Estimating Toxicity Putative Mechanisms from Smoking Residual Substances Using a Whole-Cell Bioreporter System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains
2.3. Growth Conditions
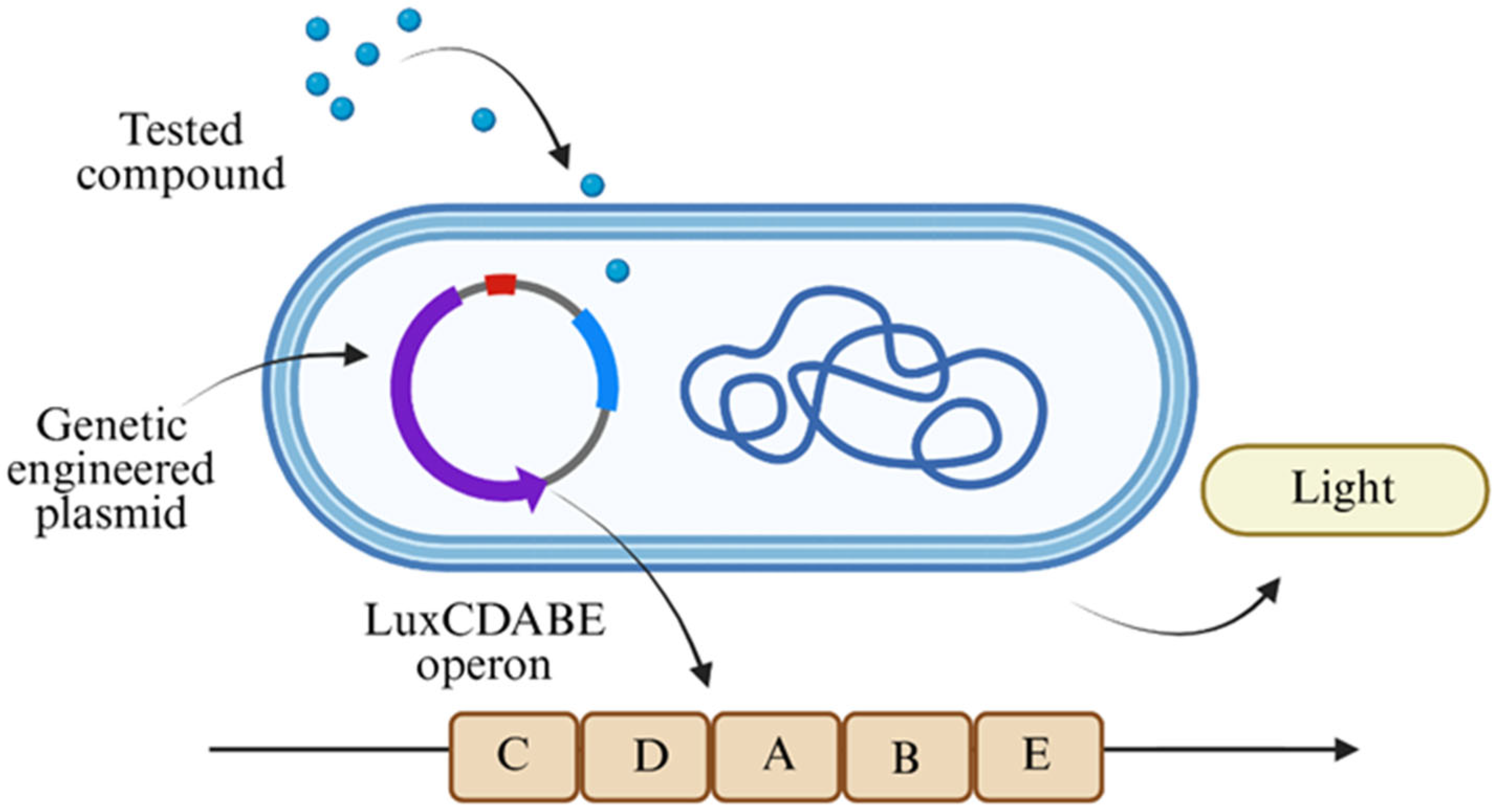
2.4. Bioluminescence Assay
2.5. Cigarette Smoke Fractions
2.6. Cumulative Test
2.7. Gas Chromatography–Mass Spectrometry Analysis
2.8. TOC Analysis
2.9. Data Analysis
3. Results and Discussion
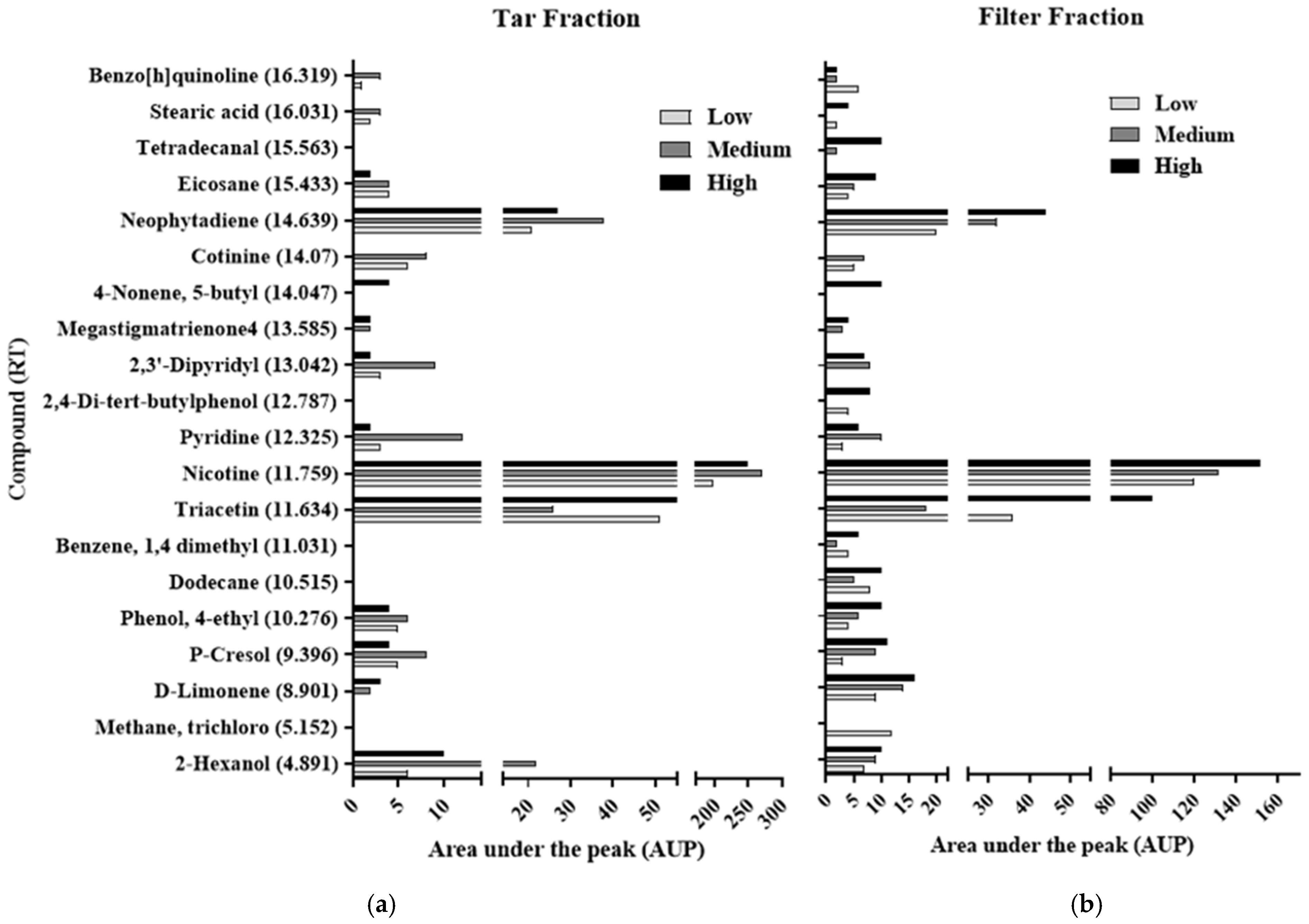
3.1. GC-MS Analysis of Cigarette Composition
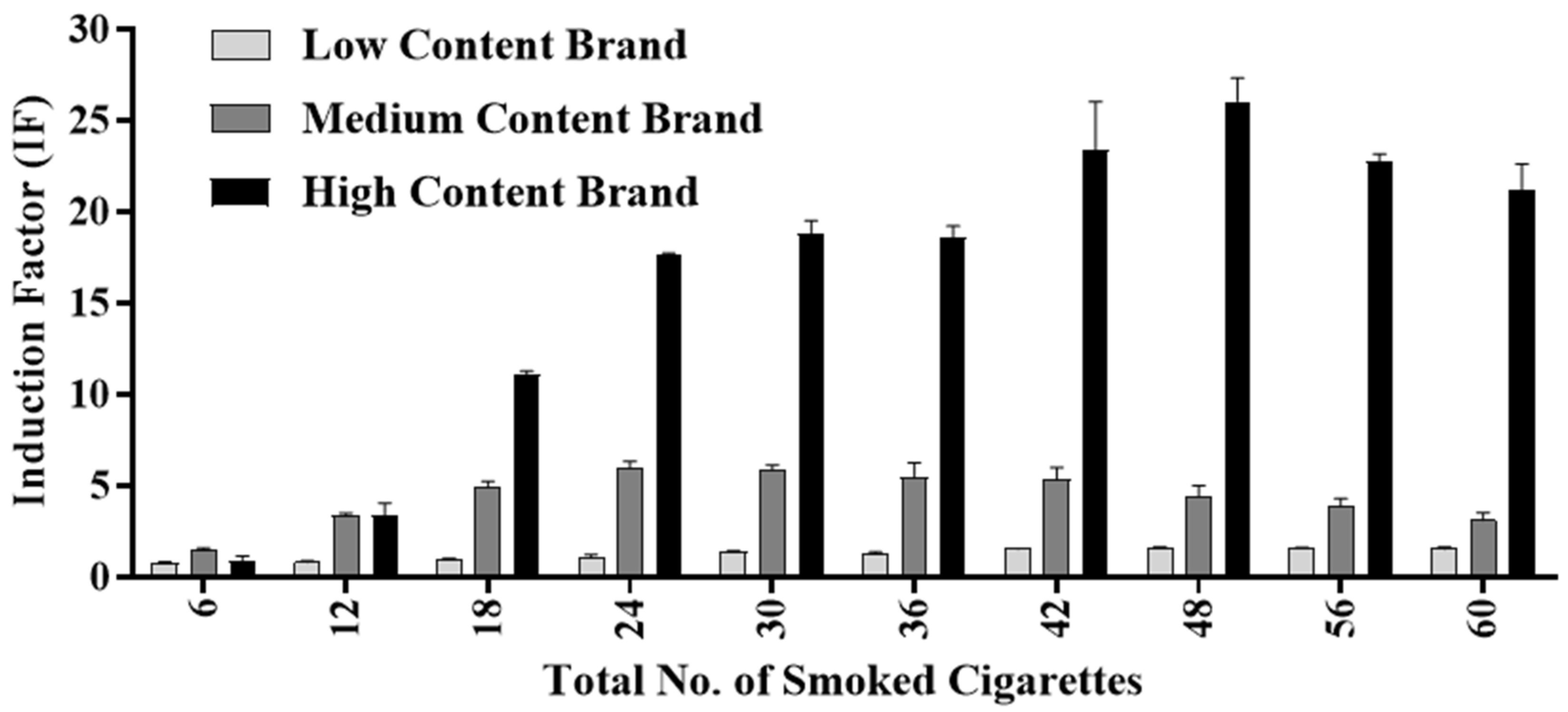
3.2. Toxicity Determination and Monitoring
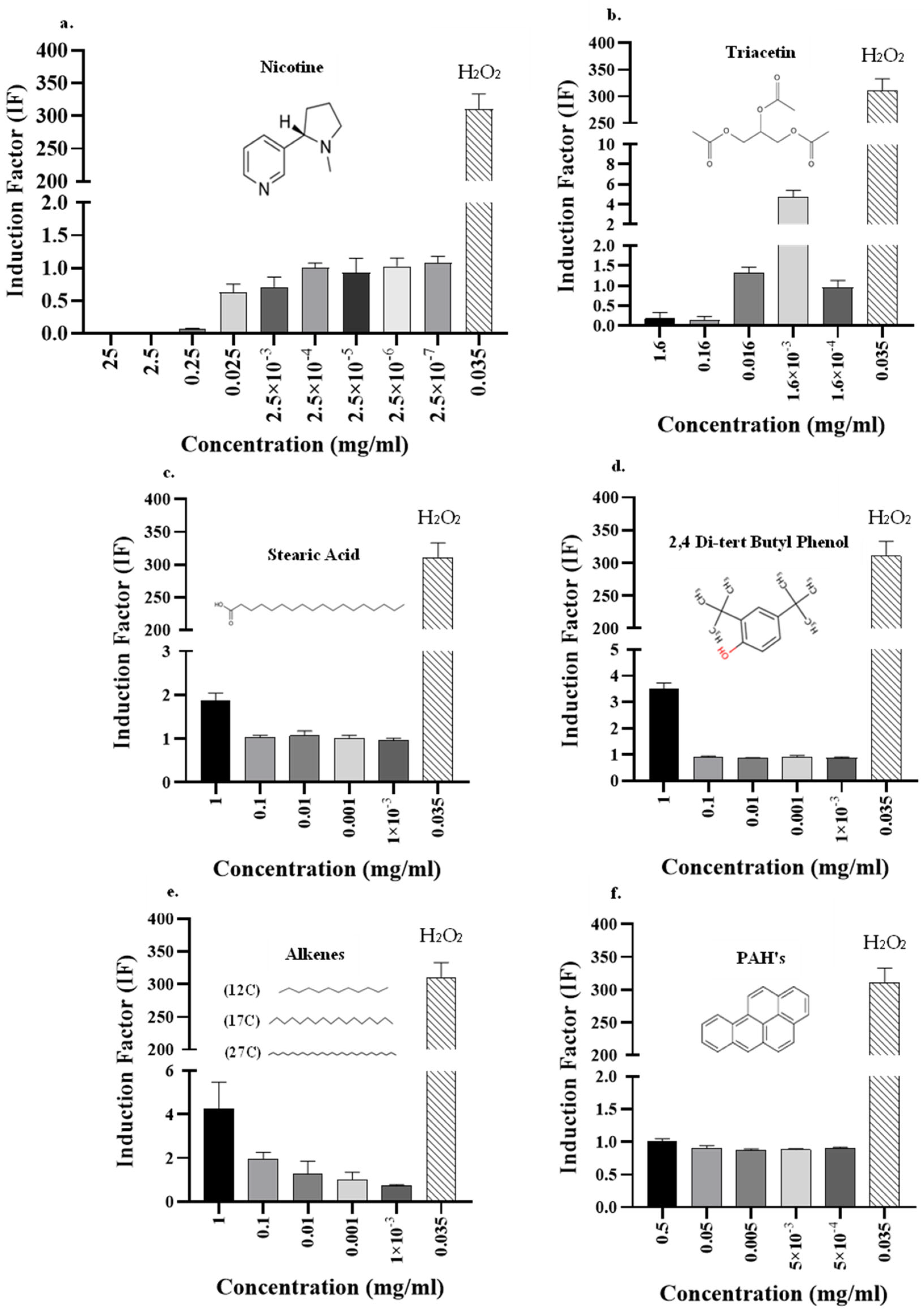
3.3. Analysis of Smoke Components Using the DPD2511 Bioreporter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COPD | Chronic obstructive pulmonary disease |
| H2O2 | Hydrogen peroxide |
| LB | Luria–Bertani |
| DDW | Double distilled water |
| OD | Optical density |
| GC-MS | Gas chromatography-mass spectroscopy |
| TOC | Total organic carbon |
| IF | Induction Factor |
| MIF | Material induction factor |
| CIF | Control induction factor |
| LCB | Low-content brand |
| MCB | Medium-content brand |
| HCB | High-content brand |
| RT | Retention time |
| AUP | Area under the peak |
| PAH | Poly-aromatic hydrocarbons |
| ROS | Reactive oxygen species |
References
- Torre, L.A.; Siegel, R.L.; Jemal, A. Lung cancer statistics. In Lung Cancer Personalized Medicine: Current Knowledge and Therapies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–19. [Google Scholar]
- Churg, A.; Cosio, M.; Wright, J.L. Mechanisms of cigarette smoke-induced COPD: Insights from animal models. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 294, L612–L631. [Google Scholar] [CrossRef]
- Hecht, S.S. Cigarette smoking: Cancer risks, carcinogens, and mechanisms. Langenbeck’s Arch. Surg. 2006, 391, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.N. The global smoking epidemic: A history and status report. Clin. Lung Cancer 2004, 5, 371–376. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion; Office on Smoking and Health. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; US Department of Health and Human Services, Public Health Service, Office of Surgeon General: Atlanta, GA, USA, 2010.
- Fowles, J.; Bates, M.; Noiton, D. The Chemical Constituents in Cigarettes and Cigarette Smoke: Priorities for Harm Reduction: A Report to the New Zealand Ministry of Health; Epidemiology and Toxicology Group, ESR: Porirua, New Zealand, 2000. [Google Scholar]
- Wang, J.; Wang, Y.; Zhou, W.; Huang, Y.; Yang, J. Impacts of cigarette smoking on blood circulation: Do we need a new approach to blood donor selection? J. Health Popul. Nutr. 2023, 42, 62. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.M. Cigarettes and cigarette smoking. Clin. Chest Med. 1991, 12, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Kosmider, B.; Messier, E.M.; Chu, H.W.; Mason, R.J. Human alveolar epithelial cell injury induced by cigarette smoke. PLoS ONE 2011, 6, e26059. [Google Scholar] [CrossRef]
- Jaccard, G.; Djoko, D.T.; Korneliou, A.; Stabbert, R.; Belushkin, M.; Esposito, M. Mainstream smoke constituents and in vitro toxicity comparative analysis of 3R4F and 1R6F reference cigarettes. Toxicol. Rep. 2019, 6, 222–231. [Google Scholar] [CrossRef]
- Johnson, M.D.; Schilz, J.; Djordjevic, M.V.; Rice, J.R.; Shields, P.G. Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3263–3304. [Google Scholar] [CrossRef]
- Andreoli, C.; Gigante, D.; Nunziata, A. A review of in vitro methods to assess the biological activity of tobacco smoke with the aim of reducing the toxicity of smoke. Toxicol. Vitr. 2003, 17, 587–594. [Google Scholar] [CrossRef]
- Esposito, E.R.; Horn, K.H.; Greene, R.M.; Pisano, M.M. An animal model of cigarette smoke-induced in utero growth retardation. Toxicology 2008, 246, 193–202. [Google Scholar] [CrossRef]
- Van Der Meer, J.R.; Belkin, S. Where microbiology meets microengineering: Design and applications of reporter bacteria. Nat. Rev. Microbiol. 2010, 8, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Belkin, S.; Smulski, D.R.; Vollmer, A.C.; Van Dyk, T.K.; LaRossa, R.A. Oxidative stress detection with Escherichia coli harboring a katG’:: Lux fusion. Appl. Environ. Microbiol. 1996, 62, 2252–2256. [Google Scholar] [CrossRef]
- Parvez, S.; Venkataraman, C.; Mukherji, S. A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ. Int. 2006, 32, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, A.C.; Belkin, S.; Smulski, D.R.; Van Dyk, T.K.; LaRossa, R.A. Detection of DNA damage by use of Escherichia coli carrying recA’:: Lux, uvrA’:: Lux, or alkA’:: Lux reporter plasmids. Appl. Environ. Microbiol. 1997, 63, 2566–2571. [Google Scholar] [CrossRef]
- Kim, B.C.; Gu, M.B. A bioluminescent sensor for high throughput toxicity classification. Biosens. Bioelectron. 2003, 18, 1015–1021. [Google Scholar] [CrossRef]
- Forbes, W.F.; Robinson, J.C.; Stanton, M. Tar and nicotine retrieval from cigarettes available in Canada. Cancer 1969, 23, 910–912. [Google Scholar] [CrossRef]
- Axelrod, T.; Eltzov, E.; Marks, R.S. Bioluminescent bioreporter pad biosensor for monitoring water toxicity. Talanta 2016, 149, 290–297. [Google Scholar] [CrossRef]
- Belkin, S.; Smulski, D.R.; Dadon, S.; Vollmer, A.C.; Van Dyk, T.K.; Larossa, R.A. A panel of stress-responsive luminous bacteria for the detection of selected classes of toxicants. Water Res. 1997, 31, 3009–3016. [Google Scholar] [CrossRef]
- Counts, M.; Morton, M.; Laffoon, S.; Cox, R.; Lipowicz, P. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul. Toxicol. Pharmacol. 2005, 41, 185–227. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.R.; da Silva, J.R.P.; Smith, G. The effect of tobacco ingredients on smoke chemistry. Part I: Flavourings and additives. Food Chem. Toxicol. 2004, 42, 3–37. [Google Scholar] [CrossRef]
- Duuren, B.L.V.; Kosak, A.I. Isolation and Identification of Some Components of Cigarette Smoke Condensate1. J. Org. Chem. 1958, 23, 473–475. [Google Scholar] [CrossRef]
- Harris, J.E. Cigarette smoke components and disease: Cigarette smoke is more than a triad of tar, nicotine, and carbon monoxide. Smok. Tob. Control Monogr. 1996, 7, 59–75. [Google Scholar]
- Brčić Karačonji, I. Facts about nicotine toxicity. Arh. Za Hig. Rada I Toksikol. 2005, 56, 363–371. [Google Scholar]
- Gladding, R.; Wartman, J.W.; Wright, J.H. Identification of neophytadiene in burley tobacco and in cigarette smoke. J. Org. Chem. 1959, 24, 1358–1359. [Google Scholar] [CrossRef]
- Quinn, M.J., Jr.; Ziolkowski, D., Jr. Wildlife Toxicity Assessment for Triacetin, in Wildlife Toxicity Assessments for Chemicals of Military Concern; Elsevier: Amsterdam, The Netherlands, 2015; pp. 291–301. [Google Scholar]
- Watts, R.J.; Washington, D.; Howsawkeng, J.; Loge, F.J.; Teel, A.L. Comparative toxicity of hydrogen peroxide, hydroxyl radicals, and superoxide anion to Escherichia coli. Adv. Environ. Res. 2003, 7, 961–968. [Google Scholar] [CrossRef]
- Kim, S.O.; Merchant, K.; Nudelman, R.; Beyer, W.F.; Keng, T.; DeAngelo, J.; Hausladen, A.; Stamler, J.S. OxyR: A molecular code for redox-related signaling. Cell 2002, 109, 383–396. [Google Scholar] [CrossRef]
- Italiani, V.C.S.; Neto, J.F.d.S.; Braz, V.S.; Marques, M.V. Regulation of catalase-peroxidase KatG is OxyR dependent and Fur independent in Caulobacter crescentus. J. Bacteriol. 2011, 193, 1734–1744. [Google Scholar] [CrossRef]
- Navarro-Pérez, M.L.; Fernández-Calderón, M.C.; Vadillo-Rodríguez, V.; Bose, A. Decomposition of growth curves into growth rate and acceleration: A novel procedure to monitor bacterial growth and the time-dependent effect of antimicrobials. Appl. Environ. Microbiol. 2022, 88, e01849-21. [Google Scholar] [CrossRef]
- Axelrod, T.; Eltzov, E.; Lerman, M.; Harpaz, D.; Marks, R.S. Cigarette smoke toxicity modes of action estimated by a bioluminescent bioreporter bacterial panel. Talanta 2021, 226, 122076. [Google Scholar] [CrossRef]
- van der Vaart, H.; Postma, D.S.; Timens, W.; Hacken, N.H.T.T. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax 2004, 59, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic aromatic hydrocarbons: Sources, toxicity, and remediation approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Crowley-Weber, C.L.; Dvorakova, K.; Crowley, C.; Bernstein, H.; Bernstein, C.; Garewal, H.; Payne, C.M. Nicotine increases oxidative stress, activates NF-κB and GRP78, induces apoptosis and sensitizes cells to genotoxic/xenobiotic stresses by a multiple stress inducer, deoxycholate: Relevance to colon carcinogenesis. Chem.-Biol. Interact. 2003, 145, 53–66. [Google Scholar] [CrossRef]
- Niaura, R. Re-thinking nicotine and its effects. Truth Initiat. 2016, 3, 24. [Google Scholar]
- He, F.; Qi, T.; Guo, S.; Wang, H.; Zhang, Z.; Liu, R.; Zong, W. Mechanistic insights into pyridine exposure induced toxicity in model Eisenia fetida species: Evidence from whole-animal, cellular, and molecular-based perspectives. Chemosphere 2023, 335, 139139. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Effect of nicotine on growth and metabolism of Streptococcus mutans. Eur. J. Oral Sci. 2012, 120, 319–325. [Google Scholar] [CrossRef]
- DuBois, A.E.; Bennett, Z.C.; Khalid, U.; Khalid, A.; Meece, R.A.; Difiore, G.J.; Gregory, R.L. Nicotine: Its stimulating and inhibitory effects on oral microorganisms. Fine Focus 2014, 1, 63–75. [Google Scholar] [CrossRef]
- Nunoshiba, T.; Obata, F.; Boss, A.C.; Oikawa, S.; Mori, T.; Kawanishi, S.; Yamamoto, K. Role of iron and superoxide for generation of hydroxyl radical, oxidative DNA lesions, and mutagenesis in Escherichia coli. J. Biol. Chem. 1999, 274, 34832–34837. [Google Scholar] [CrossRef] [PubMed]
- Aleby, S.; Von Sydow, E. The crystal structure of methyl stearate. Acta Crystallogr. 1960, 13, 487–492. [Google Scholar] [CrossRef]
- Hanzalova, K.; Rossner, P., Jr.; Sram, R.J. Oxidative damage induced by carcinogenic polycyclic aromatic hydrocarbons and organic extracts from urban air particulate matter. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2010, 696, 114–121. [Google Scholar] [CrossRef]
- Kim, Y.S.; Min, J.; Na Hong, H.; Park, J.H.; Park, K.S.; Gu, M.B. Gene expression analysis and classification of mode of toxicity of polycyclic aromatic hydrocarbons (PAHs) in Escherichia coli. Chemosphere 2007, 66, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Velali, E.; Pantazaki, A.; Besis, A.; Choli-Papadopoulou, T.; Samara, C. Oxidative stress, DNA damage, and mutagenicity induced by the extractable organic matter of airborne particulates on bacterial models. Regul. Toxicol. Pharmacol. 2019, 104, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.A.; Pan, C.-H.; Diawara, N.; Chang-Chien, G.-P.; Lin, W.-Y.; Huang, C.-T.; Ho, C.-K.; Wu, M.-T. Polycyclic aromatic hydrocarbon-induced oxidative stress and lipid peroxidation in relation to immunological alteration. Occup. Environ. Med. 2011, 68, 653–658. [Google Scholar] [CrossRef]
- Kostelli, G.; Kourea, K.; Ikonomidis, I. Effects of combustible tobacco smoking and novel tobacco products on oxidative stress: Different sides of the same coin? Curr. Opin. Toxicol. 2020, 20, 41–47. [Google Scholar] [CrossRef]
- Marcilla, A.; Martínez, I.; Berenguer, D.; Gómez-Siurana, A.; Beltrán, M. Comparative study of the main characteristics and composition of the mainstream smoke of ten cigarette brands sold in Spain. Food Chem. Toxicol. 2012, 50, 1317–1333. [Google Scholar] [CrossRef]
- Bhalla, D.K.; Hirata, F.; Rishi, A.K.; Gairola, C.G. Cigarette smoke, inflammation, and lung injury: A mechanistic perspective. J. Toxicol. Environ. Health Part B 2009, 12, 45–64. [Google Scholar] [CrossRef]
- Sørensen, S.J.; Burmølle, M.; Hansen, L.H. Making bio-sense of toxicity: New developments in whole-cell biosensors. Curr. Opin. Biotechnol. 2006, 17, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xie, Y.; Zhou, X.; Zhang, Q.; Wang, N. The effect of different tobacco tar levels on DNA damage in cigarette smoking subjects. Toxicol. Res. 2020, 9, 302–307. [Google Scholar] [CrossRef] [PubMed]


| Bacteria | Antibiotic | Temperature | ||||
|---|---|---|---|---|---|---|
| Strain | Plasmid | Name | Concentration (µg/mL) | Growth (C0) | Refreshment (C0) | Stress Sensitivity |
| DPD2794 | pGrpELux3 | Ampicillin | 100 | 37 | 30 | SOS |
| TV1061 | pRecALux3 | Ampicillin | 100 | 37 | 30 | Heat Shock (general stress) |
| DPD2511 | pKatGLux2 | Ampicillin | 100 | 37 | 30 | Oxidative (peroxides) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bar, T.; Shagan, M.; Kramarsky-Winter, E.; Marks, R.S.; Golberg, K.; Kushmaro, A. Estimating Toxicity Putative Mechanisms from Smoking Residual Substances Using a Whole-Cell Bioreporter System. Biosensors 2025, 15, 733. https://doi.org/10.3390/bios15110733
Bar T, Shagan M, Kramarsky-Winter E, Marks RS, Golberg K, Kushmaro A. Estimating Toxicity Putative Mechanisms from Smoking Residual Substances Using a Whole-Cell Bioreporter System. Biosensors. 2025; 15(11):733. https://doi.org/10.3390/bios15110733
Chicago/Turabian StyleBar, Tal, Marilou Shagan, Esti Kramarsky-Winter, Robert S. Marks, Karina Golberg, and Ariel Kushmaro. 2025. "Estimating Toxicity Putative Mechanisms from Smoking Residual Substances Using a Whole-Cell Bioreporter System" Biosensors 15, no. 11: 733. https://doi.org/10.3390/bios15110733
APA StyleBar, T., Shagan, M., Kramarsky-Winter, E., Marks, R. S., Golberg, K., & Kushmaro, A. (2025). Estimating Toxicity Putative Mechanisms from Smoking Residual Substances Using a Whole-Cell Bioreporter System. Biosensors, 15(11), 733. https://doi.org/10.3390/bios15110733







