All-Dielectric Metasurface-Based Terahertz Molecular Fingerprint Sensor for Trace Cinnamoylglycine Detection
Abstract
1. Introduction
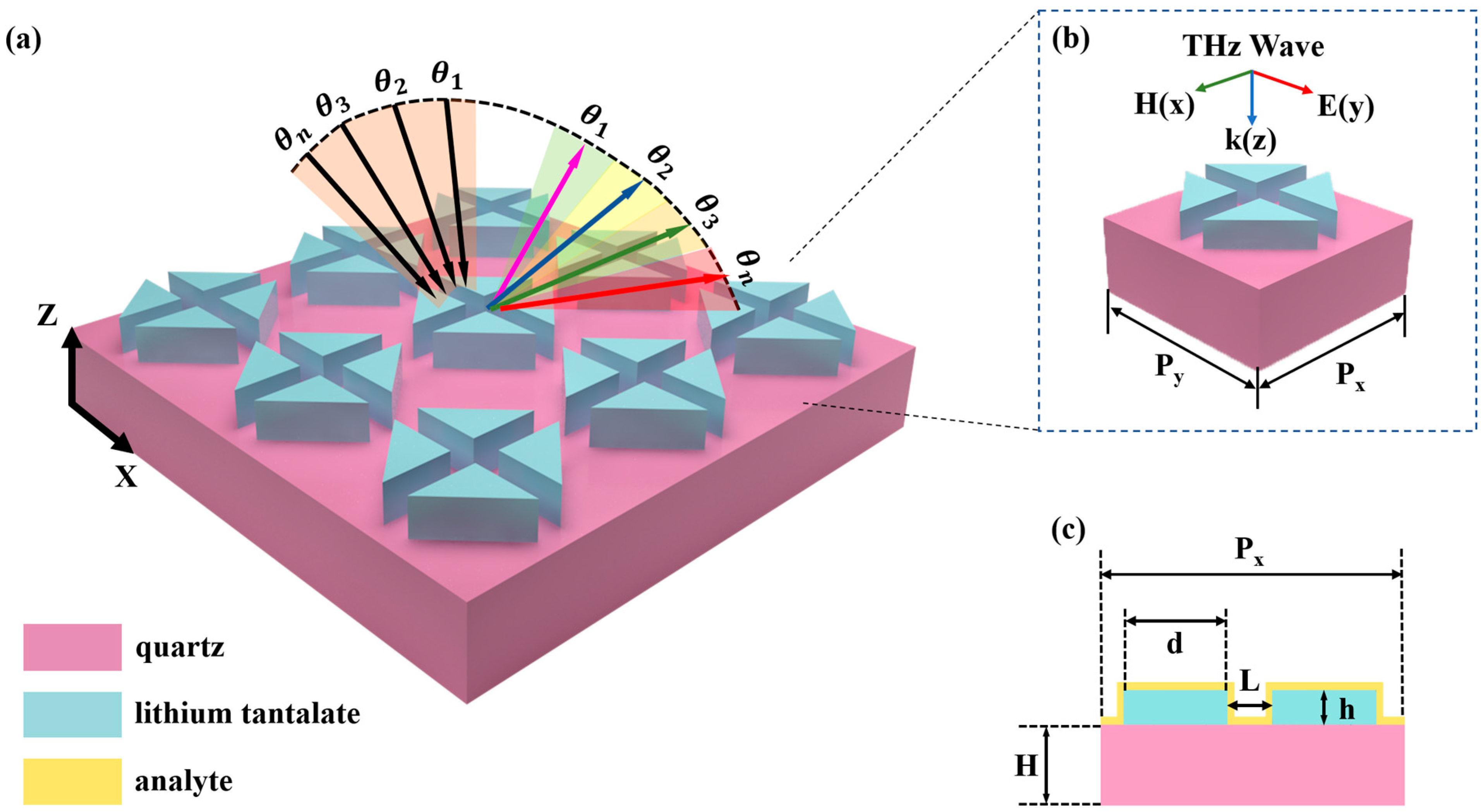
2. Structure and Design
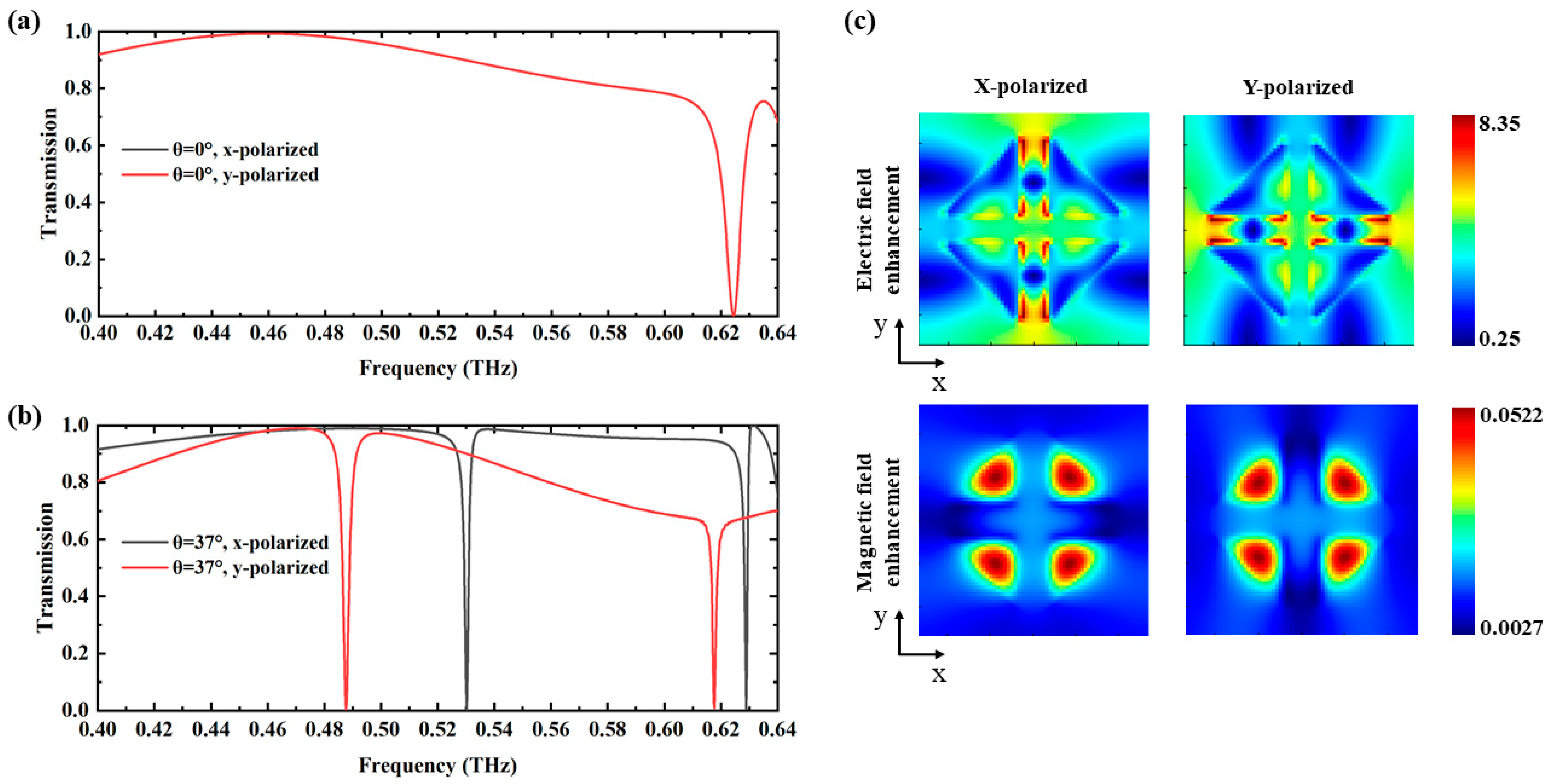
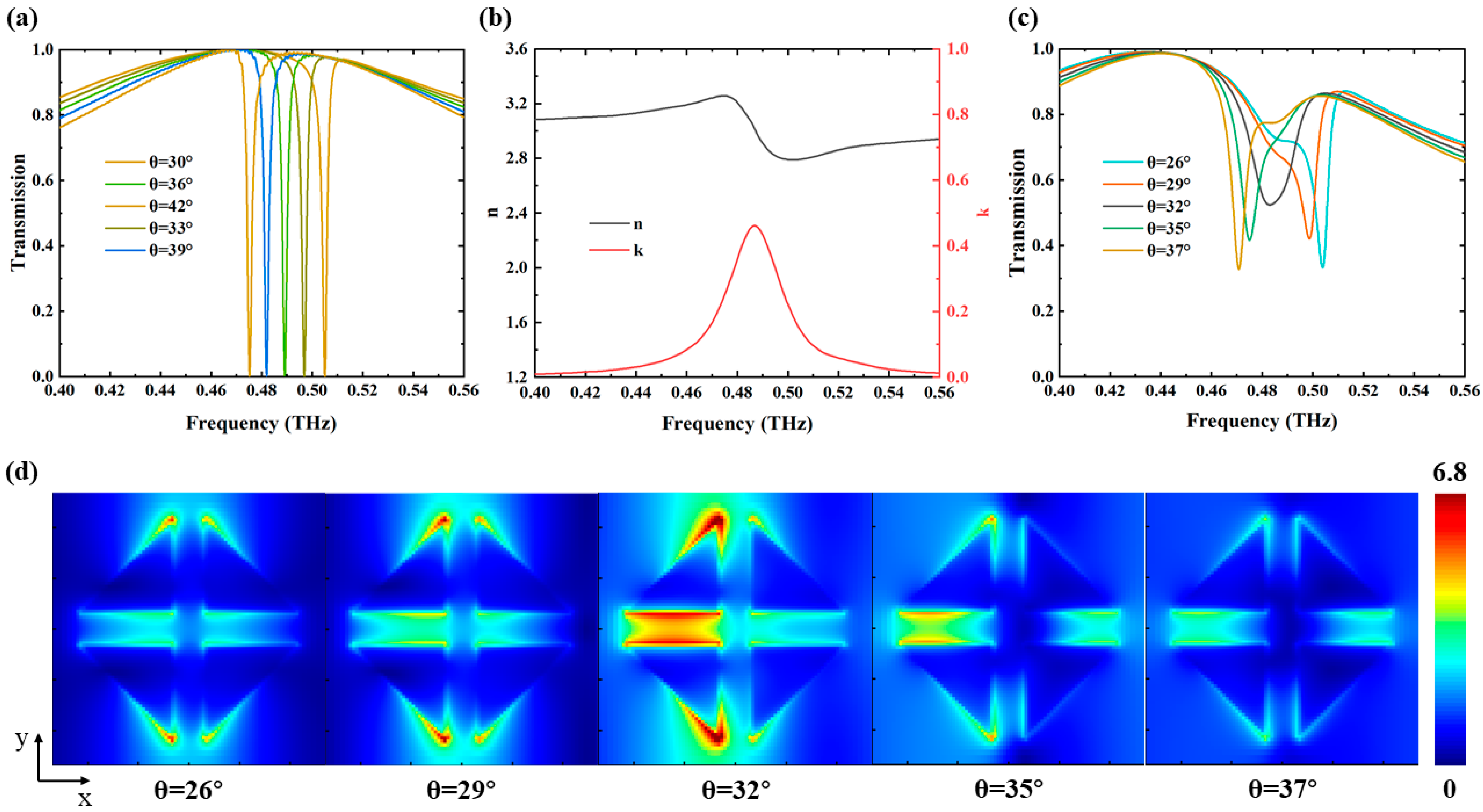
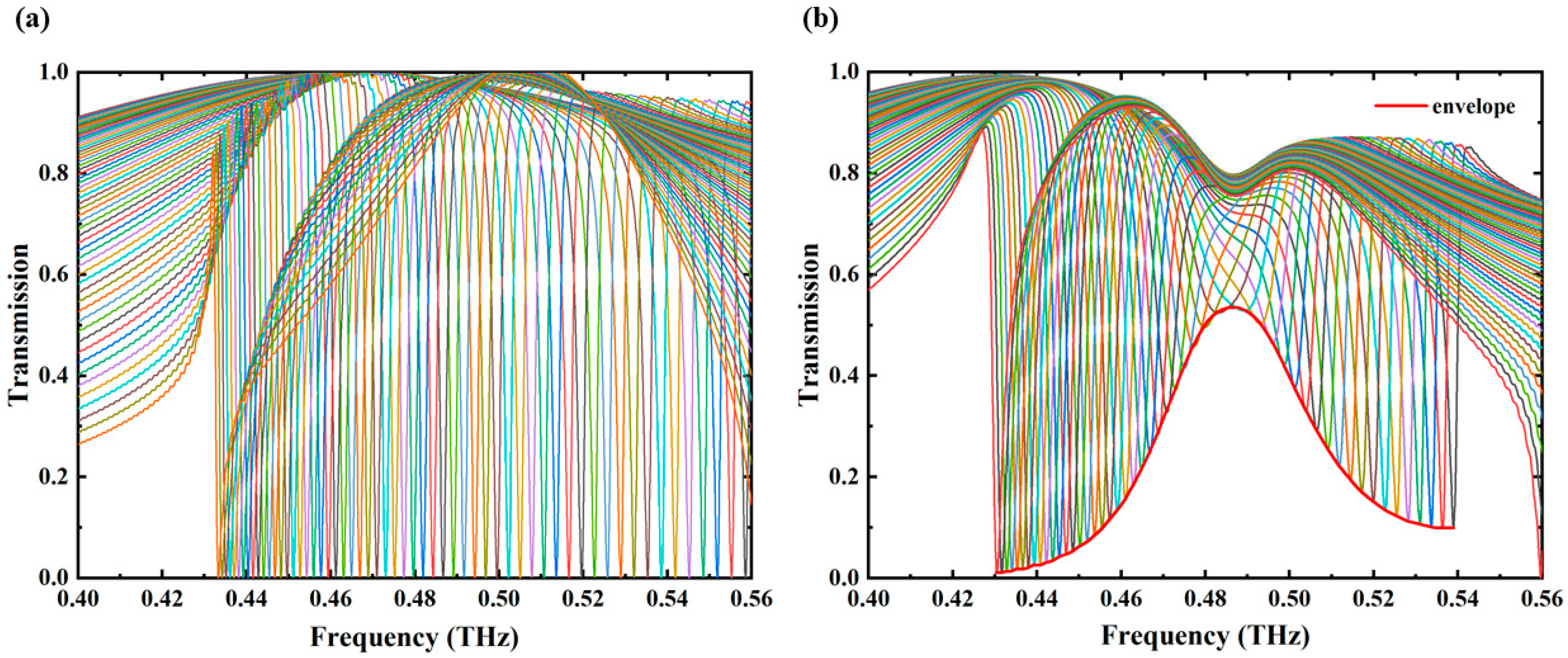
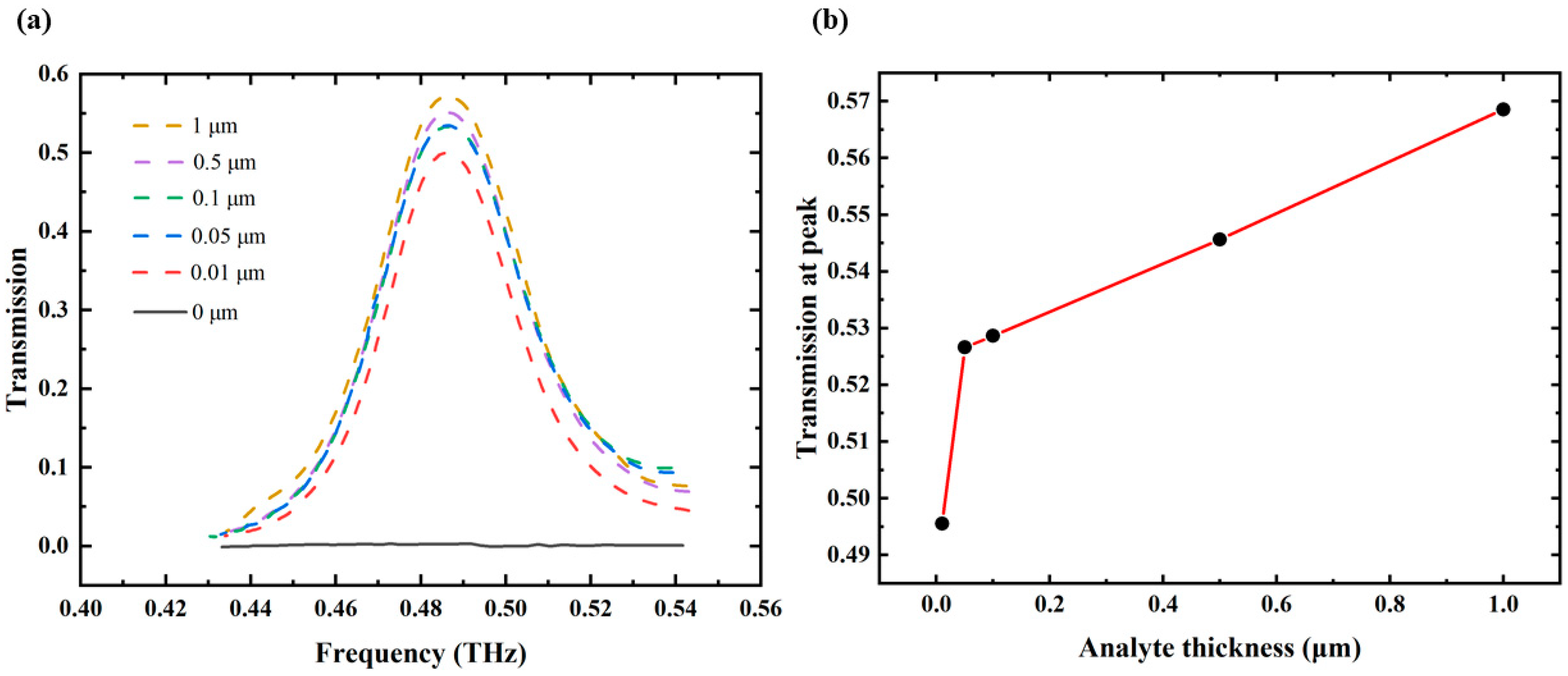
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pawar, A.Y.; Sonawane, D.D.; Erande, K.B.; Derle, D.V. Terahertz technology and its applications. Drug Invent. Today 2013, 5, 157–163. [Google Scholar] [CrossRef]
- Walther, M.; Plochocka, P.; Fischer, B.; Helm, H.; Uhd Jepsen, P. Collective vibrational modes in biological molecules investigated by terahertz time-domain spectroscopy. Biopolymers 2002, 67, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, S.; Lin, J.; Wang, W.; Chai, Z.; Sun, M.; Shi, Y.; Zhang, Y. Metasurface-based sensor with terahertz molecular fingerprint enhancement in trace additives identification. J. Phys. D Appl. Phys. 2024, 57, 235104. [Google Scholar] [CrossRef]
- Bi, H.; Yang, M.; You, R. Advances in terahertz metasurface graphene for biosensing and application. Discov. Nano 2023, 18, 63. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Mao, J.; Yang, F.; Wang, N. Metasurface-Assisted Terahertz Sensing. Sensors 2023, 23, 5902. [Google Scholar] [CrossRef] [PubMed]
- Withayachumnankul, W.; Fischer, B.M.; Abbott, D. Material thickness optimization for transmission-mode terahertz time-domain spectroscopy. Opt. Express 2008, 16, 7382–7396. [Google Scholar] [CrossRef]
- Beruete, M.; Jáuregui-López, I. Terahertz Sensing Based on Metasurfaces. Adv. Opt. Mater. 2019, 8, 1900721. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B.; Ahuja, R.; Kumar Mishra, Y. Terahertz plasmonics: The rise of toroidal metadevices towards immunobiosensings. Mater. Today 2020, 32, 108–130. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, F.; Xu, Y.; Mauk, M.G.; Qiu, X.; Tian, Z.; Zhang, L. Recent progress in terahertz biosensors based on artificial electromagnetic subwavelength structure. TrAC Trends Anal. Chem. 2023, 158, 116888. [Google Scholar] [CrossRef]
- Zhang, J.; Grischkowsky, D. Waveguide terahertz time-domain spectroscopy of nanometer water layers. Opt. Lett. 2004, 29, 1617–1619. [Google Scholar] [CrossRef]
- Cao, H.; Nahata, A. Resonantly enhanced transmission of terahertz radiation through a periodic array of subwavelength apertures. Opt. Express 2004, 12, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Sultana, J.; Biabanifard, M.; Vafapour, Z.; Nine, M.J.; Dinovitser, A.; Cordeiro, C.M.B.; Ng, B.W.H.; Abbott, D. Tunable localized surface plasmon graphene metasurface for multiband superabsorption and terahertz sensing. Carbon 2020, 158, 559–567. [Google Scholar] [CrossRef]
- Schurig, D.; Mock, J.J.; Justice, B.J.; Cummer, S.A.; Pendry, J.B.; Starr, A.F.; Smith, D.R. Metamaterial Electromagnetic Cloak at Microwave Frequencies. Science 2006, 314, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Luk’yanchuk, B.; Zheludev, N.I.; Maier, S.A.; Halas, N.J.; Nordlander, P.; Giessen, H.; Chong, C.T. The Fano resonance in plasmonic nanostructures and metamaterials. Nat. Mater. 2010, 9, 707–715. [Google Scholar] [CrossRef]
- Hajati, Y. Tunable broadband multiresonance graphene terahertz sensor. Opt. Mater. 2020, 101, 109725. [Google Scholar] [CrossRef]
- Wang, K.; Mittleman, D.M. Metal wires for terahertz wave guiding. Nature 2004, 432, 376–379. [Google Scholar] [CrossRef]
- Bohn, J.; Bucher, T.; Chong, K.E.; Komar, A.; Choi, D.Y.; Neshev, D.N.; Kivshar, Y.S.; Pertsch, T.; Staude, I. Active Tuning of Spontaneous Emission by Mie-Resonant Dielectric Metasurfaces. Nano Lett. 2018, 18, 3461–3465. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Jacob, Z. All-dielectric metamaterials. Nat. Nanotechnol. 2016, 11, 23–36. [Google Scholar] [CrossRef]
- Smith, D.R.; Vier, D.C.; Koschny, T.; Soukoulis, C.M. Electromagnetic parameter retrieval from inhomogeneous metamaterials. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2005, 71, 036617. [Google Scholar] [CrossRef]
- Sautter, J.; Staude, I.; Decker, M.; Rusak, E.; Neshev, D.N.; Brener, I.; Kivshar, Y.S. Active tuning of all-dielectric metasurfaces. ACS Nano 2015, 9, 4308–4315. [Google Scholar] [CrossRef]
- Chung, T.; Wang, H.; Cai, H. Dielectric metasurfaces for next-generation optical biosensing: A comparison with plasmonic sensing. Nanotechnology 2023, 34, 402001. [Google Scholar] [CrossRef] [PubMed]
- Bark, H.S.; Kim, G.J.; Jeon, T.-I. Transmission characteristics of all-dielectric guided-mode resonance filter in the THz region. Sci. Rep. 2018, 8, 13570. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Liu, J.; Chen, W.; Cheng, Y.-Y.; You, K.-W.; Fan, Z.-C.; Ye, Q.; Huang, P.-H.; Chen, Y.-S. Study on the enhancement mechanism of terahertz molecular fingerprint sensing. Results Phys. 2022, 39, 105766. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Qin, J. A terahertz metasurface sensor with fingerprint enhancement in a wide spectrum band for thin film detection. Nanoscale Adv. 2023, 5, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Nutley, B.P.; Farmer, P.; Caldwell, J. Metabolism of trans-cinnamic acid in the rat and the mouse and its variation with dose. Food Chem. Toxicol. 1994, 32, 877–886. [Google Scholar] [CrossRef]
- Butte, N.F. Carbohydrate and lipid metabolism in pregnancy: Normal compared with gestational diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 1256S–1261S. [Google Scholar] [CrossRef]
- Hadden, D.R.; McLaughlin, C. Normal and abnormal maternal metabolism during pregnancy. Semin. Fetal Neonatal Med. 2009, 14, 66–71. [Google Scholar] [CrossRef]
- Schneider, S.; Freerksen, N.; Röhrig, S.; Hoeft, B.; Maul, H. Gestational diabetes and preeclampsia—Similar risk factor profiles? Early Hum. Dev. 2012, 88, 179–184. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Ferrara, A.; Sacks, D.A. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: Association with increased risk of spontaneous preterm birth. Obstet. Gynecol. 2003, 102, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Hillier, T.A.; Brown, J.B. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am. J. Med. 2008, 121, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, A.J.; Spichler, E.R.; Branchtein, L.; Nucci, L.B.; Franco, L.J.; Schmidt, M.I.; Brazilian Study of Gestational Diabetes Working, G. Fasting Plasma Glucose Is a Useful Test for the Detection of Gestational Diabetes. Diabetes Care 1998, 21, 1246–1249. [Google Scholar] [CrossRef]
- Bogdanet, D.; O’Shea, P.; Lyons, C.; Shafat, A.; Dunne, F. The Oral Glucose Tolerance Test—Is It Time for a Change?—A Literature Review with an Emphasis on Pregnancy. J. Clin. Med. 2020, 9, 3451. [Google Scholar] [CrossRef]
- Kattini, R.; Hummelen, R.; Kelly, L. Early Gestational Diabetes Mellitus Screening With Glycated Hemoglobin: A Systematic Review. J. Obstet. Gynaecol. Can. 2020, 42, 1379–1384. [Google Scholar] [CrossRef]
- Adisakwattana, S. Cinnamic Acid and Its Derivatives: Mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef]
- de Boer, I.H.; Gao, X.; Bebu, I.; Hoofnagle, A.N.; Lachin, J.M.; Paterson, A.; Perkins, B.A.; Saenger, A.K.; Steffes, M.W.; Zinman, B.; et al. Biomarkers of tubulointerstitial damage and function in type 1 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000461. [Google Scholar] [CrossRef]
- Menni, C.; Zhu, J.; Le Roy, C.I.; Mompeo, O.; Young, K.; Rebholz, C.M.; Selvin, E.; North, K.E.; Mohney, R.P.; Bell, J.T.; et al. Serum metabolites reflecting gut microbiome alpha diversity predict type 2 diabetes. Gut Microbes 2020, 11, 1632–1642. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, X.; Bai, C.; Zhao, C.; Lu, G.; Xu, G. LC–MS-based metabonomics analysis. J. Chromatogr. B 2008, 866, 64–76. [Google Scholar] [CrossRef]
- Abina, A.; Korošec, T.; Puc, U.; Jazbinšek, M.; Zidanšek, A. Urinary Metabolic Biomarker Profiling for Cancer Diagnosis by Terahertz Spectroscopy: Review and Perspective. Photonics 2023, 10, 1051. [Google Scholar] [CrossRef]
- Hou, X.; Chen, X.; Li, T.; Li, Y.; Tian, Z.; Wang, M. Highly sensitive terahertz metamaterial biosensor for bovine serum albumin (BSA) detection. Opt. Mater. Express 2021, 11, 2268–2277. [Google Scholar] [CrossRef]
- Yan, S.; Xia, L.; Wei, D.; Cui, H.L.; Du, C. Terahertz biosensing of protein based on a metamaterial. In Proceedings of the 2016 IEEE International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale (3M-NANO), Chongqing, China, 18–22 July 2016; pp. 327–330. [Google Scholar]
- Geng, Z.; Zhang, X.; Fan, Z.; Lv, X.; Chen, H. A Route to Terahertz Metamaterial Biosensor Integrated with Microfluidics for Liver Cancer Biomarker Testing in Early Stage. Sci. Rep. 2017, 7, 16378. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, M.; Tang, X.; Zhang, W.; Luo, D.; Chen, M. Micro/Nano Technology for Next-Generation Diagnostics. Small Methods 2019, 4, 1900506. [Google Scholar] [CrossRef]
- Yang, J.; Long, J.; Yang, L. First-principles investigations of the physical properties of lithium niobate and lithium tantalate. Phys. B Condens. Matter 2013, 425, 12–16. [Google Scholar] [CrossRef]
- Basharin, A.A.; Kafesaki, M.; Economou, E.N.; Soukoulis, C.M.; Fedotov, V.A.; Savinov, V.; Zheludev, N.I. Dielectric Metamaterials with Toroidal Dipolar Response. Phys. Rev. X 2015, 5, 011036. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Z.; Du, Y.; Qin, J. Ultrasensitive terahertz sensing with high-Q toroidal dipole resonance governed by bound states in the continuum in all-dielectric metasurface. Nanophotonics 2021, 10, 1295–1307. [Google Scholar] [CrossRef]
- Sánchez, C.; Agulló-López, F. Transient Effects in the Room-Temperature F-Colouring of NaCl Irradiated with X- or γ-rays. Phys. Status Solidi (b) 2006, 29, 217–230. [Google Scholar] [CrossRef]
- Tuz, V.R.; Khardikov, V.V.; Kivshar, Y.S. All-Dielectric Resonant Metasurfaces with a Strong Toroidal Response. ACS Photonics 2018, 5, 1871–1876. [Google Scholar] [CrossRef]
- Dorney, T.D.; Baraniuk, R.G.; Mittleman, D.M. Material parameter estimation with terahertz time-domain spectroscopy. J. Opt. Soc. Am. A 2001, 18, 1562–1571. [Google Scholar] [CrossRef]
- Liu, B.; Chen, S.; Zhang, J.; Yao, X.; Zhong, J.; Lin, H.; Huang, T.; Yang, Z.; Zhu, J.; Liu, S.; et al. A Plasmonic Sensor Array with Ultrahigh Figures of Merit and Resonance Linewidths down to 3 nm. Adv. Mater. 2018, 30, e1706031. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Sun, M.; Wang, W.; Shi, Y. All-Dielectric Metasurface-Based Terahertz Molecular Fingerprint Sensor for Trace Cinnamoylglycine Detection. Biosensors 2024, 14, 440. https://doi.org/10.3390/bios14090440
Xu Q, Sun M, Wang W, Shi Y. All-Dielectric Metasurface-Based Terahertz Molecular Fingerprint Sensor for Trace Cinnamoylglycine Detection. Biosensors. 2024; 14(9):440. https://doi.org/10.3390/bios14090440
Chicago/Turabian StyleXu, Qiyuan, Mingjun Sun, Weijin Wang, and Yanpeng Shi. 2024. "All-Dielectric Metasurface-Based Terahertz Molecular Fingerprint Sensor for Trace Cinnamoylglycine Detection" Biosensors 14, no. 9: 440. https://doi.org/10.3390/bios14090440
APA StyleXu, Q., Sun, M., Wang, W., & Shi, Y. (2024). All-Dielectric Metasurface-Based Terahertz Molecular Fingerprint Sensor for Trace Cinnamoylglycine Detection. Biosensors, 14(9), 440. https://doi.org/10.3390/bios14090440





