Smartphone-Linked and Electricity-Free Platforms for Rapid Colorimetric Molecular Detection of Poultry Respiratory Viruses at the Point of Need
Abstract
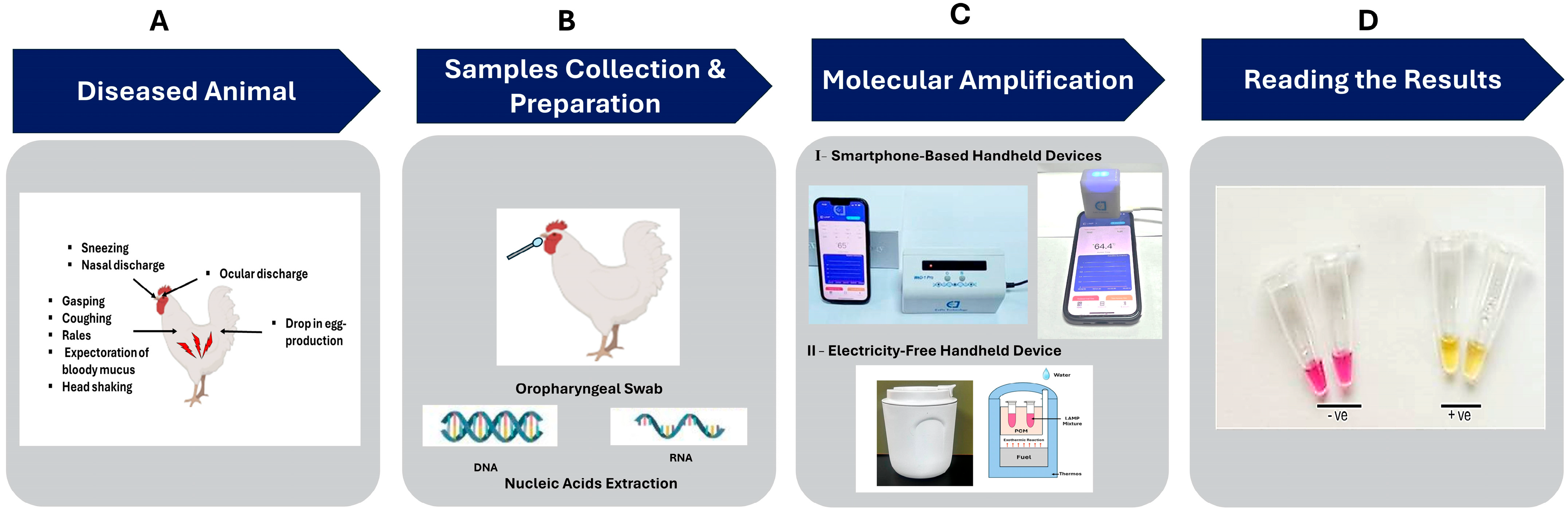
1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Synthetic cDNA Fragments of IV-Matrix Protein Gene
2.3. Spiked Clinical Samples
2.4. Nucleic Acids Extraction
2.5. LAMP Primers
2.6. Smartphone Handheld Devices
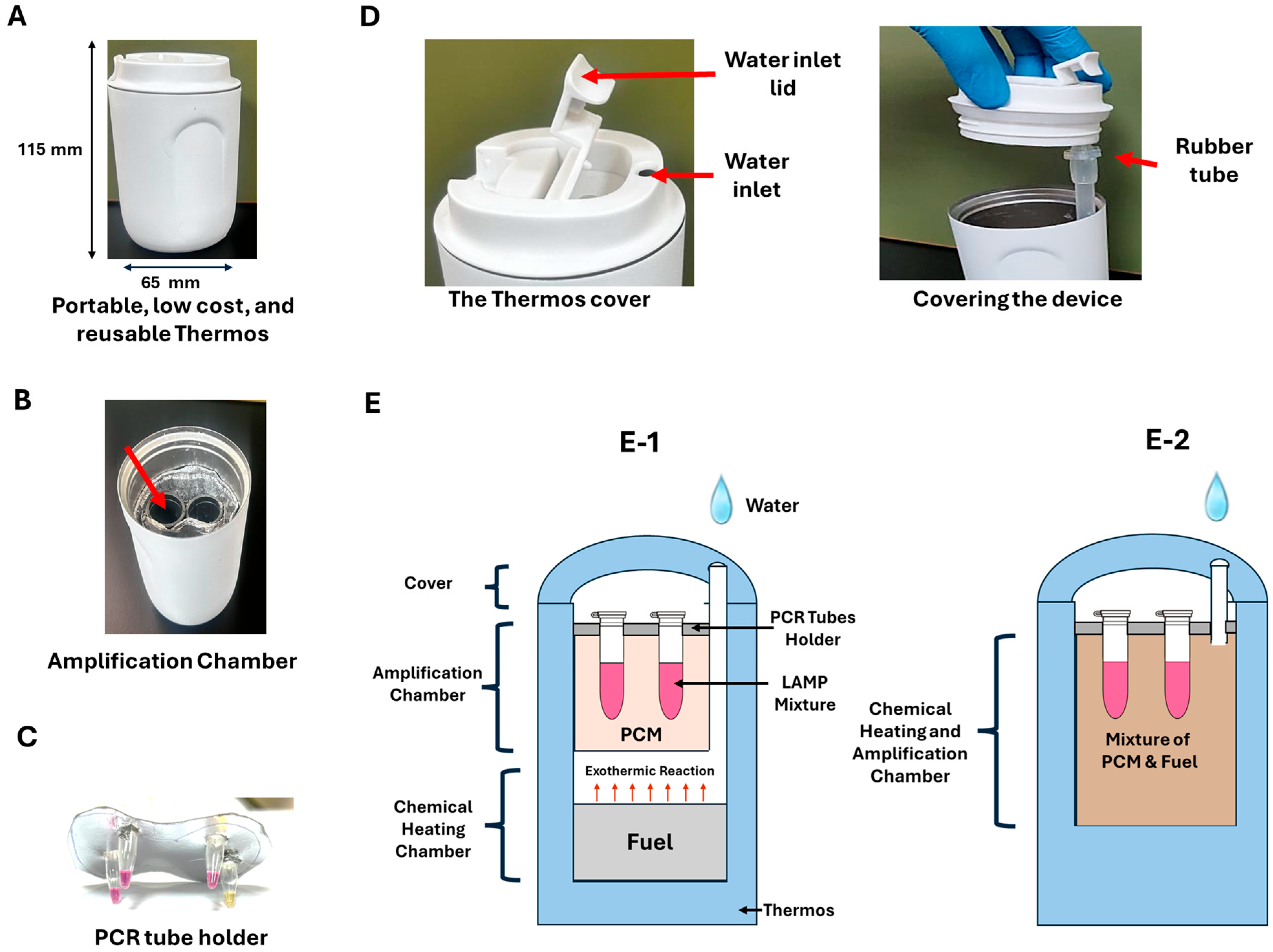
2.7. Electricity-Free Device
2.8. Fluorometric LAMP Assay
2.9. Colorimetric LAMP Assay
2.10. Analytical Sensitivity
2.11. Detection of Nucleic Acids from Spiked Clinical Samples
3. Results
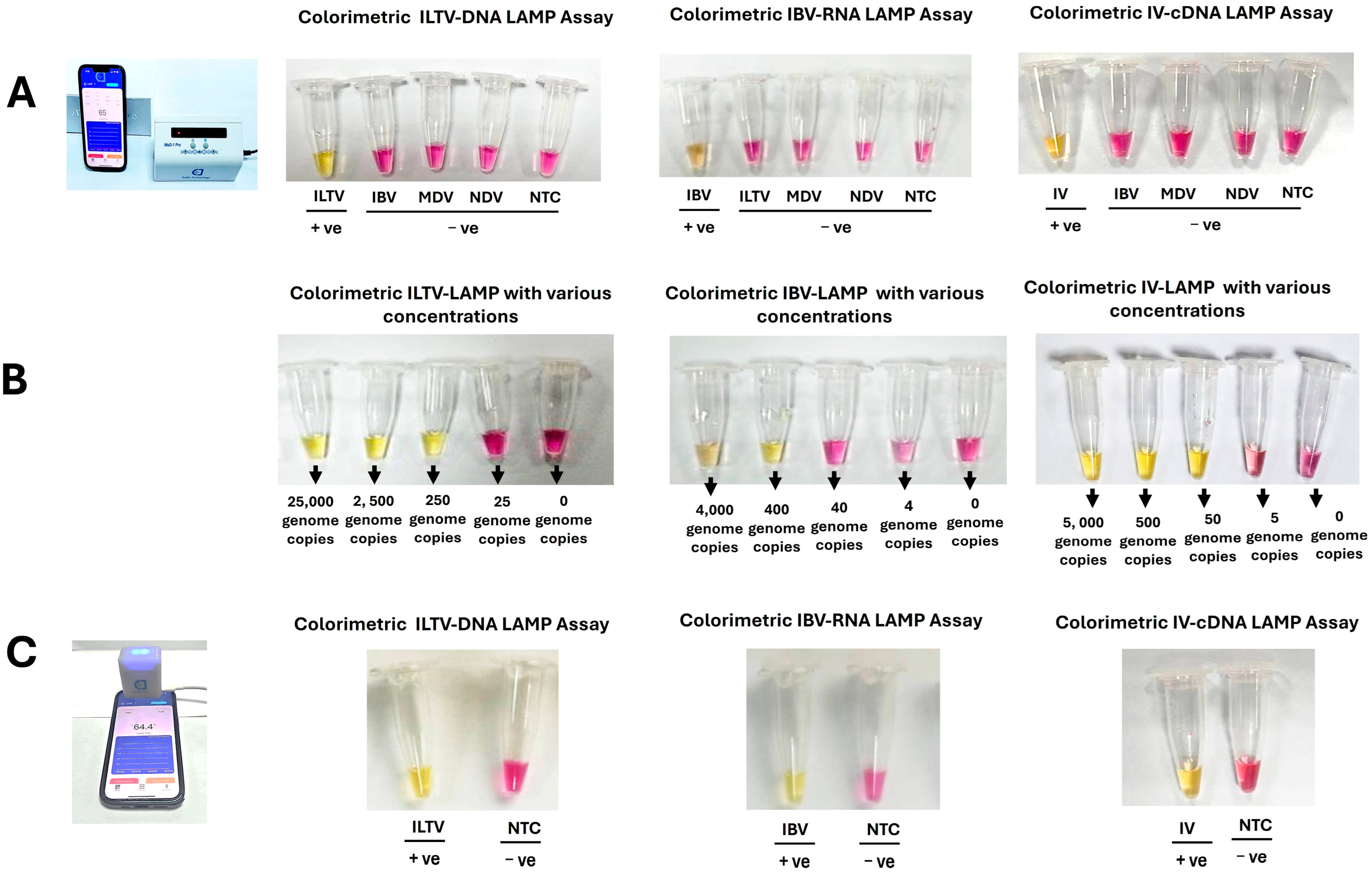
3.1. Benchtop LAMP Assays
3.2. Smartphone-Linked, Handheld Devices
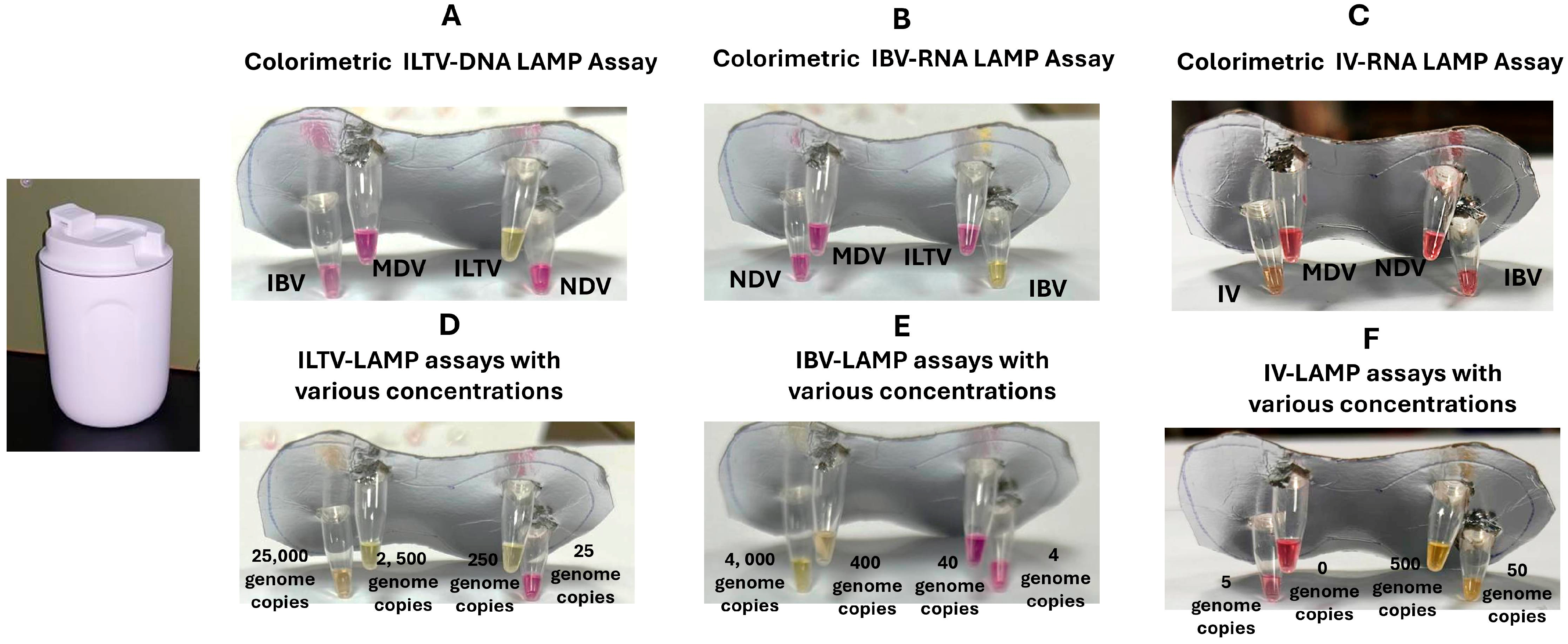
3.3. Electricity-Free Device
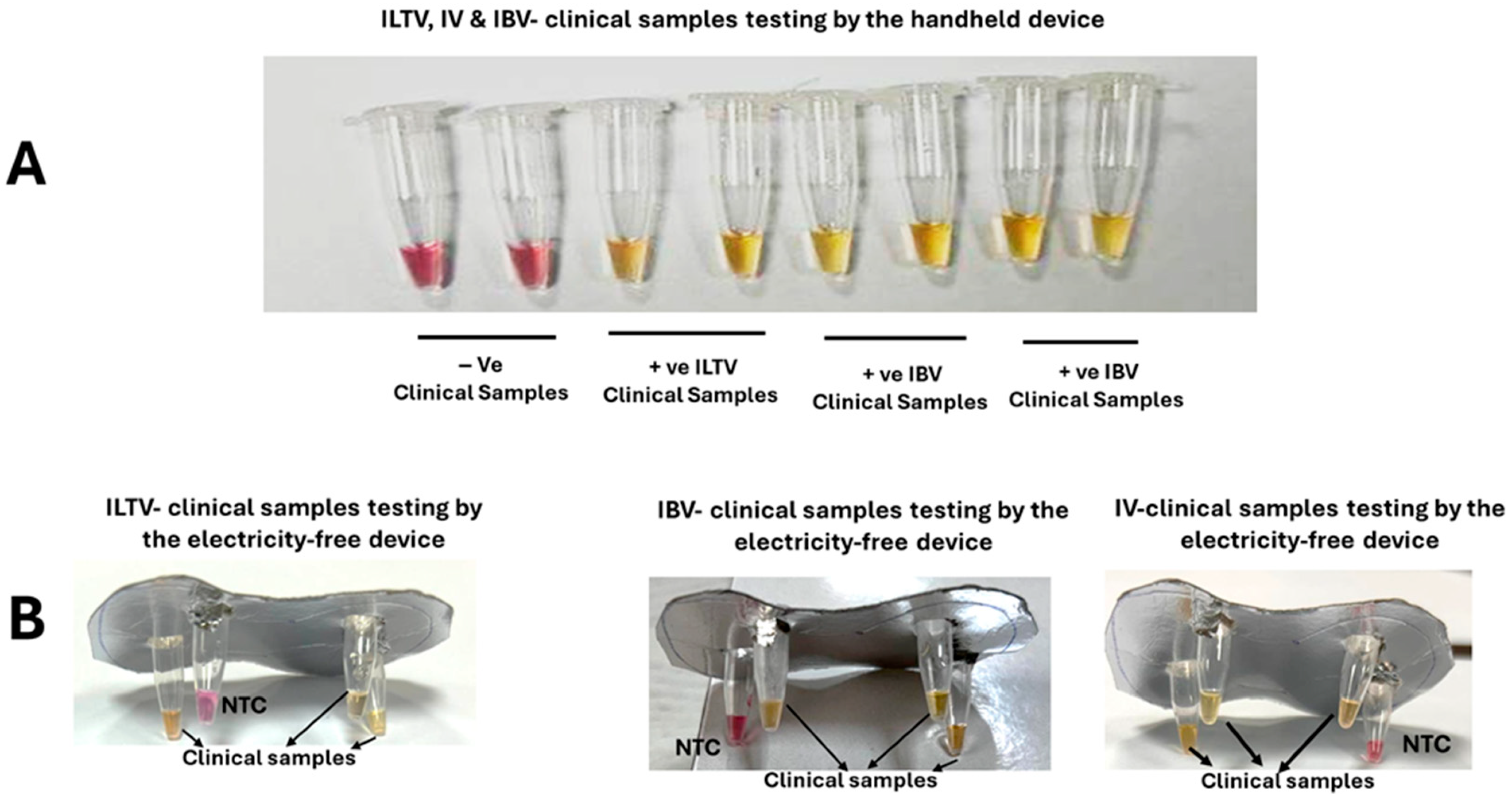
3.4. Clinical Performance of ILTV, IBV, and IV LAMP Assays
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Chronic Respiratory Diseases Collaborators. Global burden of chronic respiratory diseases and risk factors, 1990–2019: An update from the Global Burden of Disease Study 2019. eClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Muyanja, S.Z.; Siddharthan, T. Health Equity and Respiratory Diseases in Low- and Middle-Income Countries. Clin. Chest Med. 2023, 44, 623–634. [Google Scholar] [CrossRef]
- Williams, B.G.; Gouws, E.; Boschi-Pinto, C.; Bryce, J.; Dye, C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect. Dis. 2002, 2, 25–32. [Google Scholar] [CrossRef]
- World Health Organization. Pneumonia. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/pneumonia (accessed on 13 March 2025).
- 14.9 Million Excess Deaths Associated with the COVID-19 Pandemic in 2020 and 2021; World Health Organisation: Geneva, Switzerland, 2022; Available online: https://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-associated-with-the-covid-19-pandemic-in-2020-and-2021 (accessed on 1 February 2025).
- Centers for Disease Control and Prevention (CDC). Nearing Record Number of Avian Influenza Outbreaks in U.S. Poultry. 2022. Available online: https://www.cdc.gov/bird-flu/spotlights/nearing-record-number-avian-influenza.html (accessed on 15 March 2025).
- Ferragamo, M. What Is Avian Flu? Council on Foreign Relations. Available online: https://www.cfr.org/backgrounder/what-avian-flu#:~:text=The%20ongoing%20avian%20flu%20outbreak,of%20the%20disease%20in%202024 (accessed on 1 February 2025).
- Naraharisetti, R.; Weinberg, M.; Stoddard, B.; Stobierski, M.G.; Dodd, K.A.; Wineland, N.; Beal, M.; Morse, J.; Hatter, S.; Sledge, D.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection of Indoor Domestic Cats Within Dairy Industry Worker Households—Michigan, May 2024. MMWR Morb. Mortal. Wkly. Rep. 2025, 74, 61–65. [Google Scholar] [CrossRef]
- Coleman, K.K.; Bemis, I.G. Avian Influenza Virus Infections in Felines: A Systematic Review of Two Decades of Literature. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2024. [Google Scholar]
- Mostafa, A.; Naguib, M.M.; Nogales, A.; Barre, R.S.; Stewart, J.P.; García-Sastre, A.; Martinez-Sobrido, L. Avian influenza A (H5N1) virus in dairy cattle: Origin, evolution, and cross-species transmission. mBio 2024, 15, e0254224. [Google Scholar] [CrossRef]
- Van Kerkhove, M.D.; Mumford, E.; Mounts, A.W.; Bresee, J.; Ly, S.; Bridges, C.B.; Otte, J. Highly pathogenic avian influenza (H5N1): Pathways of exposure at the animal-human interface, a systematic review. PLoS ONE 2011, 6, e14582. [Google Scholar] [CrossRef]
- Davison, A.J.; Eberle, R.; Hayward, G.S.; Mcgeoch, D.J.; Minson, A.C.; Pellet, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The order herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef]
- García, M.; Spatz, S.J.; Guy, J.S. Infectious laryngotracheitis. In Diseases of Poultry, 13th ed.; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V., Eds.; Blackwell Publishing: Ames, IA, USA, 2013; pp. 161–179. [Google Scholar]
- Menendez, K.R.; García, M.; Spatz, S.; Tablante, N.L. Molecular epidemiology of infectious laryngotracheitis: A review. Avian Pathol. 2014, 43, 108–117. [Google Scholar] [CrossRef]
- García, M.; Volkening, J.; Riblet, S.; Spatz, S. Genomic sequence analysis of the United States infectious laryngotracheitis vaccine strains chicken embryo origin (CEO) and tissue culture origin (TCO). Virology 2013, 440, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, D. Coronaviruses in poultry and other birds. Avian Pathol. 2005, 34, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Jackwood, M.W.; de Wit, S. Infectious bronchitis. In Diseases of Poultry; Swayne, D.E., Glisson, J.R., McDougald, L.R., Nolan, L.K., Suarez, D.L., Nair, V., Eds.; Wiley-Blackwell Publishing: Ames, IA, USA, 2013; pp. 139–160. [Google Scholar]
- El-Tholoth, M.; Bau, H.H. Molecular Detection of Respiratory Tract Viruses in Chickens at the Point of Need by Loop-Mediated Isothermal Amplification (LAMP). Viruses 2024, 16, 1248. [Google Scholar] [CrossRef]
- Ide, P.R. Sensitivity and specificity of the fluorescent antibody technique for detection of infectious laryngotracheitis virus. Can. J. Comp. Med. 1978, 42, 54–62. [Google Scholar]
- Kotiw, M.; Wilks, C.R.; May, J.T. Differentiation of infectious laryngotracheitis virus strains using restriction endonucleases. Avian Dis. 1982, 26, 718–731. [Google Scholar] [CrossRef]
- Russell, R.G.; Turner, A.J. Characterization of infectious laryngotracheitis viruses, antigenic comparison by kinetics of neutralization and immunization studies. Can. J. Comp. Med. 1983, 47, 163–171. [Google Scholar]
- York, J.J.; Young, J.G.; Fahey, K.J. The appearance of viral antigen and antibody in the trachea of naïve and vaccinated chickens infected with infectious laryngotracheitis virus. Avian Pathol. 1989, 18, 643–658. [Google Scholar] [CrossRef]
- Shirley, M.W.; Kemp, D.J.; Sheppard, M.; Fahey, K.J. Detection of DNA from infectious laryngotracheitis virus by colourimetric analyses of polymerase chain reactions. J. Virol. Methods 1990, 30, 251–259. [Google Scholar] [CrossRef]
- Vogtlin, A.; Bruckner, L.; Ottiger, H.P. Use of polymerase chain reaction (PCR) for the detection of vaccine contamination by infectious laryngotracheitis virus. Vaccine 1999, 17, 2501–2506. [Google Scholar] [CrossRef]
- Humberd, J.; Garcia, M.; Riblet, S.M.; Resurreccion, R.S.; Brown, T.P. Detection of infectious laryngotracheitis virus in formalin-fixed, paraffin-embedded tissues by nested polymerase chain reaction. Avian Dis. 2002, 46, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, A.; Kirkpatrick, N.C.; Coppo, M.; Lee, S.W.; Devlin, J.M.; Markham, P.F.; Browning, G.F.; Noormohammadi, A.H. Development of a SYBR Green quantitative polymerase chain reaction assay for rapid detection and quantification of infectious laryngotracheitis virus. Avian Pathol. 2011, 40, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kong, C.; Cui, X.; Cui, H.; Shi, X.; Zhang, X.; Hu, S.; Hao, L.; Wang, Y. Detection of Infectious Laryngotracheitis Virus by RealTime PCR in Naturally and Experimentally Infected Chickens. PLoS ONE 2013, 8, e67598. [Google Scholar]
- Laamiri, N.; Aouini, R.; Marnissi, B.; Ghram, A.; Hmila, I. A multiplex real-time RT PCR for simultaneous detection of four most common avian respiratory viruses. Virology 2018, 515, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.K.; Brown, A.J.; Bracewell, C.D. Comparison of the haemagglutination inhibition test and the serum neutralisation test in tracheal organ cultures for typing infectious bronchitis virus strains. Avian Pathol. 1987, 16, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Callison, S.A.; Hilt, D.A.; Boynton, T.O.; Sample, B.F.; Robison, R.; Swayne, D.E.; Jackwood, M.W. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J. Virol. Methods 2006, 138, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Meir, R.; Maharat, O.; Farnushi, Y.; Simanov, L. Development of a real-time TaqMan RT-PCR assay for the detection of infectious bronchitis virus in chickens, and comparison of RT-PCR and virus isolation. J. Virol. Methods 2010, 163, 190–194. [Google Scholar] [CrossRef]
- Fellahi, S.; El Harrak, M.; Kuhn, J.H.; Sebbar, G.; Bouaiti El, A.; Khataby, K.; Fihri, O.F.; El Houadfi, M.; Ennaji, M.M. Comparison of SYBR green I real-time RT-PCR with conventional agarose gel-based RT-PCR for the diagnosis of infectious bronchitis virus infection in chickens in Morocco. BMC Res. Notes 2016, 9, 231. [Google Scholar] [CrossRef]
- Parvin, R.; Kabiraj, C.K.; Hossain, I.; Hassan, A.; Begum, J.A.; Nooruzzaman, M.; Islam, M.T.; Chowdhury, E.H. Investigation of respiratory disease outbreaks of poultry in Bangladesh using two real-time PCR-based simultaneous detection assays. Front. Vet. Sci. 2022, 9, 1036757. [Google Scholar] [CrossRef]
- Payungporn, S.; Phakdeewirot, P.; Chutinimitkul, S.; Theamboonlers, A.; Keawcharoen, J.; Oraveerakul, K.; Amonsin, A.; Poovorawan, Y. Single-step multiplex reverse transcription-polymerase chain reaction (RT-PCR) for influenza A virus subtype H5N1 detection. Viral. Immunol. 2004, 17, 588–593. [Google Scholar] [CrossRef]
- Pham, H.M.; Nakajima, C.; Ohashi, K.; Onuma, M. Loop-mediated isothermal amplification for rapid detection of Newcastle disease virus. J. Clin. Microbiol. 2005, 43, 1646–1650. [Google Scholar] [CrossRef]
- Chen, H.T.; Zhang, J.; Sun, D.H.; Ma, L.N.; Liu, X.T.; Cai, X.P.; Liu, Y.S. Development of reverse transcription loop-mediated isothermal amplification for rapid detection of H9 avian influenza virus. J. Virol. Methods 2008, 151, 200–203. [Google Scholar] [CrossRef]
- Li, Q.; Xue, C.; Qin, J.; Zhou, Q.; Chen, F.; Bi, Y.; Cao, Y. An improved reverse transcription loop-mediated isothermal amplification assay for sensitive and specific detection of Newcastle disease virus. Arch. Virol. 2009, 154, 1433–1440. [Google Scholar] [CrossRef]
- Chen, H.T.; Zhang, J.; Ma, Y.P.; Ma, L.N.; Ding, Y.Z.; Liu, X.T.; Cai, X.P.; Ma, L.Q.; Zhang, Y.G.; Liu, Y.S. Reverse transcription loop-mediated isothermal amplification for the rapid detection of infectious bronchitis virus in infected chicken tissues. Mol. Cell Probes. 2010, 24, 104–106. [Google Scholar] [CrossRef]
- El-Tholoth, M.; Mauk, M.G.; Anis, E.; Bau, H.H. A closed-tube, single-step, real time, reverse transcription-loop-mediated isothermal amplification assay for infectious bronchitis virus detection in chickens. J. Virol. Methods 2020, 284, 113940. [Google Scholar] [CrossRef]
- El-Tholoth, M.; Bai, H.; Mauk, M.G.; Anis, E.; Bau, H.H. Molecular Detection of Infectious Laryngotracheitis Virus in Chickens with a Microfluidic Chip. Animals 2021, 11, 3203. [Google Scholar] [CrossRef]
- El-Tholoth, M.; Anis, E.; Bau, H.H. Two stage, nested isothermal amplification in a single tube. Analyst 2021, 146, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Xun, G.; Lane, S.T.; Petrov, V.A.; Pepa, B.E.; Zhao, H. A rapid, accurate, scalable, and portable testing system for COVID-19 diagnosis. Nat. Commun. 2021, 12, 2905. [Google Scholar] [CrossRef]
- Sreejith, K.R.; Umer, M.; Dirr, L.; Bailly, B.; Guillon, P.; von Itzstein, M.; Soda, N.; Kasetsirikul, S.; Shiddiky, M.J.A.; Nguyen, N.T. A Portable Device for LAMP Based Detection of SARS-CoV-2. Micromachines 2021, 12, 1151. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, H.; Guo, X.; Liu, D.; Li, Z.; Sun, J.; Huang, J.; Liu, T.; Zhao, P.; Xu, H.; et al. Argonaute-integrated isothermal amplification for rapid, portable, multiplex detection of SARS-CoV-2 and influenza viruses. Biosens. Bioelectron. 2022, 207, 114169. [Google Scholar] [CrossRef]
- Meyers, E.; Park, J.; Coen, A.; Raman, L.; Heytens, S.; Rhee, J.; Padalko, E.; Cools, P. Evaluation of a smartphone-operated point-of-care device using loop-mediated isothermal amplification technology for rapid and remote detection of SARS-CoV-2. J. Med. Virol. 2023, 95, e29158. [Google Scholar] [CrossRef] [PubMed]
- Coopersmith, M.; Dijkman, R.; Bartlett, M.L.; Currie, R.; Schuurman, S.; Wit, S. Development and Laboratory Validation of Rapid, Bird-Side Molecular Diagnostic Assays for Avian Influenza Virus Including Panzootic H5Nx. Microorganisms 2025, 13, 1090. [Google Scholar] [CrossRef] [PubMed]
- Mauk, M.G.; Ansah, F.; El-Tholoth, M. Chemical Heating for Minimally Instrumented Point-of-Care (POC) Molecular Diagnostics. Biosensors 2024, 14, 554. [Google Scholar] [CrossRef]
- Li, R.J.; Mauk, M.G.; Seok, Y.; Bau, H.H. Electricity-free chemical heater for isothermal nucleic acid amplification with applications in COVID-19 home testing. Analyst 2021, 146, 4212–4218. [Google Scholar] [CrossRef]
- Xiao, M.; Tian, F.; Liu, X.; Zhou, Q.; Pan, J.; Luo, Z.; Yang, M.; Yi, C. Virus Detection: From State-of-the-Art Laboratories to Smartphone-Based Point-of-Care Testing. Adv. Sci. 2022, 9, e2105904. [Google Scholar] [CrossRef]
- Pang, F.; Zhang, T.; Dai, F.; Wang, K.; Jiao, T.; Zhang, Z.; Zhang, L.; Liu, M.; Hu, P.; Song, J. A handheld isothermal fluorescence detector for duplex visualization of aquatic pathogens via enhanced one-pot LAMP-PfAgo assay. Biosens. Bioelectron. 2024, 254, 116187. [Google Scholar] [CrossRef]
- Dai, F.; Zhang, T.; Pang, F.; Jiao, T.; Wang, K.; Zhang, Z.; Wang, N.; Xie, Z.; Zhang, Y.; Wang, Z.; et al. A compact, palm-sized isothermal fluorescent diagnostic intelligent IoT device for personal health monitoring and beyond via one-tube/one-step LAMP-CRISPR assay. Biosens. Bioelectron. 2025, 270, 116945. [Google Scholar] [CrossRef]
- Liao, S.C.; Peng, J.; Mauk, M.G.; Awasthi, S.; Song, J.; Friedman, H.; Bau, H.H.; Liu, C. Smart Cup: A Minimally-Instrumented, Smartphone-Based Point-of-Care Molecular Diagnostic Device. Sens. Actuators B Chem. 2016, 229, 232–238. [Google Scholar] [CrossRef]
- Yin, Q. Manually-Operated, Slider Cassette for Multiplexed Molecular Detection at the Point of Care. Ph.D. Dissertation, University of Pennsylvania, Philadelphia, PA, USA, 2023. [Google Scholar]
- Korver, D.R. Review: Current challenges in poultry nutrition, health, and welfare. Animal 2023, 17 (Suppl. S2), 100755. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Pandian, V.; Mauk, M.G.; Bau, H.H.; Cherry, S.; Tisi, L.C.; Liu, C. Smartphone-Based Mobile Detection Platform for Molecular Diagnostics and Spatiotemporal Disease Mapping. Anal. Chem. 2018, 90, 4823–4831. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.R.; Trus, I.; Desmarets, L.M.; Li, Y.; Theuns, S.; Nauwynck, H.J. Productive replication of nephropathogenic infectious bronchitis virus in peripheral blood monocytic cells, a strategy for viral dissemination and kidney infection in chickens. Vet. Res. 2016, 47, 70. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huo, C.; Zhao, J.; Liu, T.; Li, X.; Yan, S.; Wang, Z.; Hu, Y.; Zhang, G. Pathogenicity differences between QX-like and Mass-type infectious bronchitis viruses. Vet. Microbiol. 2018, 213, 129–135. [Google Scholar] [CrossRef]
- Zhou, J.J.; Fang, D.Y.; Fu, J.; Tian, J.; Zhou, J.M.; Yan, H.J.; Liang, Y.; Jiang, L.F. Infection and replication of avian influenza H5N1 virus in an infected human. Virus Genes 2009, 39, 76–80. [Google Scholar] [CrossRef]
- Moulin, H.R.; Liniger, M.; Python, S.; Guzylack-Piriou, L.; Ocaña-Macchi, M.; Ruggli, N.; Summerfield, A. High interferon type I responses in the lung, plasma and spleen during highly pathogenic H5N1 infection of chicken. Vet. Res. 2011, 42, 6. [Google Scholar] [CrossRef] [PubMed]
- Seok, Y.; Yin, Q.; Li, R.; Mauk, M.G.; Bai, H.; Bau, H.H. Manually Operated, Slider Cassette for Multiplexed Molecular Detection at the Point of Care. Sens. Actuators B Chem. 2022, 369, 132353. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; El-Tholoth, M.; Li, Y.; Graham-Wooten, J.; Liang, Y.; Li, J.; Li, W.; Weiss, S.R.; Collman, R.G.; Bau, H.H. Single- and Two-Stage, Closed-Tube, Point-of-Care, Molecular Detection of SARS-CoV-2. Anal. Chem. 2021, 93, 13063–13071. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Tholoth, M.; Seboussi, R.; Hussein, M.; Rahmdel, S.; Alalawi, A.; Bau, H.H. Smartphone-Linked and Electricity-Free Platforms for Rapid Colorimetric Molecular Detection of Poultry Respiratory Viruses at the Point of Need. Biosensors 2025, 15, 638. https://doi.org/10.3390/bios15100638
El-Tholoth M, Seboussi R, Hussein M, Rahmdel S, Alalawi A, Bau HH. Smartphone-Linked and Electricity-Free Platforms for Rapid Colorimetric Molecular Detection of Poultry Respiratory Viruses at the Point of Need. Biosensors. 2025; 15(10):638. https://doi.org/10.3390/bios15100638
Chicago/Turabian StyleEl-Tholoth, Mohamed, Rabiha Seboussi, Mahmoud Hussein, Salameh Rahmdel, Alanoud Alalawi, and Haim H. Bau. 2025. "Smartphone-Linked and Electricity-Free Platforms for Rapid Colorimetric Molecular Detection of Poultry Respiratory Viruses at the Point of Need" Biosensors 15, no. 10: 638. https://doi.org/10.3390/bios15100638
APA StyleEl-Tholoth, M., Seboussi, R., Hussein, M., Rahmdel, S., Alalawi, A., & Bau, H. H. (2025). Smartphone-Linked and Electricity-Free Platforms for Rapid Colorimetric Molecular Detection of Poultry Respiratory Viruses at the Point of Need. Biosensors, 15(10), 638. https://doi.org/10.3390/bios15100638




