Detecting Attomolar Concentrations of Interleukin IL-17A via Pollen-Based Nanoplasmonic Biochips
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
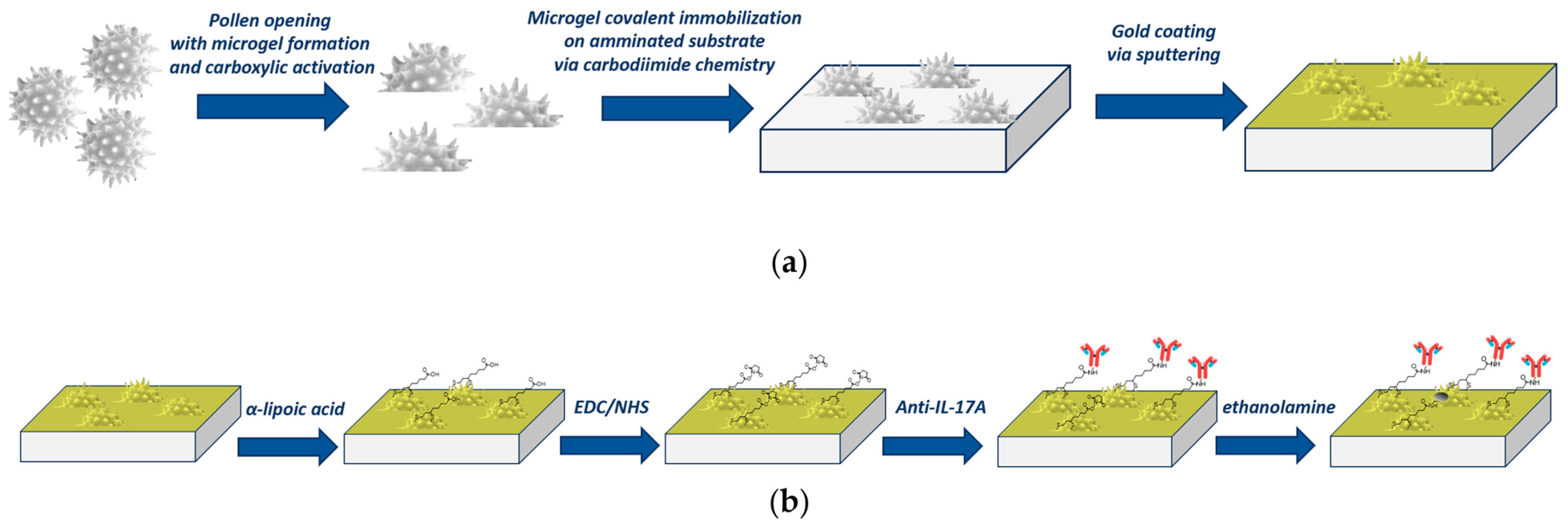
2.2. Plasmonic Pollen-Based Chip Preparation
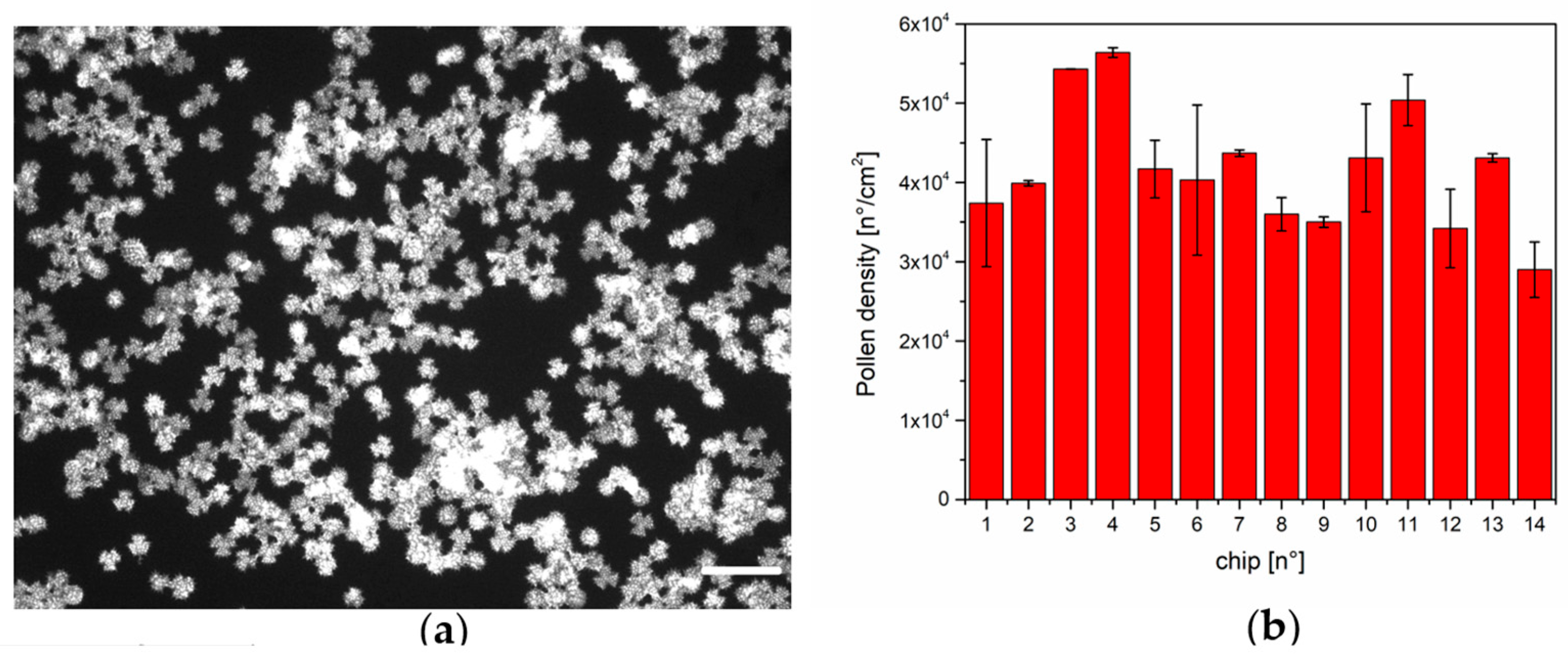
2.3. Pollen Density Estimation
2.4. Surface Functionalization of the Pollen-Based Nanoplasmonic Chip
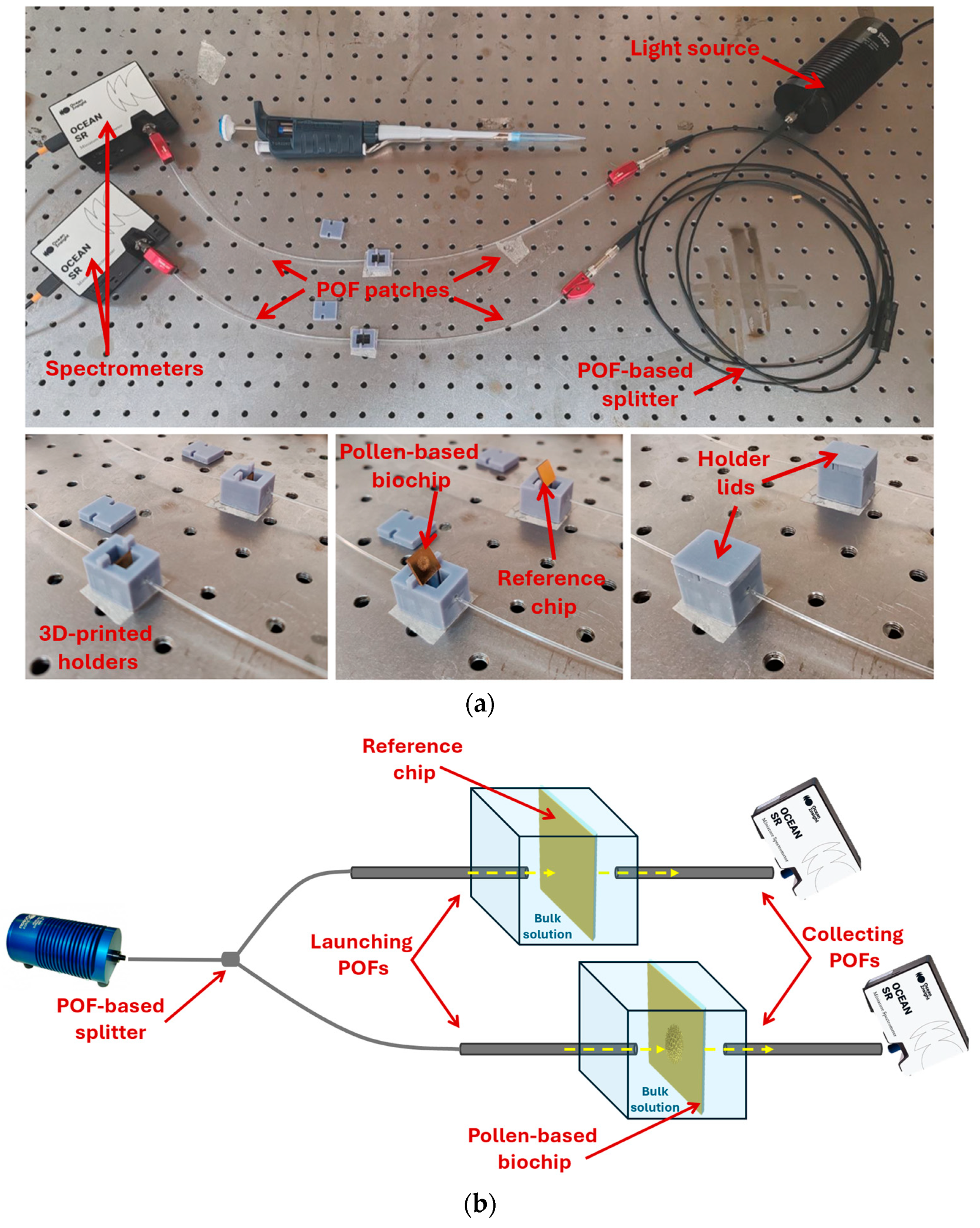
2.5. Optical Setup
2.6. Measurement Protocol and Data Analysis
3. Results and Discussion
3.1. Pollen Density Measurements
3.2. IL-17A Detection via Pollen-Based Biochips
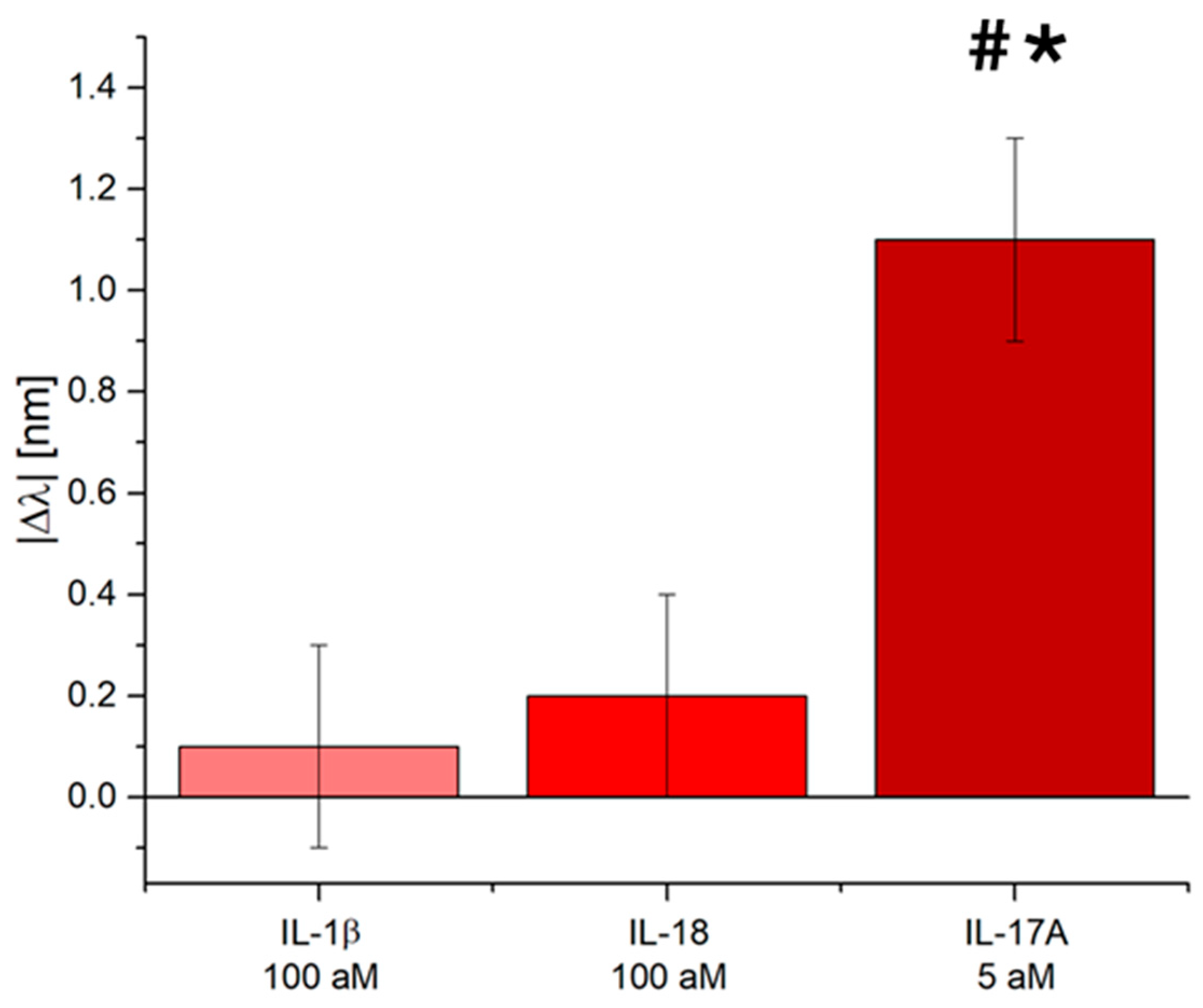
3.3. Selectivity Tests of the IL-17A Pollen-Based Plasmonic Biosensor
3.4. Discussion: Comparison with the State-of-the-Art
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzik, T.J.; Mohiddin, S.A.; Dimarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediat. Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 family of cytokines in health and disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 signaling: The yin and the yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef]
- Nanki, K.; Fujii, M.; Shimokawa, M.; Matano, M.; Nishikori, S.; Date, S.; Takano, A.; Toshimitsu, K.; Ohta, Y.; Takahashi, S.; et al. Somatic inflammatory gene mutations in human ulcerative colitis epithelium. Nature 2020, 577, 254–259. [Google Scholar] [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin. Rev. Allergy Immunol. 2018, 55, 379–390. [Google Scholar] [CrossRef]
- Potashman, M.; Haas, J.S.; Pandit, A.; Stafkey, D.; Coric, V.; Singer, W.; L’Italien, G. The impact of anti-inflammatory therapy on Parkinson’s disease incidence: A retrospective cohort study. Park. Relat. Disord. 2025, 130, 107194. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.J.A.C.; Jonkers, J.; et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of inflammatory cytokines within the breast tumor microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef]
- Enamorado, M.; Kulalert, W.; Han, S.J.; Rao, I.; Delaleu, J.; Link, V.M.; Yong, D.; Smelkinson, M.; Gil, L.; Nakajima, S.; et al. Immunity to the microbiota promotes sensory neuron regeneration. Cell 2023, 186, 607–620. [Google Scholar] [CrossRef]
- Li, X.; Bechara, R.; Zhao, J.; McGeachy, M.J.; Gaffen, S.L. IL-17 receptor–based signaling and implications for disease. Nat. Immunol. 2019, 20, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2023, 13, 1098725. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From clinical significance to quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef] [PubMed]
- Borg, K.N.; Ho, Y.P.; Zeng, S. Recent Developments on Optical Aptasensors for the Detection of Pro-Inflammatory Cytokines with Advanced Nanostructures. Adv. Opt. Mater. 2024, 12, 2400608. [Google Scholar] [CrossRef]
- Dutta, N.; Lillehoj, P.B.; Estrela, P.; Dutta, G. Electrochemical biosensors for cytokine profiling: Recent advancements and possibilities in the near future. Biosensors 2021, 11, 94. [Google Scholar] [CrossRef]
- Fattahi, Z.; Khosroushahi, A.Y.; Hasanzadeh, M. Recent progress on developing of plasmon biosensing of tumor biomarkers: Efficient method towards early stage recognition of cancer. Biomed. Pharmacother. 2020, 132, 110850. [Google Scholar] [CrossRef]
- Wei, S.C.; Hsu, M.N.; Chen, C.H. Plasmonic droplet screen for single-cell secretion analysis. Biosens. Bioelectron. 2019, 144, 111639. [Google Scholar] [CrossRef]
- Markhali, B.P.; Sriram, M.; Bennett, D.T.; Khiabani, P.S.; Hoque, S.; Tilley, R.D.; Bakthavathsalam, P.; Gooding, J.J. Single particle detection of protein molecules using dark-field microscopy to avoid signals from nonspecific adsorption. Biosens. Bioelectron. 2020, 169, 112612. [Google Scholar] [CrossRef]
- Lopez-Muñoz, G.A.; Fernández-Costa, J.M.; Ortega, M.A.; Balaguer-Trias, J.; Martin-Lasierra, E.; Ramón-Azcón, J. Plasmonic nanocrystals on polycarbonate substrates for direct and label-free biodetection of Interleukin-6 in bioengineered 3D skeletal muscles. Nanophotonics 2021, 10, 4477–4488. [Google Scholar] [CrossRef]
- Alba-Patino, A.; Russell, S.M.; Borges, M.; Pazos-Pérez, N.; Álvarez-Puebla, R.A.; De la Rica, R. Nanoparticle-based mobile biosensors for the rapid detection of sepsis biomarkers in whole blood. Nanoscale Adv. 2020, 2, 1253–1260. [Google Scholar] [CrossRef]
- Zou, B.; Lou, S.; Duan, J.; Zhou, S.; Wang, Y. Design of Raman reporter-embedded magnetic/plasmonic hybrid nanostirrers for reliable microfluidic SERS biosensors. Nanoscale 2023, 15, 8424–8431. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Piccirillo, A.; Bencivenga, D.; Arcadio, F.; Annunziata, M.; Della Ragione, F.; Guida, L.; Zeni, L.; Borriello, A. Towards a point-of-care test to cover atto-femto and pico-nano molar concentration ranges in interleukin 6 detection exploiting PMMA-based plasmonic biosensor chips. Talanta 2023, 256, 124284. [Google Scholar] [CrossRef] [PubMed]
- Barya, P.; Xiong, Y.; Shepherd, S.; Gupta, R.; Akin, L.D.; Tibbs, J.; Lee, H.; Singamaneni, S.; Cunningham, B.T. Photonic-Plasmonic Coupling Enhanced Fluorescence Enabling Digital-Resolution Ultrasensitive Protein Detection. Small 2023, 19, 2207239. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, D.; Luo, Y.; Liu, Y.; Guo, Y.; Yang, G.Z.; Qiu, G. Intraoperative assessment of microimplantation-induced acute brain inflammation with titanium oxynitride-based plasmonic biosensor. Biosens. Bioelectron. 2024, 264, 116664. [Google Scholar] [CrossRef]
- Chen, P.; Chung, M.T.; McHugh, W.; Nidetz, R.; Li, Y.; Fu, J.; Cornell, T.T.; Shanley, T.P.; Kurabayashi, K. Multiplex serum cytokine immunoassay using nanoplasmonic biosensor microarrays. ACS Nano 2015, 9, 4173–4181. [Google Scholar] [CrossRef]
- Lau, U.Y.; Saxer, S.S.; Lee, J.; Bat, E.; Maynard, H.D. Direct write protein patterns for multiplexed cytokine detection from live cells using electron beam lithography. ACS Nano 2016, 10, 723–729. [Google Scholar] [CrossRef]
- Zhu, C.; Luo, X.; Espulgar, W.V.; Koyama, S.; Kumanogoh, A.; Saito, M.; Takamatsu, H.; Tamiya, E. Real-time monitoring and detection of single-cell level cytokine secretion using LSPR technology. Micromachines 2020, 11, 107. [Google Scholar] [CrossRef]
- Kamińska, A.; Sprynskyy, M.; Winkler, K.; Szymborski, T. Ultrasensitive SERS immunoassay based on diatom biosilica for detection of interleukins in blood plasma. Anal. Bioanal. Chem. 2017, 409, 6337–6347. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Zhou, X.; Wang, Y. A paper-based SERS assay for sensitive duplex cytokine detection towards the atherosclerosis-associated disease diagnosis. J. Mater. Chem. B 2020, 8, 3582–3589. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, F.; Goldys, E.M.; Gao, F.; Liu, G. Advances in structure-switching aptasensing towards real time detection of cytokines. TrAC Trends Anal. Chem. 2018, 102, 379–396. [Google Scholar] [CrossRef]
- Cennamo, N.; Pasquardini, L.; Arcadio, F.; Zeni, L. Pollen-based natural nanostructures to realize nanoplasmonic biochips for single-molecule detection. Sens. Actuators B Chem. 2025, 422, 136404. [Google Scholar] [CrossRef]
- Jo, H.; Kim, S.K.; Youn, H.; Lee, H.; Lee, K.; Jeong, J.; Mok, J.; Kim, S.H.; Park, H.S.; Ban, C. A highly sensitive and selective impedimetric aptasensor for interleukin-17 receptor A. Biosens. Bioelectron. 2016, 81, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Smejkal, J.; Malý, P.; Kuchař, M.; Panova, N.; Semerádtová, A.; Aubrecht, P.; Štofik, M.; Malý, J. Cell immunocapture microfluidic chip based on high-affinity recombinant protein binders. Biosens. Bioelectron. 2021, 172, 112784. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chin, H.; Hwang, Y.; Fan, T.F.; Cho, N.J. A facile approach to patterning pollen microparticles for in situ imaging. Appl. Mater. Today 2020, 20, 100702. [Google Scholar] [CrossRef]
- Fan, T.F.; Park, S.; Shi, Q.; Zhang, X.; Liu, Q.; Song, Y.; Suresh, S.; Chin, H.; Bin Ibrahim, M.S.; Mokrzecka, N.; et al. Transformation of hard pollen into soft matter. Nat. Commun. 2020, 11, 1449. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Arcadio, F.; Zeni, L.; Montemurro, D.; Eramo, C.; Di Ronza, S.; Perri, C.; D’Agostino, G.; Chiaretti, G.; Porto, G.; Cennamo, N. Biochemical sensing exploiting plasmonic sensors based on gold nanogratings and polymer optical fibers. Photonics Res. 2021, 9, 1397–1408. [Google Scholar] [CrossRef]
- Sriram, M.; Markhali, B.P.; Nicovich, P.R.; Bennett, D.T.; Reece, P.J.; Hibbert, D.B.; Tilley, R.D.; Gaus, K.; Vivekchand, S.R.C.; Gooding, J.J. A rapid readout for many single plasmonic nanoparticles using dark-field microscopy and digital color analysis. Biosens. Bioelectron. 2018, 117, 530–536. [Google Scholar] [CrossRef]
- Valpapuram, I.; Candeloro, P.; Coluccio, M.L.; Parrotta, E.I.; Giugni, A.; Das, G.; Cuda, G.; Di Fabrizio, E.; Perozziello, G. Waveguiding and SERS simplified Raman spectroscopy on biological samples. Biosensors 2019, 9, 37. [Google Scholar] [CrossRef]
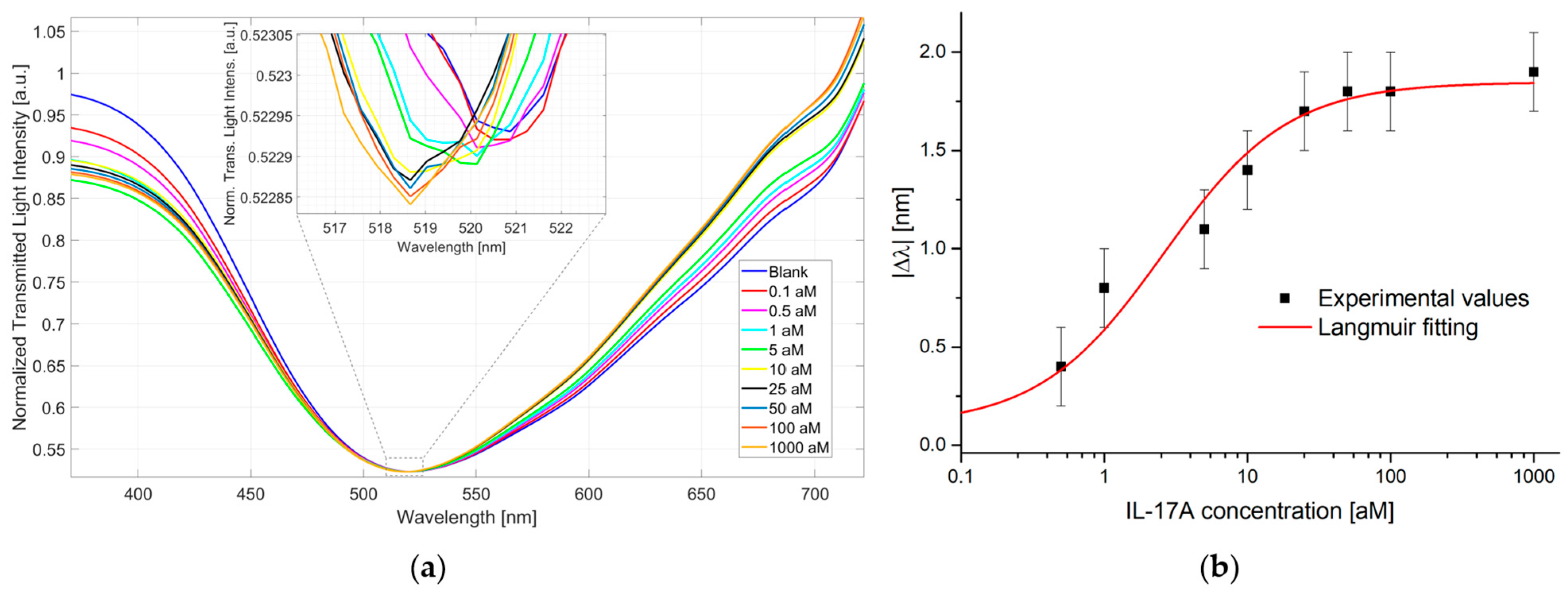
| |Δλ0| [nm] | |Δλmax| [nm] | K [aM] | R2 |
|---|---|---|---|
| 0.10 ± 0.11 | 1.85 ± 0.07 | 2.59 ± 0.77 | 0.97 |
| Biosensor Principle | Interleukin | LoD | Reference |
|---|---|---|---|
| LSPR of single gold nanoparticles | IL-6 | - | [38] |
| Optical waveguide with plasmonic surfaces based on gold nanodimers | IL-6 | - | [39] |
| LSPR microfluidic platform coupled with gold nanorods | IL-2, IL-4, IL-6, IL-10 | 20.56 pg/mL, 4.6 pg/mL, 11.29 pg/mL, 10.97 pg/mL | [25] |
| LSPR immunoassay with silver-enhanced gold nanoparticles conjugated to secondary antibodies | IL-6 | 50 pg/mL | [26] |
| Plasmonic droplet device | IL-8 | 7.2 ng/mL | [17] |
| Nanoimprinted gold-capped nanopillar structures on polymer for LSPR detection | IL-6 | 10 ng/mL | [27] |
| SERS based immunoassay | IL-8 | 6.2 pg/mL | [28] |
| Paper-based SERS assay | IL-10 | 0.1 pg/mL | [29] |
| Dark field microscopy coupled with LSPR effect of Au nanoparticles | IL-6 | 7 pg/mL | [18] |
| Plasmonic nanocrystals based in Blu-ray optical disc | IL-6 | 0.03 ng/mL | [19] |
| Plasmonic effect of Au nanoparticles on paper-based device | IL-6 | 0.1 pg/mL | [20] |
| Microfluidic SERS biosensor based on magnetic/plasmonic nanostirrer embedded with Raman reporter | IL-6 | 0.8 pg/mL | [21] |
| SPR-POF platform | IL-6 | 0.84 ng/mL | [22] |
| gold nanograting GNG | 14.28 fg/mL | ||
| Plasmonic gold nanorods coupled with photonic crystal | IL-6 | 10 fg/mL | [23] |
| Titanium oxynitride nanofilm | IL-6 | 6.3 fg/mL | [24] |
| Pollen-based biosensor | IL-17A | 6.9 ag/mL | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzano, C.; Pitruzzella, R.; Arcadio, F.; Passeggio, F.; Seggio, M.; Zeni, L.; Pasquardini, L.; Cennamo, N. Detecting Attomolar Concentrations of Interleukin IL-17A via Pollen-Based Nanoplasmonic Biochips. Biosensors 2025, 15, 161. https://doi.org/10.3390/bios15030161
Marzano C, Pitruzzella R, Arcadio F, Passeggio F, Seggio M, Zeni L, Pasquardini L, Cennamo N. Detecting Attomolar Concentrations of Interleukin IL-17A via Pollen-Based Nanoplasmonic Biochips. Biosensors. 2025; 15(3):161. https://doi.org/10.3390/bios15030161
Chicago/Turabian StyleMarzano, Chiara, Rosalba Pitruzzella, Francesco Arcadio, Federica Passeggio, Mimimorena Seggio, Luigi Zeni, Laura Pasquardini, and Nunzio Cennamo. 2025. "Detecting Attomolar Concentrations of Interleukin IL-17A via Pollen-Based Nanoplasmonic Biochips" Biosensors 15, no. 3: 161. https://doi.org/10.3390/bios15030161
APA StyleMarzano, C., Pitruzzella, R., Arcadio, F., Passeggio, F., Seggio, M., Zeni, L., Pasquardini, L., & Cennamo, N. (2025). Detecting Attomolar Concentrations of Interleukin IL-17A via Pollen-Based Nanoplasmonic Biochips. Biosensors, 15(3), 161. https://doi.org/10.3390/bios15030161










