NIR-Sensitive Squaraine Dye—Peptide Conjugate for Trypsin Fluorogenic Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Theoretical Structure Calculation
2.3. Instrumentation
2.4. Spectroscopic Measurements
2.5. Determination of Detection Limit
2.6. Fluorescence Quenching Efficiency
3. Results
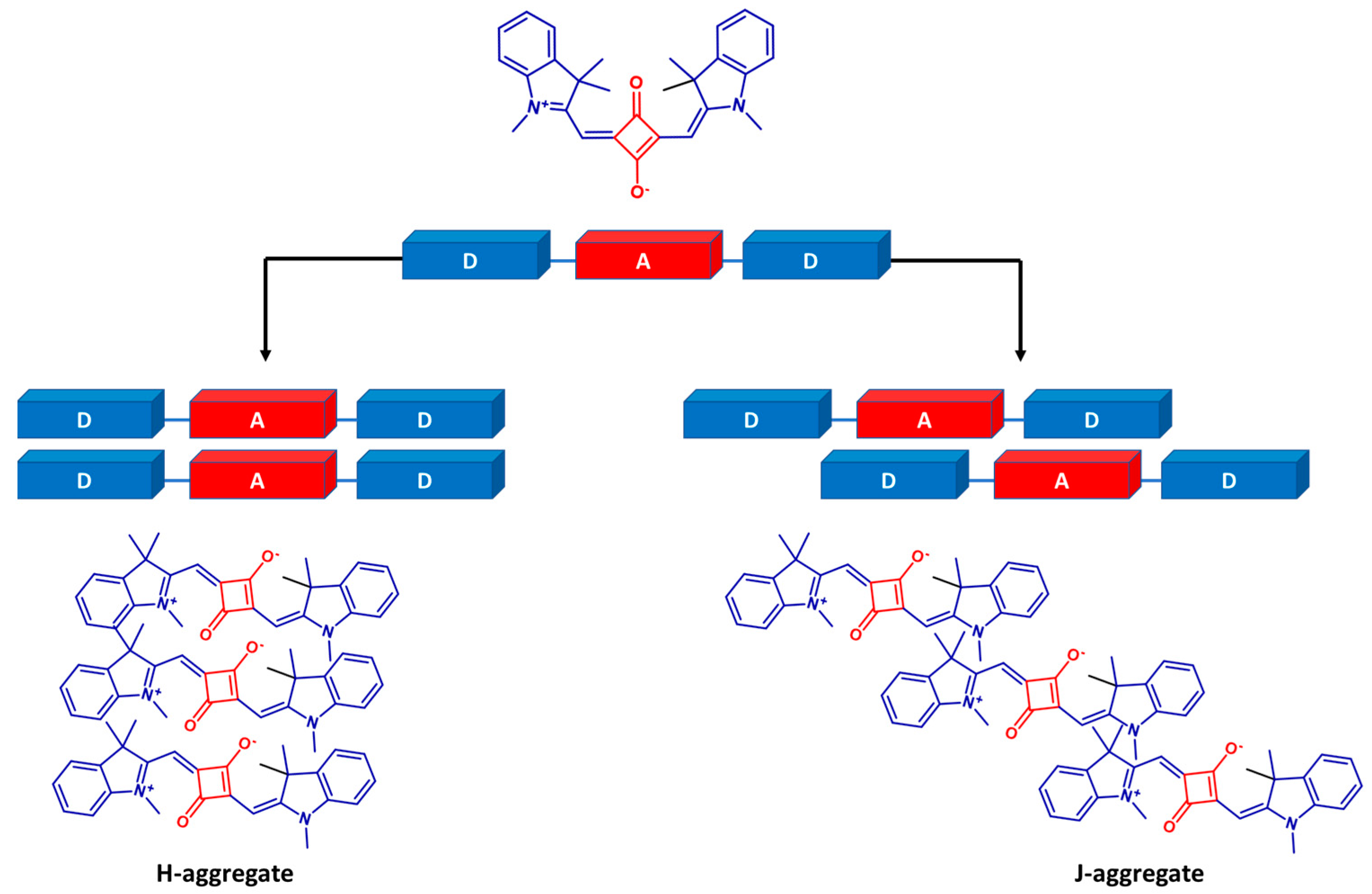
3.1. Design and Synthesis of Target Molecules
3.2. Optical Characterization of Squaraine Dyes and Dye–Peptide Conjugate
3.3. Enzymatic Hydrolysis of SQ-3-PC with Trypsin
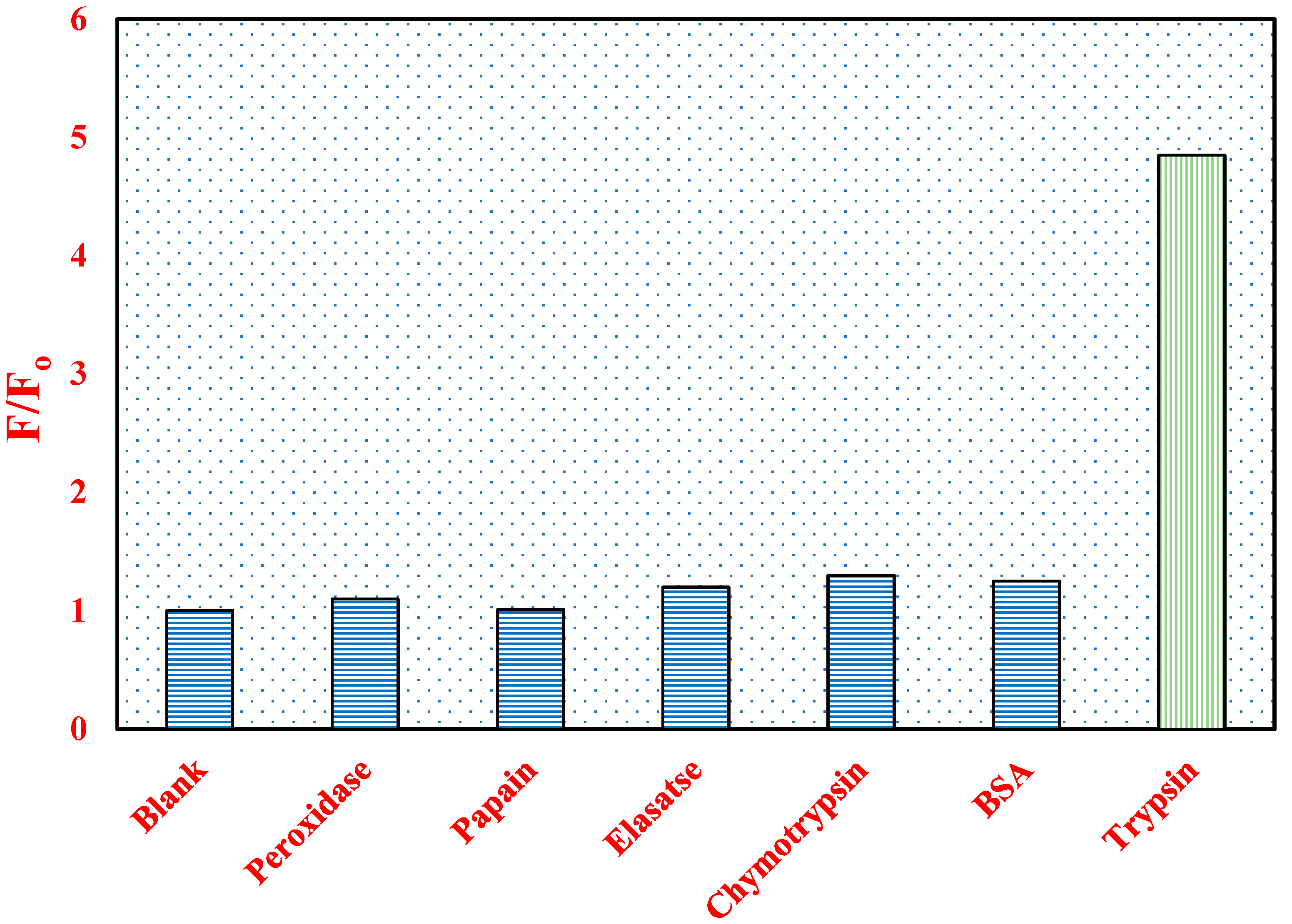
3.4. Enzyme Selectivity of the Enzyme Probe SQ-3-PC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Otín, C.; Bond, J.S. Proteases: Multifunctional Enzymes in Life and Disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef] [PubMed]
- Lasher, D.; Szabó, A.; Masamune, A.; Chen, J.-M.; Xiao, X.; Whitcomb, D.C.; Barmada, M.M.; Ewers, M.; Ruffert, C.; Paliwal, S.; et al. Protease-Sensitive Pancreatic Lipase Variants Are Associated with Early Onset Chronic Pancreatitis. Am. J. Gastroenterol. 2019, 114, 974–983. [Google Scholar] [CrossRef]
- Malla, S.R.; Krueger, B.; Wartmann, T.; Sendler, M.; Mahajan, U.M.; Weiss, F.U.; Thiel, F.G.; De Boni, C.; Gorelick, F.S.; Halangk, W.; et al. Early Trypsin Activation Develops Independently of Autophagy in Caerulein-Induced Pancreatitis in Mice. Cell. Mol. Life Sci. 2020, 77, 1811–1825. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Campbell-Thompson, M.; Kusmartseva, I.; Kaestner, K.H. Organisation of the Human Pancreas in Health and in Diabetes. Diabetologia 2020, 63, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Hoffert, U.; Rogalski, C.; Seifert, S.; Schmeling, G.; Wingertszahn, J.; Proksch, E.; Wiedow, O. Trypsin Induces Epidermal Proliferation and Inflammation in Murine. Skin. Exp. Dermatol. 2004, 13, 234–241. [Google Scholar] [CrossRef]
- Rakash, S. Role of Proteases in Cancer: A Review. Biotechnol. Mol. Biol. Rev. 2012, 7, 90–101. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Al-Obaidy, S.S.M.; Al-Kawaz, H.S.; Almashhedy, L.A.; Kadhum, M.A.; Khudhair, D.A.; Hadwan, A.M.; Hadwan, M.M. An Optimized Protocol to Assess Trypsin Activity in Biological Samples. Monatshefte Für Chem. Chem. Mon. 2023, 154, 267–277. [Google Scholar] [CrossRef]
- Park, T.; Han, M.; Schanze, K.S.; Lee, S.H. Ultrasensitive Determination of Trypsin in Human Urine Based on Amplified Fluorescence Response. ACS Sens. 2023, 8, 2591–2597. [Google Scholar] [CrossRef]
- Soreide, K.; Janssen, E.; Körner, H.; Baak, J. Trypsin in Colorectal Cancer: Molecular Biological Mechanisms of Proliferation, Invasion, and Metastasis. J. Pathol. 2006, 209, 147–156. [Google Scholar] [CrossRef]
- Gaber, A.; Johansson, M.; Stenman, U.-H.; Hotakainen, K.; Pontén, F.; Glimelius, B.; Bjartell, A.; Jirström, K.; Birgisson, H. High Expression of Tumour-Associated Trypsin Inhibitor Correlates with Liver Metastasis and Poor Prognosis in Colorectal Cancer. Br. J. Cancer 2009, 100, 1540–1548. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Torgashina, I.G.; Nemtseva, E.V.; Antashkevich, A.A.; Sasova, P.Y.; Kratasyuk, V.A. Trypsin-Based Chemoenzymatic Assay for Detection of Pollutants and Safety Assessment of Food Additives. Chemosensors 2023, 11, 237. [Google Scholar] [CrossRef]
- Woessmann, J.; Petrosius, V.; Üresin, N.; Kotol, D.; Aragon-Fernandez, P.; Hober, A.; Auf Dem Keller, U.; Edfors, F.; Schoof, E.M. Assessing the Role of Trypsin in Quantitative Plasma and Single-Cell Proteomics toward Clinical Application. Anal. Chem. 2023, 95, 13649–13658. [Google Scholar] [CrossRef] [PubMed]
- Rainio, M.; Lindström, O.; Penttilä, A.; Itkonen, O.; Kemppainen, E.; Stenman, U.-H.; Kylänpää, L. Serum Serine Peptidase Inhibitor Kazal-Type 1, Trypsinogens 1 to 3, and Complex of Trypsin 2 and A1-Antitrypsin in the Diagnosis of Severe Acute Pancreatitis. Pancreas 2019, 48, 374–380. [Google Scholar] [CrossRef]
- Boyer, A.E.; Moura, H.; Woolfitt, A.R.; Kalb, S.R.; McWilliams, L.G.; Pavlopoulos, A.; Schmidt, J.G.; Ashley, D.L.; Barr, J.R. From the Mouse to the Mass Spectrometer: Detection and Differentiation of the Endoproteinase Activities of Botulinum Neurotoxins A−G by Mass Spectrometry. Anal. Chem. 2005, 77, 3916–3924. [Google Scholar] [CrossRef]
- Kim, Y.-P.; Lee, B.S.; Kim, E.; Choi, I.S.; Moon, D.W.; Lee, T.G.; Kim, H.-S. Activity-Based Assay of Matrix Metalloproteinase on Nonbiofouling Surfaces Using Time-of-Flight Secondary Ion Mass Spectrometry. Anal. Chem. 2008, 80, 5094–5102. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D. Gel Electrophoresis of Proteolytic Enzymes. Anal. Chim. Acta 1998, 372, 173–185. [Google Scholar] [CrossRef]
- Abuknesha, R.A.; Jeganathan, F.; DeGroot, R.; Wildeboer, D.; Price, R.G. Detection of Proteases Using an Immunochemical Method with Haptenylated–Gelatin as a Solid-Phase Substrate. Anal. Bioanal. Chem. 2010, 396, 2547–2558. [Google Scholar] [CrossRef]
- Steiner, V.J.M.; Williams, D.A.; Moeller, E.M.; Melgarejo, T. Development and Validation of an Enzyme-Linked Immunosorbent Assay for Feline Trypsin-like Immunoreactivity. Am. J. Vet. Res. 2000, 61, 620–623. [Google Scholar] [CrossRef]
- Li, Y.; Bai, H.; Li, C.; Shi, G. Colorimetric Assays for Acetylcholinesterase Activity and Inhibitor Screening Based on the Disassembly−Assembly of a Water-Soluble Polythiophene Derivative. ACS Appl. Mater. Interfaces 2011, 3, 1306–1310. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, G.; Zhang, D. A Sensitive Colorimetric Label-Free Assay for Trypsin and Inhibitor Screening with Gold Nanoparticles. Analyst 2011, 136, 3136. [Google Scholar] [CrossRef]
- Gu, X.; Yang, G.; Zhang, G.; Zhang, D.; Zhu, D. A New Fluorescence Turn-on Assay for Trypsin and Inhibitor Screening Based on Graphene Oxide. ACS Appl. Mater. Interfaces 2011, 3, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Zhang, G.; Zhang, D.; Zhu, D. A New Label-Free Continuous Fluorometric Assay for Trypsin and Inhibitor Screening with Tetraphenylethene Compounds. Org. Lett. 2010, 12, 2274–2277. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Singh, P.K. Trypsin Detection Strategies: A Review. Crit. Rev. Anal. Chem. 2022, 52, 949–967. [Google Scholar] [CrossRef] [PubMed]
- Ong, I.L.H.; Yang, K.-L. Recent Developments in Protease Activity Assays and Sensors. Analyst 2017, 142, 1867–1881. [Google Scholar] [CrossRef]
- Gutiérrez, O.A.; Salas, E.; Hernández, Y.; Lissi, E.A.; Castrillo, G.; Reyes, O.; Garay, H.; Aguilar, A.; Garcia, B.; Otero, A.; et al. An Immunoenzymatic Solid-Phase Assay for Quantitative Determination of HIV-1 Protease Activity. Anal. Biochem. 2002, 307, 18–24. [Google Scholar] [CrossRef]
- Priyanka; Bila, G.; Mavileti, S.K.; Bila, E.; Negrych, N.; Gupta, S.; Tang, L.; Bilyy, R.; Pandey, S.S.; Kato, T. A Biocompatible NIR Squaraine Dye and Dye-Antibody Conjugates for Versatile Long-Term in Vivo Fluorescence Bioimaging. Mater. Adv. 2024, 5, 3940–3949. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, L.; Li, X.; Chen, H.; Wang, Z.; Yang, W.; Zhang, H.; Zhang, H. Peptide Modified Manganese-Doped Iron Oxide Nanoparticles as a Sensitive Fluorescence Nanosensor for Non-Invasive Detection of Trypsin Activity in Vitro and in Vivo. RSC Adv. 2021, 11, 2213–2220. [Google Scholar] [CrossRef]
- Cheng, M.; Zhou, J.; Zhou, X.; Xing, D. Peptide Cleavage Induced Assembly Enables Highly Sensitive Electrochemiluminescence Detection of Protease Activity. Sens. Actuators B Chem. 2018, 262, 516–521. [Google Scholar] [CrossRef]
- Zhuo, C.-X.; Wang, L.-H.; Feng, J.-J.; Zhang, Y.-D. Label-Free Fluorescent Detection of Trypsin Activity Based on DNA-Stabilized Silver Nanocluster-Peptide Conjugates. Sensors 2016, 16, 1477. [Google Scholar] [CrossRef]
- Scott, J.I.; Deng, Q.; Vendrell, M. Near-Infrared Fluorescent Probes for the Detection of Cancer-Associated Proteases. ACS Chem. Biol. 2021, 16, 1304–1317. [Google Scholar] [CrossRef]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent Progress in the Development of Near-Infrared Fluorescent Probes for Bioimaging Applications. Chem. Soc. Rev. 2013, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Khopkar, S.; Shankarling, G. Synthesis, Photophysical Properties and Applications of NIR Absorbing Unsymmetrical Squaraines: A Review. Dyes Pigment. 2019, 170, 107645. [Google Scholar] [CrossRef]
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjug. Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, Y.; Shen, K.; Lu, J.; Zhang, Z.; Yi, D.; Hao, N.; Fu, Q.; Ye, Z.; Wei, J.; et al. NIR Squaraine Dyes for Dual Colorimetric and Fluorescent Determination of Fe3+, Cu2+, and Hg2+ Ions. RSC Adv. 2023, 13, 17202–17211. [Google Scholar] [CrossRef]
- Barcenas, G.; Biaggne, A.; Mass, O.A.; Wilson, C.K.; Obukhova, O.M.; Kolosova, O.S.; Tatarets, A.L.; Terpetschnig, E.; Pensack, R.D.; Lee, J.; et al. First-Principles Studies of Substituent Effects on Squaraine Dyes. RSC Adv. 2021, 11, 19029–19040. [Google Scholar] [CrossRef]
- Chen, H.; Farahat, M.S.; Law, K.-Y.; Whitten, D.G. Aggregation of Surfactant Squaraine Dyes in Aqueous Solution and Microheterogeneous Media: Correlation of Aggregation Behavior with Molecular Structure. J. Am. Chem. Soc. 1996, 118, 2584–2594. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, Z.; Huang, Y.; Gao, Q.; Luo, C.; Mehdi, M.; Xu, Y.; Li, H.; Sun, S. A Bovine Serum Albumin and Squaraine Dye Assembly Fluorescent Probe for Pepsin Detection. Microchem. J. 2023, 186, 108361. [Google Scholar] [CrossRef]
- Butnarasu, C.; Pontremoli, C.; Moran Plata, M.J.; Barbero, N.; Visentin, S. Squaraine Dyes as Fluorescent Turn-on Probes for Mucins: A Step Toward Selectivity†. Photochem. Photobiol. 2023, 99, 562–569. [Google Scholar] [CrossRef]
- Gupta, S.; Mavileti, S.; Pandey, S.S.; Kato, T. Design and Synthesis of Novel Squaraine-Based Fluorescent Probe for Far-Red Detection of Chymotrypsin Enzyme. Molecules 2024, 29, 1282. [Google Scholar] [CrossRef]
- Gaussian.Com. Expanding the Limits of Computational Chemistry. Available online: https://gaussian.com/ (accessed on 1 August 2024).
- Mayerhöffer, U.; Fimmel, B.; Würthner, F. Bright Near-Infrared Fluorophores Based on Squaraines by Unexpected Halogen Effects. Angew. Chem. Int. Ed. 2012, 51, 164–167. [Google Scholar] [CrossRef]
- Soumya, M.S.; Abraham, A. Preclinical Evaluation of Symmetrical Iiodinated Squaraine Dye on Experimental Animal Models. J. Glycobiol. S 2013, 1. [Google Scholar] [CrossRef]
- Olsen, J.V.; Ong, S.-E.; Mann, M. Trypsin Cleaves Exclusively C-Terminal to Arginine and Lysine Residues. Mol. Cell. Proteom. 2004, 3, 608–614. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, G.; Marchena, M.; Zitnan, M.; Pandey, S.S.; Hayase, S.; Douhal, A. Femto to Millisecond Observations of Indole-Based Squaraine Molecules Photodynamics in Solution. Phys. Chem. Chem. Phys. 2012, 14, 1796–1805. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yan, Z.; Xu, H. Advances in Synthesis and Application of Near-Infrared Absorbing Squaraine Dyes. RSC Adv. 2013, 3, 7667. [Google Scholar] [CrossRef]
- Yum, J.H.; Moon, S.J.; Humphry-Baker, R.; Walter, P.; Geiger, T.; Nüesch, F.; Grätzel, M.; Nazeeruddin, M.D.K. Effect of Coadsorbent on the Photovoltaic Performance of Squaraine Sensitized Nanocrystalline Solar Cells. Nanotechnology 2008, 19, 424005. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sasabe, H.; Lu, W.; Wang, X.-F.; Kido, J.; Hong, Z.; Yang, Y. J-Aggregation of a Squaraine Dye and Its Application in Organic Photovoltaic Cells. J. Mater. Chem. C 2013, 1, 6547. [Google Scholar] [CrossRef]
- Lake-Bakaar, G.; McKavanagh, S.; Redshaw, M.; Wood, T.; Summerfield, J.A.; Elias, E. Serum Immunoreactive Trypsin Concentration after a Lundh Meal. Its Value in the Diagnosis of Pancreatic Disease. J. Clin. Pathol. 1979, 32, 1003–1008. [Google Scholar] [CrossRef]
- Artigas, J.M.G.; Garcia, M.E.; Faure, M.R.A.; Gimeno, A.M.B. Serum Trypsin Levels in Acute Pancreatic and Non-Pancreatic Abdominal Conditions. Postgrad. Med. J. 1981, 57, 219–222. [Google Scholar] [CrossRef]
- Heinrich, H.C.; Gabbe, E.E.; Ičagić, F. Immunoreaktives Serum Trypsin bei Pankreaserkrankungen. Klin. Wochenschr. 1979, 57, 1237–1238. [Google Scholar] [CrossRef]
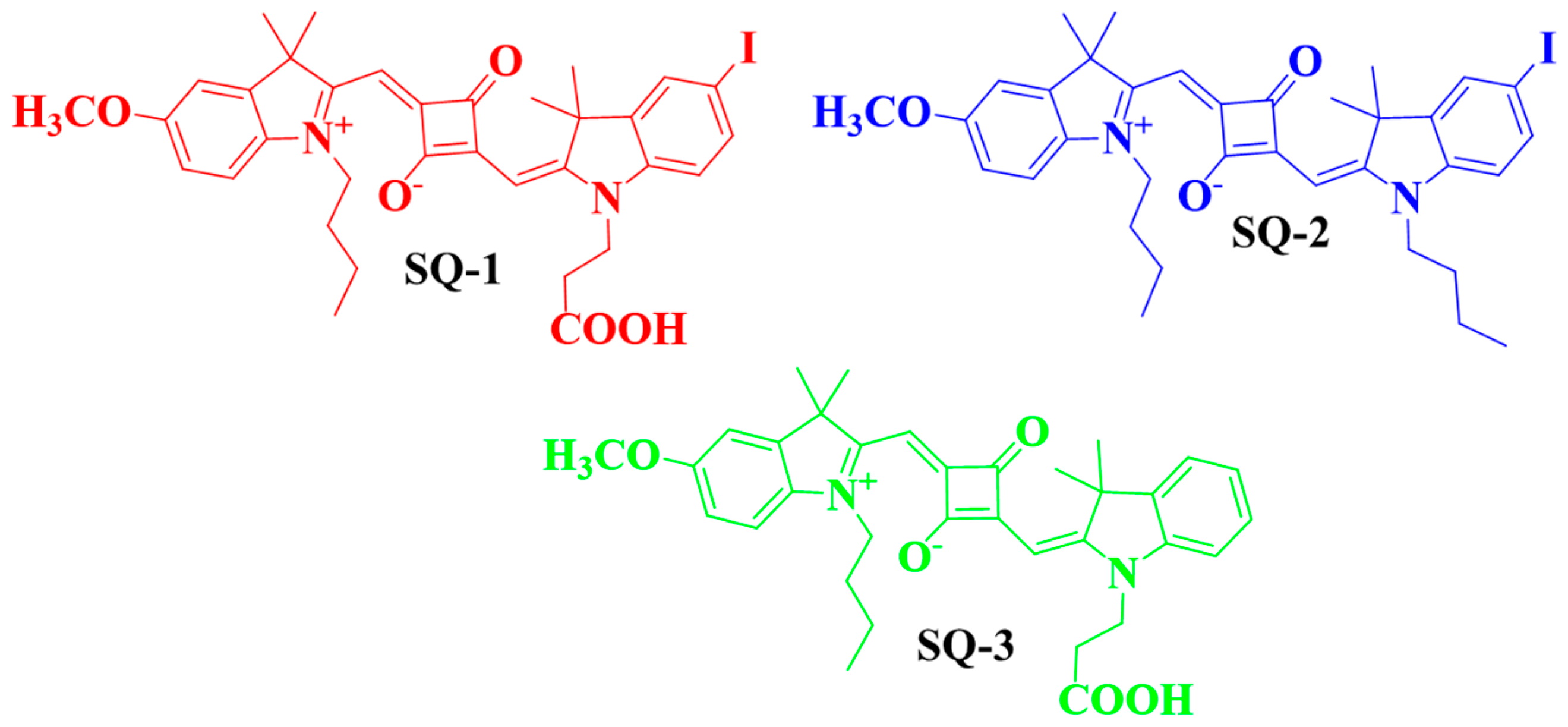
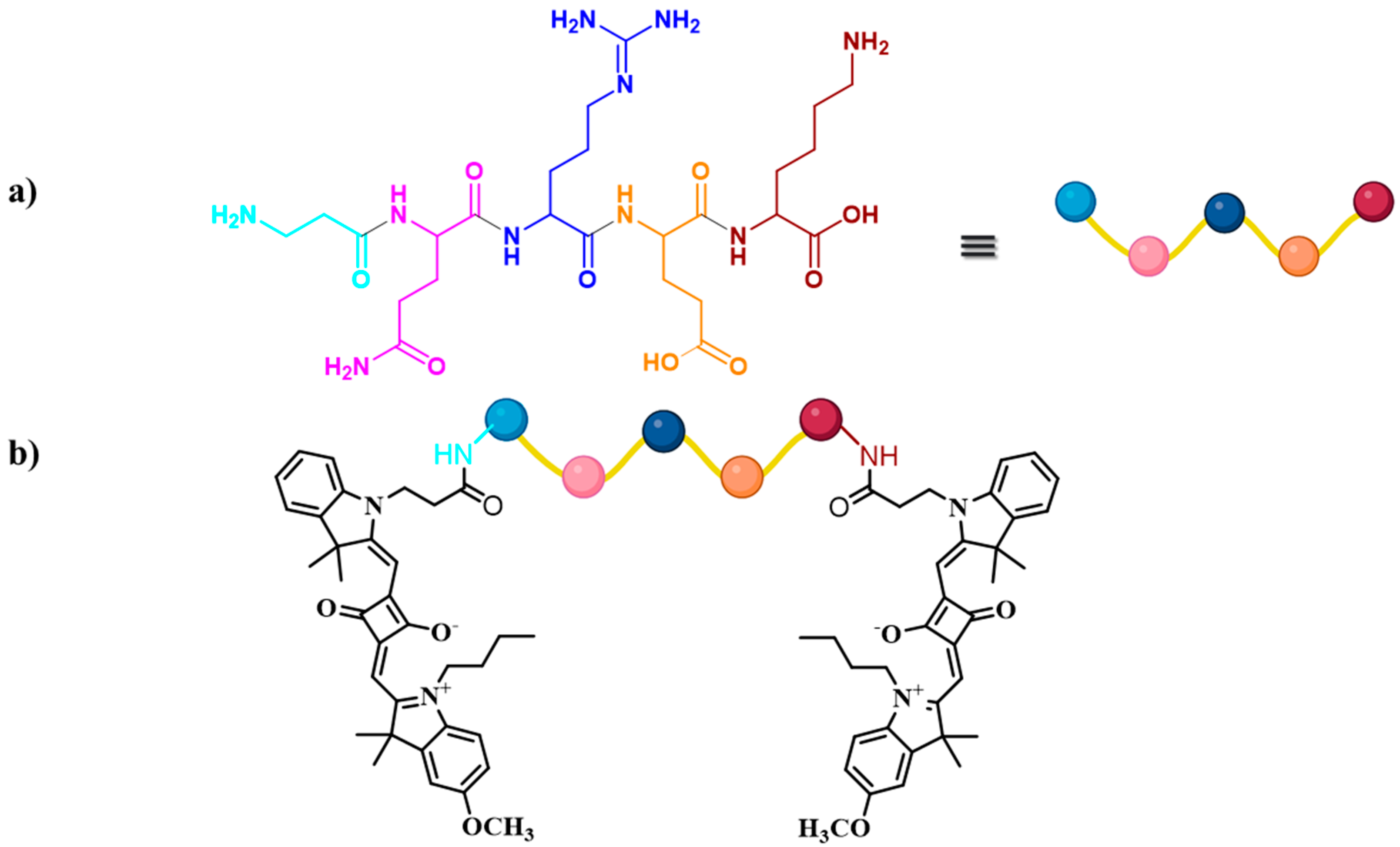
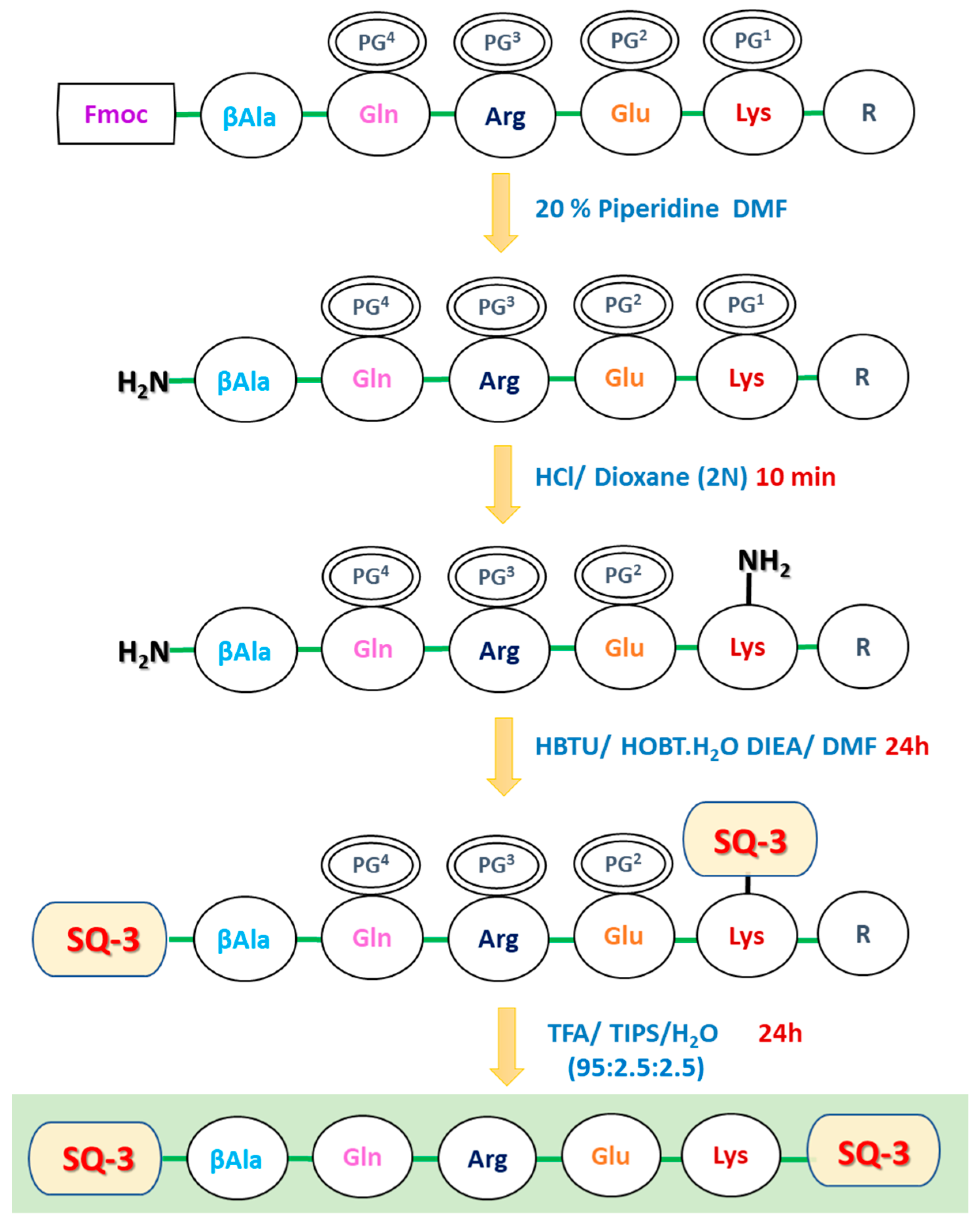
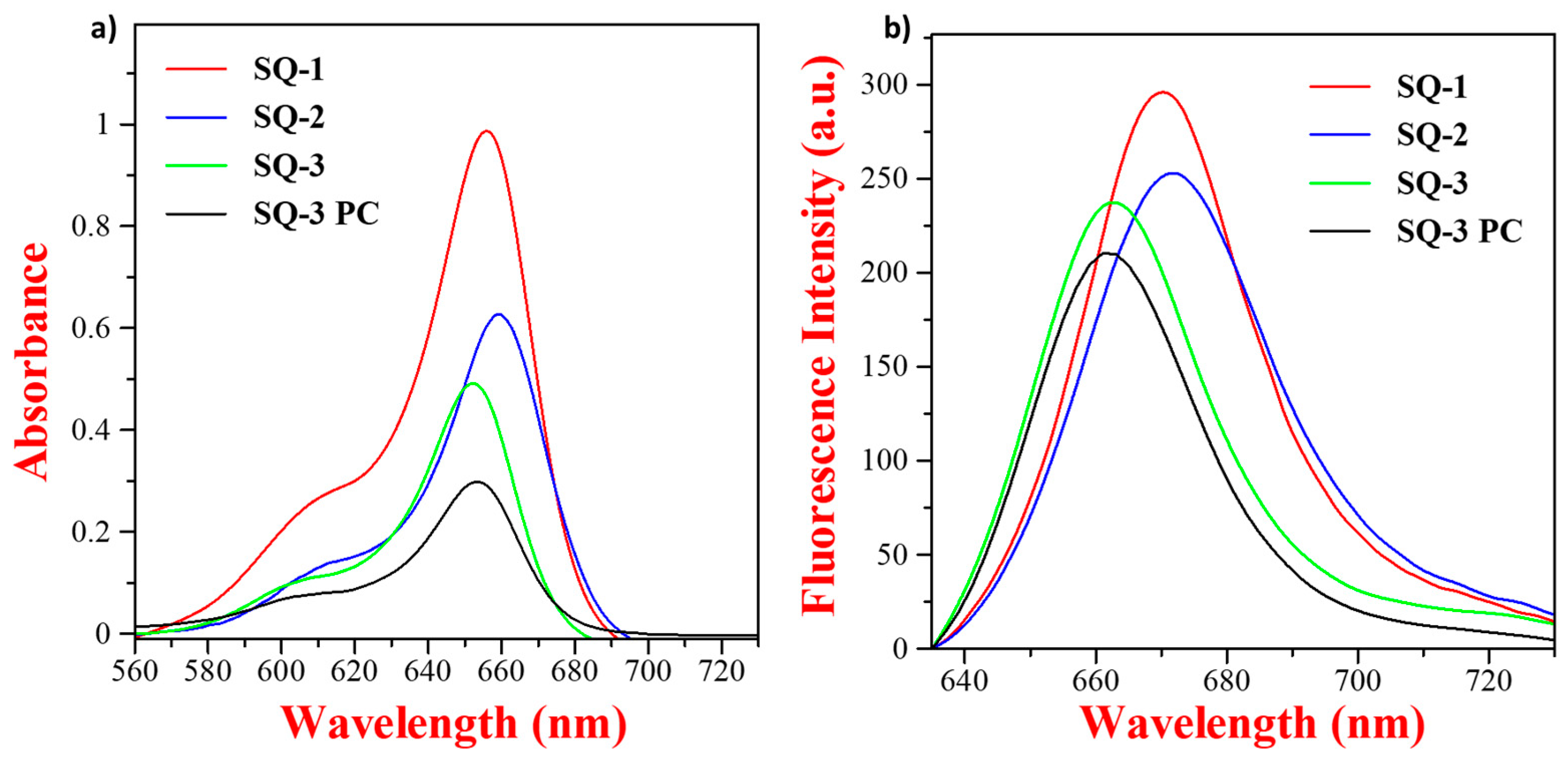
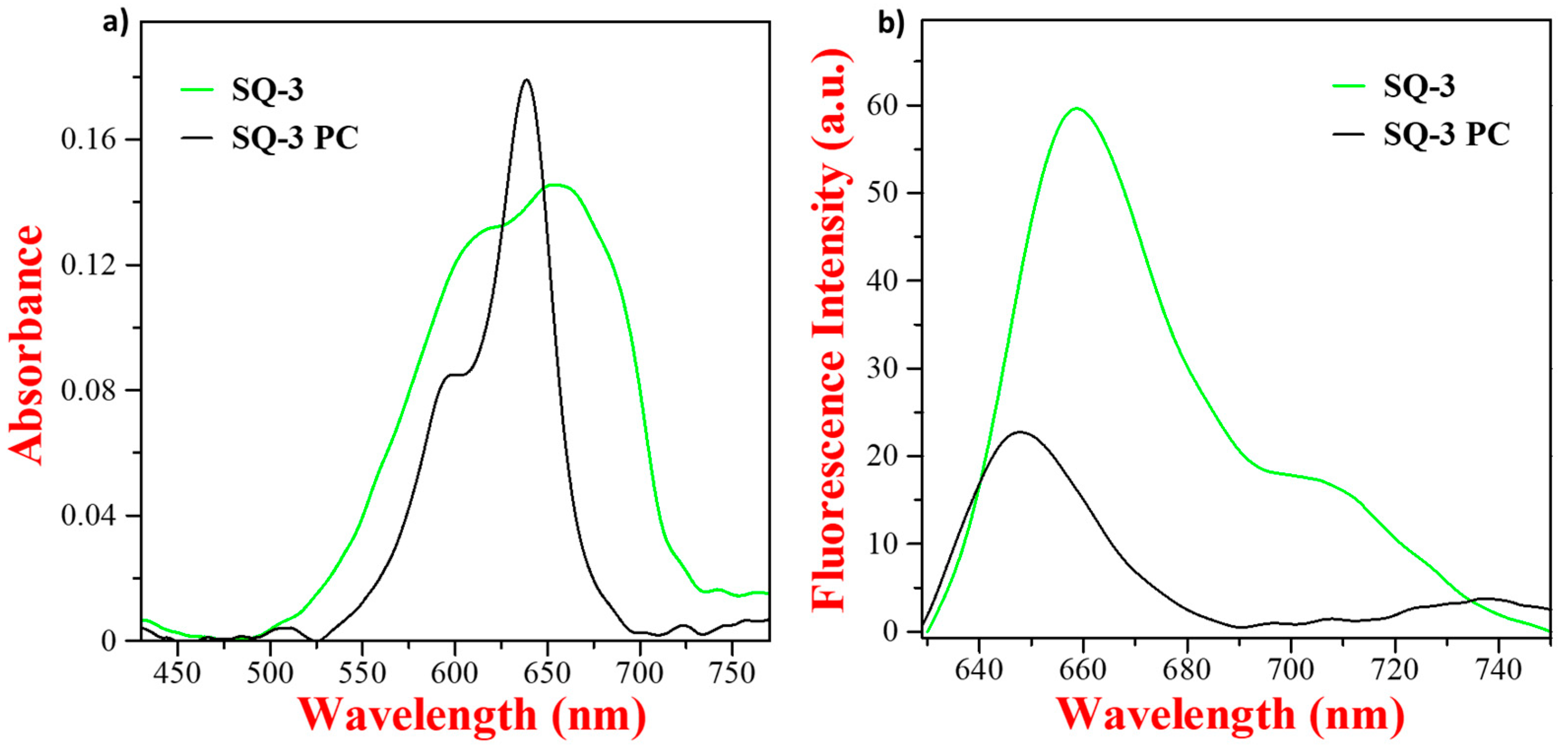
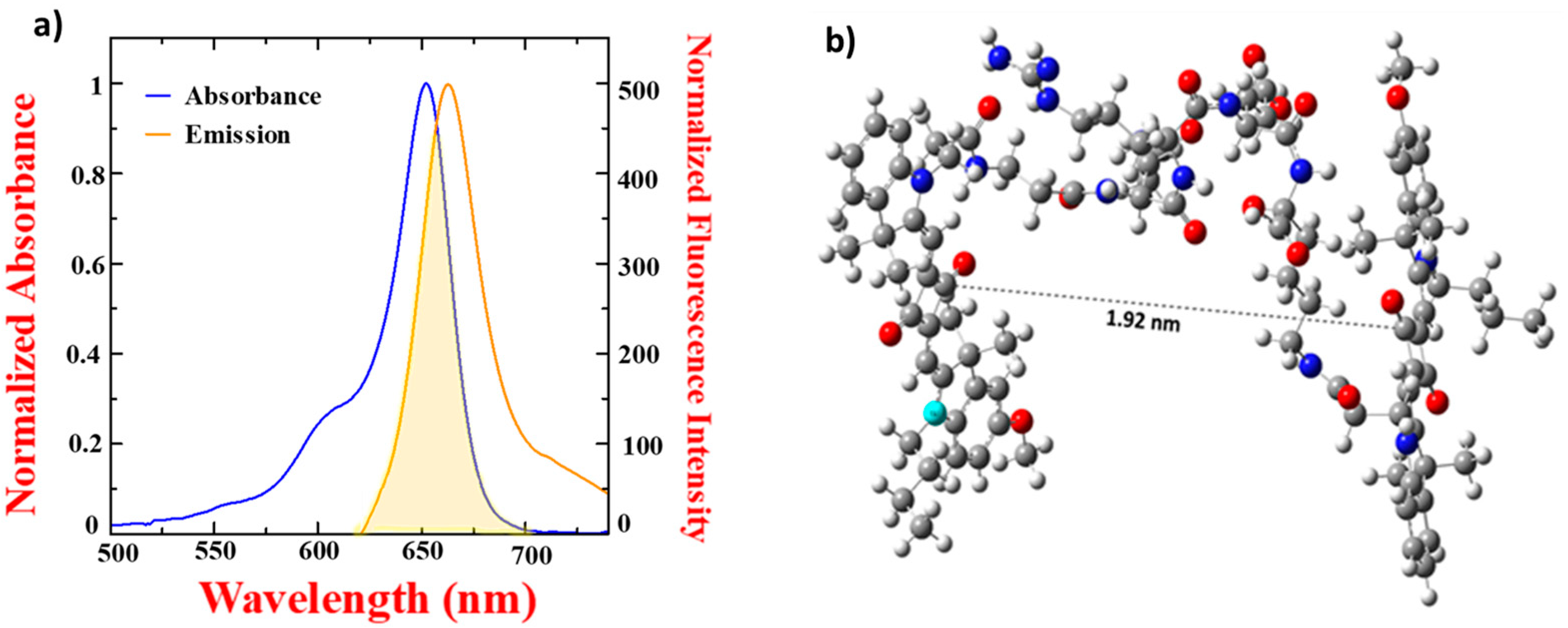

| Squaraine Dye | λabs | λem | Δ | ԑ (M−1cm−1) |
|---|---|---|---|---|
| SQ-1 | 656 nm | 671 nm | 15 | 1.98 × 105 |
| SQ-2 | 659 nm | 672 nm | 13 | 1.26 × 105 |
| SQ-3 | 652 nm | 663 nm | 11 | 1.00 × 105 |
| SQ-3 PC | 654 nm | 662 nm | 8 | 0.60 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balyan, P.; Gupta, S.; Mavileti, S.K.; Pandey, S.S.; Kato, T. NIR-Sensitive Squaraine Dye—Peptide Conjugate for Trypsin Fluorogenic Detection. Biosensors 2024, 14, 458. https://doi.org/10.3390/bios14100458
Balyan P, Gupta S, Mavileti SK, Pandey SS, Kato T. NIR-Sensitive Squaraine Dye—Peptide Conjugate for Trypsin Fluorogenic Detection. Biosensors. 2024; 14(10):458. https://doi.org/10.3390/bios14100458
Chicago/Turabian StyleBalyan, Priyanka, Shekhar Gupta, Sai Kiran Mavileti, Shyam S. Pandey, and Tamaki Kato. 2024. "NIR-Sensitive Squaraine Dye—Peptide Conjugate for Trypsin Fluorogenic Detection" Biosensors 14, no. 10: 458. https://doi.org/10.3390/bios14100458
APA StyleBalyan, P., Gupta, S., Mavileti, S. K., Pandey, S. S., & Kato, T. (2024). NIR-Sensitive Squaraine Dye—Peptide Conjugate for Trypsin Fluorogenic Detection. Biosensors, 14(10), 458. https://doi.org/10.3390/bios14100458





