Development of a Flexible Sensor-Integrated Tissue Patch to Monitor Early Organ Rejection Processes Using Impedance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrode Passivation Using Thermal Oxidation of Titanium Electrodes
2.2. Biosensor Fabrication and Assembly
2.3. Contactless Bioimpedance Sensing Using Passivated Titanium Electrodes
2.4. Organ Sample Preparation
2.5. Computational Simulation of Tissue Rejection Monitoring Using SPICE
3. Results
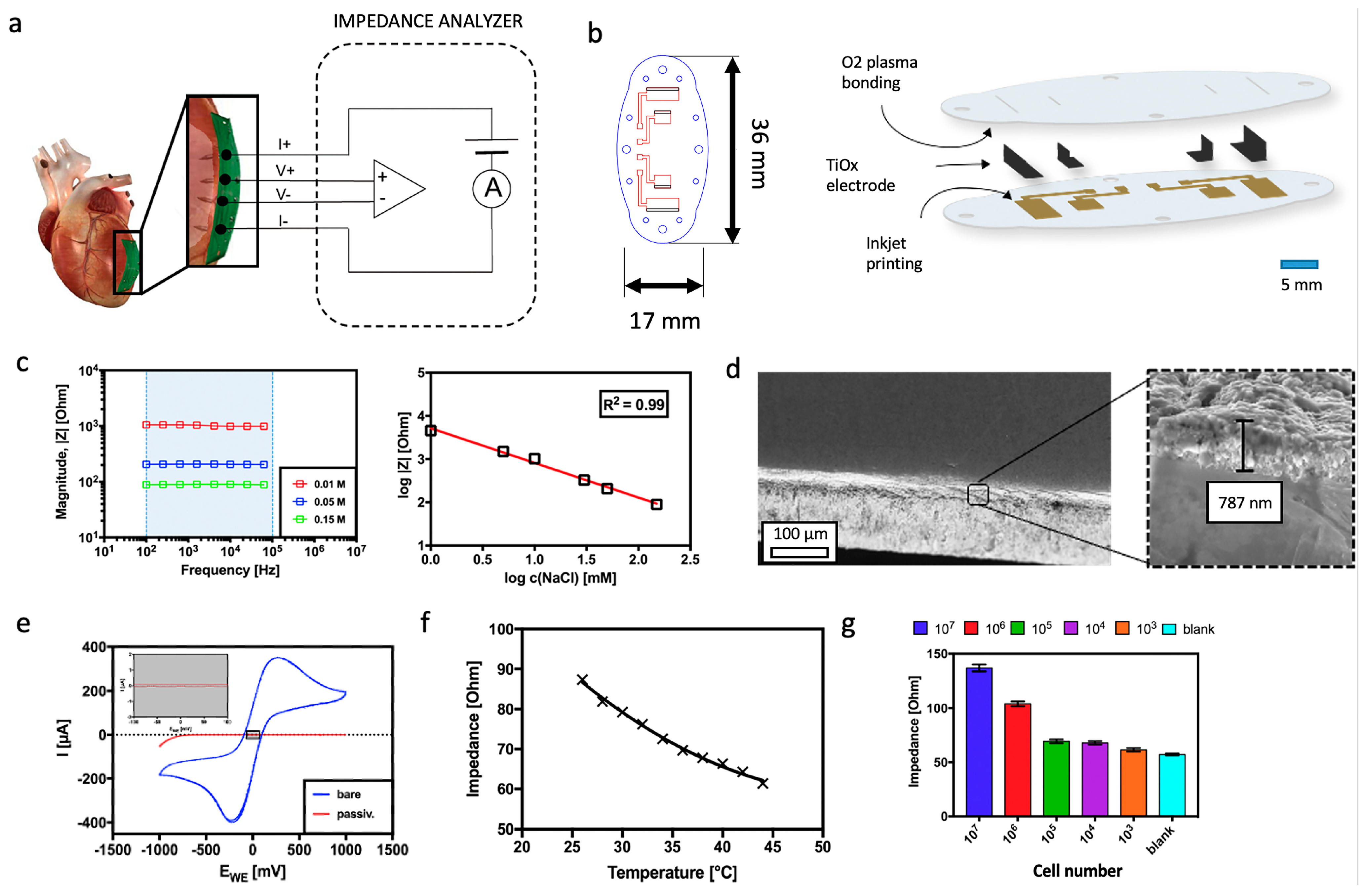
3.1. Establishment and Characterization of the Tetrapolar Biosensor Setup Consisting of Oxygen-Passivated Titanium Electrodes
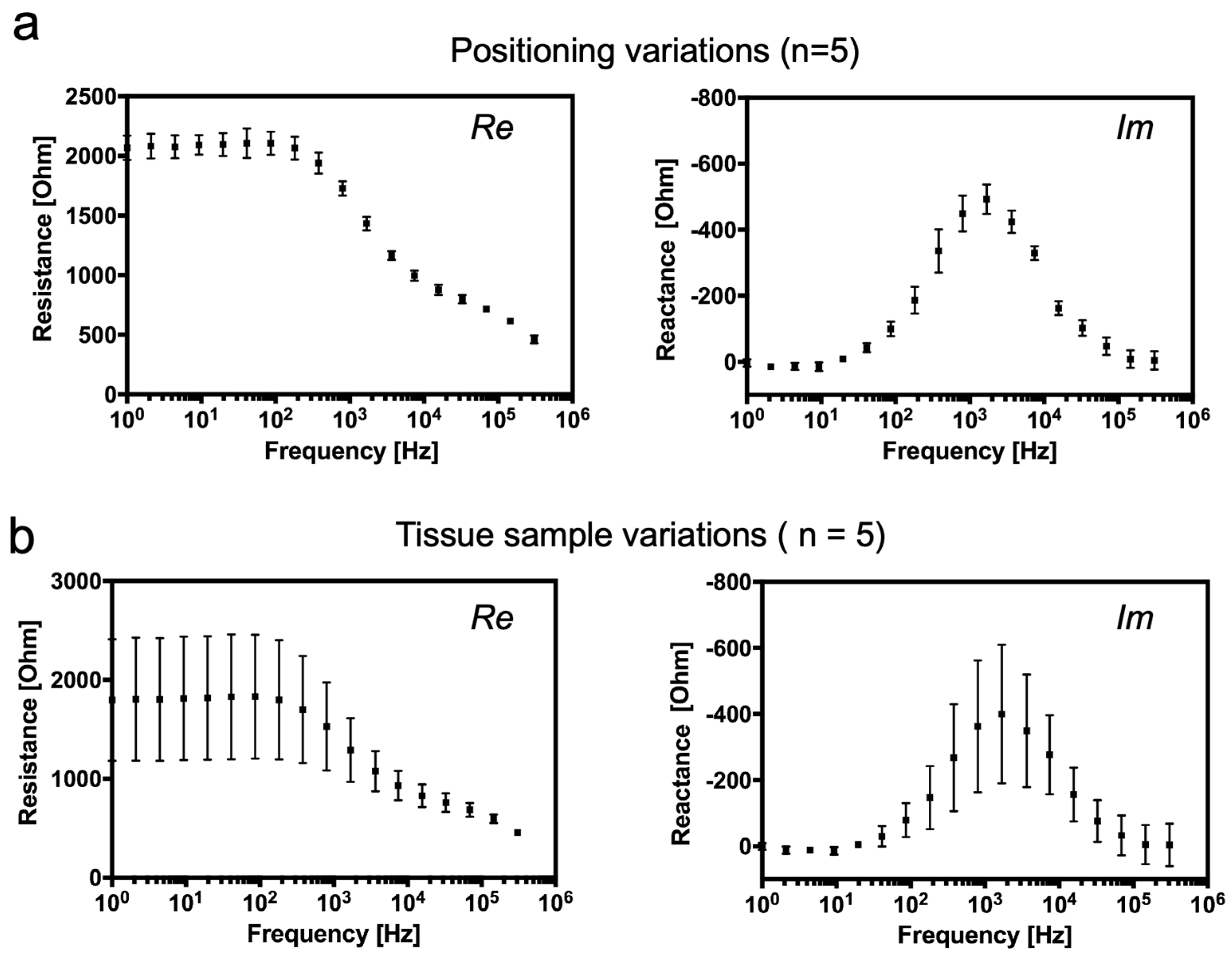
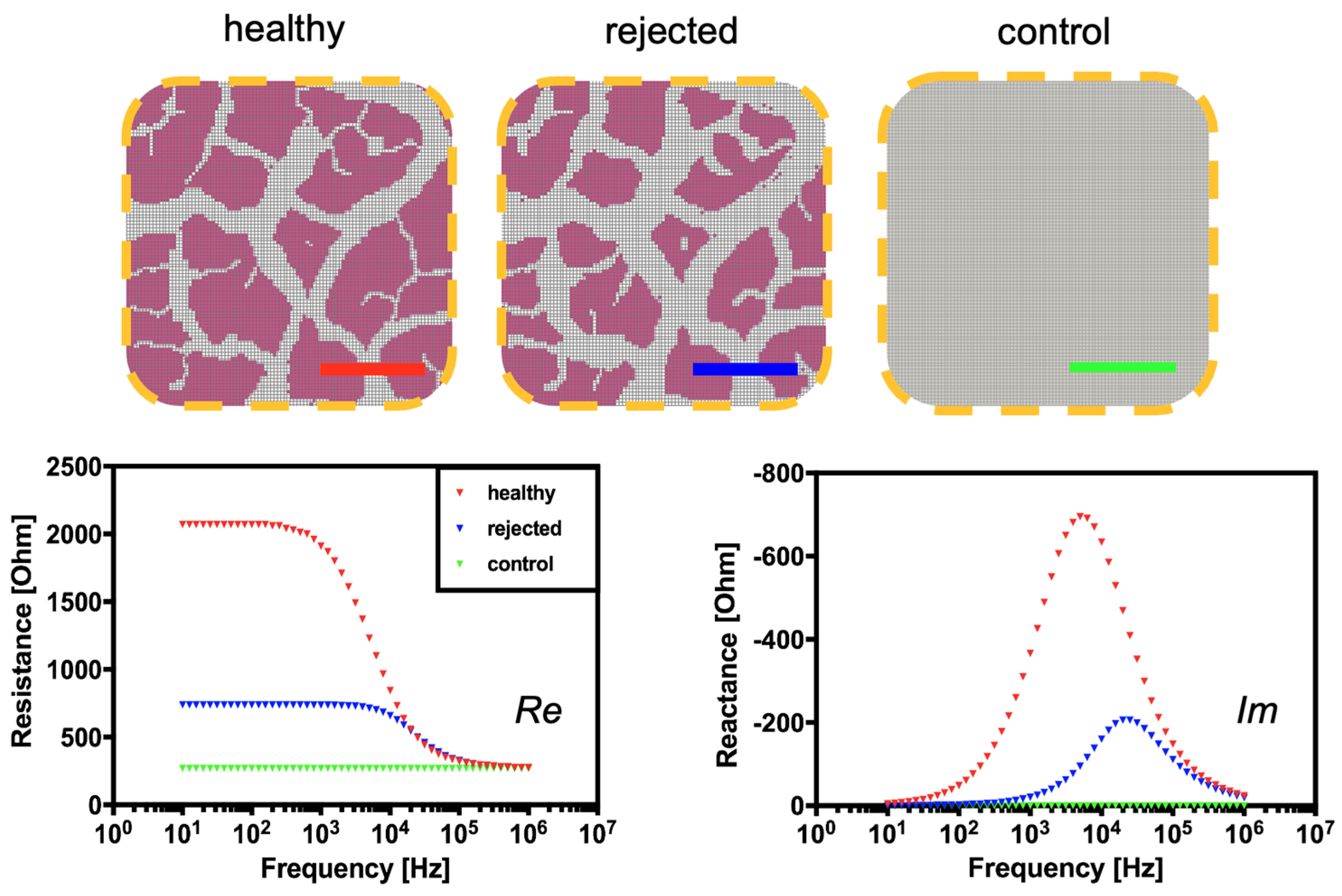
3.2. Computational and Experimental Estimation of Impedance Changes Caused by Declining Tissue Integrity
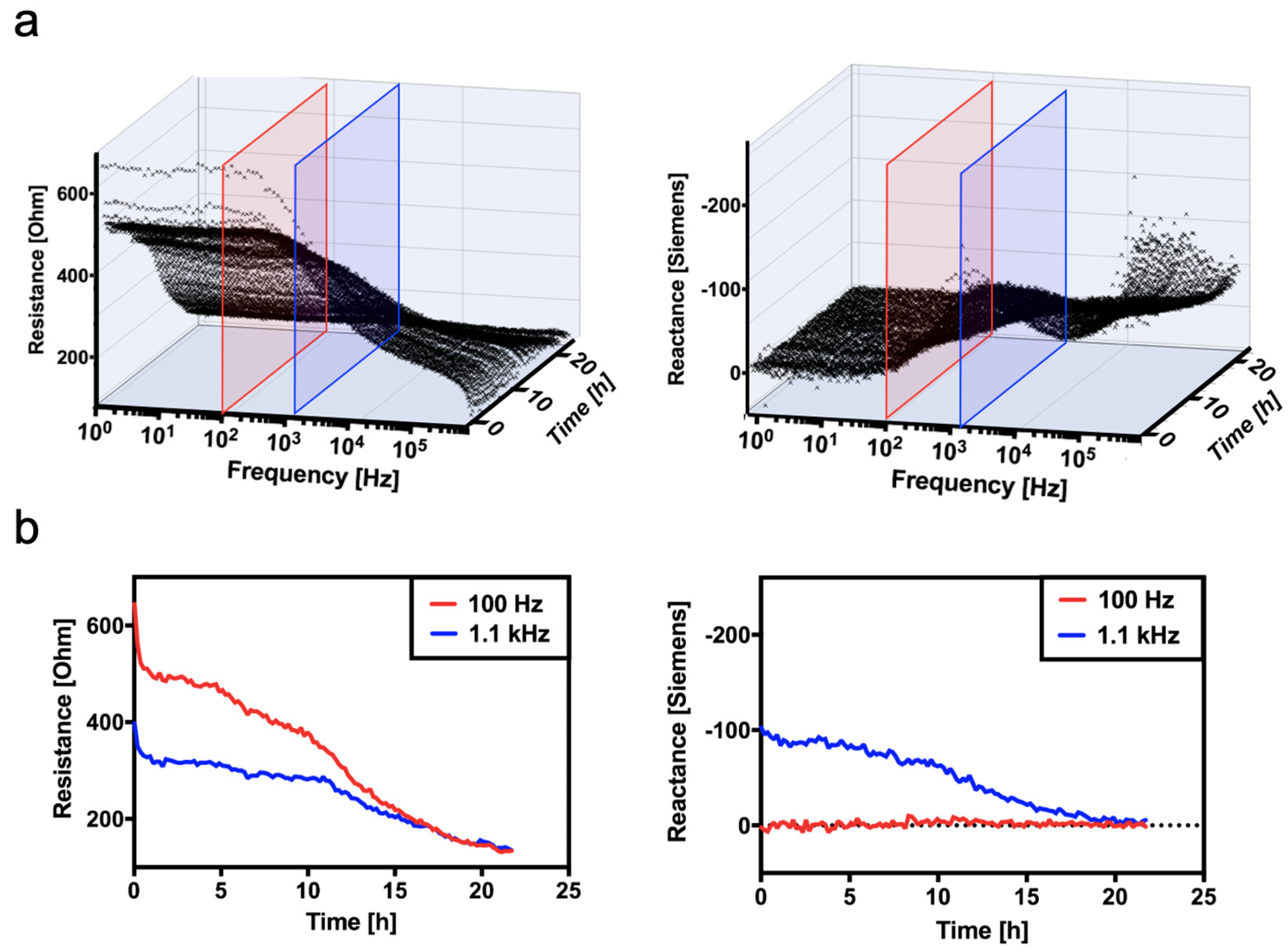
3.3. Continuous Monitoring of Structural Changes in Tissue Integrity Using a Flexible Sensor-Integrated Tissue Patch
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Johnson, N.B.; Hayes, L.D.; Brown, K.; Hoo, E.C.; Ethier, K.A. CDC National Health Report: Leading causes of morbidity and mortality and associated behavioral risk and protective factors—United States, 2005–2013. Morb. Mortal. Wkly. Rep. 2014, 63, 3–27. [Google Scholar]
- Singh, T.P.; Cherikh, W.S.; Hsich, E.; Lewis, A.; Perch, M.; Kian, S.; Hayes, D.; Potena, L.; Stehlik, J.; Zuckermann, A.; et al. Graft survival in primary thoracic organ transplant recipients: A special report from the International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2023, 42, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card. Fail. Rev. 2023, 9, e11. [Google Scholar] [CrossRef]
- Kivimäki, M.; Nyberg, S.T.; Fransson, E.I.; Heikkilä, K.; Alfredsson, L.; Casini, A.; Clays, E.; De Bacquer, D.; Dragano, N.; Ferrie, J.E.; et al. Associations of job strain and lifestyle risk factors with risk of coronary artery disease: A meta-analysis of individual participant data. Can. Med. Assoc. J. 2013, 185, 763–769. [Google Scholar] [CrossRef]
- Väisänen, D.; Kallings, L.; Andersson, G.; Wallin, P.; Hemmingsson, E.; Stenling, A.; Ekblom-Bak, E. Mediation of lifestyle-associated variables on the association between occupation and incident cardiovascular disease. Prev. Med. 2023, 167, 107411. [Google Scholar] [CrossRef]
- Hsich, E.; Singh, T.P.; Cherikh, W.S.; Harhay, M.O.; Hayes, D.; Perch, M.; Potena, L.; Sadavarte, A.; Lindblad, K.; Zuckermann, A.; et al. The International thoracic organ transplant registry of the international society for heart and lung transplantation: Thirty-ninth adult heart transplantation report—2022; focus on transplant for restrictive heart disease. J. Heart Lung Transplant. 2022, 41, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Barnard, C.N. The operation. A human cardiac transplant: An interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S. Afr. Med. J. 1967, 41, 1271–1274. [Google Scholar] [PubMed]
- Anyanwu, A.; Treasure, T. Prognosis after heart transplantation. BMJ 2003, 326, 509–510. [Google Scholar] [CrossRef]
- Awad, M.A.; Shah, A.; Griffith, B.P. Current status and outcomes in heart transplantation: A narrative review. Rev. Cardiovasc. Med. 2022, 23, 11. [Google Scholar] [CrossRef]
- Lund, L.H.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden, C.; Christie, J.D.; Dipchand, A.I.; Dobbels, F.; Goldfarb, S.B.; Levvey, B.J.; Meiser, B.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-first Official Adult Heart Transplant Report—2014; Focus Theme: Retransplantation. J. Heart Lung Transplant. 2014, 33, 996–1008. [Google Scholar] [CrossRef]
- Cunningham, K.S.; Veinot, J.P.; Butany, J. An approach to endomyocardial biopsy interpretation. J. Clin. Pathol. 2006, 59, 121–129. [Google Scholar] [CrossRef] [PubMed]
- From, A.M.; Maleszewski, J.J.; Rihal, C.S. Current Status of Endomyocardial Biopsy. Mayo Clin. Proc. 2011, 86, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Hummel, M.; Müller, J.; Wellnhofer, E.; Meyer, R.; Solowjowa, N.; Ewert, R.; Hetzer, R. Reliability of Tissue Doppler Wall Motion Monitoring After Heart Transplantation for Replacement of Invasive Routine Screenings by Optimally Timed Cardiac Biopsies and Catheterizations. Circulation 2001, 104, I-184–I-191. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.K.; Kosaraju, S.; A Arabia, F.; Roasdo, L.J.; McCarthy, M.S.; Copeland, J.G. Is it necessary to perform surveillance endomyocardial biopsies in heart transplant recipients? J. Heart Lung Transplant. 1995, 14, 1047–1051. [Google Scholar] [PubMed]
- White, J.A.; Guiraudon, C.; Pflugfelder, P.W.; Kostuk, W.J. Routine surveillance myocardial biopsies are unnecessary beyond one year after heart transplantation. J. Heart Lung Transplant. 1995, 14, 1052–1056. [Google Scholar]
- Costello, J.P.; Mohanakumar, T.; Nath, D.S. Mechanisms of chronic cardiac allograft rejection. Tex. Heart Inst. J. 2013, 40, 395–399. [Google Scholar] [PubMed]
- Kittleson, M.; Patel, J.; Rafiei, M.; Osborne, A.; Tittle, M.; Chang, D.; Czer, L.; Esmailian, F.; Trento, A.; Kobashigawa, J. Longer and Shorter Hospital Stay after Heart Transplant Both Risk Factors for Suboptimal Outcome. J. Heart Lung Transplant. 2013, 32, S263. [Google Scholar] [CrossRef]
- Porcari, A.; Baggio, C.; Fabris, E.; Merlo, M.; Bussani, R.; Perkan, A.; Sinagra, G. Endomyocardial biopsy in the clinical context: Current indications and challenging scenarios. Heart Fail. Rev. 2022, 28, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Sticker, D.; Rothbauer, M.; Charwat, V.; Steinkühler, J.; Bethge, O.; Bertagnolli, E.; Wanzenboeck, H.D.; Ertl, P. Zirconium dioxide nanolayer passivated impedimetric sensors for cell-based assays. Sens. Actuators B Chem. 2015, 213, 35–44. [Google Scholar] [CrossRef]
- Charwat, V.; Joksch, M.; Sticker, D.; Purtscher, M.; Rothbauer, M.; Ertl, P. Monitoring cellular stress responses using integrated high-frequency impedance spectroscopy and time-resolved ELISA. Analyst 2014, 139, 5271–5282. [Google Scholar] [CrossRef]
- Sun, T.; Tsuda, S.; Zauner, K.-P.; Morgan, H. On-chip electrical impedance tomography for imaging biological cells. Biosens. Bioelectron. 2010, 25, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Atefi, S.R.; Seoane, F.; Thorlin, T.; Lindecrantz, K. Stroke Damage Detection Using Classification Trees on Electrical Bioimpedance Cerebral Spectroscopy Measurements. Sensors 2013, 13, 10074–10086. [Google Scholar] [CrossRef] [PubMed]
- Schuller, P.; Rothbauer, M.; Kratz, S.R.A.; Hoell, G.; Taus, P.; Schinnerl, M.; Genser, J.; Bastus, N.; Moriones, O.H.; Puntes, V.; et al. A lab-on-a-chip system with an embedded porous membrane-based impedance biosensor array for nanoparticle risk assessment on placental Bewo trophoblast cells. Sens. Actuators B Chem. 2020, 312, 127946. [Google Scholar] [CrossRef]
- Martinez, M.G.V.; Reihs, E.I.; Stuetz, H.M.; Hafner, A.; Brandauer, K.; Selinger, F.; Schuller, P.; Bastus, N.; Puntes, V.; Frank, J.; et al. Using Rapid Prototyping to Develop a Cell-Based Platform with Electrical Impedance Sensor Membranes for In Vitro RPMI2650 Nasal Nanotoxicology Monitoring. Biosensors 2024, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Galanti, G.; Stefani, L.; Scacciati, I.; Mascherini, G.; Buti, G.; Maffulli, N. Eating and nutrition habits in young competitive athletes: A comparison between soccer players and cyclists. Transl. Med. UniSa 2015, 11, 44–47. [Google Scholar]
- Wang, Y.; Ye, Z.; Ying, Y. New Trends in Impedimetric Biosensors for the Detection of Foodborne Pathogenic Bacteria. Sensors 2012, 12, 3449–3471. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, H.S.; Frazier, A.B.; Chen, Z.G.; Shin, D.M.; Han, A. Whole-Cell Impedance Analysis for Highly and Poorly Metastatic Cancer Cells. J. Microelectromechanical Syst. 2009, 18, 808–817. [Google Scholar] [CrossRef]
- Hildebrandt, C.; Büth, H.; Cho, S.; Impidjati; Thielecke, H. Detection of the osteogenic differentiation of mesenchymal stem cells in 2D and 3D cultures by electrochemical impedance spectroscopy. J. Biotechnol. 2010, 148, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.H.; Park, J.-K. Cytotoxicity test based on electrochemical impedance measurement of HepG2 cultured in microfabricated cell chip. Anal. Biochem. 2005, 341, 308–315. [Google Scholar] [CrossRef]
- Xiao, C.; Luong, J.H.T. On-Line Monitoring of Cell Growth and Cytotoxicity Using Electric Cell-Substrate Impedance Sensing (ECIS). Biotechnol. Prog. 2003, 19, 1000–1005. [Google Scholar] [CrossRef]
- Wegener, J.; Keese, C.R.; Giaever, I. Electric Cell–Substrate Impedance Sensing (ECIS) as a Noninvasive Means to Monitor the Kinetics of Cell Spreading to Artificial Surfaces. Exp. Cell Res. 2000, 259, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Abassi, Y.A.; Jackson, J.A.; Zhu, J.; Oconnell, J.; Wang, X.; Xu, X. Label-free, real-time monitoring of IgE-mediated mast cell activation on microelectronic cell sensor arrays. J. Immunol. Methods 2004, 292, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Gemelli, N.H.A.; Scariot, E.; Camargo, A. Thermal characterization of commercially pure titanium for dental applications. Mater. Res. 2007, 10, 241–246. [Google Scholar] [CrossRef]
- Villares, A.; Pera, G.; Giner, I.; Cea, P.; López, M.C.; Martín, S. The Use of Cyclic Voltammetry To Probe the Passivation of Electrode Surfaces by Well-Ordered Self-Assembly and Langmuir–Blodgett Films. An Advanced Undergraduate Laboratory Experiment in Surface Science and Nanomaterials Chemistry. J. Chem. Educ. 2009, 86, 723. [Google Scholar] [CrossRef]
- Sticker, D. Development of Thiol-Ene-Epoxy Thermoset Microdevices with Automated Fluid Handling and Nanolayer Passivated Microstructured Impedimetric Sensor for In Vitro Cell-Based Assays. Ph.D. Thesis, Technische Universität Wien, Vienna, Austria, 2015. [Google Scholar]
- Buendía, R.; Muellner, P.; Bethge, O.; Bertagnolli, E. Monolayer: Silanization of PDMS using MPTMS. Chem. Commun. 2014, 50, 2424–2427. [Google Scholar]
- Buendia, R.; Seoane, F.; Gil-Pita, R. A novel approach for removing the hook effect artefact from Electrical Bioimpedance spectroscopy measurements. J. Phys. Conf. Ser. 2010, 224, 012126. [Google Scholar] [CrossRef]
- Grimnes, S.; Martinsen, Ø.G. Alpha-dispersion in human tissue. J. Phys. Conf. Ser. vol. 2010, 224, 012073. [Google Scholar] [CrossRef]
- McAdams, E.T.; Jossinet, J. A physical interpretation of Schwan’s limit current of linearity. Ann. Biomed. Eng. 1992, 20, 307–319. [Google Scholar] [CrossRef]
- Fishman, J.A. Infection in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2007, 357, 2601–2614. [Google Scholar] [CrossRef]
- Wu, L.; Ogawa, Y.; Tagawa, A. Electrical impedance spectroscopy analysis of eggplant pulp and effects of drying and freezing–thawing treatments on its impedance characteristics. J. Food Eng. 2008, 87, 274–280. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ertl, P.; Wladimir, T.; Sticker, D.; Schuller, P.; Rothbauer, M.; Wieselthaler, G.; Frauenlob, M. Development of a Flexible Sensor-Integrated Tissue Patch to Monitor Early Organ Rejection Processes Using Impedance Spectroscopy. Biosensors 2024, 14, 253. https://doi.org/10.3390/bios14050253
Ertl P, Wladimir T, Sticker D, Schuller P, Rothbauer M, Wieselthaler G, Frauenlob M. Development of a Flexible Sensor-Integrated Tissue Patch to Monitor Early Organ Rejection Processes Using Impedance Spectroscopy. Biosensors. 2024; 14(5):253. https://doi.org/10.3390/bios14050253
Chicago/Turabian StyleErtl, Peter, Tibor Wladimir, Drago Sticker, Patrick Schuller, Mario Rothbauer, Georg Wieselthaler, and Martin Frauenlob. 2024. "Development of a Flexible Sensor-Integrated Tissue Patch to Monitor Early Organ Rejection Processes Using Impedance Spectroscopy" Biosensors 14, no. 5: 253. https://doi.org/10.3390/bios14050253
APA StyleErtl, P., Wladimir, T., Sticker, D., Schuller, P., Rothbauer, M., Wieselthaler, G., & Frauenlob, M. (2024). Development of a Flexible Sensor-Integrated Tissue Patch to Monitor Early Organ Rejection Processes Using Impedance Spectroscopy. Biosensors, 14(5), 253. https://doi.org/10.3390/bios14050253





