Highly Sensitive Detection of Hydrogen Peroxide in Cancer Tissue Based on 3D Reduced Graphene Oxide–MXene–Multi-Walled Carbon Nanotubes Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of the 3D rGO–Ti3C2–MWCNTs Electrode
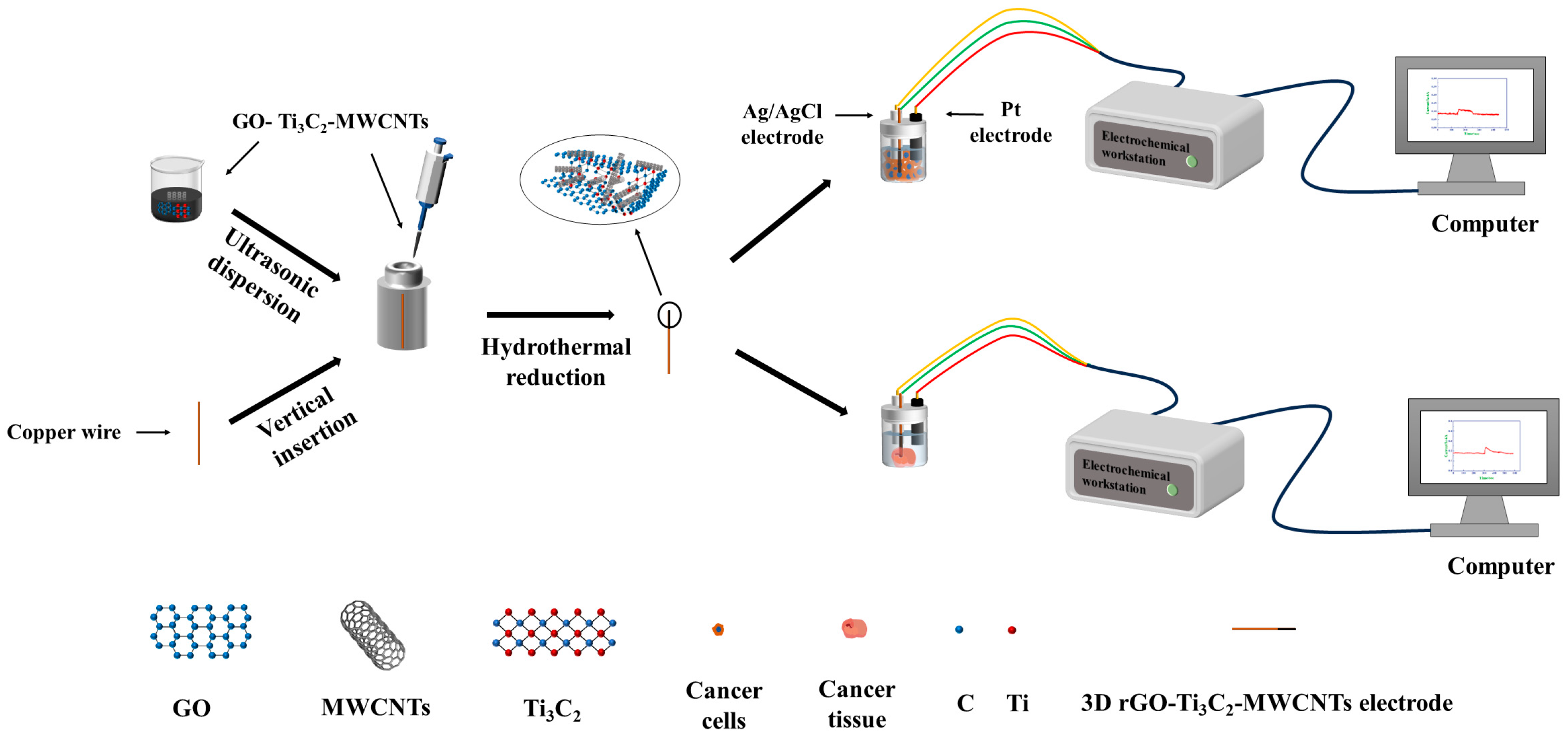
2.3. Electrochemical Characterization
2.4. Cell Culture and Animals
2.5. Detection of H2O2 in Live Cells and Tissue
3. Results and Discussion
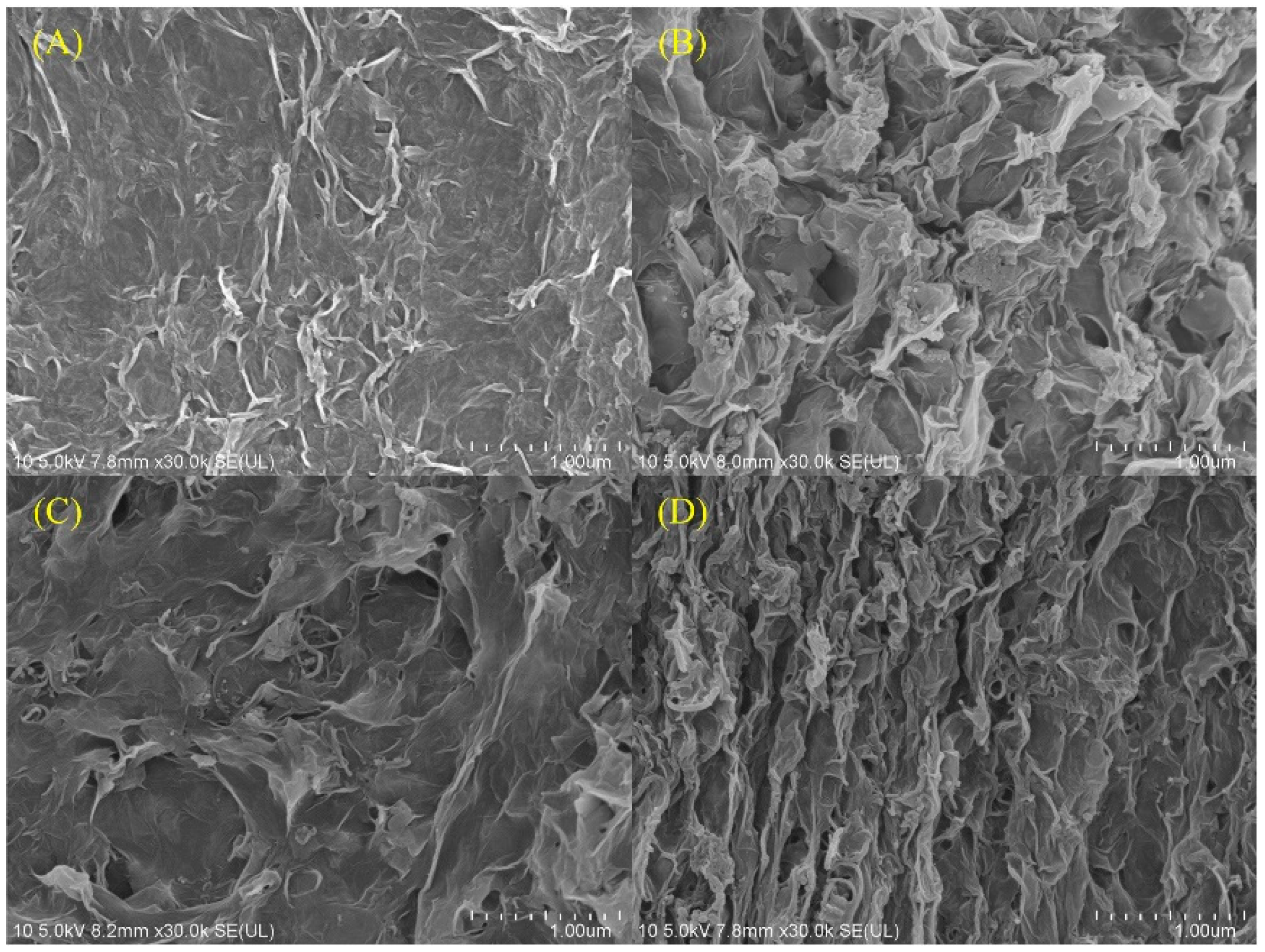
3.1. Physical Characterization
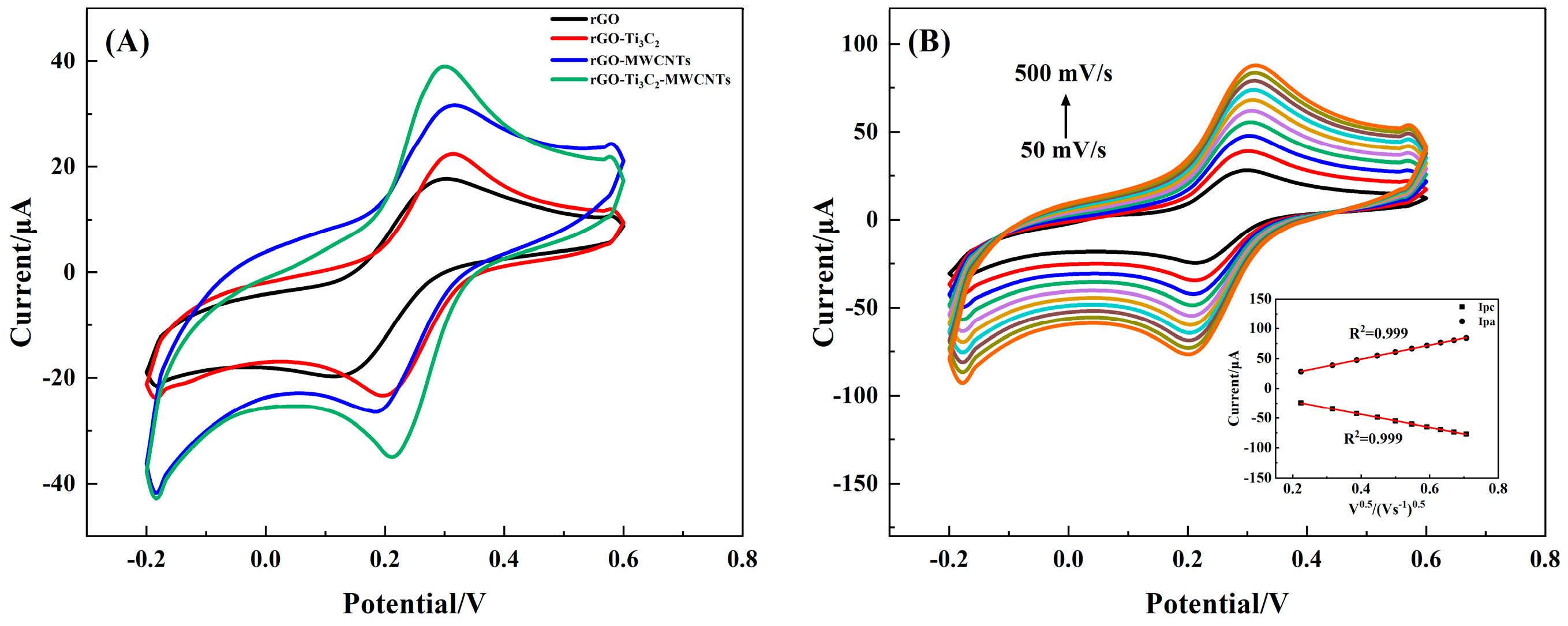
3.2. Direct Electrochemical Behavior of the 3D rGO–Ti3C2–MWCNTs Electrode
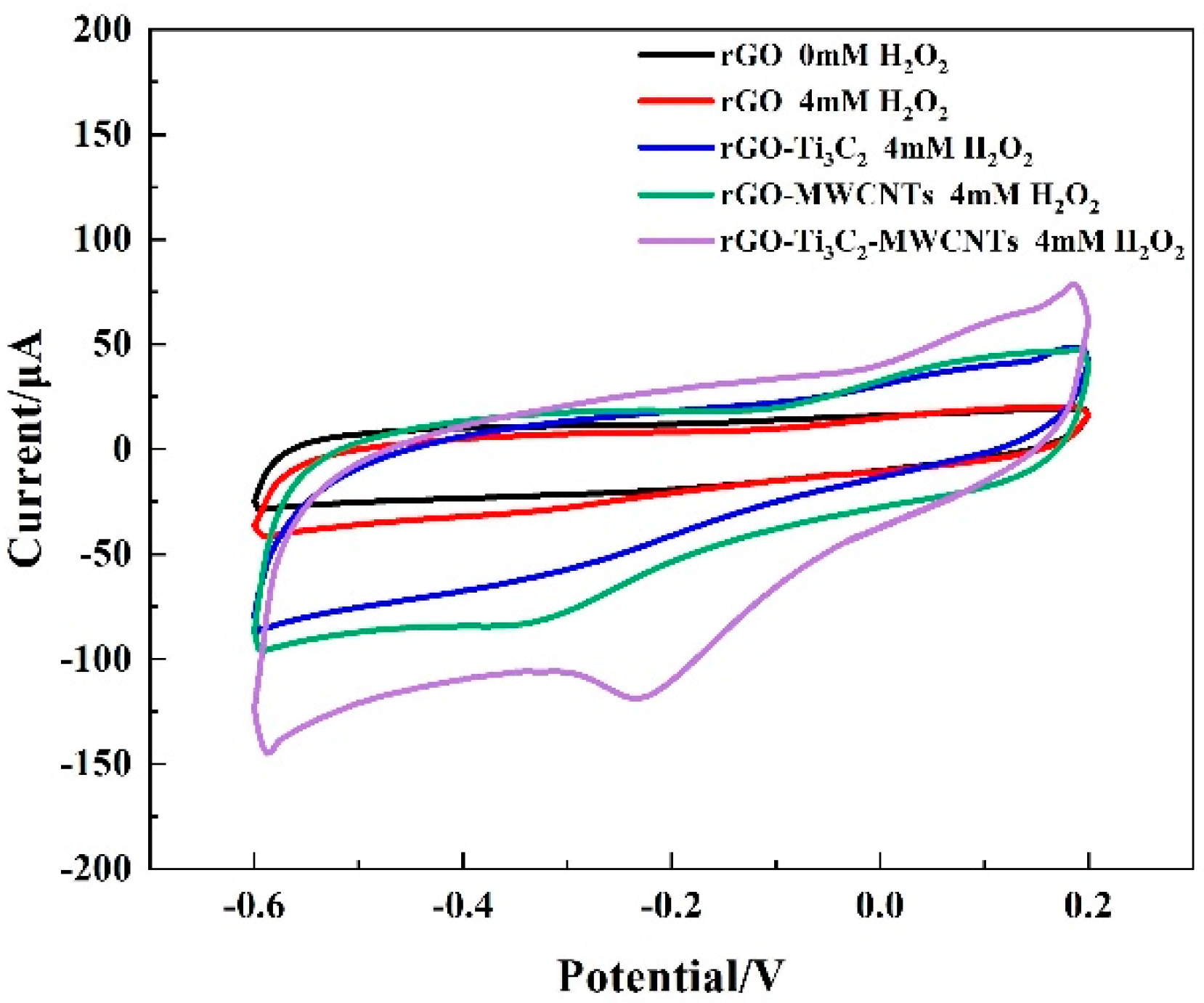
3.3. Electrocatalytic Reduction of H2O2 by the 3D rGO–Ti3C2–MWCNTs Electrode
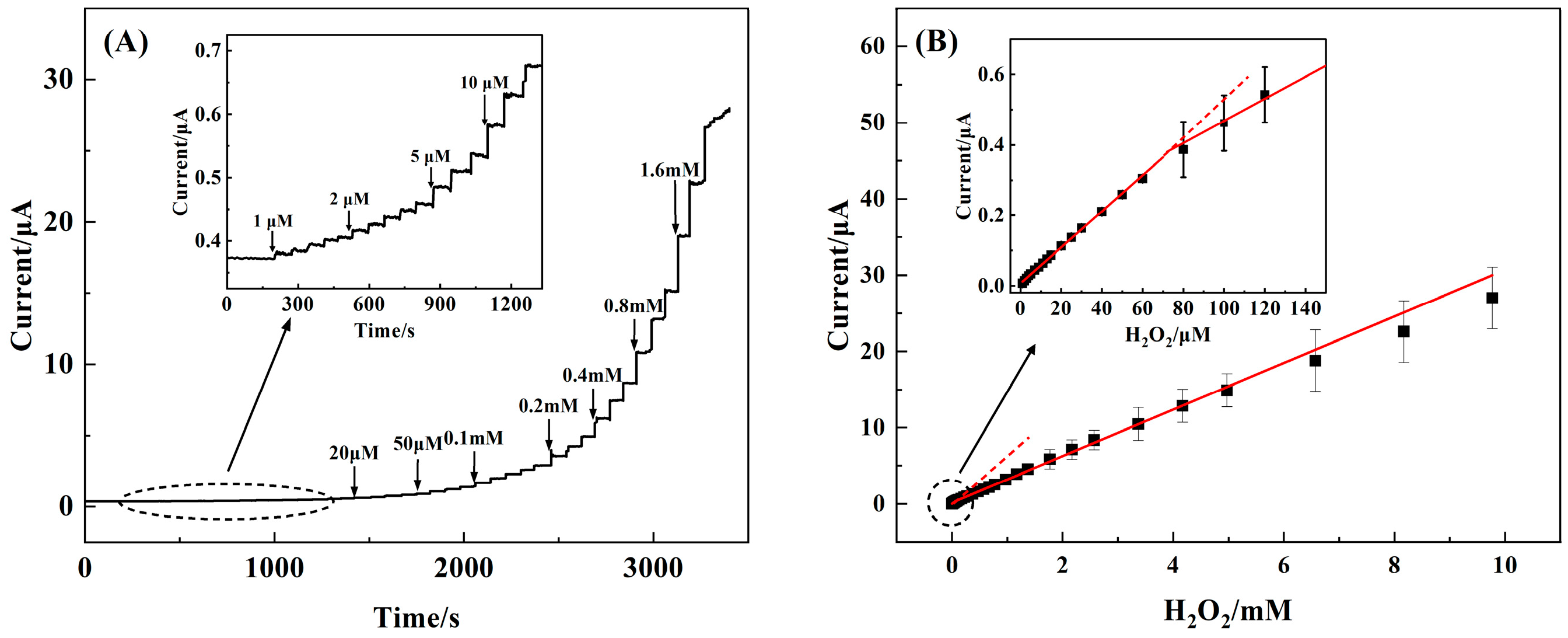
3.4. Amperometric Performance of the 3D rGO–Ti3C2–MWCNTs Electrode
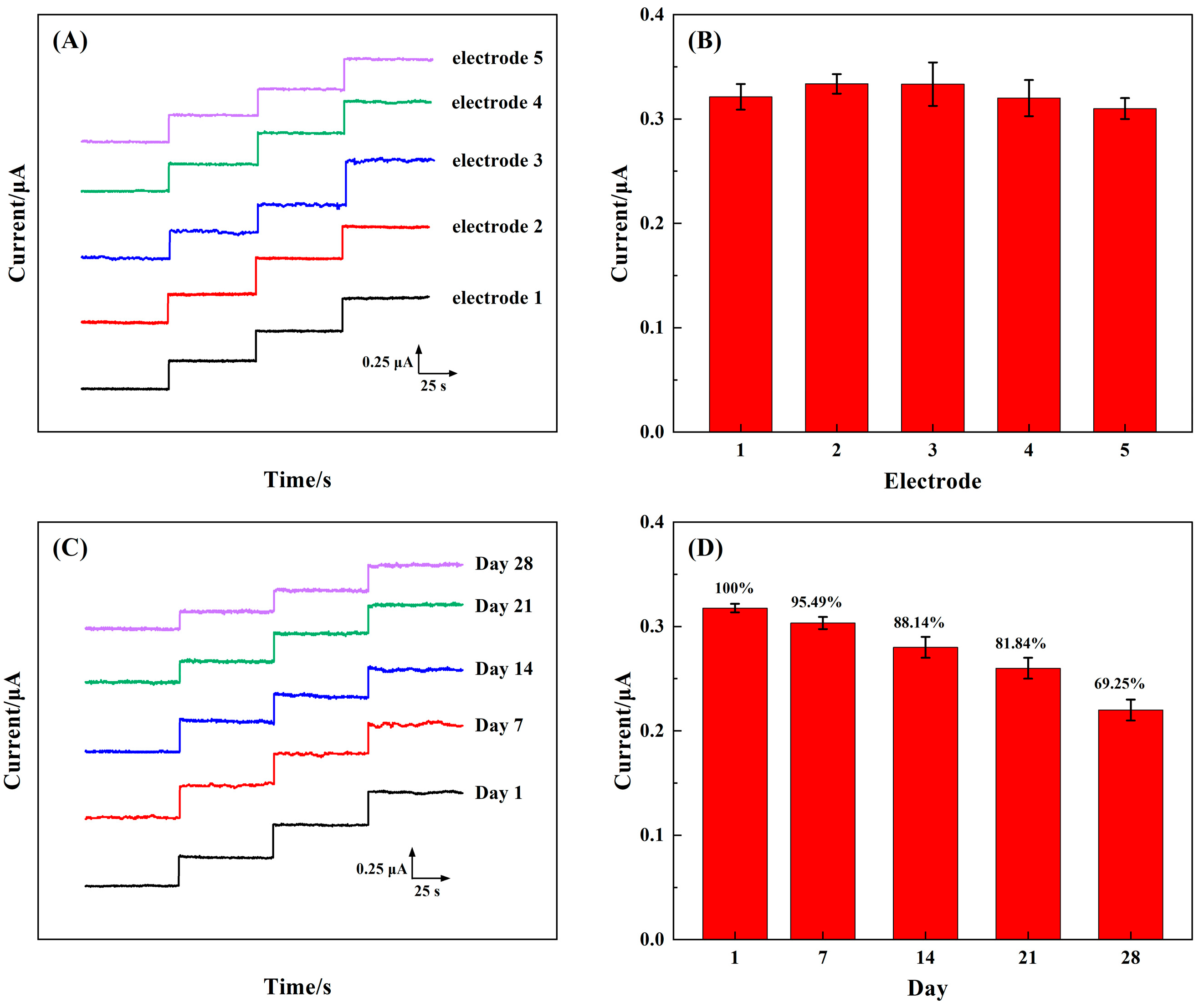
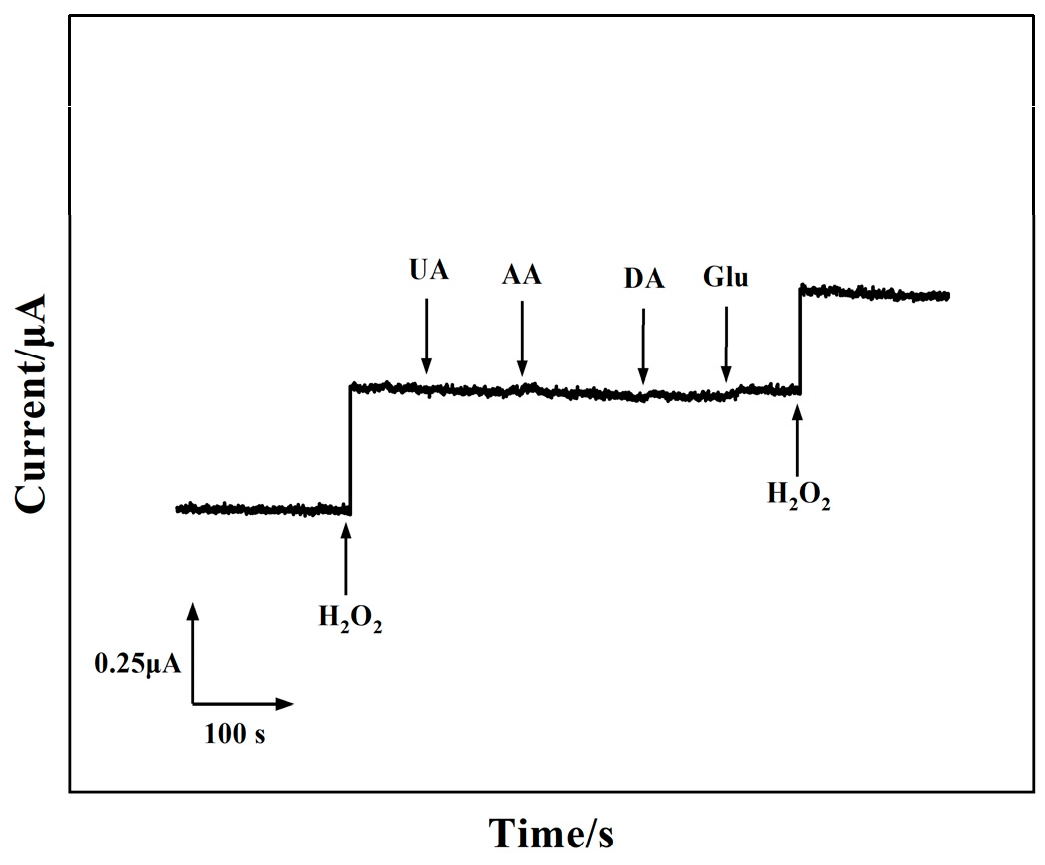
3.5. Repeatability, Stability, and Selectivity of the 3D rGO–Ti3C2–MWCNTs Electrode
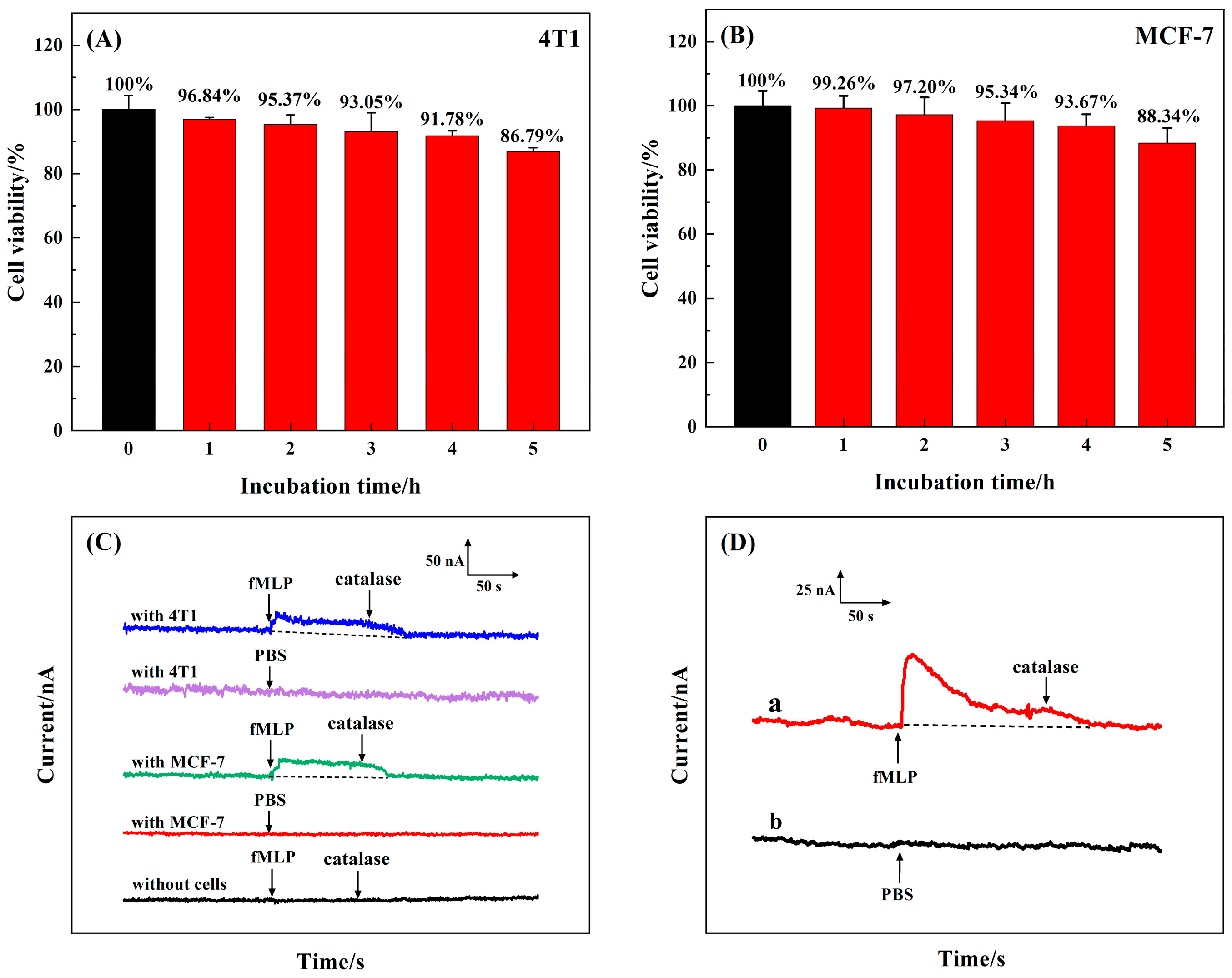
3.6. Ex Vivo Experimental Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.O.; Mirkin, C.A.; Walt, D.R.; Ismagilov, R.F.; Toner, M.; Sargent, E.H. Advancing the speed, sensitivity and accuracy of biomolecular detection using multi-length-scale engineering. Nat. Nanotechnol. 2014, 9, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Liu, Z.; Fang, X.; Chen, H.; Men, X.; Yuan, Y.; Sun, K.; Zhang, X.; Yuan, Z.; Wu, C. Enhanced Phototherapy by Nanoparticle-Enzyme via Generation and Photolysis of Hydrogen Peroxide. Nano Lett. 2017, 17, 4323. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, T.; Zhu, X.; Zu, S.; Xie, Z.; Lu, X.; Zhang, M.; Song, L.; Jin, Y. Metal–Organic Frameworks for Electrocatalytic Sensing of Hydrogen Peroxide. Molecules 2022, 27, 4571. [Google Scholar] [CrossRef]
- Cui, H.; Cui, S.; Zhang, S.; Tian, Q.; Liu, Y.; Zhang, P.; Wang, M.; Zhang, J.; Li, X. Cu-MOF/hemin: A bionic enzyme with excellent dispersity for the determination of hydrogen peroxide released from living cells. Analyst 2021, 146, 5951–5961. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Xie, J.; Zhou, D.; Pang, Z.L.; Liu, X.; Yin, M.L.; Zhang, P.; Li, Y.Y.; Liu, H.T.; Yao, X.Y.; Zhuo, S. Hydrogen peroxide sensing in body fluids and tumor cells via in situ produced redox couples on two-dimensional holey CuCo2O4 nanosheets. Mikrochim. Acta Int. J. Phys. Chem. Methods Anal. 2020, 187, 469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, J.; Sun, Y.; Wang, L.; Dong, X.; Ren, J.; He, W.; Xiao, F. Flexible nanohybrid microelectrode based on carbon fiber wrapped by gold nanoparticles decorated nitrogen doped carbon nanotube arrays: In situ electrochemical detection in live cancer cells-ScienceDirect. Biosens. Bioelectron. 2018, 100, 453–461. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, C.; Liu, H.W.; Xiong, M.; Yin, S.-Y.; Yang, Y.; Hu, X.-X.; Yin, X.; Zhang, X.-B.; Tan, W. Supramolecular assembly affording a ratiometric two-photon fluorescent nanoprobe for quantitative detection and bioimaging. Chem. Sci. 2017, 8, 8214–8220. [Google Scholar] [CrossRef]
- Su, Y.Y.; Song, H.J.; Yi, L. Recent advances in chemiluminescence for reactive oxygen species sensing and imaging analysis. Microchem. J. Devoted Appl. Microtech. All Branches Sci. 2019, 146, 83–97. [Google Scholar] [CrossRef]
- Tsukada, Y.; Yasutake, M.; Jia, D.; Kusama, Y.; Kishida, H.; Takano, T.; Tsukada, S. Real-time measurement of nitric oxide by luminol-hydrogen peroxide reaction in crystalloid perfused rat heart. Life Sci. 2003, 72, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.B.; Brahim, M.B.; Abdelhedi, R.; Samet, Y. Boron doped diamond sensor for sensitive determination of metronidazole: Mechanistic and analytical study by cyclic voltammetry and square wave voltammetry. Mater. Sci. Eng. C 2016, 59, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Hu, Y.; Wang, P.; Liu, P.; Chen, Z.; Sun, D. Electrochemical biosensor based on gold nanoflowers-encapsulated magnetic metal-organic framework nanozymes for drug evaluation with in-situ monitoring of H2O2 released from H9C2 cardiac cells-ScienceDirect. Sens. Actuators B Chem. 2020, 311, 127909. [Google Scholar] [CrossRef]
- Annalakshmi, T.W. Enzyme-free electrocatalytic sensing of hydrogen peroxide using a glassy carbon electrode modified with cobalt nanoparticle-decorated tungsten carbide. Mikrochim. Acta Int. J. Phys. Chem. Methods Anal. 2019, 186, 265. [Google Scholar] [CrossRef]
- Huang, W.; Xu, Y.; Sun, Y. Functionalized Graphene Fiber Modified With MOF-Derived Rime-Like Hierarchical Nanozyme for Electrochemical Biosensing of H. Front. Chem. 2022, 10, 873187. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Wu, Q.; Shan, Z.; Huang, Q.M. BSA-stabilized Au clusters as peroxidase mimetics for use in xanthine detection. Biosens. Bioelectron. 2011, 26, 3614–3619. [Google Scholar] [CrossRef]
- Chen, T.W.; Palanisamy, S.; Chen, S.M. Non-enzymatic sensing of hydrogen peroxide using a glassy carbon electrode modified with a composite consisting of chitosan-encapsulated graphite and platinum nanoparticles. Microchim. Acta 2016, 184, 4587–4595. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene Oxide: Intrinsic Peroxidase Catalytic Activity and Its Application to Glucose Detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Du, X.; Chen, Y.; Dong, W.; Han, B.; Chen, Q. Facile fabrication of Pt-Ag bimetallic nanoparticles decorated reduced graphene oxide for highly sensitive non-enzymatic hydrogen peroxide sensing. Talanta 2016, 159, 280–286. [Google Scholar] [CrossRef]
- George, J.M.; Antony, A.; Mathew, B. Metal oxide nanoparticles in electrochemical sensing and biosensing: A review. Mikrochim. Acta Int. J. Phys. Chem. Methods Anal. 2018, 185, 358. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.E.; Lee, C.-S.; Kim, T.W.; Jeon, S.; Kim, T.H. A Flexible and Transparent PtNP/SWCNT/PET Electrochemical Sensor for Nonenzymatic Detection of Hydrogen Peroxide Released from Living Cells with Real-Time Monitoring Capability. Biosensors 2023, 13, 704. [Google Scholar] [CrossRef]
- Prez, R.J.; Iniesta, J.; Baeza-Romero, M.T.; Valero, E. On the performance of carbon-based screen-printed electrodes for (in) organic hydroperoxides sensing in rainwater. Talanta 2021, 839, 75–82. [Google Scholar]
- Zheng, J.; Diao, J.; Jin, Y.; Ding, A.; Chen, J. An Inkjet Printed Ti3C2-GO Electrode for the Electrochemical Sensing of Hydrogen Peroxide. J. Electrochem. Soc. 2018, 165, B227–B231. [Google Scholar] [CrossRef]
- Tu, S.; Jiang, Q.; Zhang, X.; Alshareef, H.N. Large Dielectric Constant Enhancement in MXene Percolative Polymer Composites. Acs Nano 2018, 12, 3369–3377. [Google Scholar] [CrossRef]
- Shi, S.; Zhong, R.; Li, L.; Wan, C.; Wu, C. Ultrasound-assisted synthesis of graphene@MXene hybrid: A novel and promising material for electrochemical sensing. Ultrason. Sonochem. 2022, 90, 106208. [Google Scholar] [CrossRef]
- Wang, T.; Wang, C.; Xu, X.; Li, Z.; Li, D. One-Step Electrodeposition Synthesized Aunps/Mxene/ERGO for Selectivity Nitrite Sensing. Nanomaterials 2021, 11, 1892. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, H.B.; Luo, J.Q.; Wang, Q.W.; Xu, B.; Hong, S.; Yu, Z.Z. Highly Electrically Conductive Three-Dimensional Ti3C2Tx MXene/Reduced Graphene Oxide Hybrid Aerogels with Excellent Electromagnetic Interference Shielding Performances. ACS Nano 2018, 12, 11193–11202. [Google Scholar] [CrossRef] [PubMed]
- Jerome, R.; Sundramoorthy, A.K. Preparation of hexagonal boron nitride doped graphene film modified sensor for selective electrochemical detection of nicotine in tobacco sample. Anal. Chim. Acta 2020, 1132, 110–120. [Google Scholar] [CrossRef]
- Jin, L.; Wu, C.; Wei, K.; He, L.; Gao, H.; Zhang, H.; Zhang, K.; Asiri, A.M.; Alamry, K.A.; Yang, L.; et al. Polymeric Ti3C2Tx MXene Composites for Room Temperature Ammonia Sensing. ACS Appl. Nano Mater. 2020, 3, 12071–12079. [Google Scholar] [CrossRef]
- Rajendran, J.; Kannan, T.S.; Dhanasekaran, L.S.; Murugan, P.; Atchudan, R.; ALOthman, Z.A.; Ouladsmane, M.; Sundramoorthy, A.K. Preparation of 2D Graphene/MXene nanocomposite for the electrochemical determination of hazardous bisphenol A in plastic products. Chemosphere 2022, 287, 132106. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.J.; Yu, S.Q.; Shang, X.W.; Wei, X.-Y.; Wang, H.-Y.; Jiang, W.-S.; Ren, Q.-Q. A non-invasive glucose sensor based on 3D reduced graphene oxide-MXene and AuNPs composite electrode for the detection of saliva glucose. J. Appl. Electrochem. 2024, 174, 107061. [Google Scholar] [CrossRef]
- Zhao, P.; Jia, Y.; Liang, Y.; Zheng, J.; Yang, M.; Huo, D.; Wang, Y.; Hou, C. A Prussian blue-doped RGO/MXene composite aerogel with peroxidase-like activity for real-time monitoring of H2O2 secretion from living cells. Chem. Commun. 2021, 57, 9870–9873. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, C.; Peng, W.; Cao, X.; Gao, H.; Jiang, M.; Wu, Z.; Yu, C. Stretchable Electrochemical Sensor Based on a Gold Nanowire and Carbon Nanotube Network for Real-Time Tracking Cell-Released H2S. Anal. Chem. 2023, 9, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.Q.; Wu, J.; Zhang, W.C.; Wang, C.; Qin, X.; Liu, G.-C.; Li, Z.-X.; Yu, Y. Real-time in vitro detection of cellular H2O2 under camptothecin stress using horseradish peroxidase, ionic liquid, and carbon nanotube-modified carbon fiber ultramicroelectrode. Sens. Actuators B Chem. 2017, 245, 615–621. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, R.; Jia, J.; Wu, C.; Zhou, A.; Xu, J.; Zhang, X. Enhanced supercapacitive performance of delaminated two-dimensional titanium carbide/carbon nanotube composites in alkaline electrolyte. J. Power Sources 2015, 284, 38–43. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, M.; Zhou, H.; Du, X.; Du, X. Assessment of Salt Stress to Arabidopsis Based on the Detection of Hydrogen Peroxide Released by Leaves Using an Electrochemical Sensor. Int. J. Mol. Sci. 2022, 23, 12502. [Google Scholar] [CrossRef] [PubMed]
- Comşa, Ş.; Cîmpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anticancer. Res. Int. J. Cancer Res. Treat. 2015, 35, 3147–3154. [Google Scholar]
- Tao, K.; Fang, M.; Alroy, J.; Sahagian, G.G. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer 2008, 8, 228. [Google Scholar] [CrossRef]
- Chmielecki, A.; Bortnik, K.; Galczynski, S.; Padula, G.; Jerczynska, H.; Stawski, R.; Nowak, D. Exhaustive Exercise Increases Spontaneous but Not fMLP-Induced Production of Reactive Oxygen Species by Circulating Phagocytes in Amateur Sportsmen. Biology 2022, 11, 103. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, L.; Li, H.; Huang, J.; Li, Z.; Hong, W.; Wang, J.; Bai, Z.; Zhu, J. Biosensor based on bimetallic/graphene composite for non-enzymatic detection of hydrogen peroxide in living tumor cells. Biotechnol. Appl. Biochem. 2023, 70, 1024–1034. [Google Scholar] [CrossRef]
- Jing, A.; Liang, G.; Shi, H.; Yuan, Y.; Zhan, Q.; Feng, W. Three-Dimensional Holey-Graphene Architectures for Highly Sensitive Enzymatic Electrochemical Determination of Hydrogen Peroxide. J. Nanosci. Nanotechnol. 2019, 19, 7404–7409. [Google Scholar] [CrossRef]
- Alam, A.U.; Deen, M.J. Bisphenol. A Electrochemical Sensor Using Graphene Oxide and β-Cyclodextrin-Functionalized Multi-Walled Carbon Nanotubes. Anal. Chem. 2020, 92, 5532–5539. [Google Scholar] [CrossRef] [PubMed]
- Vasuki, K.; Babu, K.J.; Sheet, S.; Siva, G.; Kim, A.R.; Yoo, D.J.; Kumar, G.G. Amperometric hydrogen peroxide sensor based on the use of cofe2o4 hollow nanostructures. Microchimica Acta. 2017, 184, 2579–2586. [Google Scholar] [CrossRef]
- Amala, G.; Saravanan, J.; Yoo, D.J.; Kim, A.R.; Gnana Kumar, G. Environmentally benign one pot green synthesis of reduced graphene oxide based composites for the enzyme free electrochemical detection of hydrogen peroxide. New Journal of Chemistry. 2017, 41, 4022–4030. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Li, X.; Du, X. An electrochemical sensor based on chalcogenide molybdenum disulfide-gold-silver nanocomposite for detection of hydrogen peroxide released by cancer cells. Sensors. 2020, 20(23), 6817. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Li, T.; Ma, J.; Li, K.; Zuo, X. Silver nanoparticles selectively deposited on graphene-colloidal carbon sphere composites and their application for hydrogen peroxide sensing. Sensors: Actuators B Chemical. 2017, 239, 1205–1212. [Google Scholar] [CrossRef]
- Promsuwan, K.; Soleh, A.; Saisahas, K.; Saichanapan, J.; Thiangchanya, A.; Phonchai, A.; Limbut, W. Micro-colloidal catalyst of palladium nanoparticles on polyaniline-coated carbon microspheres for a non-enzymatic hydrogen peroxide sensor. Microchem. J. 2021, 171, 106785. [Google Scholar] [CrossRef]
- Shu, Y.; Chen, J.; Xu, Q.; Wei, Z.; Liu, F.; Lu, R.; Xu, S.; Hu, X. MoS2 nanosheet-Au nanorod hybrids for highly sensitive amperometric detection of H2O2 in living cells. Mater. Chem. B 2017, 5, 1446–1453. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Duan, Y.L.; Shao, Z.Y.; Chen, C.; Yang, M.; Lu, G.D.; Xu, W.F.; Liao, X.L. Amperometric hydrogen peroxide sensor using a glassy carbon electrode modified with a nanocomposite prepared from ferumoxytol and reduced graphene oxide decorated with platinum nanoparticles. Mikrochim. Acta Int. J. Phys. Chem. Methods Anal. 2019, 186, 386. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, X.; Yang, X.; Zhao, C.; Zhang, Y.; Qu, S.; Wu, S.; Ji, W. Reasonable design of an MXene-based enzyme-free amperometric sensing interface for highly sensitive hydrogen peroxide detection. Anal. Methods Adv. Methods Appl. 2021, 13, 2512–2518. [Google Scholar] [CrossRef]
- Lian, M.; Chen, X.; Lu, Y.; Yang, W. Self-Assembled Peptide Hydrogel as a Smart Biointerface for Enzyme-Based Electrochemical Biosensing and Cell Monitoring. Am. Chem. Soc. 2016, 8, 25036–25042. [Google Scholar] [CrossRef]
- Boobphahom, S.; Siripongpreda, T.; Zhang, D.; Qin, J.; Rattanawaleedirojn, P.; Rodthongkum, N. TiO2/MXene-PVA/GO hydrogel-based electrochemical sensor for neurological disorder screening via urinary norepinephrine detection. Mikrochim. Acta 2021, 188, 387. [Google Scholar] [CrossRef]
- Huang, T.Y.; Huang, J.H.; Wei, H.Y.; Ho, K.C.; Chu, C.W. rGO/SWCNT composites as novel electrode materials for electrochemical biosensing. Biosens. Bioelectron. 2013, 43, 173–179. [Google Scholar] [CrossRef]
- Li, Z.; Xin, Y.; Wu, W.; Fu, B.; Zhang, Z. Topotactic Conversion of Copper(I) Phosphide Nanowires for Sensitive Electrochemical Detection of H2O2 Release from Living Cells. Anal Chem. 2016, 88, 7724–7729. [Google Scholar] [CrossRef]
- Chen, S.; Shi, M.; Xu, Q.; Xu, J.; Duan, X.; Gao, Y.; Lu, L.; Gao, F.; Wang, X.; Yu, Y. Ti(3)C(2)T(x)MXene/nitrogen-doped reduced graphene oxide composite: A high-performance electrochemical sensing platform for adrenaline detection. Nanotechnology 2021, 32, 26. [Google Scholar]
- Wang, L.; Xiao, F.; Xiong, Q.; Duan, H. 2D nanomaterials based electrochemical biosensors for cancer diagnosis. Biosens. Bioelectron. 2017, 89, 136–151. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, J.-X.; Chen, D.-N.; He, J.-W.; Wang, A.-J.; Feng, J.-J. Ultrasensitive label-free electrochemical immunosensor of NT-proBNP biomarker based on branched AuPd nanocrystals/N-doped honeycombed porous carbon. Bioelectrochemistry 2022, 148, 108225. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhang, S.; Zhuang, X.; Wang, H.; Chen, D.; Luan, F.; He, T.; Qiu, Y. Preparation of gold nanoparticles supported on graphene oxide with flagella as the template for nonenzymatic hydrogen peroxide sensing. Anal. Bioanal. Chem. 2018, 410, 5915–5921. [Google Scholar] [CrossRef] [PubMed]
| Electrode | Method | Linear Range (mM) | LOD (µM) | Sensitivity (µA mM−1 cm−2) | Refs. |
|---|---|---|---|---|---|
| CoFe2O4 HS/GCE | Amperometry | 0.01–1.2 | 2.5 | 715 | [44] |
| rGO/Ag/PdNPs/GCE | Amperometry | 0.005–14.65 | 1.1 | 342 | [45] |
| PtPd/MWCNT/GCE | Amperometry | 0.0025–0.125 | 1.2 | 414.8 | [22] |
| MoS2–Au–Ag/GCE | Amperometry | 0.05–20 | 7.19 | 405.2 | [46] |
| Nafion/Gr–CCS–AgNPs/GCE | Amperometry | 0.02–5.02 | 2.49 | - | [47] |
| Pd-PANi/CMs | Amperometry | 0.002–10 | 0.7 | 234 | [48] |
| Catalase/MoS2–Au/chitosan/GCE | Amperometry | 0.0005–0.2 | 0.1 | 187.4 | [49] |
| Fer/rGO–Pt | Amperometry | 0.0004–0.01 0.0075–4.27 4.89–10.77 | 0.228 | 340 | [50] |
| MX/CS/PB/GCE | Amperometry | 0.0005–0.667 | 0.004 | - | [51] |
| rGO–Ti3C2–MWCNTs | Amperometry | 0.001–9.77 | 0.3 | 235.2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.-Q.; Li, P.; Li, H.-J.; Shang, L.-J.; Guo, R.; Sun, X.-M.; Ren, Q.-Q. Highly Sensitive Detection of Hydrogen Peroxide in Cancer Tissue Based on 3D Reduced Graphene Oxide–MXene–Multi-Walled Carbon Nanotubes Electrode. Biosensors 2024, 14, 261. https://doi.org/10.3390/bios14060261
Yu S-Q, Li P, Li H-J, Shang L-J, Guo R, Sun X-M, Ren Q-Q. Highly Sensitive Detection of Hydrogen Peroxide in Cancer Tissue Based on 3D Reduced Graphene Oxide–MXene–Multi-Walled Carbon Nanotubes Electrode. Biosensors. 2024; 14(6):261. https://doi.org/10.3390/bios14060261
Chicago/Turabian StyleYu, Shuai-Qun, Pan Li, Hao-Jie Li, Ling-Jun Shang, Rui Guo, Xu-Ming Sun, and Qiong-Qiong Ren. 2024. "Highly Sensitive Detection of Hydrogen Peroxide in Cancer Tissue Based on 3D Reduced Graphene Oxide–MXene–Multi-Walled Carbon Nanotubes Electrode" Biosensors 14, no. 6: 261. https://doi.org/10.3390/bios14060261
APA StyleYu, S.-Q., Li, P., Li, H.-J., Shang, L.-J., Guo, R., Sun, X.-M., & Ren, Q.-Q. (2024). Highly Sensitive Detection of Hydrogen Peroxide in Cancer Tissue Based on 3D Reduced Graphene Oxide–MXene–Multi-Walled Carbon Nanotubes Electrode. Biosensors, 14(6), 261. https://doi.org/10.3390/bios14060261





