Development of a Portable Cell-Based Biosensor for the Ultra-Rapid Screening for Boscalid Residues in Lettuce
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Biological Materials
2.2. Biosensor Manufacturing
2.3. Point-of-Test Setup
2.4. Standard Solutions Prepation and Measurement Process
2.5. Spiked Lettuce Samples—Sample Preparation
2.6. Experimental Design and Data Analysis
3. Results
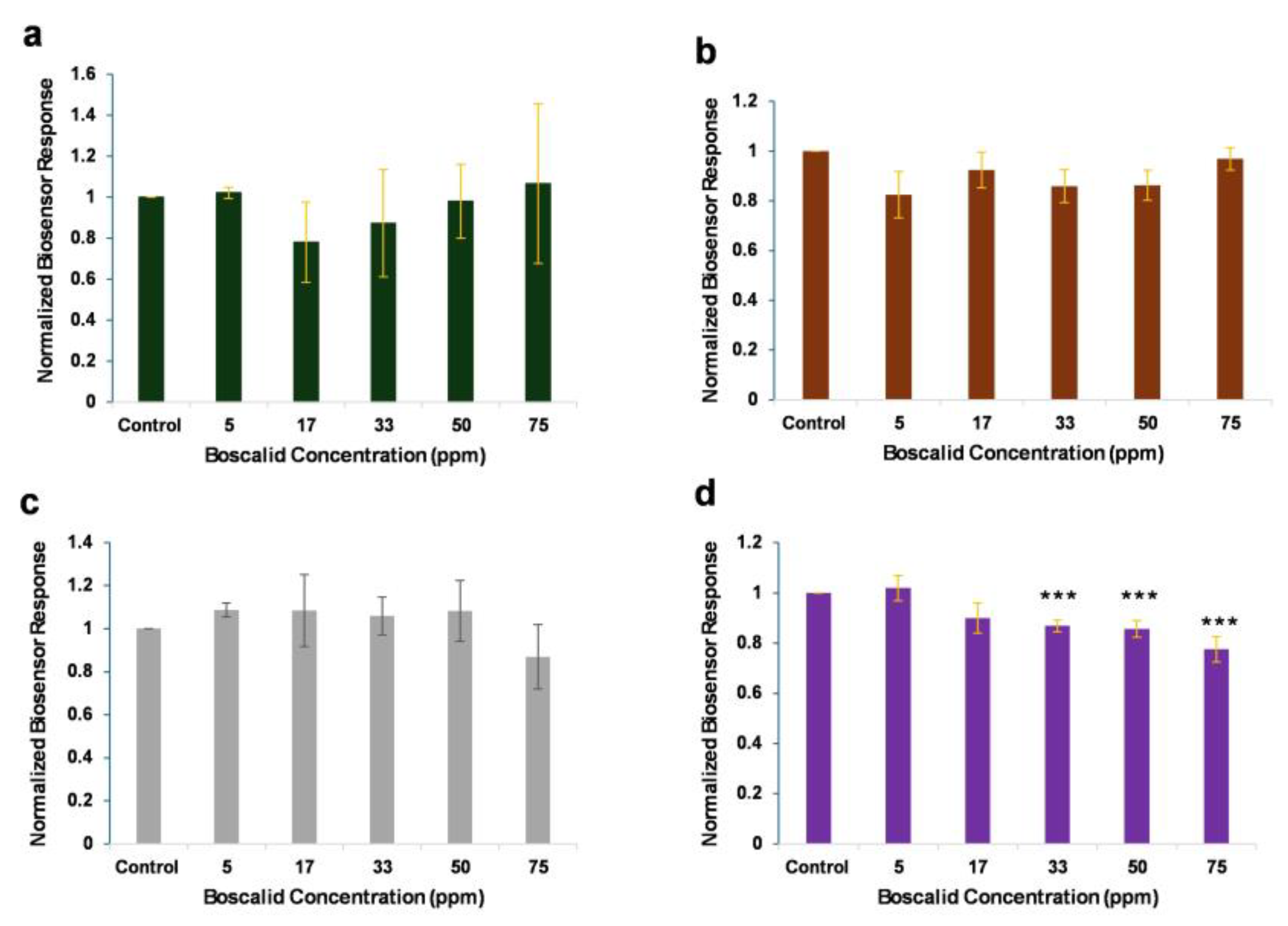
3.1. Biosensor Response to the Presence of Standard Boscalid Solutions
3.2. Proof of Biosensor Selectivity
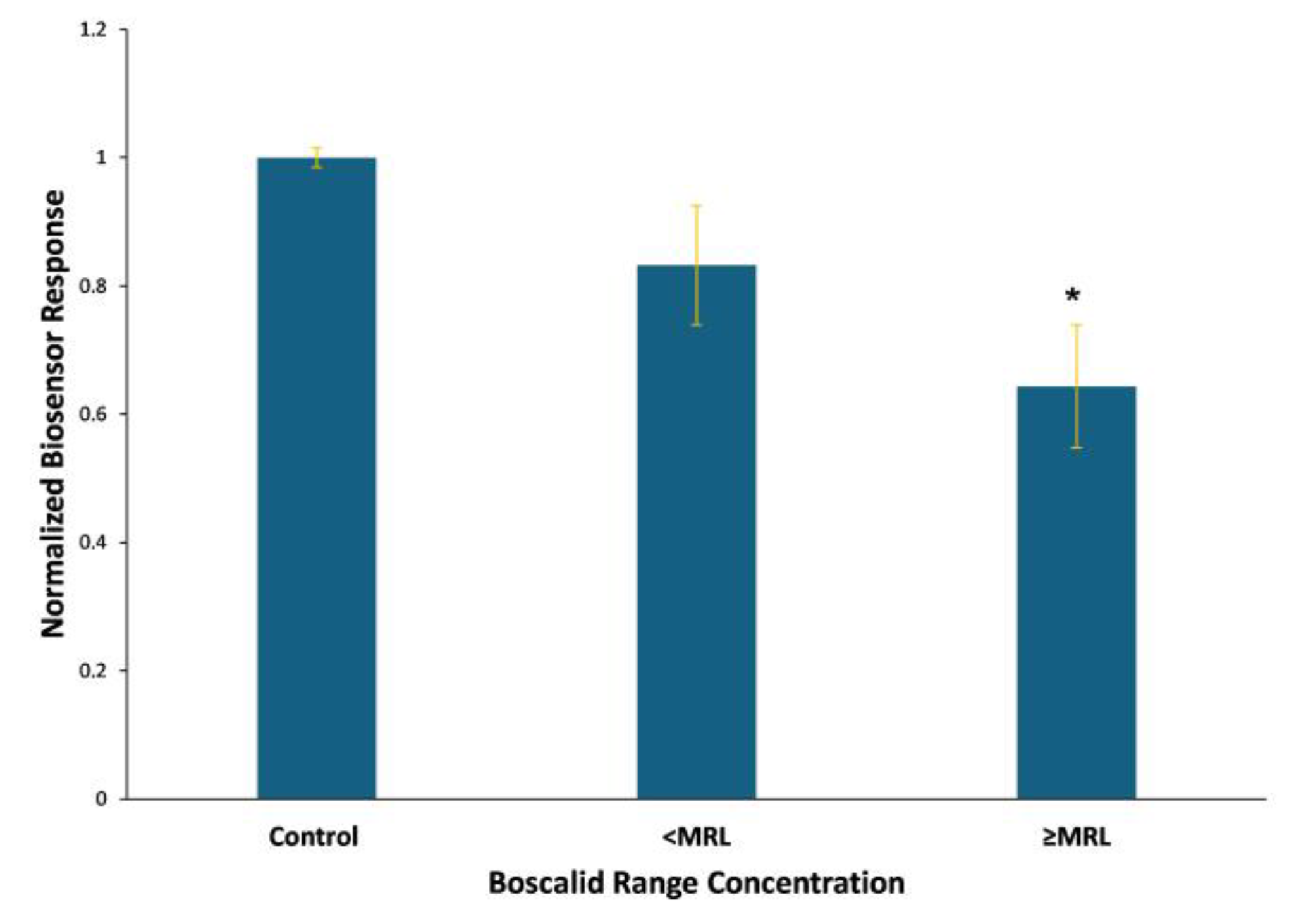
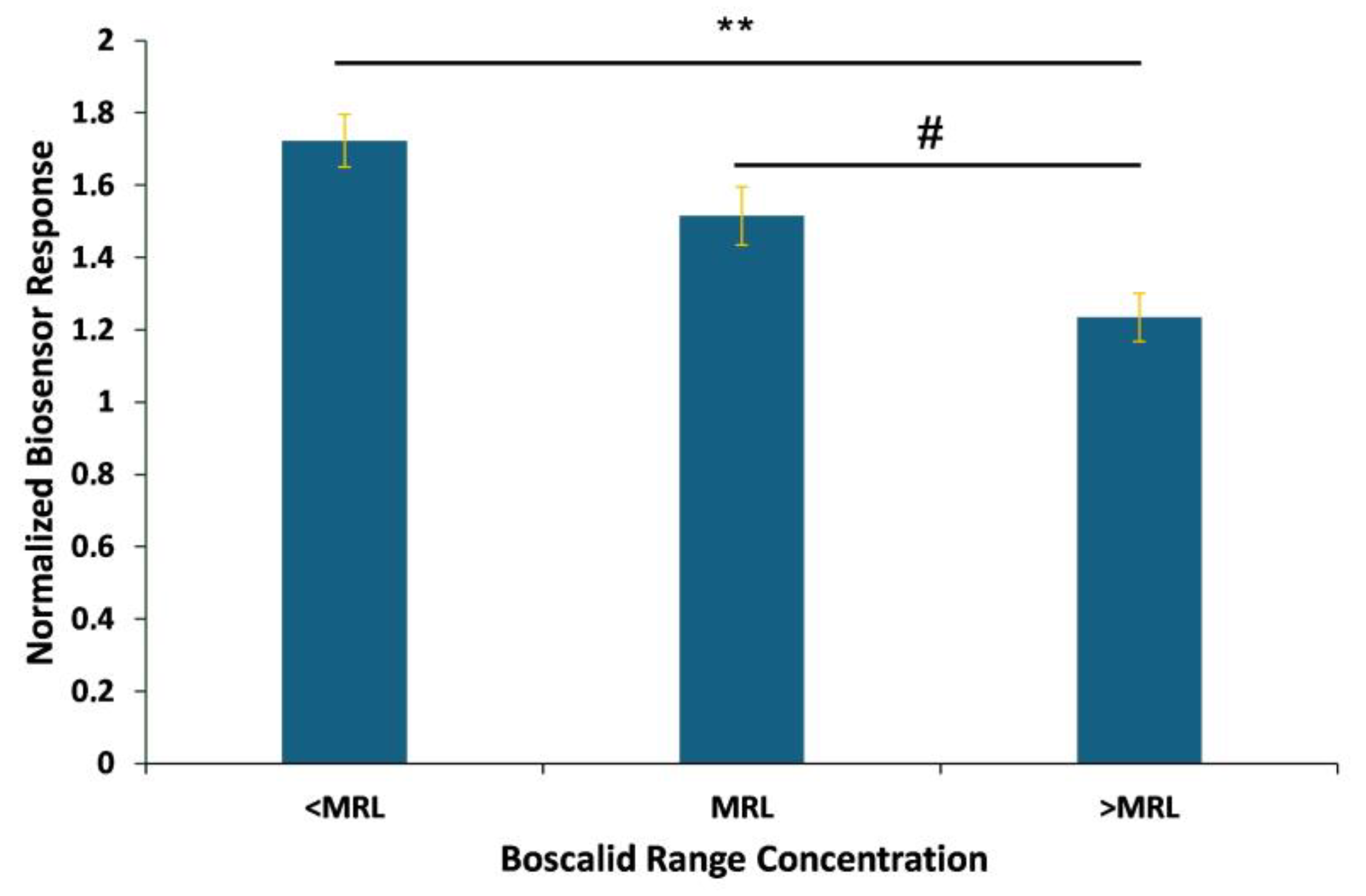
3.3. Biosensor Response to the Presence of Spiked Boscalid in Lettuce Leaf Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Eurostat. EU Sales of Pesticides Rebounded in 2021. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20230510-1 (accessed on 10 May 2023).
- Rhodes, L.A.; McCarl, B.A. An Analysis of Climate Impacts on Herbicide, Insecticide, and Fungicide Expenditures. Agronomy 2020, 10, 745. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, C.; Guo, Q.; Zhang, J.; Ruiz-Menjivar, J. The impact of agricultural chemical inputs on environment: Global evidence from informetrics analysis and visualization. Int. J. Low-Carbon Technol. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Schäfer, R.B.; Liess, M.; Altenburger, R.; Filser, J.; Hollert, H.; Roß-Nickoll, M.; Schäffer, A.; Scheringer, M. Future pesticide risk assessment: Narrowing the gap between intention and reality. Environ. Sci. Eur. 2019, 31, 21. [Google Scholar] [CrossRef]
- Anonymous Pesticide Residues. Reprot of the 1967 Joint Meeting of the FAO Working Party and the WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1968, 391, 1–43. [Google Scholar]
- Li, S.; Li, X.; Zhang, H.; Wang, Z.; Xu, H. The research progress in and perspective of potential fungicides: Succinate dehydrogenase inhibitors. Bioorg. Med. Chem. 2021, 50, 116476. [Google Scholar] [CrossRef] [PubMed]
- Abad-Fuentes, A.; Ceballos-Alcantarilla, E.; Mercader, J.V.; Agulló, C.; Abad-Somovilla, A.; Esteve-Turrillas, F.A. Determination of succinate-dehydrogenase-inhibitor fungicide residues in fruits and vegetables by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 4207–4211. [Google Scholar] [CrossRef]
- Qian, L.; Qi, S.; Cao, F.; Zhang, J.; Zhao, F.; Li, C.; Wang, C. Toxic effects of boscalid on the growth, photosynthesis, antioxidant system and metabolism of Chlorella vulgaris. Environ. Pollut. 2018, 242, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fu, L.; Tan, H.; Jiang, J.; Che, Z.; Tian, Y.; Chen, G. Resistance to Boscalid in Botrytis cinerea From Greenhouse-Grown Tomato. Plant Dis. 2021, 105, 628–635. [Google Scholar] [CrossRef]
- Matheron, M.E.; Porchas, M. Activity of Boscalid, Fenhexamid, Fluazinam, Fludioxonil, and Vinclozolin on Growth of Sclerotinia minor and S. sclerotiorum and Development of Lettuce Drop. Plant Dis. 2004, 88, 665–668. [Google Scholar] [CrossRef]
- Cherrad, S.; Charnay, A.; Hernandez, C.; Steva, H.; Belbahri, L.; Vacher, S. Emergence of boscalid-resistant strains of Erysiphe necator in French vineyards. Microbiol. Res. 2018, 216, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Landschoot, S.; Carrette, J.; Vandecasteele, M.; De Baets, B.; Höfte, M.; Audenaert, K.; Haesaert, G. Boscalid-resistance in Alternaria alternata and Alternaria solani populations: An emerging problem in Europe. Crop Prot. 2017, 92, 49–59. [Google Scholar] [CrossRef]
- Vasić, T.; Vojinović, U.; Žujović, S.; Krnjaja, V.; Živković, S.; Marković, J.; Stević, M. In vitro toxicity of fungicides with different modes of action to alfalfa anthracnose fungus, Colletotrichum destructivum. J. Environ. Sci. Health Part B 2019, 54, 964–971. [Google Scholar] [CrossRef] [PubMed]
- EFSA; Anastassiadou, M.; Bernasconi, G.; Brancato, A.; Carrasco Cabrera, L.; Ferreira, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; et al. Modification of the existing maximum residue level for boscalid in pomegranates. EFSA J. 2020, 18, e06236. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; San Martin, G.; Bruneau, E.; Hautier, L. Time-to-death approach to reveal chronic and cumulative toxicity of a fungicide for honeybees not revealed with the standard ten-day test. Sci. Rep. 2018, 8, 7241. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.; Schoville, S.; Clements, A.; Amezian, D.; Davis, T.; Sanchez-Sedillo, B.; Bradfield, C.; Huseth, A.; Groves, R. Agricultural fungicides inadvertently influence the fitness of Colorado potato beetles, Leptinotarsa decemlineata, and their susceptibility to insecticides. Sci. Rep. 2018, 8, 13282. [Google Scholar] [CrossRef]
- Aksakal, F.I. Evaluation of boscalid toxicity on Daphnia magna by using antioxidant enzyme activities, the expression of genes related to antioxidant and detoxification systems, and life-history parameters. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 237, 108830. [Google Scholar] [CrossRef]
- Qian, L.; Qi, S.; Zhang, J.; Duan, M.; Schlenk, D.; Jiang, J.; Wang, C. Exposure to Boscalid Induces Reproductive Toxicity of Zebrafish by Gender-Specific Alterations in Steroidogenesis. Environ. Sci. Technol. 2020, 54, 14275–14287. [Google Scholar] [CrossRef] [PubMed]
- Arzu, Ö.; Dilek, A.; Muhsin, K. Pesticides, Environmental Pollution, and Health. In Environmental Health Risk; Marcelo, L.L., Sonia, S., Eds.; IntechOpen: Rijeka, Croatia, 2016; Chapter 1. [Google Scholar]
- Hakme, E.; Herrmann, S.S.; Poulsen, M.E. Processing factors of pesticide residues in biscuits and their relation to the physicochemical properties of pesticides. Food Addit. Contam. Part A 2020, 37, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Hu, J. Dissipation behaviour and dietary risk assessment of boscalid, triflumizole and its metabolite (FM-6-1) in open-field cucumber based on QuEChERS using HPLC–MS/MS technique. J. Sci. Food Agric. 2018, 98, 4501–4508. [Google Scholar] [CrossRef]
- He, Y.; Meng, M.; Yohannes, W.K.; Khan, M.; Wang, M.; Abd Ei-Aty, A.M.; Hacımüftüoğlu, F.; He, Y.; Gao, L.; She, Y. Dissipation pattern and residual levels of boscalid in cucumber and soil using liquid chromatography-tandem mass spectrometry. J. Environ. Sci. Health Part B 2020, 55, 388–395. [Google Scholar] [CrossRef]
- Munitz, M.S.; Resnik, S.L.; Montti, M.I.T. Method development and validation for boscalid in blueberries by solid-phase microextraction gas chromatography, and their degradation kinetics. Food Chem. 2013, 136, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Ji, R.; Wang, X.; Yu, C.; Yu, Y.; Yang, X. Fluorescence detection of boscalid pesticide residues in grape juice. Optik 2019, 180, 236–239. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Yamasaki, T.; Harada, A.; Ohtake, T.; Adachi, K.; Iwasa, S.; Narita, H.; Miyake, S. Analysis of the Fungicide Boscalid in Horticultural Crops Using an Enzyme-Linked Immunosorbent Assay and an Immunosensor Based on Surface Plasmon Resonance. J. Agric. Food Chem. 2015, 63, 8075–8082. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Turrillas, F.A.; Mercader, J.V.; Agulló, C.; Abad-Somovilla, A.; Abad-Fuentes, A. Highly sensitive monoclonal antibody-based immunoassays for boscalid analysis in strawberries. Food Chem. 2018, 267, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Sequeira, R.; Starbird-Pérez, R.; Rojas-Carillo, O.; Vargas-Villalobos, S. What are the Main Sensor Methods for Quantifying Pesticides in Agricultural Activities? A Review. Molecules 2019, 24, 2659. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Cai, Q.; Li, Y.; Zhang, Z.; Cao, L.; Li, K.; Yang, H. Sensors Applied for the Detection of Pesticides and Heavy Metals in Freshwaters. J. Sens. 2020, 2020, 8503491. [Google Scholar] [CrossRef]
- Miyake, S.; Hirakawa, Y.; Yamasaki, T.; Watanabe, E.; Harada, A.; Iwasa, S.; Narita, H. Simultaneous Detection of Six Different Types of Pesticides by an Immunosensor Based on Surface Plasmon Resonance. Anal. Sci. 2020, 36, 335–340. [Google Scholar] [CrossRef]
- Zhang, Y.; Kuang, J.; Dong, J.; Shi, L.; Li, Q.; Zhang, B.; Shi, W.; Huang, X.; Zhu, Z.; Ma, Y.; et al. Ultra-sensitive boscalid sensors based on a β-cyclodextrin modified perfluorinated copper phthalocyanine field-effect transistor. J. Mater. Chem. C 2021, 9, 12877–12883. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kintzios, S.; Bem, F.; Mangana, O.; Nomikou, K.; Markoulatos, P.; Alexandropoulos, N.; Fasseas, C.; Arakelyan, V.; Petrou, A.L.; Soukouli, K.; et al. Study on the mechanism of Bioelectric Recognition Assay: Evidence for immobilized cell membrane interactions with viral fragments. Biosens. Bioelectron. 2004, 20, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, V.; Hotka, M.; Freissmuth, M.; Sandtner, W. An Electrophysiological Approach to Measure Changes in the Membrane Surface Potential in Real Time. Biophys. J. 2020, 118, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Kulbacka, J.; Choromańska, A.; Rossowska, J.; Weżgowiec, J.; Saczko, J.; Rols, M.P. Cell Membrane Transport Mechanisms: Ion Channels and Electrical Properties of Cell Membranes. Adv. Anat. Embryol. Cell Biol. 2017, 227, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Kokla, A.; Blouchos, P.; Livaniou, E.; Zikos, C.; Kakabakos, S.E.; Petrou, P.S.; Kintzios, S. Visualization of the membrane engineering concept: Evidence for the specific orientation of electroinserted antibodies and selective binding of target analytes. J. Mol. Recognit. 2013, 26, 627–632. [Google Scholar] [CrossRef]
- Moschopoulou, G.; Kintzios, S. Application of “membrane-engineering” to bioelectric recognition cell sensors for the ultra-sensitive detection of superoxide radical: A novel biosensor principle. Anal. Chim. Acta 2006, 573–574, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, T.; Mavrikou, S.; Denaxa, N.-K.; Paivana, G.; Roussos, P.A.; Kintzios, S. Assessment of Cypermethrin Residues in Tobacco by a Bioelectric Recognition Assay (BERA) Neuroblastoma Cell-Based Biosensor. Chemosensors 2019, 7, 58. [Google Scholar] [CrossRef]
- Mavrikou, S.; Flampouri, K.; Moschopoulou, G.; Mangana, O.; Michaelides, A.; Kintzios, S. Assessment of Organophosphate and Carbamate Pesticide Residues in Cigarette Tobacco with a Novel Cell Biosensor. Sensors 2008, 8, 2818–2832. [Google Scholar] [CrossRef] [PubMed]
- Moschopoulou, G.; Mavrikou, S.; Valdes, D.; Kintzios, S. Comparative Study of a Cell-Based and Electrochemical Biosensor for the Rapid Detection of 2,4,6-Trichloroanisole in Barrel Water Extracts. Beverages 2019, 5, 1. [Google Scholar] [CrossRef]
- Moschopoulou, G.; Vitsa, K.; Bem, F.; Vassilakos, N.; Perdikaris, A.; Blouhos, P.; Yialouris, C.; Frosyniotis, D.; Anthopoulos, I.; Mangana, O.; et al. Engineering of the membrane of fibroblast cells with virus-specific antibodies: A novel biosensor tool for virus detection. Biosens. Bioelectron. 2008, 24, 1027–1030. [Google Scholar] [CrossRef]
- Moschopoulou, G.; Valero, T.; Kintzios, S. Superoxide determination using membrane-engineered cells: An example of a novel concept for the construction of cell sensors with customized target recognition properties. Sens. Actuators B Chem. 2012, 175, 78–84. [Google Scholar] [CrossRef]
- Mavrikou, S.; Moschopoulou, G.; Zafeirakis, A.; Kalogeropoulou, K.; Giannakos, G.; Skevis, A.; Kintzios, S. An Ultra-Rapid Biosensory Point-of-Care (POC) Assay for Prostate-Specific Antigen (PSA) Detection in Human Serum. Sensors 2018, 18, 3834. [Google Scholar] [CrossRef] [PubMed]
- 2022/1324, C.R.E. Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Benzovindiflupyr, Boscalid, Fenazaquin, Fluazifop-P, Flupyradifurone, Fluxapyroxad, Fosetyl-Al, Isofetamid, Metaflumizone, Pyraclostrobin, Spirotetramat, Thiabendazole and Tolclofos-Methyl in or on Certain Products. Available online: https://eur-lex.europa.eu/eli/reg/2022/1324/oj (accessed on 28 July 2022).
- 15662, E.C.f.S.C.S.M.E. Food of Plant Origin. Multimethod for the Determination of Pesticide Residues Using GC- and LC-Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE—Modular QuEChERS-Method. Available online: https://www.cencenelec.eu/ (accessed on 5 June 2018).
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Choi, M.; Shim, J.; Park, S. Hook effect detection and detection-range-controllable one-step immunosensor for inflammation monitoring. Sens. Actuators B Chem. 2020, 304, 127408. [Google Scholar] [CrossRef]
- Horská, T.; Kocourek, F.; Stará, J.; Holý, K.; Mráz, P.; Krátký, F.; Kocourek, V.; Hajšlová, J. Evaluation of Pesticide Residue Dynamics in Lettuce, Onion, Leek, Carrot and Parsley. Foods 2020, 9, 680. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Brancato, A.; Brocca, D.; De Lentdecker, C.; Ferreira, L.; Greco, L.; Jarrah, S.; Kardassi, D.; Leuschner, R.; Lythgo, C.; et al. Review of the existing maximum residue levels for mandipropamid according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2018, 16, e05284. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce-A Comprehensive Review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef]
- Umapathi, R.; Park, B.; Sonwal, S.; Rani, G.M.; Cho, Y.; Huh, Y.S. Advances in optical-sensing strategies for the on-site detection of pesticides in agricultural foods. Trends Food Sci. Technol. 2022, 119, 69–89. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Bhardwaj, A. Biosensor Technology for Pesticides—A review. Appl. Biochem. Biotechnol. 2015, 175, 3093–3119. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi-Moghaddam, H.; Akbari Javar, H.; Garkani-Nejad, Z. Fabrication of platinum-doped NiCO2O4 nanograss modified electrode for determination of carbendazim. Food Chem. 2022, 383, 132398. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, Q.; Shi, Z.; Guo, Y.; Li, F.; Zhang, Y.; Sun, X. Sensitive Acetylcholinesterase Biosensor Based on Screen-Printed Carbon Electrode Modified with Cerium Oxide-Chitosan/Mesoporous Carbon-Chitosan for Organophosphorus Pesticide Residue Detection. Int. J. Electrochem. Sci. 2018, 13, 9231–9241. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, X.; Kong, M.; Jiang, G.; Sun, Y.; Mo, W.; Lin, T.; Ye, F.; Zhao, S. A competitive immunoassay for electrochemical impedimetric determination of chlorpyrifos using a nanogold-modified glassy carbon electrode based on enzymatic biocatalytic precipitation. Mikrochim. Acta 2020, 187, 204. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.C.; Gomes, N.O.; Calegaro, M.L.; Machado, S.A.S.; de Oliveira, T.V.; de Fátima Ferreira Soares, N.; Raymundo-Pereira, P.A. Sustainable plant-wearable sensors for on-site, rapid decentralized detection of pesticides toward precision agriculture and food safety. Biomater. Adv. 2023, 155, 213676. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, D.; Hu, Y.; Liu, S.; Wei, H.; Zheng, J.; Wang, G.; Hu, X.; Wang, C. Construction of an impedimetric immunosensor for label-free detecting carbofuran residual in agricultural and environmental samples. Food Control 2015, 53, 72–80. [Google Scholar] [CrossRef]
- Prabhakar, N.; Thakur, H.; Bharti, A.; Kaur, N. Chitosan-iron oxide nanocomposite based electrochemical aptasensor for determination of malathion. Anal. Chim. Acta 2016, 939, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Bhatnagar, A.; Bhalla, A.; Prabhakar, N. Determination of an organophosphate pesticide using antibody immobilised hybrid nanocomposites. Int. J. Environ. Anal. Chem. 2021, 101, 1485–1498. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Yamasaki, T.; Harada, A.; Iwasa, S.; Narita, H.; Miyake, S. Development of an Immunosensor Based on Surface Plasmon Resonance for Simultaneous Residue Analysis of Three Pesticides—Boscalid, Clothianidin, and Nitenpyram—In Vegetables. Anal. Sci. 2018, 34, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Mou, B.; Zuo, C.; Chen, L.; Xie, H.; Zhang, W.; Wang, Q.; Wen, L.; Gan, N. On-site Simultaneous Determination of Neonicotinoids, Carbamates, and Phenyl Pyrazole Insecticides in Vegetables by QuEChERS Extraction on Nitrogen and Sulfur co-doped Carbon Dots and Portable Mass Spectrometry. J. Chromatogr. A 2023, 1689, 463744. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.-W.; Wang, D.; Wei, N. A Whole-Cell Biosensor for Point-of-Care Detection of Waterborne Bacterial Pathogens. ACS Synth. Biol. 2021, 10, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiu, M. A short review on cell-based biosensing: Challenges and breakthroughs in biomedical analysis. J. Biomed. Res. 2020, 35, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Aynalem, B.; Muleta, D. Microbial Biosensors as Pesticide Detector: An Overview. J. Sens. 2021, 2021, 5538857. [Google Scholar] [CrossRef]
- Gui, Q.; Lawson, T.; Shan, S.; Yan, L.; Liu, Y. The Application of Whole Cell-Based Biosensors for Use in Environmental Analysis and in Medical Diagnostics. Sensors 2017, 17, 1623. [Google Scholar] [CrossRef] [PubMed]
- Feller, K.-H. Mammalian Cell-Based Biosensors. In Handbook of Cell Biosensors; Thouand, G., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–28. [Google Scholar]
- Apostolou, T.; Loizou, K.; Hadjilouka, A.; Inglezakis, A.; Kintzios, S. Newly Developed System for Acetamiprid Residue Screening in the Lettuce Samples Based on a Bioelectric Cell Biosensor. Biosensors 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moschopoulou, G.; Tsekouras, V.; Mercader, J.V.; Abad-Fuentes, A.; Kintzios, S. Development of a Portable Cell-Based Biosensor for the Ultra-Rapid Screening for Boscalid Residues in Lettuce. Biosensors 2024, 14, 311. https://doi.org/10.3390/bios14060311
Moschopoulou G, Tsekouras V, Mercader JV, Abad-Fuentes A, Kintzios S. Development of a Portable Cell-Based Biosensor for the Ultra-Rapid Screening for Boscalid Residues in Lettuce. Biosensors. 2024; 14(6):311. https://doi.org/10.3390/bios14060311
Chicago/Turabian StyleMoschopoulou, Georgia, Vasileios Tsekouras, Josep V. Mercader, Antonio Abad-Fuentes, and Spyridon Kintzios. 2024. "Development of a Portable Cell-Based Biosensor for the Ultra-Rapid Screening for Boscalid Residues in Lettuce" Biosensors 14, no. 6: 311. https://doi.org/10.3390/bios14060311
APA StyleMoschopoulou, G., Tsekouras, V., Mercader, J. V., Abad-Fuentes, A., & Kintzios, S. (2024). Development of a Portable Cell-Based Biosensor for the Ultra-Rapid Screening for Boscalid Residues in Lettuce. Biosensors, 14(6), 311. https://doi.org/10.3390/bios14060311






