Triple-Model Immunoassays with the Self-Assemblies of Three-in-One Small Molecules as Signaling Labels
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Preparation of Metal-PQQ Hybrids
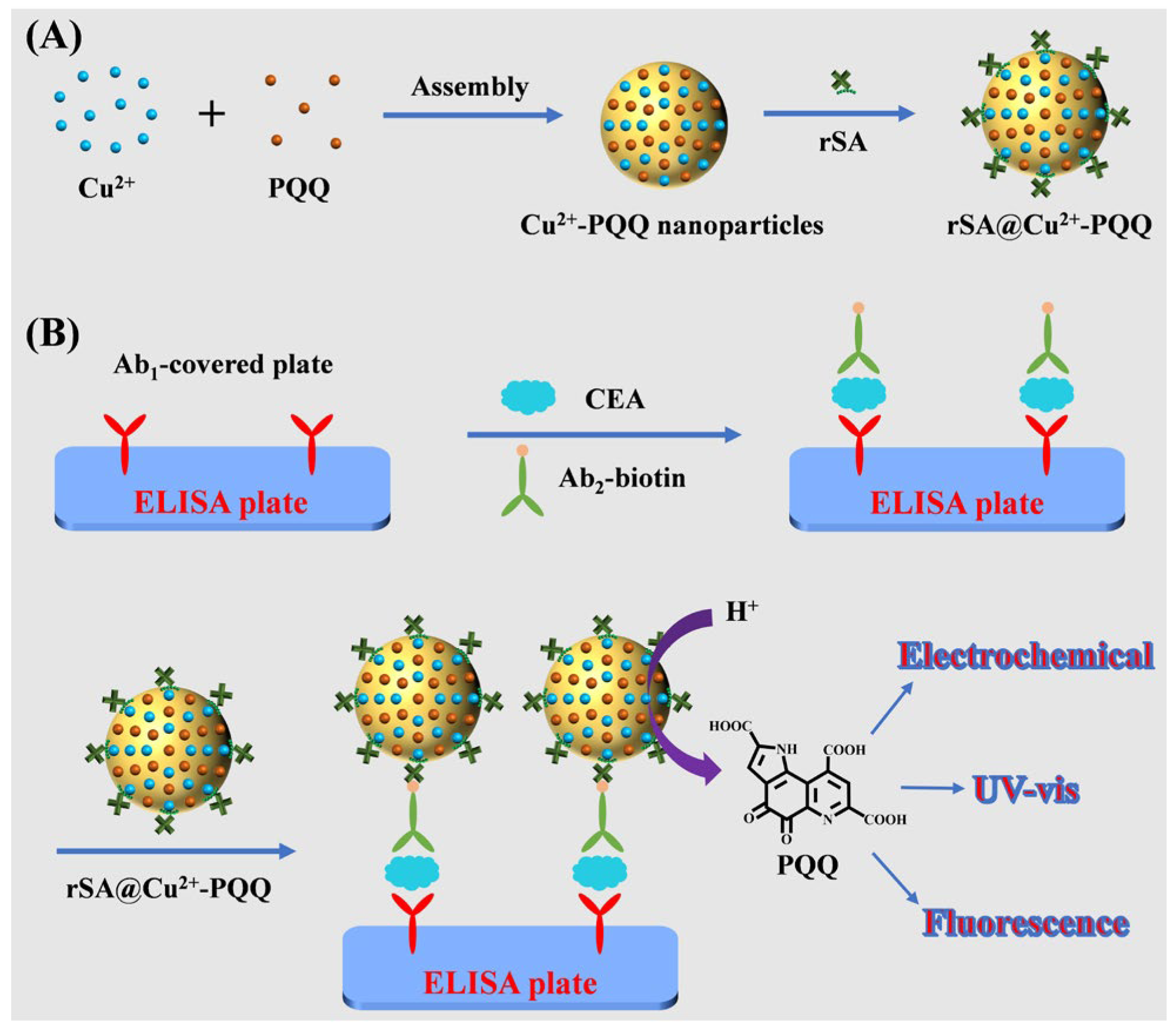
2.3. Modification of Cu-PQQ with Recombinant Streptavidin (rSA)
2.4. Procedures for CEA Detection
3. Results and Discussion
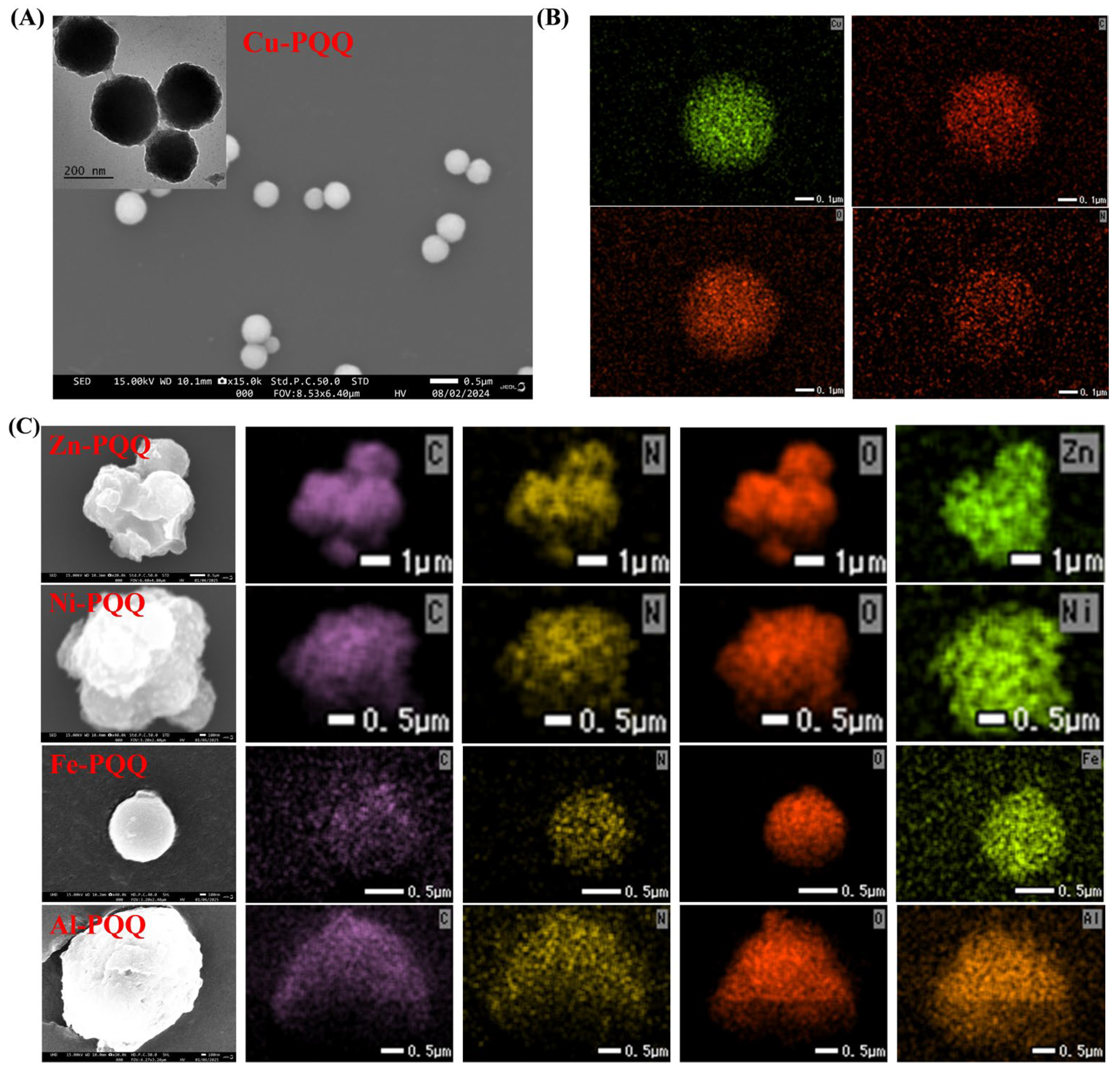
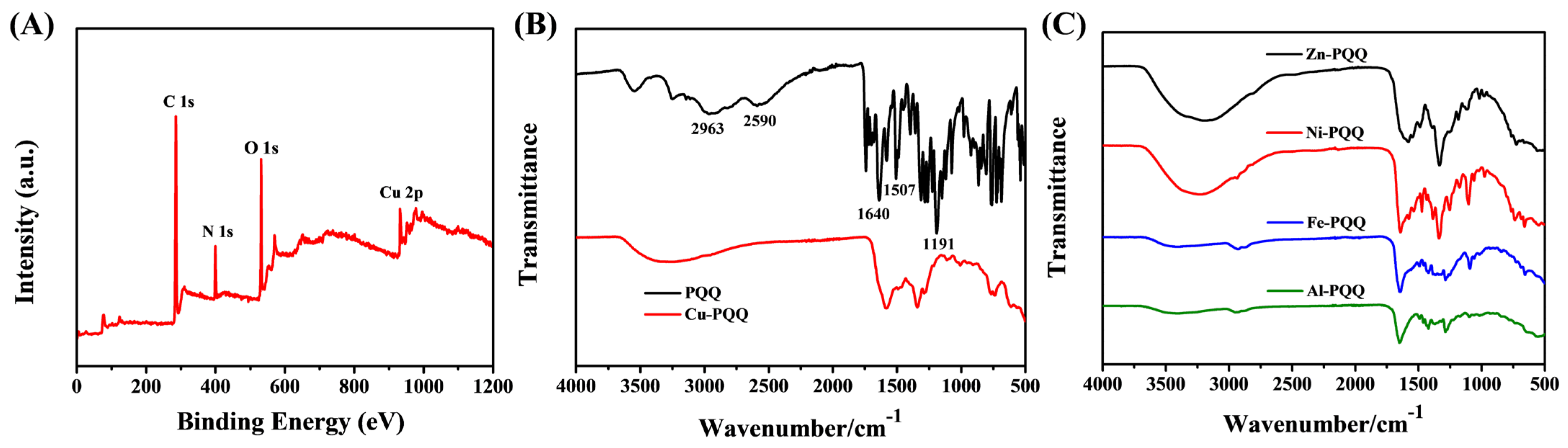
3.1. Characterization of Metal-PQQ Hybrids
3.2. Stability of Cu-PQQ
3.3. Characterization of the Interaction Between Cu-PQQ Nanoparticles and His6 Tags
3.4. Principle of Triple-Readout Immunoassays
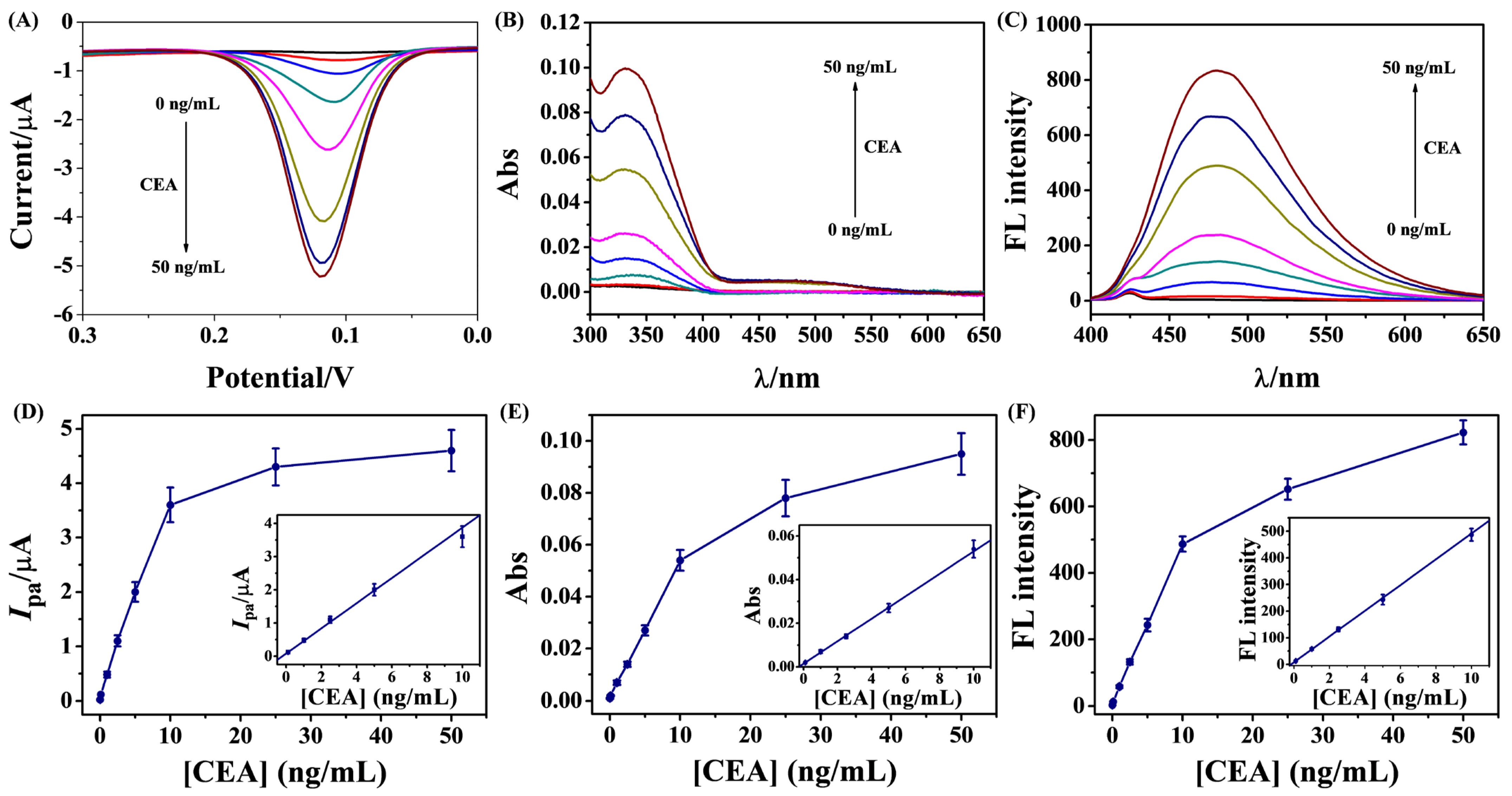
3.5. Feasibility of Triple-Readout Immunoassays
3.6. Sensitivity for CEA Detection
3.7. Selectivity, Stability, and Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farka, Z.; Juřík, T.; Kovář, D.; Trnkova, L.; Skládal, P. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Lara, S.; Perez-Potti, A. Applications of nanomaterials for immunosensing. Biosensors 2018, 8, 104. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, M. Recent progress in electrochemical immunosensors. Biosensors 2021, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Mayor, A.; Amor-Gutiérrez, O.; Costa-García, A.; de la Escosura-Muñiz, A. Nanoparticles as emerging labels in electrochemical immunosensors. Sensors 2019, 19, 5137. [Google Scholar] [CrossRef]
- Ma, X.; Ge, Y.; Xia, N. Overview of the design and application of dual-signal immunoassays. Molecules 2024, 29, 4551. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, D.; Xiao, S.; Yin, Y.; Li, L.; Wang, J.; Wu, Y.; Qiu, Y.; Dong, Y. Dual-mode aptasensors with cross validation capacity for reliability enhancement and analytical assurance. TrAC-Trends Anal. Chem. 2024, 177, 117755–117768. [Google Scholar] [CrossRef]
- Han, Q.; Wang, H.; Wang, J. Multi-mode/signal biosensors: Electrochemical integrated sensing techniques. Adv. Funct. Mater. 2024, 34, 2403122. [Google Scholar] [CrossRef]
- Saleh, R.O.; Almajidi, y.Q.; Mansouri, S.; Hammoud, A.; Rodrigues, P.; Mezan, S.O.; Maabreh, H.G.; Deorari, M.; Shakir, M.N.; Alasheqi, M.Q. Dual-mode colorimetric and fluorescence biosensors for the detection of foodborne bacteria. Clin. Chim. Acta 2024, 553, 117741. [Google Scholar] [CrossRef]
- Chen, M.; Qileng, A.; Liang, H.; Lei, H.; Liu, W.; Liu, Y. Advances in immunoassay-based strategies for mycotoxin detection in food: From single-mode immunosensors to dual-mode immunosensors. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1285–1311. [Google Scholar] [CrossRef]
- Meng, X.X.; Wang, J.; Diao, L.L.; Li, C.P. Construction of multi-mode photoelectrochemical immunoassays for accurate detection of cancer markers: Assisted with MOF-confined plasmonic nanozyme. Anal. Chem. 2024, 96, 1336–1344. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Yang, H.; Zhou, Y. Triple-readout immunoassay based on copper ion trigger for the detection of ochratoxin A. Anal. Chim. Acta 2025, 1345, 343750. [Google Scholar] [CrossRef]
- Kuang, Y.; Yuan, W.; Chen, X.; Wang, X.; Wang, X. A dual-mode immunosensor for detection of oncoprotein c-Myc via indirect competition using trimetallic nanocomposite as an excellent label. Anal. Chim. Acta 2025, 1344, 343724. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Zheng, C.; Liu, G. Fluorescent–electrochemical–colorimetric triple-model immunoassays with multifunctional metal–organic frameworks for signal amplification. Biosensors 2025, 15, 376. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Li, Y.; He, C.; Deng, D. Nanolabels prepared by the entrapment or self-assembly of signaling molecules for colorimetric and fluorescent immunoassays. Biosensors 2024, 14, 597. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.C.; Sin, K.K.; Chan, C.P.; Wong, L.W.; Renneberg, R. Biofunctionalized indigo-nanoparticles as biolabels for the generation of precipitated visible signal in immunodipsticks. Biosens. Bioelectron. 2011, 26, 3148–3153. [Google Scholar] [CrossRef]
- Song, Y.; Cai, X.; Ostermeyer, G.; Yu, J.; Du, D.; Lin, Y. Self-assembling allochroic nanocatalyst for improving nanozyme-based immunochromatographic assays. ACS Sens. 2021, 6, 220–228. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Fluorescence immunoassay based on fluorescer microparticles. Colloids Surf. B 2000, 18, 13–17. [Google Scholar] [CrossRef]
- Yan, C.; Sun, Y.; Yao, M.; Jin, X.; Yang, Q.; Wu, W. pH-responsive nanoparticles and automated detection apparatus for dual detection of pathogenic bacteria. Sens. Actuat. B: Chem. 2022, 354, 131117. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.; Ye, J.; Jiao, J.; Liu, Z.; Zhao, C.; Li, B.; Fu, Y. Metal-coordinated supramolecular self-assemblies for cancer theranostics. Adv. Sci. 2021, 8, 2101101. [Google Scholar] [CrossRef]
- Wong, K.Y.; Nie, Z.; Wong, M.S.; Wang, Y.; Liu, J. Metal–drug coordination nanoparticles and hydrogels for enhanced delivery. Adv. Mater. 2024, 36, 2404053. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Jin, X.; Liu, H.; Sun, K.; Wang, X.; Li, M.; Wang, P.; Chang, Y.; Wang, T.; et al. An encounter between metal ions and natural products: Natural products-coordinated metal ions for the diagnosis and treatment of tumors. J. Nanobiotech. 2024, 22, 726. [Google Scholar] [CrossRef]
- Li, Y.; Miao, Y.; Yang, L.; Zhao, Y.; Wu, K.; Lu, Z.; Hu, Z.; Guo, J. Recent advances in the development and antimicrobial applications of metal–phenolic networks. Adv. Sci. 2022, 9, 2202684. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Q.; Wu, F.-G.; Dai, Y.; Chen, X. Polyphenol-containing nanoparticles: Synthesis, properties, and therapeutic delivery. Adv. Mater. 2021, 33, 2007356. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Liang, S.; Deng, D.; Chang, Y.; Yi, X. Metal–phenolic networks for sensing applications. Biosensors 2025, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Roder, R.; Preiß, T.; Hirschle, P.; Steinborn, B.; Zimpel, A.; Hohn, M.; Radler, J.O.; Bein, T.; Wagner, E.; Wuttke, S.; et al. Multifunctional nanoparticles by coordinative self-assembly of His tagged units with metal−organic frameworks. J. Am. Chem. Soc. 2017, 139, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Satheeshkumar, K.; Saravana Kumar, P.; Nandhini, C.; Shanmugapriya, R.; Vennila, K.N.; Elango, K.P. A simple metal ion displacement-type turn-on fluorescent probe for the detection of halide ions in 100% water—Spectroscopic and TD-DFT investigations. Inorg. Chem. Commun. 2022, 139, 109299. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Yang, B.; Xiao, K.; Duan, H.; Zhao, H. The chemical stability of metal-organic frameworks in water treatments: Fundamentals, effect of water matrix and judging methods. Chem. Eng. J. 2022, 450, 138215. [Google Scholar] [CrossRef]
- Zhu, L.; Chang, Y.; Li, Y.; Qiao, M.; Liu, L. Biosensors based on the binding events of nitrilotriacetic acid–metal complexes. Biosensors 2023, 13, 507. [Google Scholar] [CrossRef]
- Feng, Y.; Gao, F.; Yi, X.; La, M. Optical bioassays based on the signal amplification of redox cycling. Biosensors 2024, 14, 269. [Google Scholar] [CrossRef]
- Yu, Z.-J.; Yang, T.-T.; Liu, G.; Deng, D.-H.; Liu, L. Gold nanoparticles-based colorimetric immunoassay of carcinoembryonic antigen with metal–organic framework to load quinones for catalytic oxidation of cysteine. Sensors 2024, 24, 6701. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, Y.; Fan, X.; Zhou, J.; Lv, Y.; Xia, N. Electrochemical immunosensors based on the solubility difference of electroactive probe and the dual signal amplification of nanocarrier plus redox cycling. Bioelectrochemistry 2025, 162, 108858. [Google Scholar] [CrossRef]
- Xia, N.; Gao, F.; Liu, G.; Chang, Y.; Liu, L. Pyrroloquinoline quinone exhibiting peroxidase-mimicking property for colorimetric assays. Anal. Chim. Acta 2025, 1335, 343468. [Google Scholar] [CrossRef]
- Mukai, K.; Ouchi, A.; Nakano, M. Kinetic study of the quenching reaction of singlet oxygen by pyrroloquinolinequinol (PQQH2, a reduced form of pyrroloquinolinequinone) in micellar solution. J. Agric. Food Chem. 2011, 59, 1705–1712. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Zhang, J.; Liu, J.; Wang, A.; Ding, L. Recent development of copper-based nanozymes for biomedical applications. Adv. Healthc. Mater. 2024, 13, 2302023. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, C.; Economou, A.; Petrou, P.S.; Kakabakos, S.E. Microfabricated tin Film electrodes for protein and DNA sensing based on stripping voltammetric detection of Cd(II) released from quantum dots labels. Anal. Chem. 2013, 85, 10686–10691. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, G.; Wu, H.; Lin, Y. Quantum-dot-based electrochemical immunoassay for high-throughput screening of the prostate-specific antigen. Small 2008, 4, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Timchalk, C.; Lin, Y. Magnetic electrochemical immunoassays with quantum dot labels for detection of phosphorylated acetylcholinesterase in plasma. Anal. Chem. 2008, 80, 8477–8484. [Google Scholar] [CrossRef]
- Kokkinos, C.; Angelopoulou, M.; Economou, A.; Prodromidis, M.; Florou, A.; Haasnoo, W.; Petrou, P.; Kakabakos, S. Lab-on-a-membrane foldable devices for duplex drop-volume electrochemical biosensing using quantum dot tags. Anal. Chem. 2016, 88, 6897–6904. [Google Scholar] [CrossRef]
- Xia, N.; Huang, Y.; Zhao, Y.; Wang, F.; Liu, L.; Sun, Z. Electrochemical biosensors by in situ dissolution of self-assembled nanolabels into small monomers on electrode surface. Sens. Actuat. B Chem. 2020, 325, 128777. [Google Scholar] [CrossRef]
- Shao, F.; Zhang, L.; Jiao, L.; Wang, X.; Miao, L.; Li, H.; Zhou, F. Enzyme-free immunosorbent assay of prostate specific antigen amplified by releasing pH indicator molecules entrapped in mesoporous silica nanoparticles. Anal. Chem. 2018, 90, 8673–8679. [Google Scholar] [CrossRef]
- Ren, R.; Cai, G.; Yu, Z.; Zeng, Y.; Tang, D. Metal-polydopamine framework: An innovative signal-generation tag for colorimetric immunoassay. Anal. Chem. 2018, 90, 11099–11105. [Google Scholar] [CrossRef]
- Shao, F.; Jiao, L.; Miao, L.; Wei, Q.; Li, H. A pH Indicator-linked immunosorbent assay following direct amplification strategy for colorimetric detection of protein biomarkers. Biosens. Bioelectron. 2017, 90, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Deng, D.; Mu, X.; Liu, A.; Xie, J.; Zhou, D.; Yang, P.; Xing, Y.; Liu, L. Colorimetric immunoassays based on pyrroloquinoline quinone-catalyzed generation of Fe(II)-ferrozine with tris(2-carboxyethyl)phosphine as the reducing reagent. Sens. Actuators B Chem. 2020, 306, 127571. [Google Scholar] [CrossRef]
- Jiao, L.; Yan, H.; Xu, W.; Wu, Y.; Gu, W.; Li, H.; Du, D.; Lin, Y.; Zhu, C. Self-assembly of all-inclusive allochroic nanoparticles for the improved ELISA. Anal. Chem. 2019, 91, 8461–8465. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.F.; Fu, C.; Chen, Y.T.; Fang-Ju Jou, A.; Chen, C.C.; Chou, C.; Annie Ho, J.A. Use of liposomal amplifiers in total internal reflection fluorescence fiber-optic biosensors for protein detection. Biosens. Bioelectron. 2016, 77, 1201–1207. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Xiong, S.; Li, X.; Zhan, S.; Zeng, L.; Xiong, Y. Dual-mode fluorescent and colorimetric immunoassay for the ultrasensitive detection of alpha-fetoprotein in serum samples. Anal. Chim. Acta 2018, 1038, 112–119. [Google Scholar] [CrossRef]
- Ho, J.A.; Wu, L.C.; Huang, M.R.; Lin, Y.J.; Baeumner, A.J.; Durst, R.A. Application of ganglioside-sensitized liposomes in a flow injection immunoanalytical system for the determination of cholera toxin. Anal. Chem. 2007, 79, 246–250. [Google Scholar] [CrossRef]
- Geißler, D.; Wegner, K.D.; Fischer, C.; Resch-Genger, U. Exploring simple particle-based signal amplification strategies in a heterogeneous sandwich immunoassay with optical detection. Anal. Chem. 2024, 96, 5078–5085. [Google Scholar] [CrossRef]
- Chan, C.P.; Bruemmel, Y.; Seydack, M.; Sin, K.K.; Wong, L.W.; Merisko-Liversidge, E.; Trau, D.; Renneberg, R. Nanocrystal biolabels with releasable fluorophores for immunoassays. Anal. Chem. 2004, 76, 3638–3645. [Google Scholar] [CrossRef]
- Gibson, L.E.; Wright, D.W. Sensitive method for biomolecule detection utilizing signal amplification with porphyrin nanoparticles. Anal. Chem. 2016, 88, 5928–5933. [Google Scholar] [CrossRef]
- Gao, F.; Chang, Y.; Xia, N.; Li, Y.; Liu, L. Multifunctional self-assembled nanoparticles serving as the signal labels of fluorescent immunoassays. Microchem. J. 2024, 207, 111921. [Google Scholar] [CrossRef]




| Method | Signal Label | Releasing Agent | Target | Linear Range (ng/mL) | Detection Limit (ng/mL) | Ref. |
|---|---|---|---|---|---|---|
| SWV | Cd-based QDs | HNO3 | PSA | 0–20 | 0.12 | [35] |
| SWV | Cd-based QDs | HCl | PSA | 0.5–80 | 0.2 | [36] |
| SWV | Cd-based QDs | HCl | OP-AChE | 0.3–3 × 102 | 0.15 | [37] |
| ASV | Pb/Cd-based QDs | HCl | Casein, IgG | 0–5 × 103, 0–2 × 103 | 40, 20 | [38] |
| DPV | FcFNPs | Methanol | PSA | 10−3–7.5 × 10−2 | 10−3 | [39] |
| UV-vis | TP@MSN | NaOH | PSA | 5 × 10−4–8 | 3.6 × 10−4 | [40] |
| UV-vis | TP@MPDA | NaOH | AFP | 10−2–1 | 2.3 × 10−3 | [41] |
| UV-vis | PP@cC3N4 | NaOH | CEA | 0.5–10−2 | 3.4 × 10−4 | [42] |
| UV-vis | PQQ@MSN | Phosphate | PSA | 5 × 10−3–0.5 | 10−3 | [43] |
| UV-vis | TMB NPs | Ethanol | IL-6 | 1 × 10−3–1 | 6.6 × 10−4 | [44] |
| fluorescence | FL@liposome | Not reported | IgG | 10−10–10−7 | 2 × 10−3 | [45] |
| fluorescence | FL@AuNF | NaOH | AFP | 10−5–10−2 | 2.9 × 10−8 | [46] |
| fluorescence | SRB@liposome | OG | CT | 10−10–10−8 | 6 × 10−11 | [47] |
| fluorescence | C153@PSP | Ethanol | CRP | 12–45 | 4.9 | [48] |
| fluorescence | FDA NCs | DMSO | IgG | 0–2 | 5.7 × 10−2 | [49] |
| fluorescence | TCPP NPs | NaOH | IgG | 1.5–1.5 × 102 | 0.31 | [50] |
| fluorescence | bio-PyFNPs | Methanol | AFP | 10−3–2.5 | 5 × 10−4 | [51] |
| DPV, UV-vis, fluorescence | Cu-PQQ | Acetic acid | CEA | 0.1–10 | 0.01, 0.1, 0.1 | This work |
| Added (ng/mL) | Found (ng/mL) | ||
|---|---|---|---|
| DPV | UV-Vis | Fluorescence | |
| 0.10 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 |
| 1.00 | 1.04 ± 0.10 | 1.06 ± 0.13 | 1.08 ± 0.12 |
| 5.00 | 4.86 ± 0.38 | 4.92 ± 0.41 | 5.02 ± 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Yuan, W.; Qiao, M.; Liu, L. Triple-Model Immunoassays with the Self-Assemblies of Three-in-One Small Molecules as Signaling Labels. Biosensors 2025, 15, 710. https://doi.org/10.3390/bios15110710
Yu Z, Yuan W, Qiao M, Liu L. Triple-Model Immunoassays with the Self-Assemblies of Three-in-One Small Molecules as Signaling Labels. Biosensors. 2025; 15(11):710. https://doi.org/10.3390/bios15110710
Chicago/Turabian StyleYu, Zhaojiang, Wenqi Yuan, Mingyi Qiao, and Lin Liu. 2025. "Triple-Model Immunoassays with the Self-Assemblies of Three-in-One Small Molecules as Signaling Labels" Biosensors 15, no. 11: 710. https://doi.org/10.3390/bios15110710
APA StyleYu, Z., Yuan, W., Qiao, M., & Liu, L. (2025). Triple-Model Immunoassays with the Self-Assemblies of Three-in-One Small Molecules as Signaling Labels. Biosensors, 15(11), 710. https://doi.org/10.3390/bios15110710






