The Development of Foodborne Pathogen Detection and Biosensor Design for Surface Plasmon Resonance Technology
Abstract
1. Introduction
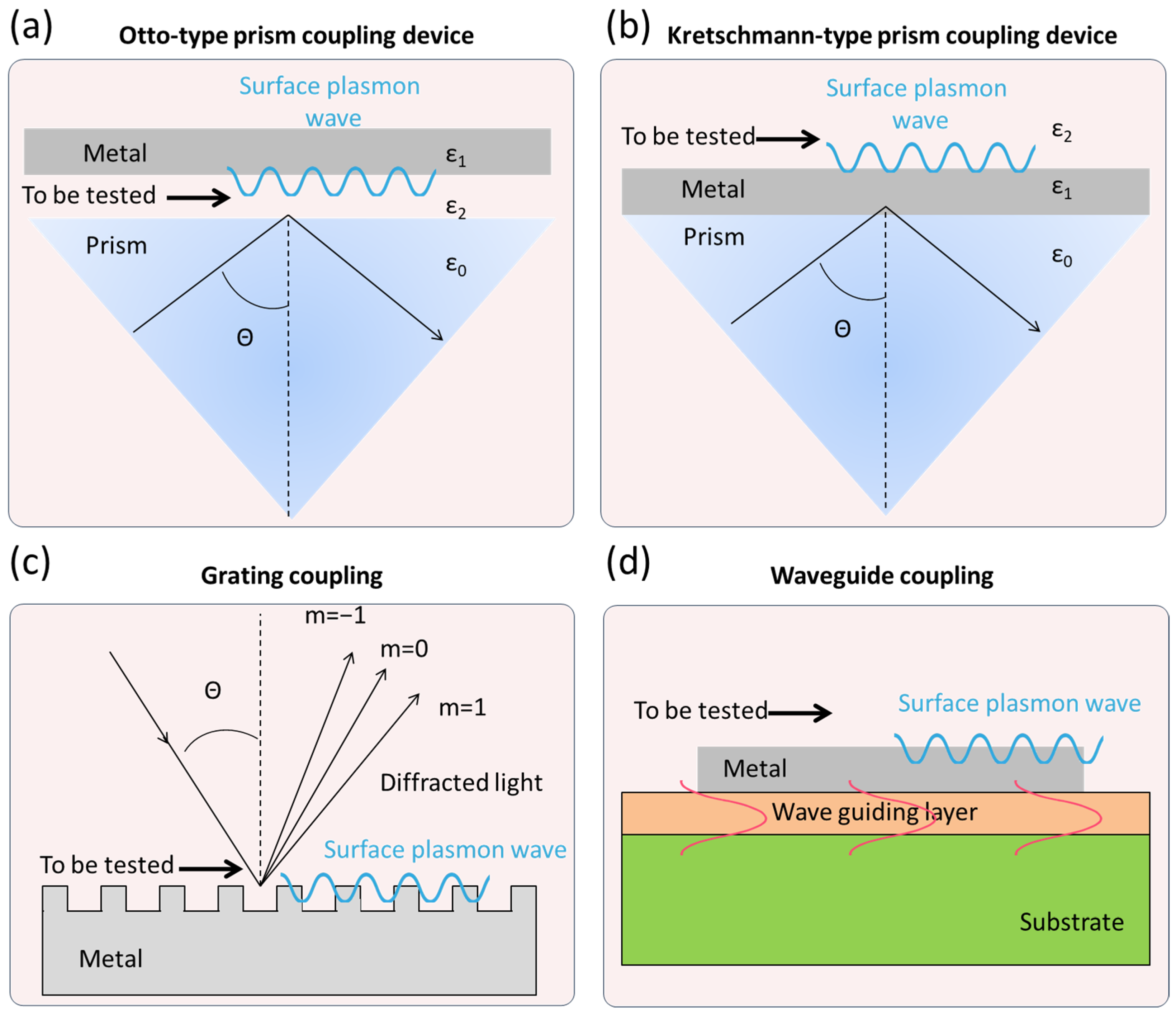
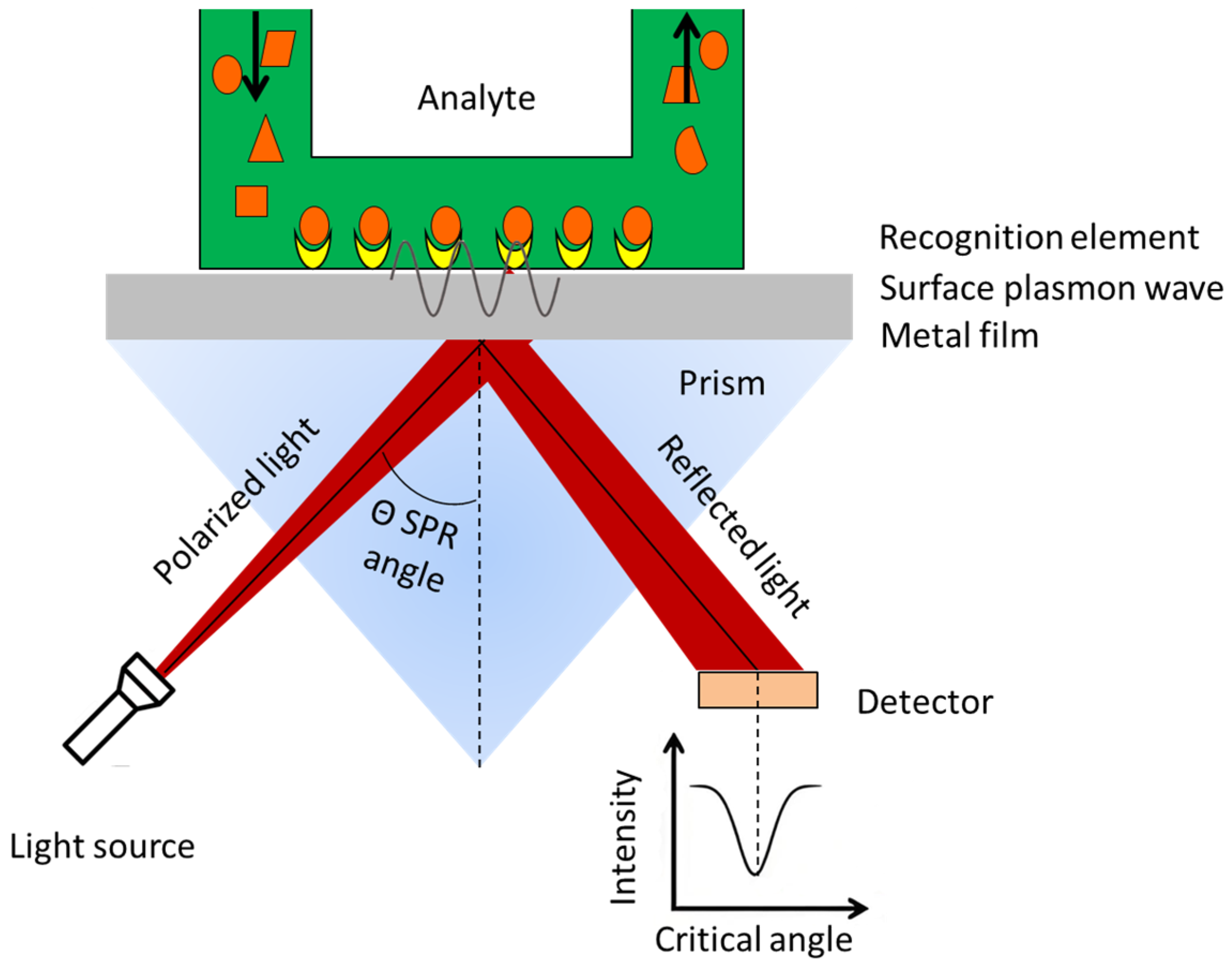
2. The Technical Fundamentals of SPR Technology
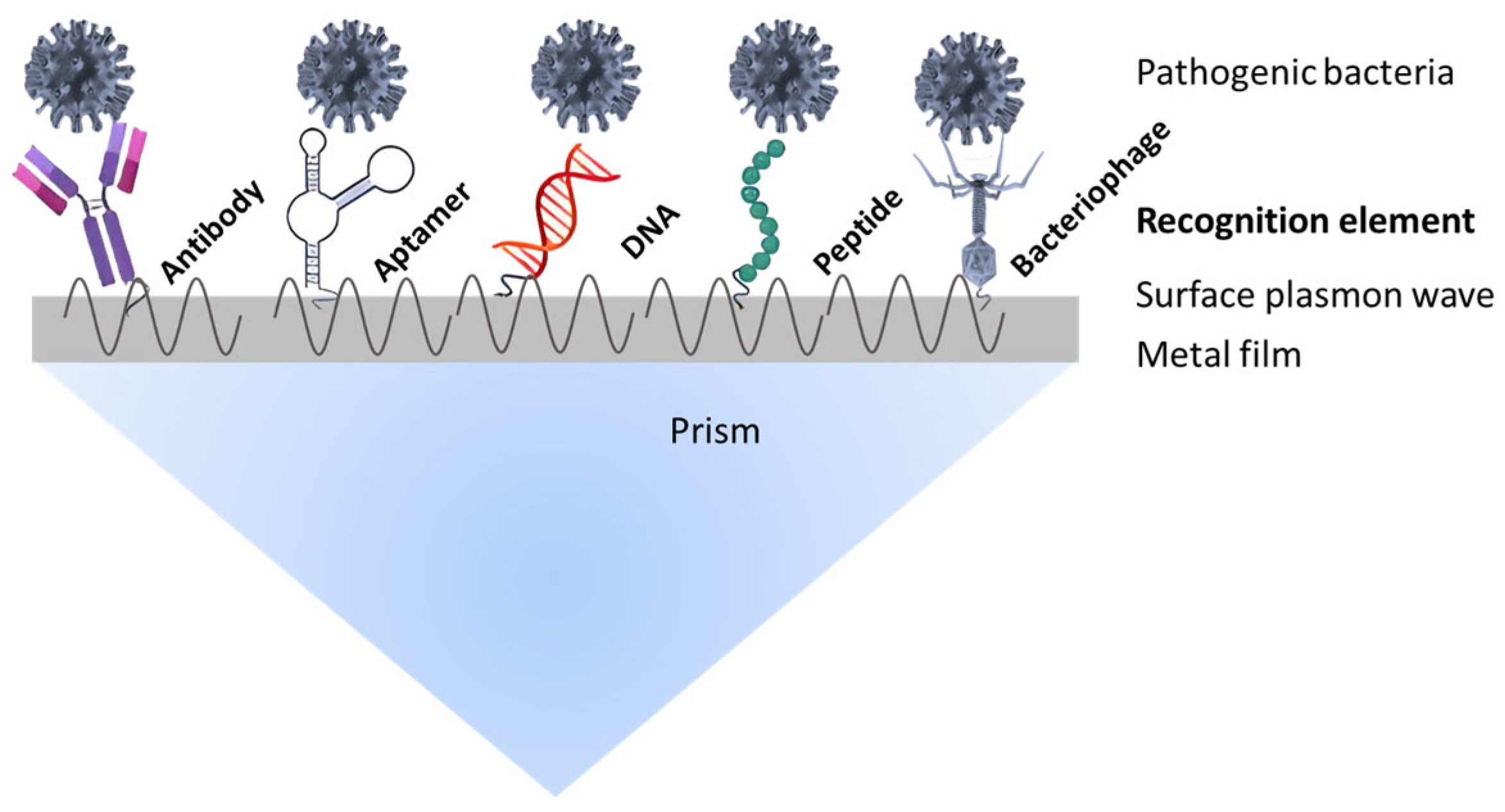
3. Classification of SPR Methods for Detecting Foodborne Pathogenic Bacteria
3.1. Direct Detection
3.1.1. Detection Using Antibodies
- Detection strategies based on nanomaterial enhancement
- Protein-based oriented immobilization and antibody conjugation strategies
- Surface modification strategies employing self-assembled monolayers (SAMs)
- Innovative SPR Sensing Strategies and Surface Functionalization Methods
- Comparative Evaluation of SPR Sensing Methodologies
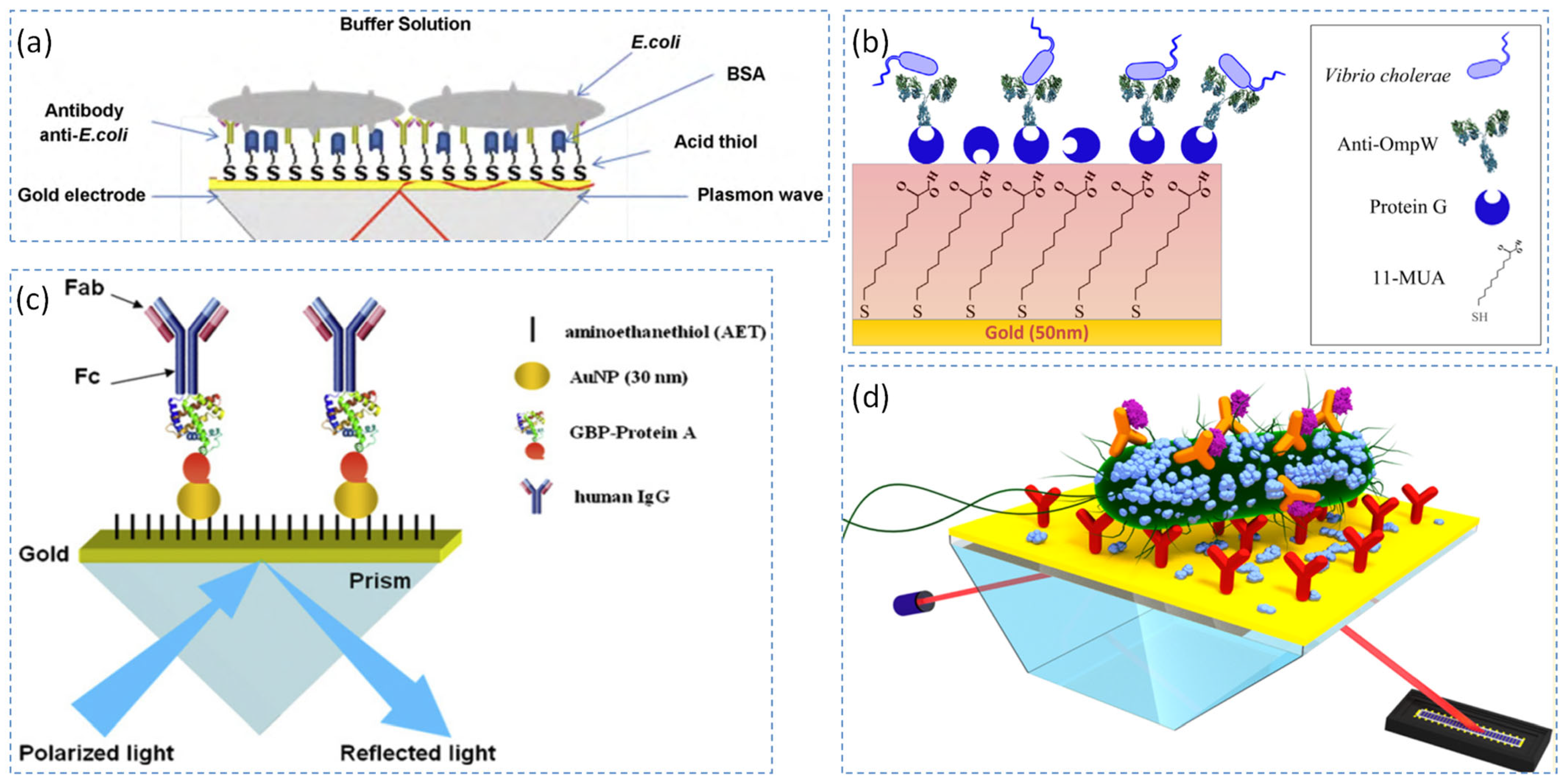
3.1.2. Detection Using Aptamers
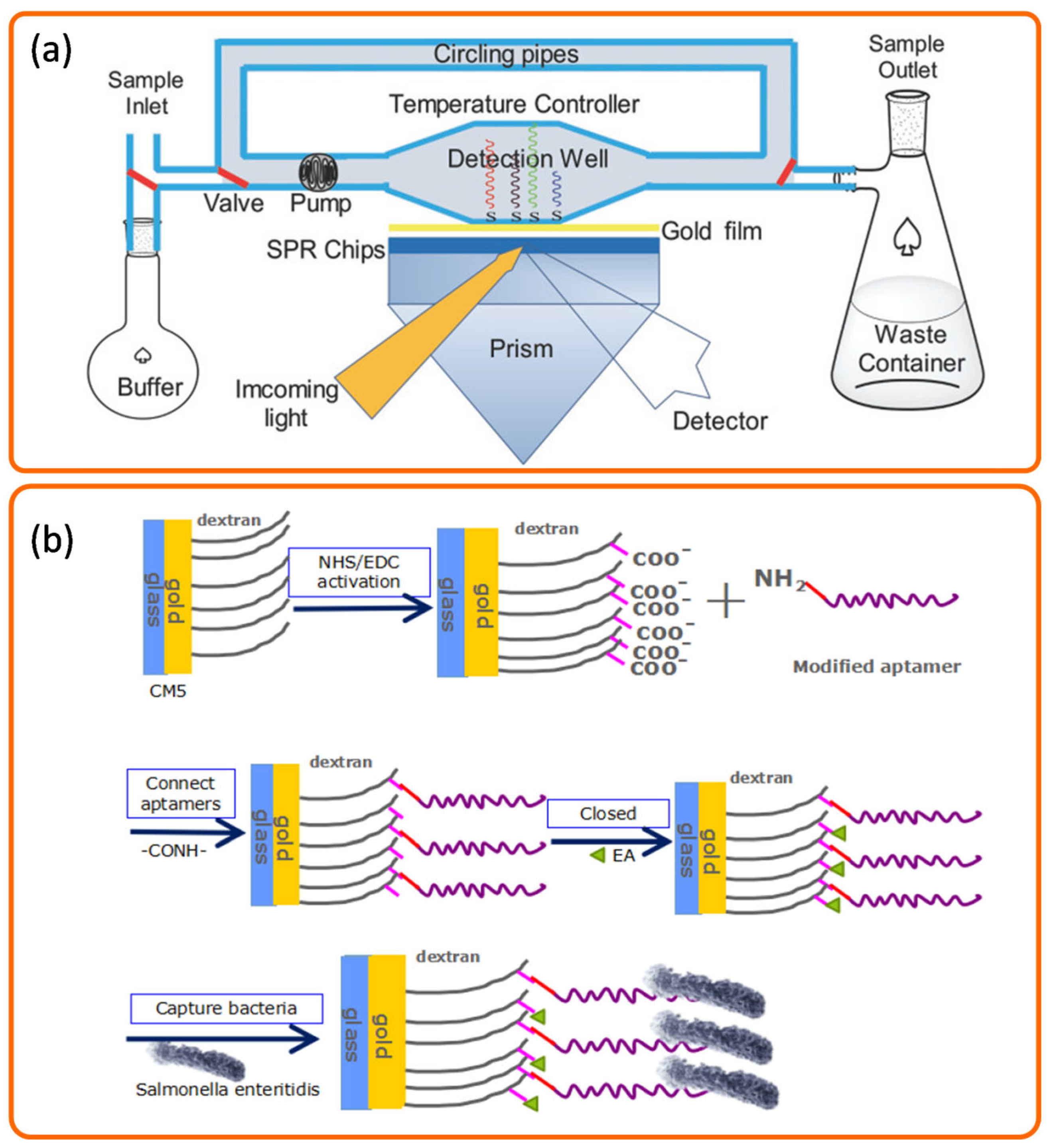
3.1.3. Detection Using Bacteriophage
3.2. Indirect Detection
Subtractive Inhibition Detection
4. Research Progress on SPR Technology in the Detection of Foodborne Pathogens
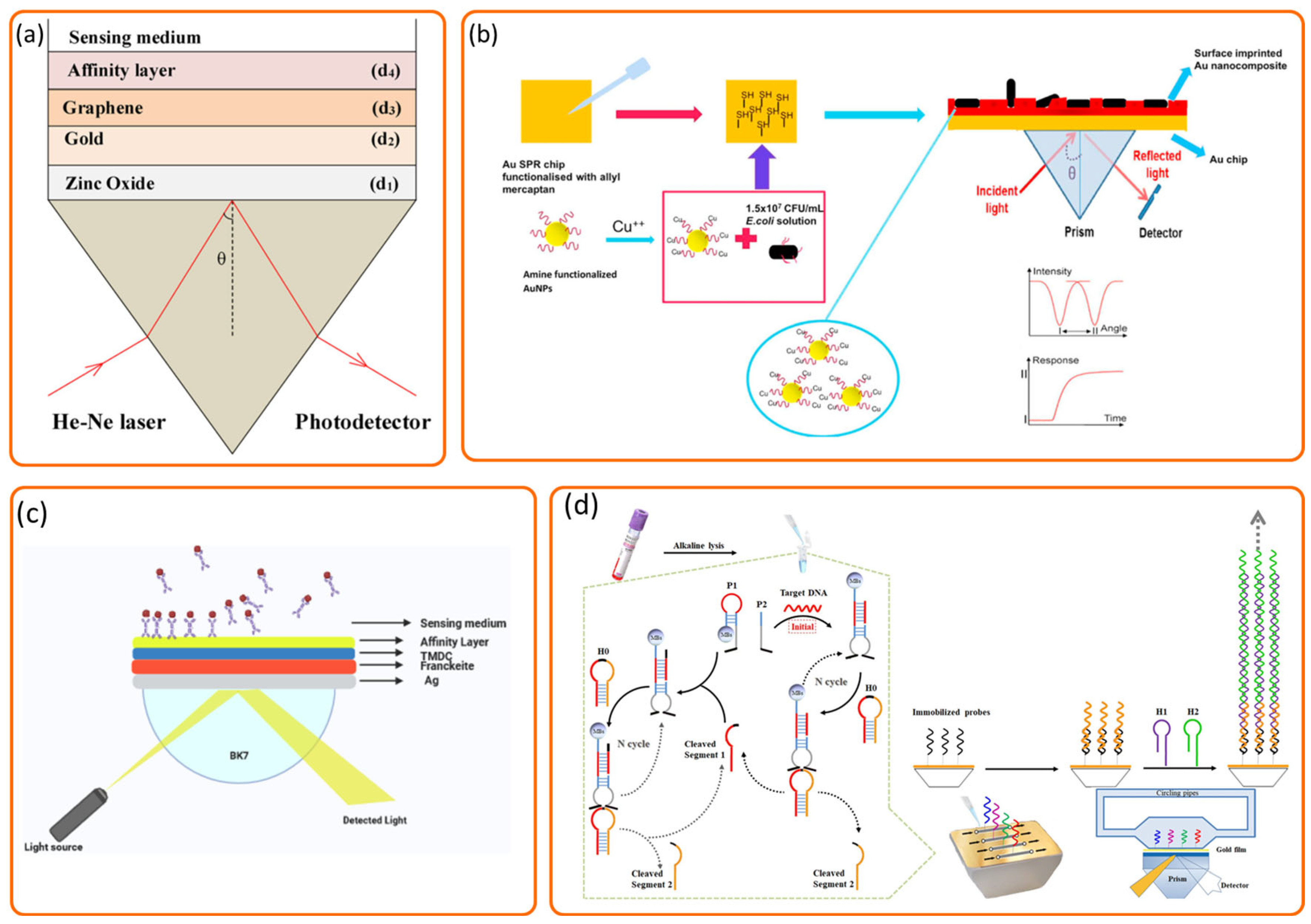
4.1. Nanomaterial Integration and Heterostructure Design for Enhanced SPR Performance
4.2. Surface Functionalization and Recognition Element Engineering for Enhanced Specificity and Affinity
4.3. Advanced Sensor Designs and Hybrid Technology Integration for Improved Efficiency and Utility
4.4. Novel SPR Methodologies for Sensitivity and Resolution Enhancement
5. Advantages and Disadvantages of SPR in Foodborne Pathogen Detection
5.1. Advantages of SPR in Foodborne Pathogen Detection
5.2. Disadvantages of SPR in Foodborne Pathogen Detection
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Qiu, M.; Zheng, M.; Zhang, J.; Yang, X.; Zhang, Y.; Zhang, W.; Man, C.; Zhao, Q.; Jiang, Y. Recent advances on emerging biosensing technologies and on-site analytical devices for detection of drug-resistant foodborne pathogens. TrAC Trends Anal. Chem. 2023, 167, 117258. [Google Scholar] [CrossRef]
- Hong, B.; Wang, W.; Li, Y.; Ma, Y.; Wang, J. Specific separation and sensitive detection of foodborne pathogens by phage-derived bacterial-binding protein-nano magnetic beads coupled with smartphone-assisted paper sensor. Biosens. Bioelectron. 2024, 247, 115911. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, A.; Pinto, V.F. Prevalence of mycotoxins in foods and decontamination. Curr. Opin. Food Sci. 2017, 14, 50–60. [Google Scholar] [CrossRef]
- Veenuttranon, K.; Lu, X.; Geng, N.; Chen, J. Recent trends in the multi-target electrochemical rapid detection of co-contaminated mycotoxins in foodstuffs. TrAC Trends Anal. Chem. 2025, 183, 118108. [Google Scholar] [CrossRef]
- Yeo, C.I.; Goh, C.H.P.; Tiekink, E.R.T.; Chew, J. Antibiotics: A “GOLDen” promise? Coord. Chem. Rev. 2024, 500, 215429. [Google Scholar] [CrossRef]
- Lake, F.B.; van Overbeek, L.S.; Baars, J.J.P.; Abee, T.; den Besten, H.M.W. Variability in growth and biofilm formation of Listeria monocytogenes in Agaricus bisporus mushroom products. Food Res. Int. 2023, 165, 112488. [Google Scholar] [CrossRef]
- Barba, F.J.; Koubaa, M.; do Prado-Silva, L.; Orlien, V.; Sant’Ana, A.D.S. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: A review. Trends Food Sci. Technol. 2017, 66, 20–35. [Google Scholar] [CrossRef]
- Bai, Z.; Xu, X.; Wang, C.; Wang, T.; Sun, C.; Liu, S.; Li, D. A comprehensive review of detection methods for Escherichia coli O157:H7. TrAC Trends Anal. Chem. 2022, 152, 116646. [Google Scholar] [CrossRef]
- Islam, N.; Nagy, A.; Garrett, W.M.; Shelton, D.; Cooper, B.; Nou, X.; Dudley, E.G. Different Cellular Origins and Functions of Extracellular Proteins from Escherichia coli O157:H7 and O104:H4 as Determined by Comparative Proteomic Analysis. Appl. Environ. Microbiol. 2016, 82, 4371–4378. [Google Scholar] [CrossRef]
- Punchihewage-Don, A.J.; Hawkins, J.; Adnan, A.M.; Hashem, F.; Parveen, S. The outbreaks and prevalence of antimicrobial resistant Salmonella in poultry in the United States: An overview. Heliyon 2022, 8, e11571. [Google Scholar] [CrossRef] [PubMed]
- Ravindhiran, R.; Sivarajan, K.; Sekar, J.N.; Murugesan, R.; Dhandapani, K. Listeria monocytogenes an Emerging Pathogen: A Comprehensive Overview on Listeriosis, Virulence Determinants, Detection, and Anti-Listerial Interventions. Microb. Ecol. 2023, 86, 2231–2251. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Y.; Pearlstein, A.J.; Wang, S.; Dillow, H.; Reed, K.; Jia, Z.; Sharma, A.; Zhou, B.; et al. Machine learning-enabled non-destructive paper chromogenic array detection of multiplexed viable pathogens on food. Nat. Food 2021, 2, 110–117. [Google Scholar] [CrossRef]
- Quan, H.; Wang, S.; Xi, X.; Zhang, Y.; Ding, Y.; Li, Y.; Lin, J.; Liu, Y. Deep learning enhanced multiplex detection of viable foodborne pathogens in digital microfluidic chip. Biosens. Bioelectron. 2024, 245, 115837. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Yao, N.; Duan, Y. Pulling G-quadruplex out of dilemma for better colorimetric performance. Sens. Actuators B Chem. 2021, 338, 129830. [Google Scholar] [CrossRef]
- Vo, T.; Paul, A.; Kumar, A.; Boykin, D.W.; Wilson, W.D. Biosensor-surface plasmon resonance: A strategy to help establish a new generation RNA-specific small molecules. Methods 2019, 167, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Naglot, S.; Aggarwal, P.; Dey, S.; Dalal, K. Estimation of Serum YKL-40 by Real-Time Surface Plasmon Resonance Technology in North-Indian Asthma Patients. J. Clin. Lab. Anal. 2017, 31, e22028. [Google Scholar] [CrossRef]
- Chen, M.; Lan, X.; Zhu, L.; Ru, P.; Xu, W.; Liu, H. PCR Mediated Nucleic Acid Molecular Recognition Technology for Detection of Viable and Dead Foodborne Pathogens. Foods 2022, 11, 2675. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, C.C.; Liu, A.; Liu, Y.; Dong, J.; Wang, Z.; Wei, W.; Liu, S. Simultaneous detection of foodborne pathogenic bacteria in milk by fluorescence immunoassay. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121830. [Google Scholar] [CrossRef]
- Cao, L.; Shi, K.; Liu, Y.; Xie, X.; Sun, X.; Dong, W.; Wang, C.; Ma, L. Identification of specific genes as molecular markers for rapid and accurate detection of oil-tea Camellia anthracnose pathogen Colletotrichum fructicola in China. Front. Microbiol. 2024, 15, 1442922. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Q.; Chi, E.; Liu, Y.; Wang, L. Pathogenic Mechanisms and Detection Technologies of Metabolic Toxins Produced by Pseudomonas cocovenenans in Food: A Review. Curr. Anal. Chem. 2025, 21, e15734110377254. [Google Scholar] [CrossRef]
- Sun, S.; Feng, Y.; Li, H.; Xu, S.; Huang, H.; Zou, X.; Lv, Z.; Yao, X.; Gui, S.; Xu, Y.; et al. A novel biosensor MDC@N-MMCNs to selective detection and elimination of foodborne bacterial pathogens. Anal. Chim. Acta 2025, 1354, 344008. [Google Scholar] [CrossRef]
- Gomes, E.; Araújo, D.; Nogueira, T.; Oliveira, R.; Silva, S.; Oliveira, L.V.N.; Azevedo, N.F.; Almeida, C.; Castro, J. Advances in whole genome sequencing for foodborne pathogens: Implications for clinical infectious disease surveillance and public health. Front. Cell. Infect. Microbiol. 2025, 15, 1593219. [Google Scholar] [CrossRef]
- Hojjat Jodaylami, M.; Masson, J.-F.; Badia, A. Surface plasmon resonance sensing. Nat. Rev. Methods Primers 2025, 5, 47. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, W.; Liu, W.; Mei, H. Resolved terahertz spectroscopy of tiny molecules employing tunable spoof plasmons in an otto prism configuration. J. Opt. 2022, 24, 045301. [Google Scholar] [CrossRef]
- Rizal, C.; Belotelov, V. Sensitivity comparison of surface plasmon resonance (SPR) and magneto-optic SPR biosensors. Eur. Phys. J. Plus 2019, 134, 435. [Google Scholar] [CrossRef]
- Murugan, D.; Tintelott, M.; Narayanan, M.S.; Vu, X.T.; Kurkina, T.; Rodriguez-Emmenegger, C.; Schwaneberg, U.; Dostalek, J.; Ingebrandt, S.; Pachauri, V. Recent Advances in Grating Coupled Surface Plasmon Resonance Technology. Adv. Opt. Mater. 2024, 12, 2401862. [Google Scholar] [CrossRef]
- Chen, T.; Xin, J.; Chang, S.J.; Chen, C.J.; Liu, J.T. Surface Plasmon Resonance (SPR) Combined Technology: A Powerful Tool for Investigating Interface Phenomena. Adv. Mater. Interfaces 2023, 10, 2202202. [Google Scholar] [CrossRef]
- Oates, T.W.H.; Wormeester, H.; Arwin, H. Characterization of plasmonic effects in thin films and metamaterials using spectroscopic ellipsometry. Prog. Surf. Sci. 2011, 86, 328–376. [Google Scholar] [CrossRef]
- Wu, Y.; Li, G.; Camden, J.P. Probing Nanoparticle Plasmons with Electron Energy Loss Spectroscopy. Chem. Rev. 2018, 118, 2994–3031. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Zeng, S. Recent Advances in Surface Plasmon Resonance for Biosensing Applications and Future Prospects. In Nanophotonics in Biomedical Engineering; Zhao, X., Lu, M., Eds.; Springer: Singapore, 2021; pp. 21–48. [Google Scholar]
- Chen, Y.; Yao, W.; Li, R.; Yang, Y.; Fan, H.; Liu, G.L.; Gao, Y.; Huang, L. Photoluminescent molecule-boosted metasurface plasmon resonance via nonradiative energy transfer. Biosens. Bioelectron. 2025, 289, 117885. [Google Scholar] [CrossRef]
- Qu, J.-H.; Dillen, A.; Saeys, W.; Lammertyn, J.; Spasic, D. Advancements in SPR biosensing technology: An overview of recent trends in smart layers design, multiplexing concepts, continuous monitoring and in vivo sensing. Anal. Chim. Acta 2020, 1104, 10–27. [Google Scholar] [CrossRef]
- Topor, C.-V.; Puiu, M.; Bala, C. Strategies for Surface Design in Surface Plasmon Resonance (SPR) Sensing. Biosensors 2023, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- Bergwerff, A.A.; Knapen, F.V. Surface Plasmon Resonance Biosensors for Detection of Pathogenic Microorganisms: Strategies to Secure Food and Environmental Safety. J. AOAC Int. 2006, 89, 826–831. [Google Scholar] [CrossRef]
- Rahmasari, L.; Jayanti, P.D.; Zurnansyah; Riswan, M.; Istiqomah, N.I.; Sari, E.K.; Aji, W.W.; Arifin, M.; Sharma, A.; Ali, D.; et al. Surface plasmon resonance-based sensor for Escherichia coli monitoring with novel green-synthesized silver/rGO/L-Histidine as an interface layer. Measurement 2025, 256, 118350. [Google Scholar] [CrossRef]
- Baccar, H.; Mejri, M.B.; Hafaiedh, I.; Ktari, T.; Aouni, M.; Abdelghani, A. Surface plasmon resonance immunosensor for bacteria detection. Talanta 2010, 82, 810–814. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Zhu, J.; Xia, B.; Wang, N.; Chen, X.; Niu, K.; Hou, J.; Jing, X.; Zhou, H. High sensitive optical fiber SPR sensor for label-free detection of Staphylococcus aureus. Microchem. J. 2024, 200, 110381. [Google Scholar] [CrossRef]
- Vaisocherová-Lísalová, H.; Víšová, I.; Ermini, M.L.; Špringer, T.; Song, X.C.; Mrázek, J.; Lamačová, J.; Scott Lynn, N.; Šedivák, P.; Homola, J. Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens. Bioelectron. 2016, 80, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Tiwari, U.K.; Pal, S.S.; Sinha, R.K. Rapid detection of Escherichia coli using fiber optic surface plasmon resonance immunosensor based on biofunctionalized Molybdenum disulfide (MoS2) nanosheets. Biosens. Bioelectron. 2019, 126, 501–509. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.; Zheng, S.; Liu, Y.; He, Z.; Luo, F. Surface plasmon resonance immunosensor for fast, highly sensitive, and in situ detection of the magnetic nanoparticles-enriched Salmonella enteritidis. Sens. Actuators B Chem. 2016, 230, 191–198. [Google Scholar] [CrossRef]
- Morlay, A.; Duquenoy, A.; Piat, F.; Calemczuk, R.; Mercey, T.; Livache, T.; Roupioz, Y. Label-free immuno-sensors for the fast detection of Listeria in food. Measurement 2017, 98, 305–310. [Google Scholar] [CrossRef]
- Bae, Y.M.; Oh, B.-K.; Lee, W.; Lee, W.H.; Choi, J.-W. Immunosensor for detection of Legionella pneumophila based on imaging ellipsometry. Mater. Sci. Eng. C 2004, 24, 61–64. [Google Scholar] [CrossRef]
- Oh, B.-K.; Lee, W.; Bae, Y.M.; Lee, W.H.; Choi, J.-W. Surface Plasmon Resonance Immunosensor for Detection of Legionella pneumophila. Biotechnol. Bioprocess Eng. 2003, 8, 112–116. [Google Scholar] [CrossRef]
- Taheri, R.A.; Rezayan, A.H.; Rahimi, F.; Mohammadnejada, J.; Mehdi, K. Development of an immunosensor using oriented immobilized anti-OmpW for sensitive detection of Vibrio cholerae by surface plasmon resonance. Biosens. Bioelectron. 2016, 86, 484–488. [Google Scholar] [CrossRef]
- Jyoung, J.-Y.; Hong, S.; Lee, W.; Choi, J.-W. Immunosensor for the detection of Vibrio cholerae O1 using surface plasmon resonance. Biosens. Bioelectron. 2006, 21, 2315–2319. [Google Scholar] [CrossRef]
- Hyeon, S.H.; Lim, W.K.; Shin, H.J. Novel surface plasmon resonance biosensor that uses full-length Det7 phage tail protein for rapid and selective detection of Salmonella enterica serovar Typhimurium. Biotechnol. Appl. Biochem. 2020, 68, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Park, T.J.; Kim, H.-S.; Kim, J.-H.; Cho, Y.-J. Directed self-assembly of gold binding polypeptide-protein A fusion proteins for development of gold nanoparticle-based SPR immunosensors. Biosens. Bioelectron. 2009, 24, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Trzaskowski, M.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Ciach, T. Detection of tuberculosis in patients with the use of portable SPR device. Sens. Actuators B Chem. 2018, 260, 786–792. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Tsao, Y.-C.; Tsai, W.-H.; Yang, Y.-W.; Yan, T.-R.; Sheu, B.-C. Development and application of side-polished fiber immunosensor based on surface plasmon resonance for the detection of Legionella pneumophila with halogens light and 850nm-LED. Sens. Actuators A Phys. 2007, 138, 299–305. [Google Scholar] [CrossRef]
- Puttharugsa, C.; Wangkam, T.; Huangkamhang, N.; Gajanandana, O.; Himananto, O.; Sutapun, B.; Amarit, R.; Somboonkaew, A.; Srikhirin, T. Development of surface plasmon resonance imaging for detection of Acidovorax avenae subsp. citrulli (Aac) using specific monoclonal antibody. Biosens. Bioelectron. 2011, 26, 2341–2346. [Google Scholar] [CrossRef]
- Yodmongkol, S.; Thaweboon, S.; Thaweboon, B.; Puttharugsa, C.; Sutapun, B.; Amarit, R.; Somboonkaew, A.; Srikhirin, T. Application of surface plasmon resonance biosensor for the detection of Candida albicans. Jpn. J. Appl. Phys. 2015, 55, 02BE03. [Google Scholar] [CrossRef]
- Zhang, X.; Tsuji, S.; Kitaoka, H.; Kobayashi, H.; Tamai, M.; Honjoh, K.i.; Miyamoto, T. Simultaneous Detection of Escherichia coli O157:H7, Salmonella enteritidis, and Listeria monocytogenes at a Very Low Level Using Simultaneous Enrichment Broth and Multichannel SPR Biosensor. J. Food Sci. 2017, 82, 2357–2363. [Google Scholar] [CrossRef]
- Meneghello, A.; Sonato, A.; Ruffato, G.; Zacco, G.; Romanato, F. A novel high sensitive surface plasmon resonance Legionella pneumophila sensing platform. Sens. Actuators B Chem. 2017, 250, 351–355. [Google Scholar] [CrossRef]
- Makhneva, E.; Farka, Z.; Skládal, P.; Zajíčková, L. Cyclopropylamine plasma polymer surfaces for label-free SPR and QCM immunosensing of Salmonella. Sens. Actuators B Chem. 2018, 276, 447–455. [Google Scholar] [CrossRef]
- Farka, Z.; Juřík, T.; Pastucha, M.; Skládal, P. Enzymatic Precipitation Enhanced Surface Plasmon Resonance Immunosensor for the Detection of Salmonella in Powdered Milk. Anal. Chem. 2016, 88, 11830–11836. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.; Chen, F.-C.; Bridgman, R.C. Detection of Salmonella Typhimurium in Romaine Lettuce Using a Surface Plasmon Resonance Biosensor. Biosensors 2019, 9, 94. [Google Scholar] [CrossRef]
- Torun, Ö.; Hakkı Boyacı, İ.; Temür, E.; Tamer, U. Comparison of sensing strategies in SPR biosensor for rapid and sensitive enumeration of bacteria. Biosens. Bioelectron. 2012, 37, 53–60. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Y.; Zhang, B.; Chen, M.; Huang, J.; Zhang, K.; Gao, W.; Fu, W.; Jiang, T.; Liao, P. Rapid label-free identification of mixed bacterial infections by surface plasmon resonance. J. Transl. Med. 2011, 9, 85. [Google Scholar] [CrossRef]
- Wang, W.-W.; Han, X.; Chu, L.-Q. Polyadenine-mediated Immobilization of Aptamers on a Gold Substrate for the Direct Detection of Bacterial Pathogens. Anal. Sci. 2019, 35, 967–972. [Google Scholar] [CrossRef]
- Zezza, F.; Pascale, M.; Mulè, G.; Visconti, A. Detection of Fusarium culmorum in wheat by a surface plasmon resonance-based DNA sensor. J. Microbiol. Methods 2006, 66, 529–537. [Google Scholar] [CrossRef]
- Di, W.T.; Du, X.W.; Pan, M.F.; Wang, J.P. The SPR detection of Salmonella enteritidis in food using aptamers as recongnition elements. IOP Conf. Ser. Mater. Sci. Eng. 2017, 231, 012114. [Google Scholar] [CrossRef]
- Daems, D.; Knez, K.; Delport, F.; Spasic, D.; Lammertyn, J. Real-time PCR melting analysis with fiber optic SPR enables multiplex DNA identification of bacteria. Analyst 2016, 141, 1906–1911. [Google Scholar] [CrossRef]
- Karoonuthaisiri, N.; Charlermroj, R.; Morton, M.J.; Oplatowska-Stachowiak, M.; Grant, I.R.; Elliott, C.T. Development of a M13 bacteriophage-based SPR detection using Salmonella as a case study. Sens. Actuators B Chem. 2014, 190, 214–220. [Google Scholar] [CrossRef]
- Masdor, N.A.; Altintas, Z.; Shukor, M.Y.; Tothill, I.E. Subtractive inhibition assay for the detection of Campylobacter jejuni in chicken samples using surface plasmon resonance. Sci. Rep. 2019, 9, 13642. [Google Scholar] [CrossRef] [PubMed]
- Gür, S.D.; Bakhshpour, M.; Denizli, A. Selective detection of Escherichia coli caused UTIs with surface imprinted plasmonic nanoscale sensor. Mater. Sci. Eng. C 2019, 104, 109869. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Barka-Bouaifel, F.; Bouckaert, J.; Yamakawa, N.; Boukherroub, R.; Szunerits, S. Graphene-Coated Surface Plasmon Resonance Interfaces for Studying the Interactions between Bacteria and Surfaces. ACS Appl. Mater. Interfaces 2014, 6, 5422–5431. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Huang, J.; Lu, X.; Wang, G.; Zhang, Z.; Yue, J.; Li, Q.; Wang, S.; Yan, J.; Deng, L.; et al. Autocatalytic MNAzyme-integrated surface plasmon resonance biosensor for simultaneous detection of bacteria from nosocomial bloodstream infection specimens. Sens. Actuators B Chem. 2021, 330, 129255. [Google Scholar] [CrossRef]
- Gasparyan, V.K.; Bazukyan, I.L. Lectin sensitized anisotropic silver nanoparticles for detection of some bacteria. Anal. Chim. Acta 2013, 766, 83–87. [Google Scholar] [CrossRef]
- Kushwaha, A.S.; Kumar, A.; Kumar, R.; Srivastava, M.; Srivastava, S.K. Zinc oxide, gold and graphene-based surface plasmon resonance (SPR) biosensor for detection of pseudomonas like bacteria: A comparative study. Optik 2018, 172, 697–707. [Google Scholar] [CrossRef]
- Janze, E.J.T.; Meshginqalam, B.; Alaei, S. A highly sensitive surface plasmon resonance biosensor using heterostructure of franckeite and TMDCs for Pseudomonas bacteria detection. Opt. Lasers Eng. 2024, 181, 108404. [Google Scholar] [CrossRef]
- Mudgal, N.; Yupapin, P.; Ali, J.; Singh, G. BaTiO3-Graphene-Affinity Layer–Based Surface Plasmon Resonance (SPR) Biosensor for Pseudomonas Bacterial Detection. Plasmonics 2020, 15, 1221–1229. [Google Scholar] [CrossRef]
- Daher, M.G.; Taya, S.A.; Colak, I.; Patel, S.K.; Olaimat, M.M.; Ramahi, O. Surface plasmon resonance biosensor based on graphene layer for the detection of waterborne bacteria. J. Biophotonics 2022, 15, e202200001. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.; Priyadarshi, N.; Pinnaka, A.K.; Soni, S.; Deep, A.; Singhal, N.K. Glycoconjugates coated gold nanorods based novel biosensor for optical detection and photothermal ablation of food borne bacteria. Sens. Actuators B Chem. 2019, 289, 207–215. [Google Scholar] [CrossRef]
- Galvan, D.D.; Parekh, V.; Liu, E.; Liu, E.L.; Yu, Q. Sensitive Bacterial Detection via Dielectrophoretic-Enhanced Mass Transport Using Surface-Plasmon-Resonance Biosensors. Anal. Chem. 2018, 90, 14635–14642. [Google Scholar] [CrossRef]
- Banerjee, A.; Rahul, R.; Thangaraj, J.; Kumar, S. High-Sensitivity SPR Fiber-Optic Biosensor with Nano-Grating Structure for Pathogenic Bacteria Detection in Drinking Water. IEEE Sens. J. 2024, 24, 36882–36890. [Google Scholar] [CrossRef]
- Roy, S.K.; Sharan, P. Design of ultra-high sensitive biosensor to detect E. coli in water. Int. J. Inf. Technol. 2019, 12, 775–780. [Google Scholar] [CrossRef]
- Taylor, A.D.; Ladd, J.; Yu, Q.; Chen, S.; Homola, J.; Jiang, S. Quantitative and simultaneous detection of four foodborne bacterial pathogens with a multi-channel SPR sensor. Biosens. Bioelectron. 2006, 22, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Tomioka, K.; Okumura, S. Optimal Conditions for the Asymmetric Polymerase Chain Reaction for Detecting Food Pathogenic Bacteria Using a Personal SPR Sensor. Appl. Biochem. Biotechnol. 2019, 187, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.M.; Satish, L.; Kushmaro, A.; Shvalya, V.; Cvelbar, U.; Abdulhalim, I. Engineering the penetration depth of nearly guided wave surface plasmon resonance towards application in bacterial cells monitoring. Sens. Actuators B Chem. 2021, 345, 130338. [Google Scholar] [CrossRef]
- Bessa Pereira, C.; Bocková, M.; Santos, R.F.; Santos, A.M.; Martins de Araújo, M.; Oliveira, L.; Homola, J.; Carmo, A.M. The Scavenger Receptor SSc5D Physically Interacts with Bacteria through the SRCR-Containing N-Terminal Domain. Front. Immunol. 2016, 7, 416. [Google Scholar] [CrossRef]
- Boulade, M.; Morlay, A.; Piat, F.; Roupioz, Y.; Livache, T.; Charette, P.G.; Canva, M.; Leroy, L.ı. Early detection of bacteria using SPR imaging and event counting: Experiments with Listeria monocytogenes and Listeria innocua. RSC Adv. 2019, 9, 15554. [Google Scholar] [CrossRef]
- Eser, E.; Ekiz, H.I. Antibody fragmentation technique for salmonella detection by SPR based biosensor. J. Biotechnol. 2017, 2565, S17–S43. [Google Scholar] [CrossRef]
- Wen, J.; Zhu, Y.; Liu, J.; He, D. Smartphone-based surface plasmon resonance sensing platform for rapid detection of bacteria. RSC Adv. 2022, 12, 13045. [Google Scholar] [CrossRef]
- Chung, K.-H.; Hwang, H.-S.; Lee, K.-Y. Kinetics of Salmonella typhimurium binding with antibody by immunoassays using a surface plasmon resonance biosensor. J. Ind. Eng. Chem. 2010, 16, 115–118. [Google Scholar] [CrossRef]
- Quintela, I.A.; Vasse, T.; Lin, C.-S.; Wu, V.C.H. Advances, applications, and limitations of portable and rapid detection technologies for routinely encountered foodborne pathogens. Front. Microbiol. 2022, 13, 1054782. [Google Scholar] [CrossRef]
- Raghu, H.V.; Kumar, N. Rapid Detection of Listeria monocytogenes in Milk by Surface Plasmon Resonance Using Wheat Germ Agglutinin. Food Anal. Methods 2020, 13, 982–991. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, H.; Meng, C.; Ding, R.; Yan, Q.; Zhao, H.; Kang, X.; Gu, D.; Pan, Z.; Jiao, X. Subtractive Inhibition Assay Based on PagN-Specific Monoclonal Antibody for the Detection of Salmonella Using Surface Plasmon Resonance. Biotechnol. J. 2025, 20, e202400616. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, Z.; Chen, J.; Huang, Z.; Wang, X.; An, H.; Duan, Y. Ω-Shaped Fiber-Optic Probe-Based Localized Surface Plasmon Resonance Biosensor for Real-Time Detection of Salmonella Typhimurium. Anal. Chem. 2018, 90, 13640–13646. [Google Scholar] [CrossRef]
- Bhandari, D.; Chen, F.-C.; Bridgman, R.C. Magnetic Nanoparticles Enhanced Surface Plasmon Resonance Biosensor for Rapid Detection of Salmonella Typhimurium in Romaine Lettuce. Sensors 2022, 22, 475. [Google Scholar] [CrossRef] [PubMed]
- Luna-Moreno, D.; Sánchez-Álvarez, A.; Islas-Flores, I.; Canto-Canche, B.; Carrillo-Pech, M.; Villarreal-Chiu, J.F.; Rodríguez-Delgado, M. Early Detection of the Fungal Banana Black Sigatoka Pathogen Pseudocercospora fijiensis by an SPR Immunosensor Method. Sensors 2019, 19, 465. [Google Scholar] [CrossRef] [PubMed]
- Pasquardini, L.; Cennamo, N.; Arcadio, F.; Perri, C.; Chiodi, A.; D’agostino, G.; Zeni, L. Immuno-SPR biosensor for the detection of Brucella abortus. Sci. Rep. 2023, 13, 22832. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, J.; Li, Y.; Luo, Q.; Xiong, X.; Shi, X.; Lv, Y.; Zhao, Q. An integrated three-signal biosensor based on phage multifunctional probe for simultaneous and ultrasensitive detection of live/dead Listeria monocytogenes. Sens. Actuators B Chem. 2025, 423, 136709. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Ding, J.; Lin, Q.; Young, G.M.; Jiang, C. Rapid identification of live and dead Salmonella by surface-enhanced Raman spectroscopy combined with convolutional neural network. Vib. Spectrosc. 2022, 118, 103332. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Q.; Saiding, Q.; An, S.; Zhou, Z.; Kong, N.; Heng, Y.J.; Abdi, R.; Tao, W. Live bacterial chemistry in biomedicine. Chem 2025, 11, 102436. [Google Scholar] [CrossRef]
- Yan, R.; Wu, Q.; Lin, G.; Chen, L.; Song, X.; Luo, S.; Situ, W. Inhibition of Salmonella typhimurium and Listeria monocytogenes in coconut juice by graphene-doped photocatalyst rGO/TiO2. Food Chem. 2025, 463, 141103. [Google Scholar] [CrossRef]
- Hirakata, Y.; Mei, R.; Morinaga, K.; Katayama, T.; Tamaki, H.; Meng, X.-Y.; Watari, T.; Yamaguchi, T.; Hatamoto, M.; Nobu, M.K. Identification and cultivation of anaerobic bacterial scavengers of dead cells. ISME J. 2023, 17, 2279–2289. [Google Scholar] [CrossRef]
- Tchatchiashvili, T.; Jundzill, M.; Marquet, M.; Mirza, K.A.; Pletz, M.W.; Makarewicz, O.; Thieme, L. CAM/TMA-DPH as a promising alternative to SYTO9/PI for cell viability assessment in bacterial biofilms. Front. Cell. Infect. Microbiol. 2025, 14, 1508016. [Google Scholar] [CrossRef]
- Nasrabadi, A.M.; An, S.; Kwon, S.-B.; Hwang, J. Investigation of live and dead status of airborne bacteria using UVAPS with LIVE/DEAD® BacLight Kit. J. Aerosol Sci. 2018, 115, 181–189. [Google Scholar] [CrossRef]
- Cao, Y.; Griffith, B.; Bhomkar, P.; Wishart, D.S.; McDermott, M.T. Functionalized gold nanoparticle-enhanced competitive assay for sensitive small-molecule metabolite detection using surface plasmon resonance. Analyst 2018, 143, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Luo, Y.; Li, D.; Li, Y.; Gong, T.; Zhao, C.; Wang, C.; Duan, R.; Yue, W. Recent advances in localized surface plasmon resonance (LSPR) sensing technologies. Nanotechnology 2025, 36, 202001. [Google Scholar] [CrossRef]
- Prajna, N.D.; Sinha, R.K. Shape-controlled Synthesis and Bulk Refractive Index Sensitivity Studies of Gold Nanoparticles for LSPR-based Sensing. Plasmonics 2024, 20, 1351–1364. [Google Scholar]
- Hang, Y.; Wang, A.; Wu, N. Plasmonic silver and gold nanoparticles: Shape- and structure-modulated plasmonic functionality for point-of-caring sensing, bio-imaging and medical therapy. Chem. Soc. Rev. 2024, 53, 2932–2971. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Fan, S.; Chen, L.; Zhou, H.; Pérez-Juste, J.; Pastoriza-Santos, I.; Wong, K.Y.; Zheng, G. Chiral Plasmonic Sensors: Fundamentals and Emerging Applications. Angew. Chem. Int. Ed. 2025, e202514816. [Google Scholar] [CrossRef]
- Orii, T.; Okazaki, T.; Yamamoto, T.; Sazawa, K.; Taguchi, A.; Sugawara, K.; Kuramitz, H. Spectroelectrochemical Fiber-Optic Sensor Based on Localized Surface Plasmon Resonance for Simultaneous Multiselective Electroactive Species Detection. Anal. Chem. 2025, 97, 12531–12539. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Gao, Y.; Zhang, W.; Wang, P.; Fang, Y.; Yang, L. Localized surface plasmon resonance (LSPR) excitation on single silver nanoring with nanoscale surface roughness. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 317, 124405. [Google Scholar] [CrossRef] [PubMed]
- Nanda, B.P.; Rani, P.; Paul, P.; Aman; Ganti, S.S.; Bhatia, R. Recent trends and impact of localized surface plasmon resonance (LSPR) and surface-enhanced Raman spectroscopy (SERS) in modern analysis. J. Pharm. Anal. 2024, 14, 100959. [Google Scholar] [CrossRef]
- Lamba, T.K.; Augustine, S.; Saini, M.; Sooraj, K.P.; Ranjan, M. LSPR anisotropy minimization by sequential growth of Ag nanoparticles on nanoripple patterned Si surface for SERS Application. Surf. Interfaces 2024, 52, 104852. [Google Scholar] [CrossRef]
- Singh, M.; Scotognella, F.; Paternò, G.M. Degenerately doped metal oxide nanocrystals for infrared light harvesting: Insight into their plasmonic properties and future perspectives. Mater. Adv. 2024, 5, 6796–6812. [Google Scholar] [CrossRef]
- Wang, W.; Yin, L.; Jayan, H.; Sun, C.; Peng, C.; Zou, X.; Guo, Z. Multiplexed optical sensors driven by nanomaterials for food and agriculture safety detection: From sensing strategies to practical challenges. TrAC Trends Anal. Chem. 2025, 193, 118428. [Google Scholar] [CrossRef]
- Waitkus, J.; Park, J.; Ndukaife, T.; Yang, S.; Du, K. Antibiotic-Mediated Plasmonic-Mie Resonance for Biosensing Applications on a Novel Silicon Nanopillar Metasurface. Adv. Mater. Interfaces 2025, 12, 2400945. [Google Scholar] [CrossRef]
- Yılmaz, F.; Çimen, D.; Denizli, A. Plasmonic sensors for point of care diagnostics. J. Pharm. Biomed. Anal. 2025, 265, 117042. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Feng, X.; Cao, Y.; Wang, Y.; Jia, M.; Wei, X.; Dong, J.; Tian, R.; Zhou, J.; Ji, H.; et al. Surface plasmon resonance based ultra-sensitive cholesterol detection using AuNPs/MPBA/β-CD functionalized gold-coated tilted fiber Bragg grating. Sens. Actuators B Chem. 2025, 443, 138227. [Google Scholar] [CrossRef]
| Technology Category | Detection Principle | Time Required | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Traditional Culture-Based Methods | Microbe isolation, culture, and identification | 3–7 days | Gold standard, accurate, quantitative, low cost | Time-consuming, labor-intensive, cannot detect VBNC states | [17] |
| Immunological Methods | Antigen–antibody reaction | Minutes–Hours | Rapid, simple, suitable for on-site screening | Potential false positives/negatives, difficult to quantify, cannot distinguish live/dead bacteria | [18] |
| Molecular Biology Methods | Amplification of specific gene fragments | Several Hours | Highly sensitive and specific, rapid, can detect hard-to-culture bacteria | Cannot distinguish live/dead bacteria, prone to contamination, expensive equipment | [19] |
| Metabolic/Phenotypic Methods | Detection of metabolic products or physical changes | Hours–2 Days | Detects live bacteria, can be automated | Difficult for direct identification, limited sensitivity, requires databases | [20] |
| Biosensors | Biological recognition + signal transduction | Minutes–30 min | Extremely fast, highly sensitive, portable, potential for real-time monitoring | Immature technology, poor stability, susceptible to interference | [21] |
| Whole Genome Sequencing | Determination of the entire genome sequence | 1–3 Days | Most comprehensive information, highest resolution, precise traceability | High cost, complex data analysis, time-consuming | [22] |
| SPR | Refractive index change from biomolecular binding | Minutes–1 h | Label-free, real-time, and dynamic monitoring, fast detection, minimal sample volume, potential for automation and high-throughput | Expensive equipment, cannot distinguish live/dead bacteria | [23] |
| LOD (Limit of Detection) | Linear Range | Foodborne Pathogens | Ref. | |
|---|---|---|---|---|
| Direct detection | 103 CFU/mL | 0–105 CFU/mL | E. coli | [36] |
| 50 CFU/mL | 50–108 CFU/mL | Staphylococcus aureus | [37] | |
| 57 CFU/mL | 1.5 × 101–1.5 × 107 CFU/mL | Salmonella sp. | [38] | |
| 94 CFU/mL | 1000–8000 CFU/mL | E. coli | [39] | |
| 14 CFU/mL | 1.4 × 101–1.4 × 109 CFU/mL | Salmonella enteritidis | [40] | |
| 43 CFU/mL | 102–105 CFU/mL | Legionella pneumophila | [42] | |
| 0.9984 CFU/mL | 3.7 × 105–3.7 × 109 CFU/mL | Vibrio cholerae O1 | [45] | |
| 104 CFU/mL | 5 × 104–5 × 107 CFU/mL | S. typhimurium | [46] | |
| 10 CFU/ml | 101–103 CFU/mL. | Legionella pneumophila | [49] | |
| 105 CFU/mL | 105–108 CFU/mL | Salmonella | [54] | |
| 104 CFU/mL | 102–106 CFU/mL | Salmonella | [55] | |
| 2 CFU/mL | 100–108 CFU/mL | Salmonella | [61] | |
| Indirect detection | 131 CFU/mL | 5–5 × 106 CFU/mL | Campylobacter jejuni | [64] |
| 1 CFU/mL | 0.5 × 101–1 × 103 CFU/mL | E. coli | [65] | |
| 102 CFU/mL | 102–109 CFU/mL | E. coli | [66] | |
| 57 CFU/mL | 1 × 102–1 × 106 CFU/mL | nBSI bacteria | [67] |
| Feature | LSPR | SPR |
|---|---|---|
| Location | Surface of metal nanoparticles | Surface of continuous, flat metal thin films |
| Plasmon Mode | Localized oscillation of electron cloud within the 3D nanostructure | Propagating wave along the 2D metal film surface, extending and oscillating at the interface |
| Excitation Method | Simple, direct excitation (e.g., using broad-spectrum light like white light) | Complex, requires wavevector matching, excited by polarized light at a specific incident angle |
| Electromagnetic Field Decay | Field intensity decays rapidly from the surface (~10–30 nm) | Longer decay length of the field intensity |
| Equipment Size and Cost | Relatively simple, miniaturized, lower cost | Complex, requires precise optical components, bulky and expensive equipment |
| Advantages and Disadvantages | Adv: Simple equipment, easy integration, higher sensitivity to changes immediately at the surface | Adv: Ultra-high sensitivity, can provide precise kinetic data |
| Dis: Generally lower absolute sensitivity than SPR, slightly weaker for quantitative kinetic analysis | Dis: Expensive equipment, bulky, more sensitive to bulk effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Yang, J.; Chen, J.; Sun, X.; Hu, W.; Liu, X. The Development of Foodborne Pathogen Detection and Biosensor Design for Surface Plasmon Resonance Technology. Biosensors 2025, 15, 774. https://doi.org/10.3390/bios15120774
Hu Y, Yang J, Chen J, Sun X, Hu W, Liu X. The Development of Foodborne Pathogen Detection and Biosensor Design for Surface Plasmon Resonance Technology. Biosensors. 2025; 15(12):774. https://doi.org/10.3390/bios15120774
Chicago/Turabian StyleHu, Ye, Jun Yang, Jian Chen, Xiaojie Sun, Wenyan Hu, and Xinmei Liu. 2025. "The Development of Foodborne Pathogen Detection and Biosensor Design for Surface Plasmon Resonance Technology" Biosensors 15, no. 12: 774. https://doi.org/10.3390/bios15120774
APA StyleHu, Y., Yang, J., Chen, J., Sun, X., Hu, W., & Liu, X. (2025). The Development of Foodborne Pathogen Detection and Biosensor Design for Surface Plasmon Resonance Technology. Biosensors, 15(12), 774. https://doi.org/10.3390/bios15120774




