Progress in the Design and Application of Chemical and Biological Sensors Based on Atom Transfer Radical Polymerization
Abstract
1. Introduction
2. Mechanisms and Types of ATRP Techniques
3. Polymeric Materials Synthesized by ATRP Techniques for Sensing Applications
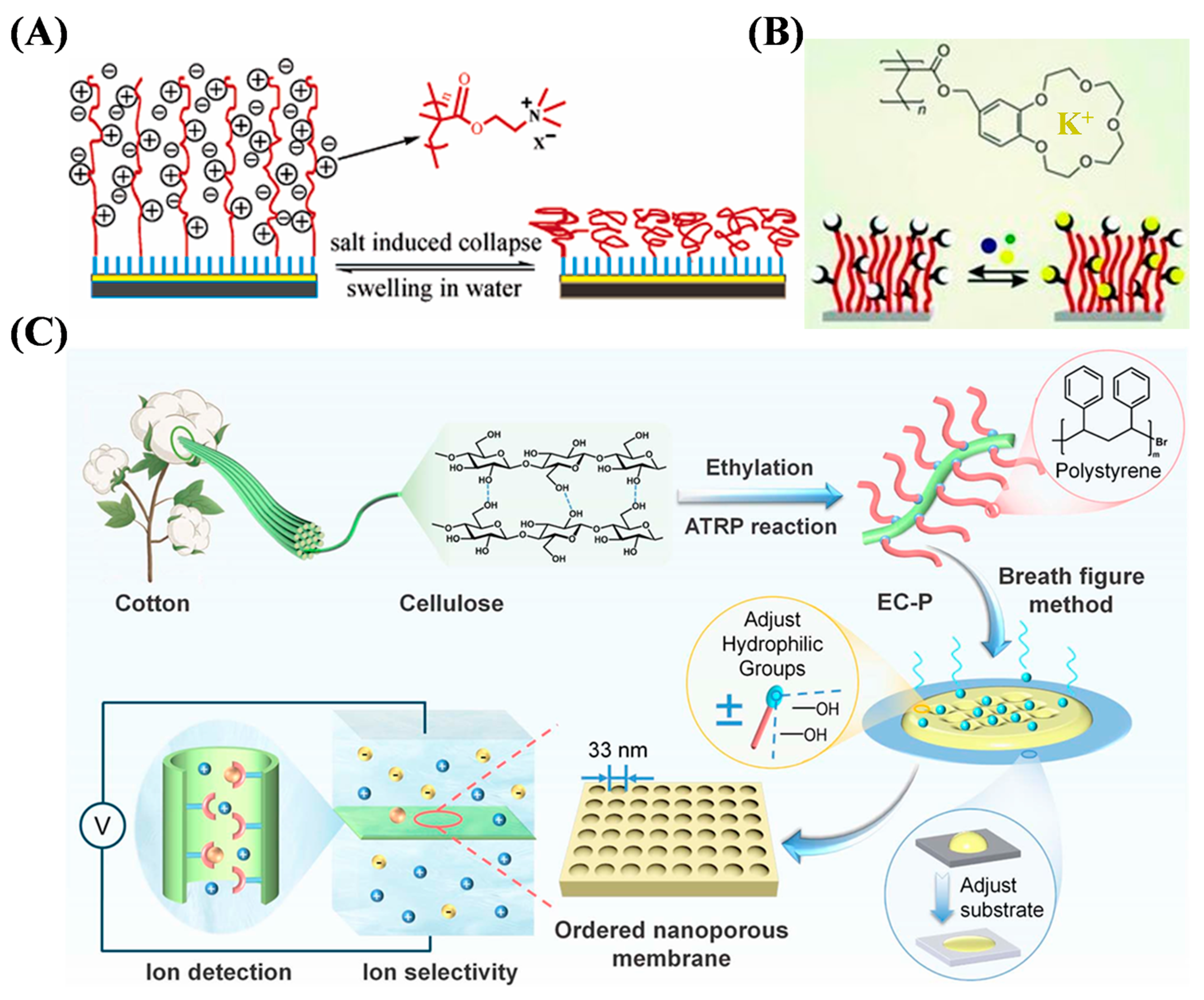
3.1. Ion Sensing
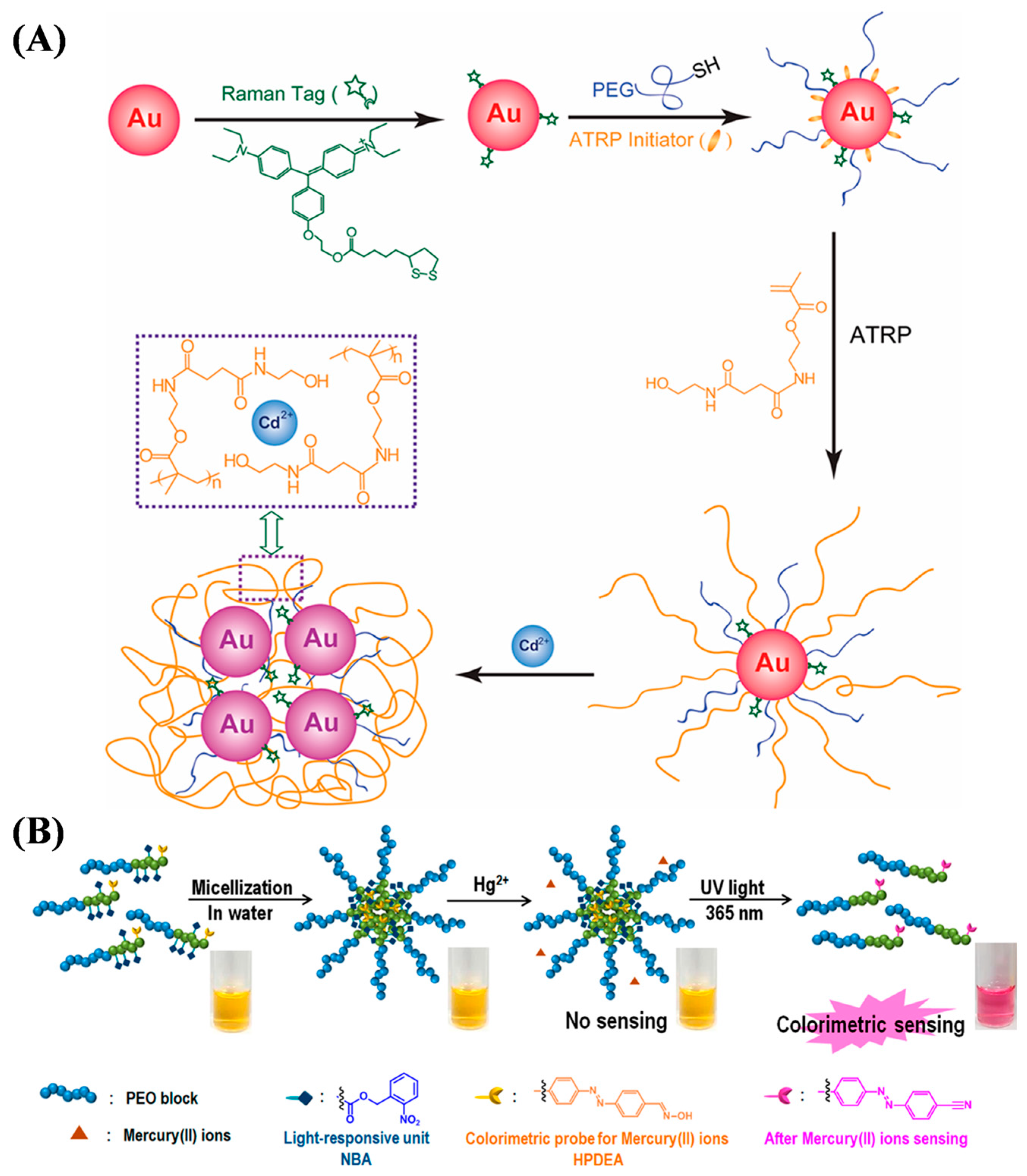
| Method | Material | Ion | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|
| QCM | Benzo-15-crown-5 polymer | K+ | 0.25–5 mM | – | [46] |
| DPASV | Polyacrylamide | Pb2+ | 3 × 10−3–2000 ng/mL | 0.37 μg/mL | [47] |
| DPASV | PAM/PMAA | Pb2+ | 10−8–0.1 mM | 2.5 pM | [48] |
| DPASV | PAN-g-GO | Hg(II) | 1 × 10−4–2 μM | 0.06 nM | [49] |
| I–V | EC-P | Cu2+ | 10−7–0.1 nM | 0.1 fM | [50] |
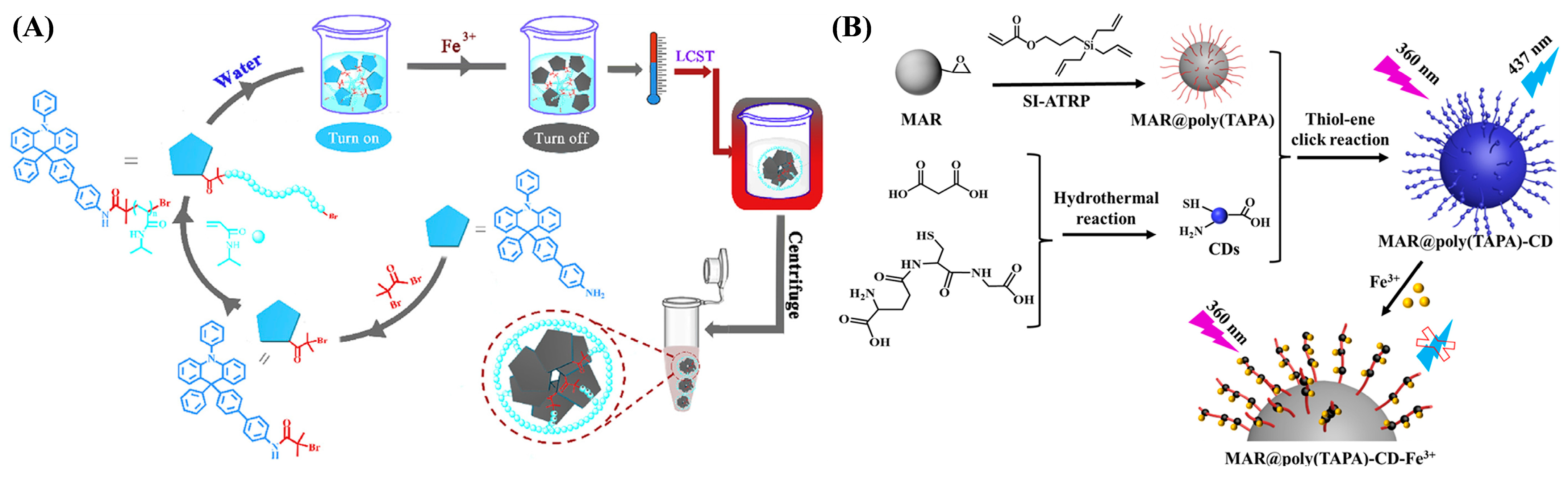
| Fluorescence | DPDHR-PNIPAM | Fe3+ | 0–0.65 μM | 1.32 μM | [51] |
| Fluorescence | MAR@poly(TAPA)-CD | Fe3+ | 10–80 nM | 9.74 nM | [52] |
| Fluorescence | PSaAEMA-co-PMPC | Zn2+ | 0–14 mM | – | [53] |
| Fluorescence | PVP-NDHIP | Al3+ | – | 3.9 nM | [31] |
| Fluorescence | Cellulose-g-PPFMA | Hg2+ | 0–10 mM | 0.5 μM | [55] |
| SERS | AuNPs | Cd2+ | 1–25 μM | 1 μM | [56] |
| Color | PEO113-b-[p(NBA10-co-FPDEA3)] | Hg(II) | 1–10 mM | 0.2 mM | [57] |
| Color | p(DMAEMA-co-HPDEA) | Hg(II) | 4 × 10−2–0.44 mM | 0.03 mM | [58] |
| Color | SP PNVCL | Fe2+ | 1.7 × 10−2–0.333 mM | 2.98 μM | [59] |
| Color | Cotton-PSMP-TMPyP | Cd2+ | 0.2–2 mM | 0.2 mM | [60] |
| LSPR | VCHR | Cu2+ | 25–400 pg/mL | 25 pg/mL | [61] |
3.2. Sensing of Small Molecules
3.2.1. Electrochemical Sensing
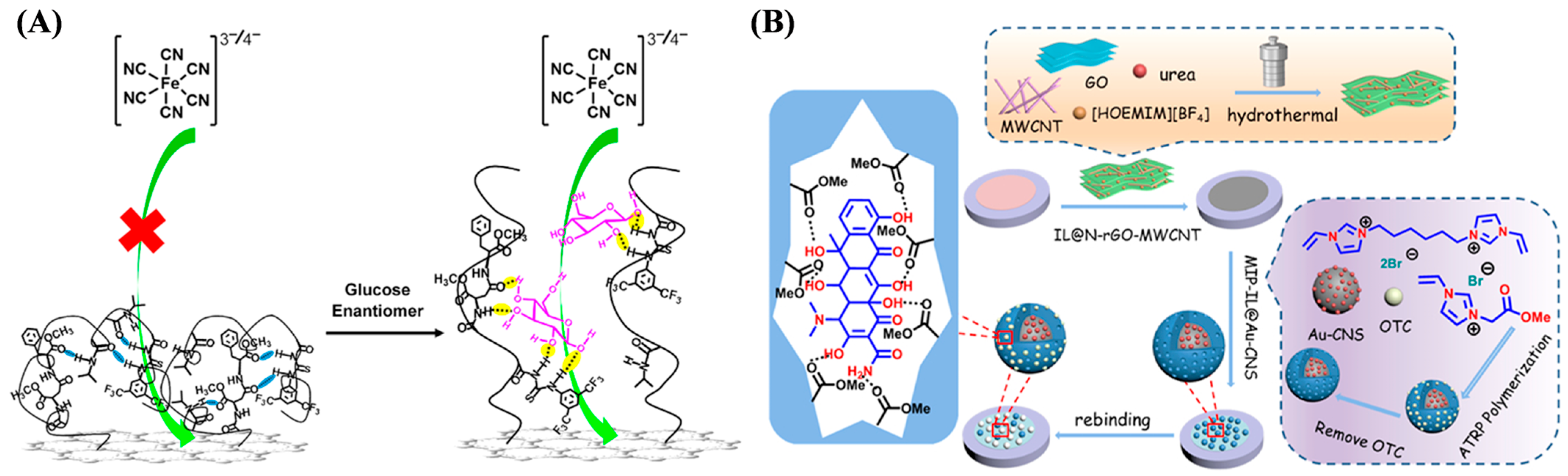
| Method/Material | Target | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|
| MIP-IL@Au-CNS | Oxytetracycline | 10−2–20 μM | 5 nM | [62] |
| MWCNTs-g-HTPB-b-PABFC | Trichlorfon | 1–106 nM | 35 nM | [63] |
| PNIPAM@SiO2 | H2O2 | 0.1–333 mM | 0.07 μM | [64] |
| MIP/AuNCs/Ni | Erythromycin | 10–108 pg/L | 3.2 pg/L | [67] |
| Polymer/graphene | D-glucose | 5 × 10−5–0.5 mM | 1 nM | [68] |
| MGO-P(4-VBA) | Glucose | 1–15 mM | 39 μM | [73] |
| Vinyl-PBA-based MIP | Dopamine | 0.04–20 μM | 96 nM | [74] |
3.2.2. Optical Sensing
| Method/Material | Target | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|
| FMIP | 2,4-D | 0–25 µM | 0.13 µM | [77] |
| FMIP | Fenvalerate | 0–80 nM | 0.068 nM | [78] |
| MIP-QD | Tetracycline | 0.5–50 μM | 0.14 μM | [79] |
| CdTe QD@MIP | 2,4-D | 1–10 μM | 0.14 μM | [80] |
| Phenylene(vinylene) polymer | Picric acid | – | 50 ppb | [81] |
| SiO2/ZnO/MIP | Cyhalothrin | 1–80 μM | 0.13 μM | [82] |
| Mn-doped ZnS QDs | Bifenthrin | 5–50 μM | 16.7 ng/mL | [83] |
| BiPO4@GO-MMIPs | Ciprofloxacin | 39–740 μg/L | 0.39 μg/L | [84] |
| SiO2-MPS@FMIP | λ-cyhalothrin | 2–80 nM | 3.7 nM | [85] |
| MAR@CD-MIP | 2,4-D | 18–72 μM | 0.35 μM | [86] |
| DSMIP@Mn3O4 | Tetracycline | 0.5–150 μM | 0.1 μM | [87] |
| Ag/CdTe/MIP | 2,6-DCP | 1–1000 nM | 1 nM | [88] |
| Cu2O@Ag-MIP | Chlorophenol | 10−5–1 mM | 5.8 nM | [89] |
| GO-g-qPDMAEMA | Enrofloxacin | 278–835 nM | 1 nM | [90] |
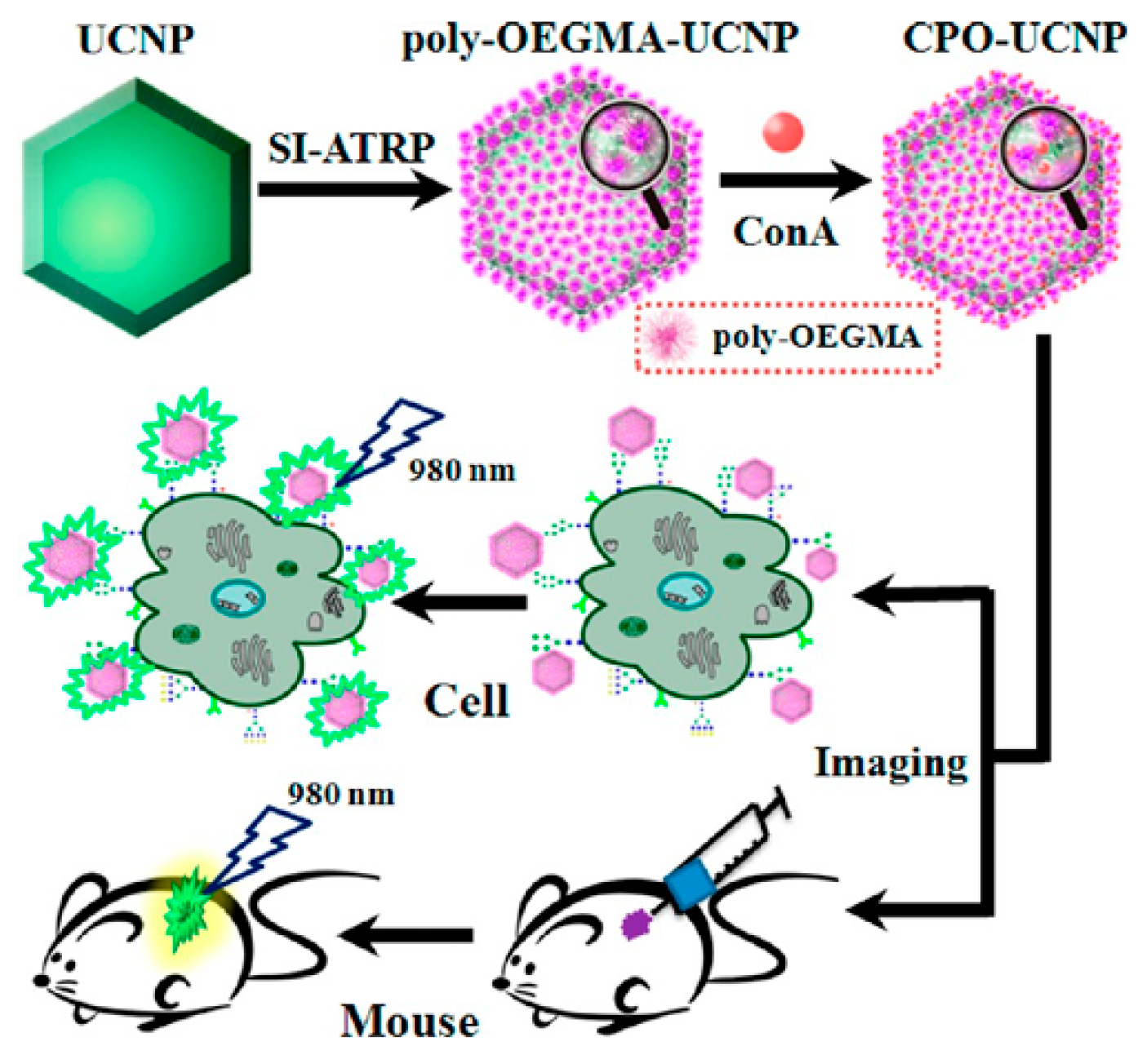
3.3. Bioimaging
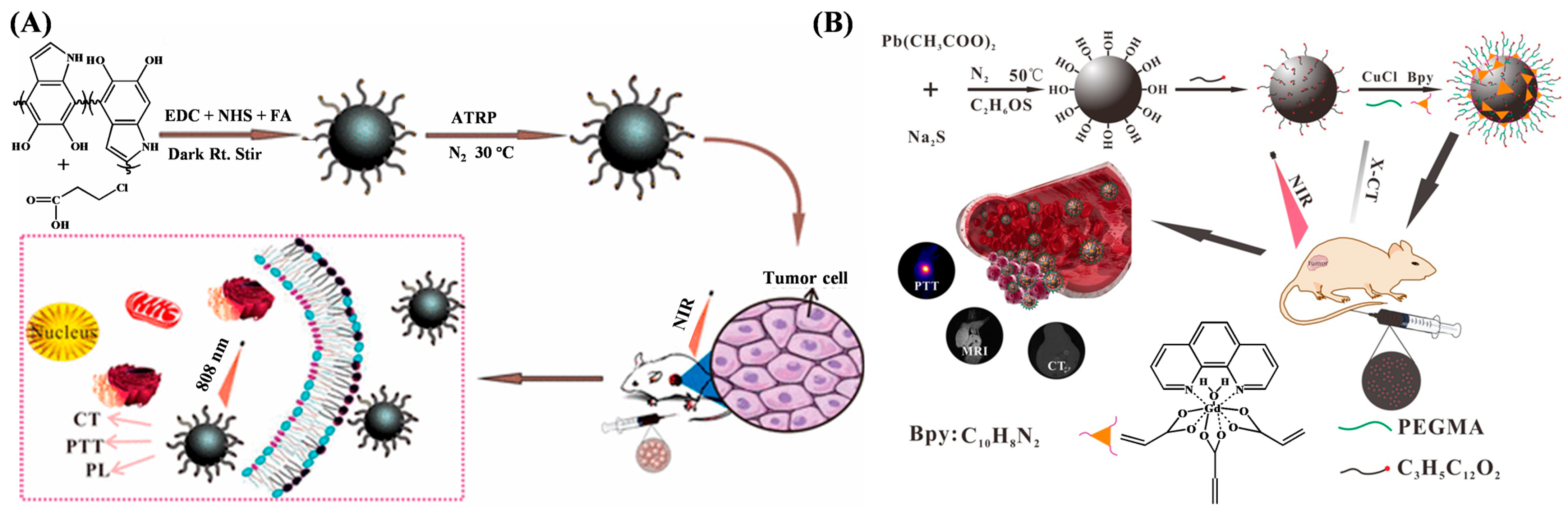
4. ATRP-Based Signal Amplification for Biosensors
4.1. Electrochemical Biosensors
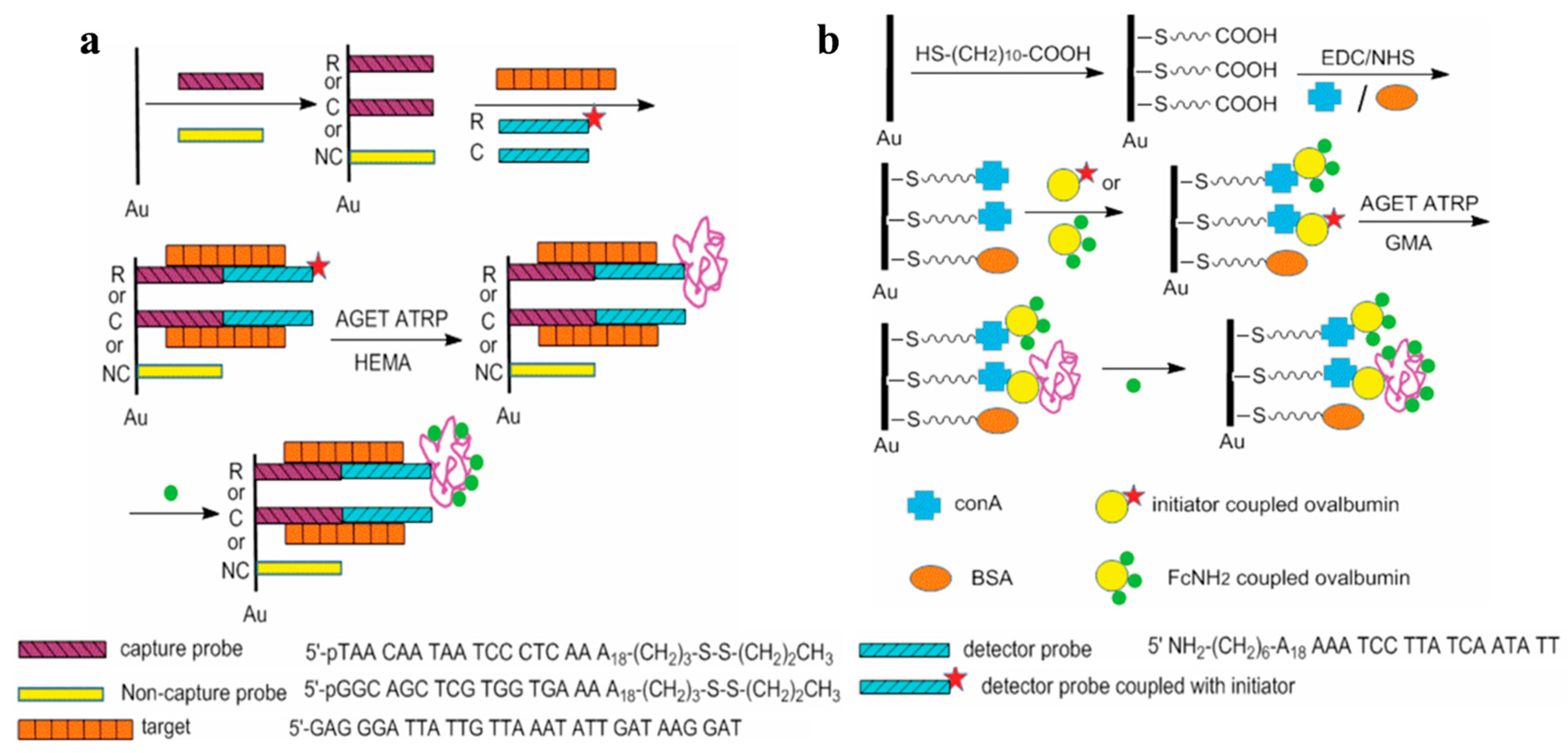
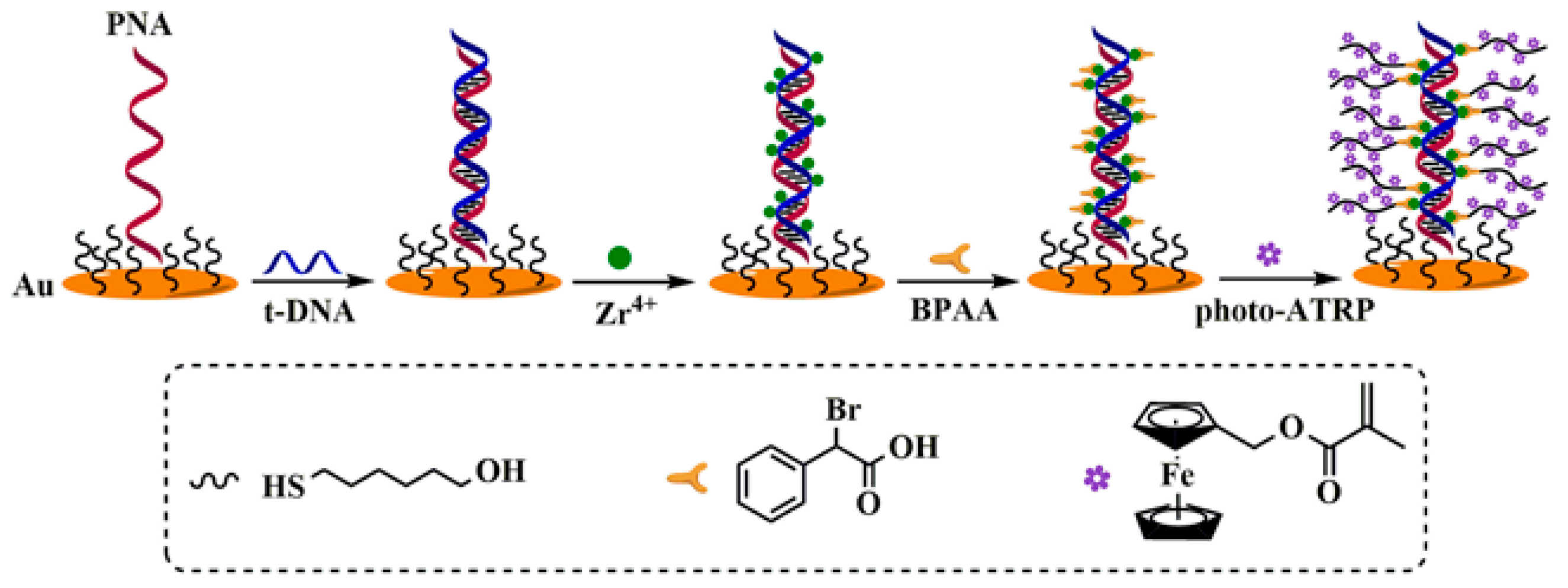
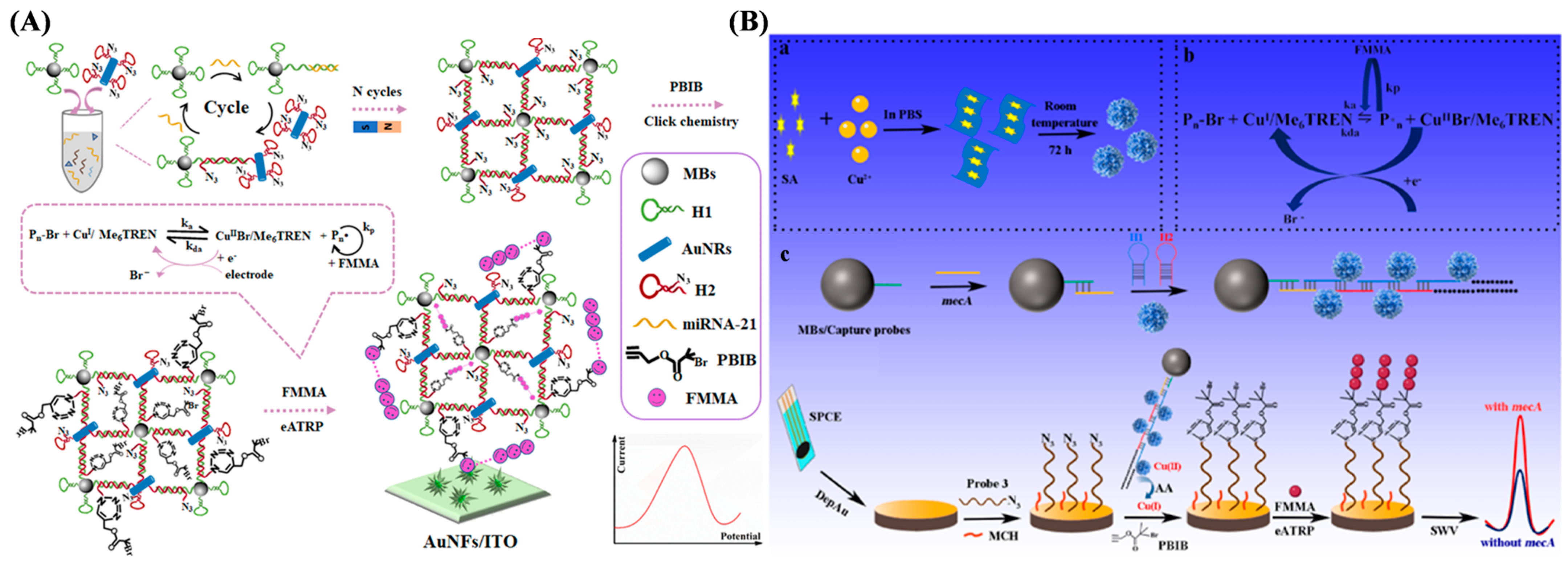
4.1.1. Electrochemical Sensing of Nucleic Acids
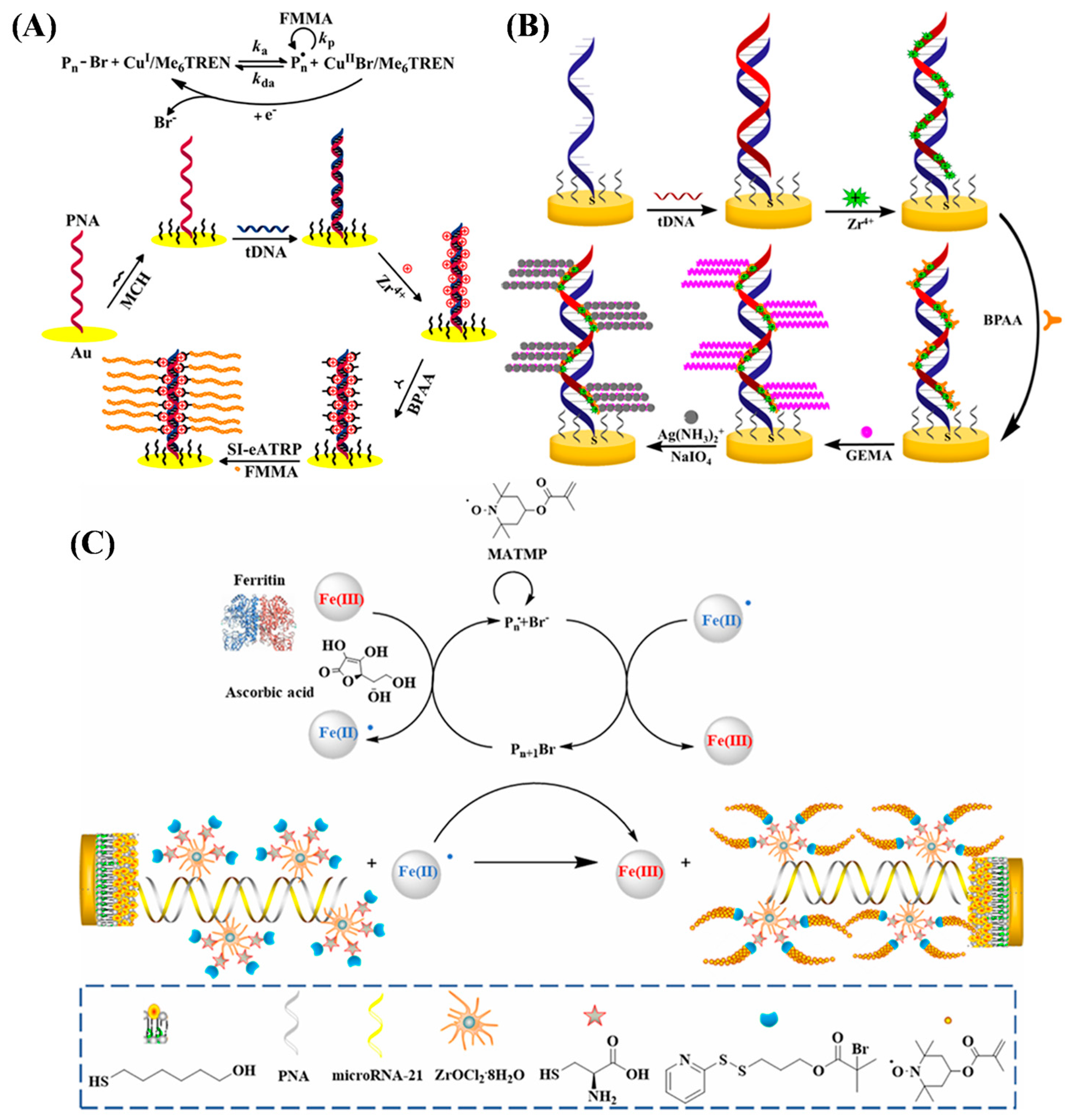
4.1.2. Electrochemical Aptasensors
4.1.3. Electrochemical Sensing of Enzymes
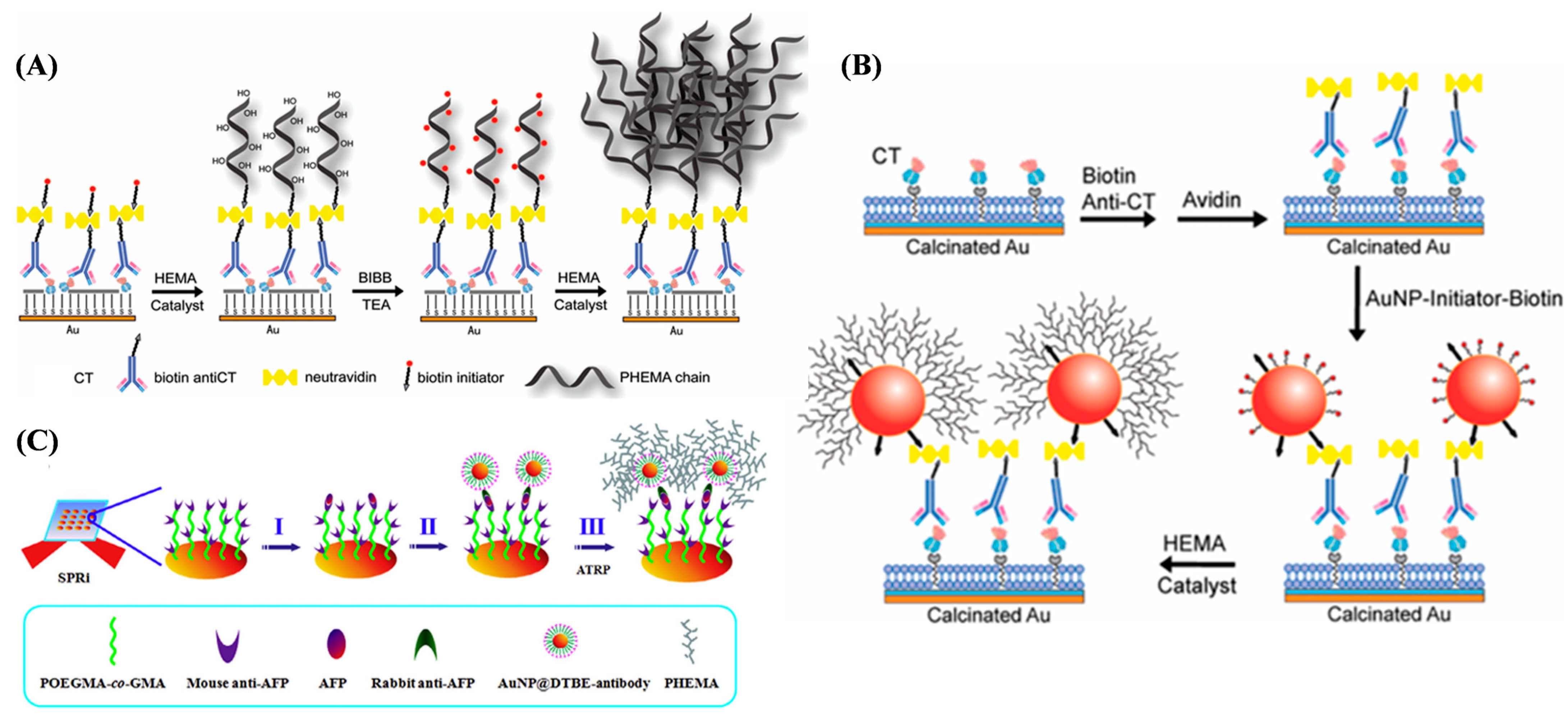
4.1.4. Electrochemical Immunoassays of Proteins and Others
| Biosensor | Analyte | Signal Amplification | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|
| Nucleic acid sensing | DNA | AGET ATRP | 0.1–1000 nM | 15 pM | [106] |
| DNA | AGET ATRP | 1–100 nM | 1 nM | [107] | |
| DNA | eATRP | 10−4–0.1 nM | 0.072 fM | [108] | |
| DNA | eATRP | 10−5–10 pM | 0.2 aM | [109] | |
| DNA | eATRP | 10−7–0.1 nM | 9.04 aM | [110] | |
| DNA | eATRP | 10−4–10 pM | 25 aM | [111] | |
| DNA | eATRP | 10−5–10 pM | 4.725 a M | [112] | |
| DNA | eATRP | 10−6–10 pM | 0.487 aM | [113] | |
| DNA | eATRP | 10−6–1 nM | 0.47 fM | [114] | |
| DNA | Hb-ATRP | 10−2–10 nM | 15.96 fM | [115] | |
| miRNA-21 | Ft-ATRP | 10−2–100 pM | 6.03 fM | [116] | |
| DNA | Photo-ATRP | 10−5–10 pM | 79 aM | [117] | |
| DNA | Photo-ATRP | 1–105 fM | 0.115 fM | [118] | |
| DNA | Photo-ATRP | 10−5–1 nM | 3.16 fM | [119] | |
| DNA | Photo-ATRP | 10−4–10 pM | 1.98 aM | [120] | |
| TMV RNA | Photo-ATRP | 0.01–10 nM | 3.5 fM | [121] | |
| RNA | PET-ATRP | 10−6–0.1 nM | 0.12 fM | [122] | |
| DNA | eATRP | 10−6–0.1 fM | 0.213 aM | [123] | |
| miRNA-141 | ATRP | 10−5–10 pM | 3.23 aM | [124] | |
| TMV RNA | eATRP | 10−4–10 nM | 2.61 fM | [128] | |
| DNA | eATRP | 10−2–10 fM | 1.954 aM | [129] | |
| miRNA-21 | Cu-ATRP | 10−8–0.1 nM | 4.96 aM | [131] | |
| miR-18a | eATRP | 10−4–50 pM | 2.5 aM | [132] | |
| miRNA-21 | eATRP | 10−9–1 nM | 0.32 aM | [133] | |
| mecA gene | eATRP | 10−4–10 pM | 0.06 fM | [134] | |
| Aptasensor | ERα | AGET ATRP | 10−5–10 ng/mL | 2.56 fg/mL | [135] |
| Bisphenol A | eATRP | 10−5–100 nM | 59 aM | [136] | |
| Acetamiprid | ATRP | 7 × 10−2–300 ng/mL | 19.26 pg/mL | [137] | |
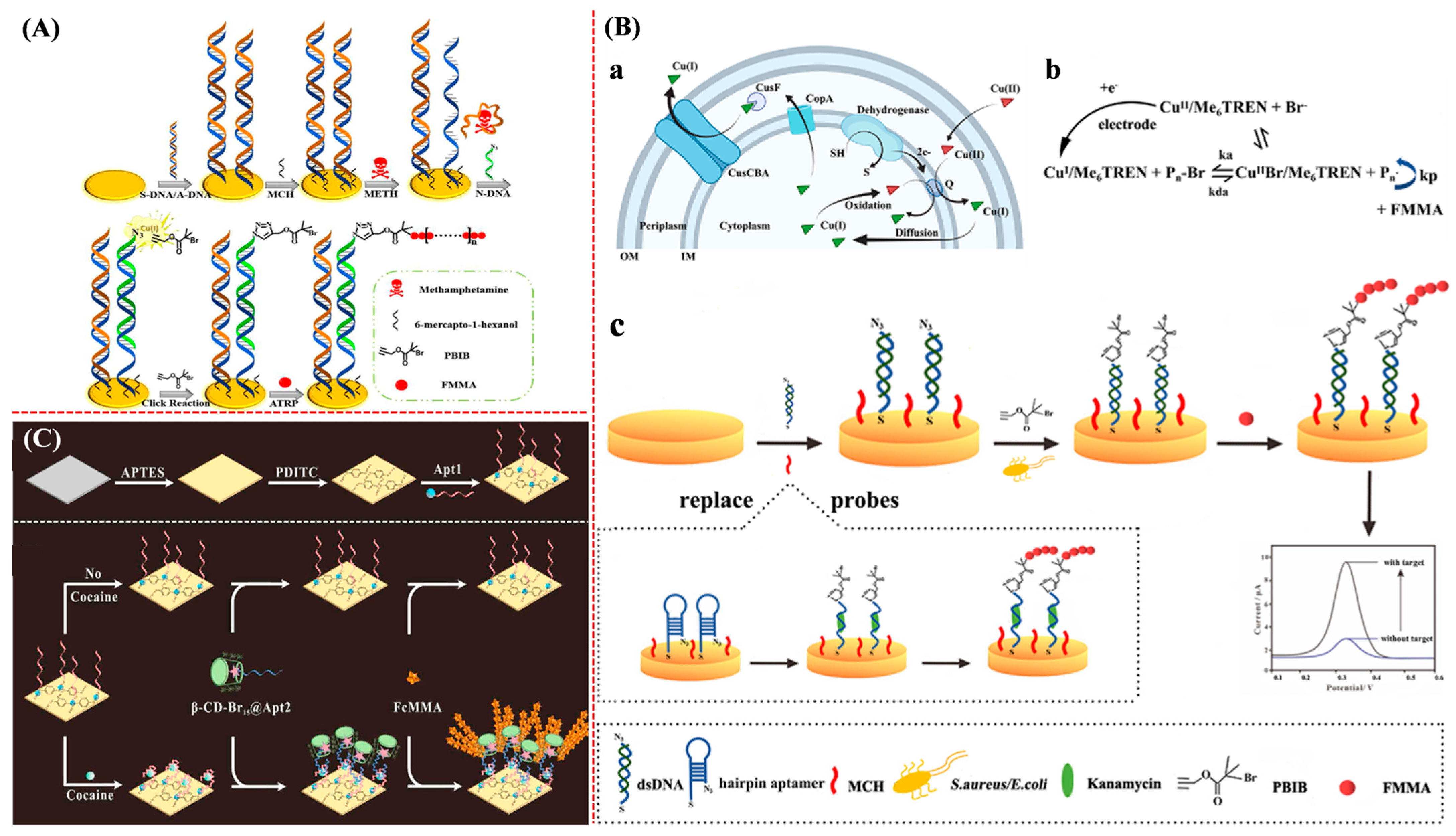
| METH | eATRP | 10−3–100 nM | 17 fM | [138] | |
| S. aureus and E. coli | eATRP | 102–107 CFU/mL | 4 and 6 CFU/mL | [139] | |
| Cocaine | AGET ATRP | 10−5–10 mg/mL | 0.0335 ng/mL | [140] | |
| Digoxin | eATRP | 1–40 pM | 0.59 pM | [141] | |
| CEA | eATRP | 10−3–102 ng/mL | 70.17 fg/mL | [142] | |
| HER2 | AGET ATRP | 10−5–10 µg/mL | 0.39 pg/mL | [143] | |
| LPS | Photo-ATRP | 10−3–0.1 pg/mL | 0.25 fg/mL | [144] | |
| AFP | eATRP | 10−3–1 ng/mL | 0.32 pg/mL | [146] | |
| Trastuzumab | eATRP | 5 × 10−2–50 ng/mL | 71.5 pg/mL | [147] | |
| Anti-Dig | eATRP | 10−3–200 nM | 1.5 pM | [148] | |
| Enzyme sensing | Tyrosinase | Photo-ATRP | 0.06–1 U/L | 0.048 U/L | [149] |
| ALP | AGET ATRP | 20–200 mU/mL | 1.64 mU/mL | [150] | |
| ALP | AGET ATRP | 5–100 mU/mL | 1.71 mU/m | [151] | |
| ALP | Photo-ATRP | 10–150 mU/mL | 2.12 mU/mL | [152] | |
| Protein kinase | eATRP | 0–140 mU/mL | 1.63 mU/mL | [153] | |
| MMP-2 | eATRP | 10−3–80 pM | 0.53 fM | [154] | |
| Trypsin | eATRP | 30–210 μU/mL | 16 μU/mL | [155] | |
| PSA | eATRP | 10−5–10 nM | 3.2 fM | [156] | |
| Thrombin | Photo-ATRP | 10−5–1 ng/mL | 4 fg/mL | [157] | |
| Immunosensor | CA153 | ATRP | 10−2–120 U/mL | 0.003 U/mL | [158] |
| CEA, AFP | PET-ATRP | 1.63 × 10−4–163, 10−4–100 ng/mL | 56.1 fg/mL and 32.8 fg/mL | [159] | |
| AFP | AGET ATRP | 10−4–100 ng/mL | 0.08 pg/mL | [160] | |
| AFP | SI-ATRP | 25–50,000 pg/mL | 0.183 pg/mL | [161] | |
| TNF-α | ATRP | 10−4–1 μg/mL | 3 pg/mL | [162] | |
| DR1 | ATRP | 5 × 10−4–5 × 102 | 0.159 pg/mL | [163] | |
| DR1 | ATRP | 10−4–102 ng/mL | 2.91 fg/mL | [164] | |
| CEA, AFP, CA125, and CA153 | AGET ATRP/HRP | 0.01–100, 0.01–100, 0.05–100, 5 × 10−2–100 ng/mL | 0.01, 0.01, 0.05, 0.05 ng/mL | [165] | |
| PSA | AGET ATRP/HRP | 5 × 10−3–20 ng/mL | 1.3 pg/mL | [166] | |
| IgG | SI-ATRP | 5–70 ng/mL | 0.3 ng/mL | [167] | |
| CYFRA 21–1 | Photo-ATRP | 10−5–1 ng/mL | 5.8 fg/mL | [168] | |
| CYFRA 21–1 | eATRP | 10−9 fg/mL–1 μg/mL | 0.8 fg/mL | [169] | |
| CA19-9 | ATRP | 10−4–100 U/mL | 39 µU/mL | [170] | |
| IgG | ATRP/HRP | 10−3–10 ng/mL | 0.73 and 0.09 pg/mL | [171] |
4.2. Optical Biosensors
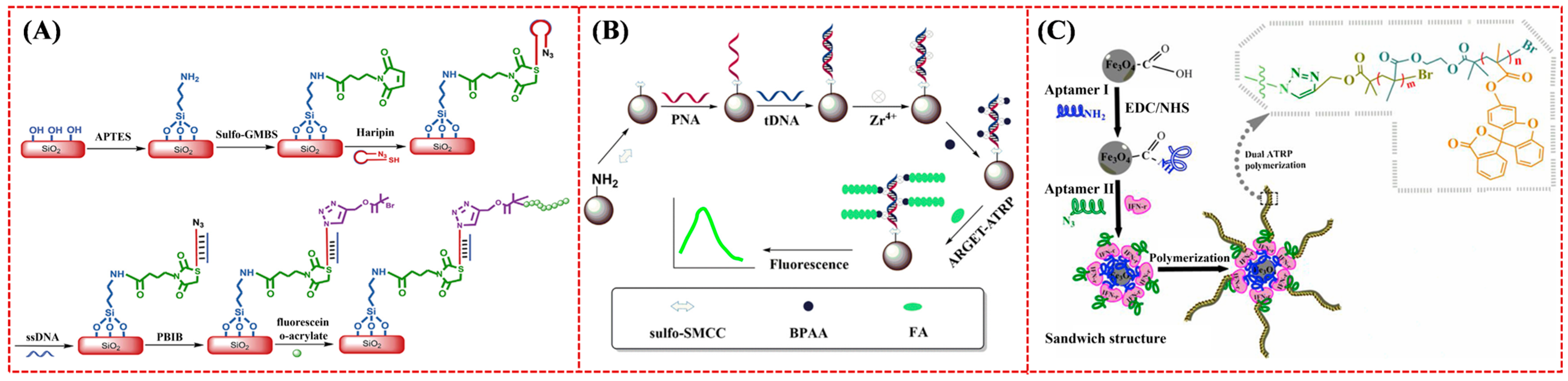
| Biosensor | Analyte | Signal Amplification | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|
| Nucleic acid sensing | DNA | ARGET ATRP | 10−7–1 nM | 23.8 aM | [194] |
| DNA | ARGET ATRP | 10−7–0.1 nM | 35.5 aM | [195] | |
| miRNA-144 | ARGET ATRP | 10−3–100 nM | 4.6 fM | [196] | |
| TMV RNA | ATRP | 10−4–10 nM | 1.14 fM | [197] | |
| DNA | REase and ATRP | 10−5–10 nM | 0.14 fM | [198] | |
| DNA | ATRP | 10−7–1 μM | 4.3 fM | [199] | |
| TMV RNA | DSN and ATRP | 10−2–100 pM | 1.03 fM | [201] | |
| DNA | Polysaccharide and ATRP | 10−7–0.1 nM | 78 aM | [202] | |
| DNA | REase and ATRP | 10−5–10 nM | 2.44 fM | [203] | |
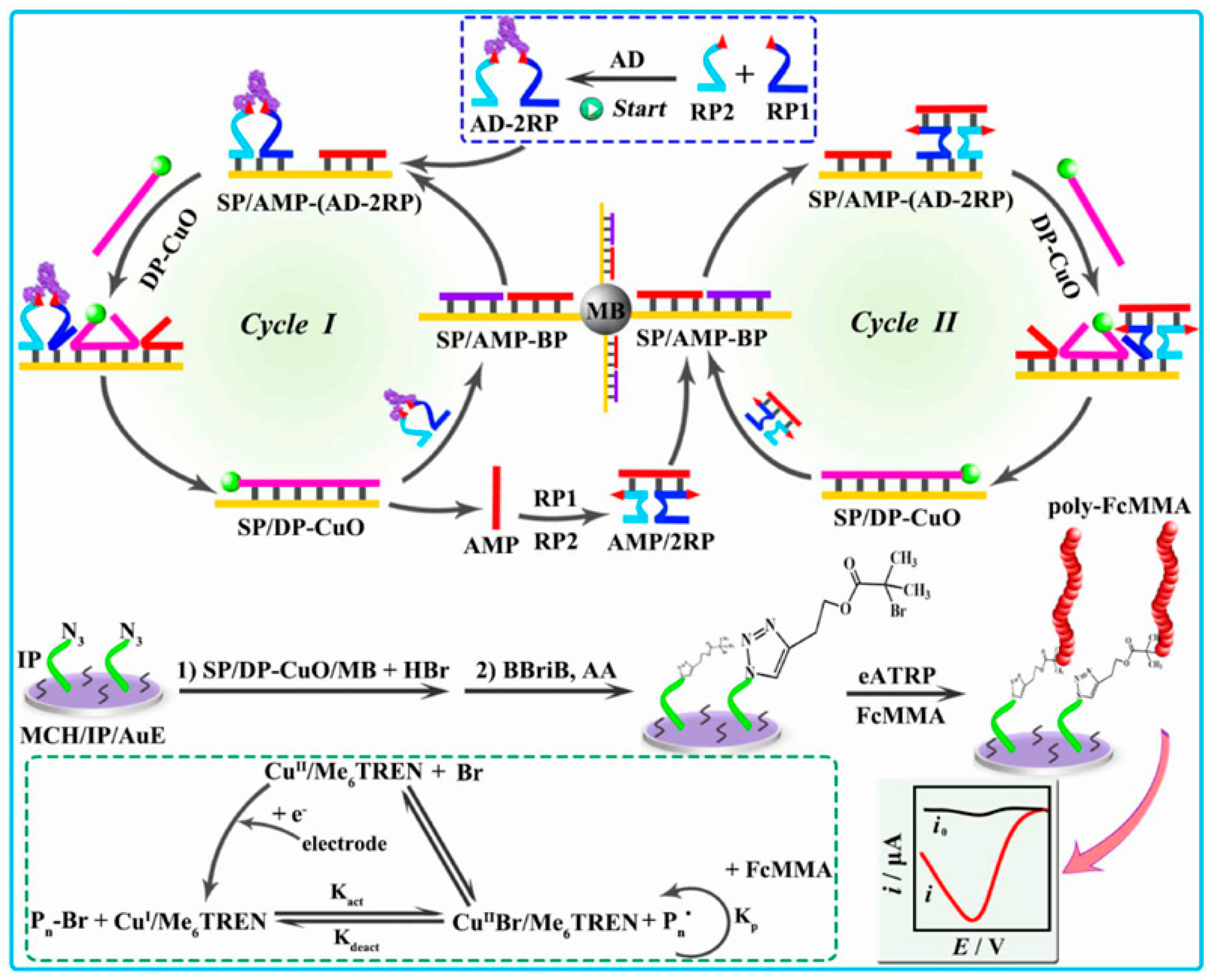
| Aptasensor | IFN-γ | Dual ATRP | 2 × 10−9–5 × 10 nM | 1.54 fM | [206] |
| OTA | ARGET ATRP | 2 × 10−3–2 × 103 ng/mL | 7.6 fg/mL | [204] | |
| BPA | ARGET ATRP | 10−4–100 nM | 6.6 fM | [205] | |
| CEA | β-CD and ATRP | 10−15–10−7 g/mL | 6.76 ag/mL | [207] | |
| Immunoassay | AFB1, OTA | ARGET ATRP | 5–250, 0.5–80 ng/mL | 426.18, 79.55 fg/mL | [208] |
| Exosome | ARGET ATRP | 5 × 104–5 × 109 exosomes/mL | 11,610 exosomes/mL | [29] | |
| CYFRA21-1 | UCNPs and ATRP | 10−3–0.1 ng/mL | 38.7 fg/mL | [209] | |
| CD81 | ATRP | 10−4–10 ng/mL | 0.067 pg/mL | [211] |
5. Conclusions, Challenges, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.-S.; Matyjaszewski, K. Controlled/”living” radical polymerization. Halogen atom transfer radical polymerization promoted by a Cu(I)/Cu(II) redox process. Macromolecules 1995, 2823, 7901–7910. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphosphine)ruthenium(II)/methylaluminum bis(2,6-di-tert-butylphenoxide) initiating system: Possibility of living radical polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Advanced materials by atom transfer radical polymerization. Adv. Mater. 2018, 30, 1706441. [Google Scholar] [CrossRef]
- Skandalis, A.; Sentoukas, T.; Selianitis, D.; Balafouti, A.; Pispas, S. Using RAFT polymerization methodologies to create branched and nanogel-type copolymers. Materials 2024, 17, 1947. [Google Scholar] [CrossRef] [PubMed]
- Siegwart, D.J.; Oh, J.K.; Matyjaszewski, K. ATRP in the design of functional materials for biomedical applications. Prog. Polym. Sci. 2012, 37, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk-Łagodzińska, M.; Plichta, A.; Dębowski, M.; Kowalczyk, S.; Iuliano, A.; Florjańczyk, Z. Recent advances in the application of ATRP in the synthesis of drug delivery systems. Polymers 2023, 15, 1234. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, S.; Ma, S.; Yan, F.; Weng, Z. Cytocompatible modification of thermoresponsive polymers on living cells for membrane proteomic isolation and analysis. Anal. Chem. 2019, 91, 3187–3194. [Google Scholar] [CrossRef]
- Yan, X.; Wei, F.; Gou, J.; Ji, M.; Hamouda, H.I.; Xue, C.; Zheng, H. Cryogel with modular and clickable building blocks: Toward the ultimate ideal macroporous medium for bacterial separation. J. Agric. Food Chem. 2024, 72, 15959–15970. [Google Scholar] [CrossRef]
- Yan, M.; Wang, X.; Zhao, Y.; Bai, Q.; Ma, S.; Bo, C.; Ou, J. Design and fabrication of acorn-like Janus molecularly imprinted materials for highly specific separation and enrichment of oxytetracycline from restaurant oily wastewater. Talanta 2025, 281, 126898. [Google Scholar] [CrossRef]
- Unsal, E.; Elmas, B.; Çağlayan, B.; Tuncel, M.; Patir, S.; Tuncel, A. Preparation of an ion-exchange chromatographic support by a “grafting from” strategy based on atom transfer radical polymerization. Anal. Chem. 2006, 78, 5868–5875. [Google Scholar] [CrossRef]
- Su, J.; He, X.; Chen, L.; Zhang, Y. A combination of “thiol−ene” click chemistry and surface initiated atom transfer radical polymerization: Fabrication of boronic acid functionalized magnetic graphene oxide composite for enrichment of glycoproteins. Talanta 2018, 180, 54–60. [Google Scholar] [CrossRef]
- Huang, C.; Tang, C.; Tang, R.; Gao, Z.; Ma, S.; Gong, B.; Ou, J. A combination of surface-initiated atom transfer radical polymerization and photo-initiated “thiol-ene” click chemistry: Fabrication of functionalized macroporous adsorption resins for enrichment of glycopeptides. J. Chromatogr. A 2023, 1689, 463774. [Google Scholar] [CrossRef]
- Yu, P.; He, X.; Zhang, L.; Mao, L. Dual recognition unit strategy improves the specificity of the adenosine triphosphate (ATP) aptamer biosensor for cerebral ATP assay. Anal. Chem. 2014, 87, 1373–1380. [Google Scholar] [CrossRef]
- Wong, A.K.Y.; Krull, U.J. Surfaces for tuning of oligonucleotide biosensing selectivity based on surface-initiated atom transfer radical polymerization on glass and silicon substrates. Anal. Chim. Acta 2009, 639, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brault, N.D.; Sundaram, H.S.; Huang, C.-J.; Li, Y.; Yu, Q.; Jiang, S. Two-layer architecture using atom transfer radical polymerization for enhanced sensing and detection in complex media. Biomacromolecules 2012, 13, 4049–4056. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wei, W.; Liu, S. Label-free electrochemical immunosensors based on surface-initiated atom radical polymerization. Biosens. Bioelectron. 2012, 38, 79–85. [Google Scholar] [CrossRef]
- Krause, J.E.; Brault, N.D.; Li, Y.; Xue, H.; Zhou, Y.; Jiang, S. Photoiniferter-mediated polymerization of zwitterionic carboxybetaine monomers for low-fouling and functionalizable surface coatings. Macromolecules 2011, 44, 9213–9220. [Google Scholar] [CrossRef]
- Huang, C.; Neoh, K.G.; Kang, E.-T. Combined ATRP and ‘click’ chemistry for designing stable tumor-targeting superparamagnetic iron oxide nanoparticles. Langmuir 2011, 28, 563–571. [Google Scholar] [CrossRef]
- Tajima, N.; Takai, M.; Ishihara, K. Significance of antibody orientation unraveled: Well-oriented antibodies recorded high binding affinity. Anal. Chem. 2011, 83, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Ozalp, V.C.; Oztekin, M.; Arica, M.Y. Rapid and label-free detection of Brucella melitensis in milk and milk products using an aptasensor. Talanta 2019, 200, 263–271. [Google Scholar] [CrossRef]
- Akkahat, P.; Mekboonsonglarp, W.; Kiatkamjornwong, S.; Hoven, V.P. Surface-grafted poly(acrylic acid) brushes as a precursor layer for biosensing applications: Effect of graft density and swellability on the detection efficiency. Langmuir 2012, 28, 5302–5311. [Google Scholar] [CrossRef]
- Hucknall, A.; Kim, D.H.; Rangarajan, S.; Hill, R.T.; Reichert, W.M.; Chilkoti, A. Simple fabrication of antibody microarrays on nonfouling polymer brushes with femtomolar sensitivity for protein analytes in serum and blood. Adv. Mater. 2009, 21, 1968–1971. [Google Scholar] [CrossRef]
- Bontempo, D.; Tirelli, N.; Feldman, K.; Masci, G.; Crescenzi, V.; Hubbell, J.A. Atom transfer radical polymerization as a tool for surface functionalization. Adv. Mater. 2002, 14, 1239–1241. [Google Scholar] [CrossRef]
- Ma, H.; Wells, M.; Beebe, T.P.; Chilkoti, A. Surface-initiated atom transfer radical polymerization of oligo(ethylene glycol) methyl methacrylate from a mixed self-assembled monolayer on gold. Adv. Funct. Mater. 2006, 16, 640–648. [Google Scholar] [CrossRef]
- Geetha, B.; Kumar, J.A.; Arthy, M.; Krithiga, T.; Kumar, G.S.; Roomi, A.B.; Shather, A.; Sillanpää, M. Conventional biosensors transformation into nanobiosensors: Spotlighting of current strategies, challenges, and recommended solutions for diverse applications. Chem. Pap. 2024, 78, 6225–6239. [Google Scholar] [CrossRef]
- Kim, S.; Sikes, H.D. Radical polymerization reactions for amplified biodetection signals. Polym. Chem. 2020, 118, 1424–1444. [Google Scholar] [CrossRef]
- Bai, Q.; Huang, C.; Ma, S.; Gong, B.; Ou, J. Rapid adsorption and detection of copper ions in water by dual-functional ion-imprinted polymers doping with carbon dots. Sep. Purif. Technol. 2023, 315, 123666. [Google Scholar] [CrossRef]
- Yang, H.; Jin, Z.; Cui, Z.; Guo, L.; Kong, J. A specific sensor system based on in-situ synthesis fluorescent polymers by ARGET ATRP achieving sensitive exosome detection. Talanta 2023, 253, 124059. [Google Scholar] [CrossRef]
- Sroka, M.; Zaborniak, I.; Chmielarz, P.; Bała, J.; Wolski, K.; Ciszkowicz, E.; Awsiuk, K.; Raczkowska, J. Grafting of multifunctional polymer brushes from a glass surface: Surface-initiated atom transfer radical polymerization as a versatile tool for biomedical materials engineering. Macromol. Chem. Phys. 2024, 225, 2300284. [Google Scholar] [CrossRef]
- Le, T.-N.; Prasannan, A.; Truong-Le, B.-T. Multifunctional fluorogenic probes from hydrazide schiff base-modified polyvinylpyrrolidone to detect Al3+ in aqueous environment and living cells. J. Photochem. Photobio. A Chem. 2023, 444, 114896. [Google Scholar] [CrossRef]
- Li, W.; Matyjaszewski, K. Star polymers via cross-linking amphiphilic macroinitiators by AGET ATRP in aqueous media. J. Am. Chem. Soc. 2009, 131, 10378–10379. [Google Scholar] [CrossRef]
- Chmielarz, P.; Fantin, M.; Park, S.; Isse, A.A.; Gennaro, A.; Magenau, A.J.; Sobkowiak, A.; Matyjaszewski, K. Electrochemically mediated atom transfer radical polymerization (eATRP). Prog. Polym. Sci. 2017, 69, 47–78. [Google Scholar] [CrossRef]
- De Bon, F.; Fantin, M.; Pereira, V.A.; Lourenço Bernardino, T.J.; Serra, A.C.; Matyjaszewski, K.; Coelho, J.F.J. Electrochemically mediated atom transfer radical polymerization driven by alternating current. Angew. Chem. Int. Ed. 2024, 63, e202406484. [Google Scholar] [CrossRef]
- Liu, S.; Ma, N.; Kong, J.; Zhang, X. Enzyme-mediated controlled polymerization and its application in biomolecular analysis. Microchem. J. 2025, 208, 112581. [Google Scholar] [CrossRef]
- Chen, G.; Guo, X.; Hu, B.; Lei, L. Heterogeneous catalysts catalyzed photo-atom transfer radical polymerization (Photo-ATRP). Macromol. Chem. Phys. 2024, 225, 2400249. [Google Scholar] [CrossRef]
- Harrisson, S.; Whitfield, R.; Anastasaki, A.; Matyjaszewski, K. Atom transfer radical polymerization. Nat. Rev. Method. Prime. 2025, 5, 2. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Current status and outlook for ATRP. Eur. Polym. J. 2024, 211, 113001. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Mozafari, M.; Saeb, M.R.; Zarrintaj, P.; Bencherif, S.A. Progress in ATRP-derived materials for biomedical applications. Prog. Mater. Sci. 2024, 143, 101248. [Google Scholar] [CrossRef]
- Fantin, M.; Isse, A.A. Improvement of electrode performance by grafting polymers via atom transfer radical polymerization (ATRP): Principles and applications. Curr. Opin. Electrochem. 2023, 40, 101313. [Google Scholar] [CrossRef]
- Dworakowska, S.; Lorandi, F.; Gorczyński, A.; Matyjaszewski, K. Toward green atom transfer radical polymerization: Current status and future challenges. Adv. Sci. 2022, 9, 2106076. [Google Scholar] [CrossRef]
- Hu, Q.; Gan, S.; Bao, Y.; Zhang, Y.; Han, D.; Niu, L. Controlled/”living” radical polymerization-based signal amplification strategies for biosensing. J. Mater. Chem. B 2020, 8, 3327–3340. [Google Scholar] [CrossRef]
- Ying, L.; Wu, Y.-F.; Yuan, L.; Liu, S.-Q. Application of atom transfer radical polymerization in biosensing. Chin. J. Anal. Chem. 2012, 40, 1797–1802. [Google Scholar] [CrossRef]
- Zhou, S.; Yuan, L.; Hua, X.; Xu, L.; Liu, S. Signal amplification strategies for DNA and protein detection based on polymeric nanocomposites and polymerization: A review. Anal. Chim. Acta 2015, 877, 19–32. [Google Scholar] [CrossRef]
- Zhou, F.; Hu, H.; Yu, B.; Osborne, V.L.; Huck, W.T.S.; Liu, W. Probing the responsive behavior of polyelectrolyte brushes using electrochemical impedance spectroscopy. Anal. Chem. 2007, 79, 176–182. [Google Scholar] [CrossRef]
- Schüwer, N.; Klok, H.A. A potassium-selective quartz crystal microbalance sensor based on crown-ether functionalized polymer brushes. Adv. Mater. 2010, 22, 3251–3255. [Google Scholar] [CrossRef]
- Sun, Y.; Du, H.; Deng, Y.; Lan, Y.; Feng, C. Preparation of polyacrylamide via surface-initiated electrochemical-mediated atom transfer radical polymerization (SI-eATRP) for Pb2+ sensing. J. Solid State Electrochem. 2016, 20, 105–113. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Zhao, M.; Liu, Y.; Zhang, J.; Lv, C. Preparation of block copolymers via metal-free visible-light-induced ATRP for the detection of lead ions. J. Appl. Polym. Sci. 2018, 135, 45863. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, N.; Chen, H.; Bai, L.; Xu, H.; Wang, W.; Yang, H.; Wei, D.; Yang, L. Fabrication of nanoprobe via AGET ATRP and photocatalytic modification for highly sensitive detection of Hg(II). React. Funct. Polym. 2019, 138, 70–78. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, X.; Wang, X.; Xiang, Z.; Li, H.; Ma, P.-C.; Qi, H. Ordered nanoporous cellulose-based membranes fabricated via breath figure method for copper ion detection. J. Mater. Chem. A 2025, 13, 11625–11636. [Google Scholar] [CrossRef]
- Cui, X.; Si, Z.; Li, Y.; Duan, Q. Synthesis of telechelic PNIPAM ended with 9,10-dihydroacridine group as a recyclable and specific Fe3+ detection fluorescent sensor. Dyes Pigments 2020, 173, 107873. [Google Scholar] [CrossRef]
- Wang, H.; Huang, C.; Ma, S.; Guo, S.; Gong, B.; Ou, J. Fabrication of bifunctional macroporous adsorption resin via grafting carbon dot and application in the detection and adsorption of iron (III) ion. Mater. Today Commun. 2023, 34, 105220. [Google Scholar] [CrossRef]
- Yan, R.; Wang, Z.; Du, Z.; Wang, H.; Cheng, X.; Xiong, J. A biomimetic fluorescent chemosensor for highly sensitive zinc(ii) detection and its application for cell imaging. RSC Adv. 2018, 8, 33361–33367. [Google Scholar] [CrossRef] [PubMed]
- Mardani, H.; Roghani-Mamaqani, H.; Shahi, S.; Salami-Kalajahi, M. Stimuli-responsive block copolymers as pH chemosensors by fluorescence emission intensification mechanism. Eur. Polym. J. 2022, 162, 110928. [Google Scholar] [CrossRef]
- Xu, L.Q.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. Rhodamine derivative-modified filter papers for colorimetric and fluorescent detection of Hg2+ in aqueous media. J. Mater. Chem. A 2013, 1, 2526. [Google Scholar] [CrossRef]
- Yin, J.; Wu, T.; Song, J.; Zhang, Q.; Liu, S.; Xu, R.; Duan, H. SERS-active nanoparticles for sensitive and selective detection of cadmium ion (Cd2+). Chem. Mater. 2011, 23, 4756–4764. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, H.-i. Polymeric micelles based on light-responsive block copolymers for the phototunable detection of mercury(II) ions modulated by morphological changes. ACS Appl. Mater. Interfaces 2018, 10, 34634–34639. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, H.-i. Thermo-tunable colorimetric detection of mercury(II) ions driven by the temperature-dependent assembly and disassembly of a block copolymer. Polym. Chem. 2019, 10, 4017–4024. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Liu, H. Dual responsive spiropyran-ended poly(N-vinyl caprolactam) for reversible complexation with metal ions. J. Polym. Res. 2019, 26, 89. [Google Scholar] [CrossRef]
- Liu, C.; Liang, X.; Liu, J.A.; Lei, X.; Zhao, X. Preparation of the porphyrin-functionalized cotton fiber for the chromogenic detection and efficient adsorption of Cd2+ ions. J. Colloid Interface Sci. 2017, 488, 294–302. [Google Scholar] [CrossRef]
- Chen, J.-K.; Zeng, X.-Y.; Cheng, C.-C.; Chen, C.-W. Fabrication of localized surface plasmon resonance sensors with scalable polyvinyltetrazole/copper cluster hybrid ring-array for Cu(II) detection. Talanta 2023, 256, 124282. [Google Scholar] [CrossRef]
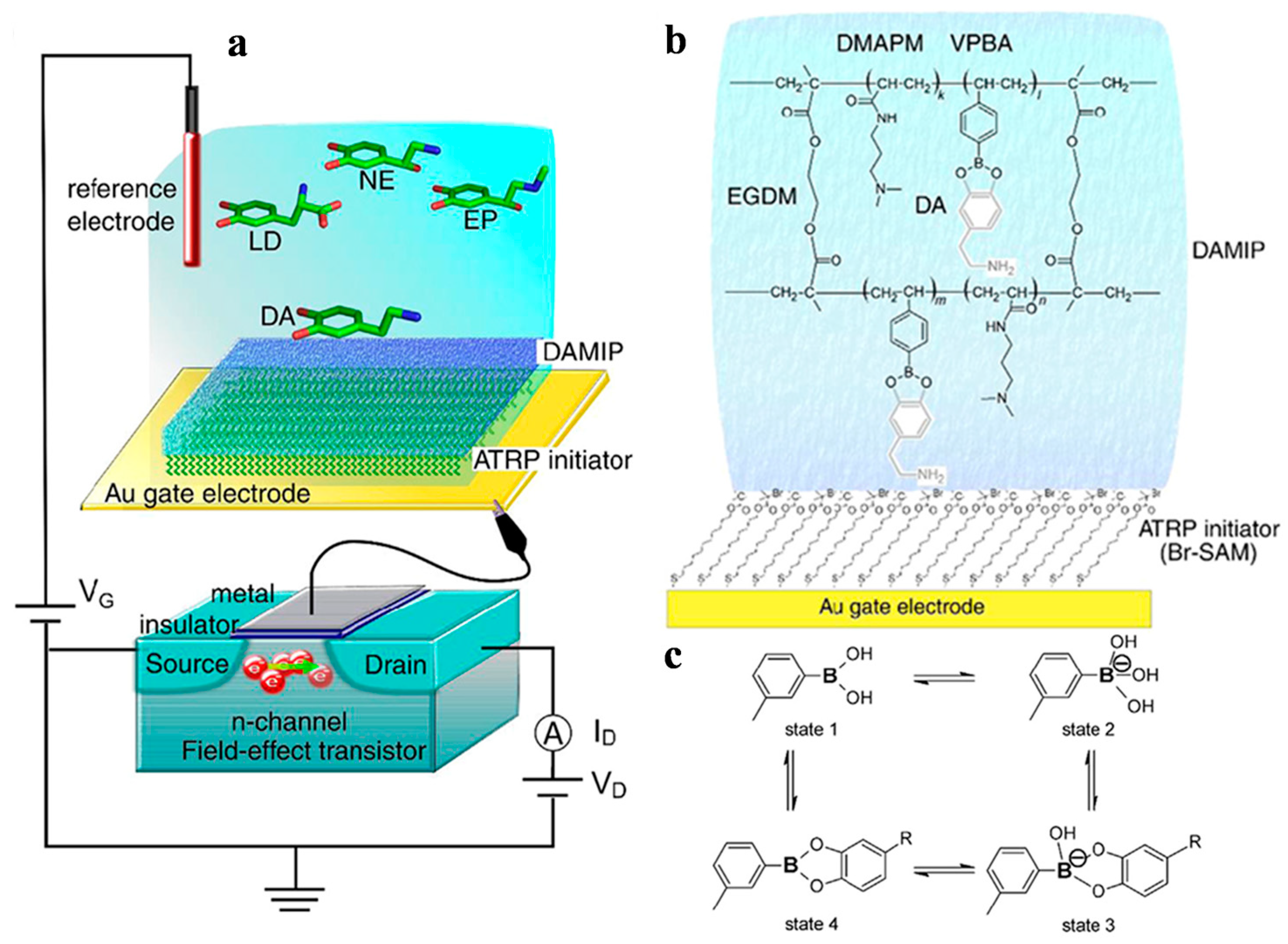
- Xu, X.; Niu, X.; Wu, S.; Zou, X.; Pan, J. A detachable and recyclable electrochemical sensor for high-performance detection of glucose based on boronate affinity. Sens. Actuat. B Chem. 2018, 268, 430–437. [Google Scholar] [CrossRef]
- Xu, F.; Cui, Z.-M.; Li, H.; Luo, Y.-L. Electrochemical determination of trace pesticide residues based on multiwalled carbon nanotube grafted acryloyloxy ferrocene carboxylates with different spacers. RSC Adv. 2017, 7, 7431–7441. [Google Scholar] [CrossRef]
- Manivannan, K.; Cheng, C.C.; Chen, J.K. Facile synthesis of poly (N-isopropylacrylamide) coated SiO2 core-shell microspheres via surface-initiated atom transfer radical polymerization for H2O2 biosensor applications. Electroanalysis 2017, 29, 1443–1450. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Yuan, S.J.; Zhu, X.L.; Neoh, K.G.; Kang, E.T. Enzyme-mediated amperometric biosensors prepared via successive surface-initiated atom-transfer radical polymerization. Biosens. Bioelectron. 2010, 25, 1102–1108. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Jin, M.; Bai, R.; Liu, Y.; Wu, Y.; Wang, W.; Feng, X.; Li, S. Fabrication of superoxide dismutase (SOD) imprinted poly(ionic liquid)s via eATRP and its application in electrochemical sensor. Electroanalysis 2020, 32, 1772–1779. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, M.; Liu, Y.; Fu, L.; Li, S.; Yang, Y. Preparation of erythromycin imprinted polymer by metal-free visible-light–induced ATRP and its application in sensor. J. Solid State Electrochem. 2018, 23, 583–590. [Google Scholar] [CrossRef]
- Ding, S.; Cao, S.; Zhu, A.; Shi, G. Wettability switching of electrode for signal amplification: Conversion of conformational change of stimuli-responsive polymer into enhanced electrochemical chiral analysis. Anal. Chem. 2016, 88, 12219–12226. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Oliveira, D.; Oliveira, A.S.R.; Mendonça, P.V.; Coelho, J.F.J.; Moreira, F.T.C.; Sales, M.G.F. An innovative approach for tailoring molecularly imprinted polymers for biosensors—Application to cancer antigen 15-3. Biosensors 2024, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Kamon, Y.; Takeuchi, T. Molecularly imprinted nanocavities capable of ligand-binding domain and size/shape recognition for selective discrimination of vascular endothelial growth factor isoforms. ACS Sens. 2018, 3, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Saeki, T.; Sunayama, H.; Kitayama, Y.; Takeuchi, T. Orientationally fabricated zwitterionic molecularly imprinted nanocavities for highly sensitive glycoprotein recognition. Langmuir 2018, 35, 1320–1326. [Google Scholar] [CrossRef]
- Yang, J.C.; Park, J. Molecular imprinting of bisphenol A on silica skeleton and gold pinhole surfaces in 2D colloidal inverse opal through thermal graft copolymerization. Polymers 2020, 12, 1892. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, Y.; Liu, Y.; Zhao, F.; Zeng, B. Kill two birds with one stone: Selective and fast removal and sensitive determination of oxytetracycline using surface molecularly imprinted polymer based on ionic liquid and ATRP polymerization. J. Hazard. Mater. 2022, 434, 128907. [Google Scholar] [CrossRef]
- Kajisa, T.; Li, W.; Michinobu, T.; Sakata, T. Well-designed dopamine-imprinted polymer interface for selective and quantitative dopamine detection among catecholamines using a potentiometric biosensor. Biosens. Bioelectron. 2018, 117, 810–817. [Google Scholar] [CrossRef]
- Nishitani, S.; Sakata, T. Polymeric nanofilter biointerface for potentiometric small-biomolecule recognition. ACS Appl. Mater. Interfaces 2019, 11, 5561–5569. [Google Scholar] [CrossRef]
- Mardani, H.; Roghani-Mamaqani, H.; Shahi, S.; Salami-Kalajahi, M. Coumarin-containing block copolymers as carbon dioxide chemosensors based on a fluorescence quenching mechanism. ACS Appl. Polym. Mater. 2022, 4, 1816–1825. [Google Scholar] [CrossRef]
- Cui, Y.; Li, X.; Wang, X.; Liu, Y.; Hu, X.; Chen, S.; Qu, X. One-pot preparation of ratiometric fluorescent molecularly imprinted polymer nanosensor for sensitive and selective detection of 2,4-dichlorophenoxyacetic acid. Sensors 2024, 24, 5039. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Gao, L.; Li, X.; Wang, L.; Yan, Y.; Che, G.; Hu, B.; Lin, X.; Song, M. A fluorescent molecularly imprinted polymer sensor synthesized by atom transfer radical precipitation polymerization for determination of ultra trace fenvalerate in the environment. RSC Adv. 2016, 6, 81346–81353. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, H.; Zhang, H. Direct and highly selective drug optosensing in real, undiluted biological samples with quantum-dot-labeled hydrophilic molecularly imprinted polymer microparticles. ACS Appl. Mater. Interfaces 2016, 8, 15741–15749. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, C.; Liu, X.; Zhang, H. Efficient synthesis of the undiluted vegetable juice-compatible hydrophilic quantum dot-labeled fluorescent molecularly imprinted polymer microspheres via facile one-pot surface-initiated ARGET ATRP. Eur. Polym. J. 2024, 219, 113422. [Google Scholar] [CrossRef]
- Duraimurugan, K.; Siva, A. Phenylene(vinylene) based fluorescent polymer for selective and sensitive detection of nitro-explosive picric acid. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3800–3807. [Google Scholar] [CrossRef]
- Li, H.; Wei, X.; Xu, Y.; Lu, K.; Zhang, Y.; Yan, Y.; Li, C. A thin shell and “sunny shape” molecular imprinted fluorescence sensor in selective detection of trace level pesticides in river. J. Alloys Comp. 2017, 705, 524–532. [Google Scholar] [CrossRef]
- Wu, X.; Lv, X.; Wang, J.; Sun, L.; Yan, Y. Surface molecular imprinted polymers based on Mn-doped ZnS quantum dots by atom transfer radical polymerization for a room-temperature phosphorescence probe of bifenthrin. Anal. Methods 2017, 9, 4609–4615. [Google Scholar] [CrossRef]
- Kumar, S.; Karfa, P.; Majhi, K.C.; Madhuri, R. Photocatalytic, fluorescent BiPO4@Graphene oxide based magnetic molecularly imprinted polymer for detection, removal and degradation of ciprofloxacin. Mater. Sci. Eng. C 2020, 111, 110777. [Google Scholar] [CrossRef]
- Gao, L.; Han, W.; Yan, Y.; Li, X.; Li, C.; Hu, B. Expeditious quantitative analysis of λ-cyhalothrin depending on fluorescence quenching of fluorescent surface molecularly imprinted sensors. Anal. Methods 2016, 8, 2434–2440. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, C.; Ma, S.; Bo, C.; Gong, B.; Ou, J. Bifunctional fluorescent molecularly imprinted resin based on carbon dot for selective detection and enrichment of 2,4-dichlorophenoxyacetic acid in lettuce. Food Chem. 2024, 439, 138167. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xia, Y.; Liu, Y.; Tang, Y.; Zhao, F.; Zeng, B. Colorimetric and electrochemical detection platforms for tetracycline based on surface molecularly imprinted polyionic liquid on Mn3O4 nanozyme. Biosens. Bioelectron. 2022, 216, 114650. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Wang, X.; Jiang, J.; Zheng, J.; Yan, Y.; Li, C. High-performance composite imprinted sensor based on the surface enhanced Raman scattering for selective detection of 2,6-dichlorophenol in water. J. Raman Spectrosc. 2017, 49, 222–229. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, M.; Zhang, J.; Wang, Q.; Li, H. A molecularly imprinted nanoprobe incorporating Cu2O@Ag nanoparticles with different morphologies for selective SERS based detection of chlorophenols. Microchim. Acta 2019, 187, 59. [Google Scholar] [CrossRef]
- Rong, X.; Gong, S.; You, J.; Geng, L.; Yang, W.; Xu, H. Highly reproducible nanofilms assembled from quaternary ammonium polymer-functionalized graphene oxide and gold nanoparticles for sensitive SERS detection of antibiotics. Appl. Surf. Sci. 2025, 688, 162390. [Google Scholar] [CrossRef]
- Vu, T.H.; Yu, B.J.; Kim, M.I. Choline oxidase-incorporated ATRP-based cerium nanogels as nanozymes for colorimetric detection of hydrogen peroxide and choline. Biosensors 2024, 14, 563. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Q.; Yang, G.; Zhang, L.; Liu, Z.; Cheng, Z.; Zhu, X. Magnetic nanomaterials with near-infrared pH-activatable fluorescence via iron-catalyzed AGET ATRP for tumor acidic microenvironment imaging. J. Mater. Chem. B 2015, 3, 2786–2800. [Google Scholar] [CrossRef]
- De Leo, T.C.; Nascimento dos Santos, S.; Del Cistia Andrade, C.; Ricci, E.; Turato, W.M.; Lopes, N.P.; Oliveira, R.S.; Bernardes, E.S.; Dias-Baruffi, M. Engineering of galectin-3 for glycan-binding optical imaging. Biochem. Biophy. Res. Commun. 2020, 521, 674–680. [Google Scholar] [CrossRef]
- Zheng, H.; Lin, H.; Chen, X.; Sui, J.; Ullah khan, M.; Ramesh Pavase, T.; Han, X.; Cao, L. Tailor-made magnetic nanocomposite with pH and thermo-dual responsive copolymer brush for bacterial separation. Food Chem. 2021, 358, 129907. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Xie, X.; Liang, Y.; Cavalcanti-Adam, E.A.; Feng, W. Compact micropatterned chip empowers undisturbed and programmable drug addition in high-throughput cell screening. Adv. Mater. 2023, 36, 2306814. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-T.; Niu, R.-X.; Guo, F.; Zhou, Z.-M.; Zhang, X.-Q.; Peng, L.; Li, Z.-K.; Wang, Z. White light emissive tetraphenylethene molecular cage-based hybrid nanoparticles for intracellular long-term imaging. ACS Appl. Nano Mater. 2024, 7, 14549–14556. [Google Scholar] [CrossRef]
- Qi, G.; Hu, F.; Kenry; Chong, K.C.; Wu, M.; Gan, Y.H.; Liu, B. Bacterium-templated polymer for self-selective ablation of multidrug-resistant bacteria. Adv. Funct. Mater. 2020, 30, 2001338. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, S.; Xue, X.; Hajizadeh, S.; Yamazaki, T.; Ye, L. Cationic polymer brushes functionalized with carbon dots and boronic acids for bacterial detection and inactivation. ACS Omega 2025, 10, 14536–14546. [Google Scholar] [CrossRef]
- He, W.; Cheng, L.; Zhang, L.; Liu, Z.; Cheng, Z.; Zhu, X. Facile fabrication of biocompatible and tunable multifunctional nanomaterials via iron-mediated atom transfer radical polymerization with activators generated by electron transfer. ACS Appl. Mater. Interfaces 2013, 5, 9663–9669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Peng, B.; Tian, F.; Qin, W.; Qian, X. Facile preparation of well-defined hydrophilic core–shell upconversion nanoparticles for selective cell membrane glycan labeling and cancer cell imaging. Anal. Chem. 2013, 86, 482–489. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, F.; Liu, C.; Yu, C.; Zhang, M.; He, Q.; Xiong, Y.; Xu, Z.; Yang, S.; Liao, G. A novel nanotheranostic agent for dual-mode imaging-guided cancer therapy based on europium complexes-grafted-oxidative dopamine. Chem. Eng. J. 2019, 357, 237–247. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, Y.; Zhong, Y.; Liao, G.; Yu, C.; Xu, Z. Polydopamine-based tumor-targeted multifunctional reagents for computer tomography/fluorescence dual-mode bioimaging-guided photothermal therapy. ACS Appl. Bio Mater. 2019, 2, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, X.; Wang, G.; Deng, W.; Shen, Q.; Jiang, R.; Wang, W.; Fan, Q.; Huang, W. A perylene diimide zwitterionic polymer for photoacoustic imaging guided photothermal/photodynamic synergistic therapy with single near-infrared irradiation. J. Mater. Chem. B 2018, 6, 3395–3403. [Google Scholar] [CrossRef]
- Xie, L.; Zuo, X.; Wang, B.; Li, D.; Chang, W.; Ji, S.; Ding, D. Micelle-like nanoparticles for drug delivery and magnetically enhanced tumor chemotherapy. ACS Biomater. Sci. Eng. 2024, 10, 7527–7538. [Google Scholar] [CrossRef]
- Zou, Y.; Jin, H.; Sun, F.; Dai, X.; Xu, Z.; Yang, S.; Liao, G. Design and synthesis of a lead sulfide based nanotheranostic agent for computer tomography/magnetic resonance dual-mode-bioimaging-guided photothermal therapy. ACS Appl. Nano Mater. 2018, 1, 2294–2305. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S.; He, L. Electrochemical biosensing using amplification-by-polymerization. Anal. Chem. 2009, 81, 7015–7021. [Google Scholar] [CrossRef]
- Qian, H.; He, L. Polymeric macroinitiators for signal amplification in AGET ATRP-based DNA detection. Sens. Actuat. B Chem. 2010, 150, 594–600. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, Q.; Sun, G.; Kong, J.; Zhang, X. Electrochemically mediated surface-initiated de novo growth of polymers for amplified electrochemical detection of DNA. Anal. Chem. 2017, 89, 9253–9259. [Google Scholar] [CrossRef]
- Sun, H.; Qiu, Y.; Liu, Q.; Wang, Q.; Huang, Y.; Wen, D.; Zhang, X.; Liu, Q.; Liu, G.; Kong, J. Ultrasensitive DNA biosensor based on electrochemical atom transfer radical polymerization. Biosens. Bioelectron. 2019, 131, 193–199. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, H.; Zheng, X.; Li, J.; Jian, L.; Feng, W.; Kong, J. Dual signal amplification by polysaccharide and eATRP for ultrasensitive detection of CYFRA 21–1 DNA. Biosens. Bioelectron. 2020, 150, 111895. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Q.; Li, M.; Feng, W.; Yang, H.; Kong, J. Dual atom transfer radical polymerization for ultrasensitive electrochemical DNA detection. Bioelectrochemistry 2020, 133, 107462. [Google Scholar] [CrossRef]
- Sun, H.; Kong, J.; Wang, Q.; Liu, Q.; Zhang, X. Dual signal amplification by eATRP and DNA-templated silver nanoparticles for ultrasensitive electrochemical detection of nucleic acids. ACS Appl. Mater. Interfaces 2019, 11, 27568–27573. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Wen, D.; Li, X.; Yang, H.; Wang, D.; Kong, J. Electrochemical CYFRA21-1 DNA sensor with PCR-like sensitivity based on AgNPs and cascade polymerization. Anal. Bioanal. Chem. 2020, 412, 4155–4163. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Wang, Q.; Kong, J.; Li, L.; Zhang, X. Electrochemically mediated in situ growth of electroactive polymers for highly sensitive detection of double-stranded DNA without sequence preference. Biosens. Bioelectron. 2018, 101, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Liu, J.; Li, L.; Huang, W.; Qiu, W.; Zhang, J.; Kong, J.; Zhang, X. Hemoglobin-catalyzed atom transfer radical polymerization for ultrasensitive electrochemical DNA detection. Biosens. Bioelectron. 2022, 213, 114485. [Google Scholar] [CrossRef]
- Ma, N.; Zhao, Y.; Li, L.; Kong, J.; Zhang, X. Ferritin-enhanced direct microRNA detection via controlled radical polymerization. Anal. Chem. 2023, 95, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, L.; Kong, J.; Zhang, X. Metal-free DNA sensor based on 10-phenylphenothiazine photo-ATRP signal amplification. Microchem. J. 2023, 191, 108816. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, S.; Ma, N.; Kong, J.; Zhang, X. Rose bengal-mediated photoinduced atom transfer radical polymerization for high sensitivity detection of target DNA. Talanta 2023, 254, 124104. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Q.; Jian, L.; Ye, S.; Zheng, X.; Kong, J. Intramolecular photoinitiator induced atom transfer radical polymerization for electrochemical DNA detection. Analyst 2020, 145, 858–864. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, H.; Liu, J.; Kong, J.; Zhang, X. A novel electrochemical biosensor for lung cancer-related gene detection based on copper ferrite-enhanced photoinitiated chain-growth amplification. Anal. Chim. Acta 2021, 1179, 338843. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Gong, P.; Liu, Y.; Feng, W.; Yang, H. Photoinduced atom transfer radical polymerization combined with click chemistry for highly sensitive detection of tobacco mosaic virus RNA. Talanta 2021, 235, 122803. [Google Scholar] [CrossRef]
- Yu, S.; Xu, Q.; Zhang, F.; Kong, J.; Zhang, X. Visible-light-driven rhodamine 6G mediated in situ poly-ferrocene strategy for highly sensitive quantification of esophageal cancer biomarkers. Talanta 2025, 295, 128291. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Yang, H.; Wang, X.; Kong, J.; Zhang, X. Highly sensitive lung cancer DNA detection via GO enhancing eATRP signal amplification. Microchem. J. 2021, 160, 105766. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, H.; Wen, D.; Wang, L.; Li, L.; Kong, J.; Zhang, X. Ultrasensitive electrochemical detection of miRNA based on polymerization signal amplification. Talanta 2021, 235, 122744. [Google Scholar] [CrossRef]
- Jazani, A.; Kapil, K.; Murata, H.; Madadi, M.; Sobieski, J.; Mocny, P.; Kim, K.; Gil, R.R.; Matyjaszewski, K. Open-vessel and scalable synthesis of linear and branched poly(meth)acrylic acid via light-mediated atom transfer radical polymerization in water. Macromolecules 2025, 58, 6190–6202. [Google Scholar] [CrossRef]
- Kapil, K.; Jazani, A.M.; Szczepaniak, G.; Murata, H.; Olszewski, M.; Matyjaszewski, K. Fully oxygen-tolerant visible-light-induced ATRP of acrylatesin water: Toward synthesis of protein-polymer hybrids. Macromolecules 2023, 56, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Jazani, A.M.; Rawls, C.; Matyjaszewski, K. Photo-RDRP for everyone: Smartphone light-induced oxygen-tolerant reversible deactivation radical polymerization. Eur. Polym. J. 2024, 202, 112631. [Google Scholar] [CrossRef]
- Guo, X.; Chen, L.; Li, P.; Li, X.; Wang, J.; Guo, L.; Yang, H. Construction of electrochemiluminescence biosensor via click chemistry and ARGET-ATRP for detecting tobacco mosaic virus RNA. Anal. Biochem. 2022, 655, 114834. [Google Scholar] [CrossRef]
- Sun, H.; Qian, L.; Kong, J.; Zhang, X. Ultra-sensitive nucleic acid detection based on target cycling of triple helix molecular switch and ATRP double signal amplification. Sens. Actuat. B: Chem. 2021, 337, 129791. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Hou, M.; Chen, L.; Wang, J.; Yang, H.; Feng, W. An electrochemical biosensor based on ARGET ATRP with DSN-assisted target recycling for sensitive detection of tobacco mosaic virus RNA. Bioelectrochemistry 2022, 144, 108037. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, S.; Tian, Z.; Kong, J.; Zhang, X. Highly sensitive detection of miRNA-21 based on hybridization chain reaction and atom transfer radical polymerization. Anal. Chim. Acta 2025, 1369, 344356. [Google Scholar] [CrossRef]
- Rezaei, H.; Hosseini, M.; Radfar, S. A dual-signaling electrochemical ratiometric strategy combining “signal-off” and “signal-on” approaches for detection of MicroRNAs. Anal. Biochem. 2021, 632, 114356. [Google Scholar] [CrossRef]
- Peng, X.; Yan, H.; Wu, Z.; Wen, W.; Zhang, X.; Wang, S. Magnetic nanobeads and de novo growth of electroactive polymers for ultrasensitive microRNA detection at the cellular level. Anal. Chem. 2020, 93, 902–910. [Google Scholar] [CrossRef]
- Huo, M.; Li, Y.; Wu, Y.; Xie, S.; Chen, D. Enzyme-free biosensor for ultrasensitive detection of mecA gene utilizing electrochemically controlled atom transfer radical polymerization triggered by copper nanoflowers enriched on DNA polymers. Talanta 2025, 284, 127231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Wang, M.; Zhang, H.; Yang, H.; Liu, Y.; Guo, L. Affinity-driven electrochemical aptasensor for Erα detection by hybrid-nanoengineering of GO-nafion-AuNPs synergistic dynamic tag growth strategy. J. Electrochem. Soc. 2025, 172, 066506. [Google Scholar] [CrossRef]
- Li, M.; Guo, Z.; Zheng, X.; Yang, H.; Feng, W.; Kong, J. An electrochemical aptasensor based on eATRP amplification for the detection of bisphenol A. Analyst 2019, 144, 5691–5699. [Google Scholar] [CrossRef]
- Li, W.; Jia, Y.; Chen, K.; Li, H.; Yang, H.; Guo, L.; Miao, M. Target-driven ratiometric electrochemical aptasensor based on polymerization and AuNP signal amplification for acetamiprid residue determination. Microch. Acta 2025, 192, 443. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.; Qiu, Y.; Kong, J.; Zhang, X. High sensitive electrochemical methamphetamine detection in serum and urine via atom transfer radical polymerization signal amplification. Talanta 2022, 238, 123026. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Yang, Y.; Bao, F.; Lu, J.; Miao, J.; Xu, Y. A universal biosensor utilizing bacteria-initiated in situ growth of electroactive polymers for bacteria-related hazards detection. Biosens. Bioelectron. 2022, 203, 114030. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Wang, M.; Qiu, Y.; Kong, J.; Zhang, X. A host guest interaction enhanced polymerization amplification for electrochemical detection of cocaine. Anal. Chim. Acta 2021, 1184, 339041. [Google Scholar] [CrossRef]
- Zhang, D.; Zhong, S.; Peng, C.; Chen, L.; Luo, J.; Li, X. Label-free electrochemical aptasensor for digoxin therapeutic monitoring in clinical samples via catalytic hairpin self-assembly and eATRP mediated signal amplification. Microchem. J. 2025, 211, 113047. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, M.; Wang, H.; Sun, S. Dual-signal amplified electrochemical biosensor based on eATRP and PEI for early detection of lung cancer. Bioelectrochemistry 2022, 148, 108224. [Google Scholar] [CrossRef]
- He, X.; Zhang, W.; Wang, Y.; Wang, Z.; Wang, J.; Li, X.; Yang, H.; Liu, Y.; Miao, M. Synergistic signal amplification for HER2 biosensing using tetrahedral DNA nanostructures and 2D transition metal carbon/nitride@Au composites via ARGET ATRP. Microchem. J. 2024, 207, 112029. [Google Scholar] [CrossRef]
- Yu, W.; Yu, S.; Zhang, F.; Xu, Q.; Zhang, X.; Kong, J. Ultrasensitive electrochemical sensor for lipopolysaccharide detection catalyzed by 3,4,9,10-perylenetetracarboxylic diimide. Anal. Chim. Acta 2025, 1352, 343926. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, S.; Wang, J.; Kong, J.; Zhang, X. Metal-free photoinduced ROMP-enabled electrochemical aptasensor for ultrasensitive detection of endotoxin. Talanta 2025, 297, 128712. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Cao, X.; Li, S.; Liang, Y.; Luo, Y.; Feng, W.; Han, D. Electrochemically controlled atom transfer radical polymerization for electrochemical aptasensing of tumor biomarkers. Anal. Chem. 2022, 94, 13516–13521. [Google Scholar] [CrossRef]
- Wan, J.; Tian, Y.; Wu, D.; Ye, Z.; Chen, S.; Hu, Q.; Wang, M.; Lv, J.; Xu, W.; Zhang, X.; et al. Site-directed electrochemical grafting for amplified detection of antibody pharmaceuticals. Anal. Chem. 2024, 96, 9278–9284. [Google Scholar] [CrossRef]
- Dou, B.; Wang, K.; Chen, Y.; Wang, P. Programmable DNA nanomachine integrated with electrochemically controlled atom transfer radical polymerization for antibody detection at picomolar level. Anal. Chem. 2024, 96, 10594–10600. [Google Scholar] [CrossRef]
- Yu, S.; Xu, Q.; Zhang, F.; Kong, J.; Zhang, X. Acridone-mediated photo-driven in situ amplification for melanoma biomarkers biosensing. Sens. Actuat. B Chem. 2025, 432, 137479. [Google Scholar] [CrossRef]
- Li, X.; Lu, J.; Li, Z.; Yang, H.; Li, W.; Liu, Y.; Miao, M. Electrochemical detection of alkaline phosphatase activity via atom transfer radical polymerization. Bioelectrochemistry 2022, 144, 107998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, P.; Lu, J.; Li, D.; Yang, H.; Li, X.; Liu, Y. A novel electrochemical platform for assay of alkaline phosphatase based on amifostine and ATRP signal amplification. Anal. Bioanal. Chem. 2022, 414, 6955–6964. [Google Scholar] [CrossRef]
- Si, F.; Zhang, Y.; Lu, J.; Hou, M.; Yang, H.; Liu, Y. A highly sensitive, eco-friendly electrochemical assay for alkaline phosphatase activity based on a photoATRP signal amplification strategy. Talanta 2023, 252, 123775. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, Q.; Jiang, C.; Zhang, J.; Kong, J.; Zhang, X. Electrochemically mediated polymerization for highly sensitive detection of protein kinase activity. Biosens. Bioelectron. 2018, 110, 52–57. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Yu, S.; Sun, H.; Wang, L.; Li, L.; Kong, J.; Zhang, X. A highly sensitive assay for matrix metalloproteinase 2 via signal amplification strategy of eATRP. Microchem. J. 2021, 164, 106015. [Google Scholar] [CrossRef]
- Hu, Q.; Bao, Y.; Gan, S.; Zhang, Y.; Han, D.; Niu, L. Electrochemically controlled grafting of polymers for ultrasensitive electrochemical assay of trypsin activity. Biosens. Bioelectron. 2020, 165, 112358. [Google Scholar] [CrossRef]
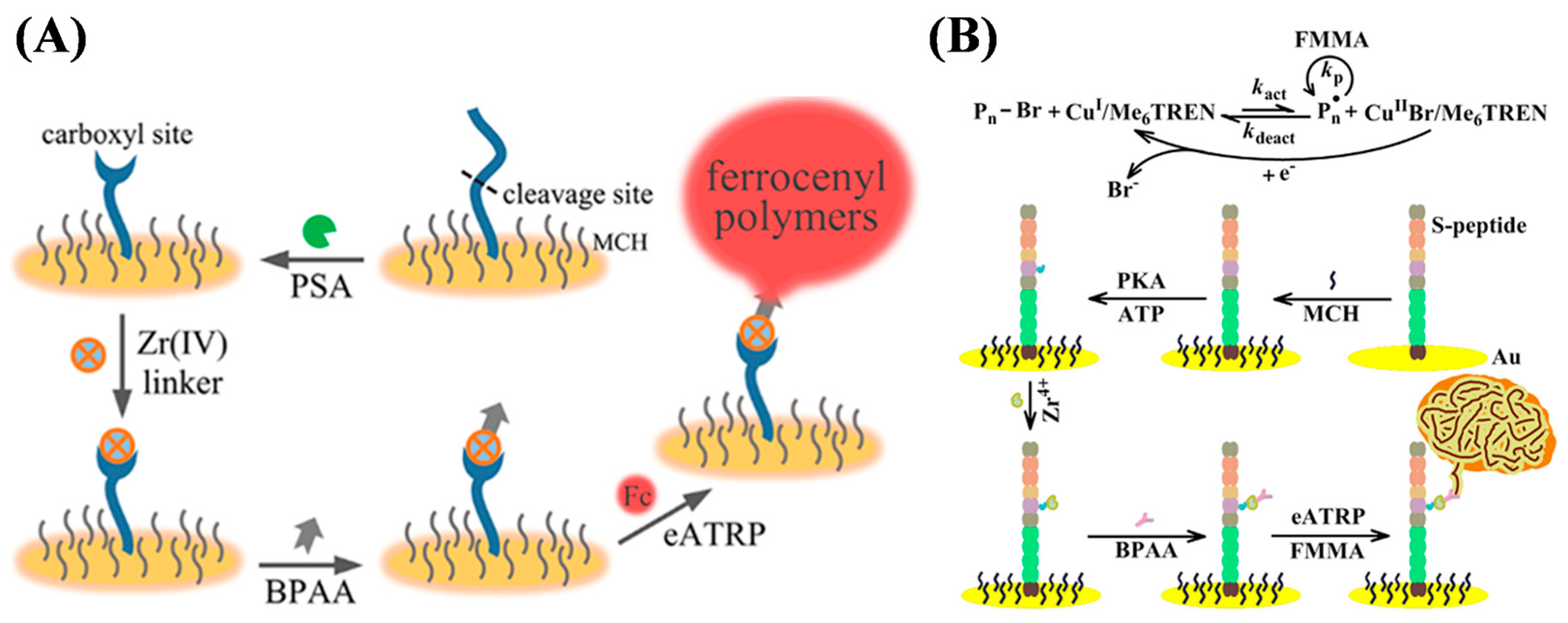
- Hu, Q.; Gan, S.; Bao, Y.; Zhang, Y.; Han, D.; Niu, L. Electrochemically controlled ATRP for cleavage-based electrochemical detection of the prostate-specific antigen. Anal. Chem. 2020, 92, 15982–15988. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, J.; Li, L.; Ma, K.; Kong, J.; Zhang, X. An electrochemical biosensor for the amplification of thrombin activity by perylene-mediated photoinitiated polymerization. Anal. Chim. Acta 2024, 1302, 342494. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Y.; Chai, Y.; Yuan, R. A super intramolecular self-enhanced electrochemiluminescence immunosensor based on polymer chains grafted on palladium nanocages. Nanoscale 2014, 6, 10316–10322. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhao, X.; Chen, H.; Bai, L.; Xu, H.; Wang, W.; Yang, H.; Wei, D.; Yang, L. Fabrication of novel electrochemical immunosensor by mussel-inspired chemistry and surface-initiated PET-ATRP for the simultaneous detection of CEA and AFP. React. Funct. Polym. 2020, 154, 104632. [Google Scholar] [CrossRef]
- Wang, N.; Wang, C.; Chen, H.; Bai, L.; Wang, W.; Yang, H.; Wei, D.; Yang, L. Facile fabrication of a controlled polymer brush-type functional nanoprobe for highly sensitive determination of alpha fetoprotein. Anal. Methods 2020, 12, 4438–4446. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, N.; Chen, H.; Bai, L.; Xu, H.; Wang, W.; Yang, H.; Wei, D.; Yang, L.; Cheng, Z. Preparation of a novel sandwich-type electrochemical immunosensor for AFP detection based on an ATRP and click chemistry technique. Polym. Chem. 2020, 11, 900–908. [Google Scholar] [CrossRef]
- Yuan, L.; Hua, X.; Wu, Y.; Pan, X.; Liu, S. Polymer-functionalized silica nanosphere labels for ultrasensitive detection of tumor necrosis factor-alpha. Anal. Chem. 2011, 83, 6800–6809. [Google Scholar] [CrossRef] [PubMed]
- Si, F.; Cui, X.; Zhang, Y.; Li, Y.; Yang, H.; Liu, Y. A novel electrochemical biosensor based on dual signal amplification of CMK-3@AuNPs and ATRP for DR1 detection. Sens. Actuat. B Chem. 2024, 403, 135080. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Y.; Zhang, H.; Yang, H.; Liu, Y.; Si, F. A novel electrochemical biosensor based on WO3@AuNPs and HBP@BIBB macromolecule-triggered ATRP for DR1 detection. New J. Chem. 2024, 48, 8106–8115. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, P.; Hui, K.M.; Kang, Y. A paper-based microfluidic electrochemical immunodevice integrated with amplification-by-polymerization for the ultrasensitive multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2014, 52, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, S.; He, L. Activators generated electron transfer for atom transfer radical polymerization for immunosensing. Biosens. Bioelectron. 2010, 26, 970–975. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, Y.; Shi, H.; Liu, S. Surface-initiated atom-transfer radical polymerization of 4-acetoxystyrene for immunosensing. Chem. Eur. J. 2010, 17, 976–983. [Google Scholar] [CrossRef]
- Si, F.; Sun, Y.; Ba, Y.; Guo, L.; Liu, Y.; Kong, J. Metal-free photochemically mediated ATRP for ultrasensitive quantification of CYFRA 21–1 for detection of early stage non-small cell lung cancer. J. Electrochem. Soc. 2022, 169, 097509. [Google Scholar] [CrossRef]
- Jian, L.; Wang, X.; Hao, L.; Liu, Y.; Yang, H.; Zheng, X.; Feng, W. Electrochemiluminescence immunosensor for cytokeratin fragment antigen 21-1 detection using electrochemically mediated atom transfer radical polymerization. Microchem. J. 2021, 188, 115. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Y.; Han, H.; Ma, Z. In situ growth of electroactive polymers via ATRP to construct a biosensing interface for tumor marker. Microchim. Acta 2021, 188, 389. [Google Scholar] [CrossRef]
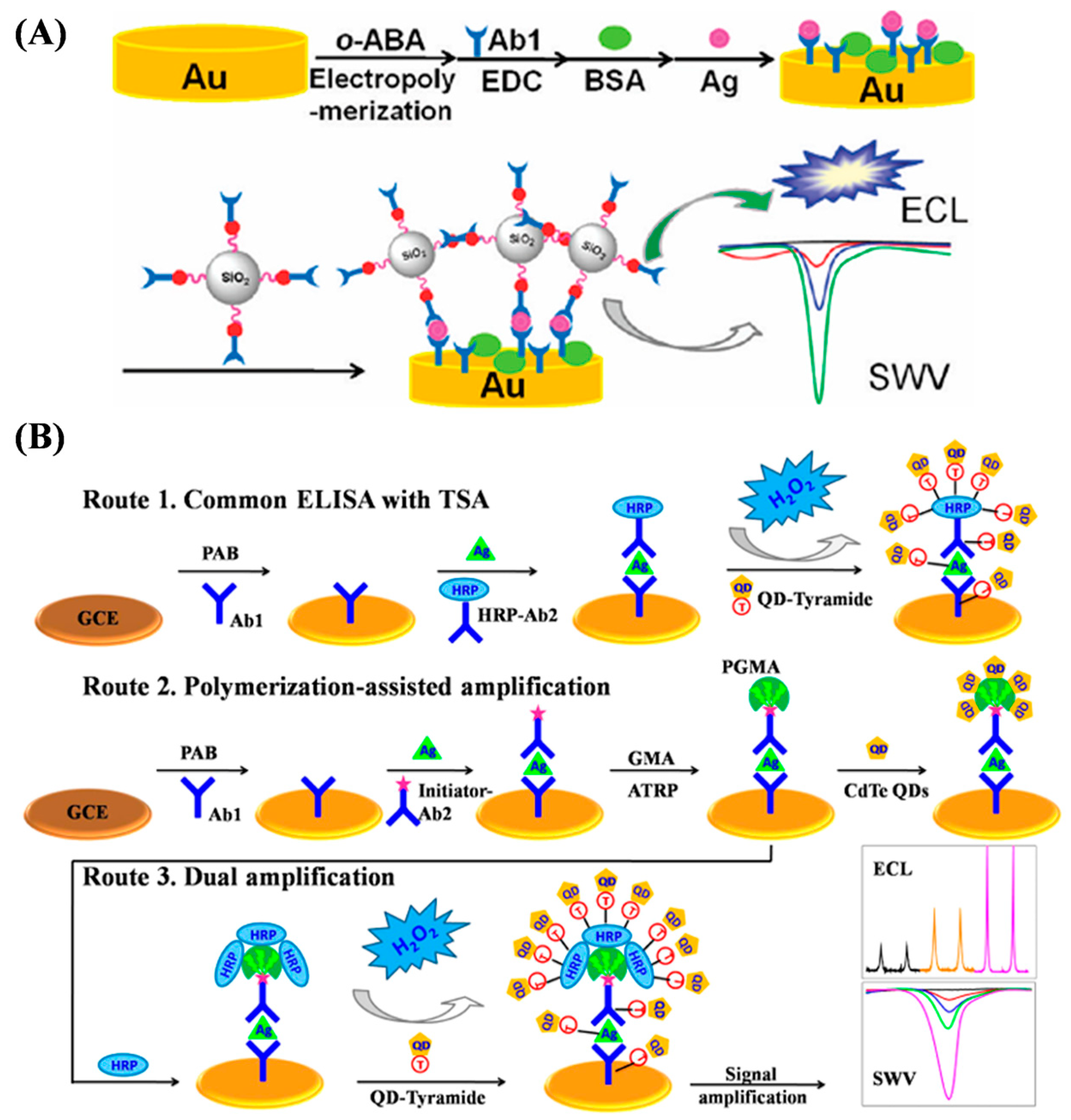
- Yuan, L.; Xu, L.; Liu, S. Integrated tyramide and polymerization-assisted signal amplification for a highly-sensitive immunoassay. Anal. Chem. 2012, 84, 10737–10744. [Google Scholar] [CrossRef]
- Ding, L.; Xiang, C.; Zhou, G. Silica nanoparticles coated by poly(acrylic acid) brushes via host-guest interactions for detecting DNA sequence of Hepatitis B virus. Talanta 2018, 181, 65–72. [Google Scholar] [CrossRef]
- Zhuang, D.; Shen, H.; Liu, G.; Yu, C.; Yang, J. A combining signal amplification of atom transfer radical polymerization and redox polymerization for visual biomolecules detection. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2791–2799. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, A.; Wang, Y.; Liu, L.; Wang, X. Atom transfer radical polymer-modified paper for improvement in protein fixation in paper-based ELISA. BMC Chem. 2019, 13, 110. [Google Scholar] [CrossRef]
- Chen, F.; Hou, S.; Li, Q.; Fan, H.; Fan, R.; Xu, Z.; Zhala, G.; Mai, X.; Chen, X.; Chen, X.; et al. Development of atom transfer radical polymer-modified gold nanoparticle-based enzyme-linked immunosorbent assay (ELISA). Anal. Chem. 2014, 86, 10021–10024. [Google Scholar] [CrossRef]
- Kitayama, Y.; Takeuchi, T. Localized surface plasmon resonance nanosensing of C-reactive protein with poly(2-methacryloyloxyethyl phosphorylcholine)-grafted gold nanoparticles prepared by surface-initiated atom transfer radical polymerization. Anal. Chem. 2014, 86, 5587–5594. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, Y.; Lu, Z.; Li, C.M. Poly[oligo(ethylene glycol) methacrylate-co-glycidyl methacrylate] brush substrate for sensitive surface plasmon resonance imaging protein arrays. Adv. Funct. Mater. 2010, 20, 3497–3503. [Google Scholar] [CrossRef]
- Kuriu, Y.; Ishikawa, M.; Kawamura, A.; Uragami, T.; Miyata, T. SPR signals of three-dimensional antibody-immobilized gel layers formed on sensor chips by atom transfer radical polymerization. Chem. Lett. 2012, 41, 1660–1662. [Google Scholar] [CrossRef]
- Chang, Y.-X.; Wang, C.-F.; Chang, C.-J.; Lu, C.-H.; Chen, J.-K. Fabrication of scalable poly(N-isopropylacrylamide)/gold nanoparticles composite ring array as LSPR sensor for label-free biosensor application. Sens. Actuat. B Chem. 2023, 375, 132875. [Google Scholar] [CrossRef]
- Anraku, Y.; Takahashi, Y.; Kitano, H.; Hakari, M. Recognition of sugars on surface-bound cap-shaped gold particles modified with a polymer brush. Colloid. Surf. B Biointerfaces 2007, 57, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Zarshenas, P.; Sedghi, R.; Nabid, M.R.; Varma, R.S. Highly selective and sensitive recognition of multi-ions in aqueous solution based on polymer-grafted nanoparticle as visual colorimetric sensor. Sci. Rep. 2024, 14, 213. [Google Scholar] [CrossRef]
- Yatabe, R.; Onodera, T.; Toko, K. Fabrication of an SPR sensor surface with antifouling properties for highly sensitive detection of 2,4,6-trinitrotoluene using surface-initiated atom transfer polymerization. Sensors 2013, 13, 9294–9304. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; He, L. Surface passivation using oligo(ethylene glycol) in ATRP-assisted DNA detection. Sens. Actuat. B Chem. 2008, 129, 225–230. [Google Scholar] [CrossRef]
- Zhao, N.; Chen, C.; Zhou, J. Surface plasmon resonance detection of ametryn using a molecularly imprinted sensing film prepared by surface-initiated atom transfer radical polymerization. Sens. Actuat. B Chem. 2012, 166–167, 473–479. [Google Scholar] [CrossRef]
- Weng, Y.-H.; Xu, L.-T.; Chen, M.; Zhai, Y.-Y.; Zhao, Y.; Ghorai, S.K.; Pan, X.-H.; Cao, S.-H.; Li, Y.-Q. In situ monitoring of fluorescent polymer brushes by angle-scanning based surface plasmon coupled emission. ACS Macro Lett. 2019, 8, 223–227. [Google Scholar] [CrossRef]
- Iwasaki, S.; Kawasaki, H.; Iwasaki, Y. Label-free specific detection and collection of C-reactive protein using zwitterionic phosphorylcholine-polymer-protected magnetic nanoparticles. Langmuir 2019, 35, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Sun, X.; Zhang, H.; Li, X.; Li, S.; Wang, Z. Development of a peptide microarray-based metal-enhanced fluorescence assay for ultrasensitive detection of multiple matrix metalloproteinase activities by using a gold nanorod-polymer substrate. Biosens. Bioelectron. 2024, 246, 115871. [Google Scholar] [CrossRef] [PubMed]
- Aied, A.; Zheng, Y.; Pandit, A.; Wang, W. DNA immobilization and detection on cellulose paper using a surface grown cationic polymer via ATRP. ACS Appl. Mater. Interfaces 2012, 4, 826–831. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.-D.; Tian, T.; Chu, L.-Q. Incorporation of multilayered silver nanoparticles into polymer brushes as 3-dimensional SERS substrates and their application for bacteria detection. Appl. Surf. Sci. 2017, 407, 185–191. [Google Scholar] [CrossRef]
- Ashraf, J.; Lau, S.; Akbarinejad, A.; Evans, C.W.; Williams, D.E.; Barker, D.; Travas-Sejdic, J. Conducting polymer-infused electrospun fibre mat modified by POEGMA brushes as antifouling biointerface. Biosensors 2022, 12, 1143. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.; Jauw, J.; Linman, M.J.; Cheng, Q. Highly sensitive detection of protein toxins by surface plasmon resonance with biotinylation-based inline atom transfer radical polymerization amplification. Anal. Chem. 2010, 82, 3679–3685. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, Q. Detection of membrane-binding proteins by surface plasmon resonance with an all-aqueous amplification scheme. Anal. Chem. 2012, 84, 3179–3186. [Google Scholar] [CrossRef]
- Hu, W.; Chen, H.; Shi, Z.; Yu, L. Dual signal amplification of surface plasmon resonance imaging for sensitive immunoassay of tumor marker. Anal. Biochem. 2014, 453, 16–21. [Google Scholar] [CrossRef]
- Zhang, J.; Ba, Y.; Liu, Q.; Zhao, L.; Wang, D.; Yang, H.; Kong, J. CuBr2/EDTA-mediated ATRP for ultrasensitive fluorescence detection of lung cancer DNA. J. Adv. Res. 2020, 22, 77–84. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Ba, Y.; Cheng, J.; Yang, H.; Cui, Y.; Kong, J.; Zhang, X. F-containing initiatior for ultrasensitive fluorescent detection of lung cancer DNA via atom transfer radical polymerization. Anal. Chim. Acta 2020, 1094, 99–105. [Google Scholar] [CrossRef]
- Ma, N.; Qiu, W.; Wei, G.; Zhang, J.; Yu, H.; Kong, J.; Zhang, X. GOX mediated oxygen tolerance ATRP for detection of esophageal cancer biomarker miRNA-144. Chem. Eng. J. 2024, 495, 153543. [Google Scholar] [CrossRef]
- Wang, H.; Ma, L.; Jin, Z.; Cui, Z.; Yang, H.; Miao, M. Highly sensitive fluorescence detection of tobacco mosaic virus RNA based on polysaccharide and ARGET ATRP double signal amplification. Talanta 2023, 257, 124360. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, L.; Hou, M.; Gao, H.; Ke, Y.; Yang, H.; Si, F. T790M mutation upconversion fluorescence biosensor via mild ATRP strategy and site-specific DNA cleavage of restriction endonuclease. Microchim. Acta 2024, 191, 148. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Q.; Wen, D.; Gao, M.; Zhang, D.; Jin, Q.; Kong, J.; Zhang, J. Ultrasensitive fluorescence detection of sequence-specific DNA via labeling hairpin DNA probes for fluorescein o-acrylate polymers. Anal. Chim. Acta 2019, 1088, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, D.; Ma, L.; Miao, M.; Liu, Y.; Kong, J. Fluorescent assay of alkaline phosphatase activity via atom transfer radical polymerization. Microchim. Acta 2022, 189, 84. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Ma, L.; Zhang, Y.; Chen, L.; Yang, H.; Liu, Y.; Guo, L. A highly sensitive fluorescence sensor for tobacco mosaic virus RNA based on DSN cycle and ARGET ATRP double signal amplification. Luminescence 2024, 39, e4804. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhao, L.; Wang, D.; Yang, H.; Kong, J. Polysaccharide-enhanced ARGET ATRP signal amplification for ultrasensitive fluorescent detection of lung cancer CYFRA 21-1 DNA. Anal. Bioanal. Chem. 2020, 412, 2413–2421. [Google Scholar] [CrossRef]
- Gao, H.; Si, G.; Wang, Z.; Liu, Y.; Yang, H.; Miao, M.; Ma, L. Enzyme-assisted upconversion fluorescence-encoded biosensing system for simultaneous detection of multiple sites EGFR mutation. Anal. Bioanal. Chem. 2024, 417, 237–250. [Google Scholar] [CrossRef]
- Cui, Z.; Guo, L.; Jin, Z.; Ma, L.; Yang, H.; Miao, M. Highly sensitive and specific assessment of ochratoxin A in herbal medicines via activator regeneration by electron transfer ATRP. New J. Chem. 2022, 46, 17479–17486. [Google Scholar] [CrossRef]
- Guo, Z.; Tang, J.; Li, M.; Liu, Y.; Yang, H.; Kong, J. An ultrasensitive fluorescent aptasensor based on truncated aptamer and AGET ATRP for the detection of bisphenol A. Anal. Bioanal. Chem. 2019, 411, 7807–7815. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Liu, Q.; Li, L.; Yang, H.; Kong, J. Ultrasensitive aptamer fluorometric detection of IFN-γ by dual atom transfer radical polymerization amplification. Sens. Actuat. B Chem. 2019, 295, 40–48. [Google Scholar] [CrossRef]
- Ba, Y.; Zhang, J.; Sun, Y.; Liu, Y.; Yang, H.; Kong, J. Novel fluorescent biosensor for carcinoembryonic antigen determination via atom transfer radical polymerization with a macroinitiator. New J. Chem. 2021, 45, 3112–3119. [Google Scholar] [CrossRef]
- Miao, M.; Guo, L.; Xue, J.; Jia, Y.; Cui, Z.; Yang, H. A controllable Y-shaped DNA structure assisted aptasensor for the simultaneous detection of AFB1 and OTA based on ARGET ATRP. J. Mater. Chem. B 2024, 12, 5861–5868. [Google Scholar] [CrossRef]
- Hou, M.; Ma, L.; Yang, H.; Si, F.; Liu, Y. Background-free and signal-amplified upconversion fluorescent biosensing platform for sensitive detection of CYFRA21-1. Talanta 2023, 262, 124659. [Google Scholar] [CrossRef]
- Guo, X.; Wang, M.; Ma, L.; Cui, Z.; Liu, Z.; Yang, H.; Liu, Y. Carboxyl porphyrin as signal molecule for sensitive fluorescent detection of aflatoxin B1 via ARGET-ATRP. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 280, 121535. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, J.; Kong, J.; Zhang, X. Fluorescence sensing of eclampsia biomarkers via the immunosorbent atom transfer radical polymerization assay. Anal. Chem. 2024, 96, 8450–8457. [Google Scholar] [CrossRef] [PubMed]
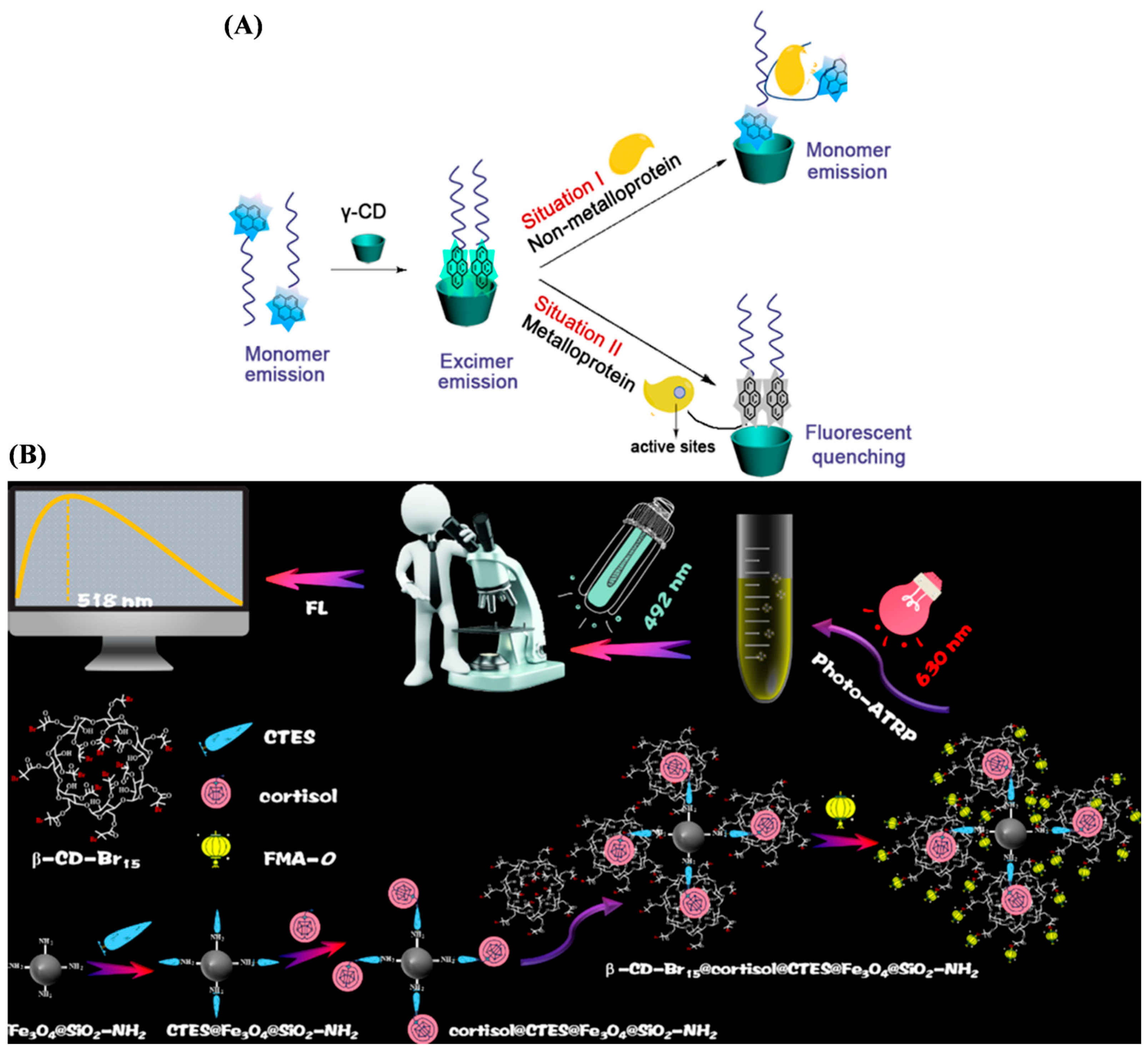
- Li, N.; Qi, L.; Qiao, J.; Chen, Y. Ratiometric fluorescent pattern for sensing proteins using aqueous polymer-pyrene/γ-cyclodextrin inclusion complexes. Anal. Chem. 2016, 88, 1821–1826. [Google Scholar] [CrossRef]
- Wang, J.; Kong, J.; Zhang, X. A fluorescent signal amplification strategy via host-guest recognition for cortisol detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 329, 125611. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, N.; Zhang, J.; Ma, K.; Kong, J.; Zhang, X. Bacteria-instructed synthesis of free radical polymers for highly sensitive detection of Escherichia coli and Staphylococcus aureus. Anal. Chim. Acta 2024, 1329, 343259. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, N.; Gao, F.; Yu, Z.; Yu, S.; Yi, X. Progress in the Design and Application of Chemical and Biological Sensors Based on Atom Transfer Radical Polymerization. Biosensors 2025, 15, 752. https://doi.org/10.3390/bios15110752
Xia N, Gao F, Yu Z, Yu S, Yi X. Progress in the Design and Application of Chemical and Biological Sensors Based on Atom Transfer Radical Polymerization. Biosensors. 2025; 15(11):752. https://doi.org/10.3390/bios15110752
Chicago/Turabian StyleXia, Ning, Fengli Gao, Zhaojiang Yu, Shuaibing Yu, and Xinyao Yi. 2025. "Progress in the Design and Application of Chemical and Biological Sensors Based on Atom Transfer Radical Polymerization" Biosensors 15, no. 11: 752. https://doi.org/10.3390/bios15110752
APA StyleXia, N., Gao, F., Yu, Z., Yu, S., & Yi, X. (2025). Progress in the Design and Application of Chemical and Biological Sensors Based on Atom Transfer Radical Polymerization. Biosensors, 15(11), 752. https://doi.org/10.3390/bios15110752






