1 | 3 to 15,000 | - | Amperometry | [122] |
| CuCo2O4/rGO | 0.00082 | 10 to 4500 and 4500 to 10,300 | - | Amperometry | [123] |
| Electrode Material | LOD (µM) | Linear Range (µM) | Sensitivity | Sensing Method | References |
|---|---|---|---|---|---|
| AuNPs/Ti3C2Tx-PDDA | 0.059 | 0.1 to 2490 and 2490 to 13,500 | - | Amperometry | [124] |
| Pd-Cu-Mo2C/GCE | 0.00035 | 0.005 to 0.165 | - | [125] | |
| Nb2C@MWCNTs-STAB | 0.022 | 0.1 to 100 and 100 to 2000 | - | DPV | [126] |
| Au@CQDs-MXene/GC | 0.078 | 1 to 500 and 500 to 3200 | - | DPV | [127] |
| NiFe-LDH/Mo2C | 0.0017 | 0.005 to 49 and 49 to 260 | 3.60 µA µM−1 cm−2 | Amperometry | [128] |
| MXene-Co/Zn ZIFs/CCE | 1.6 | 2 to 500 | 1.80180 µA mM−1 cm−2 | Amperometry | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, M.; Ali, S.; Hamdy, K.; Danishuddin; Ahmad, K.; Gautam, R.K.S. Progress in Electrode Modifiers for Nitrite Electrochemical Sensing Applications. Biosensors 2025, 15, 783. https://doi.org/10.3390/bios15120783
Aslam M, Ali S, Hamdy K, Danishuddin, Ahmad K, Gautam RKS. Progress in Electrode Modifiers for Nitrite Electrochemical Sensing Applications. Biosensors. 2025; 15(12):783. https://doi.org/10.3390/bios15120783
Chicago/Turabian StyleAslam, Mohammad, Saood Ali, Khaled Hamdy, Danishuddin, Khursheed Ahmad, and Rohit Kumar Singh Gautam. 2025. "Progress in Electrode Modifiers for Nitrite Electrochemical Sensing Applications" Biosensors 15, no. 12: 783. https://doi.org/10.3390/bios15120783
APA StyleAslam, M., Ali, S., Hamdy, K., Danishuddin, Ahmad, K., & Gautam, R. K. S. (2025). Progress in Electrode Modifiers for Nitrite Electrochemical Sensing Applications. Biosensors, 15(12), 783. https://doi.org/10.3390/bios15120783






