Exploring Innovative Approaches for the Analysis of Micro- and Nanoplastics: Breakthroughs in (Bio)Sensing Techniques
Abstract
1. Introduction
2. Current Methods for Analyzing Microplastics and Nanoplastics
2.1. Visual Identification Methods
2.2. Spectroscopy
2.3. Thermoanalytical Methods
3. Biosensors for Microplastic Detection
3.1. Electrochemical Sensing Approaches
3.2. Plasmonic Sensing Approaches
3.3. Fluorescence Biosensor Approaches
4. Prospects and Future Challenges
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Yu, K.; Zhang, H.; Liu, Y.; He, J.; Liu, X.; Jiang, J. A novel heating-assisted density separation method for extracting microplastics from sediments. Chemosphere 2020, 256, 127039. [Google Scholar] [CrossRef] [PubMed]
- Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M. Microplastics in aquatic environments: A review on occurrence, distribution, toxic effects, and implications for human health. Sci. Total Environ. 2021, 780, 146551. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Malinconico, M.; Guarneri, A.; Gardossi, L. Renewable polymers and plastics: Performance beyond the green. New Biotechnol. 2021, 60, 146–158. [Google Scholar] [CrossRef]
- Babaremu, K.O.; Okoya, S.A.; Hughes, E.; Tijani, B.; Teidi, D.; Akpan, A.; Igwe, J.; Karera, S.; Oyinlola, M.; Akinlabi, E.T. Sustainable plastic waste management in a circular economy. Heliyon 2022, 8, e09984. [Google Scholar] [CrossRef]
- Bui, X.T.; Vo, T.D.H.; Nguyen, P.T.; Nguyen, V.T.; Dao, T.S.; Nguyen, P.D. Microplastics pollution in wastewater: Characteristics, occurrence and removal technologies. Environ. Technol. Innov. 2020, 19, 101013. [Google Scholar] [CrossRef]
- Mai, L.; Bao, L.; Shi, L.; Wong, C.S.; Zeng, E.Y. A review of methods for measuring microplastics in aquatic environments. Environ. Sci. Pollut. Res. 2018, 25, 11319–11332. [Google Scholar] [CrossRef]
- Li, C.; Gan, Y.; Dong, J.; Fang, J.; Chen, H.; Quan, Q.; Liu, J. Impact of microplastics on microbial community in sediments of the Huangjinxia Reservoir—Water source of a water diversion project in western China. Chemosphere 2020, 253, 126740. [Google Scholar] [CrossRef]
- Aytan, U.; Esensoy, F.B.; Senturk, Y. Microplastic ingestion and egestion by copepods in the Black Sea. Sci. Total Environ. 2022, 806, 150921. [Google Scholar] [CrossRef]
- Hoang, V.H.; Nguyen, M.K.; Hoang, T.D.; Ha, M.C.; Huyen, N.T.T.; Bui, V.K.H.; Pham, M.T.; Nguyen, C.M.; Chang, S.W.; Nguyen, D.D. Sources, environmental fate, and impacts of microplastic contamination in agricultural soils: A comprehensive review. Sci. Total Environ. 2024, 950, 175276. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Wang, Z.; Saadé, N.K.; Ariya, P.A. Advances in Ultra-Trace Analytical Capability for Micro/Nanoplastics and Water-Soluble Polymers in the Environment: Fresh Falling Urban Snow. Environ. Pollut. 2021, 276, 116698. [Google Scholar] [CrossRef]
- Andrady, A.L.; Barnes, P.W.; Bornman, J.F.; Gouin, T.; Madronich, S.; White, C.C.; Zepp, R.G.; Jansen, M.A.K. Oxidation and fragmentation of plastics in a changing environment; from UV-radiation to biological degradation. Sci. Total Environ. 2022, 851, 158022. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Analysis and prevention of microplastics pollution in water: Current perspectives and future directions. ACS Omega 2019, 4, 6709–6719. [Google Scholar] [CrossRef]
- Ekvall, M.T.; Lundqvist, M.; Kelpsiene, E.; Šileikis, E.; Gunnarsson, S.B.; Cedervall, T. Nanoplastics formed during the mechanical breakdown of daily-use polystyrene products. Nanoscale Adv. 2018, 1, 1055–1061. [Google Scholar] [CrossRef]
- ISO/TR 21960; Plastics—Environmental Aspects—State of Knowledge and Methodologies. International Organization for Standardization: Geneva, Switzerland, 2020.
- Kokalj, A.J.; Hartmann, N.B.; Drobne, D.; Potthoff, A.; Kühnel, D. Quality of nanoplastics and microplastics ecotoxicity studies: Refining quality criteria for nanomaterial studies. J. Hazard. Mater. 2021, 415, 125751. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Microplastics in the Environment: Occurrence, Perils and Eradication. Chem. Eng. J. 2021, 408, 127317. [Google Scholar] [CrossRef]
- Cholewinski, A.; Dadzie, E.; Sherlock, C.; Anderson, W.A.; Charles, T.C.; Habib, K.; Young, S.B.; Zhao, B. Critical Review of Microplastic Degradation and Material Flow Analysis towards a Circular Economy. Environ. Pollut. 2022, 315, 120334. [Google Scholar] [CrossRef]
- Belone, M.C.L.; Kokko, M.; Sarlin, E. The effects of weathering-induced degradation of polymers in the microplastic study involving reduction of organic matter. Environ. Pollut. 2022, 308, 119669. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, T.; Liu, L.; Fan, Y.; Rao, W.; Zheng, J.; Qian, X. Distribution and sedimentation of microplastics in Taihu Lake. Sci. Total Environ. 2021, 795, 148745. [Google Scholar] [CrossRef]
- Waldschläger, K.; Brückner, M.Z.; Almroth, B.C.; Hackney, C.R.; Adyel, T.M.; Alimi, O.S.; Belontz, S.L.; Cowger, W.; Doyle, D.; Gray, A.; et al. Learning from natural sediments to tackle microplastics challenges: A multidisciplinary perspective. Earth Sci. Rev. 2022, 228, 104021. [Google Scholar] [CrossRef]
- Rajmohan, K.V.S.; Ramya, C.; Viswanathan, M.R.; Varjani, S. Plastic pollutants: Effective waste management for pollution control and abatement. Curr. Opin. Environ. Sci. Health 2019, 12, 72–84. [Google Scholar] [CrossRef]
- Liu, P.; Wu, X.; Liu, H.; Wang, H.; Lu, K.; Gao, S. Desorption of pharmaceuticals from pristine and aged polystyrene microplastics under simulated gastrointestinal conditions. J. Hazard. Mater. 2020, 392, 122346. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic contamination of packaged meat: Occurrence and associated risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Luo, X.; Shukla, T.; Gao, T.; Allen, D.; Allen, S.; Bergmann, M. Microplastics and nanoplastics pose risks on the Tibetan Plateau environment. Sci. Bull. 2023, 69, 589–592. [Google Scholar] [CrossRef]
- Peng, X.; Chen, M.; Chen, S.; Dasgupta, S.; Xu, H.; Ta, K.; Du, M.; Li, J.; Guo, Z.; Bai, S. Microplastics contaminate the deepest part of the world’s ocean. Geochem. Perspect. Lett. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Dong, H.; Wang, L.; Wang, X.; Xu, L.; Chen, M.; Gong, P.; Wang, C. Microplastics in a Remote Lake Basin of the Tibetan Plateau: Impacts of Atmospheric Transport and Glacial Melting. Environ. Sci. Technol. 2021, 55, 12951–12960. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Wang, X.; Dong, H.; Ciren, N.; Zhang, H.; Chen, X.; Zhuoga, S.; Jia, X.; Xu, L.; Zhou, Y. Microplastics in remote region of the world: Insights from the glacier of Geladandong, China. Appl. Geochem. 2024, 168, 106026. [Google Scholar] [CrossRef]
- Vercauteren, M.; Semmouri, I.; Van Acker, E.; Pequeur, E.; Van Esch, L.; Uljee, I.; Asselman, J.; Janssen, C.R. Assessment of road run-off and domestic wastewater contribution to microplastic pollution in a densely populated area (Flanders, Belgium). Environ. Pollut. 2023, 333, 122090. [Google Scholar] [CrossRef] [PubMed]
- Trindade, L.D.S.; Gloaguen, T.V.; Benevides, T.D.S.F.; Valentim, A.C.S.; Bomfim, M.R.; Santos, J.A.G. Microplastics in surface waters of tropical estuaries around a densely populated Brazilian bay. Environ. Pollut. 2023, 323, 121224. [Google Scholar] [CrossRef] [PubMed]
- Markic, A.; Niemand, C.; Bridson, J.H.; Mazouni-Gaertner, N.; Gaertner, J.C.; Eriksen, M.; Bowen, M. Double trouble in the South Pacific subtropical gyre: Increased plastic ingestion by fish in the oceanic accumulation zone. Mar. Pollut. Bull. 2018, 136, 547–564. [Google Scholar] [CrossRef]
- Alfaro-Núñez, A.; Astorga, D.; Cáceres-Farías, L.; Bastidas, L.; Soto Villegas, C.; Macay, K.; Christensen, J.H. Microplastic pollution in seawater and marine organisms across the Tropical Eastern Pacific and Galápagos. Sci. Rep. 2021, 11, 6424. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Larat, V.; Karbalaei, S.; Salamatinia, B. Microplastic and mesoplastic contamination in canned sardines and sprats. Sci. Total Environ. 2018, 612, 1380–1386. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics contamination in eggs: Detection, occurrence and status. Food Chem. 2022, 397, 133771. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G. First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks—Future research and environmental considerations. Sci. Total Environ. 2020, 726, 138580. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R.; Dobaradaran, S.; Schmidt, T.C.; Nabipour, I.; Spitz, J. Worldwide bottled water occurrence of emerging contaminants: A review of the recent scientific literature. J. Hazard. Mater. 2020, 392, 122271. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Galvão, L.D.S.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of microplastic in human placenta and meconium in a clinical setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2020, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, R.; Liang, W.; Wei, S.; Zhou, Y.; Zeng, F. Assessment of BPA and BPS exposure in the general population in Guangzhou, China—Estimation of daily intakes based on urinary metabolites. Environ. Pollut. 2022, 315, 120375. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, L.; Hirsch, T. Current challenges in nanomaterial-based sensors for online monitoring of drinking water by surface plasmon resonance. Curr. Opin. Environ. Sci. Health 2022, 26, 100326. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahashi, Y.; Kajiwara, T.; Matsukami, H.; Kuramochi, H.; Osako, M.; Suzuki, G. Identification and quantification of additive-derived chemicals in beached micro–mesoplastics and macroplastics. Mar. Pollut. Bull. 2022, 186, 114438. [Google Scholar] [CrossRef] [PubMed]
- Abouda, S.; Missawi, O.; Cappello, T.; Boughattas, I.; De Marco, G.; Maisano, M.; Banni, M. Toxicological impact of environmental microplastics and benzo[a]pyrene in the seaworm Hediste diversicolor under environmentally relevant exposure conditions. Environ. Pollut. 2022, 310, 119856. [Google Scholar] [CrossRef]
- Issaka, E.; Yakubu, S.; Sulemana, H.; Kerkula, A.; Aniagyei, O.N.-D. Current status of the direct detection of microplastics in environments and implications for toxicological effects. Chem. Eng. J. Adv. 2023, 14, 100449. [Google Scholar] [CrossRef]
- Varshney, S.; Gora, A.H.; Kiron, V.; Siriyappagouder, P.; Dahle, D.; Kögel, T.; Ørnsrud, R.; Olsvik, P.A. Polystyrene nanoplastics enhance the toxicological effects of DDE in zebrafish (Danio rerio) larvae. Sci. Total Environ. 2022, 859, 160457. [Google Scholar] [CrossRef]
- Radisic, V.; Nimje, P.S.; Bienfait, A.M.; Marathe, N.P. Marine plastics from norwegian west coast carry potentially virulent fish pathogens and opportunistic human pathogens harboring new variants of antibiotic resistance genes. Microorganisms 2020, 8, 1200. [Google Scholar] [CrossRef] [PubMed]
- Athulya, P.A.; Chandrasekaran, N. Interactions of natural colloids with microplastics in aquatic environment and its impact on FTIR characterization of polyethylene and polystyrene microplastics. J. Mol. Liq. 2022, 369, 120950. [Google Scholar] [CrossRef]
- Lofty, J.; Ouro, P.; Wilson, C.A.M.E. Microplastics in the riverine environment: Meta-analysis and quality criteria for developing robust field sampling procedures. Sci. Total Environ. 2022, 863, 160893. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments. J. Hazard. Mater. 2021, 422, 126843. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Y.; Qiu, Z.; He, Y.; Zhang, Y. Towards a fast and generalized microplastic quantification method in soil using terahertz spectroscopy. Sci. Total Environ. 2022, 841, 156624. [Google Scholar] [CrossRef] [PubMed]
- Anger, P.M.; von der Esch, E.; Baumann, T.; Elsner, M.; Niessner, R.; Ivleva, N.P. Raman microspectroscopy as a tool for microplastic particle analysis. TrAC Trends Anal. Chem. 2018, 109, 214–226. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xiong, W.; Yang, Z.; Xu, R.; Zhang, Y.; Wu, M.; Ye, Y.; Peng, H.; Tong, J.; Wang, D. Coexistence of microplastics alters the inhibitory effect of antibiotics on sludge anaerobic digestion. Chem. Eng. J. 2023, 455, 140754. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Huffer, T.; Thompson, R.C.; Hassellov, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Xu, J.L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Yusuf, A.; Sodiq, A.; Giwa, A.; Eke, J.; Pikuda, O.; Eniola, J.O.; Ajiwokewu, B.; Sambudi, N.S.; Bilad, M.R. Updated review on microplastics in water, their occurrence, detection, measurement, environmental pollution, and the need for regulatory standards. Environ. Pollut. 2022, 292, 118421. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y. Effects of microplastics on wastewater and sewage sludge treatment and their removal: A review. Chem. Eng. J. 2020, 382, 122955. [Google Scholar] [CrossRef]
- Turan, N.B.; Erkan, H.S.; Engin, G.O. Microplastics in wastewater treatment plants: Occurrence, fate and identification. Process Saf. Environ. Prot. 2021, 146, 77–84. [Google Scholar] [CrossRef]
- Fahrenfeld, N.L.; Arbuckle-Keil, G.; Beni, N.N.; Bartelt-Hunt, S.L. Source tracking microplastics in the freshwater environment. TrAC Trends Anal. Chem. 2019, 112, 248–254. [Google Scholar] [CrossRef]
- Prata, J.C.; Reis, V.; Matos, J.T.V.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). Sci. Total Environ. 2019, 690, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yu, K.; Zhao, Y. The development and application of advanced analytical methods in microplastics contamination detection: A critical review. Sci. Total Environ. 2022, 818, 151851. [Google Scholar] [CrossRef] [PubMed]
- von der Esch, E.; Kohles, A.J.; Anger, P.M.; Hoppe, R.; Niessner, R.; Elsner, M.; Ivleva, N.P. TUM-ParticleTyper: A detection and quantification tool for automated analysis of (Microplastic) particles and fibers. PLoS ONE 2020, 15, e0234766. [Google Scholar] [CrossRef] [PubMed]
- Seghers, J.; Stefaniak, E.A.; La Spina, R.; Cella, C.; Mehn, D.; Gilliland, D.; Held, A.; Jacobsson, U.; Emteborg, H. Preparation of a reference material for microplastics in water—Evaluation of homogeneity. Anal. Bioanal. Chem. 2022, 414, 385–397. [Google Scholar] [CrossRef]
- Malankowska, M.; Echaide-Gorriz, C.; Coronas, J. Microplastics in marine environment: A review on sources, classification, and potential remediation by membrane technology. Environ. Sci. Water Res. Technol. 2021, 7, 243–258. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Li, H.; Xue, H.; Tao, J.; Li, M.; Wang, F.; Li, Y.; Wang, J.; Li, S. Current advances in microplastic contamination in aquatic sediment: Analytical methods, global occurrence, and effects on elemental cycling. TrAC Trends Anal. Chem. 2023, 168, 117331. [Google Scholar] [CrossRef]
- Weisser, J.; Pohl, T.; Heinzinger, M.; Ivleva, N.P.; Hofmann, T.; Glas, K. The identification of microplastics based on vibrational spectroscopy data—A critical review of data analysis routines. TrAC Trends Anal. Chem. 2022, 148, 116535. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Investigation of microplastics in aquatic environments: An overview of the methods used, from field sampling to laboratory analysis. TrAC Trends Anal. Chem. 2018, 108, 195–202. [Google Scholar] [CrossRef]
- Renner, G.; Schmidt, T.C.; Schram, J. Analytical methodologies for monitoring micro(nano)plastics: Which are fit for purpose? Curr. Opin. Environ. Sci. Health 2018, 1, 55–61. [Google Scholar] [CrossRef]
- Guerrero-Pérez, M.O.; Patience, G.S. Experimental methods in chemical engineering: Fourier transform infrared spectroscopy—FTIR. Can. J. Chem. Eng. 2020, 98, 25–330. [Google Scholar] [CrossRef]
- Materić, D.; Kasper-Giebl, A.; Kau, D.; Anten, M.; Greilinger, M.; Ludewig, E.; van Sebille, E.; Röckmann, T.; Holzinger, R. Micro-and Nanoplastics in Alpine Snow: A New Method for Chemical Identification and (Semi)Quantification in the Nanogram Range. Environ. Sci. Technol. 2020, 54, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Nava, V.; Frezzotti, M.L.; Leoni, B. Raman Spectroscopy for the Analysis of Microplastics in Aquatic Systems. Appl. Spectrosc. 2021, 75, 1341–1357. [Google Scholar] [CrossRef] [PubMed]
- Schwaferts, C.; Niessner, R.; Elsner, M.; Ivleva, N.P. Methods for the analysis of submicrometer- and nanoplastic particles in the environment. TrAC Trends Anal. Chem. 2019, 112, 52–65. [Google Scholar] [CrossRef]
- Hendrickson, E.; Minor, E.C.; Schreiner, K. Microplastic Abundance and Composition in Western Lake Superior As Determined via Microscopy, Pyr-GC/MS, and FTIR. Environ. Sci. Technol. 2018, 52, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Min, J.; Jiang, W.; Li, Y.; Zhang, W. Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci. Total Environ. 2020, 721, 137561. [Google Scholar] [CrossRef]
- Peñalver, R.; Arroyo-Manzanares, N.; López-García, I.; Hernández-Córdoba, M. An overview of microplastics characterization by thermal analysis. Chemosphere 2019, 242, 125170. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Liu, Y.; Xia, S.; Zhao, J. Effects of exposure of polyethylene microplastics to air, water and soil on their adsorption behaviors for copper and tetracycline. Chem. Eng. J. 2021, 404, 126412. [Google Scholar] [CrossRef]
- Abbasi, S.; Jaafarzadeh, N.; Zahedi, A.; Ravanbakhsh, M.; Abbaszadeh, S.; Turner, A. Microplastics in the atmosphere of Ahvaz City, Iran. J. Environ. Sci. 2023, 126, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Zou, M.; Jia, Z.; Zhou, S.; Li, Y. Microplastics in soils: A review of methods, occurrence, fate, transport, ecological and environmental risks. Sci. Total Environ. 2020, 748, 141368. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, H.; Guo, X.; Zhang, Z.; Zhang, J.; Huang, X. Microplastic distribution and migration in soil, water and sediments in Caohai Lake under the different hydrological periods, Southwest China. Sci. Total Environ. 2022, 865, 161292. [Google Scholar] [CrossRef]
- Sillanpää, M.; Sainio, P. Release of polyester and cotton fibers from textiles in machine washings. Environ. Sci. Pollut. Res. 2017, 24, 19313–19321. [Google Scholar] [CrossRef]
- Blair, R.M.; Waldron, S.; Gauchotte-Lindsay, C. Average daily flow of microplastics through a tertiary wastewater treatment plant over a ten-month period. Water Res. 2019, 163, 114909. [Google Scholar] [CrossRef] [PubMed]
- Ashjar, N.; Keshavarzi, B.; Moore, F.; Zarei, M.; Busquets, R.; Zebarjad, S.M.; Mohammadi, Z. Microplastics (MPs) distribution in Surface Sediments of the Freidounkenar Paddy Wetland. Environ. Pollut. 2023, 317, 120799. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Nguyen, N.B.; Kim, M.K.; Le, Q.T.; Ngo, D.N.; Zoh, K.D.; Joo, S.W. Spectroscopic analysis of microplastic contaminants in an urban wastewater treatment plant from Seoul, South Korea. Chemosphere 2021, 263, 127812. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Bharath, K.M.; Muthulakshmi, A.L.; Natesan, U. Microplastic contamination around the landfills: Distribution, characterization and threats: A review. Curr. Opin. Environ. Sci. Health 2023, 31, 100422. [Google Scholar] [CrossRef]
- Kim, S.W.; Chae, Y.; Kim, D.; An, Y.J. Zebrafish can recognize microplastics as inedible materials: Quantitative evidence of ingestion behavior. Sci. Total Environ. 2019, 649, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.J.M.; Silva, A.L.P.; Gravato, C.; Pestana, J.L.T. Ingestion of small-sized and irregularly shaped polyethylene microplastics affect Chironomus riparius life-history traits. Sci. Total Environ. 2019, 672, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Srinivasalu, S.; Natesan, U.; Ayyamperumal, R.; Kalam, N.; Anbalagan, S.; Sujatha, K.; Alagarasan, C. Microplastics as an emerging threat to the freshwater ecosystems of Veeranam lake in south India: A multidimensional approach. Chemosphere 2021, 264, 128502. [Google Scholar] [CrossRef]
- Sighicelli, M.; Pietrelli, L.; Lecce, F.; Iannilli, V.; Falconieri, M.; Coscia, L.; Di Vito, S.; Nuglio, S.; Zampetti, G. Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ. Pollut. 2018, 236, 645–651. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.J.M.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M.M. Spatial and temporal distribution of microplastics in water and sediments of a freshwater system (Antuã River, Portugal). Sci. Total Environ. 2018, 633, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Bucol, L.A.; Romano, E.F.; Cabcaban, S.M.; Siplon, L.M.D.; Madrid, G.C.; Bucol, A.A.; Polidoro, B. Microplastics in marine sediments and rabbitfish (Siganus fuscescens) from selected coastal areas of Negros Oriental, Philippines. Mar. Pollut. Bull. 2020, 150, 110685. [Google Scholar] [CrossRef]
- Tammina, S.K.; Khan, A.; Rhim, J.W. Advances and prospects of carbon dots for microplastic analysis. Chemosphere 2023, 313, 137433. [Google Scholar] [CrossRef]
- Clere, I.K.; Ahmmed, F.; Peter III, J.G.; Fraser-Miller, S.J.; Gordon, K.C.; Komyakova, V.; Allan, B.J. Quantification and characterization of microplastics in commercial fish from southern New Zealand. Mar. Pollut. Bull. 2022, 184, 114121. [Google Scholar] [CrossRef]
- Di Fiore, C.; Sammartino, M.P.; Giannattasio, C.; Avino, P.; Visco, G. Microplastic contamination in commercial salt: An issue for their sampling and quantification. Food Chem. 2023, 404, 134682. [Google Scholar] [CrossRef] [PubMed]
- Mercy, F.T.; Alam, A.K.M.R.; Akbor, M.A. Abundance and characteristics of microplastics in major urban lakes of Dhaka, Bangladesh. Heliyon 2023, 9, e14587. [Google Scholar] [CrossRef]
- Kandeyaya, K.B.K.D.K.; Ranatunga, S.; Ranatunga, R.R.M.K.P. Occurrence of microplastics in some commercially important seafood varieties from Negombo, Sri Lanka. Reg. Stud. Mar. Sci. 2023, 62, 102958. [Google Scholar] [CrossRef]
- Altunışık, A. Prevalence of microplastics in commercially sold soft drinks and human risk assessment. J. Environ. Manag. 2023, 336, 117720. [Google Scholar] [CrossRef] [PubMed]
- Gniadek, M.; Dąbrowska, A. The marine nano- and microplastics characterisation by SEM-EDX: The potential of the method in comparison with various physical and chemical approaches. Mar. Pollut. Bull. 2019, 148, 210–216. [Google Scholar] [CrossRef]
- Cowger, W.; Gray, A.; Christiansen, S.H.; DeFrond, H.; Deshpande, A.D.; Hemabessiere, L.; Lee, E.; Mill, L.; Munno, K.; Ossmann, B.E.; et al. Critical Review of Processing and Classification Techniques for Images and Spectra in Microplastic Research. Appl. Spectrosc. 2020, 74, 989–1010. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.M.; Vijayaprabhakaran, K.; Devika, P.T. Baseline study on identification, characterization, distribution and abundance of microplastics in surface water from Ennore to Kovalam along the east coast of India. Phys. Chem. Earth 2023, 130, 103391. [Google Scholar] [CrossRef]
- Matluba, M.; Ahmed, M.K.; Chowdhury, K.M.A.; Khan, N.; Ashiq, M.A.R.; Islam, M.S. The pervasiveness of microplastic contamination in the gastrointestinal tract of fish from the western coast of Bangladesh. Mar. Pollut. Bull. 2023, 193, 115145. [Google Scholar] [CrossRef] [PubMed]
- Mahon, A.M.; Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Z.; Yang, L.; Gao, T.; Zhang, Y. A review of analytical methods and models used in atmospheric microplastic research. Sci. Total Environ. 2022, 828, 154487. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A.; Mielańczuk, M.; Syczewski, M. The Raman spectroscopy and SEM/EDS investigation of the primary sources of microplastics from cosmetics available in Poland. Chemosphere 2022, 308, 136407. [Google Scholar] [CrossRef]
- Hossain, M.B.; Banik, P.; Nur, A.A.U.; Rahman, T. Abundance and characteristics of microplastics in sediments from the world’s longest natural beach, Cox’s Bazar, Bangladesh. Mar. Pollut. Bull. 2021, 163, 111956. [Google Scholar] [CrossRef] [PubMed]
- Sierra, I.; Chialanza, M.R.; Faccio, R.; Carrizo, D.; Fornaro, L.; Pérez-Parada, A. Identification of microplastics in wastewater samples by means of polarized light optical microscopy. Environ. Sci. Pollut. Res. 2020, 27, 7409–7419. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S. Prevalence and physicochemical characteristics of microplastics in the sediment and water of Hashilan Wetland, a national heritage in NW Iran. Environ. Technol. Innov. 2021, 23, 101782. [Google Scholar] [CrossRef]
- Kotar, S.; McNeish, R.; Murphy-Hagan, C.; Renick, V.; Lee, C.F.T.; Steele, C.; Lusher, A.; Moore, C.; Minor, E.; Schroeder, J.; et al. Quantitative assessment of visual microscopy as a tool for microplastic research: Recommendations for improving methods and reporting. Chemosphere 2022, 308, 136449. [Google Scholar] [CrossRef]
- Mossotti, R.; Fontana, G.D.; Anceschi, A.; Gasparin, E.; Battistini, T. Preparation and analysis of standards containing microfilaments/microplastic with fibre shape. Chemosphere 2021, 270, 129410. [Google Scholar] [CrossRef]
- Kalaronis, D.; Ainali, N.M.; Evgenidou, E.; Kyzas, G.Z.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Microscopic techniques as means for the determination of microplastics and nanoplastics in the aquatic environment: A concise review. Green Anal. Chem. 2022, 3, 100036. [Google Scholar] [CrossRef]
- Rodríguez-Romeu, O.; Constenla, M.; Carrassón, M.; Campoy-Quiles, M.; Soler-Membrives, A. Are anthropogenic fibres a real problem for red mullets (Mullus barbatus) from the NW Mediterranean? Sci. Total Environ. 2020, 733, 139336. [Google Scholar] [CrossRef] [PubMed]
- Mak, C.W.; Tsang, Y.Y.; Leung, M.M.-L.; Fang, J.K.-H.; Chan, K.M. Microplastics from effluents of sewage treatment works and stormwater discharging into the Victoria Harbor, Hong Kong. Mar. Pollut. Bull. 2020, 157, 111181. [Google Scholar] [CrossRef]
- Samanta, P.; Dey, S.; Kundu, D.; Dutta, D.; Jambulkar, R.; Mishra, R.; Ghosh, A.R.; Kumar, S. An insight on sampling, identification, quantification and characteristics of microplastics in solid wastes. Trends Environ. Anal. Chem. 2022, 36, e00181. [Google Scholar] [CrossRef]
- Mbachu, O.; Jenkins, G.; Pratt, C.; Kaparaju, P. A New Contaminant Superhighway? A Review of Sources, Measurement Techniques and Fate of Atmospheric Microplastics. Water Air Soil Pollut. 2020, 231, 85. [Google Scholar] [CrossRef]
- Schür, C.; Rist, S.; Baun, A.; Mayer, P.; Hartmann, N.B.; Wagner, M. When Fluorescence Is not a Particle: The Tissue Translocation of Microplastics in Daphnia magna Seems an Artifact. Environ. Toxicol. Chem. 2019, 38, 1495–1503. [Google Scholar] [CrossRef]
- Payton, T.G.; Beckingham, B.A.; Dustan, P. Microplastic exposure to zooplankton at tidal fronts in Charleston Harbor, SC USA. Estuar. Coast. Shelf Sci. 2020, 232, 106510. [Google Scholar] [CrossRef]
- Tiwari, M.; Rathod, T.D.; Ajmal, P.Y.; Bhangare, R.C.; Sahu, S.K. Distribution and characterization of microplastics in beach sand from three different Indian coastal environments. Mar. Pollut. Bull. 2019, 140, 262–273. [Google Scholar] [CrossRef]
- Klein, M.; Fischer, E.K. Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci. Total Environ. 2019, 685, 96–103. [Google Scholar] [CrossRef]
- Lee, J.; Chae, K.J. A systematic protocol of microplastics analysis from their identification to quantification in water environment: A comprehensive review. J. Hazard. Mater. 2020, 403, 124049. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.N.V.; Löschel, L.A.; Imhof, H.K.; Löder, M.G.J.; Laforsch, C. Analysis of microplastics of a broad size range in commercially important mussels by combining FTIR and Raman spectroscopy approaches. Environ. Pollut. 2021, 269, 116147. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, W.Y.; Liao, Y.; Sun, R.; Hu, J.; Lu, Z.; Chang, M.; Yang, J.; Dai, Z.; Zhou, C.; et al. Separation of false-positive microplastics and analysis of microplastics via a two-phase system combined with confocal Raman spectroscopy. J. Hazard. Mater. 2022, 440, 129803. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Su, W.; Xu, D.; Wang, Z.; Wu, H.; Chen, B.; Wu, J. Component identification for the SERS spectra of microplastics mixture with convolutional neural network. Sci. Total Environ. 2023, 895, 165138. [Google Scholar] [CrossRef]
- Yang, C.; Niu, S.; Xia, Y.; Wu, J. Microplastics in urban road dust: Sampling, analysis, characterization, pollution level, and influencing factors. TrAC Trends Anal. Chem. 2023, 168, 117348. [Google Scholar] [CrossRef]
- Blevins, M.G. Field-Portable Dissolved Gas Sensing and Perspectives in Aqueous Microplastic Detection. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2021. [Google Scholar]
- Mazlan, N.; Shukhairi, S.S.; Husin, M.J.M.; Shalom, J.; Saud, S.N.; Sani, M.S.A.; Ong, M.C.; Mohan, N.K.N.C.; Sopian, N.A. Evaluation of microplastics isolated from sea cucumber Acaudina molpadioides in Pulau Langkawi, Malaysia. Heliyon 2023, 9, e16822. [Google Scholar] [CrossRef] [PubMed]
- Veerasingam, S.; Ranjani, M.; Venkatachalapathy, R.; Bagaev, A.; Mukhanov, V.; Litvinyuk, D.; Mugilarasan, M.; Gurumoorthi, M.; Guganathan, L.; Aboobacker, V.M.; et al. Contributions of Fourier transform infrared spectroscopy in microplastic pollution research: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2681–2743. [Google Scholar] [CrossRef]
- Teboul, E.; Orihel, D.M.; Provencher, J.F.; Drever, M.C.; Wilson, L.; Harrison, A.L. Chemical identification of microplastics ingested by Red Phalaropes (Phalaropus fulicarius) using Fourier Transform Infrared spectroscopy. Mar. Pollut. Bull. 2021, 171, 112640. [Google Scholar] [CrossRef]
- Simon, M.; Vianello, A.; Shashoua, Y.; Vollertsen, J. Accelerated weathering affects the chemical and physical properties of marine antifouling paint microplastics and their identification by ATR-FTIR spectroscopy. Chemosphere 2021, 274, 129749. [Google Scholar] [CrossRef] [PubMed]
- Basaran, B.; Özçifçi, Z.; Akcay, H.T.; Aytan, Ü. Microplastics in branded milk: Dietary exposure and risk assessment. J. Food Compos. Anal. 2023, 123, 105611. [Google Scholar] [CrossRef]
- Fadare, O.O.; Okoffo, E.D.; Olasehinde, E.F. Microparticles and microplastics contamination in African table salts. Mar. Pollut. Bull. 2021, 164, 112006. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, M.B.; Takashima, K.; Yamaguchi, S.; Tanaka, M.; Isobe, A. Microplastics on plankton samples: Multiple digestion techniques assessment based on weight, size, and FTIR spectroscopy analyses. Mar. Pollut. Bull. 2021, 173, 113027. [Google Scholar] [CrossRef]
- Stockin, K.A.; Pantos, O.; Betty, E.L.; Pawley, M.D.; Doake, F.; Masterton, H.; Palmer, E.I.; Perrott, M.R.; Nelms, S.E.; Machovsky-Capuska, G.E. Fourier transform infrared (FTIR) analysis identifies microplastics in stranded common dolphins (Delphinus delphis) from New Zealand waters. Mar. Pollut. Bull. 2021, 173, 113084. [Google Scholar] [CrossRef] [PubMed]
- Fadlelmoula, A.; Pinho, D.; Carvalho, V.H.; Catarino, S.O.; Minas, G. Fourier Transform Infrared (FTIR) Spectroscopy to Analyse Human Blood over the Last 20 Years: A Review towards Lab-on-a-Chip Devices. Micromachines 2022, 13, 187. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Li, Q.; Kolandhasamy, P.; Shi, H.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Dellisanti, W.; Leung, M.M.L.; Lam, K.W.K.; Wang, Y.; Hu, M.; Lo, H.S.; Fang, J.K.H. A short review on the recent method development for extraction and identification of microplastics in mussels and fish, two major groups of seafood. Mar. Pollut. Bull. 2023, 186, 114221. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Massarelli, C.; Uricchio, V.F. Fourier Transform Infrared Spectroscopy to Assess the Degree of Alteration of Artificially Aged and Environmentally Weathered Microplastics. Polymers 2023, 15, 911. [Google Scholar] [CrossRef]
- Sota-Uba, I.; Bamidele, M.; Moulton, J.; Booksh, K.; Lavine, B.K. Authentication of edible oils using Fourier transform infrared spectroscopy and pattern recognition methods. Chemom. Intell. Lab. Syst. 2021, 210, 104521. [Google Scholar] [CrossRef]
- Barbosa, F.; Adeyemi, J.A.; Bocato, M.Z.; Comas, A.; Campiglia, A. A critical viewpoint on current issues, limitations, and future research needs on micro- and nanoplastic studies: From the detection to the toxicological assessment. Environ. Res. 2020, 182, 109089. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in freshwater sediment: A review on methods, occurrence, and sources. Sci. Total Environ. 2021, 754, 141948. [Google Scholar] [CrossRef]
- Huda, F.R.; Richard, F.S.; Rahman, I.; Moradi, S.; Hua, C.T.Y.; Wanwen, C.A.S.; Fong, T.K.; Mujahid, A.; Müller, M. Comparison of learning models to predict LDPE, PET, and ABS concentrations in beach sediment based on spectral reflectance. Sci. Rep. 2023, 13, 6258. [Google Scholar] [CrossRef]
- Markic, A.; Bridson, J.H.; Morton, P.; Hersey, L.; Budiša, A.; Maes, T.; Bowen, M. Microplastic pollution in the intertidal and subtidal sediments of Vava’u, Tonga. Mar. Pollut. Bull. 2023, 186, 114451. [Google Scholar] [CrossRef] [PubMed]
- Asamoah, B.O.; Salmi, P.; Räty, J.; Ryymin, K.; Talvitie, J.; Karjalainen, A.K.; Kukkonen, J.V.K.; Roussey, M.; Peiponen, K.E. Optical monitoring of microplastics filtrated from wastewater sludge and suspended in ethanol. Polymers 2021, 13, 871. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Zhang, X.; Gibson, C.; Naidu, R.; Megharaj, M.; Fang, C. Identification and visualisation of microplastics/nanoplastics by Raman imaging (i): Down to 100 nm. Water Res. 2020, 174, 115658. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Marina-Montes, C.; Pérez-Arribas, L.V.; Anzano, J.; de Vallejuelo, S.F.O.; Aramendia, J.; Gómez-Nubla, L.; de Diego, A.; Madariaga, J.M.; Cáceres, J.O. Characterization of atmospheric aerosols in the Antarctic region using Raman Spectroscopy and Scanning Electron Microscopy. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2022, 266, 120452. [Google Scholar] [CrossRef]
- Matupang, D.M.; Zulkifli, H.I.; Arnold, J.; Lazim, A.M.; Ghaffar, M.A.; Musa, S.M. Tropical sharks feasting on and swimming through microplastics: First evidence from Malaysia. Mar. Pollut. Bull. 2023, 189, 114762. [Google Scholar] [CrossRef]
- Liu, M.; Mu, J.; Wang, M.; Hu, C.; Ji, J.; Wen, C.; Zhang, D. Impacts of polypropylene microplastics on lipid profiles of mouse liver uncovered by lipidomics analysis and Raman spectroscopy. J. Hazard. Mater. 2023, 458, 131918. [Google Scholar] [CrossRef] [PubMed]
- Mogha, N.K.; Shin, D. Nanoplastic detection with surface enhanced Raman spectroscopy: Present and future. TrAC—Trends Anal. Chem. 2022, 158, 116885. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, D.; Pei, J.; Fei, Y.; Ouyang, D.; Zhang, H.; Luo, Y. Identification and quantification of microplastics using Fourier-transform infrared spectroscopy: Current status and future prospects. Curr. Opin. Environ. Sci. Health 2020, 18, 14–19. [Google Scholar] [CrossRef]
- Luo, Y.; Su, W.; Rabbi, M.F.; Wan, Q.; Xu, D.; Wang, Z.; Liu, S.; Xu, X.; Wu, J. Quantitative analysis of microplastics in water environments based on Raman spectroscopy and convolutional neural network. Sci. Total Environ. 2024, 926, 171925. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Banik, S.; Biswas, R.; Yamamoto, T.; Noothalapati, H.; Mazumder, N. Raman spectroscopy for microplastic detection in water sources: A systematic review. Int. J. Environ. Sci. Technol. 2023, 20, 10435–10448. [Google Scholar] [CrossRef]
- Campanale, C.; Stock, F.; Massarelli, C.; Kochleus, C.; Bagnuolo, G.; Reifferscheid, G.; Uricchio, V.F. Microplastics and their possible sources: The example of Ofanto river in southeast Italy. Environ. Pollut. 2020, 258, 113284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, B.; Pileggi, V.; Chang, S. Methods to recover and characterize microplastics in wastewater treatment plants. Case Stud. Chem. Environ. Eng. 2022, 5, 100183. [Google Scholar] [CrossRef]
- Xue, Q.; Dong, Y.; Lu, F.; Yang, H.; Yu, G. ELM combined with differential Raman spectroscopy for the detection of microplastics in organisms. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2024, 312, 124039. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, Q.; Xing, X.; Chen, W.; She, Z.; Luo, Z. Raman spectra and surface changes of microplastics weathered under natural environments. Sci. Total Environ. 2020, 739, 139990. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Arakawa, H. A double sliding-window method for baseline correction and noise estimation for Raman spectra of microplastics. Mar. Pollut. Bull. 2023, 190, 114887. [Google Scholar] [CrossRef]
- Martí, E.; Martin, C.; Galli, M.; Echevarría, F.; Duarte, C.M.; Cózar, A. The Colors of the Ocean Plastics. Environ. Sci. Technol. 2020, 54, 6594–6601. [Google Scholar] [CrossRef]
- Van Tran, T.; Jalil, A.A.; Nguyen, T.M.; Nguyen, T.T.T.; Nabgan, W.; Nguyen, D.T.C. A review on the occurrence, analytical methods, and impact of microplastics in the environment. Environ. Toxicol. Pharmacol. 2023, 102, 104248. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, B.; Wang, H. Analytical methods for microplastics in the environment: A review. Environ. Chem. Lett. 2022, 21, 383–401. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; An, J.; Kwon, J.H. Sequential quantification of number and mass of microplastics in municipal wastewater using Fourier-transform infrared spectroscopy and pyrolysis gas chromatography-mass spectrometry. Environ. Pollut. 2023, 336, 122452. [Google Scholar] [CrossRef]
- Lou, F.; Wang, J.; Sun, C.; Song, J.; Wang, W.; Pan, Y.; Huang, Q.; Yan, J. Influence of interaction on accuracy of quantification of mixed microplastics using Py-GC/MS. J. Environ. Chem. Eng. 2022, 10, 108012. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, D.; Feng, H.; Li, Y.; Zhang, S.; Peng, C.; Wang, Y.; Sun, H.; Wang, L. Mass spectrometry detection of environmental microplastics: Advances and challenges. TrAC Trends Anal. Chem. 2024, 170, 117472. [Google Scholar] [CrossRef]
- Albignac, M.; Ghiglione, J.F.; Labrune, C.; Halle, A.T. Determination of the microplastic content in Mediterranean benthic macrofauna by pyrolysis-gas chromatography-tandem mass spectrometry. Mar. Pollut. Bull. 2022, 181, 113882. [Google Scholar] [CrossRef] [PubMed]
- Okoffo, E.D.; Ribeiro, F.; O’brien, J.W.; O’brien, S.; Tscharke, B.J.; Gallen, M.; Samanipour, S.; Mueller, J.F.; Thomas, K.V. Identification and quantification of selected plastics in biosolids by pressurized liquid extraction combined with double-shot pyrolysis gas chromatography–mass spectrometry. Sci. Total Environ. 2020, 715, 136924. [Google Scholar] [CrossRef]
- Wenzel, M.; Schoettl, J.; Pruin, L.; Fischer, B.; Wolf, C.; Kube, C.; Renner, G.; Schram, J.; Schmidt, T.C.; Tuerk, J. Determination of atmospherically deposited microplastics in moss: Method development and performance evaluation. Green Anal. Chem. 2023, 7, 100078. [Google Scholar] [CrossRef]
- Sorolla-Rosario, D.; Llorca-Porcel, J.; Pérez-Martínez, M.; Lozano-Castelló, D.; Bueno-López, A. Microplastics’ analysis in water: Easy handling of samples by a new Thermal Extraction Desorption-Gas Chromatography-Mass Spectrometry (TED-GC/MS) methodology. Talanta 2023, 253, 123829. [Google Scholar] [CrossRef]
- Caldwell, J.; Taladriz-Blanco, P.; Lehner, R.; Lubskyy, A.; Ortuso, R.D.; Rothen-Rutishauser, B.; Petri-Fink, A. The micro-, submicron-, and nanoplastic hunt: A review of detection methods for plastic particles. Chemosphere 2022, 293, 133514. [Google Scholar] [CrossRef]
- Yang, L.; Kang, S.; Luo, X.; Wang, Z. Microplastics in drinking water: A review on methods, occurrence, sources, and potential risks assessment. Environ. Pollut. 2024, 348, 123857. [Google Scholar] [CrossRef]
- Goedecke, C.; Dittmann, D.; Eisentraut, P.; Wiesner, Y.; Schartel, B.; Klack, P.; Braun, U. Evaluation of thermoanalytical methods equipped with evolved gas analysis for the detection of microplastic in environmental samples. J. Anal. Appl. Pyrolysis 2020, 152, 104961. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Takeda, H.; Kinoshita, K.; Takeuchi, M.; Takayanagi, T.; Teramae, N.; Pipkin, W.; Matsui, K.; Watanabe, A.; Watanabe, C. Direct analysis of airborne microplastics collected on quartz filters by pyrolysis-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2023, 171, 105946. [Google Scholar] [CrossRef]
- da Costa, J.P.; Reis, V.; Paço, A.; Costa, M.; Duarte, A.C.; Rocha-Santos, T. Micro(nano)plastics—Analytical challenges towards risk evaluation. TrAC Trends Anal. Chem. 2019, 111, 173–184. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Kintzi, A.; Muñoz, K.; Schaumann, G.E. A simple method for the selective quantification of polyethylene, polypropylene, and polystyrene plastic debris in soil by pyrolysis-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2020, 147, 104803. [Google Scholar] [CrossRef]
- Cai, H.; Xu, E.G.; Du, F.; Li, R.; Liu, J.; Shi, H. Analysis of environmental nanoplastics: Progress and challenges. Chem. Eng. J. 2021, 410, 128208. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Pyrolysis gas chromatography-mass spectrometry in environmental analysis: Focus on organic matter and microplastics. TrAC Trends Anal. Chem. 2020, 130, 115964. [Google Scholar] [CrossRef]
- Xu, Y.; Ou, Q.; Wang, X.; Hou, F.; Li, P.; van der Hoek, J.P.; Liu, G. Assessing the Mass Concentration of Microplastics and Nanoplastics in Wastewater Treatment Plants by Pyrolysis Gas Chromatography-Mass Spectrometry. Environ. Sci. Technol. 2023, 57, 3114–3123. [Google Scholar] [CrossRef] [PubMed]
- Funck, M.; Yildirim, A.; Nickel, C.; Schram, J.; Schmidt, T.C.; Tuerk, J. Identification of microplastics in wastewater after cascade filtration using Pyrolysis-GC–MS. MethodsX 2020, 7, 100778. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Okoffo, E.D.; O’Brien, J.W.; Fraissinet-Tachet, S.; O’Brien, S.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Mueller, J.F.; Galloway, T.; et al. Quantitative Analysis of Selected Plastics in High-Commercial-Value Australian Seafood by Pyrolysis Gas Chromatography Mass Spectrometry. Environ. Sci. Technol. 2020, 54, 9408–9417. [Google Scholar] [CrossRef]
- Vagner, M.; Boudry, G.; Courcot, L.; Vincent, D.; Dehaut, A.; Duflos, G.; Huvet, A.; Tallec, K.; Zambonino-Infante, J.L. Experimental evidence that polystyrene nanoplastics cross the intestinal barrier of European seabass. Environ. Int. 2022, 166, 107340. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, Y.; He, S.; Chi, H.Y.; Li, Z.C.; Zhou, X.X.; Yan, B. Quantification of Nanoplastic Uptake in Cucumber Plants by Pyrolysis Gas Chromatography/Mass Spectrometry. Environ. Sci. Technol. Lett. 2021, 8, 633–638. [Google Scholar] [CrossRef]
- Zhou, X.X.; He, S.; Gao, Y.; Li, Z.C.; Chi, H.Y.; Li, C.J.; Wang, D.J.; Yan, B. Protein Corona-Mediated Extraction for Quantitative Analysis of Nanoplastics in Environmental Waters by Pyrolysis Gas Chromatography/Mass Spectrometry. Anal. Chem. 2021, 93, 6698–6705. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Bai, Q.; Sheng, X.; Li, P.; Zheng, R.; Yu, S.; Liu, J. Influence of particle characteristics, heating temperature and time on the pyrolysis product distributions of polystyrene micro- and nano-plastics. J. Chromatogr. A 2022, 1682, 463503. [Google Scholar] [CrossRef]
- Bouzid, N.; Anquetil, C.; Dris, R.; Gasperi, J.; Tassin, B.; Derenne, S. Quantification of Microplastics by Pyrolysis Coupled with Gas Chromatography and Mass Spectrometry in Sediments: Challenges and Implications. Microplastics 2022, 1, 229–239. [Google Scholar] [CrossRef]
- Wahl, A.; Le Juge, C.; Davranche, M.; El Hadri, H.; Grassl, B.; Reynaud, S.; Gigault, J. Nanoplastic occurrence in a soil amended with plastic debris. Chemosphere 2021, 262, 127784. [Google Scholar] [CrossRef] [PubMed]
- Dessì, C.; Dessì, C.; Okoffo, E.D.; O’Brien, J.W.; Gallen, M.; Samanipour, S.; Kaserzon, S.; Rauert, C.; Wang, X.; Thomas, K.V. Plastics contamination of store-bought rice. J. Hazard. Mater. 2021, 416, 125778. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Ainali, N.M.; Kalaronis, D.; Kontogiannis, A.; Evgenidou, E.; Kyzas, G.Z.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Microplastics in the environment: Sampling, pretreatment, analysis and occurrence based on current and newly-exploited chromatographic approaches. Sci. Total Environ. 2021, 794, 148725. [Google Scholar] [CrossRef] [PubMed]
- Alprol, A.E.; Gaballah, M.S.; Hassaan, M.A. Micro and Nanoplastics analysis: Focus on their classification, sources, and impacts in marine environment. Reg. Stud. Mar. Sci. 2021, 42, 101625. [Google Scholar] [CrossRef]
- Ishimura, T.; Iwai, I.; Matsui, K.; Mattonai, M.; Watanabe, A.; Robberson, W.; Cook, A.M.; Allen, H.L.; Pipkin, W.; Teramae, N.; et al. Qualitative and quantitative analysis of mixtures of microplastics in the presence of calcium carbonate by pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2021, 157, 105188. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Insa, S.; Arxé, M.; Buttiglieri, G.; Rodríguez-Mozaz, S.; Barceló, D. Analysis of microplastics in the environment: Identification and quantification of trace levels of common types of plastic polymers using pyrolysis-GC/MS. MethodsX 2023, 10, 102143. [Google Scholar] [CrossRef] [PubMed]
- Matsueda, M.; Mattonai, M.; Iwai, I.; Watanabe, A.; Teramae, N.; Robberson, W.; Ohtani, H.; Kim, Y.M.; Watanabe, C. Preparation and test of a reference mixture of eleven polymers with deactivated inorganic diluent for microplastics analysis by pyrolysis-GC–MS. J. Anal. Appl. Pyrolysis 2021, 154, 104993. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A. Advances in microplastics detection: A comprehensive review of methodologies and their effectiveness. TrAC Trends Anal. Chem. 2023, 170, 117440. [Google Scholar] [CrossRef]
- Dümichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.K.; Senz, R.; Braun, U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Eisentraut, P.; Dümichen, E.; Ruhl, A.S.; Jekel, M.; Albrecht, M.; Gehde, M.; Braun, U. Two Birds with One Stone—Fast and Simultaneous Analysis of Microplastics: Microparticles Derived from Thermoplastics and Tire Wear. Environ. Sci. Technol. Lett. 2018, 5, 608–613. [Google Scholar] [CrossRef]
- Becker, R.; Altmann, K.; Sommerfeld, T.; Braun, U. Quantification of microplastics in a freshwater suspended organic matter using different thermoanalytical methods—outcome of an interlaboratory comparison. J. Anal. Appl. Pyrolysis 2020, 148, 104829. [Google Scholar] [CrossRef]
- Goedecke, C.; Eisentraut, P.; Altmann, K.; Elert, A.M.; Bannick, C.G.; Ricking, M.; Obermaier, N.; Barthel, A.K.; Schmitt, T.; Jekel, M.; et al. Development of a Routine Screening Method for the Microplastic Mass Content in a Wastewater Treatment Plant Effluent. Front. Environ. Chem. 2022, 3, 844633. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Shao, Y.; Ray, S.S.; Wang, B.; Zhao, Z.; Yu, B.; Zhang, X.; Li, W.; Ding, J.; et al. A review on the occurrence, detection methods, and ecotoxicity of biodegradable microplastics in the aquatic environment: New cause for concern. TrAC Trends Anal. Chem. 2024, 178, 117832. [Google Scholar] [CrossRef]
- Shruti, V.C.; Kutralam-Muniasamy, G. Migration testing of microplastics in plastic food-contact materials: Release, characterization, pollution level, and influencing factors. TrAC Trends Anal. Chem. 2024, 170, 117421. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Trzebiatowska, P.J.; Knez, E.; Zaleska-Medynska, A.; Grembecka, M. Microplastics in food—a critical approach to definition, sample preparation, and characterisation. Food Chem. 2023, 418, 135985. [Google Scholar] [CrossRef] [PubMed]
- Mansa, R.; Zou, S. Thermogravimetric analysis of microplastics: A mini review. Environ. Adv. 2021, 5, 100117. [Google Scholar] [CrossRef]
- Fang, C.; Sobhani, Z.; Zhang, D.; Zhang, X.; Gibson, C.T.; Tang, Y.; Luo, Y.; Megharaj, M.; Naidu, R. Capture and characterisation of microplastics printed on paper via laser printer’s toners. Chemosphere 2021, 281, 130864. [Google Scholar] [CrossRef]
- Parida, D.; Sangtani, R.; Bala, K. Microplastics: The stemming environmental challenge and the quest for the missing mitigation strategies. Int. Biodeterior. Biodegrad. 2023, 179, 105581. [Google Scholar] [CrossRef]
- Meyers, N.; Catarino, A.I.; Declercq, A.M.; Brenan, A.; Devriese, L.; Vandegehuchte, M.; De Witte, B.; Janssen, C.; Everaert, G. Microplastic detection and identification by Nile red staining: Towards a semi-automated, cost- and time-effective technique. Sci. Total Environ. 2022, 823, 153441. [Google Scholar] [CrossRef] [PubMed]
- Giardino, M.; Balestra, V.; Janner, D.; Bellopede, R. Automated method for routine microplastic detection and quantification. Sci. Total Environ. 2022, 859, 160036. [Google Scholar] [CrossRef]
- Mansouri, S. Recent developments of (bio)-sensors for detection of main microbiological and non-biological pollutants in plastic bottled water samples: A critical review. Talanta 2024, 274, 125962. [Google Scholar] [CrossRef] [PubMed]
- Presti, D.L.; Zaltieri, M.; Bravi, M.; Morrone, M.; Caponero, M.A.; Schena, E.; Sterzi, S.; Massaroni, C. A Wearable System Composed of FBG-Based Soft Sensors for Trunk Compensatory Movements Detection in Post-Stroke Hemiplegic Patients. Sensors 2022, 22, 1386. [Google Scholar] [CrossRef] [PubMed]
- Parolo, C.; Sena-Torralba, A.; Bergua, J.F.; Calucho, E.; Fuentes-Chust, C.; Hu, L.; Rivas, L.; Álvarez-Diduk, R.; Nguyen, E.P.; Cinti, S.; et al. Tutorial: Design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 2020, 15, 3788–3816. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F. Perspective—An Age of Sensors. ECS Sens. Plus 2022, 1, 011601. [Google Scholar] [CrossRef]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- del Valle, M. Sensors as green tools in analytical chemistry. Curr. Opin. Green Sustain. Chem. 2021, 31, 100501. [Google Scholar] [CrossRef]
- Sumitha, M.S.; Xavier, T.S. Recent advances in electrochemical biosensors—A brief review. Hybrid Adv. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Chadha, U.; Bhardwaj, P.; Agarwal, R.; Rawat, P.; Agarwal, R.; Gupta, I.; Panjwani, M.; Singh, S.; Ahuja, C.; Selvaraj, S.K.; et al. Recent progress and growth in biosensors technology: A critical review. J. Ind. Eng. Chem. 2022, 109, 21–51. [Google Scholar] [CrossRef]
- Singh, S.; Wang, J.; Cinti, S. Review—An Overview on Recent Progress in Screen-Printed Electroanalytical (Bio)Sensors. ECS Sens. Plus 2022, 1, 023401. [Google Scholar] [CrossRef]
- Tang, Y.; Hardy, T.J.; Yoon, J.Y. Receptor-based detection of microplastics and nanoplastics: Current and future. Biosens. Bioelectron. 2023, 234, 115361. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, J.F.; Rojas, D.; Escarpa, A. Electrochemical Sensing Directions for Next-Generation Healthcare: Trends, Challenges, and Frontiers. Anal. Chem. 2020, 93, 167–183. [Google Scholar] [CrossRef]
- Ganesh, P.S.; Kim, S.Y. Electrochemical sensing interfaces based on novel 2D-MXenes for monitoring environmental hazardous toxic compounds: A concise review. J. Ind. Eng. Chem. 2022, 109, 52–67. [Google Scholar] [CrossRef]
- Popenda, A.; Wiśniowska, E.; Manuel, C. Biosensors in environmental analysis of microplastics and heavy metal compounds—A review on current status and challenges. Desalin. Water Treat. 2024, 319, 100456. [Google Scholar] [CrossRef]
- Chalyan, T.; Ottevaere, H.; Pasquardini, L. Optical biosensors: From working principles to detection methods of label-free devices. In Biophotonics and Biosensing; Elsevier: Amsterdam, The Netherlands, 2024; pp. 15–48. [Google Scholar]
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.S.; Singh, R.; Kumar, S.; Jha, R.; Nedoma, J.; Martinek, R.; Marques, C. The role of smart optical biosensors and devices on predictive analytics for the future of aquaculture systems. Opt. Laser Technol. 2024, 177, 111049. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Zeng, Z.; Zeng, G.; Xiao, R.; Wang, Y.; Hu, Y.; Tang, L.; Feng, C. Sensors for the environmental pollutant detection: Are we already there? Coord. Chem. Rev. 2020, 431, 213681. [Google Scholar] [CrossRef]
- Singh, S.; Podder, P.S.; Russo, M.; Henry, C.; Cinti, S. Tailored point-of-care biosensors for liquid biopsy in the field of oncology. Lab Chip 2022, 23, 44–61. [Google Scholar] [CrossRef]
- Hasan, M.; Sakib, M.N.; Sabroj, R.B.; Rahman, M.Z. Advanced nanomaterial-based biosensors for clinical diagnosis, environmental protection and industrial fermentation. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2024; pp. 30–47. [Google Scholar]
- Ganesh, P.S.; Elugoke, S.E.; Lee, S.H.; Kim, S.Y.; Ebenso, E.E. Smart and emerging point of care electrochemical sensors based on nanomaterials for SARS-CoV-2 virus detection: Towards designing a future rapid diagnostic tool. Chemosphere 2024, 352, 141269. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Durairaj, S.; Prins, S.; Chen, A. Nanomaterial-based electrochemical sensors and biosensors for the detection of pharmaceutical compounds. Biosens. Bioelectron. 2021, 175, 112836. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Exploring the potential of ionic liquid-based electrochemical biosensors for real-time biomolecule monitoring in pharmaceutical applications: From lab to life. Results Eng. 2023, 20, 101533. [Google Scholar] [CrossRef]
- Erdem, A.; Yildiz, E.; Senturk, H.; Maral, M. Implementation of 3D printing technologies to electrochemical and optical biosensors developed for biomedical and pharmaceutical analysis. J. Pharm. Biomed. Anal. 2023, 230, 115385. [Google Scholar] [CrossRef] [PubMed]
- Shalileh, F.; Sabahi, H.; Golbashy, M.; Dadmehr, M.; Hosseini, M. Recent developments in DNA nanostructure-based biosensors for the detection of melamine adulteration in milk. Microchem. J. 2023, 195, 109316. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Eltzov, E. Enhancing food safety: A low-cost biosensor for Bacillus licheniformis detection in food products. Talanta 2024, 276, 126152. [Google Scholar] [CrossRef] [PubMed]
- Hjort, R.G.; Pola, C.C.; Soares, R.R.; Oliveira, D.A.; Stromberg, L.; Claussen, J.C.; Gomes, C.L. Advances in Biosensors for Detection of Foodborne Microorganisms, Toxins, and Chemical Contaminants. In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2024; pp. 372–384. [Google Scholar]
- Chakraborty, A.; Bardhan, S.; Das, S.; Chowdhury, B.R. Development of biosensors for application in industrial biotechnology. In Metagenomics to Bioremediation; Elsevier: Amsterdam, The Netherlands, 2023; pp. 737–753. [Google Scholar]
- Kukrety, S.; Godbole, V.; Bisht, M.; Pal, M.K. Application of biosensors in agriculture and food industry. In Biosensors for Foodborne Pathogens Detection; Elsevier: Amsterdam, The Netherlands, 2024; pp. 265–276. [Google Scholar]
- Raucci, A.; Miglione, A.; Lenzi, L.; Fabbri, P.; Di Tocco, J.; Massaroni, C.; Presti, D.L.; Schena, E.; Pifferi, V.; Falciola, L.; et al. Characterization and application of porous PHBV-based bacterial polymers to realize novel bio-based electroanalytical (bio)sensors. Sens. Actuators B Chem. 2022, 379, 133178. [Google Scholar] [CrossRef]
- Laad, M.; Ghule, B. Removal of toxic contaminants from drinking water using biosensors: A systematic review. Groundw. Sustain. Dev. 2023, 20, 100888. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Chopra, C.; Singh, R.; Hong, J.C.; Kadam, U.S. CRISPR/Cas12a-based biosensors for environmental monitoring and diagnostics. Environ. Technol. Innov. 2024, 34, 103625. [Google Scholar] [CrossRef]
- Mahendran, G.; Savitha, T.; Khalifa, A.Y.Z.; Sharma, A.; Sankaranarayanan, A. Evaluation of environment by microbial sensors. In Bioprospecting of Microbial Diversity; Elsevier: Amsterdam, The Netherlands, 2022; pp. 407–424. [Google Scholar]
- Pollard, M.; Hunsicker, E.; Platt, M. A tunable three-dimensional printed microfluidic resistive pulse sensor for the characterization of algae and microplastics. ACS Sens. 2020, 5, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Colson, B.C.; Michel, A.P. Flow-through quantification of microplastics using impedance spectroscopy. ACS Sens. 2021, 6, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.C.; Midón, J.; Vidal, A.B.; Ciomaga, C.; Laborda, F. Detection, quantification, and characterization of polystyrene microplastics and adsorbed bisphenol A contaminant using electroanalytical techniques. Microchim. Acta 2023, 190, 203. [Google Scholar] [CrossRef]
- Zhao, J.; Ruan, Y.; Zheng, Z.; Li, Y.; Sohail, M.; Hu, F.; Ling, J.; Zhang, L. Gold nanoparticles-anchored peptides enable precise colorimetric estimation of microplastics. iScience 2023, 26, 106823. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Zhang, H.; Zhang, C.; Chen, Y.; Li, C.; Hu, Y.; Yu, X.; Zhang, B.; Lin, X. A Nanozymatic-Mediated Smartphone Colorimetric Sensing Platform for the Detection of Dimethyl Phthalate (DMP) and Dibutyl Phthalate (DBP). Biosensors 2023, 13, 919. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, F.; Xia, C.; You, Q.; Chen, X.; Li, Y.; Lin, W.; Guo, L.; Fu, F. Copper nanoparticles incorporated nitrogen-rich carbon nitride as laccase-like nanozyme for colorimetric detection of bisphenol a released from microplastics. Microchem. J. 2023, 190, 108682. [Google Scholar] [CrossRef]
- Seggio, M.; Arcadio, F.; Cennamo, N.; Zeni, L.; Bossi, A.M. A plasmonic gold nano-surface functionalized with the estrogen receptor for fast and highly sensitive detection of nanoplastics. Talanta 2024, 267, 125211. [Google Scholar] [CrossRef]
- Wei, X.F.; Bohlén, M.; Lindblad, C.; Hedenqvist, M.; Hakonen, A. Microplastics Generated from a Biodegradable Plastic in Freshwater and Seawater. Water Res. 2021, 198, 117123. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; He, L.; Jiang, S.; Chen, J.; Zhou, C.; Qu, J.; Lu, Y.; Hong, P.; Sun, S.; Li, C. In Situ Surface-Enhanced Raman Spectroscopy for Detecting Microplastics and Nanoplastics in Aquatic Environments. Sci. Total Environ. 2020, 728, 138449. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.X.; Liu, R.; Hao, L.T.; Liu, J.F. Identification of Polystyrene Nanoplastics Using Surface Enhanced Raman Spectroscopy. Talanta 2021, 221, 121552. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, K.; Wang, W.; Wei, Y.; Lai, L. Quantitative and Sensitive Analysis of Polystyrene Nanoplastics down to 50 nm by Surface-Enhanced Raman Spectroscopy in Water. J. Hazard. Mater. 2022, 429, 128388. [Google Scholar] [CrossRef] [PubMed]
- Kihara, S.; Chan, A.; In, E.; Taleb, N.; Tollemache, C.; Yick, S.; McGillivray, D.J. Detecting Polystyrene Nanoplastics Using Filter Paper-Based Surface-Enhanced Raman Spectroscopy. RSC Adv. 2022, 12, 20519–20522. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Su, W.; Lu, H.; Luo, Y.; Yi, T.; Wu, J.; Wu, H.; Yin, C.; Chen, B. Gold Nanoparticle Doped Flexible Substrate for Microplastics SERS Detection. Phys. Chem. Chem. Phys. 2022, 24, 12036–12042. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Kim, D.; Kwon, G.; Lee, K.; Oh, C.S.; Kim, U.J.; You, J. Detection of Nanoplastics Based on Surface-Enhanced Raman Scattering with Silver Nanowire Arrays on Regenerated Cellulose Films. Carbohydr. Polym. 2021, 272, 118470. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, S.; Su, J.; Li, S.; Lv, X.; Chen, J.; Lai, Y.; Zhan, J. Identification of Trace Polystyrene Nanoplastics Down to 50 Nm by the Hyphenated Method of Filtration and Surface-Enhanced Raman Spectroscopy Based on Silver Nanowire Membranes. Environ. Sci. Technol. 2022, 56, 10818–10828. [Google Scholar] [CrossRef]
- Yin, R.; Ge, H.; Chen, H.; Du, J.; Sun, Z.; Tan, H.; Wang, S. Sensitive and Rapid Detection of Trace Microplastics Concentrated through Au-Nanoparticle-Decorated Sponge on the Basis of Surface-Enhanced Raman Spectroscopy. Environ. Adv. 2021, 5, 100096. [Google Scholar] [CrossRef]
- Mao, H.; Liu, M.; Cao, Z.; Ji, C.; Sun, Y.; Liu, D.; Wu, S.; Zhang, Y.; Song, X.M. Poly(4-Vinylphenylboronic Acid) Functionalized Polypyrrole/Graphene Oxide Nanosheets for Simultaneous Electrochemical Determination of Catechol and Hydroquinone. Appl. Surf. Sci. 2017, 420, 594–605. [Google Scholar] [CrossRef]
- Yin, D.; Liu, J.; Bo, X.; Guo, L. Cobalt-Iron Selenides Embedded in Porous Carbon Nanofibers for Simultaneous Electrochemical Detection of Trace of Hydroquinone, Catechol and Resorcinol. Anal. Chim. Acta 2020, 1093, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Jebril, S.; Garciıa-Moreno, M.D.V.; PalaciosSantander, J.M.; Dridi, C.; Cubillana-Aguilera, L. Development of a Cost-Effective and Sustainable Nanoplatform Based on a Green Gold Sononanoparticles/ Carbon Black Nanocomposite for High-Performance Simultaneous Determination of Nanoplastics. Environ. Sci. Nano 2022, 9, 3126–3138. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Wu, Z.; Wen, W.; Zhang, X.; Wang, S. Electrochemical Sensor Based on Confined Synthesis of Gold Nanoparticles @ Covalent Organic Frameworks for the Detection of Bisphenol A. Anal. Chim. Acta 2023, 1239, 340743. [Google Scholar] [CrossRef] [PubMed]
- Naik, T.S.K.; Singh, S.; Pavithra, N.; Varshney, R.; Uppara, B.; Singh, J.; Khan, N.A.; Singh, L.; Arshad, M.Z.; Ramamurthy, P.C. Advanced Experimental Techniques for the Sensitive Detection of a Toxic Bisphenol A Using UiO-66-NDC/GO-Based Electrochemical Sensor. Chemosphere 2022, 311, 137104. [Google Scholar] [CrossRef]
- Radha, A.; Wang, S.-F. Designing Hybrid Lanthanum Stannate/Functionalized Halloysite Nanotubes as Electrode Material for Electrochemical Detection of 4- (Methylamino)Phenol (Metol) in Environmental Samples. ACS Sustain. Chem. Eng. 2023, 11, 5072–5081. [Google Scholar] [CrossRef]
- Goulart, L.A.; Gonçalves, R.; Correa, A.A.; Pereira, E.C.; Mascaro, L.H. Synergic Effect of Silver Nanoparticles and Carbon Nanotubes on the Simultaneous Voltammetric Determination of Hydroquinone, Catechol, Bisphenol A and Phenol. Microchim. Acta 2018, 185, 12. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G.; Raril, C.; Tighezza, A.M.; Albaqami, M.D.; Sillanpää, M. Electrochemically Polymerized Glutamine-Activated Graphite Paste Surface as a Green Biosensor for Sensitive Catechol Detection in Water Samples. J. Mater. Sci. Mater. Electron. 2023, 34, 533. [Google Scholar] [CrossRef]
- Veerapandi, G.; Govindan, R.; Sekar, C. Quick and Accurate Determination of Hazardous Phenolic Compounds Using CaCu2O3 Nanorods Based Electrochemical Sensor. Chemosphere 2022, 313, 137370. [Google Scholar] [CrossRef]
- Manjunatha, J.G.A. Surfactant Enhanced Graphene Paste Electrode as an Effective Electrochemical Sensor for the Sensitive and Simultaneous Determination of Catechol and Resorcinol. Chem. Data Collect. 2019, 25, 100331. [Google Scholar] [CrossRef]
- Uchida, A.; Kitayama, Y.; Takano, E.; Ooya, T.; Takeuchi, T. Supraparticles comprised of molecularly imprinted nanoparticles and modified gold nanoparticles as a nanosensor platform. RSC Adv. 2013, 3, 25306–25311. [Google Scholar] [CrossRef]
- Shaikh, H.; Sener, G.; Memon, N.; Bhanger, M.I.; Nizamani, S.M.; Üzek, R.; Denizli, A. Molecularly imprinted surface plasmon resonance (SPR) based sensing of bisphenol A for its selective detection in aqueous systems. Anal. Methods 2015, 7, 4661–4670. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, R.; Liao, S.; Miao, Y.; Zhang, B.; Wang, F.; Yang, H. In situ reduced silver nanoparticles embedded molecularly imprinted reusable sensor for selective and sensitive SERS detection of Bisphenol A. Appl. Surf. Sci. 2018, 457, 323–331. [Google Scholar] [CrossRef]
- Pan, R.; Hu, K.; Jiang, D.; Samuni, U.; Mirkin, M.V. Electrochemical resistive-pulse sensing. J. Am. Chem. Soc. 2019, 141, 19555–19559. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.; Maugi, R.; Platt, M. Multi-resistive pulse sensor microfluidic device. Analyst 2022, 147, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Blevins, M.G.; Allen, H.L.; Colson, B.C.; Cook, A.M.; Greenbaum, A.Z.; Hemami, S.S.; Hollmann, J.; Kim, E.; LaRocca, A.A.; Markoski, K.A.; et al. Field-portable microplastic sensing in aqueous environments: A perspective on emerging techniques. Sensors 2021, 21, 3532. [Google Scholar] [CrossRef]
- Shimizu, K.; Sokolov, S.V.; Kätelhön, E.; Holter, J.; Young, N.P.; Compton, R.G. In situ Detection of Microplastics: Single Microparticle-electrode Impacts. Electroanalysis 2017, 29, 2200–2207. [Google Scholar] [CrossRef]
- Davies, C.D.; Crooks, R.M. Focusing, sorting, and separating microplastics by serial faradaic ion concentration polarization. Chem. Sci. 2020, 11, 5547–5558. [Google Scholar] [CrossRef]
- Wang, S.; Xu, M.; Jin, B.; Wünsch, U.J.; Su, Y.; Zhang, Y. Electrochemical and microbiological response of exoelectrogenic biofilm to polyethylene microplastics in water. Water Res. 2022, 211, 118046. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Vasudevan, N. Detection of phthalate esters in PET bottled drinks and lake water using esterase/PANI/CNT/CuNP based electrochemical biosensor. Anal. Chim. Acta 2020, 1135, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Gongi, W.; Touzi, H.; Sadly, I.; Ben Ouada, H.; Tamarin, O.; Ben ouada, H. A Novel Impedimetric Sensor Based on Cyanobacterial Extracellular Polymeric Substances for Microplastics Detection. J. Polym. Environ. 2022, 30, 4738–4748. [Google Scholar] [CrossRef]
- Baumgarten, L.G.; Freitas, A.A.; Santana, E.R.; Winiarski, J.P.; Dreyer, J.P.; Vieira, I.C. Graphene and gold nanoparticle-based bionanocomposite for the voltammetric determination of bisphenol A in (micro)plastics. Chemosphere 2023, 334, 139016. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Sun, H.; Zhang, Z.; Qian, Y.; Zhu, X.; Qu, J. A sensitive biosensor based on carbon nanohorn/rhodamine B for toxicity detection of polystyrene microplastics and typical pollutants. Microchem. J. 2023, 193, 109036. [Google Scholar] [CrossRef]
- Shan, Y.; Han, Y.; Yao, X.; Liu, T.; Liu, Y.; Chu, Z.; Jin, W. A novel Prussian blue/PANI nanostructure-based biosensor for ultrasensitive determination of trace hydroquinone. Sens. Actuators B Chem. 2023, 393, 134137. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Wu, Y.; Huang, B.; Xu, L.; Yang, J.; Liang, B.; Han, L. Construction of bacterial laccase displayed on the microbial surface for ultrasensitive biosensing of phenolic pollutants with nanohybrids-enhanced performance. J. Hazard. Mater. 2023, 452, 131265. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, X.; Sun, Y.; Chen, B.; Hu, F.; Guo, C.; Yang, T. Recent Advances in Colorimetric Sensors Based on Gold Nanoparticles for Pathogen Detection. Biosensors 2023, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Parmigiani, M.; Albini, B.; Pellegrini, G.; Genovesi, M.; De Vita, L.; Pallavicini, P.; Dacarro, G.; Galinetto, P.; Taglietti, A. Surface-Enhanced Raman Spectroscopy Chips Based on Silver Coated Gold Nanostars. Nanomaterials 2022, 12, 3609. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Gold and Silver Nanoparticle-Based Colorimetric Sensors: New Trends and Applications. Chemosensors 2021, 9, 305. [Google Scholar] [CrossRef]
- Hong, J.; Lee, B.; Park, C.; Kim, Y. A Colorimetric Detection of Polystyrene Nanoplastics with Gold Nanoparticles in the Aqueous. Phase. Sci. Total Environ. 2022, 850, 158058. [Google Scholar] [CrossRef]
- Behera, A.; Mahapatra, S.R.; Majhi, S.; Misra, N.; Sharma, R.; Singh, J.; Singh, R.P.; Pandey, S.S.; Singh, K.R.; Kerry, R.G. Gold nanoparticle assisted colorimetric biosensors for rapid polyethylene terephthalate (PET) sensing for sustainable environment to monitor microplastics. Environ. Res. 2023, 234, 116556. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Mahdi, M.A. Design and Optimization of Surface Plasmon Resonance Spectroscopy for Optical Constant Characterization and Potential Sensing Application: Theoretical and Experimental Approaches. Photonics 2021, 8, 361. [Google Scholar] [CrossRef]
- Estevez, M.-C.; Otte, M.A.; Sepulveda, B.; Lechuga, L.M. Trends and challenges of refractometric nanoplasmonic biosensors: A review. Anal. Chim. Acta 2014, 806, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Tuoriniemi, J.; Moreira, B.; Safina, G. Determining Number Concentrations and Diameters of Polystyrene Particles by Measuring the Effective Refractive Index of Colloids Using Surface Plasmon Resonance. Langmuir 2016, 32, 10632–10640. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Narasimha, G.V.; Chen, Y.C.; Chen, J.K.; Dong, G.C. Measurement of Low Concentration of Micro-Plastics by Detection of Bioaffinity-Induced Particle Retention Using Surface Plasmon Resonance Biosensors. Biosensors 2021, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Hur, H.; Kim, Y.; Shin, S.; Woo, H.; Choi, J.; Lee, H.H. Peptide specific nanoplastic detection based on sandwich typed localized surface plasmon resonance. Nanomaterials 2021, 11, 2887. [Google Scholar] [CrossRef]
- Schmidt, M.S.; Hübner, J.; Boisen, A. Large Area Fabrication of Leaning Silicon Nanopillars for Surface Enhanced Raman Spectroscopy. Adv. Mater. 2012, 24, 11–18. [Google Scholar] [CrossRef]
- Mikac, L.; Rigó, I.; Himics, L.; Toli, A.; Ivanda, M.; Veres, M. Surface-Enhanced Raman Spectroscopy for the Detection of Microplastics. Appl. Surf. Sci. 2023, 608, 155239. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Lee, H.; Han, S.; Kang, T.H.; Kim, D.; Kang, T.; Choi, I. Colloidal Multiscale Assembly via Photothermally Driven Convective Flow for Sensitive In-Solution Plasmonic Detections. Small 2022, 18, e2201075. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Cheng, H.; Jones, R.; Feng, Y.; Gong, K.; Li, K.; Fang, X.; Tahir, M.A.; Valev, V.K.; Zhang, L. Surface-Enhanced Raman Spectroscopy Facilitates the Detection of Microplastics <1 Mm in the Environment. Sci. Technol. Environ. 2020, 54, 15594–15603. [Google Scholar]
- Lê, Q.T.; Ly, N.H.; Kim, M.K.; Lim, S.H.; Son, S.J.; Zoh, K.D.; Joo, S.W. Nanostructured Raman Substrates for the Sensitive Detection of Submicrometer-Sized Plastic Pollutants in Water. J. Hazard. Mater. 2020, 402, 123499. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, N.; Choi, Y.; Kim, J.; Hwang, H.; Kim, C.; Lee, H.Y.; Kim, S.; Kim, J.S.; Lee, H.H.; et al. Peptide-Decorated Microneedles for the Detection of Microplastics. Biosensors 2024, 14, 140. [Google Scholar] [CrossRef]
- Woo, H.; Kang, S.H.; Kwon, Y.; Choi, Y.; Kim, J.; Ha, D.H.; Tanaka, M.; Okochi, M.; Kim, J.S.; Kim, H.K.; et al. Sensitive and specific capture of polystyrene and polypropylene microplastics using engineered peptide biosensors. RSC Adv. 2022, 12, 7680–7688. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, R.F.; Wypych, A.; Pérez-garcía, P.; Danso, D.; Chow, J.; Streit, W.R. An Ultra-Sensitive Comamonas thiooxidans Biosensor for the Degradation. Appl. Environ. Microbiol. 2023, 89, e01603-22. [Google Scholar] [CrossRef]
- Puhakka, E.; Santala, V. Method for acrylic acid monomer detection with recombinant biosensor cells for enhanced plastic degradation monitoring from water environments. Mar. Pollut. Bull. 2022, 178, 113568. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors-sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.H.; Hefnawy, A.; Hazeem, L.J.; Rashdan, S.A.; Abd-Rabboh, H.S. Current perspectives, challenges, and future directions in the electrochemical detection of microplastics. RSC Adv. 2024, 14, 2134–2158. [Google Scholar] [CrossRef]
- Hill, R.T. Plasmonic biosensors. Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 152–168. [Google Scholar] [CrossRef]
- Gupta, B.D.; Verma, R.K. Surface plasmon resonance-based fiber optic sensors: Principle, probe designs, and some applications. J. Sens. 2009, 2009, 979761. [Google Scholar] [CrossRef]
- Koyun, A.; Ahlatcolu, E.; Koca, Y.; Kara, S. Biosensors and their principles. In A Roadmap of Biomedical Engineers and Milestones, 1st ed.; Kara, S., Ed.; InTech—Janeza Trdine: Rijeka, Croatia, 2012; pp. 117–142. [Google Scholar]
- Dey, T.K.; Uddin, E.; Jamal, M. Detection and removal of microplastics in wastewater: Evolution and impact. Environ. Sci. Pollut. Res. 2021, 28, 16925–16947. [Google Scholar] [CrossRef]
- Schiano, M.E.; Abduvakhidov, A.; Varra, M.; Albrizio, S. Aptamer-based biosensors for the analytical determination of Bisphenol A in foodstuffs. Appl. Sci. 2022, 12, 3752. [Google Scholar] [CrossRef]
- Lim, H.J.; Song, H.; Son, A. Multi-target aptamer assay for endocrine-disrupting phthalic acid ester panel screening in plastic leachates. Chemosphere 2024, 359, 142366. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wu, J.; Eda, S.; Chen, J.; Chen, W.; Zheng, L. Rapid capacitive detection of femtomolar levels of bisphenol A using an aptamer-modified disposable microelectrode array. Microchim. Acta 2015, 182, 2361–2367. [Google Scholar] [CrossRef]
- Xue, F.; Wu, J.; Chu, H.; Mei, Z.; Ye, Y.; Liu, J.; Zhang, R.; Peng, C.; Zheng, L.; Chen, W. Electrochemical aptasensor for the determination of bisphenol A in drinking water. Microchim. Acta 2012, 180, 109–115. [Google Scholar] [CrossRef]
- Peltola, E.; Aarva, A.; Sainio, S.; Heikkinen, J.J.; Wester, N.; Jokinen, V.; Koskinen, J.; Laurila, T. Biofouling affects the redox kinetics of outer and inner sphere probes on carbon surfaces drastically differently–implications to biosensing. Phys. Chem. Chem. Phys. 2020, 22, 16630–16640. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, W.; Gao, L.; Yao, S.Q. Emerging biosensing and transducing techniques for potential applications in point-of-care diagnostics. Chem. Sci. 2022, 13, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
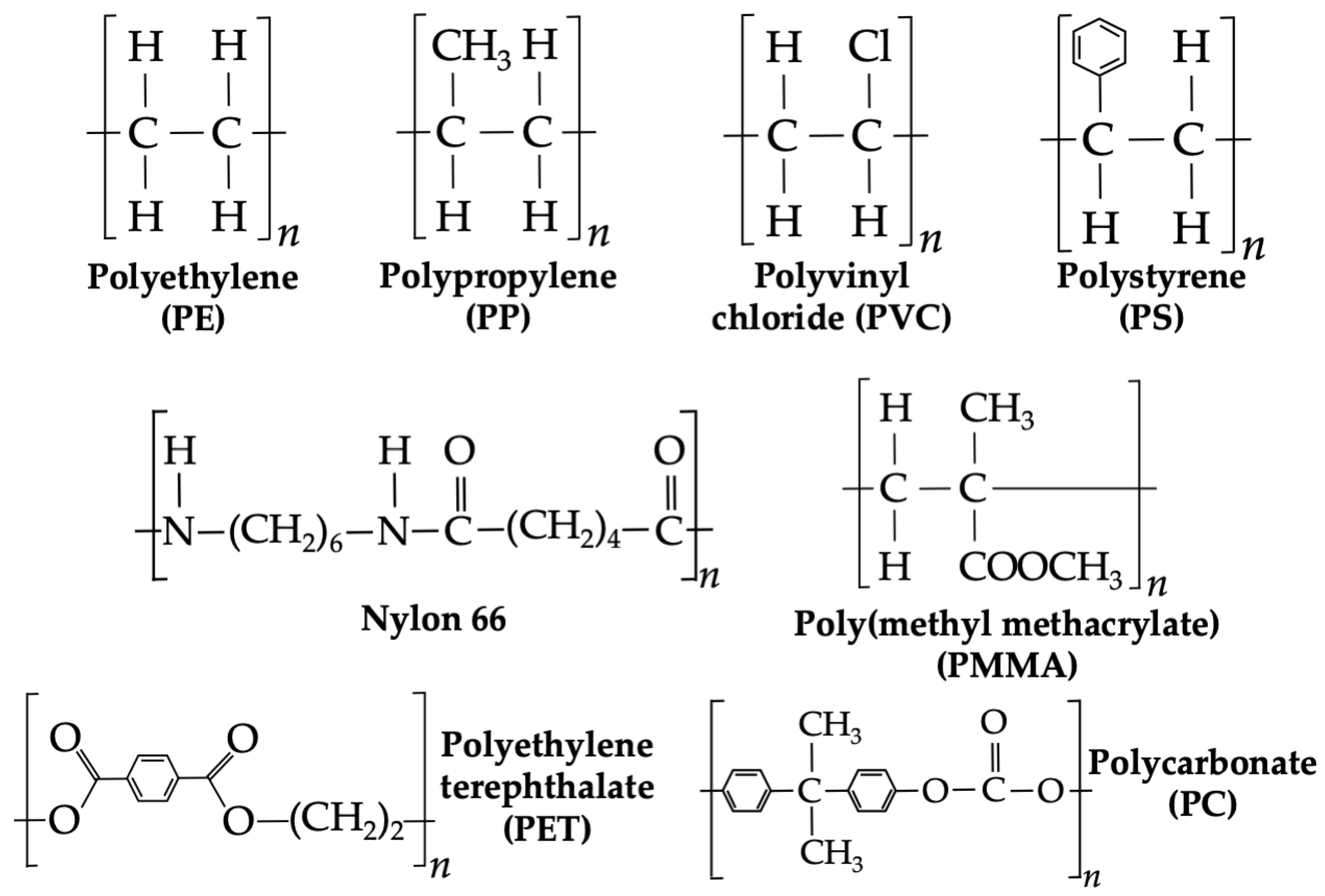

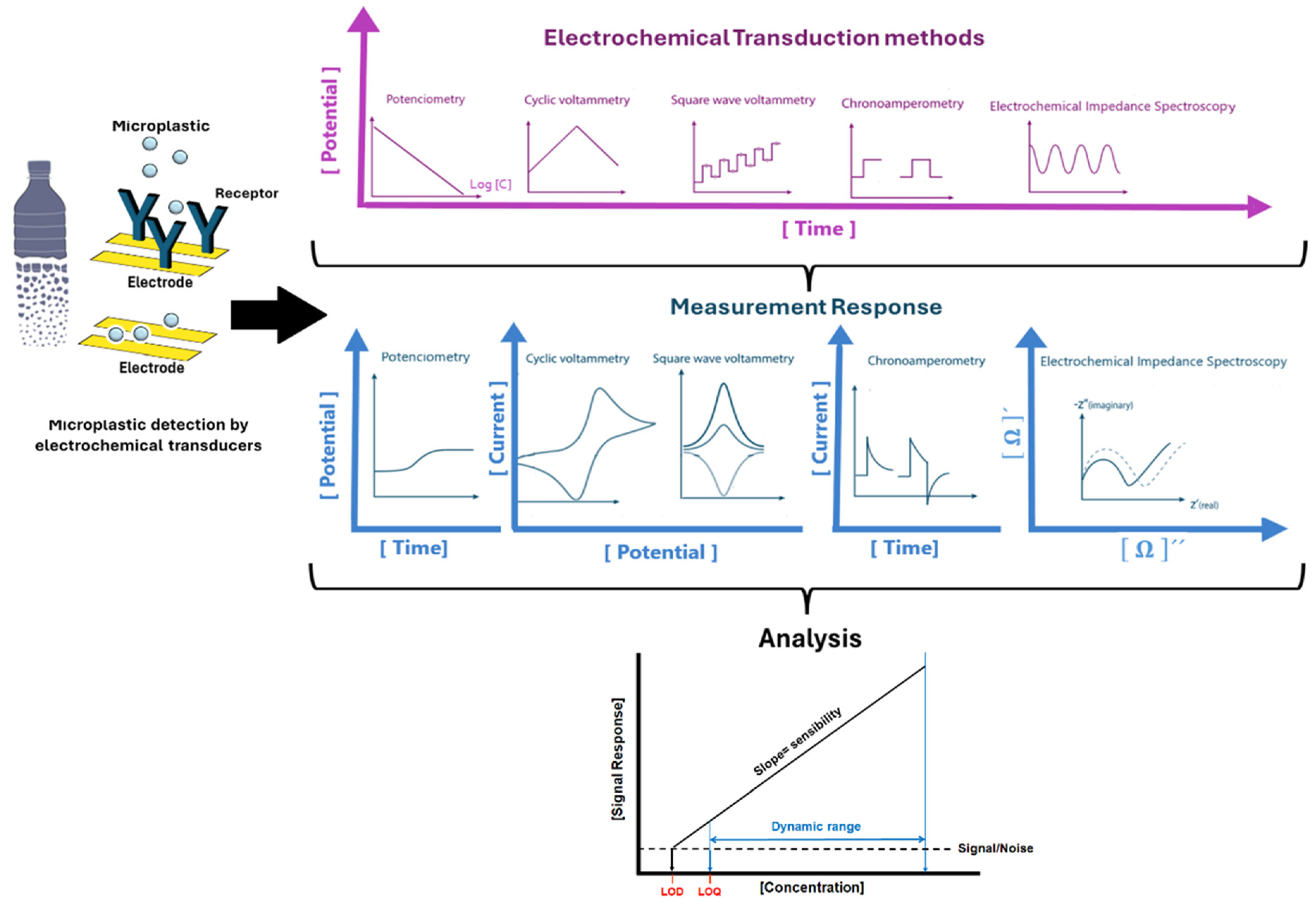
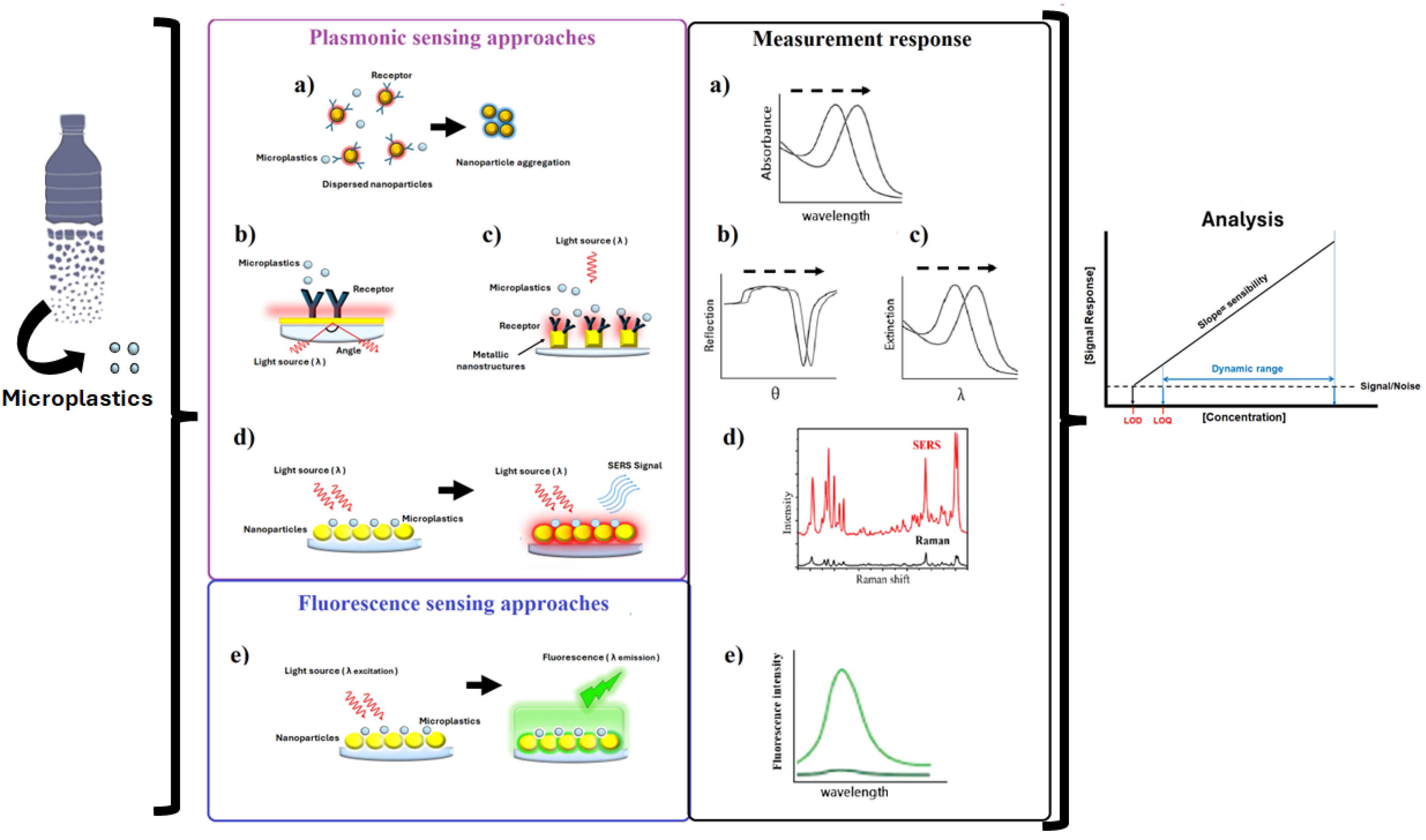
| Method | Advantages | Limitations |
|---|---|---|
| Stereomicroscope | Rapid and facile technique. Can identify shape, color, and size. | It is impossible to determine plastic particles’ composition, additives, and nature. There are no available data on transparent and small-size particles. Morphological estimation may lead to miscalculation of microplastic numbers. |
| SEM | High-resolution images with good clarity. If bound with EDS, elemental analysis is possible. No gas and sputtering when combined with ESEM mode. STEM mode can identify small particles. Sample treatment is not necessary. | Costly equipment. Longer duration for analysis. Composition cannot be identified. |
| Fluorescence microscope | Easy and immediate visualization of microplastics. A facile strategy for the detection of transparent particles. | Ultraviolet radiation can be toxic and harmful. Chemical additives of the plastic particles can misinterpret the result. |
| Fourier transform infrared spectrophotometer (FT-IR) | Facile sample preparation and no pretreatment. Chemical composition can be identified. The fingerprint region can reveal the distinction between plastic particles. If ATR is attached, solid, liquid, film, and powder samples can be analyzed. Less than 20 µm size particles can be identified using µ-FTIR Non-destructive method. | Costly equipment. The detection factor is limited by wavelength radiation. Time consumption for every particle identification. |
| Raman spectroscopy | A technique to identify smaller size microplastics (1 mm). Non-destructive, gaseous films, solids, and single-crystal samples can be analyzed. | It is an expensive and time-consuming technique. Interference may come from pigments and fragments released by adhesive polymers. |
| Thermal GC-MS | Unknown plastic particles can be identified based on the mass particle. | Number and size information is not detectable. |
| Inductively coupled plasma–mass spectrometry (ICP-MS) | Several particles’ chemical properties, density, and mass concentration can be identified easily. | It is a costly and destructive technique. |
| Laser direct infrared spectroscopy (LDIR) | Detect particles up to 20 µm in soil, groundwater, ocean, and biological tissues. | Costly equipment. Smaller size (<20 µm) particles cannot be identified. |
| Micro- and Nanoplastic Detection | ||||
|---|---|---|---|---|
| Method | Analyte | Limit of Detection | Measurement Conditions | Reference |
| Resistive pulse sensors | Microparticles 21.9 µm | 6.52 × 10−4 particles/mL | Salt concentrations ranging from 2.5 × 10−4 to 0.1 M Sample: teabags | [242] |
| Impedance spectroscopy Chronoamperometry | Polyethylene particles 212–1000 mm Polystyrene 0.1 to 10 µm | 5–500 ng/L | Carbon fiber wire electrode Sample: tap water Carbon electrodes with ferrocene as mediator Sample: water | [243,244]. |
| Colorimetric | Polyethylene terephthalate particles Dimethyl phthalate (DMP) and dibutyl phthalate (DBP) Bisphenol A | 2.5–15 mg/L DMP: 0.1 μg/L DBP: 0.5 μg/L 0.09 µg/mL | Gold nanoparticles with anchored peptides Platinum–gold nanoparticles coupled to antibodies for DMP and DBP Sample: baijiu and other plastic-bottled beverages Copper nanoparticles with a carbon nitride skeleton and triazole groups (Cu-g-C3N5) Sample: water | [245,246,247] |
| Surface plasmon resonance (SPR) | Poly(methyl methacrylate) nanoparticles | 0.39 ng/mL | SPR platform with a polymer-based gold nanograting Water Sample: seawater | [248] |
| Localized surface plasmon resonance (LSPR) | Polystyrene particles | - | Gold nanoparticles (Au NPs) with bio-mimicked peptide probes | [245] |
| Plasmon-enhanced fluorescence (PEF) | Low-density polyethylene (LDPE), poly(butylene adipate-co-terephthalate) (PBAT), and epoxy resins from 0.8 to 2.5 µm | - | Gold nanopillar substrates Sample: miliQ water | [249] |
| Surface-enhanced Raman spectroscopy (SERS) | Polystyrene 100, 500 nm PE 10 µm PP 10 µm Polystyrene 1 µm, 50 nm Polystyrene 50 to 2 µm Polystyrene 20 and 200 nm Polyethylene terephthalate 10, 15, 20 µm Polystyrene 84–630 nm Polystyrene (PS) from 50 nm to 1 µm and poly(methyl methacrylate)/PMMA 500 nm Polystyrene (PS), polyethylene terephthalate (PET), polyethylene (PE), polyvinyl chloride (PVC), polypropylene (PP), and polycarbonates (PCs) from 80 to 150 µm | 40 µg/mL 5 µg/mL PS 50 nm: 12.5 µg/mL PS 100 nm: 6.25 µg/mL PS 200 nm: 25 µg/mL PS 500 nm: 25 µg/mL PS 1 µm: 12.5–25 µg/mL PS 20 nm: 10 µg/mL PS 200 nm: 1 µg/mL 100 µg/mL PS 84 nm: 100 µg/mL PS 444 nm: 50 µg/mL PS 630 nm: 100 µg/mL PS 84 nm: 500 µg/mL PS 444 nm: 500 µg/mL PS 630 nm: 500 µg/mL PS 50 nm: 10–4 µg/mL PMMA 500 nm: 10–3 µg/mL PS: 1 µg/mL | Silver nanoparticles Sample: seawater Silver nanoparticles Sample: river water Silver nanoparticles Sample: real water Gold nanoparticles Sample: seawater Filter paper with gold nanoparticles Sample: tap water and pond water Silver nanowires on cellulose Gold nanorods on cellulose Silver nanowire array Sample: seafood, market water, and seawater Gold nanoparticle-decorated sponge Sample: seawater, river water, snow water, and rainwater | [250,251,252,253,254,255,256,257] |
| Released Harmful Compounds | ||||
| Method | Analyte | Limit of Detection | Measurement Conditions | Reference |
| Differential pulse voltammetry | Catechol Hydroquinone Hydroquinone Catechol Resorcinol Catechol Hydroquinone Resorcinol Bisphenol A Bisphenol A 4-(methylamino)phenol | 0.96 µM 0.56 µM 0.13 µM 0.15 µM 1.36 µM 1.70 µM 5.10 µM 4.50 µM 1.0 µM 0.025 µM 0.0021 µM | Electrode: poly(4-vinylphenylboronic acid)-functionalized polypyrrole/graphene oxide nanosheets Sample: tap water Electrode: cobalt–iron selenides/porous carbon nanofibers/graphene carbon electrode Sample Lake water Electrode: Carbon electrode modified by carbon black/gold sononanoparticle nanocomposite (CB/AuSNPs) Sample: tap, dam, and swamp water Electrode: gold nanoparticles/1,3,5-triformylphloroglucinol and benzidine covalent organic frameworks/graphene carbon electrode Sample: lake water Electrode: metal–organic framework/graphene oxide/carbon paste electrode Sample: lake, tap, and drinking water | [258,259,260,261,262,263] |
| Square-wave voltammetry | Catechol Hydroquinone Bisphenol A Phenol | 0.20 0.16 2.40 3.0 | Electrode: silver nanoparticles/multi-walled carbon nanotubes/graphene carbon electrodes Sample: tap water | [264] |
| Cyclic voltammetry | Catechol 2-aminophenol 2-chlorophenol 2-nitrophenol Catechol | 0.045 µM 0.0057 µM 0.0013 µM 0.0010 µM 0.106 µM | Electrode: glutamine-activated graphite paste electrode Sample: Water samples Electrode: CaCu2O3 nanorod-shaped/graphene carbon electrode Sample: tap water and agricultural water Electrode: sodium dodecyl sulfate modified graphene paste electrode Sample: tap water | [265,266,267] |
| Colorimetric | Dimethyl phthalate (DMP) and dibutyl phthalate (DBP) Bisphenol A | DMP: 0.1 μg/L DBP: 0.5 μg/L 0.09 mg/L | Platinum–gold nanoparticles coupled to antibodies for DMP and DBP Sample: baijiu and other plastic-bottled beverages Copper nanoparticles with a carbon nitride skeleton and triazole groups (Cu-g-C3N5) Water Sample: water | [246,247] |
| Localized surface plasmon resonance (LSPR) | Bisphenol A | 0.0010 µM | Gold nanoparticle-modified | [268] |
| Surface plasmon resonance | Bisphenol A | 0.0087 µM | molecular imprinted polymers based on monomer ethylene glycol dimethacrylate-N-methacryloyl-L-phenylalanine-vinyl imidazole Sample: water Time of response: 5 min | [269] |
| Surface-enhanced Raman spectroscopy (SERS) | Bisphenol A | 0.05 µM | Molecular imprinted polymers based on monomer methacrylic acid Time of response: 20 min | [270] |
| Transduction Principle | Sensing Technology | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Electrochemical sensors | Voltammetry | Low-cost production of electrodes and microelectronic circuits. A straightforward read-out and processing device. Multiplexing capability. | Conditions of pH and ionic strength in the sample significantly affect the sensor’s performance. The miniaturization of sensor devices tends to increase the signal-to-noise ratio. The lifetime of electrodes diminishes due to fouling effects. Redox molecules tend to be employed for reaction at the working electrode. | [303] |
| Resistive pulse sensor | Allows a high concentration detection. A straightforward read-out and processing device. Suitable for different types of plastics. | The sample’s pH and ionic strength significantly affect the sensor’s performance. Restricted size range. | [304] | |
| Impedance spectroscopy | A straightforward read-out and processing device. Suitable for different types of plastics. | The sample’s pH and ionic strength significantly affect the sensor’s performance. Sophisticated manufacturing and data processing. Comparatively poor recovery rate. | [304] | |
| Plasmonic sensors | SPR | Instruments and chips are already well established in the market (mainly for biological assays). Allows label-free detection schemes (i.e., no addition of fluorescent tags). Highly sensitive to the refractive index and allows multiplexing detection. | The prism is a drawback in miniaturization attempts. Detects refractive index changes close to the metal film surface (extending up to 200 nm). Temperature control is needed to produce stable SPR signals. | [305] |
| LSPR | Multiplexing and miniaturization capability. Tuning detection by varying the nanoparticles’ size, shape, and composition. Use of different wavelengths that do not overlap with the spectral of natural chromophores in the samples. | The sensors are susceptible to the refractive index of the surrounding medium. The experiments need to ensure that the binding of the target molecule happens within the sensing volume when it involves bulky molecules. | [306] | |
| Plasmon-enhanced fluorescence | Present good signal-noise ratio. Damage of sensing elements due to prolonged exposure to incident light. Allows discrimination of plastics with different sizes and different compositions. | Complex instrumentation. No studies have been reported in real samples for microplastic detection. Slow response time due to the diffusion effect of analytes. | [307] | |
| Colorimetric methods | The plasmon resonant nanostructures can be used as fluorophore tags. Easy to use. No expensive instrumentation required. Allows fast qualitative screening tests. | Unable to provide reliable quantitative measurements. | [307] | |
| Surface-enhanced Raman spectroscopy | Provides simultaneous quantitative and qualitative detection by combining the Raman fingerprints of different analytes and plasmonic nanostructures of the systems (required for the differentiation of MP size and composition). Allows real-time data processing by the combination of fiber optics and microfluidic circuits. | Lack of a standard methodology for sample preparation and a standardized procedure of analysis. It is challenging to perform accurate detection in the field. No matrix effects have been established. | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Rivera, D.M.; Quintanilla-Villanueva, G.E.; Luna-Moreno, D.; Sánchez-Álvarez, A.; Rodríguez-Delgado, J.M.; Cedillo-González, E.I.; Kaushik, G.; Villarreal-Chiu, J.F.; Rodríguez-Delgado, M.M. Exploring Innovative Approaches for the Analysis of Micro- and Nanoplastics: Breakthroughs in (Bio)Sensing Techniques. Biosensors 2025, 15, 44. https://doi.org/10.3390/bios15010044
Rivera-Rivera DM, Quintanilla-Villanueva GE, Luna-Moreno D, Sánchez-Álvarez A, Rodríguez-Delgado JM, Cedillo-González EI, Kaushik G, Villarreal-Chiu JF, Rodríguez-Delgado MM. Exploring Innovative Approaches for the Analysis of Micro- and Nanoplastics: Breakthroughs in (Bio)Sensing Techniques. Biosensors. 2025; 15(1):44. https://doi.org/10.3390/bios15010044
Chicago/Turabian StyleRivera-Rivera, Denise Margarita, Gabriela Elizabeth Quintanilla-Villanueva, Donato Luna-Moreno, Araceli Sánchez-Álvarez, José Manuel Rodríguez-Delgado, Erika Iveth Cedillo-González, Garima Kaushik, Juan Francisco Villarreal-Chiu, and Melissa Marlene Rodríguez-Delgado. 2025. "Exploring Innovative Approaches for the Analysis of Micro- and Nanoplastics: Breakthroughs in (Bio)Sensing Techniques" Biosensors 15, no. 1: 44. https://doi.org/10.3390/bios15010044
APA StyleRivera-Rivera, D. M., Quintanilla-Villanueva, G. E., Luna-Moreno, D., Sánchez-Álvarez, A., Rodríguez-Delgado, J. M., Cedillo-González, E. I., Kaushik, G., Villarreal-Chiu, J. F., & Rodríguez-Delgado, M. M. (2025). Exploring Innovative Approaches for the Analysis of Micro- and Nanoplastics: Breakthroughs in (Bio)Sensing Techniques. Biosensors, 15(1), 44. https://doi.org/10.3390/bios15010044







