Immobilization of Horseradish Peroxidase onto Montmorillonite/Glucosamine–Chitosan Composite for Electrochemical Biosensing of Polyphenols
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Pennyroyal and Lemon Verbena Extracts
2.3. Equipment
2.4. Electrochemical Measurement Procedures and Statistical Analysis of the Response
2.5. Preparation of Na+-Mt/GA-Chit Composite
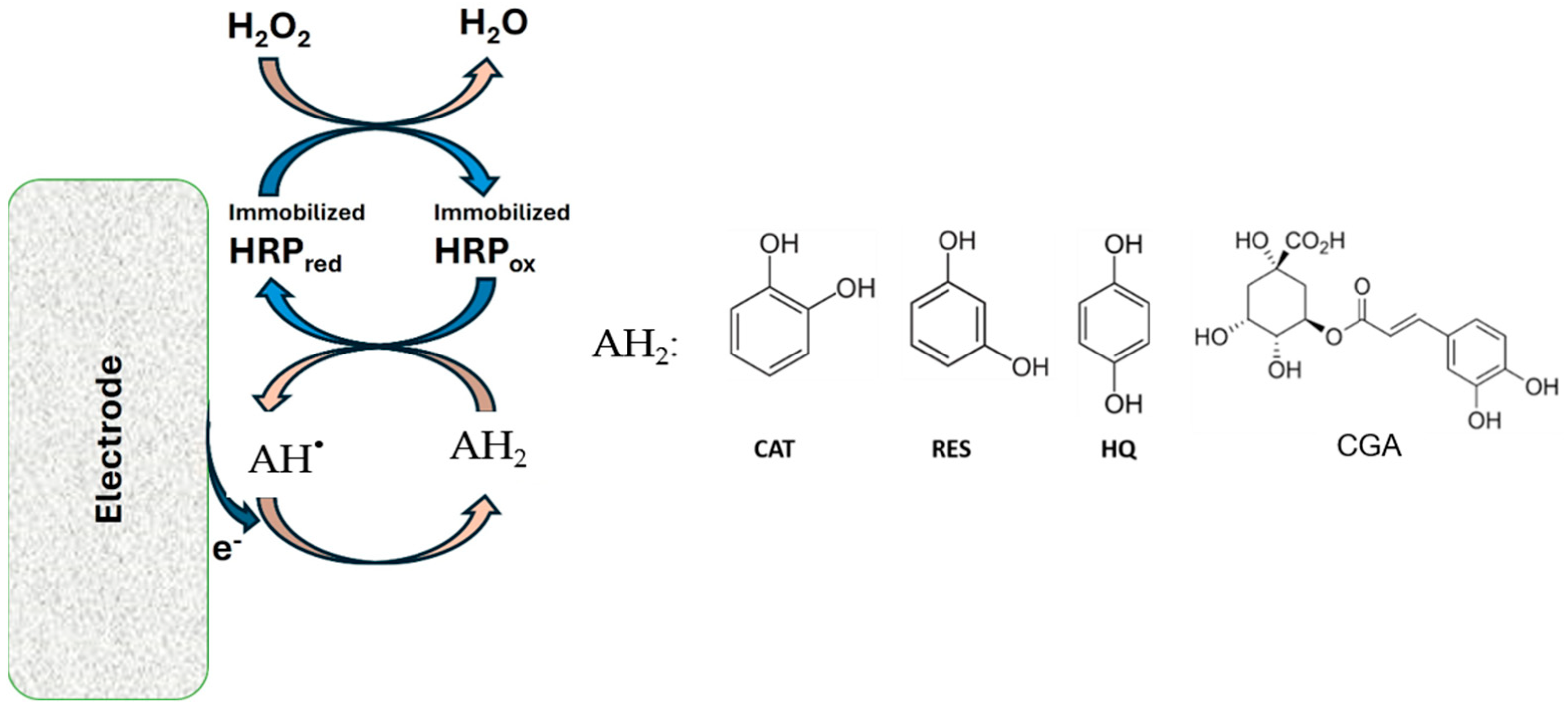
2.6. Surface Modifications and Construction of the Bioelectrode
3. Results and Discussion
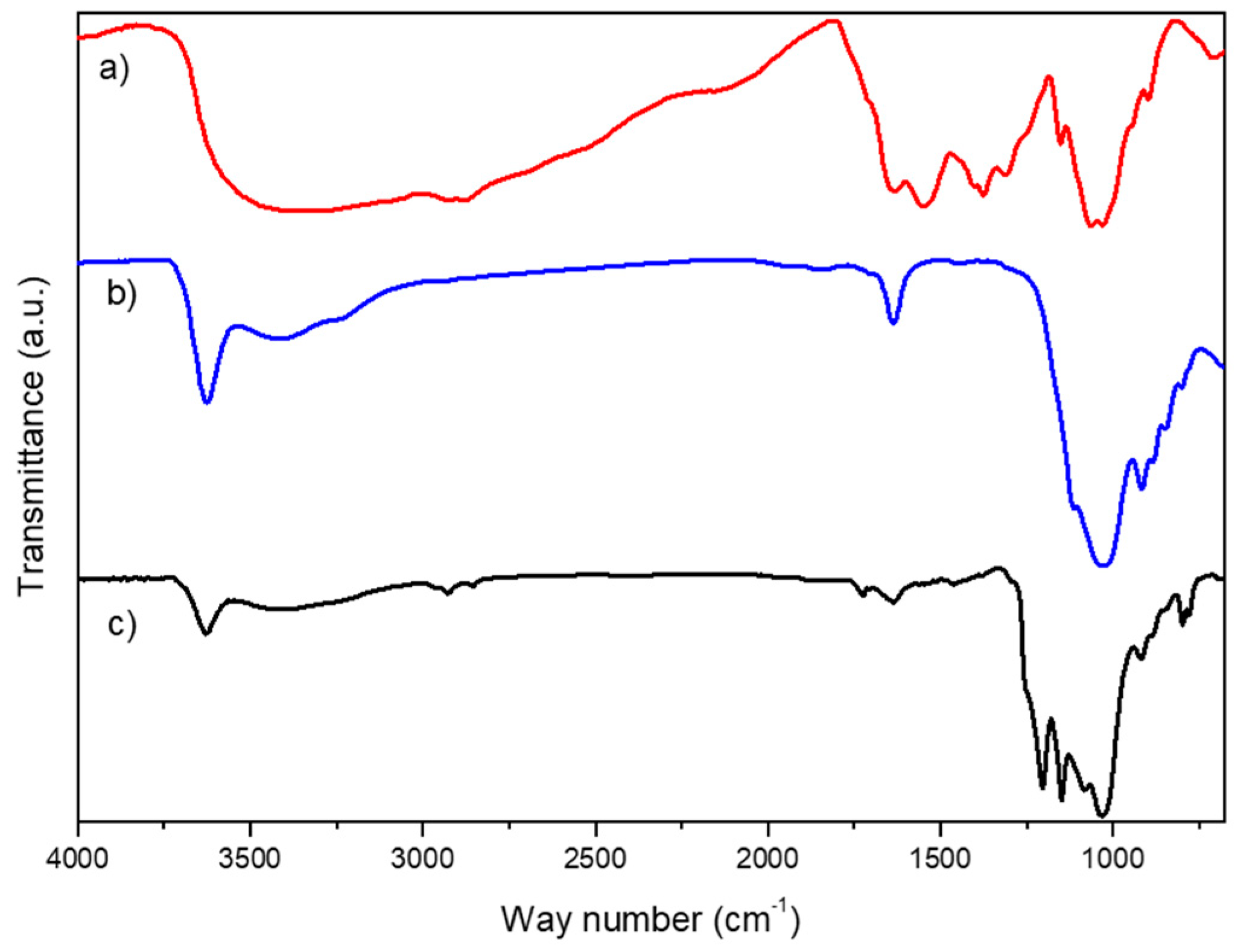
3.1. Characterization of Na+-Mt/GA-CHIT Composite
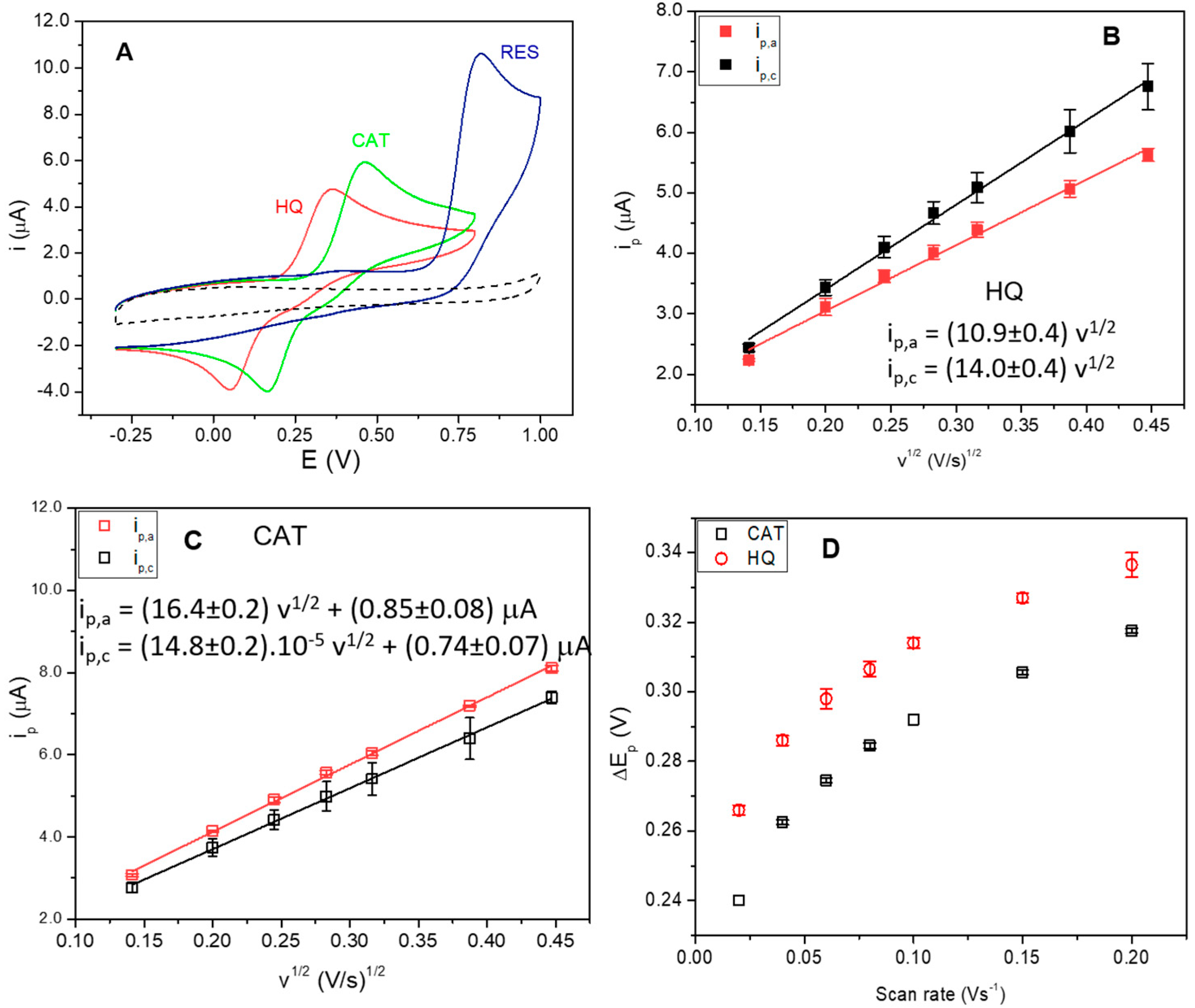
3.2. Electrochemical Behavior of Polyphenols at GCE Modified with the Na+-Mt/CHIT Composite
3.3. Selection of the Composite Proportion and HRP Adsorption Methodology
3.4. Chronoamperometric Response of Phenolic Compounds
| Substrate | Sensitivity (μA mM−1) | LOD * | Linear Range (μM) | Time of Response (s) | Reference |
|---|---|---|---|---|---|
| HQ | (1.6 ± 0.0) × 102 | (74 ± 8) nM | 0.22–29 | 12 ± 1 | This work |
| HQ | ~2.5 ** | 1.26 μM | 5.0–30 | - | [50] |
| HQ | 15.32 | 0.6 μM | 1.6–15 | - | [51] |
| HQ | 218 ± 2 | 1.6 nM | up to 120 | 27 ± 3 | [20] |
| HQ | 8 | 6.42 μM | 16–240 | 2 | [43] |
| CGA | (1.2 ± 0.1) × 102 | (26 ± 3) nM | 0.08–9 | 9 ± 1 | This work |
| CGA | 132 | 2.7 nM | up to 4.2 | - | [20] |
| CGA | 22.7 | 0.7 μM | 1–50 | - | [45] |
| CAT | (16 ± 2) | (0.74 ± 0.09) μM | 2.2–210 | 14 ± 1 | This work |
| CAT | 0.28 | 0.16 μM | 20–32.5 | - | [49] |
| CAT | 18 | 0.22 μM | 0.5–8 | - | [51] |
| CAT | 2 | 0.93 μM | 1.6–8 | 2 | [52] |
| RES | (3.7 ± 0.3) | (3.3 ± 0.2) μM | 9.9–70 | 11 ± 1 | This work |
| RES | 16 | - | - | - | [53] |
3.5. Determination of Polyphenols in Pennyroyal and Lemon Verbena Plant Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mueed, A.; Shibli, S.; Al-Quwaie, D.A.; Ashkan, M.F.; Alharbi, M.; Alanazi, H.; Binothman, N.; Aljadani, M.; Majrashi, K.A.; Huwaikem, M.; et al. Extraction, Characterization of Polyphenols from Certain Medicinal Plants and Evaluation of Their Antioxidant, Antitumor, Antidiabetic, Antimicrobial Properties, and Potential Use in Human Nutrition. Front. Nutr. 2023, 10, 1125106. [Google Scholar] [CrossRef] [PubMed]
- Guemari, F.; Laouini, S.E.; Rebiai, A.; Bouaa, A.; Tliba, A.; Barhoum, A. UV-Visible Spectroscopic Technique for Prediction of Total Polyphenol Contents for a Bunch of Medicinal Plant Extracts. Res. Sq. 2022, 22, 1–17. [Google Scholar] [CrossRef]
- Lubaina, A.S.; Renjith, P.R.; Roshni, A.S. Identification and Quantification of Polyphenols from Pineapple Peel by High Performance Liquid Chromatography Analysis. Adv. Zool. Bot. 2020, 8, 431–438. [Google Scholar] [CrossRef]
- Arena, K.; Cacciola, F.; Miceli, N.; Taviano, M.F.; Cavò, E.; Murphy, R.E.; Dugo, P.; Mondello, L. Determination of the Polyphenolic Content of Berry Juices Using Focusing-Modulated Comprehensive Two-Dimensional Liquid Chromatography Coupled to Mass Spectrometry Detection. Anal. Bioanal. Chem. 2023, 415, 2371–2382. [Google Scholar] [CrossRef]
- Tulli, F.; Lemos, M.L.; Gutiérrez, D.R.; Rodríguez, S.d.C.; de Mishima, B.A.L.; Paz Zanini, V.I. Electrochemical and Spectrophotometric Methods for Polyphenol and Ascorbic Acid Determination in Fruit and Vegetable Extracts. Food Technol. Biotechnol. 2020, 58, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, M.; Moroni, S.; Klonos, P.; Kyritsis, A.; Bikiaris, D.N.; Lamprou, D.A. 3D-Printed Hydrogels Based on Amphiphilic Chitosan Derivative Loaded with Levofloxacin for Wound Healing Applications. Int. J. Polym. Mater. Polym. Biomater. 2024, 13, 1–18. [Google Scholar] [CrossRef]
- Shrestha, R.; Thenissery, A.; Khupse, R.; Rajashekara, G. Strategies for the Preparation of Chitosan Derivatives for Antimicrobial, Drug Delivery, and Agricultural Applications: A Review. Molecules 2023, 28, 7659. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Tsai, C.F.; Li, C.F. Preparation and Characterization of Water-Soluble Chitosan Produced by Maillard Reaction. Fish. Sci. 2006, 72, 1096–1103. [Google Scholar] [CrossRef]
- Vanden Braber, N.L.; Díaz Vergara, L.I.; Morán Vieyra, F.E.; Borsarelli, C.D.; Yossen, M.M.; Vega, J.R.; Correa, S.G.; Montenegro, M.A. Physicochemical Characterization of Water-Soluble Chitosan Derivatives with Singlet Oxygen Quenching and Antibacterial Capabilities. Int. J. Biol. Macromol. 2017, 102, 200–207. [Google Scholar] [CrossRef]
- Vanden Braber, N.L.; Novotny Nuñez, I.; Bohl, L.; Porporatto, C.; Nazar, F.N.; Montenegro, M.A.; Correa, S.G. Soy Genistein Administered in Soluble Chitosan Microcapsules Maintains Antioxidant Activity and Limits Intestinal Inflammation. J. Nutr. Biochem. 2018, 62, 50–58. [Google Scholar] [CrossRef]
- Gulotta, F.A.; Montenegro, M.A.; Vergara Diaz, L.; Arata Badano, J.; Ferreyra, N.F.; Paz Zanini, V.I. Chitosan-Based Maillard Products for Enzyme Immobilization in Multilayers Structure: Its Application in Electrochemical Sensing. Microchem. J. 2023, 190, 108689–108696. [Google Scholar] [CrossRef]
- Pavón, E.; Martín-Rodríguez, R.; Perdigón, A.C.; Alba, M.D. New Trends in Nanoclay-Modified Sensors. Inorganics 2021, 9, 43. [Google Scholar] [CrossRef]
- Phongphut, A.; Chayasombat, B.; Cass, A.E.G.; Sirisuk, A.; Phisalaphong, M.; Prichanont, S.; Thanachayanont, C. Clay/Au Nanoparticle Composites as Acetylcholinesterase Carriers and Modified-Electrode Materials: A Comparative Study. Appl. Clay Sci. 2020, 194, 105704–105714. [Google Scholar] [CrossRef]
- Apetrei, R.M.; Camurlu, P. The Effect of Montmorillonite Functionalization on the Performance of Glucose Biosensors Based on Composite Montmorillonite/PAN Nanofibers. Electrochim. Acta 2020, 353, 136484–136495. [Google Scholar] [CrossRef]
- Songurtekin, D.; Yalcinkaya, E.E.; Ag, D.; Seleci, M.; Demirkol, D.O.; Timur, S. Histidine Modified Montmorillonite: Laccase Immobilization and Application to Flow Injection Analysis of Phenols. Appl. Clay Sci. 2013, 86, 64–69. [Google Scholar] [CrossRef]
- Lozzi, I.; Calamai, L.; Fusi, P.; Bosetto, M.; Stotzky, G. Interaction of Horseradish Peroxidase with Montmorillonite Homoionic to Na+ and Ca2+: Effects on Enzymatic Activity and Microbial Degradation. Soil Biol. Biochem. 2001, 33, 1021–1028. [Google Scholar] [CrossRef]
- Yilmaz, Y.Y.; Yalcinkaya, E.E.; Demirkol, D.O.; Timur, S. 4-Aminothiophenol-Intercalated Montmorillonite: Organic-Inorganic Hybrid Material as an Immobilization Support for Biosensors. Sens. Actuators B Chem. 2020, 307, 127665–127674. [Google Scholar] [CrossRef]
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Esti, M. Papain Covalently Immobilized on Chitosan–Clay Nanocomposite Films: Application in Synthetic and Real White Wine. Nanomaterials 2020, 10, 1622. [Google Scholar] [CrossRef] [PubMed]
- Alcântara, A.C.S.; Darder, M. Building Up Functional Bionanocomposites from the Assembly of Clays and Biopolymers. Chem. Rec. 2018, 18, 696–712. [Google Scholar] [CrossRef]
- Sarkar, T.; Narayanan, N.; Solanki, P.R. Polymer–Clay Nanocomposite-Based Acetylcholine Esterase Biosensor for Organophosphorous Pesticide Detection. Int. J. Environ. Res. 2017, 11, 591–601. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N.; Cui, J. “Smart” Chemistry and Its Application in Peroxidase Immobilization Using Different Support Materials. Int. J. Biol. Macromol. 2018, 119, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Felisardo, R.J.A.; Luque, A.M.; Silva, Q.S.; Soares, C.M.F.; Fricks, A.T.; Lima, Á.S.; Cavalcanti, E.B. Biosensor of Horseradish Peroxidase Immobilized onto Self-Assembled Monolayers: Optimization of the Deposition Enzyme Concentration. J. Electroanal. Chem. 2020, 879, 114784. [Google Scholar] [CrossRef]
- Sellami, K.; Couvert, A.; Nasrallah, N.; Maachi, R.; Abouseoud, M.; Amrane, A. Peroxidase Enzymes as Green Catalysts for Bioremediation and Biotechnological Applications: A Review. Sci Total Environ. 2022, 806, 150500. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.; Costa, V.C.; Felício, R.C. Comparative Study of Nanostructured Matrices Employed in the Development of Biosensors Based on HRP Enzyme for Determination of Phenolic Compounds. Electroanalysis 2015, 27, 1572–1578. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Romero-Molina, L.; Martínez-López, A.; Rato, A.E.; Heredia, F.J.; Hernández-Hierro, J.M.; Escudero-Gilete, M.L.; González-Miret, M.L. Control of the Extractable Content of Bioactive Compounds in Coffee Beans by near Infrared Hyperspectral Imaging. LWT 2020, 134, 110201–110208. [Google Scholar] [CrossRef]
- Hudz, N.; Yezerska, O.; Shanaida, M.; Sedláčková, V.H.; Wieczorek, P.P. Application of the Folin-Ciocalteu Method to the Evaluation of Salvia Sclarea Extracts. Pharmacia 2019, 66, 209–215. [Google Scholar] [CrossRef]
- Tetyana, P.; Shumbula, P.M.; Njengele-Tetyana, Z. Biosensors: Design, Development and Applications. In Nanopores; Ameen, S., Akhtar, M.S., Shin, H.-S., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Monvisade, P.; Siriphannon, P. Chitosan Intercalated Montmorillonite: Preparation, Characterization and Cationic Dye Adsorption. Appl. Clay Sci. 2009, 42, 427–431. [Google Scholar] [CrossRef]
- Paluszkiewicz, C.; Stodolak, E.; Hasik, M.; Blazewicz, M. FT-IR Study of Montmorillonite-Chitosan Nanocomposite Materials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 784–788. [Google Scholar] [CrossRef]
- Gavalyan, V.B. Synthesis and Characterization of New Chitosan-Based Schiff Base Compounds. Carbohydr. Polym. 2016, 145, 37–47. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; He, J.; Peng, W.; Cao, Y.; Liu, J.; Huang, Y.; Fan, G. Chitosan-Induced Self-Assembly of Montmorillonite Nanosheets along the End-Face for Methylene Blue Removal from Water. Int. J. Biol. Macromol. 2023, 227, 952–961. [Google Scholar] [CrossRef]
- Hu, C.; Deng, Y.; Hu, H.; Duan, Y.; Zhai, K. Adsorption and Intercalation of Low and Medium Molar Mass Chitosans on/in the Sodium Montmorillonite. Int. J. Biol. Macromol. 2016, 92, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, N.; Zhang, Q.; Wang, K.; Fu, Q.; Zhang, X. Preparation and Properties of Chitosan Nanocomposites with Nanofillers of Different Dimensions. Polym. Degrad. Stab. 2009, 94, 124–131. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Zhao, Y.; Bai, H.; Huang, M.; Zhang, T.; Song, S. High-Performance Two-Dimensional Montmorillonite Supported-Poly(Acrylamide-Co-Acrylic Acid) Hydrogel for Dye Removal. Environ. Pollut. 2020, 257, 113574–113585. [Google Scholar] [CrossRef] [PubMed]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer-Clay Nanocomposites Based on Chitosan Intercalated in Montmorillonite. Chem. Mater. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Geuna, A.; Alvarez, M.; Satti, A.J. Adsorbent Composites of Montmorillonite and Chitosan of Different Molecular Weight, Obtained by Gamma Irradiation. J. Environ. Chem. Eng. 2022, 10, 107080–107090. [Google Scholar] [CrossRef]
- Borralleras, P.; Segura, I.; Aranda, M.A.G.; Aguado, A. Influence of Experimental Procedure on D-Spacing Measurement by XRD of Montmorillonite Clay Pastes Containing PCE-Based Superplasticizer. Cem. Concr. Res. 2019, 116, 266–272. [Google Scholar] [CrossRef]
- Wang, S.F.; Shen, L.; Tong, Y.J.; Chen, L.; Phang, I.Y.; Lim, P.Q.; Liu, T.X. Biopolymer Chitosan/Montmorillonite Nanocomposites: Preparation and Characterization. Polym. Degrad. Stab. 2005, 90, 123–131. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Noomun, K.; Khunthon, S.; Limpanart, S. Influence of Chitosan Characteristics on the Properties of Biopolymeric Chitosan–Montmorillonite. Prog. Nat. Sci. Mater. Int. 2012, 22, 502–508. [Google Scholar] [CrossRef]
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of Enzymes on Clay Minerals for Biocatalysts and Biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- Liu, J.J.; Kim, J.G.; Kim, H.B.; Abeysinghe, S.; Lin, Y.W.; Baek, K. Covalent Immobilizing Horseradish Peroxidase on Electrochemically-Functionalized Biochar for Phenol Removal. Chemosphere 2023, 312, 137218. [Google Scholar] [CrossRef]
- Luo, X.L.; Xu, J.J.; Zhang, Q.; Yang, G.J.; Chen, H.Y. Electrochemically Deposited Chitosan Hydrogel for Horseradish Peroxidase Immobilization through Gold Nanoparticles Self-Assembly. Biosens. Bioelectron. 2005, 21, 190–196. [Google Scholar] [CrossRef]
- Besharati Vineh, M.; Saboury, A.A.; Poostchi, A.A.; Rashidi, A.M.; Parivar, K. Stability and Activity Improvement of Horseradish Peroxidase by Covalent Immobilization on Functionalized Reduced Graphene Oxide and Biodegradation of High Phenol Concentration. Int. J. Biol. Macromol. 2018, 106, 1314–1322. [Google Scholar] [CrossRef]
- Betancor, L.; López-Gallego, F.; Hidalgo, A.; Alonso-Morales, N.; Dellamora-Ortiz, G.; CesarMateo, G.D.O.; Fernández-Lafuente, R.; Guisán, J.M. Different Mechanisms of Protein Immobilization on Glutaraldehyde Activated Supports: Effect of Support Activation and Immobilization Conditions. Enzym. Microb. Technol. 2006, 39, 877–882. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in Bio-Catalysts Design: A Useful Crosslinker and a Versatile Tool in Enzyme Immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Zaak, H.; Peirce, S.; de Albuquerque, T.L.; Sassi, M.; Fernandez-Lafuente, R. Exploiting the Versatility of Aminated Supports Activated with Glutaraldehyde to Immobilize β-Galactosidase from Aspergillus Oryzae. Catalysts 2017, 7, 250. [Google Scholar] [CrossRef]
- Melo, M.N.; Pereira, F.M.; Rocha, M.A.; Ribeiro, J.G.; Diz, F.M.; Monteiro, W.F.; Ligabue, R.A.; Severino, P.; Fricks, A.T. Immobilization and Characterization of Horseradish Peroxidase into Chitosan and Chitosan/PEG Nanoparticles: A Comparative Study. Process Biochem. 2020, 98, 160–171. [Google Scholar] [CrossRef]
- Aybastier, Ö.; Şahin, S.; Işik, E.; Demir, C. Determination of Total Phenolic Content in Prunella L. by Horseradish Peroxidase Immobilized onto Chitosan Beads. Anal. Methods 2011, 3, 2289–2297. [Google Scholar] [CrossRef]
- Duarte, R.R.; De Fátima Giarola, J.; Da Silva, D.N.; Saczk, A.A.; Teixeira Tarley, C.R.; Ribeiro, E.S.; Pereira, A.C. Development of Electrochemical HRP-MWCNT-Based Screen-Printed Biosensor for the Determination of Phenolic Compounds in Effluent from Washing Coffee Beans. Rev. Virtual Quim. 2021, 13, 43–60. [Google Scholar] [CrossRef]
- Mossanha, R.; Ramos, M.K.; Santos, C.S.; Pessoa, C.A. Mixed Self-Assembled Monolayers of Mercaptoundecanoic Acid and Thiolactic Acid for the Construction of an Enzymatic Biosensor for Hydroquinone Determination. J. Electrochem. Soc. 2015, 162, B145–B151. [Google Scholar] [CrossRef]
- Topcu Sulak, M.; Erhan, E.; Keskinler, B. Amperometric Phenol Biosensor Based on Horseradish Peroxidase Entrapped PVF and PPy Composite Film Coated GC Electrode. Appl. Biochem. Biotechnol. 2010, 160, 856–867. [Google Scholar] [CrossRef]
- Korkut, S.; Keskinler, B.; Erhan, E. An Amperometric Biosensor Based on Multiwalled Carbon Nanotube-Poly(Pyrrole)-Horseradish Peroxidase Nanobiocomposite Film for Determination of Phenol Derivatives. Talanta 2008, 76, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.; Jiang, X.; Chen, Z.; Lin, X. Horseradish Peroxidase Biosensor Based on Layer-by-Layer Technique for the Determination of Phenolic Compounds. Sens. Actuators B Chem. 2006, 114, 774–780. [Google Scholar] [CrossRef]
- Lopes Da Silva, A.R.; Jhones Dos Santos, A.; Martínez-Huitle, C.A. Electrochemical Measurements and Theoretical Studies for Understanding the Behavior of Catechol, Resorcinol and Hydroquinone on the Boron Doped Diamond Surface. RSC Adv. 2018, 8, 3483–3492. [Google Scholar] [CrossRef] [PubMed]
- Purich, D.L. Chapter 5—Initial-Rate Kinetics of One-Substrate Enzyme-Catalyzed Reactions. In Enzyme Kinetics: Catalysis & Control; Purich, D.L., Ed.; Elsevier: Boston, MA, USA, 2010; pp. 287–334. ISBN 978-0-12-380924-7. [Google Scholar] [CrossRef]
- Wu, L.; Yin, W.; Tang, K.; Li, D.; Shao, K.; Zuo, Y.; Ma, J.; Liu, J.; Han, H. Enzymatic Biosensor of Horseradish Peroxidase Immobilized on Au-Pt Nanotube/Au-Graphene for the Simultaneous Determination of Antioxidants. Anal. Chim. Acta 2016, 933, 89–96. [Google Scholar] [CrossRef]
- Spychalska, K.; Zając, D.; Cabaj, J. Electrochemical Biosensor for Detection of 17β-Estradiol Using Semi-Conducting Polymer and Horseradish Peroxidase. RSC Adv. 2020, 10, 9079–9087. [Google Scholar] [CrossRef]





| Sample | Zeta Potential (mV) * | |
|---|---|---|
| Na+-Mt | (−86 ± 2) | |
| GA-CHIT | (+39 ± 2) | |
| Na+-Mt/GA-CHIT composite | Mass ratio, 4:1 | Mass ratio, 20:1 |
| (−69 ± 4) | (−87 ± 4) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccoli, M.B.; Gulotta, F.A.; Montenegro, M.A.; Vanden Braber, N.L.; Paz Zanini, V.I.; Ferreyra, N.F. Immobilization of Horseradish Peroxidase onto Montmorillonite/Glucosamine–Chitosan Composite for Electrochemical Biosensing of Polyphenols. Biosensors 2024, 14, 278. https://doi.org/10.3390/bios14060278
Piccoli MB, Gulotta FA, Montenegro MA, Vanden Braber NL, Paz Zanini VI, Ferreyra NF. Immobilization of Horseradish Peroxidase onto Montmorillonite/Glucosamine–Chitosan Composite for Electrochemical Biosensing of Polyphenols. Biosensors. 2024; 14(6):278. https://doi.org/10.3390/bios14060278
Chicago/Turabian StylePiccoli, María Belén, Florencia Alejandra Gulotta, Mariana Angélica Montenegro, Noelia Luciana Vanden Braber, Verónica Irene Paz Zanini, and Nancy Fabiana Ferreyra. 2024. "Immobilization of Horseradish Peroxidase onto Montmorillonite/Glucosamine–Chitosan Composite for Electrochemical Biosensing of Polyphenols" Biosensors 14, no. 6: 278. https://doi.org/10.3390/bios14060278
APA StylePiccoli, M. B., Gulotta, F. A., Montenegro, M. A., Vanden Braber, N. L., Paz Zanini, V. I., & Ferreyra, N. F. (2024). Immobilization of Horseradish Peroxidase onto Montmorillonite/Glucosamine–Chitosan Composite for Electrochemical Biosensing of Polyphenols. Biosensors, 14(6), 278. https://doi.org/10.3390/bios14060278






