Mn Oxide Nanowire/ZIF-8 Composites with Multiple Enzyme-like Activities for Enantioselective Glutamate Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
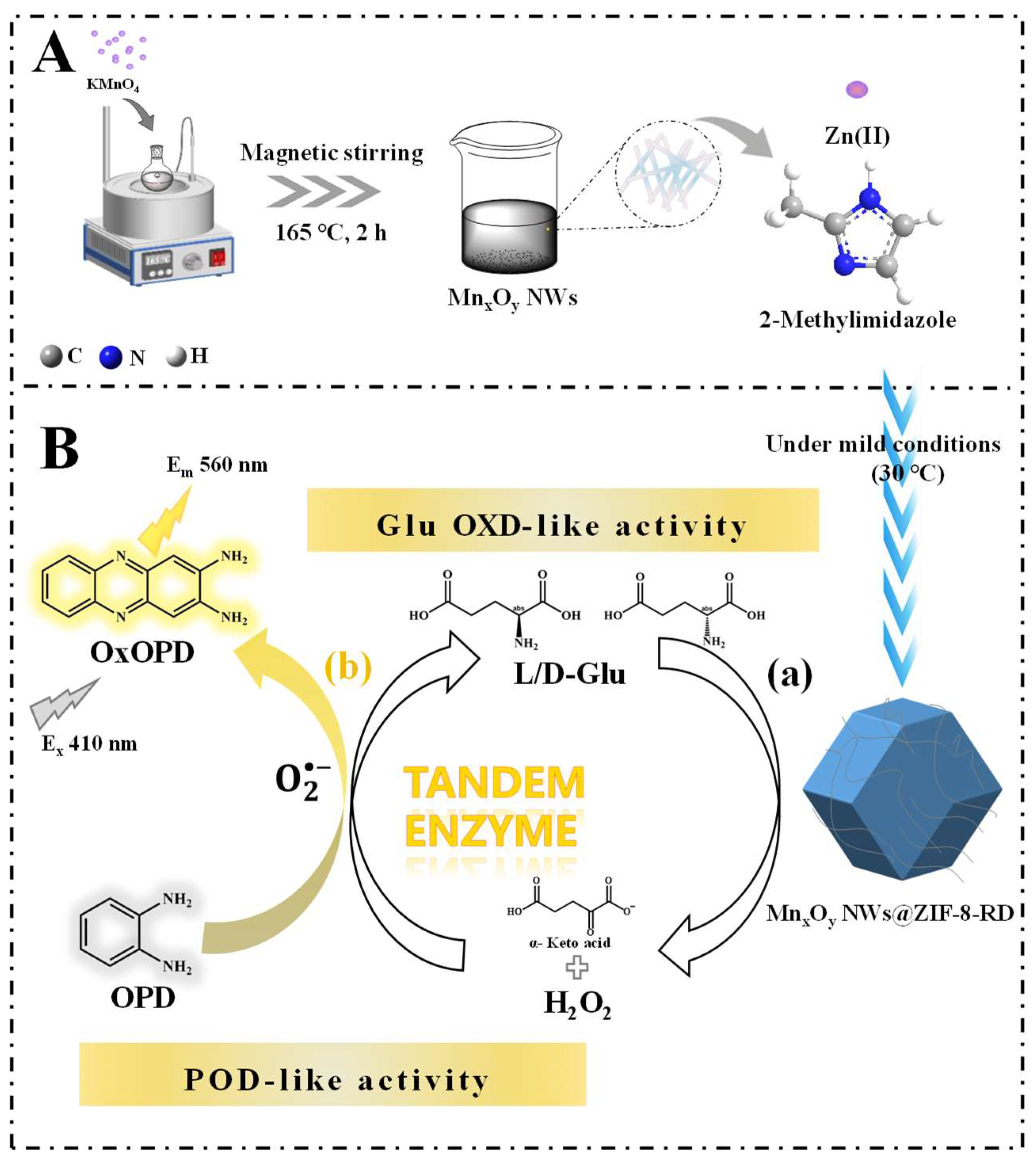
2.3. Synthesis of MnxOy NWs
2.4. Synthesis of MnxOy NWs@ZIF-8-RD
3. Results and Discussion
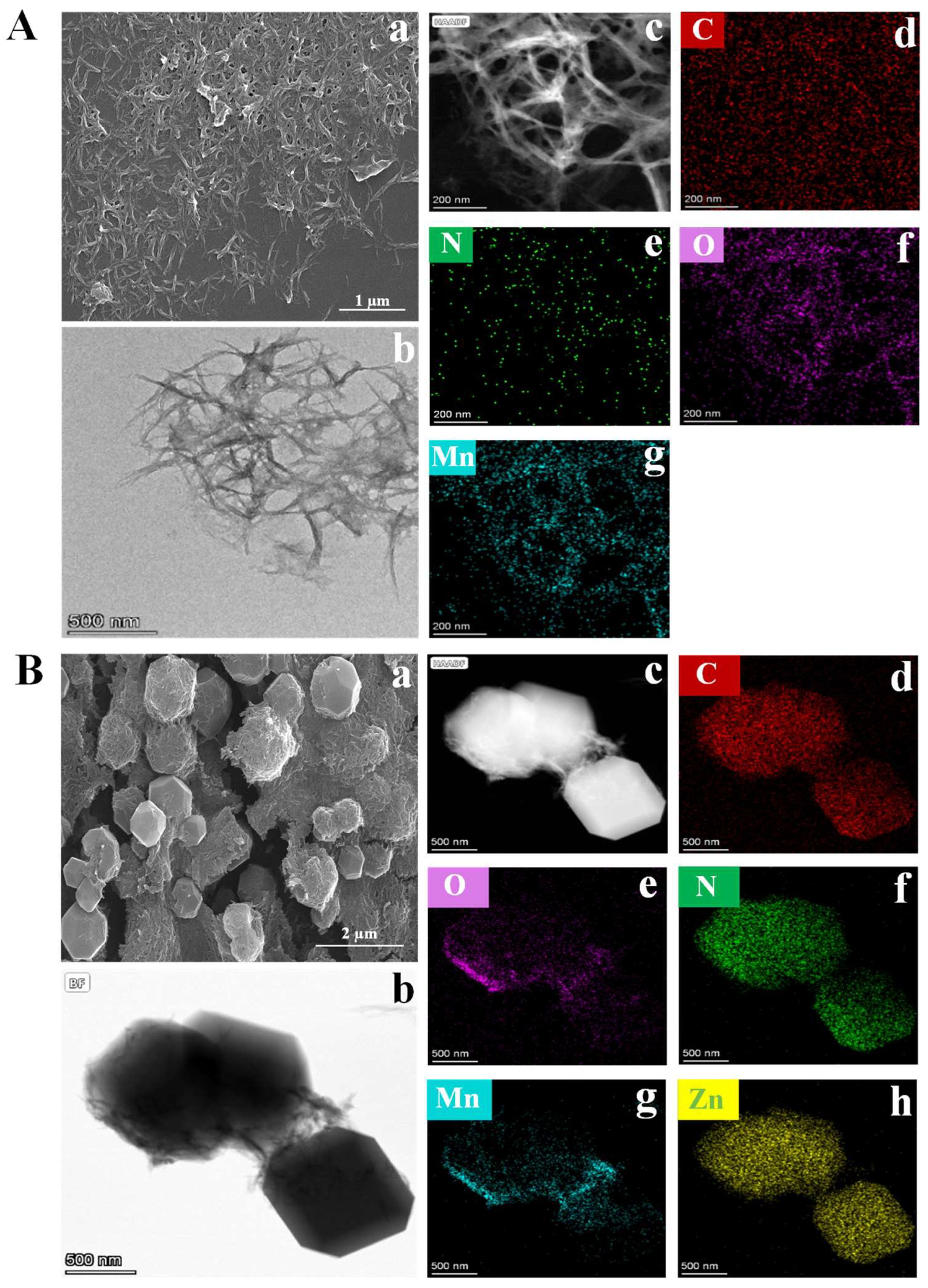
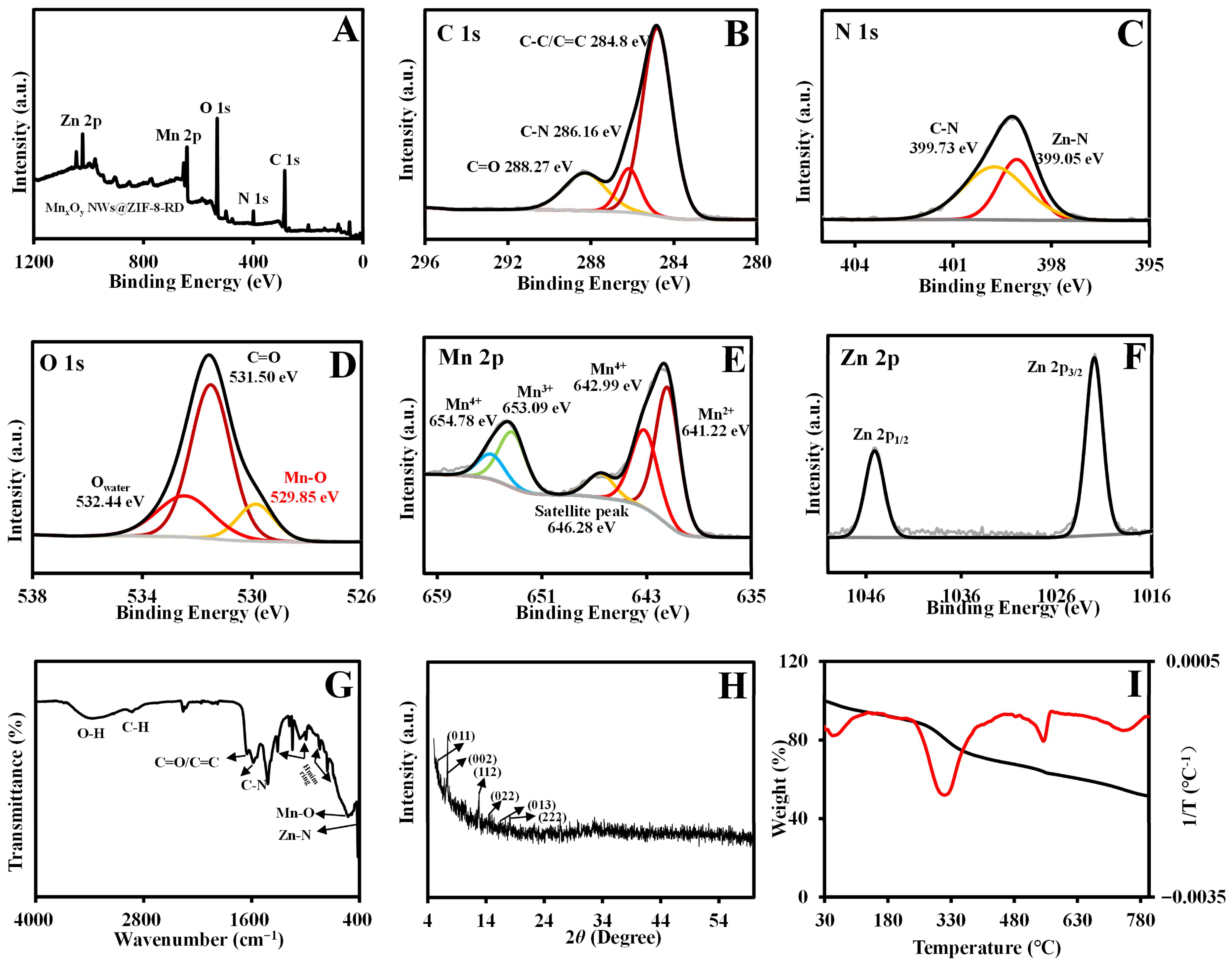
3.1. Characterization of MnxOy NWs and MnxOy NWs@ZIF-8-RD
3.2. Optimization of Experimental Conditions for Glu Enantiomers Recognition
3.2.1. The Effect of MnxOy NWs Concentration
3.2.2. The Effect of Zn(II)/Hmim Ratio
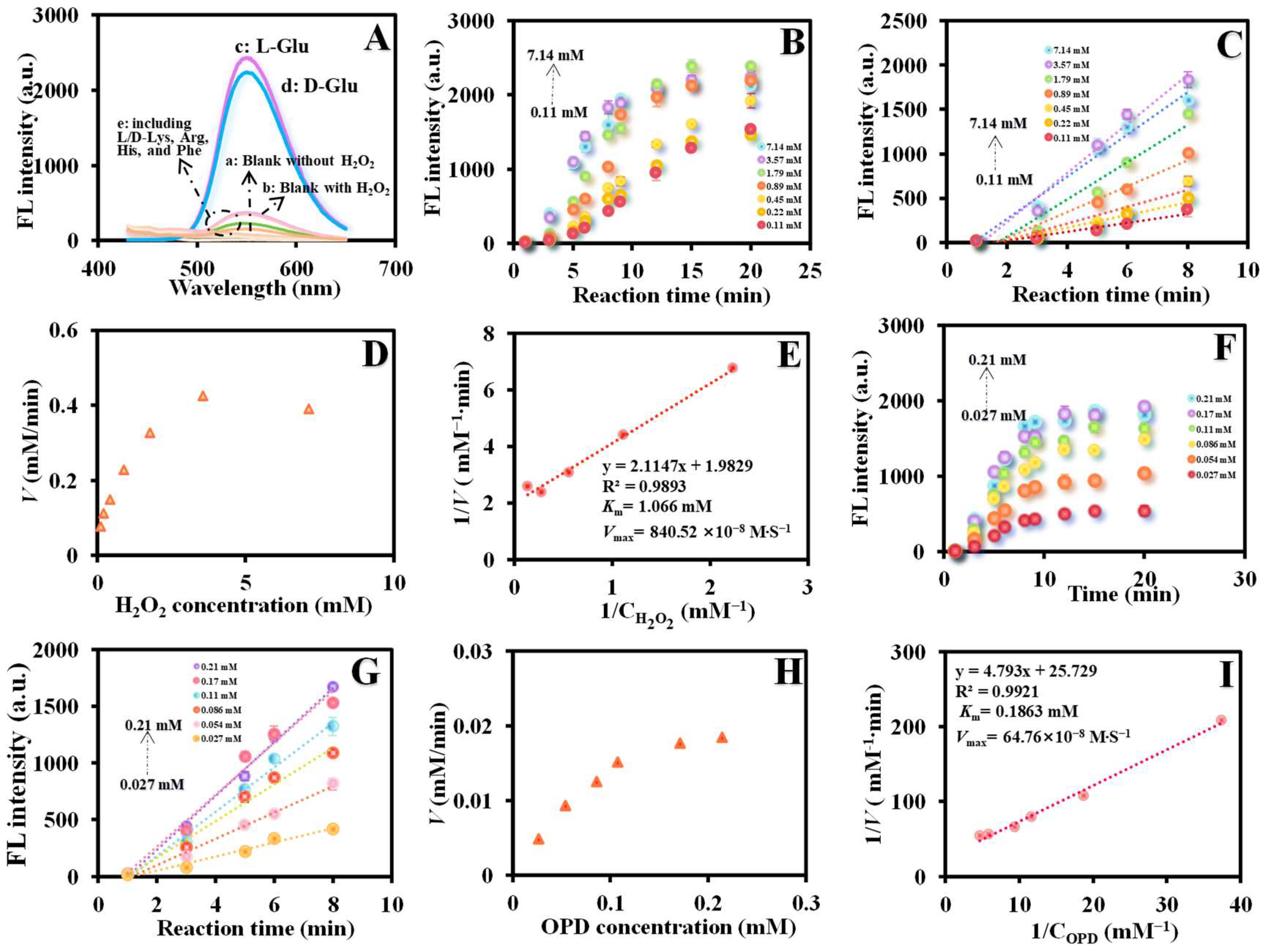
3.3. Enzyme Kinetic
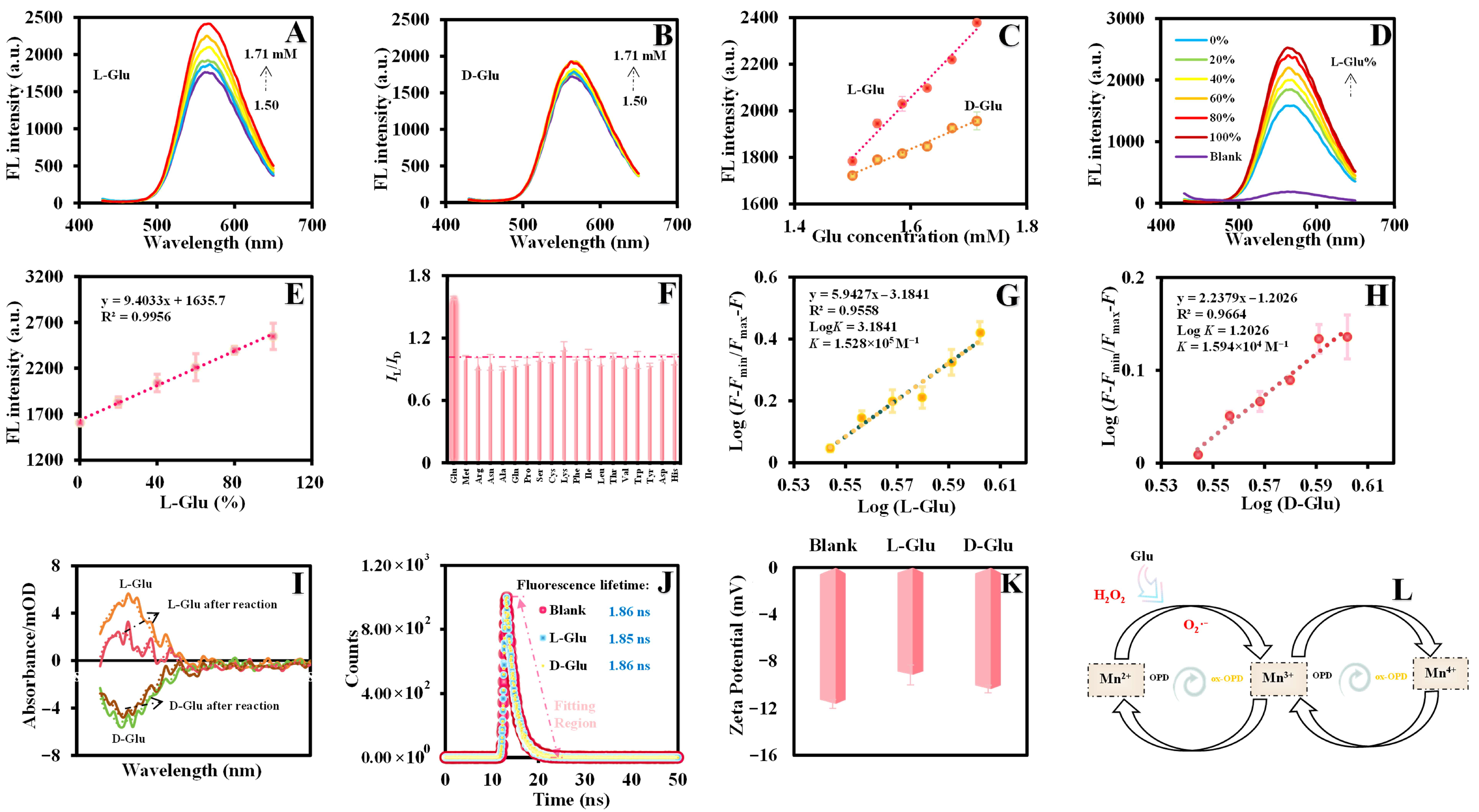
3.4. Enantiomeric Differentiation of L/Glu
3.5. Chiral Recognition Mechanism
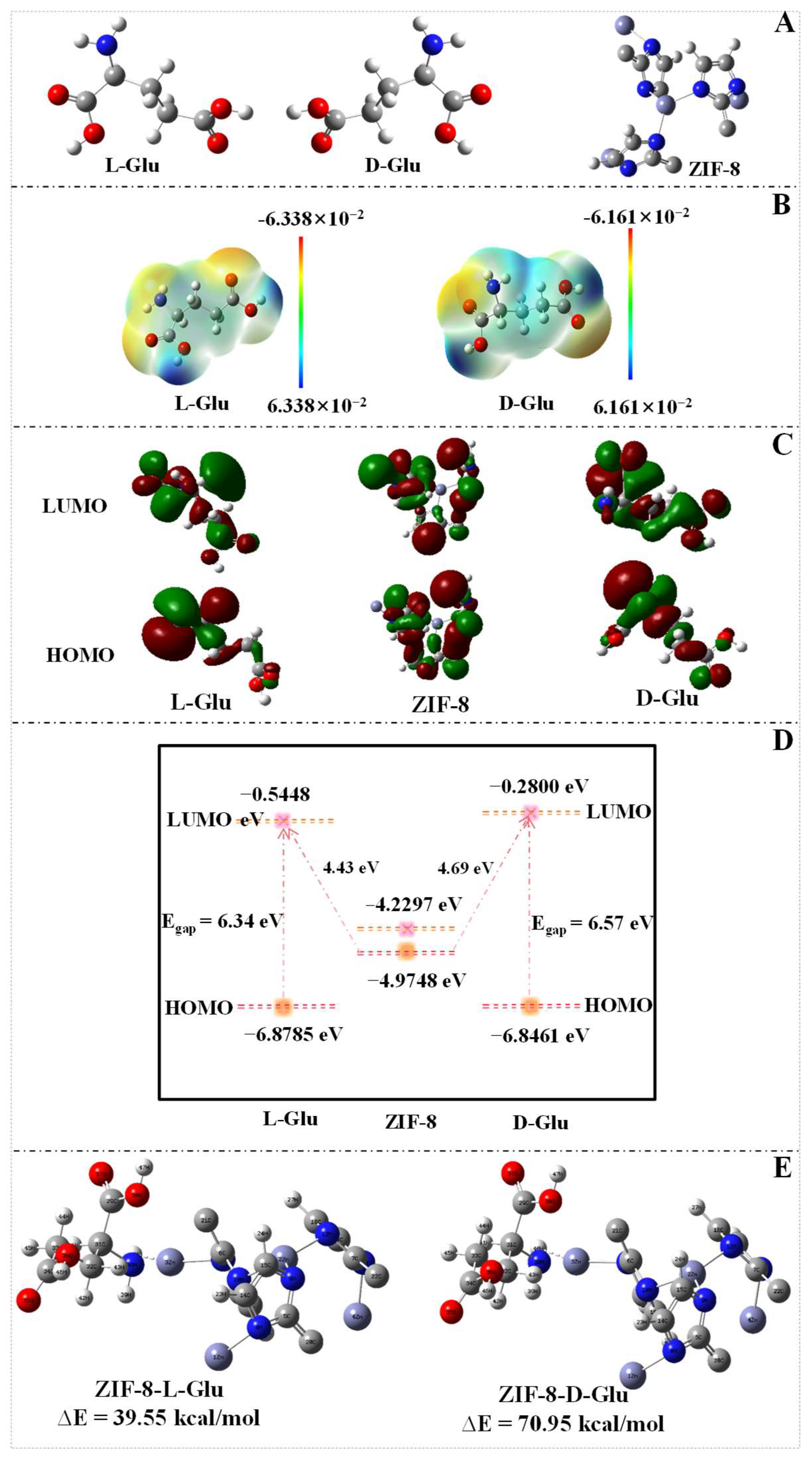
3.6. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, X.; Song, J.; Wu, Q.; Lv, H. Chiral carbon quantum dots as fluorescent probe for rapid chiral recognition of isoleucine enantiomers. Anal. Chim. Acta 2021, 1184, 339012. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, J.; Zhu, X. One-step hydrothermal preparation of chiral carbon quantum dots and enantioselective sensing of glutamine enantiomeric isomers. Luminescence 2024, 39, e4639. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kou, M.; Quan, K.; Wang, J.; Zhang, H.; Ihara, H.; Takafuji, M.; Qiu, H. Enantioselective glutamic acid discrimination and nanobiological imaging by chiral fluorescent silicon nanoparticles. Anal. Chem. 2024, 96, 2173–2182. [Google Scholar] [CrossRef]
- Li, D.; Guan, T.; He, Y.; Liu, F.; Yang, A.; HE, Q.; Shen, Z.; Xin, M. A chiral sensor based on weak measurement for the determination of Proline enantiomers in diverse measuring circumstances. Biosens. Bioelectron. 2018, 110, 103–109. [Google Scholar] [CrossRef]
- Wei, S.; Liu, B.; Shi, X.; Cui, S.; Zhang, H.; Lu, P.; Guo, H.; Wang, B.; Sun, G.; Jiang, C. Gadolinium (III) doped carbon dots as dual-mode sensor for the recognition of dopamine hydrochloride and glutamate enantiomers with logic gate operation. Talanta 2023, 252, 123865. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Chen, X.; Zhang, J.; Yuan, L. Gas chromatographic separation of enantiomers on novel chiral stationary phases. Trac-Trend Anal. Chem. 2020, 124, 115808. [Google Scholar] [CrossRef]
- Scriba, G.K. Differentiation of enantiomers by capillary electrophoresis. Top. Curr. Chem. 2013, 340, 209–275. [Google Scholar] [CrossRef]
- Liu, M.; Chen, L.; Tian, T.; Zhang, Z.; Li, X. Identification and quantitation of enantiomers by capillary electrophoresis and circular dichroism independent of single enantiomer standard. Anal. Chem. 2019, 91, 13803–13809. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Jiang, W.; Liu, H.; Sun, B. Chiroptical-responsive nanoprobe for the optosensing of chiral amino acids. Microchim. Acta 2022, 189, 184. [Google Scholar] [CrossRef]
- Jiang, W.; He, R.; Lv, H.; He, X.; Wang, L.; Wei, Y. Chiral sensing of tryptophan enantiomers based on the enzyme mimics of β-cyclodextrin-modified sulfur quantum dots. ACS Sens. 2023, 8, 4264–4271. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Qian, Z.; Li, W.; Li, C.; Hu, Y.; Yang, F. A portable personal glucose meter method for enzyme activity detection and inhibitory activity evaluation based on alkaline phosphatase-mediated reaction. Anal. Bioanal. Chem. 2021, 413, 2457–2466. [Google Scholar] [CrossRef]
- Chen, G.; Chai, T.; Wang, J.; Yang, F. Recent advances in the colorimetric and fluorescence analysis of bioactive small-molecule compounds based on the enzyme-like activity of nanomaterials. J. Pharmaceut. Biomed. 2023, 236, 115695. [Google Scholar] [CrossRef]
- Chen, G.; Chai, T.; Zhang, H.; Yang, F. Applications of mild-condition synthesized metal complexes with enzyme-like activity in the colorimetric and fluorescence analysis. Coordin. Chem. Rev. 2024, 508, 215761. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, Z.; Sun, T.; Li, B.; Wu, Z. Manganese-based nanozymes: Preparation, catalytic mechanisms, and biomedical applications. Adv. Healthc. Mater. 2022, 11, e202201733. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Chen, G.; Chai, T.; Li, J.; Chen, H.; Yang, F. Construction of a novel cuboid-shape Mn-urea nanozyme with arsenic (v)-enhanced oxidase-like activity as a colorimetric probe for the selective detection of inorganic arsenic. CrystEngComm 2024, 26, 2641–2651. [Google Scholar] [CrossRef]
- Song, H.; Xu, L.; Chen, M.; Cui, Y.; Wu, C.; Qiu, J.; Xu, L.; Cheng, G.; Hu, X. Recent progresses in the synthesis of MnO2 nanowire and its application in environmental catalysis. RSC Adv. 2021, 11, 35494–35513. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, X.; Wang, W.; Shao, Z. Recent advances in metal-organic framework derivatives as oxygen catalysts for zinc-air batteries. Batter. Supercaps 2019, 2, 272–289. [Google Scholar] [CrossRef]
- Zhao, B.; Yang, H.; Mao, J.; Shi, J. MOF-derived hollow-open hierarchically porous carbon spheres for enzyme encapsulation and biocatalysis. Chem. Eng. J. 2025, 505, 158972. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, W.; Liu, Z.; Wang, L.; Zheng, K.; Li, W.; Qin, C. Starch of oat derived nanostructured Fe/Mn bimetallic carbon materials for sulfamethoxazole degradation via peroxymonosulfate activation. Int. J. Biol. Macromol. 2024, 256, 128400. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Yu, D.; Qin, C.; Deng, J.; Wang, X.; Ge, B.; Huang, F. Bimetallic zeolitic imidazolate frameworks Co/ZIF-8 crystals as carbonic anhydrase-mimicking nanozyme. Colloids Surf. A 2024, 685, 133227. [Google Scholar] [CrossRef]
- Wang, F.; Buhro, W.E. Surfactant-mediated solution-liquid-solid (SLS) growth of phase-pure wurtzite CdS quantum wires. Chem. Mater. 2024, 36, 10307–10318. [Google Scholar] [CrossRef]
- Luo, D.; Chang, C.; Hu, Z. One-step encapsulation of TBAB in ZIF-8 for CO2 fixation: Revealing the synergistic mechanism between TBAB and ZIF-8. ACS Catal. 2024, 14, 11101–11112. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Wu, H.; Mu, L. Persistence and recovery of ZIF-8 and ZIF-67 phytotoxicity. Environ. Sci. Technol. 2021, 55, 15301–15312. [Google Scholar] [CrossRef]
- Amur, S.A.; Sharma, B.P.; Soomro, N.A.; Khuhro, Q.; Tariq, M.; Liang, H.; Kazi, M.; Nur-e-Alam, M. Synthesis, characterization, density functional theory study, antibacterial activity and molecular docking of zeolitic imidazolate framework-8. Appl. Organomet. Chem. 2025, 39, e7826. [Google Scholar] [CrossRef]
- O’Flynn, B.G.; Mittag, T. A new phase for enzyme kinetics. Nat. Chem. Biol. 2021, 17, 628–630. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, S.; Yoon, Y.; Lee, M. A resorufin-based fluorescent turn-on probe responsive to nitroreductase activity and its application to bacterial detection. Dyes Pigments 2019, 171, 107779. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.; Huang, C.; Su, W.; Xiao, Q. Amino-functionalized graphene quantum dots based ratiometric fluorescent nanosensor for ultrasensitive and highly selective recognition of horseradish peroxidase. Sens. Actuators B Chem. 2016, 234, 255–263. [Google Scholar] [CrossRef]
- Li, Y.; Gu, X.; Zhao, J.; Xi, F. Fabrication of a ratiometric fluorescence sensor based on carbon dots as both luminophores and nanozymes for the sensitive detection of hydrogen peroxide. Molecules 2022, 27, 7379. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Bain, D.; Chakraborty, S.; Kolay, S.; Patra, A. Copper nanocluster (Cu23 NC)-based biomimetic system with peroxidase activity. ACS Sustain. Chem. Eng. 2020, 8, 18335–18344. [Google Scholar] [CrossRef]
- Kergaravat, S.V.; Pividori, M.I.; Hernandez, S.R. Evaluation of seven cosubstrates in the quantification of horseradish peroxidase enzyme by square wave voltammetry. Talanta 2012, 88, 468–476. [Google Scholar] [CrossRef]
- Xu, M.; Liu, D.; Yang, J.; Zhu, Q.; Wang, Y.; Sha, J. MIL-100 (Fe) metal-organic framework nanospheres embedded in graphene matrixes for xanthine fluorescence sensing. ACS Appl. Nano Mater. 2021, 4, 7172–7181. [Google Scholar] [CrossRef]
- Yang, D.; Li, Q.; Tammina, S.K.; Gao, Z.; Yang, Y. Cu-CDs/H2O2 system with peroxidase-like activities at neutral pH for the co-catalytic oxidation of o-phenylenediamine and inhibition of catalytic activity by Cr (III). Sens. Actuators B Chem. 2020, 319, 128273. [Google Scholar] [CrossRef]
- Kang, B.; Park, G.; Kim, S.H.; Lee, D.; Oh, S.S. Noncovalent minimal assembly of exogenous histamine with hemin cofactor as a peroxidase-mimicking cooperative catalyst. iScience 2022, 25, 105257. [Google Scholar] [CrossRef]
- Sadiq, S.; Khan, I.; Humayun, M.; Wu, P.; Khan, A.; Khan, S.; Khan, A.; Khan, S.; Alanazi, A.F.; Bououdina, M. Synthesis of metal-organic framework-based ZIF-8@ZIF-67 nanocomposites for antibiotic decomposition and antibacterial activities. ACS Omega 2023, 8, 49244–49258. [Google Scholar] [CrossRef]
- Xu, X.; Ma, T.; Gao, Q.; Tan, M.; Cen, B.; Hu, Q.; Gao, L.; Yang, Z. A multiple enzyme-mimicking Ag/Fe-ZIF nanozyme for efficient inactivation and colorimetric sensing of foodborne pathogens. Chem. Eng. J. 2025, 524, 169506. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Slobodnik, K.; Struble, L.R.; Lutz, W.E.; Coates, L.; Weiss, K.L.; Myles, D.A.A.; Kroll, T.; Borgstahl, G.E.O. Revealing the atomic and electronic mechanism of human manganese superoxide dismutase product inhibition. Nat. Commun. 2024, 15, 5973. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Zhang, Y.; Zhang, Y.; Dong, W. A highly sensitive and selective bis (salamo)-type fluorescent chemosensor for identification of Cu2+ and the continuous recognition of S2−, Arginine and Lysine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117927. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fei, X.; Liu, H.; Gao, J.; Nie, J.; Wang, Y.; Tian, Z.; He, C.; Wang, J.; Ji, C.; et al. Fluorescence and optical activity of chiral CdTe quantum dots in their interaction with amino acids. ACS Nano 2020, 14, 4196–4205. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Ghosh, D.C. On the electrophilic character of molecules through its relation with electronegativity and chemical hardness. Int. J. Mol. Sci. 2012, 13, 2160–2175. [Google Scholar] [CrossRef]
- Yu, J.; Su, N.Q.; Yang, W. Describing chemical reactivity with frontier molecular orbitalets. JACS Au 2022, 2, 1383–1394. [Google Scholar] [CrossRef]
- Prasad, G.; Tamang, S.; Jha, S.; Bhattacharyya, N.K.; Biswas, J. A theoretical insight into graphene-based materials: A DFT study. Results Surf. Interfaces 2025, 18, 100463. [Google Scholar] [CrossRef]
- Ganiev, B.; Mardonov, U.; Kholikova, G. Molecular structure, HOMO-LUMO, MEP-–Analysis of triazine compounds using DFT (B3LYP) calculations. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Sharma, B.P.; Adhikari Subin, J.; Panthi, K.P.; Pandey, S.K.; Ahamad, A.; Sharma, M.L. Chemical synthesis, characterization, and computational investigation of two schiff bases derived from 3-(2-hydroxyphenyl)-4-amino-4H-1,2,4-triazole-5-thiol and their corresponding oxovanadiu (IV) complexes. J. Nepal Chem. Soc. 2023, 43, 70–90. [Google Scholar] [CrossRef]
- Sharma, B.P.; Subin, J.A.; Marasini, B.P.; Adhikari, R.; Pandey, S.K.; Sharma, M.L. Triazole based schiff bases and their oxovanadium (IV) complexes: Synthesis, characterization, antibacterial assay, and computational assessments. Heliyon 2023, 9, e15239. [Google Scholar] [CrossRef] [PubMed]
- Rezvan, V.H. Molecular structure, HOMO-LUMO, and NLO studies of some quinoxaline 1,4-dioxide derivatives: Computational (HF and DFT) analysis. Results Chem. 2024, 7, 101437. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, C.; Shang, S.; Gao, W.; Zeng, P.; Jiang, S. Flexible, reusable SERS substrate derived from ZIF-67 by adjusting LUMO and HOMO and its application in identification of bacteria. ACS Appl. Mater. Interfaces 2020, 12, 49452–49463. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Y.; Xie, S.; Chen, H.; Ding, Q.; Zhang, W.; Zhang, L. A solid phase extraction column based on SiO2 @ZIF-8 for efficient analysis of domoic acid toxins in the seawater environment: Experiments and DFT calculations on adsorption behaviour. Anal. Methods 2023, 15, 6590–6602. [Google Scholar] [CrossRef]
- Sapkota, A.; Slade, T.J.; Huyan, S.; Nepal, N.K.; Wilde, J.M.; Furukawa, N.; Lapidus, S.H.; Wang, L.L.; Budko, S.L.; Canfield, P.C. First-order structural phase transition at low temperature in GaPt5P and its rapid enhancement with pressure. Phys. Rev. B 2024, 110, 024112. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, C.; Blankenfeldt, W.; Pessler, F.; Büssow, K. Effect of pH and buffer on substrate binding and catalysis by cis-aconitate decarboxylase. Sci. Rep. 2025, 15, 5076. [Google Scholar] [CrossRef]
- Li, T.; Wang, X.; Wang, Y.; Zhang, Y.; Li, S.; Liu, W.; Liu, S.; Liu, Y.; Xing, H.; Otake, K.I.; et al. Microenvironmental modulation breaks intrinsic pH limitations of nanozymes to boost their activities. Nat. Commun. 2024, 15, 10861. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, Y.; Fan, Y.; Gong, L.; Zhu, X.; Zhang, Y.; Liu, M.; Yao, S. The pH-dependent multiple nanozyme activities of copper-cerium dioxide and its application in regulating intracellular oxygen and hydrogen peroxide levels. J. Colloid Interface Sci. 2023, 654, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, N.; Wang, J. Electrochemical synthesis of (poly)dimethoxyaniline on glassy carbon electrodes and their applications in the detection of L- and D-glutamic acids. J. Electrochem. Soc. 2019, 166, B3066–B3071. [Google Scholar] [CrossRef]
- Ci, Q.; Liu, J.; Qin, X.; Han, L.; Li, H.; Yu, H.; Lim, K.L.; Zhang, C.W.; Li, L.; Huang, W. Polydopamine dots-based fluorescent nanoswitch assay for reversible recognition of glutamic acid and Al3+ in human serum and living cell. ACS Appl. Mater. Interfaces 2018, 10, 35760–35769. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhang, S.; Lang, Q.; Song, J.; Han, L.; Liu, A. Amperometric L-glutamate biosensor based on bacterial cell-surface displayed glutamate dehydrogenase. Anal. Chim. Acta 2015, 884, 83–89. [Google Scholar] [CrossRef] [PubMed]
| Enzyme Mimics | H2O2 | OPD | Specific Activity (μmol·min−1·mg−1) | TOF (min) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Km (mM) | Vmax (10−8 M/S) | Km (mM) | Vmax (10−8 M/S) | ||||
| CDs (Colorimetry) | 0.7568 | 1.086 | 6.81 | 0.76 | – | – | [28] |
| Cu23 NC (Colorimetry) | 1.0 | 0.00467 | 1.09 | 0.02 | – | 12.05 | [29] |
| HRP (Colorimetry) | 0.15 | 0.077 | 1.800 | 0.120 | – | 1.32 | [30] |
| MIL-3DG-75 (Fluorometry) | 0.029 | 0.011 | 49.5 | 18 | – | – | [31] |
| Cu-CDs (Fluorometry) | – | – | 0.588 | 4.256 | – | – | [32] |
| Hemin-histamine pair | 1.37 | 5.29 | 1.63 (ABTS) | 11.70 (ABTS) | 36.45 | 1.758 | [33] |
| Hemin | – | – | 2.33 (ABTS) | 0.97 (ABTS) | 0.63 | 0.144 | |
| MnxOy NWs@ZIF-8-RD (Fluorometry) | 1.066 | 840.52 | 0.1863 | 64.76 | 1.284 (Composite material); 24.69 (Mn total); 17.30 (MnxOy) | 1.358 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.-Y.; Luo, M.-L.; Dai, J.-J.; Yang, F.-Q. Mn Oxide Nanowire/ZIF-8 Composites with Multiple Enzyme-like Activities for Enantioselective Glutamate Sensing. Biosensors 2025, 15, 771. https://doi.org/10.3390/bios15120771
Chen G-Y, Luo M-L, Dai J-J, Yang F-Q. Mn Oxide Nanowire/ZIF-8 Composites with Multiple Enzyme-like Activities for Enantioselective Glutamate Sensing. Biosensors. 2025; 15(12):771. https://doi.org/10.3390/bios15120771
Chicago/Turabian StyleChen, Guo-Ying, Mao-Ling Luo, Jing-Jing Dai, and Feng-Qing Yang. 2025. "Mn Oxide Nanowire/ZIF-8 Composites with Multiple Enzyme-like Activities for Enantioselective Glutamate Sensing" Biosensors 15, no. 12: 771. https://doi.org/10.3390/bios15120771
APA StyleChen, G.-Y., Luo, M.-L., Dai, J.-J., & Yang, F.-Q. (2025). Mn Oxide Nanowire/ZIF-8 Composites with Multiple Enzyme-like Activities for Enantioselective Glutamate Sensing. Biosensors, 15(12), 771. https://doi.org/10.3390/bios15120771






