Enhanced Electrochemiluminescence from Ruthenium-Tagged Immune Complex at Flexible Chains for Sensitive Analysis of Glutamate Decarboxylase Antibody
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
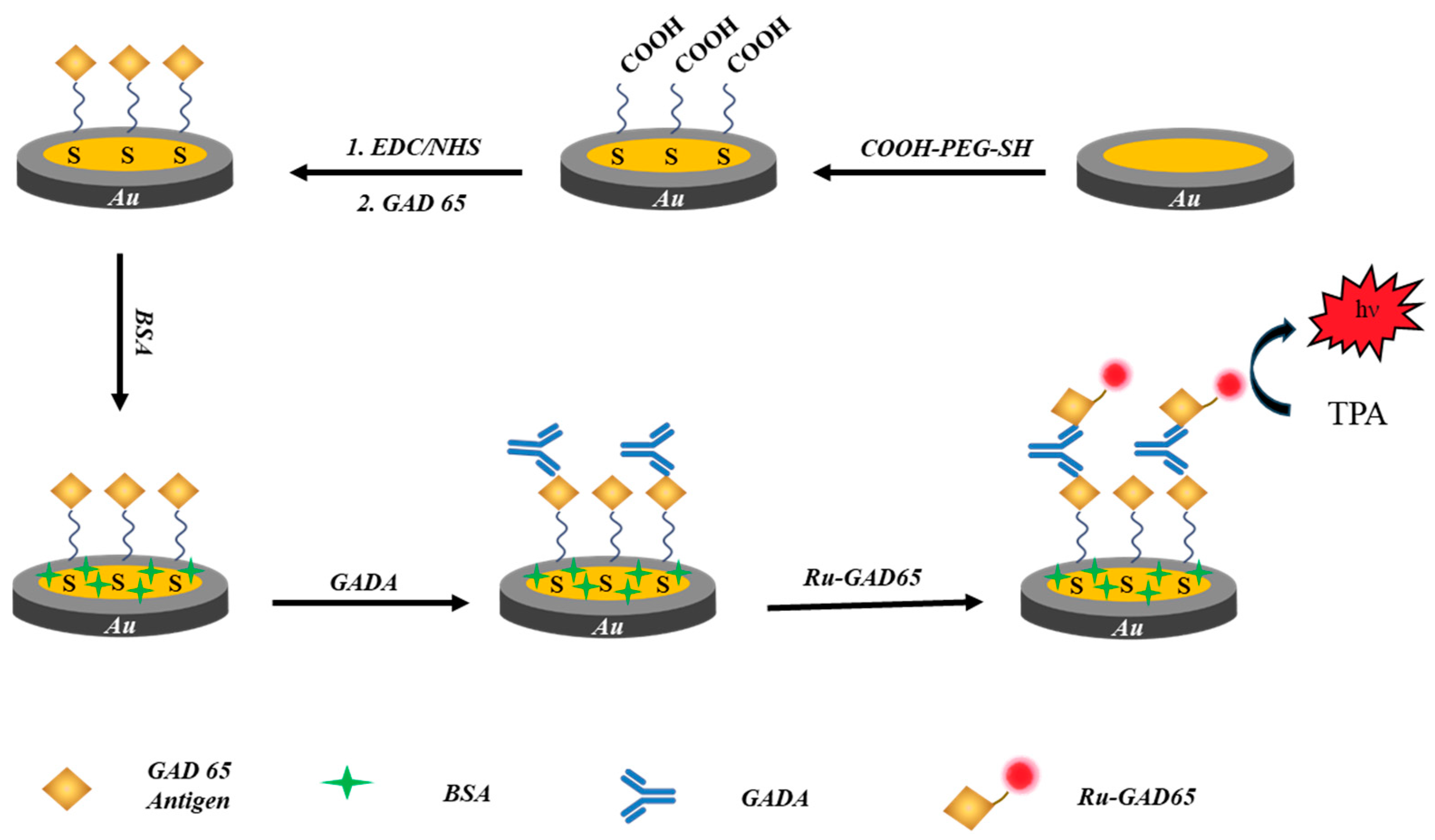
2.2. Fabrication of the Immunosensor
2.3. Electrochemical Characterization of the Immunosensor
2.4. ECL Measurements
3. Results
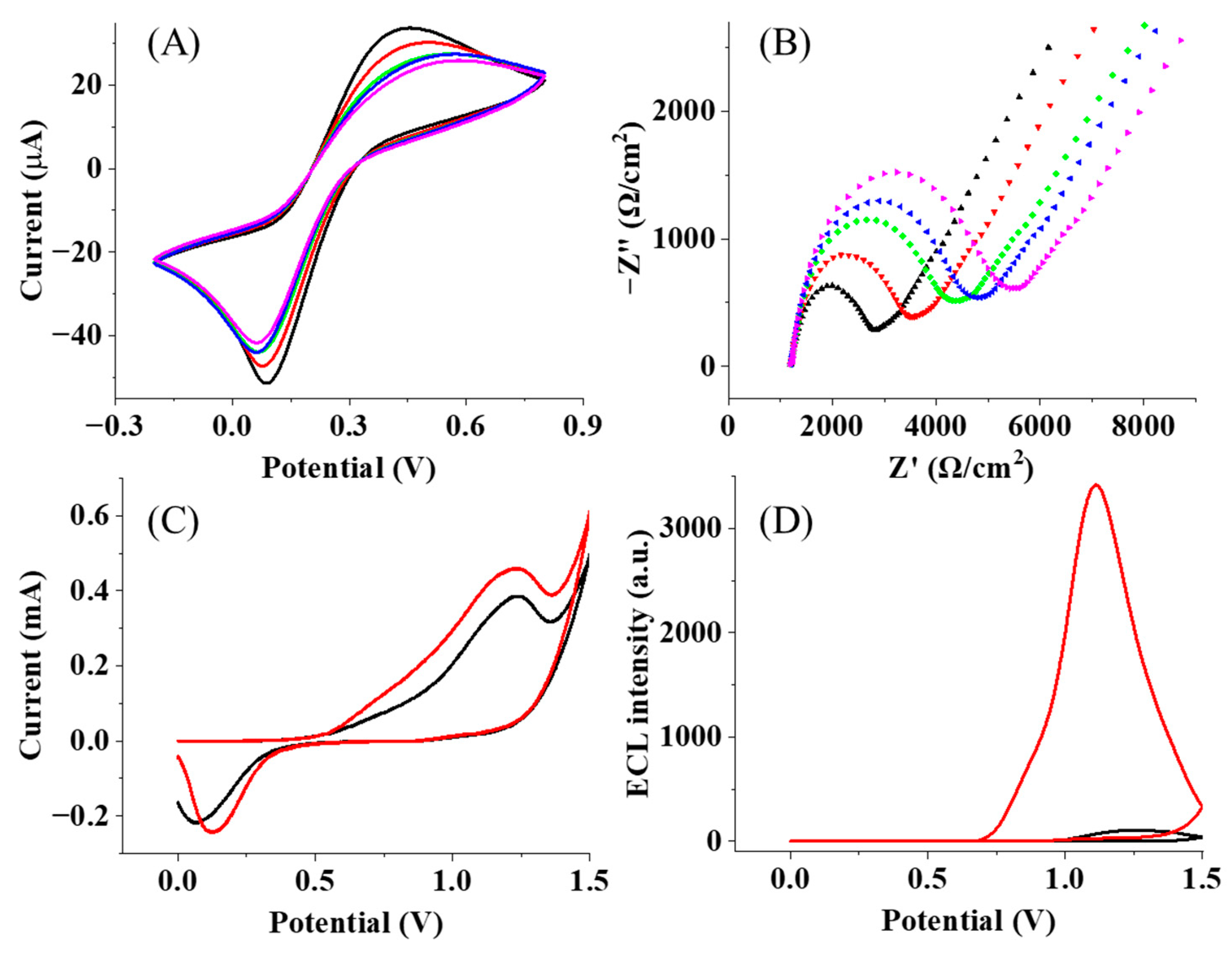
3.1. Characterization of the Immune Complex at Flexible Chains
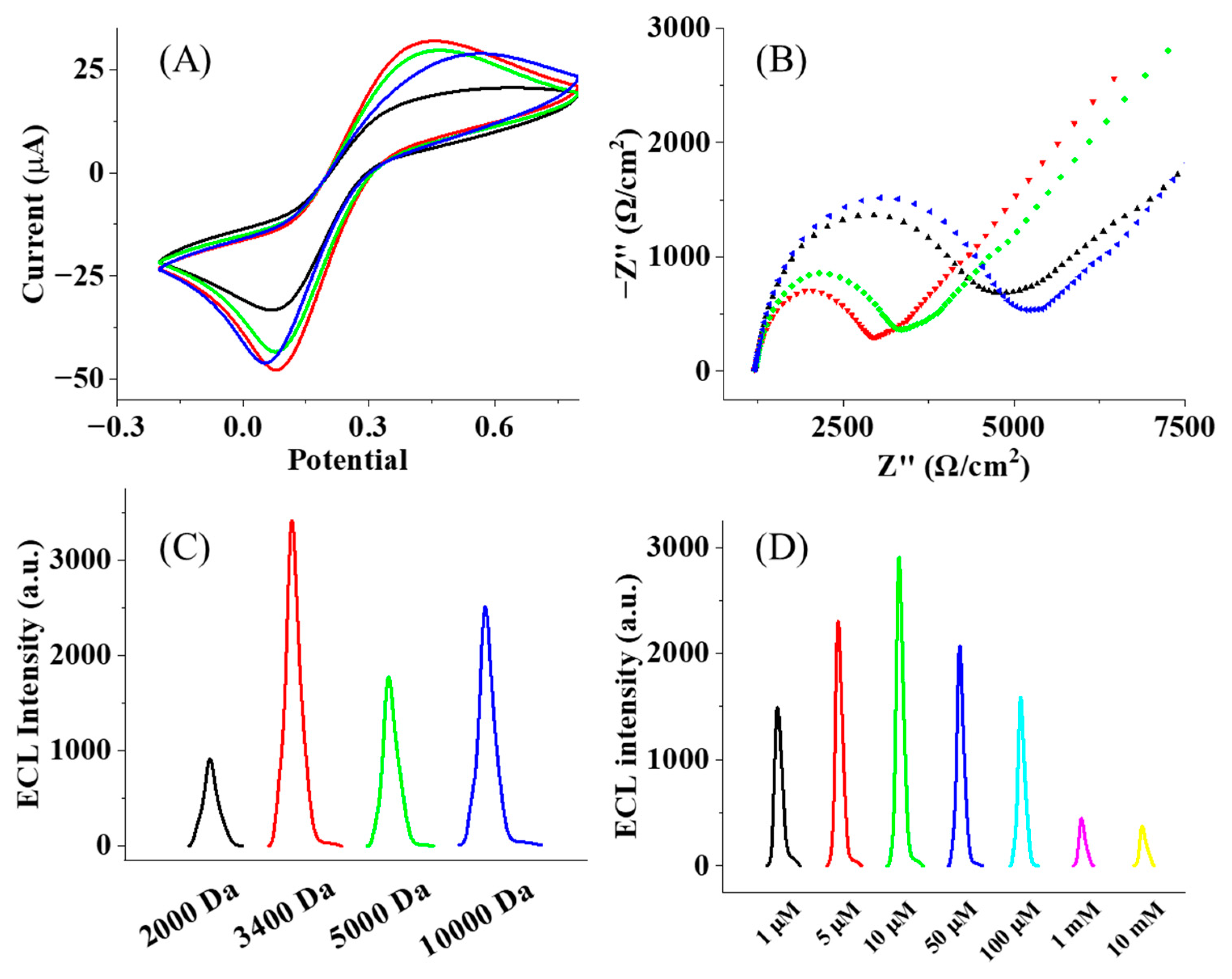
3.2. Optimization of the Fabrication Process
3.3. Detection of GADA in Serum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Syed, F.Z. Type 1 Diabetes Mellitus. Ann. Intern. Med. 2022, 175, ITC33–ITC48. [Google Scholar] [CrossRef]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 Diabetes Mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.J.; Morgan, N.G. Heterogeneity and Endotypes in Type 1 Diabetes Mellitus. Nat. Rev. Endocrinol. 2023, 19, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xie, Z.; Huang, G.; Zhou, Z. Incidence and Trend of Type 1 Diabetes and the Underlying Environmental Determinants. Diabetes Metab. Res. 2019, 35, e3075. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, Z.-H.; Qiang, H.; Wu, J.; Shen, M.; Zhang, L.; Lyu, J. Trends in the Incidence of Diabetes Mellitus: Results from the Global Burden of Disease Study 2017 and Implications for Diabetes Mellitus Prevention. BMC Public Health 2020, 20, 1415. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global Trends in Diabetes Complications: A Review of Current Evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Twig, G. The Impact of Childhood and Adolescent Obesity on Cardiovascular Risk in Adulthood: A Systematic Review. Curr. Diab Rep. 2018, 18, 91. [Google Scholar] [CrossRef]
- Chiang, Y.; Tsay, P.; Chen, C.; Hsu, C.; Yu, H.; Chang, C.; Lo, F.; Moons, P. A Delphi Study on the Healthcare Needs of Patients with Type 1 Diabetes during the Transition from Adolescence to Adulthood: Consensus among Patients, Primary Caregivers, and Healthcare Providers. Int. J. Environ. Res. Public Health 2021, 18, 7149. [Google Scholar] [CrossRef]
- Lernmark, Å.; Larsson, H.E. Immune Therapy in Type 1 Diabetes Mellitus. Nat. Rev. Endocrinol. 2013, 9, 92–103. [Google Scholar] [CrossRef]
- Mathieu, C.; Lahesmaa, R.; Bonifacio, E.; Achenbach, P.; Tree, T. Immunological Biomarkers for the Development and Progression of Type 1 Diabetes. Diabetologia 2018, 61, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, E.; Noveiry, B.B.; Rezaei, N. The Relationship between GAD65 Autoantibody and the Risk of T1DM Onset. J. Diabetes Metab. Disord. 2022, 21, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Saiz, A.; Dalmau, J. GAD Antibodies in Neurological Disorders—Insights and Challenges. Nat. Rev. Neurol. 2020, 16, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Jia, X.; Vartak, T.; Miao, D. Improving Clinical Utility of GAD65 Autoantibodies by Electrochemiluminescence Assay and Clinical Phenotype When Identifying Autoimmune Adult-Onset Diabetes. Diabetologia 2021, 64, 2052–2060. [Google Scholar] [CrossRef]
- Takagi, M.; Ishigaki, Y.; Uno, K.; Sawada, S.; Imai, J.; Kaneko, K.; Hasegawa, Y.; Yamada, T.; Tokita, A.; Iseki, K.; et al. Cognitive Dysfunction Associated with Anti-Glutamic Acid Decarboxylase Autoimmunity: A Case-Control Study. BMC Neurol. 2013, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, X.; Liang, H.; Cheng, Y.; Huang, G.; Zhou, Z. Pathophysiological Characteristics in Patients with Latent Autoimmune Diabetes in Adults Using Clamp Tests: Evidence of a Continuous Disease Spectrum of Diabetes. Acta Diabetol. 2019, 56, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qian, L.; Liu, Q.; Zou, J.; Zhou, Y.; Yang, T.; Huang, G.; Zhou, Z.; Liu, Y. Glutamic Acid Decarboxylase Autoantibody Detection by Electrochemiluminescence Assay Identifies Latent Autoimmune Diabetes in Adults with Poor Islet Function. Diabetes Metab. J. 2020, 44, 260. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Xing, Z.; Ma, C.; Zhu, J.-J. A Close Look at Mechanism, Application, and Opportunities of Electrochemiluminescence Microscopy. Chem. Biomed. Imaging 2023, 1, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Abdussalam, A.; Xu, G. Recent Advances in Electrochemiluminescence Luminophores. Anal. Bioanal. Chem. 2022, 414, 131–146. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y. Recent Progress of Novel Electrochemiluminescence Nanoprobes and Their Analytical Applications. Front. Chem. 2021, 8, 626243. [Google Scholar] [CrossRef] [PubMed]
- Fereja, T.H.; Du, F.; Wang, C.; Snizhko, D.; Guan, Y.; Xu, G. Electrochemiluminescence Imaging Techniques for Analysis and Visualizing. J. Anal. Test. 2020, 4, 76–91. [Google Scholar] [CrossRef]
- Rebeccani, S.; Zanut, A.; Santo, C.I.; Valenti, G.; Paolucci, F. A Guide Inside Electrochemiluminescent Microscopy Mechanisms for Analytical Performance Improvement. Anal. Chem. 2022, 94, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, R.; Yang, X.; Qi, H.; Zhang, C. Recent Advances in Electrogenerated Chemiluminescence Biosensing Methods for Pharmaceuticals. J. Pharm. Anal. 2019, 9, 9–19. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, C. Electrogenerated Chemiluminescence Biosensing. Anal. Chem. 2020, 92, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Rebeccani, S.; Wetzl, C.; Zamolo, V.A.; Criado, A.; Valenti, G.; Paolucci, F.; Prato, M. Electrochemiluminescent Immunoassay Enhancement Driven by Carbon Nanotubes. Chem. Commun. 2021, 57, 9672–9675. [Google Scholar] [CrossRef]
- Zhao, C.; Niu, L.; Wang, X.; Sun, W. A Simple and Convenient Electrochemiluminescence Immunoassay by Using Gold Nanoparticles as Both Label and Co-Reactant. Bioelectrochemistry 2020, 135, 107585. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, Y.; Cao, Z.; Su, B. Imaging Analysis Based on Electrogenerated Chemiluminescence. J. Anal. Test. 2017, 1, 14. [Google Scholar] [CrossRef]
- Yin, F.; Sun, Q.; Huang, X.; Wu, G.; Zhang, Y.; Shen, Y. Recent Progress in Signal Enhancement of Nanomaterials-Based Electrochemiluminescence Systems. TrAC Trends Anal. Chem. 2023, 169, 117376. [Google Scholar] [CrossRef]
- Jin, Z.; Zhu, X.; Wang, N.; Li, Y.; Ju, H.; Lei, J. Electroactive Metal–Organic Frameworks as Emitters for Self-Enhanced Electrochemiluminescence in Aqueous Medium. Angew. Chem. Int. Ed. 2020, 59, 10446–10450. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-L.; Zhang, J.-Q.; Tang, Z.-L.; Zhuo, Y.; Chai, Y.-Q.; Yuan, R. Near-Infrared Aggregation-Induced Enhanced Electrochemiluminescence from Tetraphenylethylene Nanocrystals: A New Generation of ECL Emitters. Chem. Sci. 2019, 10, 4497–4501. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Ma, C.; Wang, L.; Cao, Y.; Chen, H.; Zhu, J. A Novel Electrochemiluminescence Janus Emitter for Dual-Mode Biosensing. Adv. Funct. Mater. 2022, 32, 2200863. [Google Scholar] [CrossRef]
- Li, B.; Huang, X.; Lu, Y.; Fan, Z.; Li, B.; Jiang, D.; Sojic, N.; Liu, B. High Electrochemiluminescence from Ru(Bpy)3 2+ Embedded Metal–Organic Frameworks to Visualize Single Molecule Movement at the Cellular Membrane. Adv. Sci. 2022, 9, 2204715. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, X.; Wang, S.; Li, B.; Liu, B. Nanoconfinement-Enhanced Electrochemiluminescence for in Situ Imaging of Single Biomolecules. ACS Nano 2023, 17, 3809–3817. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wu, H.; Chen, R.; Chi, Y. Strong Electrochemiluminescence Emission from Oxidized Multiwalled Carbon Nanotubes. Small 2019, 15, 1901550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Song, X.; Fan, D.; Liu, X.; Wang, H.; Wei, Q.; Wu, D. Highly Efficient Signal On/Off Electrochemiluminescence Gel Aptasensor Based on a Controlled Release Strategy for the Sensitive Detection of Prostate Specific Antigen. Anan. Chem. 2023, 95, 5695–5701. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, Y.; Pan, J.; Chen, T.; Xing, X.; Zhang, W.; Lu, Z. Plasmon-Enhanced Electrochemiluminescence at the Single-Nanoparticle Level. Angew. Chem. Int. Ed. 2023, 62, e202214103. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Choi, J.-P.; Bard, A.J. Electrogenerated Chemiluminescence 69: The Tris(2,2‘-bipyridine)ruthenium(II), (Ru(bpy)32+)/Tri-n-propylamine (TPrA) System RevisitedA New Route Involving TPrA•+ Cation Radicals. J. Am. Chem. Soc. 2002, 124, 14282–14506. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Bard, A.J. Electrogenerated chemiluminescence. 66. The role of direct coreactant oxidation in the ruthenium tris(2,2′)bipyridyl/tripropylamine system and the effect of halide ions on the emission intensity. Anal. Chem. 2000, 72, 3223–3232. [Google Scholar] [CrossRef]
- Anne, A.; Demaille, C.; Moiroux, J. Terminal Attachment of Polyethylene Glycol (PEG) Chains to a Gold Electrode Surface. Cyclic Voltammetry Applied to the Quantitative Characterization of the Flexibility of the Attached PEG Chains and of Their Penetration by Mobile PEG Chains. Macromolecules 2002, 35, 5578–5586. [Google Scholar] [CrossRef]
- Hotchen, C.E.; Maybury, I.J.; Nelson, G.W.; Foord, J.S.; Holdway, P.; Marken, F. Amplified Electron Transfer at Poly-Ethylene-Glycol (PEG) Grafted Electrodes. Phys. Chem. Chem. Phys. 2015, 17, 11260–11268. [Google Scholar] [CrossRef]
- Steentjes, T.; Jonkheijm, P.; Huskens, J. Electron Transfer Processes in Ferrocene-Modified Poly(Ethylene Glycol) Monolayers on Electrodes. Langmuir 2017, 33, 11878–11883. [Google Scholar] [CrossRef] [PubMed]
- Cattani, G.; Vogeley, L.; Crowley, P.B. Structure of a PEGylated Protein Reveals a Highly Porous Double-Helical Assembly. Nat. Chem. 2015, 7, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.T.; Stubbs, V.R.; Soto, C.M.; Martin, B.D.; White, B.J.; Taitt, C.R. Reduction of Non-Specific Protein Adsorption Using Poly(Ethylene) Glycol (PEG) Modified Polyacrylate Hydrogels In Immunoassays for Staphylococcal Enterotoxin B Detection. Sensors 2009, 9, 645–655. [Google Scholar] [CrossRef]
- Van Aelst, S.; Gillard, P.; Weets, I.; Dillaerts, D.; Billen, J.; Mathieu, C.; Bossuyt, X. Pancreas Islet Cell-Specific Antibody Detection by ELISA. J. Appl. Lab. Med. 2022, 7, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Katahira, M.; Ogata, H.; Ito, T.; Miwata, T.; Goto, M.; Nakamura, S.; Takashima, H. Association of Autoimmune Thyroid Disease with Anti-GAD Antibody ELISA Test Positivity and Risk for Insulin Deficiency in Slowly Progressive Type 1 Diabetes. J. Diabetes Res. 2018, 2018, 1847430. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qian, L.; Zhang, Q.; Li, Y.; Liu, Y.; Jiang, D. Enhanced Electrochemiluminescence from Ruthenium-Tagged Immune Complex at Flexible Chains for Sensitive Analysis of Glutamate Decarboxylase Antibody. Biosensors 2025, 15, 47. https://doi.org/10.3390/bios15010047
Zhang Y, Qian L, Zhang Q, Li Y, Liu Y, Jiang D. Enhanced Electrochemiluminescence from Ruthenium-Tagged Immune Complex at Flexible Chains for Sensitive Analysis of Glutamate Decarboxylase Antibody. Biosensors. 2025; 15(1):47. https://doi.org/10.3390/bios15010047
Chicago/Turabian StyleZhang, Yuyao, Li Qian, Qian Zhang, Yu Li, Yu Liu, and Dechen Jiang. 2025. "Enhanced Electrochemiluminescence from Ruthenium-Tagged Immune Complex at Flexible Chains for Sensitive Analysis of Glutamate Decarboxylase Antibody" Biosensors 15, no. 1: 47. https://doi.org/10.3390/bios15010047
APA StyleZhang, Y., Qian, L., Zhang, Q., Li, Y., Liu, Y., & Jiang, D. (2025). Enhanced Electrochemiluminescence from Ruthenium-Tagged Immune Complex at Flexible Chains for Sensitive Analysis of Glutamate Decarboxylase Antibody. Biosensors, 15(1), 47. https://doi.org/10.3390/bios15010047




