Electrochemical Glucose Sensor Based on Dual Redox Mediators
Abstract
1. Introduction
2. Experiment Section
2.1. Materials and Reagents
2.2. Apparatus
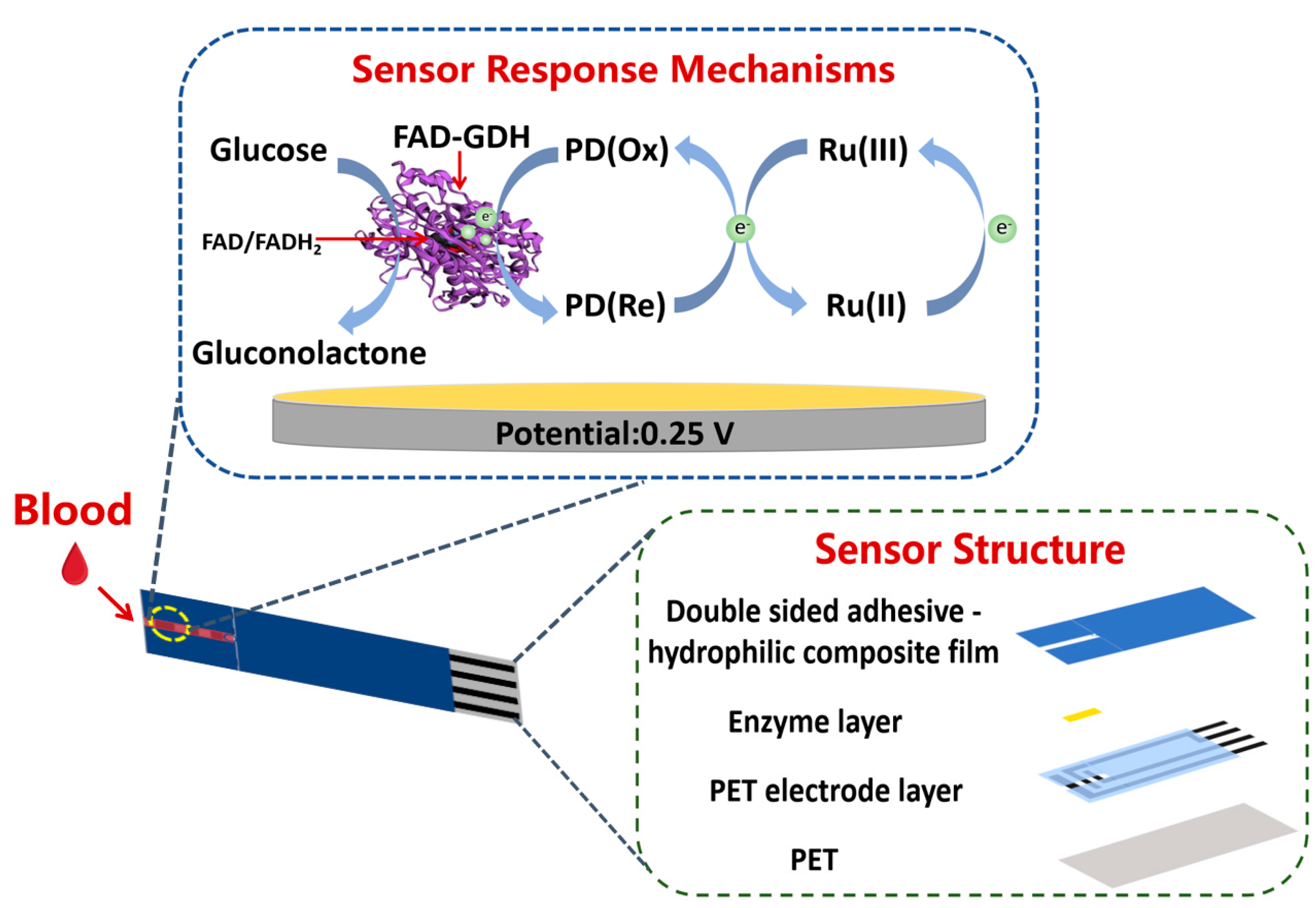
2.3. Sensor Construction
2.4. Evaluation of Electrochemical Performance of the Sensor
2.5. Blood Measurement
3. Results and Discussion
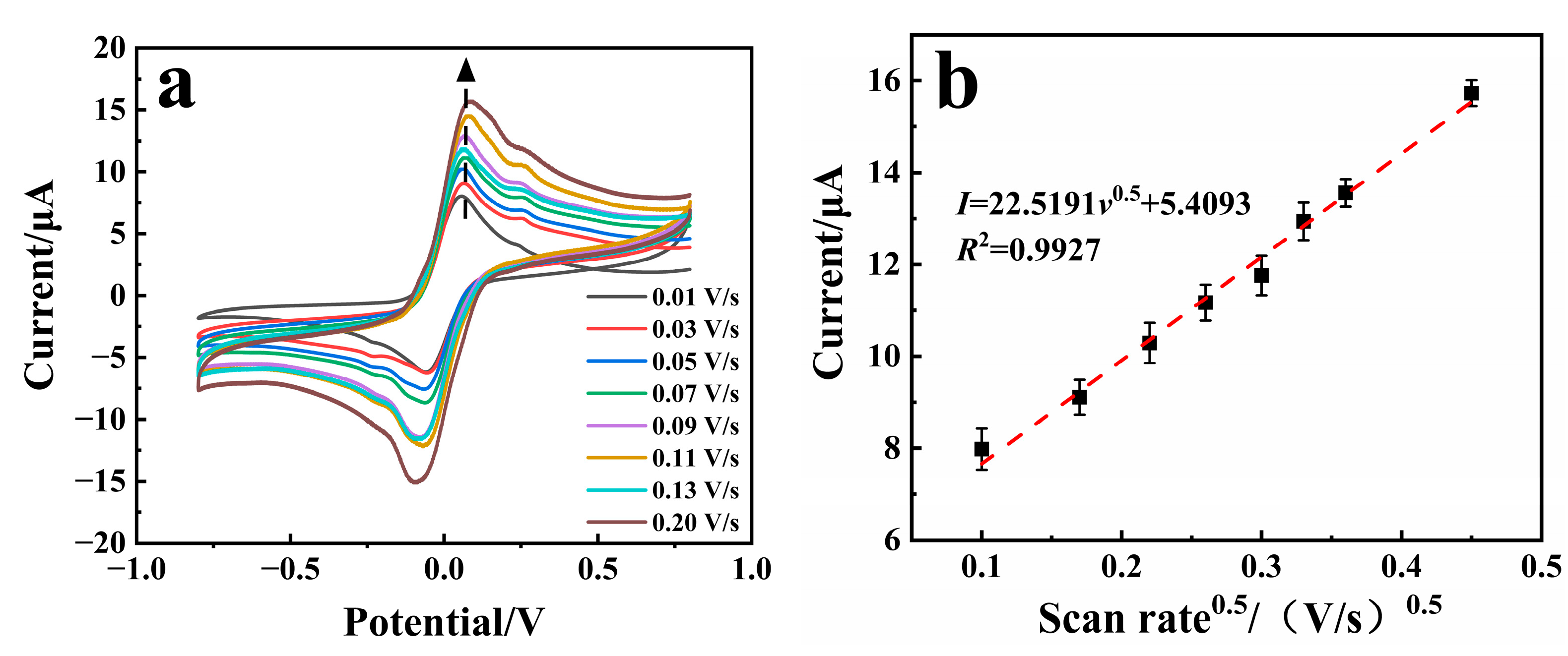
3.1. Electrochemical Study of the Sensor
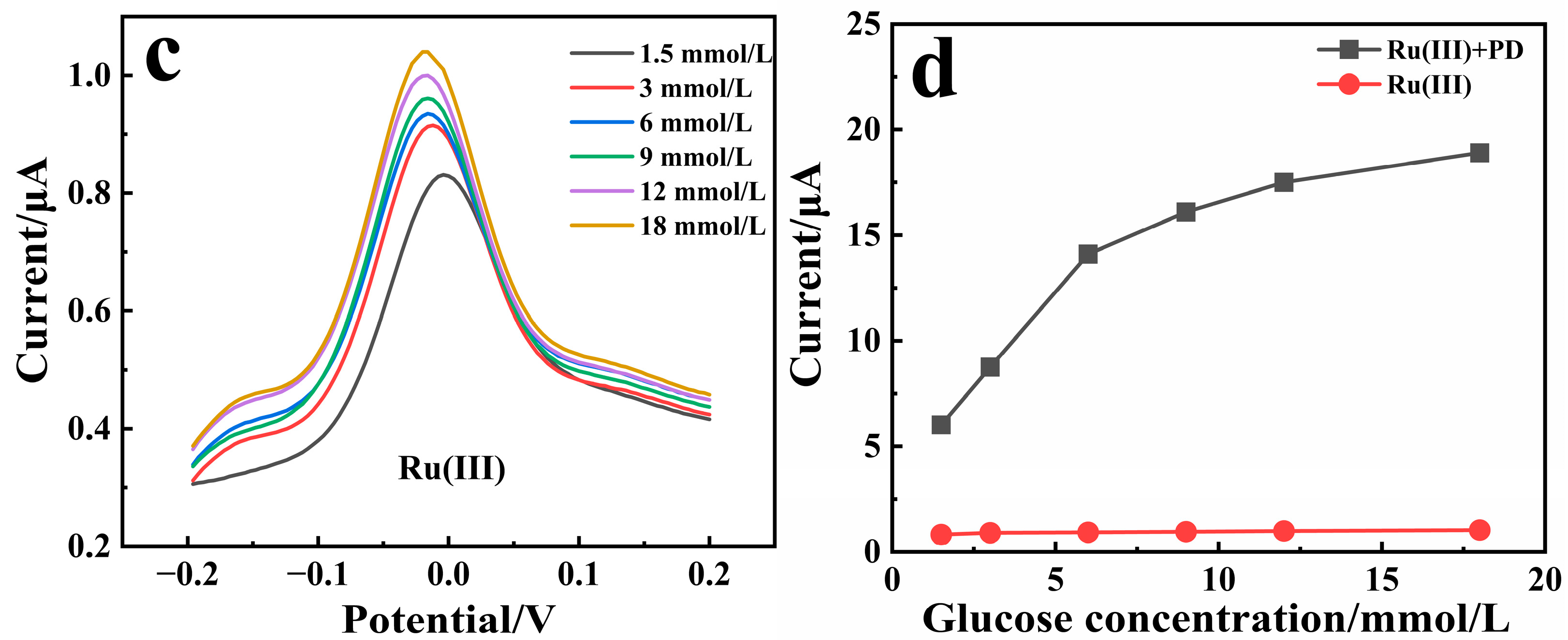
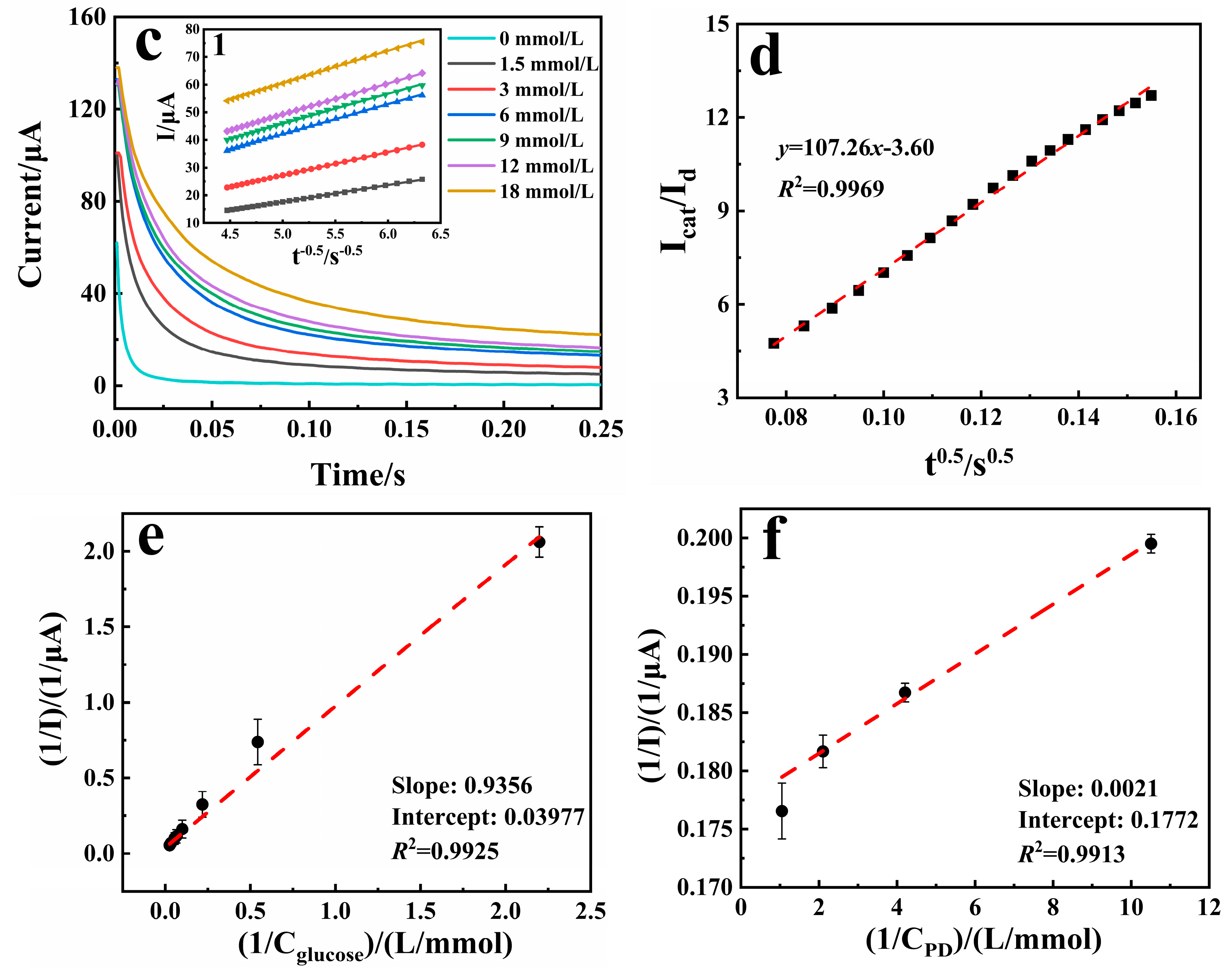
3.2. Catalytic Activity Study of the Sensors
3.3. Optimization of Experimental Conditions
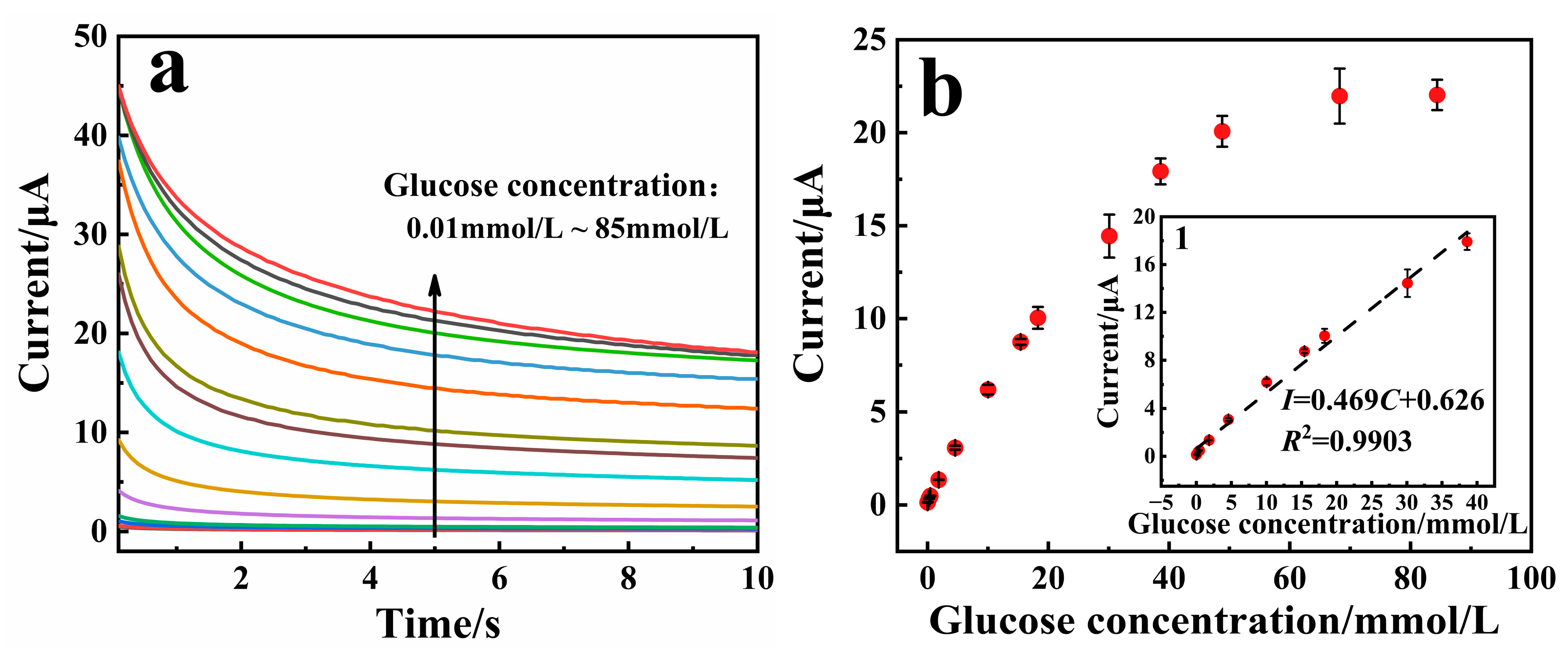
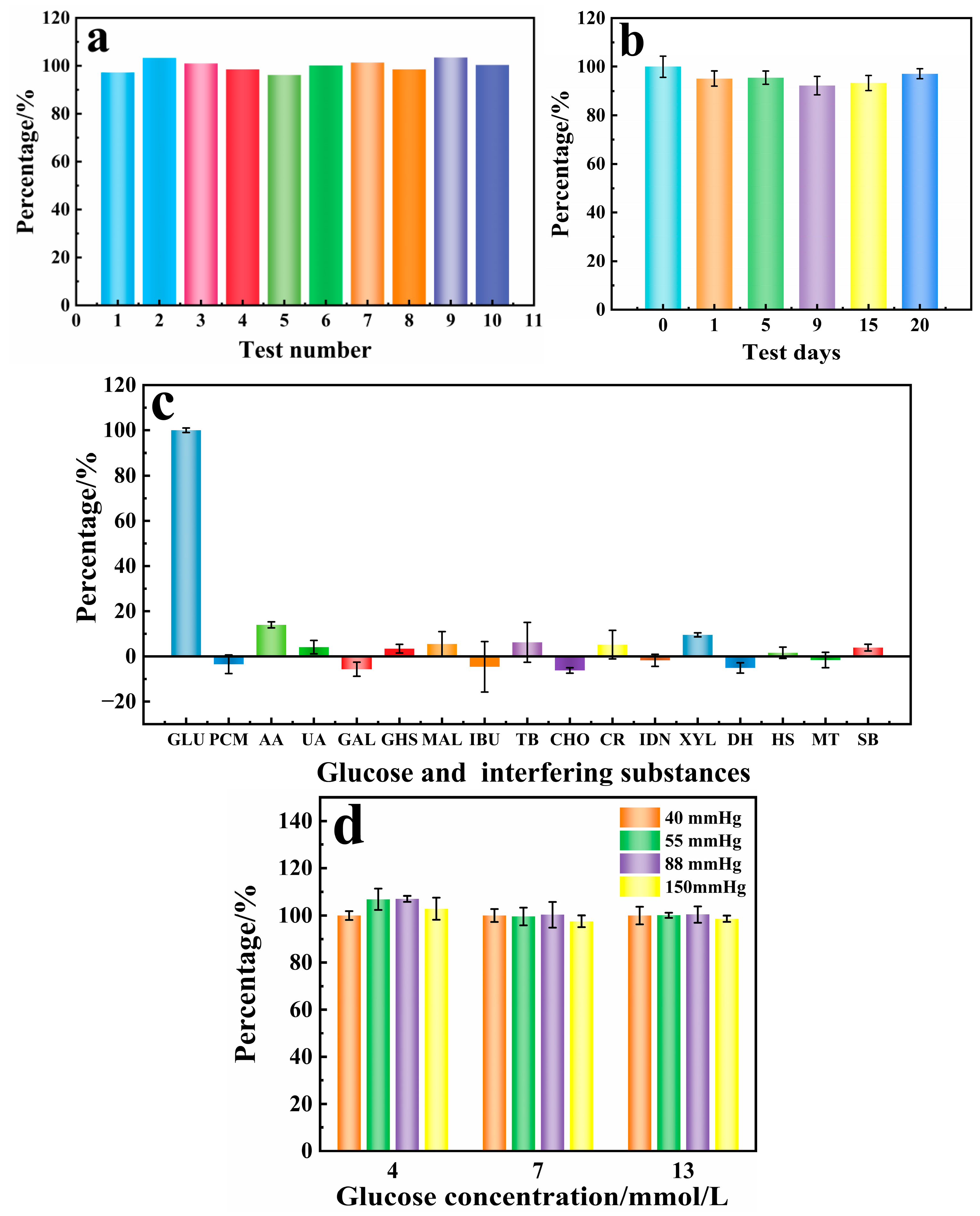
3.4. Analytical Performance of the Sensor
3.5. Spiking Recovery Experiments in Real Samples (Venous Blood)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukhtar, Y.; Galalain, A.; Yunusa, U. A Modern Overview on Diabetes Mellitus: A Chronic Endocrine Disorder. Eur. J. Biol. 2020, 5, 1–14. [Google Scholar] [CrossRef]
- Taxirovich, D.A.; Ulugbekovna, R.N.; Abduxalimova, Y.J. Status and Prospects for the Fight Against Diabetes Mellitus. Educ. Res. Univers. Sci. 2024, 3, 4–9. [Google Scholar]
- Das, S.K.; Nayak, K.K.; Krishnaswamy, P.R.; Kumar, V.; Bhat, N. Electrochemistry and Other Emerging Technologies for Continuous Glucose Monitoring Devices. ECS Sens. Plus 2022, 1, 031601. [Google Scholar]
- Amor-Gutiérrez, O.; Costa-Rama, E.; Fernández-Abedul, M.T. Based Enzymatic Electrochemical Sensors for Glucose Determination. Sensors 2022, 22, 6232. [Google Scholar] [CrossRef]
- Setford, S.; Phillips, S.; Cameron, H.; Grady, M. Clinical Accuracy of a Glucose Oxidase-Based Blood Glucose Test-Strip Across Extremes of Oxygen Partial Pressure. J. Diabetes Sci. Technol. 2024, 18, 1445–1451. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A. Glucose Biosensors in Clinical Practice: Principles, Limits and Perspectives of Currently Used Devices. Theranostics 2022, 12, 493–511. [Google Scholar] [CrossRef]
- Morshed, J.; Nakagawa, R.; Hossain, M.M.; Nishina, Y.; Tsujimura, S. Disposable Electrochemical Glucose Sensor Based on Water-Soluble Quinone-Based Mediators with Flavin Adenine Dinucleotide-Dependent Glucose Dehydrogenase. Biosens. Bioelectron. 2021, 189, 113357. [Google Scholar] [CrossRef]
- Imran, H.; Alam, A.; Dharuman, V.; Lim, S. Fabrication of Enzyme-Free and Rapid Electrochemical Detection of Glucose Sensor Based on Zno Rod and Ru Doped Carbon Nitride Modified Gold Transducer. Nanomaterials 2022, 12, 1778. [Google Scholar] [CrossRef]
- Cui, G.; Yoo, J.H.; Woo, B.W.; Kim, S.S.; Cha, G.S.; Nam, H. Disposable Amperometric Glucose Sensor Electrode with Enzyme-Immobilized Nitrocellulose Strip. Talanta 2001, 54, 1105–1111. [Google Scholar] [CrossRef]
- Deng, H.; Teo, A.K.L.; Gao, Z. An Interference-Free Glucose Biosensor Based on a Novel Low Potential Redox Polymer Mediatoro. Sens. Actuators B. Chem. 2014, 191, 522–528. [Google Scholar] [CrossRef]
- Nakabayashi, Y.; Hirosaki, Y.; Yamauchi, O. Dipolar Ruthenium (II) Ammine Complexes as Electron Transfer Mediators of Amperometric Glucose Sensors. Bioelectrochemistry 2006, 69, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Loew, N.; Tsugawa, W.; Nagae, D.; Kojima, K.; Sode, K. Mediator Preference of Two Different FAD-Dependent Glucose Dehydrogenases Employed in Disposable Enzyme Glucose Sensors. Sensors 2017, 17, 2636. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Tsujimura, S. Glucose Sensor Strip Using Flavin Adenine Dinucleotide-Dependent Glucose Dehydrogenase with Quinones as Redox Mediators. Sens. Mater. 2022, 34, 3141–3146. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Simanaityte, R.; Ramanaviciene, A.; Glumbokaite, L.; Ramanavicius, A. Reagent-Less Amperometric Glucose Biosensor Based on a Graphite Rod Electrode Layer-by-Layer Modified with 1,10-Phenanthroline-5,6-Dione and Glucose Oxidase. Talanta 2017, 171, 204–212. [Google Scholar] [CrossRef]
- Zor, E.; Oztekin, Y.; Mikoliunaite, L.; Voronovic, J.; Ramanaviciene, A.; Anusevicius, Z.; Bingol, H.; Ramanavicius, A. 1,10-Phenanthroline-5,6-Dione and 9,10-Phenanthrenequinone as Redox Mediators for Amperometric Glucose Biosensors. J. Solid State Electrochem. 2014, 18, 1529–1536. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Liu, Y.; Jin, Z.; Zhao, Q.; Yang, F.; Xiao, D. Sensitive and Selective Determination of GSH Based on the ECL Quenching of Ru (II) 1,10-Phenanthroline-5,6-Dione Complex. Biosens. Bioelectron. 2016, 77, 182–187. [Google Scholar] [CrossRef]
- Fujihara, T.; Okamura, R.; Wada, T.; Tanaka, K. Coordination Ability of 1,10-Phenanthroline-5,6-Dione: Syntheses and Redox Behavior of a Ru (II) Complex with an O-quinoid Moiety and of Bridged Ru (II)–M (II) Complexes (M = Pd, Pt). Dalton Trans. 2003, 16, 3221–3226. [Google Scholar] [CrossRef]
- Karimi, M.A.; Hatefi-Mehrjardi, A.; Mazloum, A.M.; Ardakani, B.R.; Mashhadizadeh, H.M.; Sargazi, S. Electrocatalytic Determination of Chlorpromazine Drug Using Alizarin Red S as a Mediator on the Glassy Carbon Electrode. Russ. J. Electrochem. 2011, 47, 34–41. [Google Scholar] [CrossRef]
- Durand, F.; Limoges, B.; Mano, N.; Mavré, F.; Miranda-Castro, R.; Savéant, J.M. Effect of Substrate Inhibition and Cooperativity on the Electrochemical Responses of Glucose Dehydrogenase. Kinetic Characterization of Wild and Mutant Types. J. Am. Chem. Soc. 2011, 133, 12801–12809. [Google Scholar] [CrossRef]
- Radomska, M.; Rutkowska, I.A.; Kowalewska, B.; Cox, J.A.; Kulesza, P.J. Development and Kinetic Characterization of Hierarchical Bioelectrocatalytic System Utilizing a Redox Mediator, Functionalized Carbon Nanotubes and an Enzyme for Glucose Oxidation. J. Electroanal. Chem. 2019, 832, 417–425. [Google Scholar] [CrossRef]
- Kowalewska, B.; Kulesza, P.J. Application of Tetrathiafulvalene-Modified Carbon Nanotubes to Preparation of Integrated Mediating System for Bioelectrocatalytic Oxidation of Glucose. Electroanalysis 2009, 21, 351–359. [Google Scholar] [CrossRef]
- Liaudet, E.; Battaglini, F.; Calvo, E.J. Electrochemical Study of Sulphonated Ferrocenes as Redox Mediators in Enzyme Electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1990, 293, 55–68. [Google Scholar] [CrossRef]
- Tsujimura, S.; Kojima, S.; Kano, K.; Ikeda, T.; Sato, M.; Sanada, H.; Omura, H. Novel FAD-Dependent Glucose Dehydrogenase for a Dioxygen-Insensitive Glucose Biosensor. Biosci. Biotechnol. Biochem. 2006, 70, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.N.; Beden, N.; Leech, D.; Sygmund, C.; Ludwig, R.; Gorton, L. Characterization of Different FAD-Dependent Glucose Dehydrogenases for Possible Use in Glucose-Based Biosensors and Biofuel Cells. Anal. Bioanal. Chem. 2012, 402, 2069–2077. [Google Scholar] [CrossRef]
- Chen, Z.; Wright, C.; Dincel, O.; Chi, T.Y.; Kameoka, J. A Low-Cost Paper Glucose Sensor with Molecularly Imprinted Polyaniline Electrode. Sensors 2020, 20, 1098. [Google Scholar] [CrossRef]
- Ehrani, F.; Reiner, L.; Bavarian, B. Rapid Prototyping of a High Sensitivity Graphene Based Glucose Sensor Strip. PLoS ONE 2015, 10, e0145036. [Google Scholar]
- Wang, Y.; Wang, X.; Lu, W.; Yuan, Q.; Zheng, Y.; Yao, B. A thin Film Polyethylene Terephthalate (PET) Electrochemical Sensor for Detection of Glucose in Sweat. Talanta 2019, 198, 86–92. [Google Scholar] [CrossRef]
- Kant, T.; Shrivas, K.; Tapadia, K.; Devi, R.; Ganesan, V.; Deb, M.K. Inkjet-Printed Paper-Based Electrochemical Sensor with Gold Nano-Ink for Detection of Glucose in Blood Serum. New J. Chem. 2021, 45, 8297–8305. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Glumbokaite, L.; Ramanaviciene, A.; Ramanavicius, A. Reagent-Less Amperometric Glucose Biosensor Based on Nanobiocomposite Consisting of Poly(1,10-Phenanthroline-5,6-Dione), Poly(Pyrrole-2-Carboxylic Acid), Gold Nanoparticles and Glucose Oxidase. Microchem. J. 2020, 154, 104665. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanaviciene, A. Reagentless Glucose Biosensor Based on Combination of Platinum Nanostructures and Polypyrrole Layer. Biosensors 2024, 14, 134. [Google Scholar] [CrossRef]
- Lin, M.J.; Wu, C.C.; Chang, K.S. Effect of Poly-l-Lysine Polycation on the Glucose Oxidase/Ferricyanide Composite-Based Second-Generation Blood Glucose Sensors. Sensors 2019, 19, 1448. [Google Scholar] [CrossRef] [PubMed]
- Hallaj, R.; Mohammadian, N.; Ghaderi, S.; Navaee, A. Nonenzymatic and Low Potential Glucose Sensor Based on Electrodeposited Ru-Nanofilm From Ionic Liquid Electrolyte. Mater. Sci. Eng. B 2020, 261, 114666. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Kaminskas, A.; Gayda, G.; Ramanaviciene, A. Towards a Self-Powered Amperometric Glucose Biosensor Based on a Single-Enzyme Biofuel Cell. Biosensors 2024, 14, 138. [Google Scholar] [CrossRef] [PubMed]


| Redox Mediator | D0/cm2/s | Km/mmol/L | Reference |
|---|---|---|---|
| MWCNTs/PyBA-NMP | / | 18 | [20] |
| TTF/CNTs | / | 21.4 | [21] |
| Ferrocene monosulphonate | 4.0 × 10−6 | 88 | [22] |
| Fe(CN)63− | 6.7 × 10−6 | 65 | [23] |
| PD/Ru(III) | 6.88 × 10−6 | 0.01 | This work |
| Sensor | Linear Range/mmol/L | LOD/mmol/L | Sensitivity/μA·L/(mmol·cm2) | Reference |
|---|---|---|---|---|
| MIP-PANI Paper Sensors | 2.2~11.1 | 1.1713 | / | [25] |
| SCT/PRG/CuNPs | 0.1~0.6 | 0.025 | 1101.3 | [26] |
| PGE glucose sensor | 0.02~1.11 | 0.0027 | 22.05 | [27] |
| AuNP-PPE | 0.05~35 | 10.0 | / | [28] |
| MWCNT/PyBA-GOx-NMP | 10~12 | 0.3 | 12.1 | [20] |
| PPD/(AuNP)PPCA-GOx | 0.2~150.0 | 0.08 | / | [29] |
| GR/PtNS/PD/GOx | 16.5 | 0.198 | 10.1 | [30] |
| GR/PtNS/PD/GOx/Ppy | 39.0 | 0.561 | 5.31 | |
| FIC/αPLL/GOx-SPCE | 2.8~27.5 | 2.3 | 212.1 | [31] |
| RuNCs/ITO | 0.02~1.2 | 0.02 | / | [32] |
| PD/Ru(III) | 0.01~38.6 | 0.007 | 38 | This work |
| Real Samples | Initial Glucose/mmol/L | Spiked/mmol/L | Detected/mmol/L | Recovery/% | RSD/% |
|---|---|---|---|---|---|
| Human venous blood | 5 | 2 | 7.0 ± 0.1 | 99.5 ± 2.1 | 2.2% |
| 5 | 10.3 ± 0.1 | 107 ± 2.4 | 2.2% | ||
| 10 | 15.6 ± 0.3 | 106 ± 3.5 | 3.2% | ||
| 15 | 19.5 ± 0.2 | 96.9 ± 1.3 | 1.3% | ||
| 20 | 25.3 ± 0.6 | 102 ± 2.9 | 3.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, C.; Zhang, Y.; Liu, Y.; Wen, L.; Yang, H.; Huang, X.; Yang, M.; Xu, B. Electrochemical Glucose Sensor Based on Dual Redox Mediators. Biosensors 2025, 15, 9. https://doi.org/10.3390/bios15010009
Quan C, Zhang Y, Liu Y, Wen L, Yang H, Huang X, Yang M, Xu B. Electrochemical Glucose Sensor Based on Dual Redox Mediators. Biosensors. 2025; 15(1):9. https://doi.org/10.3390/bios15010009
Chicago/Turabian StyleQuan, Changyun, Yue Zhang, Yuanyuan Liu, Liping Wen, Haixia Yang, Xueqin Huang, Minghui Yang, and Binjie Xu. 2025. "Electrochemical Glucose Sensor Based on Dual Redox Mediators" Biosensors 15, no. 1: 9. https://doi.org/10.3390/bios15010009
APA StyleQuan, C., Zhang, Y., Liu, Y., Wen, L., Yang, H., Huang, X., Yang, M., & Xu, B. (2025). Electrochemical Glucose Sensor Based on Dual Redox Mediators. Biosensors, 15(1), 9. https://doi.org/10.3390/bios15010009






