Noble-Metal-Free MIL-101(Cr)@rGO for Formaldehyde SERS Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
2.3. Synthesis of Reduced Graphene Oxide rGO
2.4. Synthesis of MIL-101(Cr)
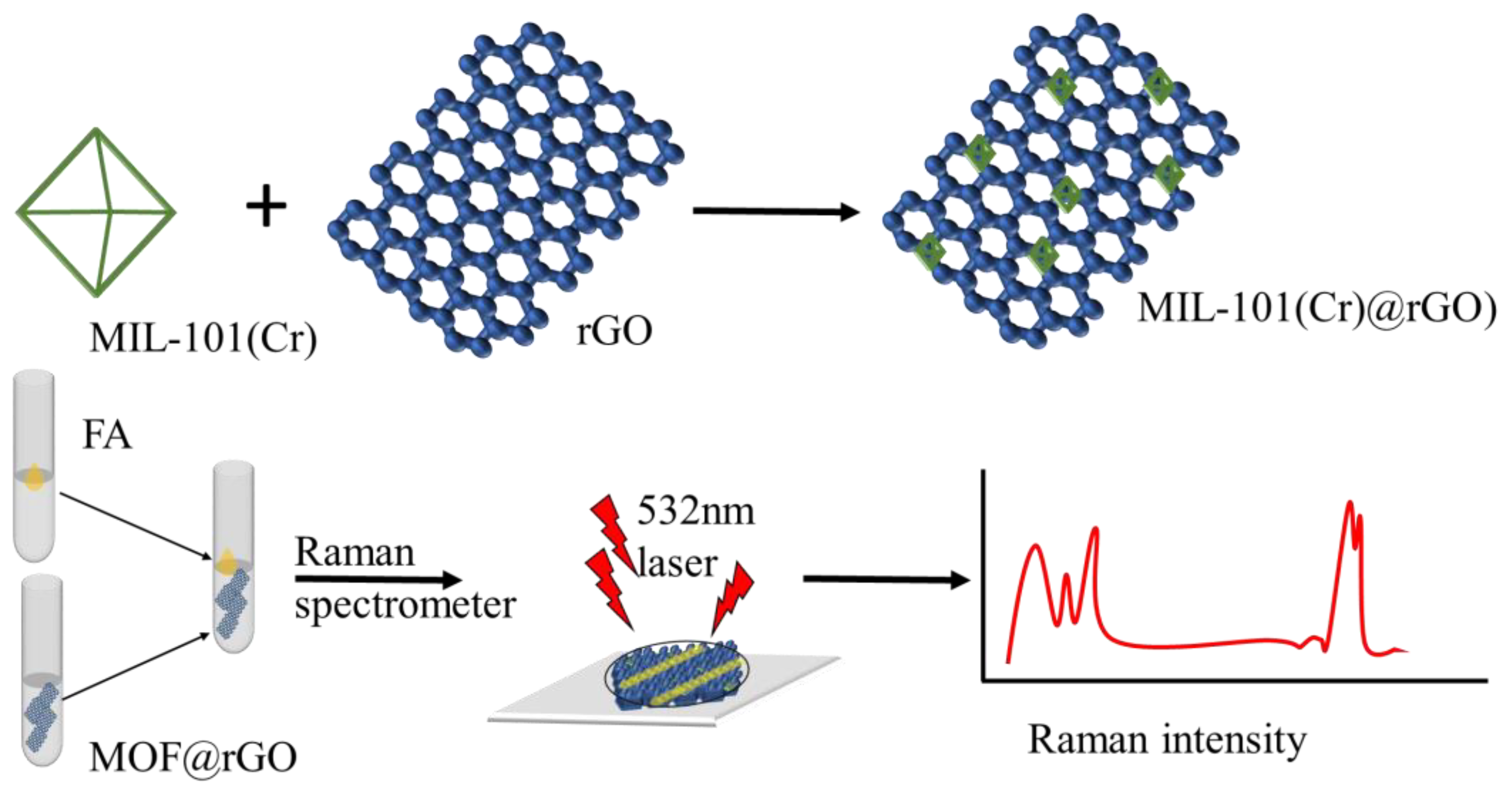
2.5. Synthesis of Complex MIL-101(Cr)@rGO
3. Results and Discussions
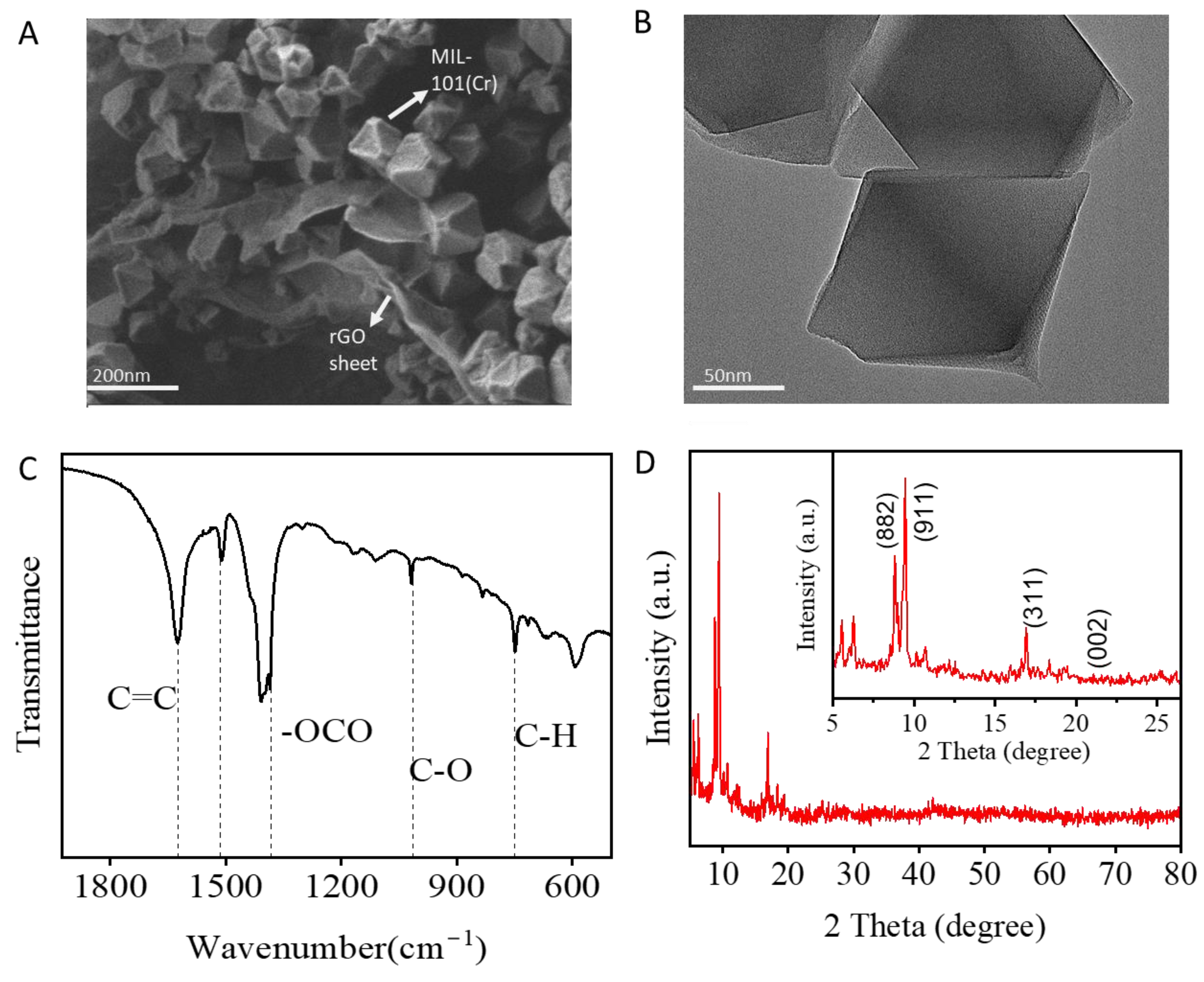
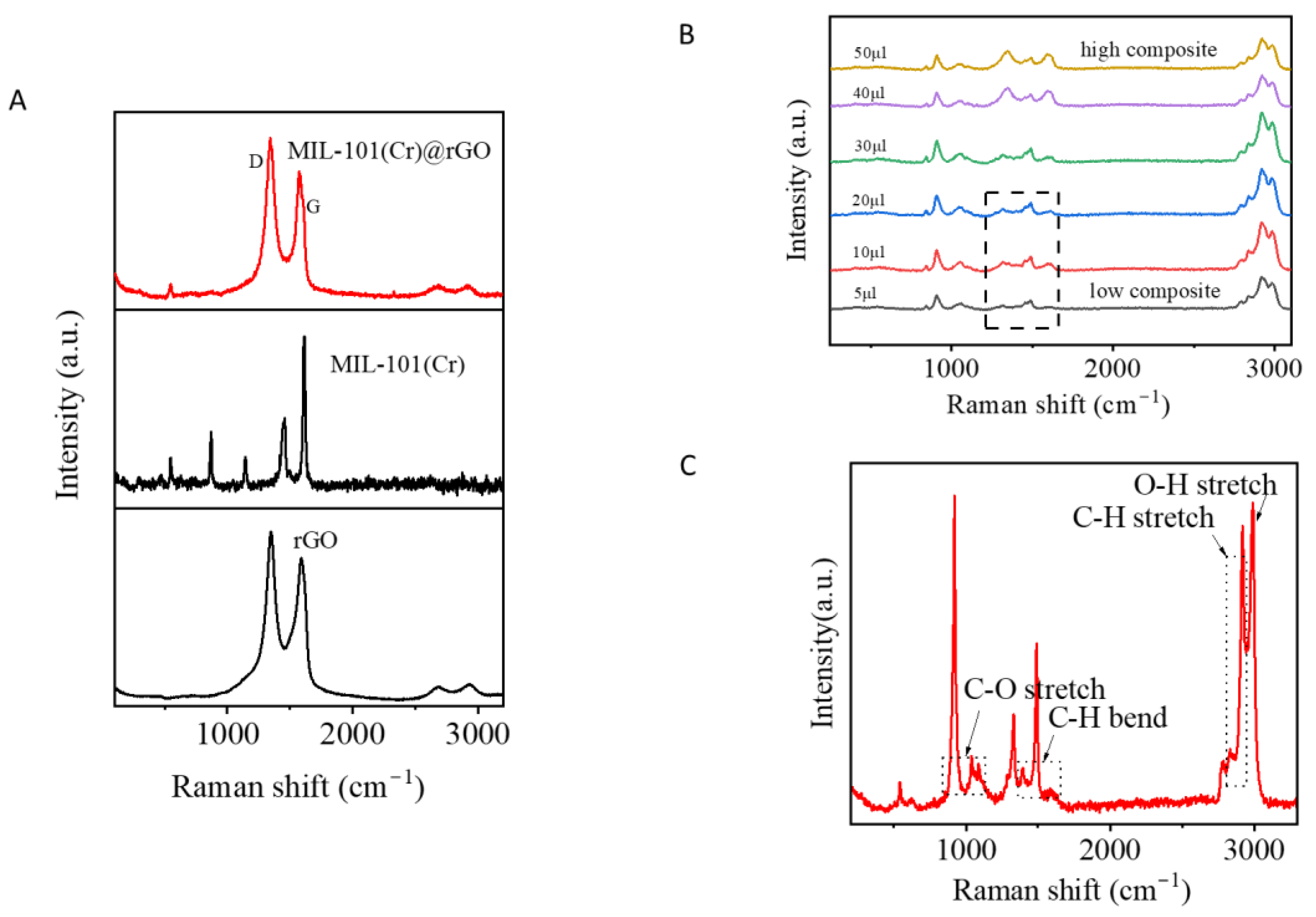
3.1. Preparation and Characterization of MIL-101(Cr)@rGO
3.2. Optimization
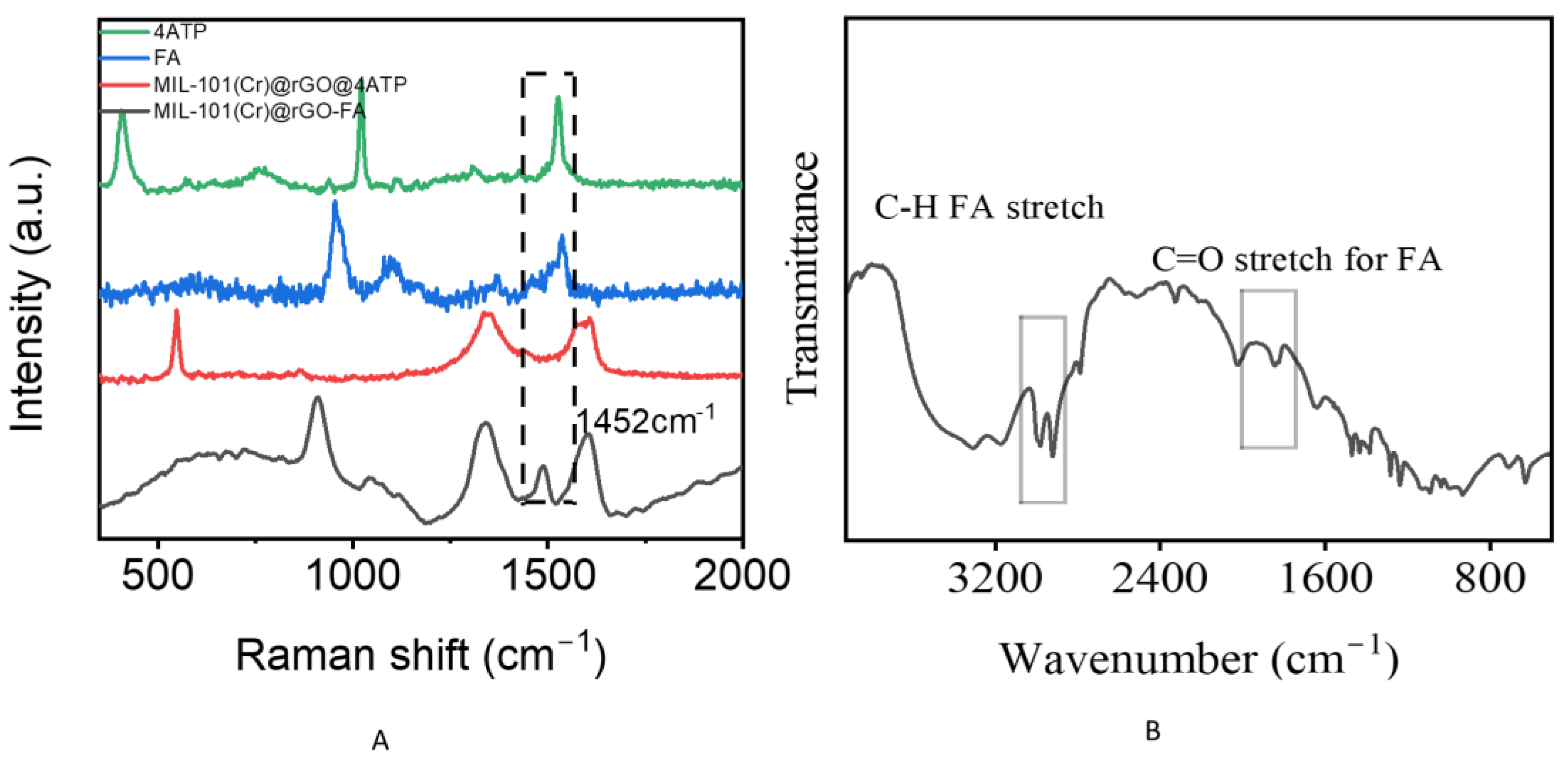
3.3. Possible Mechanism
3.4. Detection of VOC Concentrate Formaldehyde (Raman)
3.5. Stability and Reproducibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VOCS | Volatile organic compounds |
| SERS | Surface Enhanced Raman Spectroscopy |
| rGO | Reduced Graphene Oxide |
| FA | Formaldehyde |
| MOF | Metal Organic Framework |
| GERS | Graphene Enhanced Raman Spectroscopy |
| PPM | parts per million |
| RSD | relative standard deviation |
| LOD | Limit of detection |
| CM | Chemical enhancement |
| EM | Electromagnetic enhancement |
References
- Rosario, W.; Singh, P.K.; Tiwari, A.; Jain, U.; Avasthi, D.K.; Chauhan, N. Nanomaterial-based VOC sensing applications and a deep dive into their developmental trends. J. Mater. Chem. A 2024, 12, 9979–10011. [Google Scholar] [CrossRef]
- McNair, H.M.; Miller, J.M.; Snow, N.H. Basic Gas Chromatography; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Yuan, Z.; Bariya, M.; Fahad, H.M.; Wu, J.; Han, R.; Gupta, N.; Javey, A. Trace-level, multi-gas detection for food quality assessment based on decorated silicon transistor arrays. Adv. Mater. 2020, 32, 1908385. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, K.; Zou, M.; Maniyara, R.A.; Bowen, T.A.; Schrecengost, J.R.; Jain, A.; Zhou, D.; Dong, C.; Yu, Z. Tuning the fermi level of graphene by two-dimensional metals for raman detection of molecules. ACS Nano 2024, 18, 8876–8884. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liao, J.; Wang, D.; Li, G. Fabrication of gold nanoparticle-embedded metal–organic framework for highly sensitive surface-enhanced Raman scattering detection. Anal. Chem. 2014, 86, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xue, F.; Sun, Q.; Yue, R.; Lin, D. Adsorption of volatile organic compounds by metal-organic frameworks MOF-177. J. Environ. Chem. Eng. 2013, 1, 713–718. [Google Scholar] [CrossRef]
- Xue, D.-X.; Belmabkhout, Y.; Shekhah, O.; Jiang, H.; Adil, K.; Cairns, A.J.; Eddaoudi, M. Tunable Rare Earth fcu-MOF Platform: Access to Adsorption Kinetics Driven Gas/Vapor Separations via Pore Size Contraction. J. Am. Chem. Soc. 2015, 137, 5034–5040. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Mizrahi Rodriguez, K.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-based membranes for gas separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Chen, J.; Guo, L.; Chen, L.; Qiu, B.; Hong, G.; Lin, Z. Sensing of hydrogen sulfide gas in the Raman-silent region based on gold nano-bipyramids (Au NBPs) encapsulated by zeolitic imidazolate framework-8. ACS Sens. 2020, 5, 3964–3970. [Google Scholar] [CrossRef]
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C. Structure of graphene and its disorders: A review. Sci. Technol. Adv. Mater. 2018, 19, 613–648. [Google Scholar] [CrossRef]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Dalfovo, M.C.; Lacconi, G.I.; Moreno, M.N.; Yappert, M.C.; Sumanasekera, G.U.; Salvarezza, R.C.; Ibañez, F.J. Synergy between graphene and au nanoparticles (heterojunction) towards quenching, improving raman signal, and UV light sensing. ACS Appl. Mater. Interfaces 2014, 6, 6384–6391. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Chu, J.; Zhao, H.; Xu, P.; Sun, M. Recent progress in the applications of graphene in surface-enhanced Raman scattering and plasmon-induced catalytic reactions. J. Mater. Chem. C 2015, 3, 9024–9037. [Google Scholar] [CrossRef]
- Lai, H.; Xu, F.; Zhang, Y.; Wang, L. Recent progress on graphene-based substrates for surface-enhanced Raman scattering applications. J. Mater. Chem. B 2018, 6, 4008–4028. [Google Scholar] [CrossRef]
- Ling, X.; Xie, L.; Fang, Y.; Xu, H.; Zhang, H.; Kong, J.; Dresselhaus, M.S.; Zhang, J.; Liu, Z. Can graphene be used as a substrate for Raman enhancement? Nano Lett. 2010, 10, 553–561. [Google Scholar] [CrossRef]
- Ling, X.; Zhang, J. First-layer effect in graphene-enhanced Raman scattering. Small 2010, 6, 2020–2025. [Google Scholar] [CrossRef]
- Liu, Z.; He, W.; Zhang, Q.; Shapour, H.; Bakhtari, M.F. Preparation of a GO/MIL-101 (Fe) composite for the removal of methyl orange from aqueous solution. ACS Omega 2021, 6, 4597–4608. [Google Scholar] [CrossRef]
- Huazhen, D.; Wei, D.; Zhenfei, G.; Dan, L.; Dawei, L. SERS-based chip for discrimination of formaldehyde and acetaldehyde in aqueous solution using silver reduction. Microchim. Acta 2019, 186, 175. [Google Scholar] [CrossRef]
- Naqvi, T.K.; Srivastava, A.K.; Kulkarni, M.M.; Siddiqui, A.M.; Dwivedi, P.K. Silver nanoparticles decorated reduced graphene oxide (rGO) SERS sensor for multiple analytes. Appl. Surf. Sci. 2019, 478, 887–895. [Google Scholar] [CrossRef]
- Gil, B.; Keshavarz, M.; Wales, D.; Darzi, A.; Yeatman, E. Orthogonal Surface-Enhanced Raman Scattering/Field-Effect Transistor Detection of Breast and Colorectal Cancer-Derived Exosomes using Graphene as a Tag-Free Diagnostic Template. Adv. NanoBiomed Res. 2023, 3, 2300055. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Yu, T.-H.; Ho, C.-H.; Wu, C.-Y.; Chien, C.-H.; Lin, C.-H.; Lee, S. Metal–organic frameworks: A novel SERS substrate. J. Raman Spectrosc. 2013, 44, 1506–1511. [Google Scholar] [CrossRef]
- Xie, D.; Wang, R.; Fu, J.; Zhao, Z.; Li, M. AuNPs@MIL-101 (Cr) as a SERS-Active Substrate for Sensitive Detection of VOCs. Front. Bioeng. Biotechnol. 2022, 10, 921693. [Google Scholar] [CrossRef]
- Bromberg, L.; Diao, Y.; Wu, H.; Speakman, S.A.; Hatton, T.A. Chromium (III) terephthalate metal organic framework (MIL-101): HF-free synthesis, structure, polyoxometalate composites, and catalytic properties. Chem. Mater. 2012, 24, 1664–1675. [Google Scholar] [CrossRef]
- Sibnath Kayal, B.S.; Chakraborty, A. Study of metal-organic framework MIL-101(Cr) for natural gas (methane) storage and compare with other MOFs (metal-organic frameworks). Energy Chem. 2015, 91, 772–781. [Google Scholar] [CrossRef]
- Li, B.; Dong, X.; Wang, H.; Ma, D.; Tan, K.; Shi, Z.; Chabal, Y.J.; Han, Y.; Li, J. Functionalized metal organic frameworks for effective capture of radioactive organic iodides. Faraday Discuss. 2017, 201, 47–61. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Tikhani, F.; Shabanian, M.; Movahedi, F.; Moghari, S.; Akbari, V.; Gabrion, X.; Laheurte, P.; Vahabi, H.; Saeb, M.R. Synthesis, characterization, and high potential of 3D metal-organic framework (MOF) nanoparticles for curing with epoxy. J. Alloys Compd. 2020, 829, 154547. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.; Jain, Y.; Kumari, M.; Gupta, R.; Sharma, S.K.; Sachdev, K. Synthesis and Characterization of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) for Gas Sensing Application. Macromol. Symp. 2017, 376, 1700006. [Google Scholar] [CrossRef]
- Su, Y.; Lu, M.; Su, R.; Zhou, W.; Xu, X.; Li, Q. A 3D MIL-101@rGO composite as catalyst for efficient conversion of straw cellulose into valuable organic acid. Chin. Chem. Lett. 2022, 33, 2573–2578. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Seop Kim, H. Fast and fully-scalable synthesis of reduced graphene oxide. Sci. Rep. 2015, 5, 10160. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Hur, W.; Kang, H.; Jun, B.-H. In vivo surface-enhanced Raman scattering techniques: Nanoprobes, instrumentation, and applications. Light Sci. Appl. 2025, 14, 79. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Ma, C.; Luo, S.; Xu, M.; Wu, Z.; Li, W.; Liu, S. Luminescent Transparent Wood Based on Lignin-derived Carbon Dots as a Building Material for Dual-channel, Real-time and Visual Detection of Formaldehyde Gas. ACS Appl. Mater. Interfaces 2020, 12, 36628–36638. [Google Scholar] [CrossRef]
- Sinha, R.K. Surface-enhanced Raman Scattering and Localized Surface Plasmon Resonance Detection of Aldehydes Using 4-ATP Functionalized Ag Nanorods. Plasmonics 2023, 18, 241–253. [Google Scholar] [CrossRef]
- Hu, X.; Wang, T.; Wang, L.; Dong, S. Surface-Enhanced Raman Scattering of 4-Aminothiophenol Self-Assembled Monolayers in Sandwich Structure with Nanoparticle Shape Dependence: Off-Surface Plasmon Resonance Condition. J. Phys. Chem. C 2007, 111, 6962–6969. [Google Scholar] [CrossRef]
- Shao, M.; Ji, C.; Tan, J.; Du, B.; Zhao, X.; Yu, J.; Man, B.; Xu, K.; Zhang, C.; Li, Z. Ferroelectrically modulate the Fermi level of graphene oxide to enhance SERS response. Opto-Electron. Adv. 2023, 6, 230094. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, B.; Ma, Z.; Wang, H.; Cheng, Z.; Xu, J. Microgravimetric Modeling—A New Method for Extracting Adsorption Parameters of Functionalized MIL-101(Cr). Nanomaterials 2023, 13, 2072. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Dong, M.; Zhao, T. Advances in Metal-Organic Frameworks MIL-101(Cr). Int. J. Mol. Sci. 2022, 23, 9396. [Google Scholar] [CrossRef] [PubMed]
- Androulidakis, C.; Kotsidi, M.; Gorgolis, G.; Pavlou, C.; Sygellou, L.; Paterakis, G.; Koutroumanis, N.; Galiotis, C. Multi-functional 2D hybrid aerogels for gas absorption applications. Sci. Rep. 2021, 11, 13548. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.Y.; Yang, S.Y.; Wu, W.J.; Lin, C.T. A Room-Temperature Operation Formaldehyde Sensing Material Printed Using Blends of Reduced Graphene Oxide and Poly(methyl methacrylate). Sensors 2015, 15, 28842–28853. [Google Scholar] [CrossRef]
- Zhou, J.; Tong, Y.; He, Y.; Tu, P.; Xue, B.; Cheng, Y.; Cen, J.; Zheng, Y.; Ni, J.; Li, X. Role of Lewis Acids of MIL-101(Cr) in the Upgrading of Ethanol to n-Butanol. Front. Chem. Eng. 2020, 2, 586142. [Google Scholar] [CrossRef]
- Busca, G.; Lamotte, J.; Lavalley, J.C.; Lorenzelli, V. FT-IR study of the adsorption and transformation of formaldehyde on oxide surfaces. J. Am. Chem. Soc. 1987, 109, 5197–5202. [Google Scholar] [CrossRef]
- Tan, T.L.; Lebron, G.B. The high resolution FTIR spectrum of the ν2 band of formaldehyde isotopomer H213CO. J. Mol. Spectrosc. 2011, 270, 79–82. [Google Scholar] [CrossRef]
- Mauney, D.T.; Mosley, J.D.; Madison, L.R.; McCoy, A.B.; Duncan, M.A. Infrared spectroscopy and theory of the formaldehyde cation and its hydroxymethylene isomer. J. Chem. Phys. 2016, 145, 174303. [Google Scholar] [CrossRef]
- GOV.UK. Formaldehyde: General Information. 2024. Available online: https://www.gov.uk/government/publications/formaldehyde-properties-incident-management-and-toxicology/formaldehyde-general-information (accessed on 20 April 2025).
- Wang, P.; Zou, X.; Tan, H.; Wu, S.; Jiang, L.; Zhu, G. Ultrathin ZIF-8 film containing polyoxometalate as an enhancer for selective formaldehyde sensing. J. Mater. Chem. C 2018, 6, 5412–5419. [Google Scholar] [CrossRef]
- Yu, L.-Q.; Wang, L.-Y.; Su, F.-H.; Hao, P.-Y.; Wang, H.; Lv, Y.-K. A gate-opening controlled metal-organic framework for selective solid-phase microextraction of aldehydes from exhaled breath of lung cancer patients. Microchim. Acta 2018, 185, 307. [Google Scholar] [CrossRef]
- Gaca-Zając, K.Z.; Smith, B.R.; Nordon, A.; Fletcher, A.J.; Johnston, K.; Sefcik, J. Investigation of IR and Raman spectra of species present in formaldehyde-water-methanol systems. Vib. Spectrosc. 2018, 97, 44–54. [Google Scholar] [CrossRef]
- Olar, G.C.; Cotet, L.C.; Baia, L.; Mihis, A.; Baia, M. SERS-active substrates based on graphene oxide or reduced graphene oxide and silver nanoparticles. Mater. Today Proc. 2021, 45, 4096–4099. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, A.K.; Dwivedi, P.K.; Jha, S.K. Metal-free Nitrogen-doped reduced graphene oxide (N-rGO) SERS platform for detecting Rhodamine B. Sens. Actuators A Phys. 2025, 383, 116263. [Google Scholar] [CrossRef]
- Muntean, C.M.; Cuibus, D.; Boca, S.; Falamas, A.; Tosa, N.; Brezeştean, I.A.; Bende, A.; Barbu-Tudoran, L.; Moldovan, R.; Bodoki, E.; et al. Gold vs. Silver Colloidal Nanoparticle Films for Optimized SERS Detection of Propranolol and Electrochemical-SERS Analyses. Biosensors 2023, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Matikainen, A.; Nuutinen, T.; Itkonen, T.; Heinilehto, S.; Puustinen, J.; Hiltunen, J.; Lappalainen, J.; Karioja, P.; Vahimaa, P. Atmospheric oxidation and carbon contamination of silver and its effect on surface-enhanced Raman spectroscopy (SERS). Sci. Rep. 2016, 6, 37192. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalumansi, H.S.; Pi, F.; Li, J.; Jiang, G. Noble-Metal-Free MIL-101(Cr)@rGO for Formaldehyde SERS Detection. Biosensors 2025, 15, 703. https://doi.org/10.3390/bios15100703
Nalumansi HS, Pi F, Li J, Jiang G. Noble-Metal-Free MIL-101(Cr)@rGO for Formaldehyde SERS Detection. Biosensors. 2025; 15(10):703. https://doi.org/10.3390/bios15100703
Chicago/Turabian StyleNalumansi, Harriet Sonia, Fuwei Pi, Jingkun Li, and Guoyong Jiang. 2025. "Noble-Metal-Free MIL-101(Cr)@rGO for Formaldehyde SERS Detection" Biosensors 15, no. 10: 703. https://doi.org/10.3390/bios15100703
APA StyleNalumansi, H. S., Pi, F., Li, J., & Jiang, G. (2025). Noble-Metal-Free MIL-101(Cr)@rGO for Formaldehyde SERS Detection. Biosensors, 15(10), 703. https://doi.org/10.3390/bios15100703




