Effects of Raman Labeling Compounds on the Stability and Surface-Enhanced Raman Spectroscopy Performance of Ag Nanoparticle-Embedded Silica Nanoparticles as Tagging Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of SiO2@Ag NPs
2.3. Synthesis of Various Types of RLCs
2.4. Reaction under pH Conditions
2.5. Reaction under Temperature Conditions
2.6. Instruments and Measurement
3. Results and Discussion
3.1. Characteristics of SiO2@Ag NPs
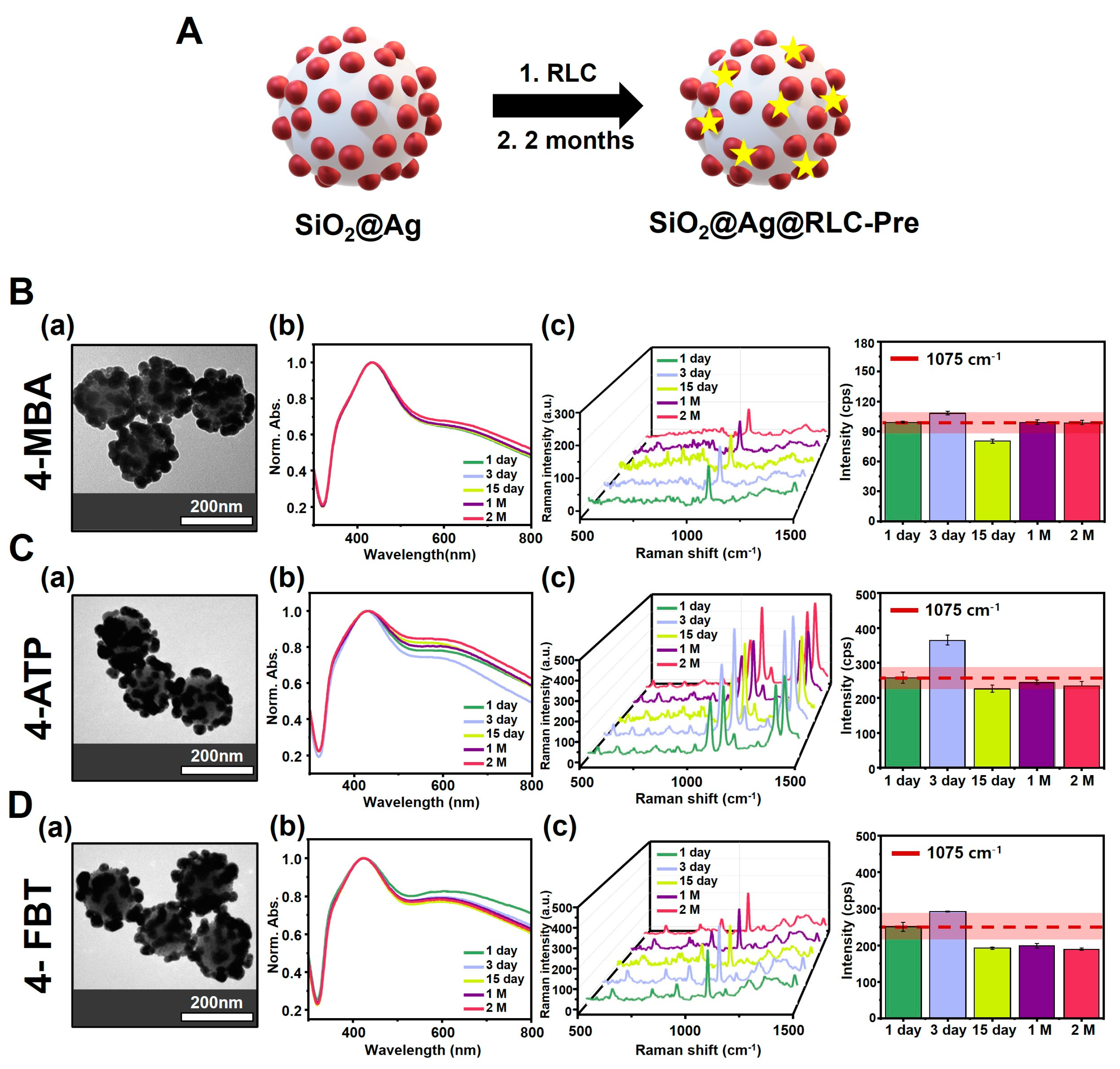
3.2. Comparison of Particle Changes over Time for SiO2@Ag NPs
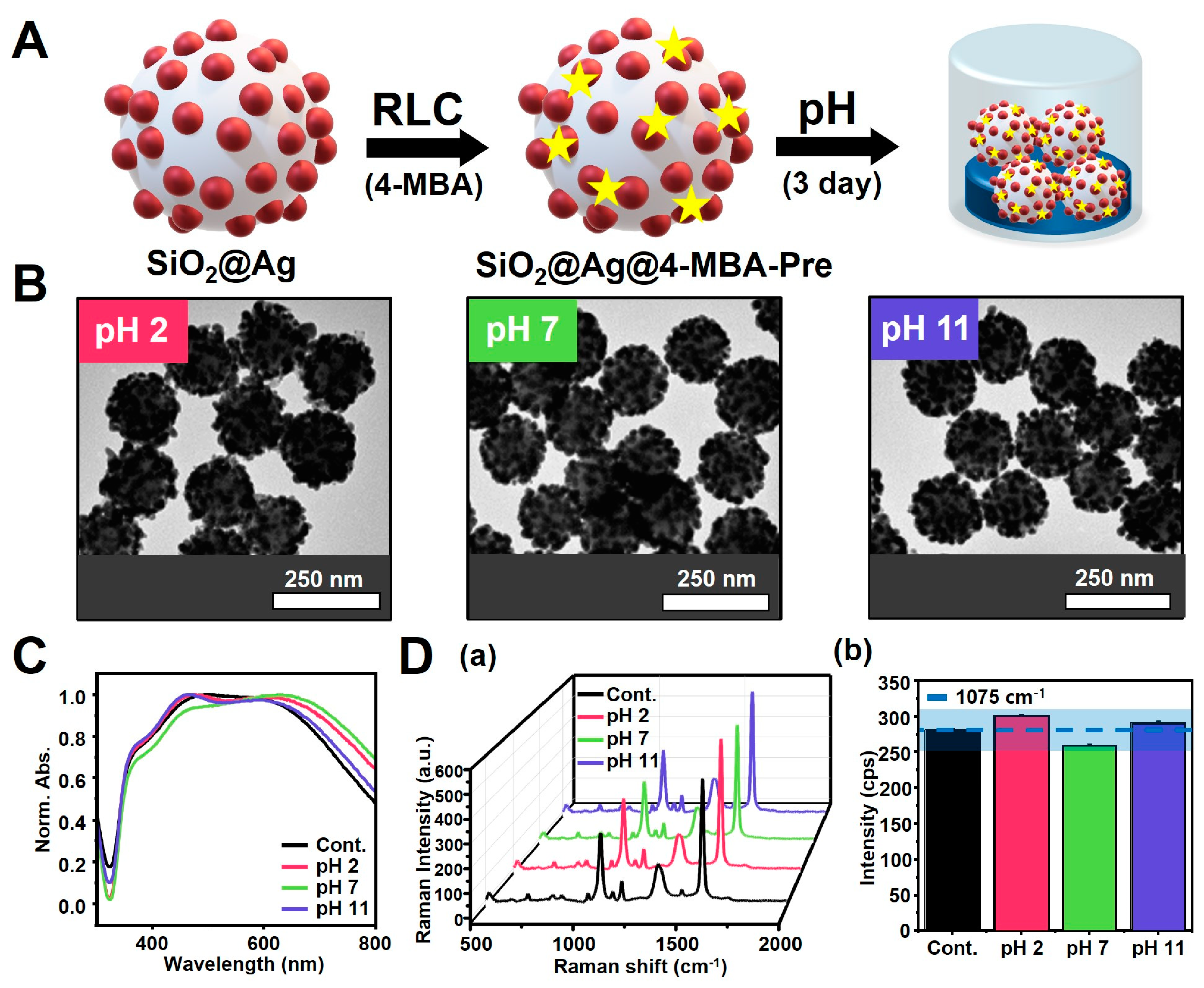
3.3. Reaction of SiO2@Ag NPs under pH Conditions
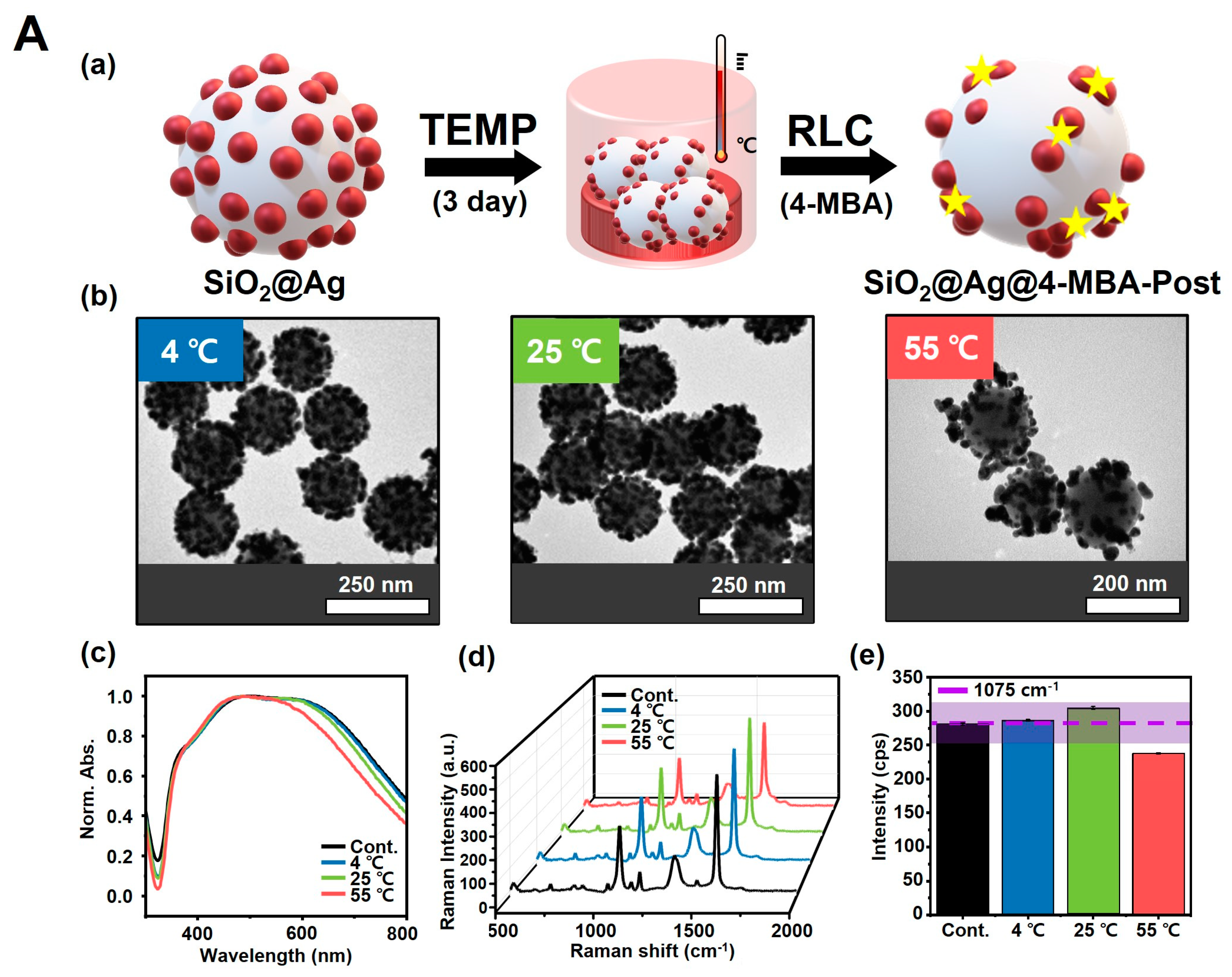
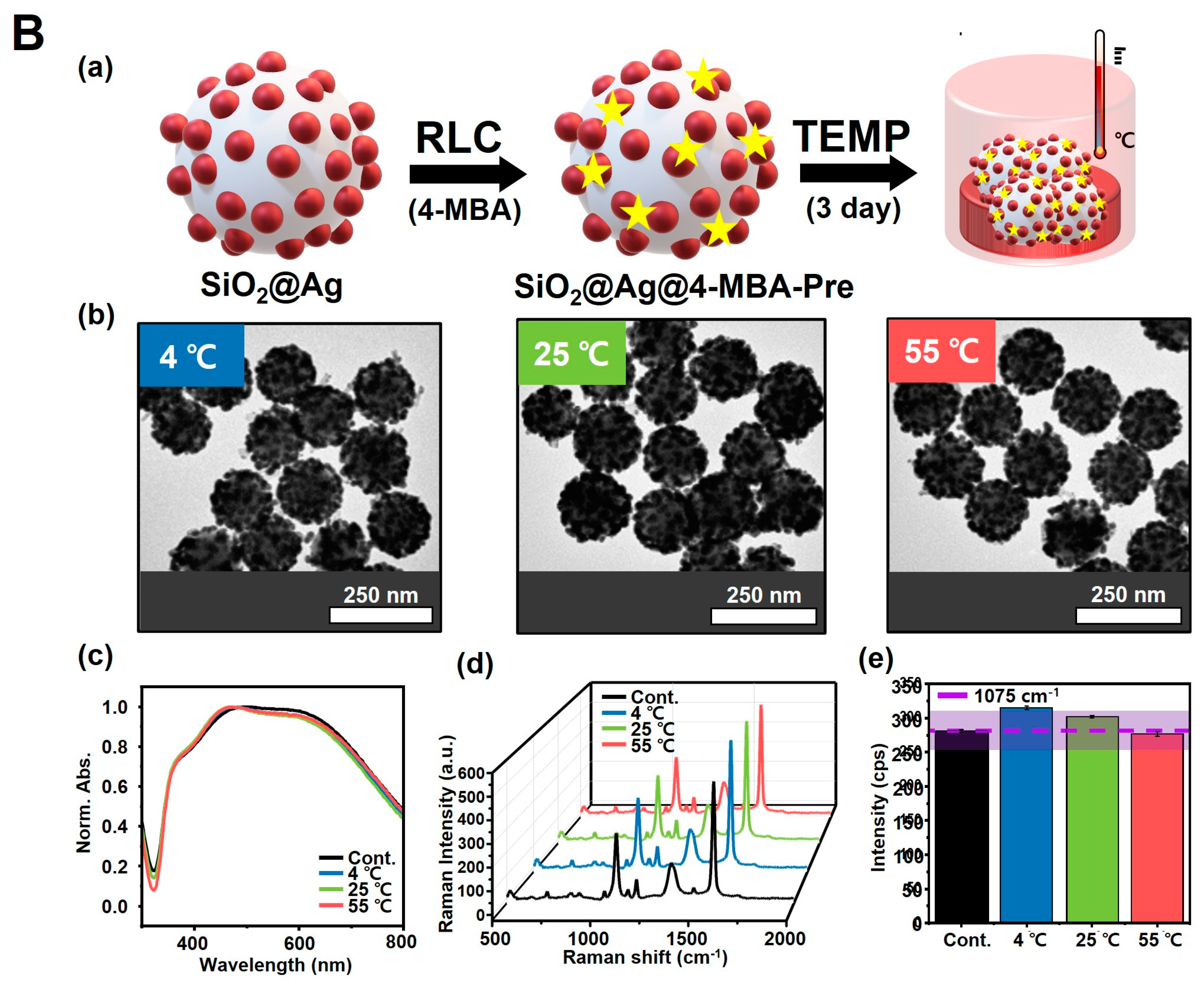
3.4. Reaction of SiO2@Ag NPs under Temperature Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Schlucker, S. Rational design and synthesis of SERS labels. Analyst 2013, 138, 2224–2238. [Google Scholar] [CrossRef] [PubMed]
- Bogle, K.A.; Dhole, S.D.; Bhoraskar, V.N. Silver nanoparticles: Synthesis and size control by electron irradiation. Nanotechnology 2006, 17, 3204–3208. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Tian, S.; Hong, T. Novel Surface-Enhanced Raman Spectroscopy Techniques for DNA, Protein and Drug Detection. Sensors 2019, 19, 1712. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yu, Q.; Zhang, X.; Du, X.; Gong, H.; Jiang, H. Synthesis and application of surface enhanced Raman scattering (SERS) tags of Ag@SiO2 core/shell nanoparticles in protein detection. J. Mater. Chem. 2012, 22, 7767–7774. [Google Scholar] [CrossRef]
- Sun, J.; Gong, L.; Wang, W.; Gong, Z.; Wang, D.; Fan, M. Surface-enhanced Raman spectroscopy for on-site analysis: A review of recent developments. Luminescence 2020, 35, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, J.; Li, Y.; Wang, B.; Yang, S.; Chen, W.; Wu, X.; Guo, J.; Ma, X. SERS substrate fabrication for biochemical sensing: Towards point-of-care diagnostics. J. Mater. Chem. B 2021, 9, 8378–8388. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Mecarini, F.; Gentile, F.; De Angelis, F.; Mohan Kumar, H.; Candeloro, P.; Liberale, C.; Cuda, G.; Di Fabrizio, E. Nano-patterned SERS substrate: Application for protein analysis vs. temperature. Biosens. Bioelectron. 2009, 24, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Gangishetty, M.K.; Scott, R.W.; Kelly, T.L. Thermal degradation mechanism of triangular Ag@SiO2 nanoparticles. Dalton Trans. 2016, 45, 9827–9834. [Google Scholar] [CrossRef]
- Jerczynski, K.; Muszynska, J.; Demirci, G.; Cetinkaya, O.; Filipczak, P.; Nowaczyk, G.; Grobelny, J.; Matyjaszewski, K.; Kozanecki, M.; Pietrasik, J. Hybrid silver nanoparticles with controlled morphology as efficient substrates for surface-enhanced Raman scattering. Polymer 2023, 285, 126363. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, S.; Radjenovic, P.M.; Tian, Z.Q.; Li, J.F. Core-Shell Nanostructure-Enhanced Raman Spectroscopy for Surface Catalysis. Acc. Chem. Res. 2020, 53, 729–739. [Google Scholar] [CrossRef]
- Hahm, E.; Jeong, D.; Cha, M.G.; Choi, J.M.; Pham, X.H.; Kim, H.M.; Kim, H.; Lee, Y.S.; Jeong, D.H.; Jung, S.; et al. beta-CD Dimer-immobilized Ag Assembly Embedded Silica Nanoparticles for Sensitive Detection of Polycyclic Aromatic Hydrocarbons. Sci. Rep. 2016, 6, 26082. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Koo, K.M.; Trau, M.; Shen, A.-G.; Hu, J.-M. Watching SERS glow for multiplex biomolecular analysis in the clinic: A review. Appl. Mater. Today 2019, 15, 431–444. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Xu, R.; Li, J.; Fu, C.; Shi, W.; Chen, J. Recent advances in ratiometric surface-enhanced Raman spectroscopy sensing strategies. Microchem. J. 2024, 199, 110127. [Google Scholar] [CrossRef]
- Kim, H.-M.; Jeong, S.; Hahm, E.; Kim, J.; Cha, M.G.; Kim, K.-M.; Kang, H.; Kyeong, S.; Pham, X.-H.; Lee, Y.-S.; et al. Large scale synthesis of surface-enhanced Raman scattering nanoprobes with high reproducibility and long-term stability. J. Ind. Eng. Chem. 2016, 33, 22–27. [Google Scholar] [CrossRef]
- Chisanga, M.; Stuible, M.; Gervais, C.; L’Abbé, D.; Cass, B.; Bisson, L.; Pelletier, A.; Lord-Dufour, S.; Durocher, Y.; Boudreau, D.; et al. SERS-based assay for multiplexed detection of cross-reactivity and persistence of antibodies against the spike of the native, P.1 and B.1.617.2 SARS-CoV-2 in non-hospitalised adults. Sens. Diagn. 2022, 1, 851–866. [Google Scholar] [CrossRef]
- Xie, W.; Qiu, P.; Mao, C. Bio-imaging, detection and analysis by using nanostructures as SERS substrates. J. Mater. Chem. 2011, 21, 5190–5202. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, J.-S.; Choi, H.; Lee, S.-M.; Jun, B.-H.; Yu, K.-N.; Kuk, E.; Kim, Y.-K.; Jeong, D.H.; Cho, M.-H. Nanoparticle probes with surface enhanced Raman spectroscopic tags for cellular cancer targeting. Anal. Chem. 2006, 78, 6967–6973. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.L.; Ying, Y.L.; Yu, R.J.; Long, Y.T. In situ food-borne pathogen sensors in a nanoconfined space by surface enhanced Raman scattering. Mikrochim. Acta 2021, 188, 201. [Google Scholar] [CrossRef]
- Zhang, H.; Yi, Y.; Zhou, C.; Ying, G.; Zhou, X.; Fu, C.; Zhu, Y.; Shen, Y. SERS detection of microRNA biomarkers for cancer diagnosis using gold-coated paramagnetic nanoparticles to capture SERS-active gold nanoparticles. RSC Adv. 2017, 7, 52782–52793. [Google Scholar] [CrossRef]
- Guerrini, L.; Pazos-Perez, N.; Garcia-Rico, E.; Alvarez-Puebla, R. Cancer characterization and diagnosis with SERS-encoded particles. Cancer Nanotechnol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Kim, T.H.; Kim, H.-M.; Lee, S.H.; Lee, S.C.; Kang, H.; Lee, H.-Y.; Jeong, D.H.; Choi, H.S.; et al. Enzyme-amplified SERS immunoassay with Ag-Au bimetallic SERS hot spots. Nano Res. 2020, 13, 3338–3346. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, D.; Li, M.; Yin, D.; Wang, S.; Wang, J. Ag@Au Core(-)Shell Porous Nanocages with Outstanding SERS Activity for Highly Sensitive SERS Immunoassay. Sensors 2019, 19, 1554. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jeong, S.; Yang, J.K.; Jo, A.; Lee, H.; Heo, E.H.; Jeong, D.H.; Jun, B.H.; Chang, H.; Lee, Y.S. Template-Assisted Plasmonic Nanogap Shells for Highly Enhanced Detection of Cancer Biomarkers. Int. J. Mol. Sci. 2021, 22, 1752. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Yang, J.K.; Noh, M.S.; Jo, A.; Jeong, S.; Lee, M.; Lee, S.; Chang, H.; Lee, H.; Jeon, S.J.; et al. One-step synthesis of silver nanoshells with bumps for highly sensitive near-IR SERS nanoprobes. J. Mater. Chem. B 2014, 2, 4415–4421. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, L.; Wei, G.; Jiang, T.; Zhou, J. SERS-based immunoassay using a core–shell SiO2@Ag immune probe and Ag-decorated NiCo2O4 nanorods immune substrate. RSC Adv. 2016, 6, 708–715. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Wang, M.; Petti, L.; Jiang, T.; Jia, Z.; Xie, S.; Zhou, J. SERS-based multiplex immunoassay of tumor markers using double SiO2@Ag immune probes and gold-film hemisphere array immune substrate. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 48–58. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, J.; Chen, Y. Preparation of Monolayer Photonic Crystals from Ag Nanobulge-Deposited SiO2 Particles as Substrates for Reproducible SERS Assay of Trace Thiol Pesticide. Nanomaterials 2020, 10, 1205. [Google Scholar] [CrossRef] [PubMed]
- Cobley, C.M.; Skrabalak, S.E.; Campbell, D.J.; Xia, Y. Shape-Controlled Synthesis of Silver Nanoparticles for Plasmonic and Sensing Applications. Plasmonics 2009, 4, 171–179. [Google Scholar] [CrossRef]
- Dhanalekshmi, K.I.; Magesan, P.; Sangeetha, K.; Zhang, X.; Jayamoorthy, K.; Srinivasan, N. Preparation and characterization of core-shell type Ag@SiO2 nanoparticles for photodynamic cancer therapy. Photodiagnosis Photodyn. Ther. 2019, 28, 324–329. [Google Scholar] [CrossRef]
- Vu, X.H.; Dien, N.D.; Ha Pham, T.T.; Van Truong, N.; Ca, N.X.; Van Thu, V. Tunable LSPR of silver/gold bimetallic nanoframes and their SERS activity for methyl red detection. RSC Adv. 2021, 11, 14596–14606. [Google Scholar] [CrossRef]
- Thai, D.V.; Pham, V.B.; Sai, C.D.; Nguyen, T.H.G.; Tran, T.D.; Tran, T.H.; Nguyen, T.T.; Nguyen, T.D.; Bui, H.V. Synthesis of SiO2@Ag Nanocomposite for Investigating Metal-Enhanced Fluorescence and Surface-Enhanced Raman Spectroscopy. J. Fluoresc. 2024, 1–10. [Google Scholar]
- Jun, B.H.; Kim, G.; Jeong, S.; Noh, M.S.; Pham, X.H.; Kang, H.; Cho, M.H.; Kim, J.H.; Lee, Y.S.; Jeong, D.H. Silica Core-based Surface-enhanced Raman Scattering (SERS) Tag: Advances in Multifunctional SERS Nanoprobes for Bioimaging and Targeting of Biomarkers#. Bull. Korean Chem. Soc. 2015, 36, 963–978. [Google Scholar] [CrossRef]
- Cho, H.S.; Noh, M.S.; Kim, Y.H.; Namgung, J.; Yoo, K.; Shin, M.S.; Yang, C.H.; Kim, Y.J.; Yu, S.J.; Chang, H.; et al. Recent Studies on Metal-Embedded Silica Nanoparticles for Biological Applications. Nanomaterials 2024, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Bock, S.; Choi, Y.S.; Kim, M.; Yun, Y.; Pham, X.H.; Kim, J.; Seong, B.; Kim, W.; Jo, A.; Ham, K.M.; et al. Highly sensitive near-infrared SERS nanoprobes for in vivo imaging using gold-assembled silica nanoparticles with controllable nanogaps. J. Nanobiotechnology 2022, 20, 130. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.V.; Ferreira, M.J.; Silva, R.; Santos, H.A.; Silva, F.; Pereira, C.M. Long time effect on the stability of silver nanoparticles in aqueous medium: Effect of the synthesis and storage conditions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 19–25. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Hahm, E.; Pham, X.-H.; Kang, H.; Jeong, D.-H.; Chang, H.; Jun, B.-H. Mercury Ion-Responsive Coalescence of Silver Nanoparticles on a Silica Nanoparticle Core for Surface-Enhanced Raman Scattering Sensing. ACS Appl. Nano Mater. 2023, 6, 23469–23476. [Google Scholar] [CrossRef]
- Wu, H.; Huang, J.; Liu, Y. Polysulfone ultrafiltration membrane incorporated with Ag-SiO(2) nanohybrid for effective fouling control. J. Water Health 2017, 15, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.; Wu, Y.; Hao, X.; Zhang, L. Fabrication of SiO2@Ag@SiO2 core–shell microspheres and thermal stability investigation. Colloids Surf. A Physicochem. Eng. Asp. 2011, 386, 135–140. [Google Scholar] [CrossRef]
- Lee, C.; Shin, K.; Lee, Y.J.; Jung, C.; Lee, H.M. Effects of shell thickness on Ag-Cu2O core-shell nanoparticles with bumpy structures for enhancing photocatalytic activity and stability. Catal. Today 2018, 303, 313–319. [Google Scholar] [CrossRef]
- Park, S.-Y.; Han, K.; O’Neill, D.B.; Mul, G. Stability of Ag@SiO2 core–shell particles in conditions of photocatalytic overall water-splitting. J. Energy Chem. 2017, 26, 309–314. [Google Scholar] [CrossRef]
- Hahm, E.; Cha, M.G.; Kang, E.J.; Pham, X.H.; Lee, S.H.; Kim, H.M.; Kim, D.E.; Lee, Y.S.; Jeong, D.H.; Jun, B.H. Multilayer Ag-Embedded Silica Nanostructure as a Surface-Enhanced Raman Scattering-Based Chemical Sensor with Dual-Function Internal Standards. ACS Appl. Mater. Interfaces 2018, 10, 40748–40755. [Google Scholar] [CrossRef]
- Kang, H.; Jeong, S.; Park, Y.; Yim, J.; Jun, B.H.; Kyeong, S.; Yang, J.K.; Kim, G.; Hong, S.; Lee, L.P.; et al. Near-Infrared SERS Nanoprobes with Plasmonic Au/Ag Hollow-Shell Assemblies for In Vivo Multiplex Detection. Adv. Funct. Mater. 2013, 23, 3719–3727. [Google Scholar] [CrossRef]
- Feng, H.-J.; Chen, Y.; Tang, F.-Q.; Ren, J. Synthesis and characterization of monodispersed SiO2/Y2O3:Eu3+ core–shell submicrospheres. Mater. Lett. 2006, 60, 737–740. [Google Scholar] [CrossRef]
- Ni, C.; Zhao, J.; Xia, X.; Wang, Z.; Zhao, X.; Yang, J.; Zhang, N.; Yang, Y.; Zhang, H.; Gao, D. Constructing a Ring-like Self-Aggregation SERS Sensor with the Coffee Ring Effect for Ultrasensitive Detection and Photocatalytic Degradation of the Herbicides Paraquat and Diquat. J. Agric. Food Chem. 2022, 70, 15296–15310. [Google Scholar] [CrossRef] [PubMed]
- Tamta, S.; Dahiya, A.; Kumar, P.S. Modified Stöber synthesis of SiO2@Ag nanocomposites and their enhanced refractive index sensing applications. Phys. B Condens. Matter 2022, 638, 413971. [Google Scholar] [CrossRef]
- Pham, X.H.; Hahm, E.; Kim, H.M.; Shim, S.; Kim, T.H.; Jeong, D.H.; Lee, Y.S.; Jun, B.H. Silver Nanoparticle-Embedded Thin Silica-Coated Graphene Oxide as an SERS Substrate. Nanomaterials 2016, 6, 176. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.W.; Liu, S.H.; Wang, X.L.; Qian, X.F.; Yin, J.; Zhu, Z.K. Silver nanocrystals by hyperbranched polyurethane-assisted photochemical reduction of Ag+. Mater. Chem. Phys. 2003, 81, 104–107. [Google Scholar] [CrossRef]
- Jain, P.K.; El-Sayed, M.A. Plasmonic coupling in noble metal nanostructures. Chem. Phys. Lett. 2010, 487, 153–164. [Google Scholar] [CrossRef]
- Ronavari, A.; Belteky, P.; Boka, E.; Zakupszky, D.; Igaz, N.; Szerencses, B.; Pfeiffer, I.; Konya, Z.; Kiricsi, M. Polyvinyl-Pyrrolidone-Coated Silver Nanoparticles-The Colloidal, Chemical, and Biological Consequences of Steric Stabilization under Biorelevant Conditions. Int. J. Mol. Sci. 2021, 22, 8673. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Ramirez, O.U.; Valenzuela-Salas, L.M.; Blanco-Salazar, A.; Rodriguez-Arenas, J.A.; Mier-Maldonado, P.A.; Garcia-Ramos, J.C.; Bogdanchikova, N.; Pestryakov, A.; Toledano-Magana, Y. Antitumor Activity against Human Colorectal Adenocarcinoma of Silver Nanoparticles: Influence of [Ag]/[PVP] Ratio. Pharmaceutics 2021, 13, 1000. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Wang, X.; Ding, S. Mechanisms of PVP in the preparation of silver nanoparticles. Mater. Chem. Phys. 2005, 94, 449–453. [Google Scholar] [CrossRef]
- Fernando, I.; Zhou, Y. Impact of pH on the stability, dissolution and aggregation kinetics of silver nanoparticles. Chemosphere 2019, 216, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.; Pradell, T.; Crespo, D.; Calderón-Moreno, J.M. Stable silver colloidal dispersions using short chain polyethylene glycol. Colloids Surf. A Physicochem. Eng. Asp. 2007, 303, 184–190. [Google Scholar] [CrossRef]
- Jiang, H.; Moon, K.-S.; Zhang, Z.; Pothukuchi, S.; Wong, C.P. Variable Frequency Microwave Synthesis of Silver Nanoparticles. J. Nanoparticle Res. 2006, 8, 117–124. [Google Scholar] [CrossRef]
- Jiang, Z.-J.; Liu, C.-Y. Seed-mediated growth technique for the preparation of a silver nanoshell on a silica sphere. J. Phys. Chem. B 2003, 107, 12411–12415. [Google Scholar] [CrossRef]
- Granbohm, H.; Larismaa, J.; Ali, S.; Johansson, L.S.; Hannula, S.P. Control of the Size of Silver Nanoparticles and Release of Silver in Heat Treated SiO2-Ag Composite Powders. Materials 2018, 11, 80. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-H.; Cho, H.-S.; Kim, Y.-H.; Yoo, K.; Lim, J.; Hahm, E.; Rho, W.Y.; Kim, Y.J.; Jun, B.-H. Effects of Raman Labeling Compounds on the Stability and Surface-Enhanced Raman Spectroscopy Performance of Ag Nanoparticle-Embedded Silica Nanoparticles as Tagging Materials. Biosensors 2024, 14, 272. https://doi.org/10.3390/bios14060272
Yang C-H, Cho H-S, Kim Y-H, Yoo K, Lim J, Hahm E, Rho WY, Kim YJ, Jun B-H. Effects of Raman Labeling Compounds on the Stability and Surface-Enhanced Raman Spectroscopy Performance of Ag Nanoparticle-Embedded Silica Nanoparticles as Tagging Materials. Biosensors. 2024; 14(6):272. https://doi.org/10.3390/bios14060272
Chicago/Turabian StyleYang, Cho-Hee, Hye-Seong Cho, Yoon-Hee Kim, Kwanghee Yoo, Jaehong Lim, Eunil Hahm, Won Yeop Rho, Young Jun Kim, and Bong-Hyun Jun. 2024. "Effects of Raman Labeling Compounds on the Stability and Surface-Enhanced Raman Spectroscopy Performance of Ag Nanoparticle-Embedded Silica Nanoparticles as Tagging Materials" Biosensors 14, no. 6: 272. https://doi.org/10.3390/bios14060272
APA StyleYang, C.-H., Cho, H.-S., Kim, Y.-H., Yoo, K., Lim, J., Hahm, E., Rho, W. Y., Kim, Y. J., & Jun, B.-H. (2024). Effects of Raman Labeling Compounds on the Stability and Surface-Enhanced Raman Spectroscopy Performance of Ag Nanoparticle-Embedded Silica Nanoparticles as Tagging Materials. Biosensors, 14(6), 272. https://doi.org/10.3390/bios14060272





